Abstract
Sarcopenia and cachexia are muscle wasting syndromes associated with aging and with many chronic diseases such as congestive heart failure (CHF), diabetes, cancer, chronic obstructive pulmonary disease and chronic kidney disease (CKD). While mechanisms are complex these conditions are often accompanied by elevated angiotensin II (Ang II). Patients with advanced CHF or CKD often have increased Ang II levels and cachexia, and angiotensin-converting enzyme (ACE) inhibitor treatment improves weight loss. We found that Ang II infusion in rodents leads to skeletal muscle wasting. Ang II increases cytokines and circulating hormones such as tumor necrosis factor-α, interleukin-6, serum amyloid-A and glucocorticoids, which regulate muscle protein synthesis and degradation. Ang II-induced muscle wasting is caused by alterations in insulin-like growth factor-1 signaling, enhanced muscle protein breakdown via the ubiquitin-proteasome system, and decreased appetite resulting from downregulation of hypothalamic orexigenic neuropeptides such as Npy and orexin. Ang II also inhibits 5′ AMP-activated protein kinase (AMPK) activity and disrupts normal energy balance via activation of AMPK phosphatase PP2Cα. Furthermore, Ang II inhibits skeletal muscle stem (satellite) cell proliferation, leading to lowered muscle regenerative capacity. Distinct satellite cell angiotensin receptor subtypes have different effects on different stages of differentiation and are critical for regulation of muscle regeneration. These data suggest that the renin-angiotensin system (RAS) plays a critical role in mechanisms underlying cachexia in chronic disease states, and is a promising target for the treatment of muscle atrophy in patients with diseases such as CHF and CKD.
Introduction
Patients with cachexia, or wasting syndrome, develop weight loss, muscle atrophy, fatigue, weakness, and often loss of appetite without actively trying to lose weight. Cachexia patients are defined as those that lose more than 5% of body weight over 12 months or less in the presence of a chronic disease such as congestive heart failure (CHF), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD) and cancer. 10–30% of the patients with these diseases develop cachexia, and it affects more than 5 million people in the United States 1. Cachexia is a multifactorial disease and, importantly, nutritional support cannot fully reverse the syndrome. In cachexia conditions, the degradation of myofibrillar proteins is increased and protein synthesis is decreased, leading to the rapid loss of muscle mass. Weight loss and reduced muscle mass are associated with a reduction in quality of life and increased mortality. Thus cachexia is a major public health issue, and the development of interventions to block or attenuate this process would have significant therapeutic benefits in a wide array of chronic diseases.
Mechanisms and potential therapies for cachexia
Among the candidate mediators of cachexia that have been investigated, proinflammatory cytokine tumor necrosis factor-α (TNF-α) is the most prominent and well characterized factor. TNF-α has been shown to induce cachexia in mice,2 and to cause myotube atrophy in vitro via activation of E3 ubiquitin ligases.3 Although many rodent tumor models of cancer cachexia showed increased TNF-α,4 the relevance of TNF-α to human cancer cachexia is unclear. Maltoni et al found that circulating levels of TNF-α in cancer cachexia patients had no correlation with weight loss and anorexia.5 Furthermore, a clinical trial designed to block TNF-α signaling using anti-TNF-α antibody (infliximab) in cancer cachexia patients closed early because the treatment prevent or palliate cancer-associated weight loss, and patients developed greater fatigue and worse global quality of life scores.6
Another candidate mediator of cachexia is interleukin-6 (IL-6). It has been shown that different kinds of cancer cells secrete IL-6 and that circulating levels of IL-6 correlate with weight loss in cancer patients in some, 7,8 but not all,5 studies. Strassmann et al showed that increasing levels of IL-6 in tumor bearing mice correlated with the development of cachexia, and that an antibody against IL-6, but not against TNF-α, suppressed cachexia development.9 However, a clinical trial of IL-6 antibody in weight-losing lung cancer patients did not have significant effect on loss of lean body mass, although anorexia, fatigue and anemia were prevented.10
Myostatin and Activin A are the most recent and promising target molecules related to cancer cachexia. Myostatin and Activin A are members of the transforming growth factor-β (TGF-β) family, and are both upregulated in patients with various kinds of wasting diseases.11 Animals and humans with null mutations of myostatin show dramatic muscle hypertrophy12,13 and blockade of Activin-A restored regenerative capacity of human myoblasts in the presence of high cytokines (TNF-α or IL-1β)14. Myostatin and Activin-A signal through the common receptor, Activin type II receptor B (ActRIIB). To inhibit both myostatin and Activin A signaling at the same time, soluble ActRIIB-Fc decoy protein (sActRIIB) was developed. Treatment of tumor-bearing mice with sActRIIB prevented cachexia development without affecting the tumor growth, and prolonged survival.15 Thus blockade of ActRIIB signaling seems to be a very promising treatment of cancer cachexia and clinical trials are ongoing to treat patients with sarcopenia and cachexia.16
Cachectic patients with CHF showed increased growth hormone (GH) levels with lower insulin-like growth factor-1 (IGF-1), suggesting GH resistance.17 Also, it has been shown that CHF patients had higher glucose and insulin levels, an indication of insulin resistance.18 Since CHF patients have higher Ang II levels and Ang II causes insulin resistance in skeletal muscle by inhibiting insulin-stimulated GLUT4 translocation,19,20 it was postulated that blockade of Ang II could benefit skeletal muscle function in chronic diseases with high levels of Ang II. There are 2 main pharmacological approaches to target the effects of Ang II: inhibiting the formation of Ang II by angiotensin converting enzyme inhibitor (ACEi) and blocking the AT1R by angiotensin receptor blocker (ARB). Because alternative ACE-independent pathways of Ang II formation exist, the use of ARB could be more specific in targeting Ang II-mediated muscle wasting. The ACEi enalapril was shown to reduce the risk of weight loss in CHF patients 21 and ACEi helped maintain body weight but not muscle strength in patients with CHF or hypertension.22 Elderly patients without heart failure on antihypertensive treatment with ACEi had higher muscle mass than patients receiving other anti-hypertensive therapy.23 In addition, insulin sensitivity was improved by losartan and lisinopril in hypertensive patients.24 There have been a few clinical trials designed to protect muscle wasting by blocking Ang II. Ark Therapeutics has completed a phase III clinical trial treating cancer cachexia patients with the ACE inhibitor imidapril. Imidapril prevented weight loss in non-small cell lung cancer and colorectal cancer, but not in pancreatic cancer, but its effect to prevent weight loss did not reach statistical significance when the data were combined. The company discontinued development of the product after the failed clinical trial, although it remains convinced of the value of this approach.25,26 Blockade of AT1R signaling could be another approach to prevent weight loss in cachexia patients, but Merck & Co. Inc., which marketed losartan as Cozaar, has no plans to develop it for new indications in the U.S., since it went off patent.26
One of the potential reasons why clinical trials of Ang II blockade have so far been unsuccessful is that the underlying mechanisms of renin-angiotensin system-mediated muscle wasting is not fully understood. For instance, Ang II and other angiotensins act on different subtypes of receptors such as AT1R, AT2R, Mas and IRAP.27 Because ACEi blocks conversion of Ang I to Ang II, ACEi treatment results in a decrease of Ang II, whereas Ang I level is inceased. On the other hand, blockade of AT1R by ARB results in a compensatory increase of Ang II, which may activate AT2R-mediated signaling. Thus, it is critical to understand the multiple signaling pathways that mediate the effect of the RAS. Current progress in understanding the role of the RAS in muscle wasting is summarized and discussed below.
Ang II and muscle wasting
It was first demonstrated that Ang II infusion in the rat caused a significant loss of body weight through 2 independent mechanisms, a reduction of food intake and increased proteolysis in skeletal muscle.28 Both of these effects were completely prevented by losartan, but not by the vasodilator hydralazine, showing that Ang II causes wasting through the AT1R and that its effect is independent of blood pressure regulation. It was found that Ang II caused an increase of muscle protein breakdown via the ubiquitin-proteasome system (UPS). Accelerated proteolysis via the UPS plays a major role in muscle atrophy in several different types of cachexia.29 The muscle specific E3 ubiquitin ligases atrogin-1 and muscle RING finger-1 (MuRF-1) were identified as genes strongly upregulated in different muscle atrophy conditions, and knockout mice for either of these genes partially prevented muscle wasting.30 In Ang II-induced muscle wasting, expression of atrogin-1 and MuRF-1, levels of ubiquitin-conjugated proteins and 20S proteasome activity were robustly increased.31–33
In the Ang II-induced wasting condition, it was also found a decrease of skeletal muscle IGF-1 signaling which is the main anabolic pathway in skeletal muscle.28,34 IGF-1 modulates muscle size via autocrine and paracrine signals, by directly stimulating protein anabolism in myofibers and by activation of satellite cell proliferation.35 IGF-1 signals through PI3K/Akt and induces muscle hypertrophy by stimulating GSK and mTOR kinases, which regulate protein translation.36 Multiple studies have shown the involvement of IGF-1/PI3K/Akt signaling in muscle cell size regulation and atrophy. For instance, inhibition of PI3K and expression of dominant-negative Akt reduce the size of myotubes in vitro,37 in mice deficient for Akt1 and Akt2 have smaller muscle size 38 and activation of Akt in rat muscle prevents denervation-induced atrophy.36,39 The authors utilized a transgenic mouse strain in which IGF-1 is overexpressed under the control of a skeletal muscle specific promoter 40 and showed that the local increase of IGF-1 could prevent Ang II-induced muscle wasting.31 Interestingly, although Ang II rapidly increased both atrogin-1 and MuRF-1 expression, IGF-1 prevented only the increase in atrogin-1.32 These data may be consistent with the current evidence suggesting the distinct roles of atrogin-1 and MuRF-1 in muscle wasting.41 Myofibrillar proteins have been identified as the target of MuRF-142,43 and it is suggested that MuRF-1 is likely involved in skeletal muscle proteolysis. On the other hand, atrogin-1 has been shown to target MyoD, the regulator of myogenesis, and eIF-e, the eukaryotic initiation factor of protein synthesis, suggesting that its main role is the regulation of protein synthesis.44,45 Although precise signaling pathways whereby Ang II and IGF-1 regulate Atrogin-1 and MuRF-1 remain to be elucidated, it is of note that atrogin-1 and MuRF-1 are regulated by distinct mechanisms.46,47 In summary, Ang II and IGF-1 have opposing roles in regulating muscle protein synthesis and degradation. Disruption of IGF-1 signaling by Ang II plays a critical role in Ang II-induced atrophy, and local activation of IGF-1 signaling can prevent Ang II-induced muscle wasting.
There have been studies reporting that the effect of Ang II to cause muscle wasting is via the direct action of Ang II on skeletal muscle cells and is indirectly mediated by other circulating factors. It has been shown that Ang II directly acts on cultured muscle cells and induces proteolysis via the UPS pathway.48,49 On the other hand, it has been demonstrated that multiple circulating hormones and cytokines mediate Ang II’s action on skeletal muscle. Glucocorticoids are required for activation of the UPS in acidosis and diabetes, and glucocorticoid inhibition restored Ang II-induced loss of muscle mass.31 After Ang II infusion, there is an increase of circulating IL-6 and serum amyloid-A (SAA), and blockade of IL-6/SAA prevented Ang II-induced wasting.50 These studies suggest that the catabolic effect of Ang II on skeletal muscle in vivo is, at least in part, mediated via intermediate molecules activated by Ang II.
Ang II and oxidative stress
Reactive oxygen species (ROS) play an important role in Ang II-induced signaling in different cell types, contributing to cardiac myocyte and vascular smooth muscle cell hypertrophy, endothelial dysfunction, hypertension, and insulin resistance.51,52 Ang II has been shown to induce ROS generation in skeletal muscle, 53,54 and ROS contributes to disuse muscle atrophy.55 NAPDH oxidase and mitochondria are major sources of ROS in atrophied skeletal muscles.56,57 Oxidative stress induces proteolysis and atrophy via several different mechanisms: (1) calcium overload and activation of calcium-activated proteases such as calpain; (2) stimulation of the 20S proteasome system via activation of caspase-3; (3) activation of E3 ubiquitin ligases atrogin-1 and MuRF-1. Ang II-induced proteolysis is prevented by antioxidants in myotubes 49, and genetic or pharmacological inhibition of NADPH oxidase blocked Ang II-induced 20S proteasome activity and muscle wasting.33 Ang II also increases mitochondrial ROS formation58,59 and it has been speculated that NADPH oxidase-induced ROS could directly stimulate the mitochondria.59 However, the mitochondrial-targeted antioxidant Mito-TEMPO failed to prevent Ang II-induced muscle wasting, suggesting that mitochondrial ROS are not directly involved.60 These data suggest that specific targeting of ROS and NADPH oxidase could be a beneficial, novel therapy to treat Ang II-induced wasting.
Ang II and energy balance
It has been proposed that a common set of molecular mechanisms underlie muscle wasting in different chronic diseases. DNA microarray analysis in different atrophying conditions revealed that the group of genes required for ATP production and late steps in glycolysis is commonly downregulated.61 These changes would suppress muscle’s capacity to utilize glucose and reduce muscle energy production. Reduced glucose utilization has been observed in the setting of cancer and renal failure, and thus disruption of metabolic homeostasis could be one of the mechanisms involved in development of cachexia.
Yoshida and Delafontaine analyzed metabolic changes in the Ang II-induced wasting condition and found that Ang II depletes skeletal muscle ATP content and causes muscle wasting likely via induction of mitochondrial dysfunction.60 This reduction of ATP is caused by decreased activity of AMPK, a cellular sensor of energy status. When the cellular energy status is low (high AMP:ATP ratio) AMPK activates ATP synthesis, and data indicate that Ang II causes muscle wasting in part by preventing skeletal muscle homeostatic capacity to maintain energy balance. The AMPK activator 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) reversed Ang II-induced inhibition of AMPK, leading to restoration of ATP levels and inhibition of the Ang II-induced muscle wasting. AICAR also blocked Ang II-induced E3 ubiquitin ligases atrogin-1 and MuRF-1 expression. Contrary to these authors’ findings, in S6 kinase-1 deficient mice there was increased AMP levels, AMPK upregulation and muscle atrophy. 62 In these mice, AMPK inhibition restores muscle cell growth and sensitivity to nutrient signals. Also, it has been reported that AMPK-mediated phosphorylation of FoxO activates E3 ubiquitin ligase expression in muscle cell culture in vitro.63 However, in Ang II infused animals the net effect of AMPK activation by AICAR is Akt activation and inhibitory phosphorylation of FoxO1, which could explain the ability of AICAR to abrogate Ang II-mediated E3 ubiquitin ligase induction. Importantly, the authors found that the Ang II-induced reduction of AMPK activity is mediated by upregulation of the upstream phosphatase PP2Cα, and PP2Cα knockdown restored mitochondrial function and muscle wasting in Ang II infused animals.64 Although the precise mechanism whereby Ang II inhibits AMPK via upregulation of PP2Cα remains to be elucidated, these data suggest a therapeutic potential of targeting PP2Cα in chronic wasting conditions with increased Ang II levels.
Ang II reduces appetite
In 2008, Evans et al65 proposed the diagnosis of cachexia to be based on at least 5% weight loss in 12 months or less in the presence of underlying illness, plus 3 of the 5 following criteria: 1) Decreased muscle strength, 2) Fatigue, 3) Anorexia, 4) Low-fat free mass index and 5) Abnormal biochemistry (increased inflammatory markers (CRP, IL-6), anemia and low serum albumin). As can be seen in this definition, anorexia is frequently, if not always, associated with wasting, and anorexia and loss of body fat is a powerful predictor of mortality in cancer cachexia patients.66 Food intake is regulated by complex mechanisms that involve the actions of hypothalamic orexigenic/anorexigenic neuropeptides and circulating factors secreted from peripheral organs (e.g. adipose tissues and gastrointestinal tract). These authors found that Ang II causes wasting through 2 different mechanisms: increased protein catabolism in skeletal muscle and loss of food intake.28 Pair-feeding experiments were performed, in which a group of animals received an identical amount of food as Ang II-infused animals, and found that approximately 80% loss of body weight in Ang II-infused animals is due to reduced food intake. Consistent with these data, AT1R deficient mice are hyperphagic and obese. Furthermore, multiple studies have shown that intracerebroventricular (icv) infusion of Ang II caused reduced food intake and changes in orexigenic/anorexigenic neuropeptides such as agouti-related protein (AgRP), proopiomelanocortin (POMC), thyrotropin-releasing hormone (TRH), CRH, neuropeptide-Y (Npy) and orexin,67–70 suggesting that systemically increased Ang II in chronic diseases could directly act on hypothalamic neurons to regulate food intake by modulating orexigenic/anorexigenic neuropeptide expressions. Indeed, it has been shown that the AT1R is expressed in multiple hypothalamic neurons, including the lateral hypothalamic area, paraventricular nucleus, retrochiasmatic area and perifornical nucleus.71
RAS and muscle regeneration
Skeletal muscle has a remarkable ability to maintain its homeostasis against injury or wasting by activating a well orchestrated regenerative response to repair damaged myofibers. Injury leads to activation and proliferation of mitotically quiescent mononuclear cells, satellite cells, which form myoblasts, terminally differentiate and fuse to form multinucleated myotubes 72. Muscle atrophy occurs in a variety of pathophysiological conditions, including disuse, denervation, starvation, sarcopenia and cachexia, but the response of satellite cells in these conditions is not well characterized. In cancer cachexia animal models, it has been suggested that there is less regeneration and possibly a reduction of satellite cell function.73–75 The most well characterized atrophy-associated regeneration condition is sarcopenia. It has been shown that aged satellite cells display reduced proliferative response and regenerative capacity 76,77. Satellite cell proliferation is regulated by Notch signaling and lowered Notch activity is responsible for the reduced proliferative capacity of aged satellite cells.76,78 In the aged skeletal muscle, there is an increase of TGF-β expression and activated p-Smad3 counteracts Notch and inhibits cell cycle progression, thus causing lower satellite cell proliferative capacity.79 Also, fibroblast growth factor-2 (FGF2) expression is increased in aged skeletal muscle and increased FGF2 disrupts satellite cell quiescence and self-renewing activity,77 which leads to lower satellite cell regenerative capacity. Importantly, impaired regeneration in aged mice is reversible by exposure to a young circulation,80 and growth differentiation factor-11 (GDF11) has recently been identified as a factor that maintains satellite cells in a “young” state.81 Although it is not clear whether the same mechanisms could lead to lower satellite cell function in chronic disease states, studies in aged muscle clearly indicate that systemic changes strongly affect satellite cell regenerative capacity. Therefore, identifying mechanisms whereby chronic diseases lead to lower satellite cell function would have the therapeutic potential to reverse the reduction in muscle regeneration seen in cachexia conditions.
Multiple studies have suggested a role of Ang II in regulating satellite cell function, and considering the potential involvement of Ang II in muscle wasting in many chronic diseases, 48,82–85 Ang II could be a systemic factor that affects satellite cell function in disease states. However, the consequence of an increase in Ang II on satellite cell function is controversial. Cohn et al86 first reported that the effect of AT1R blockade by losartan improved muscle regeneration in mouse models of myopathy through suppression of TGF-β. Consistent with this study, it is reported that losartan improved muscle regeneration and decreased fibrosis after laceration-induced injury.87 Burks et al showed that losartan treatment blocked TGF-β signaling and losartan-treated mice developed significantly less fibrosis and exhibited improved muscle function after cardiotoxin-induced injury. In addition, immobilized mice treated with losartan were protected against loss of muscle mass. Interestingly, however, this muscle wasting-protective effect of losartan was not mediated by TGF-β, but by increased IGF-1/Akt/mTOR signaling, suggesting AT1R signaling modulates different signaling cascades in regeneration and atrophy.88 On the other hand, ACE inhibition or genetic ablation of AT1R have been reported to impair skeletal muscle regeneration.89 Murphy et al also reported that muscle regenerative capacity was impaired in AT1R knockout mice, although they found muscle strength and locomoter activity were paradoxically enhanced.90 Since none of these studies directly analyzed the effect of Ang II on satellite cells in vivo, animals were infused with Ang II in the setting of muscle injury-induced regeneration.91 It was found that Ang II infusion suppressed Notch signaling in satellite cells, leading to impaired satellite cell proliferation and reduced regeneration. Importantly, quiescent and proliferating satellite cells highly express AT1R, whereas it declines after cells are differentiated into myotubes. These data are consistent with a suppressive role for Ang II on satellite cell function and muscle regeneration. The conflicting data reported for the function of Ang II on satellite cells could be because of the different experimental settings utilizing pharmacological inhibitors or genetic deletion of the AT1R. Caution should be exercised when analyzing RAS effects resulting from inhibition of 1 part of the entire system. The RAS includes multiple angiotensins and receptors, and it is not fully understood how different angiotensin ligands and receptors act in orchestration. For instance, inhibition of Ang II production by ACEi would result in an increase of its precursor Ang I, whereas blockade of Ang II signaling by AT1R blocker would increase Ang II through a compensatory mechanism. Indeed, the authors recently found that another Ang II receptor AT2R positively regulates satellite cell differentiation and fusion process.92 Considering the antagonistic action of AT1R and AT2R,93 these data indicate that AT1R and AT2R counteract each other and regulate different stages of satellite cell differentiation processes. Furthermore, Acuña et al reported that another angiotensin ligand Ang (1–7) restored muscle strength through inhibition of TGF-β.94 Although complete mechanisms underlying RAS-mediated regulation of satellite cell function are complex and remain to be elucidated, these studies suggest that the RAS, via the actions of multiple angiotensin ligands and their receptors, regulates satellite cell function in physiological conditions, and that alterations in RAS effects in pathophysiological states may lead to impaired satellite cell function.
Future prospects
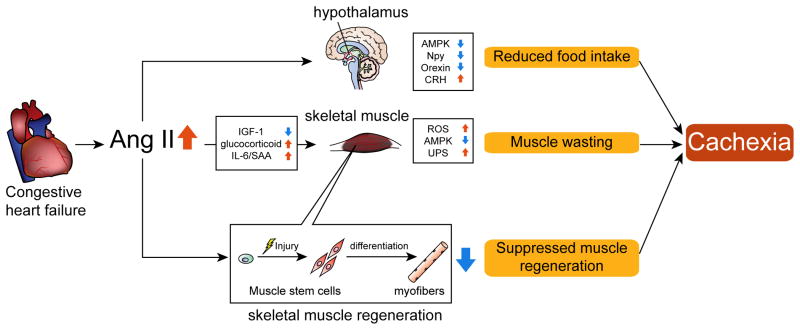
There have been a growing number of studies related to cachexia and our understanding of underlying mechanisms of loss of muscle mass in chronic disease conditions has made substantial progress in recent years. Approaches to prevent or attenuate cachexia are urgently needed and several promising therapies are under investigation in clinical trials. A major challenge underlying the development of cachexia treatment is the complex and multifactorial nature of the disease. It is unlikely that 1 therapeutic intervention could be effective in all the cachexia conditions associated with different chronic diseases. The RAS is activated in many chronic diseases such as CHF, CKD, COPD and cancer and in this manuscript the authors have summarized and discussed the involvement of the RAS in the development of muscle wasting. Studies have shown that Ang II induces muscle wasting through multiple mechanisms: (1) Increased protein breakdown via reduced IGF-1 and increased cytokine signaling such as glucocorticoid and IL-6; (2) Increased oxidative stress via activation of NADPH oxidase; (3) Impaired energy balance via inhibition of AMPK; (4) Reduced appetite via alteration of orexigenic/anorexigenic neuropeptide expression in the hypothalamus; (5) Inhibition of satellite cell function and muscle regeneration. It is likely that Ang II causes muscle wasting via a combination of these effects (Fig. 1), and recent evidence suggests that other RAS components play important roles in skeletal muscle physiology. Future studies are required to elucidate the RAS-mediated regulation of skeletal muscle and satellite cell function to connect these findings to the development of effective therapies for cachexia.
Fig. 1.
Ang II-induced muscle wasting: potential mechanisms of cardiac cachexia.
In CHF patients, there is an increase of Ang II. Increased Ang II causes a reduction of IGF-1 and increased glucocorticoids and IL-6/SAA, which result in muscle wasting. In skeletal muscle, there is a increase of ROS, reduction of AMPK and increased UPS, all of which result in muscle proteolysis. Ang II also acts on hypothalamic neurons to reduce appetite via alterations of orexigenic/anorexigenic neuropeptide expression. Reduced appetite leads to muscle wasting due to insufficient energy intake to maintain muscle mass. Ang II prevents satellite cell proliferation and skeletal muscle regeneration via inhibition of Notch signaling. The combination of Ang II-induced muscle wasting, reduced food intake and lower muscle regeneration lead to the development of cachexia.
Acknowledgments
Sources of Support
This research was supported by grants from the National Institutes of Health (NIH)/National Heart, Lung and Blood Institute (R01-HL070241 and R01-HL080682) and the NIH/National Institute of General Medical Sciences (P20-GM103629, P30-GM103337 and U54-GM104940).
Footnotes
Disclosure
Authors have nothing to disclose.
References
- 1.Morley JE, Thomas DR, Wilson M-MG. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006 Apr;83(4):735–43. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 2.Oliff A, Defeo-Jones D, Boyer M, Martinez D, Kiefer D, Vuocolo G, et al. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987 Aug 14;50(4):555–63. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- 3.Li Y-P, Chen Y, John J, Moylan J, Jin B, Mann DL, et al. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J Federation of American Societies for Experimental Biology. 2005 Mar;19(3):362–70. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovera M, García-Martínez C, López-Soriano J, Carbó N, Agell N, López-Soriano FJ, et al. Role of TNF receptor 1 in protein turnover during cancer cachexia using gene knockout mice. Mol Cell Endocrinol. 1998 Jul 25;142(1–2):183–9. doi: 10.1016/s0303-7207(98)00105-1. [DOI] [PubMed] [Google Scholar]
- 5.Maltoni M, Fabbri L, Nanni O, Scarpi E, Pezzi L, Flamini E, et al. Serum levels of tumour necrosis factor alpha and other cytokines do not correlate with weight loss and anorexia in cancer patients. Support Care Cancer. 1997 Mar;5(2):130–5. doi: 10.1007/BF01262570. [DOI] [PubMed] [Google Scholar]
- 6.Jatoi A, Ritter HL, Dueck A, Nguyen PL, Nikcevich DA, Luyun RF, et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9) Lung Cancer. 2010 May;68(2):234–9. doi: 10.1016/j.lungcan.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott HR, McMillan DC, Crilly A, McArdle CS, Milroy R. The relationship between weight loss and interleukin 6 in non-small-cell lung cancer. Br J Cancer. 1996 Jun;73(12):1560–2. doi: 10.1038/bjc.1996.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moses AGW, Maingay J, Sangster K, Fearon KCH, Ross JA. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncol Rep. 2009 Apr;21(4):1091–5. doi: 10.3892/or_00000328. [DOI] [PubMed] [Google Scholar]
- 9.Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest American Society for Clinical Investigation. 1992 May;89(5):1681–4. doi: 10.1172/JCI115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayliss TJ, Smith JT, Schuster M, Dragnev KH, Rigas JR. Expert Opin Biol Ther. 12. Vol. 11. Informa UK, Ltd; London: 2011. Dec, A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer; pp. 1663–8. [DOI] [PubMed] [Google Scholar]
- 11.Han HQ, Zhou X, Mitch WE, Goldberg AL. Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol. 2013 Oct;45(10):2333–47. doi: 10.1016/j.biocel.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, et al. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007 May 25;3(5):e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Eng J Med. 2004 Jun 24;350(26):2682–8. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 14.Trendelenburg AU, Meyer A, Jacobi C, Feige JN, Glass DJ. Skeletal Muscle. 1. Vol. 2. BioMed Central Ltd; 2012. TAK-1/p38/nNFκB signaling inhibits myoblast differentiation by increasing levels of Activin A; p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010 Aug 20;142(4):531–43. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015 Jan;14(1):58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 17.Anker SD, Volterrani M, Pflaum CD, Strasburger CJ, Osterziel KJ, Doehner W, et al. Acquired growth hormone resistance in patients with chronic heart failure: implications for therapy with growth hormone. J Am Coll Cardiol. 2001 Aug;38(2):443–52. doi: 10.1016/s0735-1097(01)01385-7. [DOI] [PubMed] [Google Scholar]
- 18.Suskin N, McKelvie RS, Burns RJ, Latini R, Pericak D, Probstfield J, et al. Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur Heart J. 2000 Aug;21(16):1368–75. doi: 10.1053/euhj.1999.2043. [DOI] [PubMed] [Google Scholar]
- 19.Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am J Physiol Endocrinol Metab. 2008 Feb;294(2):E345–51. doi: 10.1152/ajpendo.00456.2007. [DOI] [PubMed] [Google Scholar]
- 20.Csibi A, Communi D, Müller N, Bottari SP. Angiotensin II inhibits insulin-stimulated GLUT4 translocation and Akt activation through tyrosine nitration-dependent mechanisms. PLoS ONE. 2010;5(4):e10070. doi: 10.1371/journal.pone.0010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anker SD, Negassa A, Coats AJS, Afzal R, Poole-Wilson PA, Cohn JN, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003 Mar 29;361(9363):1077–83. doi: 10.1016/S0140-6736(03)12892-9. [DOI] [PubMed] [Google Scholar]
- 22.Schellenbaum GD, Smith NL, Heckbert SR, Lumley T, Rea TD, Furberg CD, et al. Weight loss, muscle strength, and angiotensin-converting enzyme inhibitors in older adults with congestive heart failure or hypertension. J Am Geriatr Soc. 2005 Nov;53(11):1996–2000. doi: 10.1111/j.1532-5415.2005.53568.x. [DOI] [PubMed] [Google Scholar]
- 23.Di Bari M, van de Poll-Franse LV, Onder G, Kritchevsky SB, Newman A, Harris TB, et al. Antihypertensive medications and differences in muscle mass in older persons: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004 Jun;52(6):961–6. doi: 10.1111/j.1532-5415.2004.52265.x. [DOI] [PubMed] [Google Scholar]
- 24.Paolisso G, Balbi V, Gambardella A, Varricchio G, Tortoriello R, Saccomanno F, et al. Lisinopril administration improves insulin action in aged patients with hypertension. J Hum Hypertens. 1995 Jul;9(7):541–6. [PubMed] [Google Scholar]
- 25.Schanze N, Springer J. J Cachexia Sarcopenia Muscle. 2. Vol. 3. Springer; 2012. Jun, Evidence for an effect of ACE inhibitors on cancer cachexia; pp. 139–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osherovich L. New muscle for ARB strategy. SciBX: Science-Business eXchange. Nature Publishing Group. 2011 May;26(21):4. [Google Scholar]
- 27.Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. 2008 Sep;264(3):224–36. doi: 10.1111/j.1365-2796.2008.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest. 1996 Jun 1;97(11):2509–16. doi: 10.1172/JCI118698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventadour S, Attaix D. Mechanisms of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care. 2006 Nov;18(6):631–5. doi: 10.1097/01.bor.0000245731.25383.de. [DOI] [PubMed] [Google Scholar]
- 30.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001 Nov 23;294(5547):1704–8. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 31.Song Y-H, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. J Clin Invest. 2. Vol. 115. American Society for Clinical Investigation; 2005. Feb, Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting; pp. 451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida T, Semprun-Prieto L, Sukhanov S, Delafontaine P. IGF-1 prevents ANG II-induced skeletal muscle atrophy via Akt- and Foxo-dependent inhibition of the ubiquitin ligase atrogin-1 expression. Am J Physiol Heart Circ Physiol. 2010 May;298(5):H1565–70. doi: 10.1152/ajpheart.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semprun-Prieto LC, Sukhanov S, Yoshida T, Rezk BM, Gonzalez-Villalobos RA, Vaughn C, et al. Angiotensin II induced catabolic effect and muscle atrophy are redox dependent. Biochem Biophys Res Comm. 2011 Jun 3;409(2):217–21. doi: 10.1016/j.bbrc.2011.04.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brink M, Price SR, Chrast J, Bailey JL, Anwar A, Mitch WE, et al. Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology. 2001 Apr;142(4):1489–96. doi: 10.1210/endo.142.4.8082. [DOI] [PubMed] [Google Scholar]
- 35.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008 Jun;23:160–70. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 36.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001 Nov;3(11):1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 37.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001 Nov;3(11):1009–13. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 38.Peng X-D, Xu P-Z, Chen M-L, Hahn-Windgassen A, Skeen J, Jacobs J, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003 Jun 1;17(11):1352–65. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci USA. 2002 Jul 9;99(14):9213–8. doi: 10.1073/pnas.142166599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001 Feb;27(2):195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 41.Attaix D, Baracos VE. MAFbx/Atrogin-1 expression is a poor index of muscle proteolysis. Curr Opin Clin Nutr Metab Care. 2010 May;13(3):223–4. doi: 10.1097/MCO.0b013e328338b9a6. [DOI] [PubMed] [Google Scholar]
- 42.Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007 Nov;6(5):376–85. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol. 2009 Jun 15;185(6):1083–95. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem. 2005 Jan 28;280(4):2847–56. doi: 10.1074/jbc.M411346200. [DOI] [PubMed] [Google Scholar]
- 45.Lagirand-Cantaloube J, Offner N, Csibi A, Leibovitch MP, Batonnet-Pichon S, Tintignac LA, et al. The initiation factor eIF3-f is a major target for Atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J. 2008 Mar 20;27(8):1266–76. doi: 10.1038/emboj.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLoughlin TJ, Smith SM, DeLong AD, Wang H, Unterman TG, Esser KA. FoxO1 induces apoptosis in skeletal myotubes in a DNA-binding-dependent manner. Am J Physiol Cell Physiol. 2009 Sep;297(3):C548–55. doi: 10.1152/ajpcell.00502.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Senf SM, Dodd SL, Judge AR. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am J Physiol Cell Physiol. 2010 Jan;298(1):C38–45. doi: 10.1152/ajpcell.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanders PM, Russell ST, Tisdale MJ. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer. 2005 Aug 22;93(4):425–34. doi: 10.1038/sj.bjc.6602725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell ST, Sanders PM, Tisdale MJ. Angiotensin II directly inhibits protein synthesis in murine myotubes. Cancer Lett. 2006 Jan;231(2):290–4. doi: 10.1016/j.canlet.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, et al. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol. 2009 Mar;20(3):604–12. doi: 10.1681/ASN.2008060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000 Mar 17;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 52.Manrique C, Lastra G, Gardner M, Sowers JR. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am. 2009 May;93(3):569–82. doi: 10.1016/j.mcna.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao W, Swanson SA, Ye J, Li X, Shelton JM, Zhang W, et al. Reactive oxygen species impair sympathetic vasoregulation in skeletal muscle in angiotensin II-dependent hypertension. Hypertension. 2006 Oct;48(4):637–43. doi: 10.1161/01.HYP.0000240347.51386.ea. [DOI] [PubMed] [Google Scholar]
- 54.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GME, Clark SE, et al. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006 Nov 17;281(46):35137–46. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- 55.Powers SK, Wiggs MP, Duarte JA, Zergeroglu AM, Demirel HA. Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab. 2012 Jul 1;303(1):E31–9. doi: 10.1152/ajpendo.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, et al. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol. 2007 Sep;293(3):R1159–68. doi: 10.1152/ajpregu.00767.2006. [DOI] [PubMed] [Google Scholar]
- 57.Whitehead NP, Yeung EW, Froehner SC, Allen DG. Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS ONE. 2010;5(12):e15354. doi: 10.1371/journal.pone.0015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000 Mar;20(3):645–51. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 59.Kimura B, Sumners C, Phillips MI. Changes in skin angiotensin II receptors in rats during wound healing. Biochem Biophys Res Comm. 1992 Sep 16;187(2):1083–90. doi: 10.1016/0006-291x(92)91308-d. [DOI] [PubMed] [Google Scholar]
- 60.Tabony AM, Yoshida T, Galvez S, Higashi Y, Sukhanov S, Chandrasekar B, et al. Angiotensin II upregulates protein phosphatase 2Cα and inhibits AMP-activated protein kinase signaling and energy balance leading to skeletal muscle wasting. Hypertension. 2011 Oct;58(4):643–9. doi: 10.1161/HYPERTENSIONAHA.111.174839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004 Jan;18(1):39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 62.Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, et al. S6 Kinase Deletion Suppresses Muscle Growth Adaptations to Nutrient Availability by Activating AMP Kinase. Cell Metab. 2007 Jun;5(6):476–87. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 63.NAKASHIMA K, YAKABE Y. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci Biotechnol Biochem. 2007 Jul;71(7):1650–6. doi: 10.1271/bbb.70057. [DOI] [PubMed] [Google Scholar]
- 64.Tabony AM, Yoshida T, Sukhanov S, Delafontaine P. Protein phosphatase 2C-alpha knockdown reduces angiotensin II-mediated skeletal muscle wasting via restoration of mitochondrial recycling and function. Skeletal Muscle. 2014;4(1):20. doi: 10.1186/2044-5040-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008 Dec;27(6):793–9. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 66.Fouladiun M, Körner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG. Cancer. 10. Vol. 103. Wiley Subscription Services, Inc, A Wiley Company; 2005. May 15, Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care--correlations with food intake, metabolism, exercise capacity, and hormones; pp. 2189–98. [DOI] [PubMed] [Google Scholar]
- 67.Porter JP, Anderson JM, Robison RJ, Phillips AC. Effect of central angiotensin II on body weight gain in young rats. Brain Res. 2003 Jan 3;959(1):20–8. doi: 10.1016/s0006-8993(02)03676-4. [DOI] [PubMed] [Google Scholar]
- 68.Porter JP, Potratz KR. Effect of intracerebroventricular angiotensin II on body weight and food intake in adult rats. Am J Physiol Regul Integr Comp Physiol. 2004 Aug;287(2):R422–8. doi: 10.1152/ajpregu.00537.2003. [DOI] [PubMed] [Google Scholar]
- 69.de Kloet AD, Krause EG, Scott KA, Foster MT, Herman JP, Sakai RR, et al. Central angiotensin-II has catabolic action at white and brown adipose tissue. Am J Physiol Endocrinol Metab. 2011 Aug 23; doi: 10.1152/ajpendo.00307.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshida T, Semprun-Prieto L, Wainford RD, Sukhanov S, Kapusta DR, Delafontaine P. Angiotensin II reduces food intake by altering orexigenic neuropeptide expression in the mouse hypothalamus. Endocrinology. 2012 Mar;153(3):1411–20. doi: 10.1210/en.2011-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol. 1997 Oct;18(4):383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- 72.Yablonka-Reuveni Z, Day K. Skeletal muscle stem cells in the spotlight: the satellite cell. In: Cohen IS, Gaudette GR, editors. Regenerating the Heart. Springer; 2011. pp. 173–200. [Google Scholar]
- 73.Mehl KA, Davis JM, Berger FG, Carson JA. Myofiber degeneration/regeneration is induced in the cachectic ApcMin/+ mouse. J Appl Physiol. 2005 Dec;99(6):2379–87. doi: 10.1152/japplphysiol.00778.2005. [DOI] [PubMed] [Google Scholar]
- 74.Penna F, Costamagna D, Fanzani A, Bonelli G, Baccino FM, Costelli P. PLoS ONE. 10. Vol. 5. Public Library of Science; 2010. Oct 27, Muscle Wasting and Impaired Myogenesis in Tumor Bearing Mice Are Prevented by ERK Inhibition; p. e13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Costelli P, Muscaritoli M, Bossola M, Moore-Carrasco R, Crepaldi S, Grieco G, et al. Skeletal muscle wasting in tumor-bearing rats is associated with MyoD down-regulation. Int J Oncol. 2005 Jun;26(6):1663–8. doi: 10.3892/ijo.26.6.1663. [DOI] [PubMed] [Google Scholar]
- 76.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003 Nov 28;302(5650):1575–7. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 77.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012 Oct 18;490(7420):355–60. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002 Sep;3(3):397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 79.Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008 Jul 24;454(7203):528–32. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005 Feb 17;433(7027):760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 81.Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014 May 9;344(6184):649–52. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Argilés JM, López-Soriano FJ, Busquets S. Novel approaches to the treatment of cachexia. Drug Discov Today. 2008 Jan;13(1–2):73–8. doi: 10.1016/j.drudis.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 83.Doehner W, Haehling von S, Anker SD, Lainscak M. Neurohormonal activation and inflammation in chronic cardiopulmonary disease: a brief systematic review. Wien Klin Wochenschr. 2009 Jun;121(9–10):293–6. doi: 10.1007/s00508-009-1194-7. [DOI] [PubMed] [Google Scholar]
- 84.Haehling von S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009 Mar;121(3):227–52. doi: 10.1016/j.pharmthera.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 85.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009 Apr;89(2):381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 86.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007 Feb;13(2):204–10. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med. 2008 Aug;36(8):1548–54. doi: 10.1177/0363546508315470. [DOI] [PubMed] [Google Scholar]
- 88.Burks TN, Andres-Mateos E, Marx R, Mejias R, van Erp C, Simmers JL, et al. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med. 2011 May 11;3(82):82ra37. doi: 10.1126/scitranslmed.3002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnston APW, Baker J, Bellamy LM, McKay BR, De Lisio M, Parise G. Regulation of muscle satellite cell activation and chemotaxis by angiotensin II. PLoS ONE. 2010;5(12):e15212. doi: 10.1371/journal.pone.0015212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy KT, Allen AM, Chee A, Naim T, Lynch GS. Disruption of muscle renin-angiotensin system in AT1a−/− mice enhances muscle function despite reducing muscle mass but compromises repair after injury. Am J Physiol Regul Integr Comp Physiol. 2012 Aug;303(3):R321–31. doi: 10.1152/ajpregu.00007.2012. [DOI] [PubMed] [Google Scholar]
- 91.Yoshida T, Galvez S, Tiwari S, Rezk BM, Semprun-Prieto L, Higashi Y, et al. Angiotensin II inhibits satellite cell proliferation and prevents skeletal muscle regeneration. J Biol Chem. 2013 Aug 16;288(33):23823–32. doi: 10.1074/jbc.M112.449074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoshida T, Huq TS, Delafontaine P. Angiotensin type 2 receptor signaling in satellite cells potentiates skeletal muscle regeneration. J Biol Chem. 2014 Sep 19;289(38):26239–48. doi: 10.1074/jbc.M114.585521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berk BC. Angiotensin type 2 receptor (AT2R): a challenging twin. Sci STKE. 2003 May;6(181):2003. PE16. doi: 10.1126/stke.2003.181.pe16. [DOI] [PubMed] [Google Scholar]
- 94.Acuna MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, et al. Restoration of muscle strength in dystrophic muscle by angiotensin-1–7 through inhibition of TGF- signalling. Human Molecular Genetics. 2014 Feb 7;23(5):1237–49. doi: 10.1093/hmg/ddt514. [DOI] [PubMed] [Google Scholar]