Abstract
The isolation of OXA-48-producing Enterobacteriaceae has increased dramatically in Mediterranean countries in the past 10 years, and has recently emerged in Asia. Between January 2012 and May 2014, a total of 760 carbapenem non-susceptible Klebsiella pneumoniae (CnSKP) isolates were collected during a Taiwan national surveillance. Carbapenemases were detected in 210 CnSKP isolates (27.6%), including 162 KPC-2 (n = 1), KPC-3, KPC-17, and NDM-1 (n = 1 each), OXA-48 (n = 4), IMP-8 (n = 18), and VIM-1 (n = 24). The four bla OXA-48 CnSKP isolates were detected in late 2013. Herein we report the emergence OXA-48-producing K. pneumoniae isolates in Taiwan. PFGE analysis revealed that the four isolates belonged to three different pulsotypes. Three isolates harboured bla CTX-M genes and belonged to MLST type ST11. In addition, the plasmids belonged to the incompatibility group, IncA/C. One isolate belonged to ST116 and the plasmid incompatibility group was non-typeable. The sequence upstream of the bla OXA-48 gene in all four isolates was identical to pKPOXA-48N1, a bla OXA-48-carrying plasmid. This is the first report of OXA-48-producing Enterobacteriaceae in Taiwan and the second report to identify bla OXA-48 on an IncA/C plasmid in K. pneumoniae. Given that three isolates belong to the same pandemic clone (ST11) and possess the IncA/C plasmid and similar plasmid digestion profile that indicated the role of clonal spread or plasmid for dissemination of bla OXA-48 gene, the emergence of OXA-48-producing K. pneumoniae in Taiwan is of great concern.
Background
Different from the third and fourth generations of cephalosporins, carbapenems are currently more consistently active against Enterobacteriaceae because carbapenems are not inactivated by extended-spectrum β-lactamases (ESBLs) or plasmid-encoded AmpC cephalosporinases. Resistance to carbapenems is relatively rare; therefore, this class of drugs is considered the last option for the treatment of severe infections.
Carbapenemases are described as chromosomally-encoded β-lactamases prior to the identification of plasmid-encoded IMP-1, OXA-23 (ARI-1), and KPC-2. The emergence of plasmids containing carbapenemase genes, including KPC-type (class A), as well as IMP-,VIM-, and NDM-types (class B), is considered a serious threat as the emergence of plasmids containing carbapenemase genes facilitates the dissemination of carbapenem resistance [1], and the carbapenemases hydrolyze almost all β-lactams. Indeed, plasmid-borne carbapenemases have been isolated worldwide, including Taiwan [1–4]. After the first identification of a Klebsiella pneumoniae strain expressing plasmid-encoded class D carbapenemase OXA-48 in Istanbul [5], the incidence of OXA-48 expression by Enterobacteriaceae has increased dramatically in Mediterranean countries [6]. In 2012, a 4-year surveillance program was initiated in Taiwan to investigate carbapenem resistance mechanisms, especially with respect to plasmid-borne carbapenemases in K. pneumoniae and Escherichia coli. Herein we report the identification of the first four isolates expressing OXA-48 during this surveillance program.
Methods
Bacterial strains and susceptibility testing
The participating hospitals in the national survey identified imipenem or meropenem non-susceptible K. pneumoniae and E. coli isolates and sent the isolates to a reference laboratory at the National Health Research Institutes of Taiwan. A total of 760 imipenem- or meropenem-non-susceptible K. pneumoniae and 144 imipenem- or meropenem-non-susceptible E. coli isolates were consecutively collected from 18 hospitals (11 medical centers and 7 regional hospitals) between January 2012 and April 2014. The participating hospitals in the surveillance program distributed in all regions of the country. The regional hospitals can handle most diseases and injuries; however, medical centers provide services with more specialists, especially critical care specialist and specialists who can diagnose and treat immune-compromised patients. All the isolates were from individual cases.
This study was approved by the Institutional Review Boards of all participating hospitals, including Kaohsiung Medical University Chung-Ho Memorial Hospital (KMUH-IRB-20110328), Linkou Chang Gung Memorial Hospital (1003399B), Chung Shan Medical University Hospital (CSMUH-CS-12187), and National Taiwan University Hospital (201110043RB).
The isolates were obtained as part of routine hospital care procedures, and written informed consent for participation in the study was waived. The primary screening for carbapenem resistance was performed by the individual participating hospitals. Further confirmation of antimicrobial susceptibility was determined using the broth micro-dilution method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [7].
Minimum inhibitory concentrations (MICs) for carbapenems (ertapenem, imipenem, meropenem, and doripenem) and other antimicrobial agents, including cefazolin, cefotaxime, cefoxitin, cefepime, ciprofloxacin, amikacin, gentamicin, trimethoprim-sulfamethoxazole (SXT), colistin, and tigecycline, were determined using the broth micro-dilution method (Sensititre; Trek Diagnostic Systems, Cleveland, OH, USA). CLSI M100-S24 [8] interpretive breakpoints were used to interpret the MIC results for all antimicrobial agents studied, with the exception of tigecycline and colistin. The Food and Drug Administration (FDA) breakpoint was used for tigecycline and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoint was used for colistin.
Detection of genes encoding carbapenemases, AmpC, and ESBLs
Carbapenemase genes (e.g., class B families [bla IMP, bla VIM, bla NDM, bla GIM, bla SPM, and bla SIM], class A families [bla NMC, bla IMI, bla SME, bla KPC, and bla GES], and class D [bla OXA-48]), plasmid-encoding AmpC (e.g., bla CMY, bla DHA, bla FOX, and bla ACT) [9], and ESBL genes (e.g., bla CTX-M, bla TEM, and bla SHV) were detected by PCR amplification [4]. The amplicons were sequenced, and the entire sequence of each gene was compared to the sequences in the GenBank nucleotide database at http://www.ncbi.nlm.nih.gov/blast/.
Characteristics of bla OXA-48-containing plasmids
Plasmid conjugation was performed using E. coli J53 AzR as the recipient strain. The recipients and bla OXA-48–carrying donor samples were separately inoculated into brain-heart infusion broth and incubated at 37°C for 4 h. The samples were then mixed at a ratio of 10:1 (donor: recipient [v: v]) for an overnight incubation at 37°C. A 0.1-mL aliquot of the overnight broth mixture was spread onto a MacConkey agar plate containing sodium azide (100 μg/mL) and imipenem (1 μg/mL). The plasmids were extracted from these conjugants using a standard alkaline lysis method, and the fingerprints of the plasmids were generated by digestion with EcoRI (New England Biolabs, Beverly, MA, USA), as previously described [10]. Plasmid incompatibility was determined by a PCR-based replicon typing scheme, as previously described [11]. PCR and sequencing of the upstream of bla OXA-48 gene were performed using the following primers in this study: OXA-48F, 5’-CGCATCTTGTTGTCCAAGTG-3’; and OXA-48R, 5’-TCGAGCATCAGCATTTTGTC-3’. The full sequence length was 1012 bp.
Pulsed-field gel electrophoresis (PFGE)
Total DNA was prepared and digested with XbaI (New England Biolabs), as suggested by the manufacturer. The restriction fragments were separated by PFGE in a 1% agarose gel (Bio-Rad, Hercules, CA, USA) with 0.5× TBE buffer (45mM Tris, 45mM boric acid, and 1.0mM EDTA [pH 8.0]) for 22 h at 200 V and 14°C, with ramp times of 2–40 s using a CHEF Mapper apparatus (Bio-Rad Laboratories, Richmond, CA, USA). The Dice coefficient was used to calculate similarities, and the unweighted pair-group method with the arithmetic mean (UPGMA) was used for the cluster analysis using the BioNumerics software (version 5.10; Applied Maths, St-Martens-Latem, Belgium).
Multi-locus Sequence Type
Multi-locus sequence typing (MLST) was performed on the four OXA-48-positive K. pneumoniae isolates according to the protocol described on the K. pneumoniae MLST website (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumonia.html). MLST results were typed according to the international K. pneumoniae MLST database, which was created in 2005 at the Pasteur Institute in Paris, France.
Results
An overall description of the findings in the surveillance program
Of 760 carbapenem non-susceptible K. pneumoniae (CnSKP) isolates, the resistant rates to imipenem, meropenem, doripenem, and ertapenem were 74.7%, 68.3%, 67.3%, and 92.3%, respectively. Carbapenemases were detected in 210 CnSKP isolate (27.6%), including 162 KPC-2, 4 OXA-48, 18 IMP-8, 24 VIM-1, and 1 each of KPC-3, KPC-17, and NDM-1. Almost all KPC-2 CnSKP isolates had the same PFGE type and belonged to MLST ST11. The four bla OXA-48 CnSKP isolates were detected in late 2013.
Case descriptions
None of the patients had a travel history abroad. Isolates 1 and 2 were acquired in one hospital in central Taiwan. The isolates possessed the same genes, including bla OXA-48, bla CTX-M-14, bla TEM-31, and bla SHV-11. Patient 1 was an 85-year-old female who suffered from pneumonia with poorly controlled type 2 diabetes. She was admitted to another hospital before being transferred. The use of antibiotics was not known. Patient 2 was a 78-year-old female who had sepsis, hospital-acquired pneumonia, and a catheter-related infection. The patient was treated with ertapenem for 7 days prior to isolation of the resistant strain. Patients 1 and 2 had overlapping stays in the same unit, which suggested an epidemiologic relationship between cases 1 and 2. Isolates 3 and 4 were isolated from two different medical centers within the same city in northern Taiwan; however, there were no patient transfers between these two centers for the two cases. Isolate 3, which carried bla OXA-48, bla CTX-M-15, bla TEM-1, and bla SHV-11, was obtained from an 82-year-old hospitalized patient who had pneumonia and was transferred from another hospital. Isolate 4 was obtained from a 56-year-old outpatient who had a toothache and had received treatment for dental caries in a community clinic. Isolate 4 had no other extended-spectrum cephalosporinase genes and no permeability defects; isolate 4 was susceptible to the late generation cephalosporins, but resistant to carbapenems. No epidemiologic linkage was found between cases in northern and central Taiwan.
Susceptibilities and extended spectrum β-lactamases of four OXA-48 producers
The antimicrobial susceptibility pattern and the β-lactamases genes carried by the four K. pneumoniae isolates carrying bla OXA-48 are presented in Table 1. Isolate 4 (from case 4) harboured bla OXA-48 alone and was resistant to ampicillin, cefazolin, piperacillin-tazobactam, ticarcillin-clavulanate, ertapenem, imipenem, meropenem, and doripenem. Isolate 4 had intermediate resistance to cefuroxime and was susceptible to cefotaxime, ceftazidime, cefepime, aztreonam, cefoxitin, gentamicin, amikacin, nalidixic acid, ciprofloxacin, trimethoprim-sulfamethoxazole, colistin and tigecycline (Table 1). Isolates 1 and 2 harboured bla OXA-48, bla CTX-M-14, and bla TEM-31, and were resistant to all of the above antibiotics except colistin and tigecycline. Isolate 3 encoded bla OXA-48 and bla CTX-M-15, and was resistant to all of the above antibiotics, except tigecycline (Table 1).
Table 1. Genetic features of four bla OXA-48 Klebsiella pneumoniae.
| Isolate | Specimen | β-lactam MICs (μg/mL) | ST type | Non-β-lactamassociated resistance | Associated β-lactamases | Inc | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ERT | IMP | MEM | CAZ | CTX | ||||||
| 1 | sputum | ≥8 | ≥8 | ≥8 | ≥32 | ≥64 | 11 | Gm, Ak, Q, SXT | CTX-M-14, TEM-31, SHV-11 | IncA/C |
| 2 | urine | ≥8 | ≥8 | ≥8 | 16 | ≥64 | 11 | Gm, Ak, Q, SXT | CTX-M-14, TEM-31, SHV-11 | IncA/C |
| 3 | urine | ≥8 | ≥8 | ≥8 | ≥32 | ≥64 | 11 | Gm, Ak, Q, SXT, Cs | CTX-M-15, TEM-1, SHV-11 | IncA/C |
| 4 | urine | ≥8 | ≥8 | 4 | ≤1 | ≤1 | 116 | none | SHV-1 | NT |
ERT: ertapenem; IMP: imipenem; MER: meropenem; CAZ: ceftazidime; CTX: ceftaxime; Gm: gentamicin; Ak: amikacin; Cs: colistin; MIC: minimum inhibitory concentration; SXT: trimethoprim-sulfamethoxazole; Q: fluoroquinolones; NT: not-typeable
Plasmid digestion profile
Molecular analysis with PCR and plasmid extraction of the E. coli conjugants revealed that bla OXA-48 and bla CTX-M were detected on the same plasmid in isolates 1–3. The plasmid belongs to the incompatibility group, IncA/C (Table 1). The incompatibility group of the plasmid from isolate 4 was not typeable by the PCR-based replicon typing method. The plasmids of conjugants of isolates 1, 2, and 3 exhibited similar restriction profiles (EcoRI digested; Fig 1). The upstream (950 bp) of all 4 plasmids were identical to pKPOXA-48N1 (GenBank database accession number KC757416) [12].
Fig 1. EcoRI-digested plasmid digestion profile of four OXA-48-producing Klebsiella pneumoniae isolates.
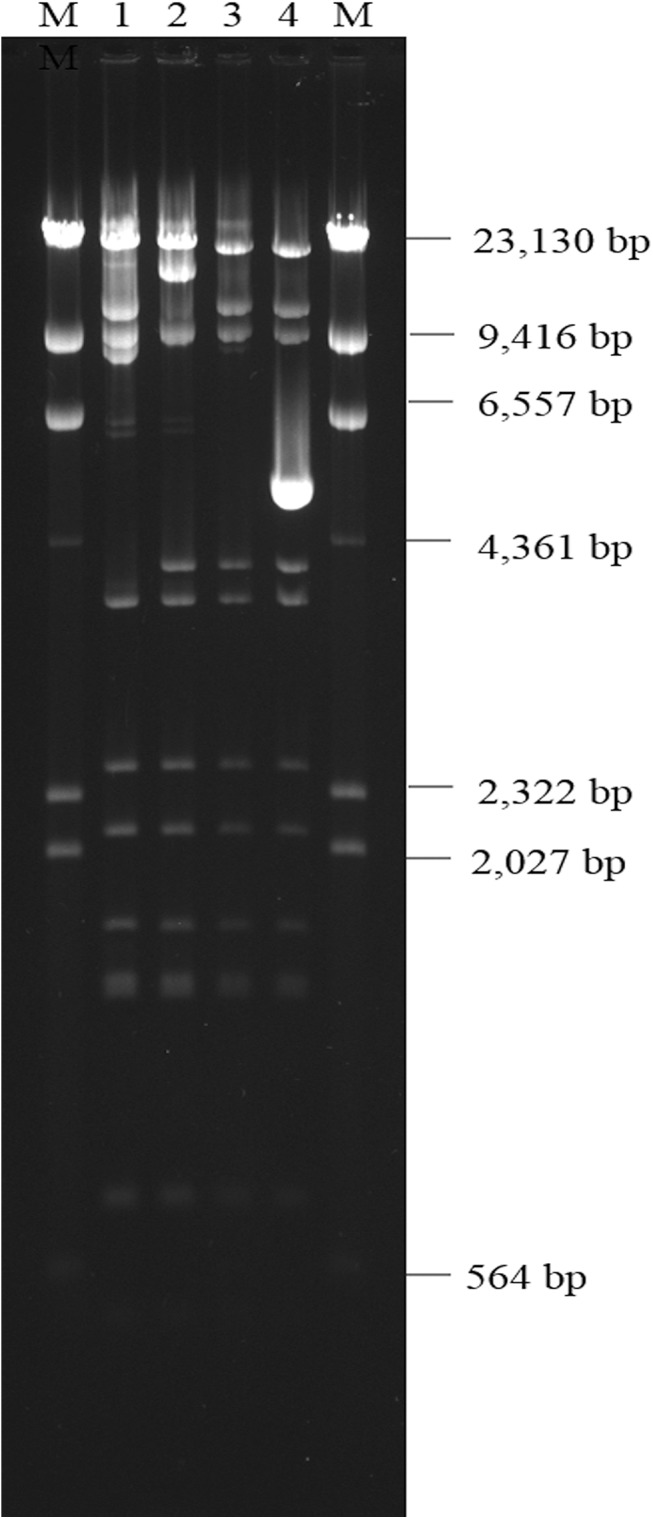
M, marker.
PFGE and MLST analyses
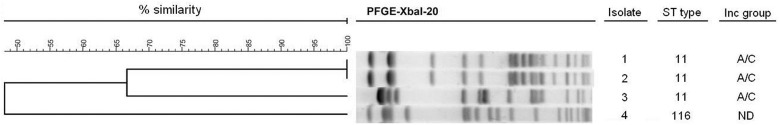
As shown in Fig 2, PFGE revealed 3 clones; isolates 1 and 2 were the same clone and distinct from isolates 3 and 4. Analysis of the ST type revealed that isolates 1–3 belong to ST11, and isolate 4 belongs to ST116 (Table 1 and Fig 2).
Fig 2. Dendrogram of XbaI-digested genomic DNA of four OXA-48 producing Klebsiella pneumoniae isolates.
Discussion
Because bla OXA-48 was first detected in K. pneumoniae in Turkey [5], it has spread rapidly throughout the Middle East and subsequently in Europe, North Africa, North America, and Asia. Most OXA-48-producing isolates were K. pneumoniae; however, OXA-48 was also identified in other Enterobacteriaceae, such as E. coli, Enterobacter spp., Klebsiella oxytoca, Citrobacter freundii, and Serratia marcecens [13].
This is the first report of OXA-48-producing K. pneumoniae in Taiwan. All four isolates appeared in late 2013, but belonged to three different pulsotypes. Moreover, with the exception of cases 1 and 2, no epidemiologic linkage was identified between the 4 cases. The absence of bla OXA-48 detection in the national surveillance program from 2012–2013 suggests that bla OXA-48 is a new resistance gene; however, the epidemiologic data did not reveal that this carbapenemase gene was transmitted from other countries.
Previous studies reported that most bla OXA-48 genes were located on a 62 kb self-conjugative plasmid in Enterobacteriaceae (pOXA-48a from the K. pneumoniae 11978 isolate from Turkey or plasmid pKPOXA-48N1) [12]. In 2013, a new 160-kb bla OXA-48-encoding conjugative plasmid, pKPOXA-48N2, was characterized from K. pneumoniae [12]. The flanking region containing bla OXA-48 in Taiwan isolates was identical to that observed in pKPOXA-48N1 [12]. Comparative analysis of the upstream region of three kinds of bla OXA-48-carrying plasmids (pOXA-48a, pKPOXA-48N1, and our bla OXA-48 plasmids) revealed that the plasmids have identical 164 bp sequences before the bla OXA-48 start codon, including IS1999 insertion sequences. Further upstream of this 164 bp sequence was a difference between pOXA-48a and pKPOXA-48N1. The sequence of our bla OXA-48 carrying plasmids at approximately 950 bp upstream was identical to pKPOXA-48N1. Moreover, three plasmids exhibited very similar restriction profiles (EcoRI) over 60-kb, and matched the predicted digestion profile of pKPOXA-48N1. The plasmid digestion profile of that obtained from isolate 4 also had a similar restriction profile with the exception of containing one additional fragment of approximately 5-Kb. The results indicate the role of plasmids in the dissemination of bla OXA-48. Approximately 80% of OXA-48 producers co-expressed ESBLs [14]; many OXA-48-producing isolates also carried CTX-M-15 [14–16]. In our OXA-48-producing isolates, three isolates also expressed CTX-M enzymes. According to previous surveillance data, the IncA/C-type plasmid was associated with bla CTX-M-15(12.7%) in Asian countries [17]. In this study, three bla OXA-48-containing plasmids also harboured bla CTX-M genes and belonged to the IncA/C group. Although most bla CTX-M-15 were associated with the IncFIIA plasmid, the IncA/C plasmid has also been shown to be related with bla CTX-M, and presumably the dissemination of CTX-M enzymes [17, 18]. Unlike the epidemic bla OXA-48 plasmid incompatibility type, IncL/M [19], this is the second study to identify bla OXA-48 on an IncA/C plasmid [20].
In an 11-year (2001–2011) molecular epidemiologic study of OXA-48 in Europe and North Africa, ST101 was the most frequently observed, followed by ST395 and ST15 in K. pneumoniae; ST11 was rarely detected [16]. In other regions, however, ST11 was the most prevalent ST type for multi-resistant K. pneumoniae [21]. OXA-48-producing isolates have been reported in Turkey, Argentina, and France [15,22]. In Greece, an OXA-48-producing ST11 clone caused an outbreak [23]. With the exception of one isolate (ST116), three of the four OXA-48 producers identified in Taiwan belonged to ST11, the predominant sequence type outside Europe and North Africa. ST11 has been found in association with different ESBLs, primarily CTX-M-15, CTX-M-14, and SHV-5 [24, 25]. ST11 is also the predominant sequence type of imipenem non-susceptible K. pneumoniae isolates with resistance mechanisms other than OXA-48 in Taiwan [3]. Our report identified ST11 to be the major sequence type for OXA-48 producers.
In conclusion, we identified the first cluster of OXA-48 carbapenem-resistant K. pneumoniae in Taiwan. Although identification of OXA-48 producers is still sporadic in Asia, studies have predicted that OXA-group enzymes will successfully spread in the near future [26]. The association between OXA-48 and CTX-M could potentially lead to pan-β-lactam resistance [14]. Considering the high prevalence of ST11 K. pneumoniae and incorporation into the IncA/C plasmid with pandemic CTX-M enzymes, the emergence of OXA-48 is of great concern.
Data Availability
Due to ethical and legal restrictions, anonymized patient-level data are available upon request from the corresponding author.
Funding Statement
This work was supported MOHW103-CDC- C-114-134504 from the Center for Diseases Control, Taiwan and by Kaohsiung Medical University Research Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Queenan AM, Bush K (2007) Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 20: 440–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiu SK, Wu TL, Chuang YC, Lin JC, Fung CP, Lu PL, et al. (2013) National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: the emergence and rapid dissemination of KPC-2 carbapenemase. PLoS One 8: e69428 10.1371/journal.pone.0069428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma L, Lu PL, Siu LK, Hsieh MH (2013) Molecular typing and resistance mechanisms of imipenem-non-susceptible Klebsiella pneumoniae in Taiwan: results from the Taiwan surveillance of antibiotic resistance (TSAR) study, 2002–2009. J Med Microbiol 62: 101–107. 10.1099/jmm.0.050492-0 [DOI] [PubMed] [Google Scholar]
- 4. Ma L, Siu LK, Lin JC, Wu TL, Fung CP, Wang JT, et al. (2013) Updated molecular epidemiology of carbapenem-non-susceptible Escherichia coli in Taiwan: first identification of KPC-2 or NDM-1-producing E. coli in Taiwan. BMC Infect Dis 13: 599 10.1186/1471-2334-13-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poirel L, Heritier C, Tolun V, Nordmann P (2004) Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother 48: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poirel L, Potron A, Nordmann P (2012) OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67: 1597–1606. 10.1093/jac/dks121 [DOI] [PubMed] [Google Scholar]
- 7. Cockerill FR, Wikler MA, Alder J, Dudley MN, Eliopoulos GM (2012) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Approved standard-Ninth Edition Wayne: Clinical and Laboratory Standards Institute; 62 p. [Google Scholar]
- 8. Patel JB, Cockerill FR, Alder J, Bradford PA, Eliopoulos GM (2014) Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. Wayne: Clinical and Laboratory Standards Institute; 226 p. [Google Scholar]
- 9. Alvarez M, Tran JH, Chow N, Jacoby GA (2004) Epidemiology of conjugative plasmid-mediated AmpC beta-lactamases in the United States. Antimicrob Agents Chemother 48: 533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kado CI, Liu ST (1981) Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol 145: 1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. (2005) Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63: 219–228. [DOI] [PubMed] [Google Scholar]
- 12. Berger S, Alauzet C, Aissa N, Henard S, Rabaud C, Bonnet R, et al. (2013) Characterization of a new blaOXA-48-carrying plasmid in Enterobacteriaceae. Antimicrob Agents Chemother 57: 4064–4067. 10.1128/AAC.02550-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nordmann P, Dortet L, Poirel L (2012) Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends in Molecular Medicine 18: 263–272. 10.1016/j.molmed.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 14. Potron A, Nordmann P, Rondinaud E, Jaureguy F, Poirel L (2013) A mosaic transposon encoding OXA-48 and CTX-M-15: towards pan-resistance. J Antimicrob Chemother 68: 476–477. 10.1093/jac/dks397 [DOI] [PubMed] [Google Scholar]
- 15. Liapis E, Pantel A, Robert J, Nicolas-Chanoine MH, Cavalie L, van der Mee-Marquet N, et al. (2014) Molecular epidemiology of OXA-48-producing Klebsiella pneumoniae in France. Clin Microbiol Infect. 20: O1121–1123. 10.1111/1469-0691.12727 [DOI] [PubMed] [Google Scholar]
- 16. Potron A, Poirel L, Rondinaud E, Nordmann P (2013) Intercontinental spread of OXA-48 beta-lactamase-producing Enterobacteriaceae over a 11-year period, 2001 to 2011. Euro Surveill 18. pii: 20549. [DOI] [PubMed] [Google Scholar]
- 17. Lee MY, Ko KS, Kang CI, Chung DR, Peck KR, Song JH. (2011) High prevalence of CTX-M-15-producing Klebsiella pneumoniae isolates in Asian countries: diverse clones and clonal dissemination. Int J Antimicrob Agents 38: 160–163. 10.1016/j.ijantimicag.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 18. Chouchani C, El Salabi A, Marrakchi R, Ferchichi L, Walsh TR. (2012) Characterization of IncA/C conjugative plasmid harboring bla TEM-52 and bla CTX-M-15 extended-spectrum β-lactamases in clinical isolates of Escherichia coli in Tunisia. Eur J Clin Microbiol Infect Dis 31: 1081–1087. 10.1007/s10096-011-1410-z [DOI] [PubMed] [Google Scholar]
- 19. Poirel L, Bonnin RA, Nordmann P (2012) Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother 56: 559–562. 10.1128/AAC.05289-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ktari S, Mnif B, Louati F, Rekik S, Mezghani S, Mahjoubi F, et al. (2011) Spread of Klebsiella pneumoniae isolates producing OXA-48 beta-lactamase in a Tunisian university hospital. J Antimicrob Chemother 66: 1644–1646. 10.1093/jac/dkr181 [DOI] [PubMed] [Google Scholar]
- 21. Woodford N, Turton JF, Livermore DM (2011) Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev 35: 736–755. 10.1111/j.1574-6976.2011.00268.x [DOI] [PubMed] [Google Scholar]
- 22. Lascols C, Peirano G, Hackel M, Laupland KB, Pitout JD (2013) Surveillance and molecular epidemiology of Klebsiella pneumoniae isolates that produce carbapenemases: first report of OXA-48-like enzymes in North America. Antimicrob Agents Chemother 57: 130–136. 10.1128/AAC.01686-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Voulgari E, Zarkotou O, Ranellou K, Karageorgopoulos DE, Vrioni G, Mamali V, et al. (2013) Outbreak of OXA-48 carbapenemase-producing Klebsiella pneumoniae in Greece involving an ST11 clone. J Antimicrob Chemother 68: 84–88. 10.1093/jac/dks356 [DOI] [PubMed] [Google Scholar]
- 24. Ko KS, Lee JY, Baek JY, Suh JY, Lee MY, Choi JY, et al. (2010) Predominance of an ST11 extended-spectrum beta-lactamase-producing Klebsiella pneumoniae clone causing bacteraemia and urinary tract infections in Korea. J Med Microbiol 59: 822–828. 10.1099/jmm.0.018119-0 [DOI] [PubMed] [Google Scholar]
- 25. Hrabak J, Empel J, Bergerova T, Fajfrlik K, Urbaskova P, Kern-Zdanowicz I, et al. (2009) International clones of Klebsiella pneumoniae and Escherichia coli with extended-spectrum beta-lactamases in a Czech hospital. J Clin Microbiol 47: 3353–3357. 10.1128/JCM.00901-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evans BA, Amyes SG (2014) OXA beta-lactamases. Clin Microbiol Rev 27: 241–263. 10.1128/CMR.00117-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to ethical and legal restrictions, anonymized patient-level data are available upon request from the corresponding author.


