Abstract
Introduction
China has the largest population of Parkinson’s disease (PD) patients; however few etiological studies of PD have been conducted in China.
Methods
The Shanghai Women’s Health Study recruited 74,941 women in urban Shanghai, aged 40 to 70, from 1996 to 2000. Self-reported PD cases were invited for a neurological examination and diagnoses were made by a movement disorder specialist.
Results
This cohort had very few smokers (2.7%), alcohol drinkers (2.3%), and postmenopausal hormone users (4.3%); however, tea drinking (29.9%) and exposure to tobacco smoke from husbands (61.8%) were common. A total of 301 participants reported PD diagnosis during the follow-up. The diagnosis was confirmed in 76 (57%) of the 133 clinically examined patients. An additional 19 (53%) PD cases were identified out of 36 participants who self-confirmed the diagnosis and provided a history on PD symptoms and treatments. As expected, increasing age was strongly associated with PD risk. Further, PD risk appears to be inversely associated with exposures to second-hand tobacco smoke from husbands and tea drinking, and positively with education, although none of these reached statistical significance. The age-adjusted odds ratio (OR) was 0.7 (95% confidence interval: 0.4–1.1) for participants whose husbands were current smokers at baseline and 0.8 (0.5–1.3) for ever tea-drinkers. Compared with primary education or lower, the age-adjusted OR was 1.3 (0.7–2.4) for middle school and 1.6 (1.0–2.7) for high school or above.
Conclusion
PD research in this unique cohort is feasible and, with extended follow-up, will allow for prospective PD etiological research in China.
Keywords: Parkinson’s disease/Parkinsonism, risk factors in epidemiology, cohort studies
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease and affects more than 1% of the elderly population. The causes for late-onset sporadic PD are largely unknown, and likely involve both genetic and environmental factors and interactions among these factors [1]. To date, most PD etiological studies have been conducted in the United States and western European countries. Research efforts in China have been limited, despite the fact that China has the largest elderly population and is believed to have the largest PD population worldwide [2, 3]. PD epidemiological research in China will have important public health and economic impacts. Further, such research may potentially lead to the identification of environmental or genetic risk factors that are less common in western countries [4, 5] and may facilitate cross-cultural comparison of study results from different regions of the world [4, 6]. We therefore conducted PD research in a well-established large community-based prospective cohort in China: the Shanghai Women’s Health Study (SWHS).
Methods
The SWHS cohort was a joint effort by Vanderbilt University, the Shanghai Cancer Institute, and the US National Cancer Institute to investigate diet and lifestyle risk factors for cancers among Chinese women [7]. Details of this cohort were published previously [7]. From 1996 to 2000, the SWHS recruited 74,941 Chinese women, ages 40 to 70, from seven communities in the Changning district of Shanghai with an overall consent rate of 92%. At baseline, in-person interviews collected information on demographics, dietary habits, lifestyle, reproductive and medical histories, and residential and employment histories from each women, as well as lifestyle and medical history for her husband. All interviews were tape-recorded and randomly checked by quality control staff to monitor the quality of the interview. In addition, 87.7% of the study participants donated urine and 75.8% donated blood, and among those who did not donate blood, 49.3% provided buccal cells for genetic research [7].
The comprehensive baseline survey included questions that are potentially useful for PD research. Participants were queried about their smoking and tea drinking habit as well as the smoking habit of their husbands. Ever smoking was defined as at least one cigarette per day continuously for six months or longer, and tea drinking was defined as continuous drinking of at least three times a week for six months or longer. Education level was ascertained with the following categorical choices: no formal education, elementary, junior high, high, professional high education, college or above, unknown. In addition, these women also provided information on their reproductive characteristics, including oral contraceptive use and use of post-menopausal hormones. After the baseline survey, this cohort has been followed by in-person interviews every 2–3 years to update exposures and to ascertain the occurrence of cancer and selected chronic diseases with response rates consistently over 92%. Participants were first asked in the third follow-up survey in 2004–2007 whether they had ever been told by a physician that they had PD, and those who answered yes or probably yes further reported the year and month of diagnosis. Similar questions were included in the fourth follow-up survey (2008–2011) to ascertain PD diagnosis since last follow-up. These two follow-up surveys identified a total of 301 self-reported PD diagnoses: 220 from the third follow-up survey and 81 additional from the fourth follow-up.
For the current study, a field coordinator from the Shanghai Cancer Institute visited the home of self-reported PD patients and invited them to a clinical examination at the Hua-Shan hospital of Fudan University, one of the leading hospitals in China on neurology clinical care and research. During the clinical examination, a neurologist took medical and neurological history, conducted a video-taped structured examination for PD diagnosis, and collected blood and urine samples. The patient was then examined by a senior movement disorder specialist, and based on all information collected, the movement disorder specialist made the PD diagnosis according to established diagnostic criteria [8, 9]. For self-reported cases that refused clinical examination, a neurologist from the Hua-Shan hospital visited each participant’s home and completed a screening questionnaire that collected information on PD diagnostic history, including where the diagnosis was made, primary clinical symptoms, and responsiveness to dopaminergic therapy. These data were subsequently reviewed by the senior movement disorder specialist who made a determination regarding PD diagnosis.
The current analysis was limited to women who participated in the third or the fourth follow-up surveys (n=71,600). We first described the basic population characteristics of cohort participants and self-reported cases. Means and standard deviations were presented for continuous variables and proportions for categorical variables. We also examined age (in 5 year groups), education (primary school or under, middle school, high school or above), smoking status (never, ever), husband’s smoking status (never, ever / current), tea drinking (never, ever), and oral contraceptive use (never, ever) in relation to PD risk. Odds ratios (OR) and 95% confidence intervals (CI) were derived from unconditional logistic regression models, adjusting for age as a continuous variable when appropriate. Self-reported PD patients whose diagnoses were not confirmed were excluded from these analyses. Statistical analysis was performed by using the Statistical Analysis Systems (SAS) release 9.3 (SAS Institute, Cary, NC, USA) with two-tailed α of 0.05.
All SWHS participants provided written consent at the baseline survey; participants who underwent the clinical examination further provided written consent for the clinical examination. The study protocol was approved by the Institutional Review Boards of the US National Institute of Environmental Health Sciences, the Shanghai Hua-Shan hospital, Vanderbilt University, and the Shanghai Cancer Institute.
Results
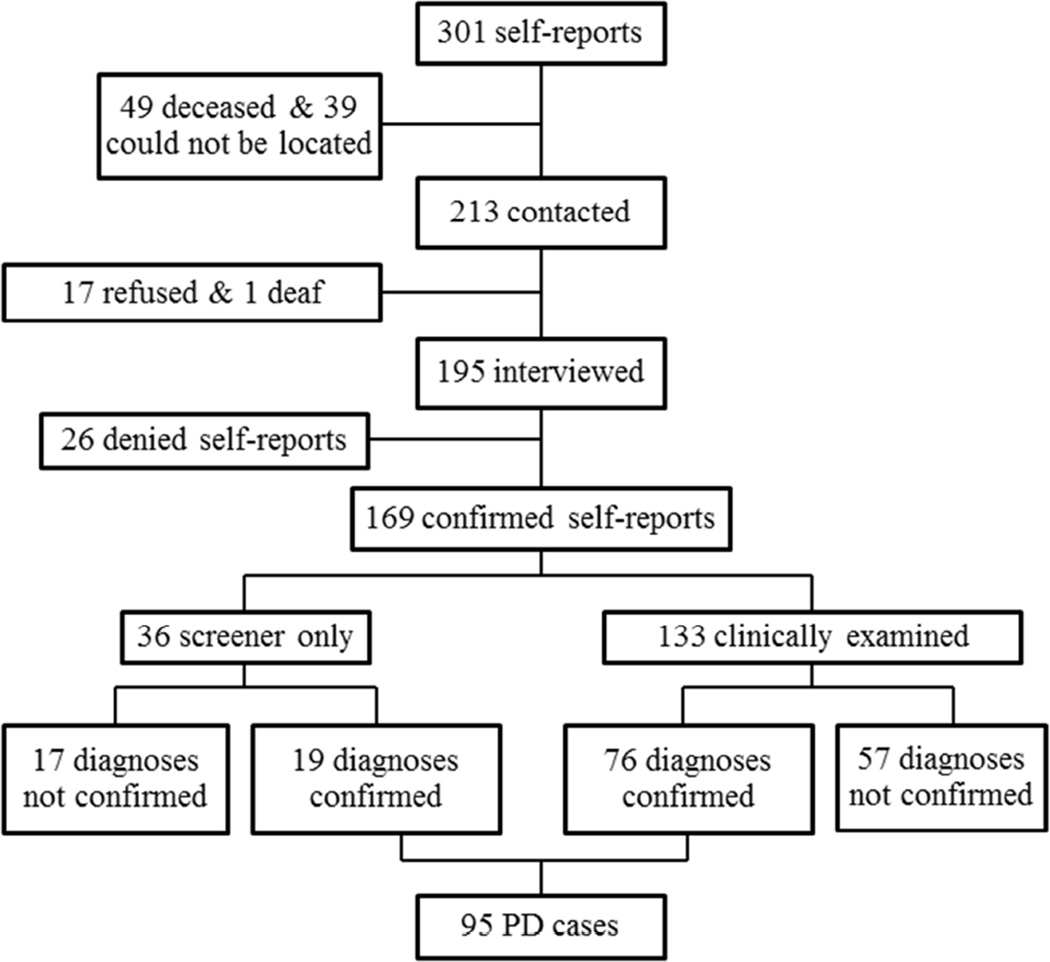
Figure 1 shows the disease confirmation process. Of the 301 women who self-reported PD, 49 had died, 39 could not be located, 17 refused participation, and 1 was deaf and unable to communicate. Of the remaining195 self-reported patients, 26 denied ever having a diagnosis of PD, 36 refused an examination but completed the screening interview and 133 were clinically examined. PD was confirmed in 76 of those clinically examined. For those whose PD diagnosis was not confirmed at the clinical examination, 34 had essential tremor, 8 secondary parkinsonisms (4 vascular, 2 drug-induced, 1 neurotoxicant induced, 1 other), 4 progressive supranuclear palsy, 4 enhanced physiological tremor, 2 dementia, 2 dystonia, 1 ischemic stroke,1 spinocerebellar ataxia and 1 had no neurological disease. Of the 36 potential cases who only provided medical history data, 19 were considered to have PD after review by the movement disorder specialist. Therefore a total of 95 PD patients were confirmed and the prevalence (/100,000) was 71.8 for ages <55 years, 124.8 for ages 55–64 years, 256.3 for ages 65–74 years, and 106.7 for ages 75–84 years.
Figure 1.
Flowchart of PD confirmation.
Table 1 presents population characteristics of all cohort participants and self-reported cases with various PD confirmation results. The average age of cohort participants was 52.0 years at study baseline, and few were ever smokers or alcohol drinkers, or had ever used postmenopausal hormones. However, about 30% of these women drank tea and 62% had a husband that ever smoked. In comparison to the entire cohort, confirmed PD cases were older at baseline and more likely to have a primary school education or lower, but they were less likely to smoke cigarettes, drink tea or have a husband that ever smoked or “currently” smoked at study baseline.
Table 1.
Population characteristics of cohort participants and potential cases of Parkinson’s disease
| All participants | Self-reported cases (n=301) | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinically examined | Screener only | Not contacted |
||||||
| All | PD confirmed | No PD | All | PD confirmed | No PD | |||
| Number of participants* | 71600 | 133 | 76 | 57 | 36 | 19 | 17 | 132 |
| Baseline age, years (mean±SD) | 52.0±9.0 | 59.8±7.1 | 59.4±7.3 | 60.4±6.9 | 63.0±5.7 | 62.8±6.6 | 63.3±4.8 | 63.6±6.0 |
| Education level, % | ||||||||
| Primary/under | 21.1 | 36.8 | 38.2 | 35.1 | 50.0 | 31.6 | 70.6 | 49.2 |
| Middle school | 37.4 | 29.3 | 25.0 | 35.1 | 19.4 | 21.1 | 17.7 | 20.5 |
| High school/above | 41.6 | 33.8 | 36.8 | 29.8 | 30.6 | 47.4 | 11.8 | 30.3 |
| Married, % | 90.0 | 87.2 | 86.8 | 87.7 | 91.7 | 89.5 | 94.1 | 84.9 |
| Ever cigarette smoking, % | 2.7 | 3.0 | 1.3 | 5.3 | 0 | 0 | 0 | 7.6 |
| Husband ever smoking* | 61.8 (64067) | 52.6 (116) | 50.0 (66) | 56.0 (50) | 54.6 (33) | 52.9 (17) | 56.3 (16) | 53.6 (112) |
| Current smoking | 52.8 (64067) | 37.1 (116) | 30.3 (66) | 46.0 (50) | 30.3 (33) | 35.3 (17) | 25.0 (16) | 33.0 (112) |
| Ever alcohol drinking, (%) | 2.3 | 3.8 | 2.6 | 5.3 | 2.8 | 0 | 5.9 | 3.0 |
| Ever tea drinking, (%) | 29.9 | 21.8 | 21.1 | 22.8 | 16.7 | 15.8 | 17.7 | 11.4 |
| Ever contraceptive use, (%) | 20.6 | 21.1 | 19.7 | 22.8 | 25.0 | 31.6 | 17.7 | 17.4 |
| Ever use of HRT, (%) | 4.3 (35030) | 2.7 (111) | 3.2 (62) | 2.0 (49) | 8.6 (35) | 11.1 (18) | 5.9 (17) | 2.4 (127) |
| Blood collected, (%) | 76.3 | 80.5 | 79.0 | 82.5 | 55.6 | 57.9 | 52.9 | 74.2 |
| Urine collected, (%) | 88.3 | 93.2 | 92.1 | 94.7 | 88.9 | 84.2 | 94.1 | 86.4 |
Denominators are provided when there are missing observations. For education, only 12 had missing in the entire cohort, none among self-reported PD cases. 12 missing on education, 616 on marriage, 7533 on husband smoking, 30 on HRT use. HRT was among postmenopausal women only.
The risk for PD clearly increased with age (Table 2). After adjusting for baseline age, higher educational level tended to be associated with greater PD risk: the OR was 1.0 (reference) for primary school or below, 1.3 (0.7–2.3) for middle school, and 1.6 (1.0–2.7) for high school or above. But this association was attenuated in the sensitivity analysis limited to participants younger than age 65 at the cohort’s baseline survey. On the other hand, smoking, tea drinking, and exposure to second-hand smoke from husband tended to be associated with lower risk. However, none of these associations reached statistical significance.
Table 2.
Age-adjusted odds ratios and 95%confidence intervals of PD according to selected baseline characteristics
| PD cases |
Participants free of PD |
OR (95%CI) | OR (95%CI) in sensitivity analysis* |
|
|---|---|---|---|---|
| Baseline age, | ||||
| <55 | 26 | 45248 | 1.0 (reference) | |
| 55–59 | 13 | 7257 | 3.1 (1.6–6.1) | |
| 60–64 | 27 | 9142 | 5.1 (3.0–8.8) | |
| ≥65 | 29 | 9655 | 5.2 (3.1–8.9) | |
| Education level, | ||||
| Primary or under | 35 | 14938 | 1.0 (reference) | |
| Middle school | 23 | 26683 | 1.3 (0.7, 2.4) | 1.2 (0.6, 2.5) |
| High school or above | 37 | 29669 | 1.6 (1.0, 2.7) | 1.2 (0.7, 2.4) |
| Smoking | ||||
| Never | 94 | 69359 | 1.0 (reference) | |
| Ever | 1 | 1943 | 0.2 (0.03, 1.6) | 0.5 (0.06,3.3) |
| Husband smoking | ||||
| Never | 41 | 24350 | 1.0 (reference) | |
| Ever | 42 | 39459 | 0.8 (0.5, 1.3) | 0.9 (0.5, 1.5) |
| Current | 26 | 33743 | 0.7 (0.4, 1.1) | 0.7 (0.4, 1.3) |
| Tea drinking | ||||
| Never | 76 | 49958 | 1.0 (reference) | |
| Ever | 19 | 21344 | 0.8 (0.5–1.3) | 0.8 (0.4, 1.4) |
| Oral contraceptive use | ||||
| Never | 74 | 56636 | 1.0 (reference) | |
| Ever | 21 | 14666 | 1.1 (0.7–1.8) | 1.1 (0.6, 1.9) |
Analyses limited to 66 PD cases and 61,640 individuals without PD who were younger than 65 years of age at study baseline.
Overall, the clinical characteristics of the 76 clinically confirmed PD cases are consistent with typical late-onset PD (Table 3). All but one of the self-identified patients whose PD diagnosis was not confirmed after the clinical examination turned out to have some other neurologic disorders, mostly essential tremor or secondary Parkinsonism. We documented clear differences in diagnostic accuracy by the level of hospitals and the specialty of physicians. Compared with self-reported patients whose diagnoses were not confirmed, confirmed cases were much more likely to receive their previous diagnosis from one of the municipal-level hospitals in Shanghai (82.9% in confirmed vs. 47.4% in unconfirmed) and from a movement disorder specialist at PD specialty clinics (36.8% vs. 8.8%). If we use the final diagnosis by the movement disorder specialist on our research team as the “gold standard”, 70.0% of the treating physician’s diagnoses made at the municipal-level hospitals were accurate as compared with 38.7% of the diagnoses made at the lower level district hospitals. Similarly, the clinical diagnosis was 84.9% accurate at the PD specialty clinics as compared to 57.5% at the general neurology department.
Table 3.
Clinical characteristics of clinically confirmed Parkinson cases and those whose diagnosis was not confirmed
| Clinical characteristics | Clinically examined | |
|---|---|---|
| PD Confirmed | PD not confirmed | |
| Number of participants | 76 | 57 |
| Age at examination, years (mean±SD) | 71.9±7.3 | 72.9±6.8 |
| Diagnosis hospital, N (%) | ||
| Municipal level | 63 (82.9) | 27 (47.4) |
| District level | 12 (15.8) | 19 (33.3) |
| Missing | 1 (1.3) | 11 (19.3) |
| Specialty of clinics, (%) | ||
| PD specialty clinics | 28 (36.8) | 5 (8.8) |
| Neurology | 46 (60.5) | 34 (59.6) |
| Others | 1 (1.3) | 7 (12.3) |
| Missing | 1 (1.3) | 11 (19.3) |
| Age at diagnosis, years (mean±SD) | 63.5 ± 8.0 | 63.4 ± 9.5 |
| Age at first symptoms, years (mean±SD) | 62.1 ± 8.5 | 60.6 ± 10.7 |
| Duration at enrollment, years (mean±SD) | 8.4 ± 4.4 | 9.3 ± 6.7 |
| Bradykinesia*, (%) | 72/73 (98.6) | 18/48 (37.5) |
| Rest tremor*, (%) | 66/73 (90.4) | 40/53 (75.5) |
| Muscle rigidity*, (%) | 62/73 (84.9) | 13/47 (27.7) |
| Postural instability*, (%) | 39/65 (60.0) | 9/44 (20.5) |
| Asymmetric onset*, (%) | 72/74 (97.3) | 40/54 (74.1) |
| Ever treated by*, (%) | ||
| Levodopa | 72/75 (96.0) | 32/52 (61.5) |
| Dopamine agonist | 20/74 (27.0) | 0/54 (0) |
| Amantadine | 36 (47.4) | 7/56 (12.5) |
| Other medications for PD | 37/74 (50.0) | 13/56 (23.2) |
| Response to dopaminergic therapy*, (%) | 70/70 (100) | 7/22 (31.8) |
Denominators are provided when there are missing observations.
Discussion
A recent study estimated that China had 2 million PD patients or 48% of all PD patients from the world’s fifteen most populous countries [3]; this number is expected to increase to 5 million by the year 2030. Epidemiological studies in China are therefore needed to understand the causes of PD and to reduce disease burden [2, 4]. To the best of our knowledge, this study is among the first efforts to establish a large population-based prospective study for PD research in China [10].
Potential PD cases from this cohort were first identified by self-report, and then followed by a direct clinical examination or a review of medical history by a movement disorder specialist. Through the fourth follow-up in 2011, we documented 95 PD cases in this cohort. Although it is difficult to directly compare the prevalence of PD across studies due to differences in study settings and methods [1], the prevalence of PD in this cohort was lower than that in most surveys in China that often used door to door survey and two stage disease confirmation [11].
An inverse association with smoking is the most robust epidemiology finding for PD in Western populations; however, the nature of this observation has been debated [1], largely due to concerns on personality and reverse causation [12]. This may be less of a concern in this cohort as very few (2.3%) women ever smoked. In contrast, most (61.8%) of the participants’ husbands were smokers. Although the current sample size is limited, our preliminary result suggests that second-hand smoke exposure may be associated with a lower risk for PD among these women. This observation is consistent with data from two previous studies [13, 14] and thus lends support for potential biological explanation(s) for the smoking-PD relationship [15]. We also found that PD patients are more likely to report higher education level than other cohort participants after accounting for age, a finding consistent with a population-based study in the US [16]. However, explanations for this observation are not straightforward as education may be a surrogate for various lifestyle, occupational or environmental exposures, health consciousness, and access to quality health care. Further the association was attenuated when the analyses was limited to participants younger than age 65 years at baseline, suggesting potential bias that older PD cases with low education level were less likely to participate in case confirmation.
The Shanghai Women’s Health Study offers a unique opportunity to study PD etiology in China. The cohort infrastructure has been well-established over the past 15 years by leading US and Chinese cancer epidemiologists and oncologists. The size of this cohort is comparable to that of the larger population-based US cohorts [17–22]. All cohort participants were from a single administrative district in Shanghai and thus make a clinical examination of self-identified PD patients more feasible than most of the large population-based US cohorts [18–22].
More importantly, the cohort has collected information on a range of environmental exposures from diet and lifestyle to occupation and residential histories as well as blood and urine samples. These exposure data and biospecimens will become increasingly valuable with future follow-ups and incident PD case accumulation. Further, the exposure profile of this population is rather unique. Factors known to be associated with PD risk such as active smoking and coffee drinking are largely absent in this population, which provides a relatively clean population in which to study other risk factors. In contrast, some other exposures are prevalent. For example, data from Western countries suggest that organochlorines may increase PD risk [23–25], and a previous study among Shanghai women found much higher plasma levels of organochlorines than those reported in Western countries [26]. Finally, diet in this population was characterized by high-consumption of tofu, certain vegetables, and fish and low intake of red meat [7, 27]. Dietary patterns high in legume, plant-based foods and fish have been linked to lower risk for PD in both the US [28] and Japan [29].
Several clinical observations are worth mentioning. Of clinically examined cases, only 57% of the previous PD diagnoses were confirmed. The clinical diagnostic accuracy was high for diagnoses made at PD specialty clinics and at municipal level hospitals, but was poor otherwise. Further, overall only 36.8% of PD patients received their clinical diagnosis from movement disorder specialists at PD specialty clinics. Most erroneous self-reports turned out to be essential tremor which has a more benign clinical course and a better prognosis than PD [30]. These errors might be the result of misdiagnosis by physicians, but also could be due to the confusion of patients and family members who did not seek diagnostic confirmation by well-trained specialists. Therefore, potential PD patients in China should be educated to actively seek diagnostic confirmation by movement disorder specialists. This also implies that population-based studies on PD in China require diagnostic confirmations by experienced movement disorder specialists.
The current study also has several limitations. Due to the size of this cohort, we had to rely on self-reported physician diagnosis to identify potential PD patients for later clinical work-up. We therefore inevitably missed some cases who failed to report their diagnoses. Further, about 30% of self-reported cases were either deceased or could not be located and therefore they could not be reached for diagnostic confirmation. These, together with possible under-diagnosis of PD in Chinese communities [2, 4], led to under-ascertainment of PD cases in this cohort. This under-ascertainment has limited our ability to examine etiological hypotheses for PD, and might have biased the analysis on education. Finally, the study was conducted in Shanghai, therefore the findings may not be readily generalizable to less developed areas of China.
In summary, preliminary results from the Shanghai Parkinson Study prove the feasibility of conducting prospective PD research in China. Such research not only has the potential to identify environmental and genetic risk factors among Chinese, but also may provide insights into PD etiology in general.
Highlights.
Chinese populations have unique characteristics for Parkinson etiological research
Among Chinese women, passive smoking may be associated with a lower risk for Parkinson disease
A substantial portion of the self-reported Parkinson diagnoses in China could not be clinically confirmed; diagnostic confirmation by a movement disorder specialist is needed.
Acknowledgements
The authors also thank participants of the Shanghai Women’s Health Study for their continuous support and Mrs. Mary Watson and Yi Lu from Social & Scientific Systems, Inc. for help on data collection.
Funding: The study was supported by the Intramural Research Program of NIH, the National Institute of Environmental Health Sciences (Z01-ES-101986) and in part by NIH Grant R37CA070867 and UM1CA182910.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Roles: 1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
Chen, Ding, Wang, Yang: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
Ding, Wang: 1A, 1B, 1C, 3B
Zhao, Meng, Li, Gao, Shu, Tanner, Hong: 1B, 1C, 3B
Full Financial Disclosures of all Authors for the Past Year: Caroline M. Tanner MD, PhD, is an employee of the University of California-San Francisco and the San Francisco Veterans Affairs Medical Center. She serves on the Scientific Advisory Boards of the Michael J. Fox Foundation and the National Spasmodic Dysphonia Association as a voluntary consultant, and has provided paid consulting services to Pfizer Pharmaceuticals. She receives grant support from the Michael J. Fox Foundation, the Parkinson’s Disease Foundation, the Department of Defense and the National Institutes of Health.
All authors reported no relevant conflict of interest.
References
- 1.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol. 2011;26(Suppl 1):S1–S58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 2.Zhang ZX, Roman GC, Hong Z, Wu CB, Qu QM, Huang JB, Zhou B, Geng ZP, Wu JX, Wen HB, Zhao H, Zahner GE. Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet. 2005;365:595–597. doi: 10.1016/S0140-6736(05)17909-4. [DOI] [PubMed] [Google Scholar]
- 3.Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 4.Rocca WA. Prevalence of Parkinson's disease in China. Lancet Neurol. 2005;4:328–329. doi: 10.1016/S1474-4422(05)70079-X. [DOI] [PubMed] [Google Scholar]
- 5.Tan LC, Koh W-P, Yuan J-M, Wang R, Au W-L, Tan JH, Tan E-K, Yu MC. Differential Effects of Black versus Green Tea on Risk of Parkinson's Disease in the Singapore Chinese Health Study. Am J Epidemiol. 2008;167:553–560. doi: 10.1093/aje/kwm338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rastogi T, Hildesheim A, Sinha R. Opportunities for cancer epidemiology in developing countries. Nat Rev Cancer. 2004;4:909–917. doi: 10.1038/nrc1475. [DOI] [PubMed] [Google Scholar]
- 7.Zheng W, Chow W-H, Yang G, Jin F, Rothman N, Blair A, Li H-L, Wen W, Ji B-T, Li Q, Shu X-O, Gao Y-T. The Shanghai Women's Health Study: Rationale, Study Design, and Baseline Characteristics. Am J Epidemiol. 2005;162:1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 8.Hughes A, Daniel S, Kilford L, Lees A. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Nie ZY, Liu Y, Chen W, Xin SM, Sun XD, Fan JH, Liu YH, Gao XH, Lu LQ, Como P, McDermott MP, Qiao YL, Kieburtz K. The prevalence of PD in a nutritionally deficient rural population in China. Acta Neurol Scand. 2005;112:29–35. doi: 10.1111/j.1600-0404.2005.00434.x. [DOI] [PubMed] [Google Scholar]
- 11.Ma CL, Su L, Xie JJ, Long JX, Wu P, Gu L. The prevalence and incidence of Parkinson's disease in China: a systematic review and meta-analysis. J Neural Transm. 2014;121:123–134. doi: 10.1007/s00702-013-1092-z. [DOI] [PubMed] [Google Scholar]
- 12.Menza M. The personality associated with Parkinson's disease. Curr Psychiatry Rep. 2000;2:421–426. doi: 10.1007/s11920-000-0027-1. [DOI] [PubMed] [Google Scholar]
- 13.Mellick GD, Gartner CE, Silburn PA, Battistutta D. Passive smoking and Parkinson disease. Neurology. 2006;67:179–180. doi: 10.1212/01.wnl.0000223618.53129.56. [DOI] [PubMed] [Google Scholar]
- 14.Searles Nielsen S, Gallagher LG, Lundin JI, Longstreth WT, Jr, Smith-Weller T, Franklin GM, Swanson PD, Checkoway H. Environmental tobacco smoke and Parkinson's disease. Mov Disord. 2012;27:293–296. doi: 10.1002/mds.24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quik M. Smoking, nicotine and Parkinson's disease. Trends in Neurosciences. 2004;27:561–568. doi: 10.1016/j.tins.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Frigerio R, Elbaz A, Sanft KR, Peterson BJ, Bower JH, Ahlskog JE, Grossardt BR, de Andrade M, Maraganore DM, Rocca WA. Education and occupations preceding Parkinson disease: a population-based case-control study. Neurology. 2005;65:1575–1583. doi: 10.1212/01.wnl.0000184520.21744.a2. [DOI] [PubMed] [Google Scholar]
- 17.Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, Tanner CM, Masaki KH, Blanchette PL, Curb JD, Popper JS, White LR. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Mosley TH, Alonso A, Huang X. Plasma Urate and Parkinson's Disease in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 2009;169:1064–1069. doi: 10.1093/aje/kwp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ton TG, Jain S, Boudreau R, Thacker EL, Strotmeyer ES, Newman AB, Longstreth WT, Checkoway H. Post hoc Parkinson's disease: identifying an uncommon disease in the Cardiovascular Health Study. Neuroepidemiology. 2010;35:241–249. doi: 10.1159/000319895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Huang X, Guo X, Mailman RB, Park Y, Kamel F, Umbach DM, Xu Q, Hollenbeck A, Schatzkin A, Blair A. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74:878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson JR, Shalat SL, Buckley B, Winnik B, O'Suilleabhain P, Diaz-Arrastia R, Reisch J, German DC. Elevated Serum Pesticide Levels and Risk of Parkinson Disease. Arch Neurology. 2009;66:870–875. doi: 10.1001/archneurol.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross GW, Duda JE, Abbott RD, Pellizzari E, Petrovitch H, Miller DB, O'Callaghan JP, Tanner CM, Noorigian JV, Masaki K, Launer L, White LR. Brain organochlorines and Lewy pathology: the Honolulu-Asia Aging Study. Mov Disord. 2012;27:1418–1424. doi: 10.1002/mds.25144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisskopf MG, Knekt P, O'Reilly EJ, Lyytinen J, Reunanen A, Laden F, Altshul L, Ascherio A. Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology. 2010;74:1055–1061. doi: 10.1212/WNL.0b013e3181d76a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SA, Dai Q, Zheng W, Gao YT, Blair A, Tessari JD, Tian Ji B, Shu XO. Association of serum concentration of organochlorine pesticides with dietary intake and other lifestyle factors among urban Chinese women. Environ Int. 2007;33:157–163. doi: 10.1016/j.envint.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Cai H, Shu XO, Gao YT, Li H, Yang G, Zheng W. A prospective study of dietary patterns and mortality in Chinese women. Epidemiology. 2007;18:393–401. doi: 10.1097/01.ede.0000259967.21114.45. [DOI] [PubMed] [Google Scholar]
- 28.Gao X, Chen H, Fung TT, Logroscino G, Schwarzschild MA, Hu FB, Ascherio A. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. 2007;86:1486–1494. doi: 10.1093/ajcn/86.5.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okubo H, Miyake Y, Sasaki S, Murakami K, Tanaka K, Fukushima W, Kiyohara C, Tsuboi Y, Yamada T, Oeda T, Shimada H, Kawamura N, Sakae N, Fukuyama H, Hirota Y, Nagai M. Dietary patterns and risk of Parkinson's disease: a case-control study in Japan. Eur J Neurol. 2012;19:681–688. doi: 10.1111/j.1468-1331.2011.03600.x. [DOI] [PubMed] [Google Scholar]
- 30.Louis ED. Essential tremor. Lancet Neurol. 2005;4:100–110. doi: 10.1016/S1474-4422(05)00991-9. [DOI] [PubMed] [Google Scholar]