Abstract
Background
Large-scale animal feeding operations compromise regional air quality in the rural United States through emission of pollutants such as ammonia gas. Exposure to airborne pollution from animal feeding operations may cause pediatric asthma exacerbations in surrounding communities.
Objectives
To describe spatial and temporal patterns in ambient ammonia concentrations in an agricultural region, and to investigate associations between short-term fluctuations in ammonia and subsequent changes in respiratory health in children with asthma.
Methods
For 13 months in the Yakima Valley of Washington State, 14 monitors sampled ammonia in outdoor air for 24-hour periods every 6 days. School-age children with asthma (n=51) were followed for two health outcomes: biweekly reports of asthma symptoms and quick relief medication usage, and daily measurements of forced expiratory volume in one second (FEV1). We assessed associations between each outcome and ammonia using generalized estimating equations.
Results
24-hour ammonia concentrations varied from 0.2 to 238.1 μg/m3 during the study period and displayed a strong correlation with proximity to animal feeding operations. FEV1% was 3.8% lower (95% CI: 0.2, 7.3) per interquartile increase in one-day lagged ammonia concentration and 3.0% lower (95% CI: 0.5, 5.8) for two-day lagged concentration. We observed no associations between self-reported asthma symptoms or medication usage and estimated ammonia exposure.
Conclusions
Ammonia concentrations were elevated in this community and strongly predicted by proximity to animal feeding operations. Ammonia's association with acute lung function decrements in children with asthma in the surrounding community may be causal or, alternatively, ammonia may be a marker for other pollutants from animal feeding operations associated with respiratory effects.
Introduction
In recent decades the industrialization of commercial agriculture has affected environmental quality in the rural United States, in part due to the rise of large-scale facilities for confinement of livestock and poultry, termed animal feeding operations (AFOs).1 The largest such operations house over a hundred thousand animals in a relatively small area free of vegetation, and substantial amounts of animal manure are generated continuously on site. Liquid and solid animal wastes combine to form a slurry that is commonly stored at animal feeding operation sites in waste lagoons and may eventually be applied to adjacent growing fields as fertilizer. During manure storage as well as application to farmlands, a variety of chemical and biologic pollutants are released to the atmosphere, and resultant impacts on regional air quality have been observed.1-5
Several components of emissions from animal feeding operations may cause or exacerbate respiratory disease for nearby residents, including ammonia gas. Ammonia is water soluble and dissolves in the mucosa of the upper respiratory system upon inhalation, causing eye, nose, and throat irritation,6 a suggested trigger for lower respiratory tract dysfunction, including bronchospasm.7 In addition, ammonia may penetrate to the lower respiratory tract following absorption to particulate matter.8 Animal studies show that ammonia causes epithelial damage and impairment of the lung's mucociliary clearance mechanism, potentially leading to increased susceptibility to inhaled particles and infectious organisms.6 Elevated ambient ammonia can affect the health and well-being of nearby residents according to non-toxicological mechanisms as well. Ammonia has a strong, unpleasant odor that contributes to the overall malodor in vicinity of animal feeding operations, often intense enough to register community complaints.9,10 Malodor can lead to feelings of unease, stress, and agitation in exposed individuals and may cause subsequent respiratory effects at concentrations below established toxicity thresholds.11
Previous epidemiologic investigations of community-level exposures to animal feeding operations and respiratory health are limited to cross-sectional studies,12-17 with the exception of one longitudinal, repeated measures study of healthy adults living near swine feeding operations.18,19 Though some cross-sectional studies have included pediatric subjects, none involved repeated measures of respiratory health for assessment of time-varying asthma health and none included direct measurements of ambient ammonia in the community.12-14,17 Here we describe results of a longitudinal study of pediatric asthma exacerbations using health and environmental data collected from the Aggravating Factors of Asthma in a Rural Environment (AFARE) project set in the Yakima Valley, an agricultural region of Washington State characterized by a high density of large dairy operations.
The primary objectives of our investigation were to describe spatial and temporal patterns in community-level ammonia concentrations in the Yakima Valley, and to estimate associations between time-varying ammonia concentrations outside subjects' homes and short-term fluctuations in asthma health, measured as daily lung function and biweekly reports of symptom and medication use. In all cases, our analyses are focused upon ambient concentrations of a single contaminant, ammonia; however, any observed associations may be due to ammonia itself or to one or more components of AFO airborne emissions, for which ammonia is a marker.
Methods
Study setting
AFARE was conducted within El Proyecto Bienestar (EPB), a community-based participatory research partnership seeking to protect the health of agricultural workers and their families in the Yakima Valley. Project partners include the University of Washington Pacific Northwest Center for Agricultural Safety and Health (PNASH); Yakima Valley Farm Workers Clinic (YVFWC), a network of federally qualified health clinics serving migrant and seasonal farmworker families as well as other underserved populations in the region; and the Northwest Communities Education Center which includes Radio KDNA, a Spanish language public radio station that provides support and education for the Latino community in the Yakima Valley. There is a high density of large-scale agricultural operations in the region including many dairy feeding operations, largely concentrated in the southern half of the valley.
Study subjects
Beginning in August 2010, AFARE subjects were recruited from the YVFWC Asthma Program.20 Inclusion criteria included being of school age and having no serious illnesses other than asthma. A total of 59 children were enrolled in the AFARE study; however, for the analyses described here we consider only the 51 children who participated for at least three months during the period of air monitoring (September 2011 through October 2012). At enrollment all subjects underwent skin prick testing to 22 common and locally relevant inhalant allergens in order to assess atopy (grass mix, Johnson grass, wheat, kochia, Russian thistle, cockroach, pigweed, mite mix, oak, cottonwood, alfalfa, cat, alder, sagebrush, horse, Western juniper, dog, mixed mold, mouse, Western ragweed, smut mix and cow). Families also completed a health history survey to describe clinical features of past and present asthma health. Research activities were approved by the University of Washington Institutional Review Board, and informed consent was obtained from all children prior to participation. Research materials for all stages of the AFARE study, from recruitment through data collection, were available in both Spanish and English.
Identification of AFOs
We used the WA State Department of Agriculture (WSDA) database of dairy operations registered in 2012 through the Dairy Nutrient Management Program (DNMP) to identify animal feeding operations in the region. We systematically inspected aerial photography images of the entire study region available online via Google Maps and Google Earth for characteristics of animal feeding operations such as large dirt areas containing cattle, feeding and milking shelters, and animal waste storage ponds in order to confirm -- and when necessary, correct -- the location of the 67 dairy operations registered with the DNMP. We also identified additional non-dairy operations, including heifer, beef, and poultry operations not included in the WSDA database. The area of all land that appeared to be part of each operation was estimated in units of m2 and the geographic location was approximated as the center of the facility.
Outcome variables
Biweekly Asthma Symptom Survey
At approximately two-week intervals, phone interviews with either the child or an adult family member were conducted. Interviewees were asked to recall the one week period prior to the interview date in their responses. The interview included five questions about asthma symptoms (nighttime waking, shortness of breath, limitation of activities, wheezing, and morning asthma symptoms). A sixth question ascertained short-acting bronchodilator frequency of use as average number of “puffs” per day.
Daily home lung function tests
At the time of enrollment, each child received a PikoNET PiKo-1 handheld peak flow meter with digital memory (nSpire Health, Inc; Longmont, CO) and was instructed in proper use of the device according to guidelines of the American Thoracic Society. Children were asked to use the peak flow meter twice daily, in the morning and evening, every day of the study and to withhold use of short-acting bronchodilator medication in the period prior. At approximately six-week intervals, a staff member from the YVFWC Asthma Program visited participants and uploaded peak flow meter measurements from the participant's device. During a 12-month AFARE follow-up visit with the research team at clinics and immediately prior to the start of ammonia monitoring, each subject's technique and ability to produce an error-free measurement was observed, and subjects were retrained in peak flow meter use if necessary. FEV1 measurements from the peak flow meter were converted into percent of predicted values (FEV1%) based on standard reference equations.21 Values of FEV1% that were implausibly high (above 150%) or low (below 30%) as well as measurements that were flagged by the device as potential errors were omitted from analysis. FEV1 measurements were not available for all subjects on every day enrolled in the study on account of several factors, including imperfect compliance as well as technical problems with the equipment used to upload PFM data during home visits. To the best of our knowledge, loss of data in the field due to technical difficulties was completely random to health and exposure status of subjects.
Ammonia measurements and meteorology
We have described the design, deployment and performance of the air sampling device previously.22 Fourteen devices were placed outside the homes of a subset of the AFARE participants that were selected based on property accessibility and security as well as consideration of overall spatial variability across the study region. Four monitors were moved during the air monitoring period because study subjects changed residences (n=3) or the family dropped out of the study (n=1), and one monitor was destroyed in a home fire three months prior to the end of the study and was not replaced. The monitoring device was constructed to collect multiple air samples simultaneously for analysis of multiple contaminants for 24-hour periods at six-day intervals, including ammonia, PM2.5, total dust, and various pesticide compounds. Only ammonia concentrations are discussed in this report due to lack of sample analysis data for other contaminants. Devices actively sampled outdoor air for ammonia using a silica bead sampling tube (SKC Inc; Eighty Four, PA) with flow rates ranging from 0.10-0.32 L/min according to the Occupational Safety and Health Administration (OSHA) method ID-188. Flow rates of sampling pumps were calibrated once a month and were adjusted to meet the intended flow rate range when necessary. Sampling tubes were transported to the University of Washington Environmental Health Laboratory at 0°C, where they were desorbed with deionized water and analyzed for ammonium ion using ion chromatography. Ammonia masses below the limit of detection (either 1 or 0.5 μg, depending on the date of analysis) were approximated as (limit of detection)/2.
Two local weather stations central to the study region provided data on 24-hour average temperature, relative humidity and precipitation (Washington State University AgWeatherNet stations at Toppenish and Snipes).
Statistical analysis
We assessed relationships between ammonia concentrations and animal feeding operation (AFO) proximity using four metrics calculated relative to each monitor location: distance to nearest animal feeding operation, count of operations within a 5 km buffer, count of operations within a 10 km buffer, and a weighted sum of regional operations calculated according to Equation 1:
| (Equation 1) |
We calculated Pearson correlation coefficients (r) to assess pairwise correlations between each of the four estimates of animal feeding operation exposure and median ammonia measured at the 18 monitoring sites.
We modeled associations between time-varying ammonia exposure and each of the outcome measures separately using generalized estimating equations (GEE) with an exchangeable correlation matrix.23 For analyses of FEV1%, lagged relationships ranging from 0 (same day) to 5 days were investigated. We estimated ammonia exposure at homes without monitors using the ammonia concentrations measured at nearest neighbor monitors. In contrast, we did not attempt to extrapolate ammonia measurements to days without readings, because the temporal variability in measured ammonia did not appear to vary in a predictable manner. Instead, only the available ammonia measurements and FEV1 readings corresponding to specific lag relationships (i.e. same day, lag 1, lag 2, etc.) were analyzed even though this greatly reduced the amount of data available for analysis.
For the six outcomes derived from biweekly interviews about asthma symptoms and medication usage, responses to each of the six questions were dichotomized as no symptom/medication use versus any symptom/medication use reported. The odds ratio for report of each symptom or medication use was estimated using GEE in association with the weekly average ammonia concentrations. Weekly average ammonia concentrations were estimated by identifying all ammonia measurements made at the five nearest neighbor monitors for a subject's home between eight days and one day prior to the interview date and averaging these measurements by the inverse square distance between home and monitor site. (Inverse distance weighting was not possible for FEV1 analysis because there was a one day difference in sampling dates for monitors in the northern versus southern half of the study region.) In all epidemiologic models, the mean outcome was modeled to be linear in response to the primary exposure of interest, and we presented the final results as effect sizes per interquartile range (IQR) increases. In sensitivity analysis, the main analyses were repeated following restriction to children living within the median distance to the nearest air monitor (1.0 km).
Covariates included in models as potential confounders were selected a priori based on existing evidence of relationships between the covariate and both respiratory health and exposure: temperature and relative humidity (averaged over the week prior for outcomes related to reported asthma symptoms) as well as two variables related to temporal trends, days elapsed in study and seasonality, represented by cubic splines. Also included were subject-specific characteristics potentially associated with asthma: sex, age, atopy, use of inhaled corticosteroids at baseline, BMI at baseline and presence of adult smoker in household.
Model diagnostics were performed to determine whether the central assumptions of GEE were violated. Specifically, plots of residuals versus the linear predictor were inspected, and the possibility of influential subjects was explored using the “leave one out” method. These analyses did not indicate the presence of any issues related to model assumptions. Analyses were repeated using linear mixed models (LMM), and in all cases produced similar results to those obtained with GEE.
All analyses were performed using Stata 12.0 IC (StataCorp LP; College Station, TX), with the exception of distance and nearest neighbor determinations, which were conducted using R (version 2.14.2, The R Foundation for Statistical Computing).
Results
Cohort characteristics
Of the 51 children enrolled in AFARE in the second year, many were from low-income families with one or more adults employed as a farm worker, and self-identified as Hispanic/Latino (Table 1). The average age at enrollment was 10.3 years. The majority of children were taking controller medications at the time of enrollment (i.e., inhaled corticosteroids and/or leukotriene antagonists), and 38 subjects (74%) were identified to be atopic on the basis of skin prick testing performed at baseline. Only 7 children (14%) lived with at least one adult smoker. Based on a clinical exam performed at baseline, nearly half of the subjects (49%) were classified as overweight, defined as BMI-for-age above the 85th percentile.
Table 1. Characteristics of AFARE children (n=51).
| N (49%) | |
|---|---|
| Demographics | |
| Female | 25 (49%) |
| Household income <=$15k/yeara | 20 (39%) |
| Born outside US | 10 (20%) |
| Hispanic/Latino ethnicity | 47 (92%) |
| Parent(s) employed as farm worker | 23 (45%) |
| Asthma and general health at baseline | |
| Medication use at baseline: | |
| Inhaled corticosteroids (IC) only | 22 (43%) |
| Leukotriene antagonist (LTRA) only | 2 (4%) |
| Both IC and LTRA | 14 (27%) |
| Ever hospitalized with asthma | 34 (67%) |
| Unscheduled visit for asthma to urgent care or ED in 12 months prior to enrollment | 39 (76%) |
| Atopicb | 38 (74%) |
| At least one adult smoker in household | 7 (14%) |
| BMI for age >85th percentile | 25 (49%) |
Two subjects did not respond.
Indicated by positive skin prick test to at least one of 22 common inhalant allergens.
Abbreviations: BMI = body mass index; ED = emergency department
Longitudinal health data
In the period during which residential air monitors collected samples for ammonia analysis, subjects completed an average of 20 interviews each. Each individual symptom was reported to have occurred in fewer than 50% of the interviews. An average of 87 FEV1 measurements were collected for each child. The average completion rate, calculated from the number of days on which FEV1 readings were available relative to the period of study enrollment for each subject, was estimated to be 30%. This value does not reflect true compliance rates, however, as it was substantially reduced by time periods during which data was lost due to technical difficulties. We thoroughly explored whether the probability of missingness in a given week was associated with each subject's asthma health during a particular week or over the entire study period (assessed with results from symptom interviews), lung function prior to and following periods of missing data, and exposure status, and found no relationships (data not shown.)
FEV1 increased for the cohort across the study period on average, with the population-average lung function growth estimated to be 0.26 L per year (s.d. = 0.37 L). Across the overall study period, the average FEV1% was 81% (s.d. = 17%).
Community ammonia concentrations
There were 18 locations where monitoring was conducted throughout the Yakima Valley region (Figure 1). For AFARE subjects without monitors placed at their homes, the distance from subject home to the nearest AFARE monitor ranged from 0.01 to 9 km. 24-hour ammonia concentrations varied from 0.2 to 238.1 μg/m3 during the study period and the median ammonia measured at each site ranged from 2.9 to 72.7 μg/m3, with higher concentrations observed in the southern region of the study area (Figure 2). Concentrations at each site exhibited a high degree of temporal variability on short time scales (i.e. days to weeks). Ammonia concentrations were weakly and negatively correlated with both daily wind speed (r=-0.29) and direction (defined as degrees on a 360° wind rose; r=-0.22), weakly correlated with relative humidity (r=0.17) but not correlated with temperature or precipitation (r<0.05). Some evidence for seasonal patterns in ammonia concentrations was observed, with slightly elevated concentrations measured during the winter months (Table 2).
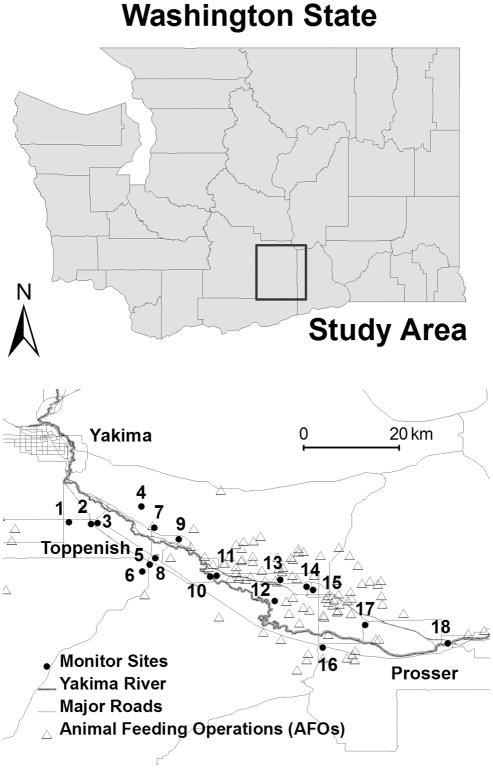
Figure 1.
Map of study region, including 18 monitoring locations and 97 animal feeding operations (AFOs). Locations of monitors are jittered in random directions and distances (up to 1 km) to protect subject identities.
Figure 2.
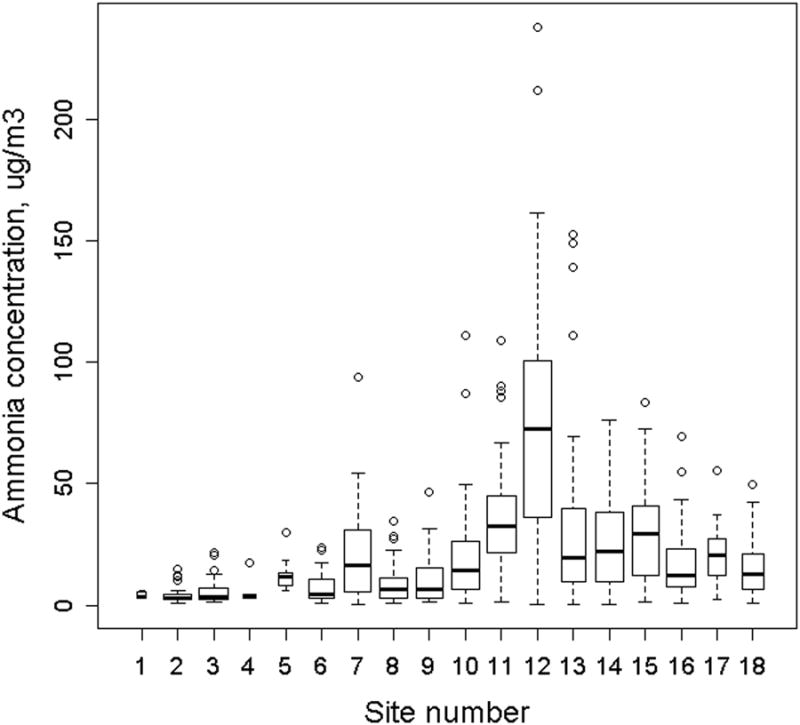
24-hour average ammonia concentrations (n=814) measured at six-day intervals over a 14 month sampling period. Monitoring sites are numbered east to west across the study area. The width of each box is inversely proportional to the number of measurements made at that site.
Table 2. 24-hour average ammonia (NH3) concentrations by season.
| Season | Median NH3 (μg/m3) | IQR |
|---|---|---|
| Dec, Jan, Feb | 17.2 | 3.8, 40.8 |
| Mar, Apr, May | 11.6 | 3.5, 22.1 |
| Jun, Jul, Aug | 11.8 | 3.5, 27.8 |
| Sep, Oct, Nov | 12.9 | 4.3, 31.2 |
Abbreviations: NH3 = ammonia; IQR = interquartile range
Our scan of aerial images of the region identified 97 likely animal feeding operation sites, ranging in size from 6500 to 900,000 m2 in estimated surface area (mean area = 190,000 m2). Median ammonia measured at each monitoring site exhibited a correlation with each measure of animal feeding operation proximity (Table 3). Distance to the nearest facility explained the smallest amount of variability in site median ammonia (R2 = 0.56), while the sum of inverse distances accounted for 78% of the variability in ammonia. Each of the four metrics of proximity to animal feeding operations displayed a high degree of pairwise correlation.
Table 3. Median ammonia (NH3) and metrics of Animal Feeding Operation (AFO) proximity: Correlation matrix.
| Log (median NH3 (μg/m3)) | Distance to nearest AFO (km) | # AFOs < 5 km | # AFOs < 10 km | Σ log(area)/distance for all AFOs in region (Equation 1) | |
|---|---|---|---|---|---|
| Log (median NH3 (μg/m3)) | 1.00 | ||||
| Distance to nearest AFO (km) | -0.75 | 1.00 | |||
| # AFOs < 5 km | 0.84 | -0.64 | 1.00 | ||
| # AFOs < 10 km | 0.80 | -0.64 | 0.97 | 1.00 | |
| Σ log(area)/distance for all AFOs in region | 0.88 | -0.73 | 0.98 | 0.97 | 1.00 |
Abbreviations: NH3 = ammonia; AFO = animal feeding operation
Ammonia exposure and asthma morbidity
We found no relationship between reported asthma symptoms or medication usage and the weekly ammonia exposure estimated for the week prior to the interview date (Table 4). Expanding the exposure window to include two and four weeks prior to the interview data had no meaningful effect on the results.
Table 4. Odds of specific asthma symptoms associated with estimate weekly ammonia.
| Symptom or medication use | OR (95%CI)a |
|---|---|
| Limitation of activities | 1.1 (0.79, 1.4) |
| Wheezing | 0.99 (0.77, 1.3) |
| Nighttime waking | 0.92 (0.76, 1.3) |
| Shortness of breath | 1.1 (0.86, 1.3) |
| Symptoms worse in morning | 0.88 (0.75, 1.0) |
| Use of short-acting “relief” medication | 0.97 (0.82, 1.2) |
Abbreviations: IQR, interquartile increase; OR, odds ratio
OR is the odds ratio for report of any symptom/medication use in week prior associated with an IQR increase in weekly ammonia (18 μg/m3).
Ammonia exposure was associated with FEV1% at various lag intervals, with FEV1% being lower following days of elevated ammonia (Figure 3). FEV1% was decreased by 3.8% (96%CI: 0.2, 7.3) per IQR increase in ammonia one day following ammonia measurement and decreased by 3.0% (96%CI: 0.5, 5.8) two days later. When we restricted analyses to children living within 1.0 km of an air monitor (n=23), associations were larger in magnitude (i.e. more negative) but generally less precise. FEV1% decreased 6.3% (95%CI: 2.3%, 10%) two days after exposure, for example, for the subset of children living near monitors.
Figure 3.
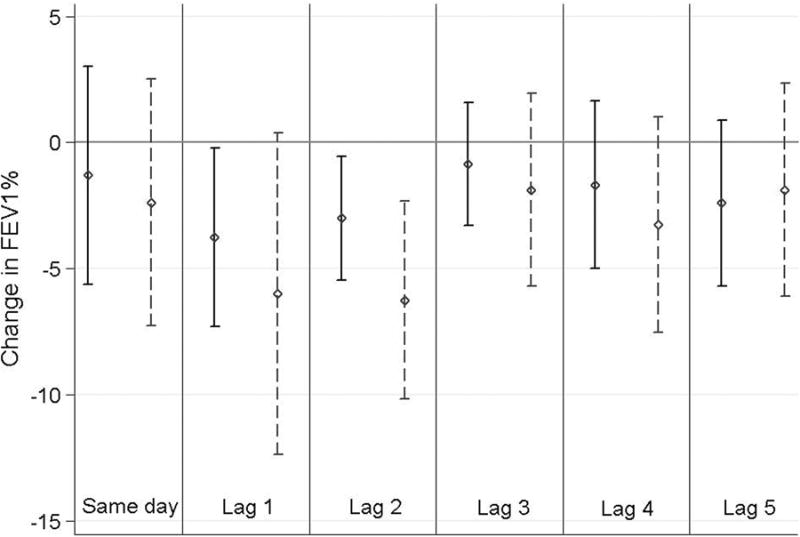
Associations between FEV1% and ammonia (NH3) concentrations measured at the nearest neighbor monitors. For each lag relationship, results for the entire cohort (solid line) as well as those obtained after restriction to subjects living within 1 km of the nearest monitor (dashed line) are displayed. Point estimates and 95% CI represent changes associated with an IQR increase in 24-hour average ammonia (25 μg/m3). Abbreviations: FEV1% = forced expiratory volume in 1 second as percent of predicted; IQR = interquartile range; GEE = generalized estimating equations; BMI = body mass index; CI = confidence interval
Discussion
Our results provide unique insight into population-level ammonia exposures occurring in an agricultural community and potential effects on the respiratory health of children with asthma. We found that ambient ammonia concentrations were elevated in the southern half of the Yakima Valley where most animal feeding operations were located. At the monitoring site with the highest density of surrounding feeding operation, the 75th percentile of 24-hour ammonia concentrations was 101 μg/m3; this can be compared to the EPA reference concentration for chronic inhalation exposure of 100 μg/m3. The annual average ammonia concentrations in our study region was 19 μg/m3, comparable to concentrations reported by some investigators in similar study regions.4,16,24,25
Our findings indicate that geographic differences in ammonia concentrations were explained in large part by proximity to animal operations, with 77% of the between site variability in time-averaged ammonia concentrations explained by a distance- and size-weighted measure of proximity (Equation 1). This is not surprising given that the majority of anthropogenic ammonia emissions worldwide are attributable to animal production facilities, which release ammonia during the production and handling of animal manure, as well as during application of liquid and solid wastes to nearby fields.1 We found that ambient concentrations were slightly higher in winter months, the time of year when fertilization occurs in this region, even though ammonia emission rates from animal feeding operations generally increase at higher temperatures.26 We observed a high degree of temporal variability in ammonia concentrations with six day sampling intervals, a feature that is important to consider in design of epidemiologic studies to estimate associations between ambient ammonia exposure and short-term health effects.
We found no evidence that increased ammonia exposure was linked to worsened asthma health as measured by self-reported symptoms and medication usage. In contrast, we found associations between a measure of lung function, FEV1%, and estimated ammonia exposure at subjects' homes. Effect sizes were largest in magnitude and most precise in relation to ammonia measured one and two days prior to lung function measurements, supporting our hypothesis that short term respiratory effects would occur. In sensitivity analysis, we restricted analysis to children who lived within 1 km of the nearest air monitor in order to address the hypothesis that non-differential error increases with distance from the nearest monitoring site. As predicted, this restriction resulted in larger estimates of effect. Non-differential exposure measurement error associated with using measurements made at distant monitors is likely to bias measured associations with health towards the null and/or increase the imprecision of results.
Our findings may reflect a true causal relationship between ammonia exposure and decreased lung function, although further studies are necessary to infer causality. One previous study of pediatric respiratory health and ambient ammonia took place in a region with a high density of fertilizer production plants, where mean ambient ammonia concentrations ranged from 54 to 113 μg/m3, exceeding most exposure levels experienced by our cohort.27 No differences were found in FEV1 of children living in high ammonia communities when compared to children in a “control” neighborhood with no known ammonia sources. However, lung function was assessed only twice for the cohort, and the concentrations of ammonia in the control area were elevated as well, ranging from 30 to 63 μg/m3. We have found no other published studies of population-level exposures to ambient ammonia and pediatric respiratory health or examining effects on a vulnerable subgroup such as children with asthma. A cross-sectional study of healthy adults residing near biodegradable waste sites did report a relationship between modeled exposure to ambient ammonia and increased frequency of self-reported respiratory and sensory effects, including coughing.28
Ammonia may serve as a marker for the complex airborne emissions from animal feeding operations, and the observed decreases in lung function may have resulted from exposure to one or more co-pollutants with established respiratory system toxicity, such as endotoxin, particulate matter, or hydrogen sulfide.29-31 Occupational studies of animal feeding operation workers have established a strong link between respiratory disease and exposure to air pollutants from these operations.32-34 Regardless of whether the decreases in lung function reported here were caused by exposure to ammonia or to one or more of other the toxicants emitted from animal feeding operations, our findings add to the existing body of evidence for a causal relationship between community-level animal feeding operation exposure and respiratory disease.35-37 Previous investigations have described relationships between prevalence of childhood asthma, either lifetime or current, and exposures to animal feeding operations;12-14,17 however, all previous epidemiologic studies involving pediatric subjects have been cross-sectional in design. To our knowledge, we have conducted the first longitudinal, repeated measures study of community-level ammonia exposures and respiratory health, as well as the first study of this design to address exposures to animal feeding operations in relation to pediatric health. A longitudinal repeated measures study is the ideal design to investigate exposures and outcomes that vary in time, and the within-subject comparisons help mitigate the influence of between-subject confounding. This is important in the context of proximity to animal feeding operations and asthma morbidity because residence on or near a farm raising animals may be associated with subject characteristics related to risk of respiratory disease, such as socioeconomic status, atopy, and early-life exposures to a farming environment.38-39
Despite these strengths, we acknowledge several limitations. As mentioned previously, it is uncertain whether the relationships with lung function are due to ammonia or other components of animal feeding operation emissions. Second, our exposure assessment was based solely on children's residences; we did not collect information about time-activity patterns. It is possible that children were less likely to play outdoors on days of higher ammonia exposures, but we are unable to account for such avoidance behavior in our analyses. In addition, our weekly estimates of ammonia exposure assigned to each symptom survey were based on only one, and sometimes two, measurement(s) of ammonia made at some time in the week prior to the interview. Because ammonia concentrations vary substantially on a time scale of days in this study region, this method is likely influenced by a large degree of nondifferential measurement error. This nondifferential error may have masked any true relationships between asthma symptoms and ammonia exposure by biasing results towards the null and/or increasing the standard errors association with effect estimates. Another important limitation of our analysis of symptoms and medication use is that outcome variables were dichotomized in analysis, and therefore analysis may be underpowered to detect any true associations in comparison to analyses of continuous outcome measures, such as lung function. Finally, substantial outcome misclassification likely results from the fact that symptoms and medication use were reported by subjects and caregivers, and therefore influenced by subjective perceptions. Our statistical approach, focused on within-subject rather than between-subject differences in reported symptoms and medication, use may have helped reduce the influence of this misclassifications.
Limitations of our analysis of FEV1% and ammonia include potential bias on account of missing FEV1 data. Results of GEE in analysis of correlated data is susceptible to bias when missingness mechanisms are not missing completely at random.23 Linear mixed models are more robust to such a bias, and in sensitivity analysis we found that results of either modeling technique to be similar.
In conclusion, we present preliminary evidence that children living near dairy operations in rural Washington State are exposed to elevated levels of ammonia likely attributable to air pollution from animal feeding operations. We found that exposure to elevated ammonia or any of numerous co-pollutants correlated with ammonia concentrations may be associated with lung function decrements in the following days for children with asthma. These potential health effects are important to consider in an environmental justice framework, as populations living near animal feeding operations in the US are more likely to be low income and belong to racial/ethnic minority groups38. Further research into impacts of emissions from animal feeding operations on regional air quality and community health is warranted.
Acknowledgments
We are grateful for the participation of research subjects and their families, and for the work of Ginger Ellingson, project coordinator. Two companies (ALK and Lincoln Diagnostics) donated supplies for skin prick testing.
Funding sources: This research was supported with grant number 5R21ES17906-2 from the National Institute of Environmental Health Sciences (NIEHS). CL was supported by training grant number HD052462-01 from the NIH.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Aneja VP, Schlesinger WH, Erisman JW. Effects of agriculture upon the air quality and climate: research, policy, and regulations. Environ Sci Technol Lett. 2009;43(12):4234–4240. doi: 10.1021/es8024403. [DOI] [PubMed] [Google Scholar]
- 2.National Research Council (U.S.) Air emissions from animal feeding operations: Current knowledge, future needs. Washington, D.C: National Academies Press; 2003. [Google Scholar]
- 3.Thorne PS, Ansley AC, Perry SS. Concentrations of bioaerosols, odors, and hydrogen sulfide inside and downwind from two types of swine livestock operations. J Occup Environ Hyg. 2009;6(4):211–220. doi: 10.1080/15459620902729184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams DAL, Breysse PN, McCormack MC, Diette GB, McKenzie S, Geyh AS. Airborne cow allergen, ammonia and particulate matter at homes vary with distance to industrial scale dairy operations: an exposure assessment. Environ Health. 2011;10 doi: 10.1186/1476-069X-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dungan RS, Leytem AB. Ambient endotoxin concentrations and assessment of offsite transport at open-lot and open-freestall dairies. J Environ Qual. 2012;41(5):1702–1702. doi: 10.2134/jeq2010.0363. [DOI] [PubMed] [Google Scholar]
- 6.Roney N. Toxicological Profile for Ammonia. Atlanta, GA: Dept of Health & Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 2004. [PubMed] [Google Scholar]
- 7.Shusterman D. The effects of air pollutants and irritants on the upper airway. Proc Am Thorac Soc. 2011;8(1):101–5. doi: 10.1513/pats.201003-027RN. [DOI] [PubMed] [Google Scholar]
- 8.Donham KJ, Cumro D, Reynolds S. Synergistic effects of dust and ammonia on the occupational health effects of poultry production workers. J Agromedicine. 2002;8(2):57–76. doi: 10.1300/J096v08n02_09. [DOI] [PubMed] [Google Scholar]
- 9.Morrow SM, O'Quin J, Hoet AE, Wilkins JR, 3rd, DeGraves F, Smith KA. Complaints associated with animal feeding facilities as reported to Ohio local health departments, 2006-2008. J Environ Health. 2013 May;75(9):8–13. [PubMed] [Google Scholar]
- 10.Fry JP, Laestadius LI, Grechis C, Nachman KE, Neff RA. Investigating the role of state permitting and agriculture agencies in addressing public health concerns related to industrial food animal production. PLoS One. 2014;9(2):e89870. doi: 10.1371/journal.pone.0089870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shusterman D. Odor-associated health complaints: competing explanatory models. Chem Senses. 2001;26(3):339–43. doi: 10.1093/chemse/26.3.339. [DOI] [PubMed] [Google Scholar]
- 12.Merchant JA, Naleway AL, Svendsen ER, et al. Asthma and farm exposures in a cohort of rural Iowa children. Environ Health Perspect. 2005;113(3):350–356. doi: 10.1289/ehp.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirabelli MC, Wing S, Marshall SW, Wilcosky TC. Asthma symptoms among adolescents who attend public schools that are located near confined swine feeding operations. Pediatrics. 2006;118(1):E66–E75. doi: 10.1542/peds.2005-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigurdarson ST, Kline JN. School proximity to concentrated animal feeding operations and prevalence of asthma in students. Chest. 2006;129(6):1486–1491. doi: 10.1378/chest.129.6.1486. [DOI] [PubMed] [Google Scholar]
- 15.Radon K, Schulze A, Ehrenstein V, van Strien RT, Praml G, Nowak D. Environmental exposure to confined animal feeding operations and respiratory health of neighboring residents. Epidemiology. 2007;18(3):300–308. doi: 10.1097/01.ede.0000259966.62137.84. [DOI] [PubMed] [Google Scholar]
- 16.Schulze A, Roemmelt H, Ehrenstein V, et al. Effects on Pulmonary Health of Neighboring Residents of Concentrated Animal Feeding Operations: Exposure Assessed Using Optimized Estimation Technique. Arch Environ Occup Health. 2011;66(3):146–154. doi: 10.1080/19338244.2010.539635. [DOI] [PubMed] [Google Scholar]
- 17.Pavilonis BT, Sanderson WT, Merchant JA. Relative exposure to swine animal feeding operations and childhood asthma prevalence in an agricultural cohort. Environ Res. 2013;(122):74–80. doi: 10.1016/j.envres.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schinasi L, Horton RA, Guidry VT, Wing S, Marshall SW, Morland KB. Air Pollution, Lung Function, and Physical Symptoms in Communities Near Concentrated Swine Feeding Operations. Epidemiology. 2011;22(2):208–215. doi: 10.1097/EDE.0b013e3182093c8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merchant JA. Advancing industrial livestock production: health effects research and sustainability. Epidemiology. 2011;22(2):216–8. doi: 10.1097/EDE.0b013e318209d3a4. [DOI] [PubMed] [Google Scholar]
- 20.Postma JM, Smalley K, Ybarra V, Kieckhefer G. The Feasibility and Acceptability of a Home-Visitation, Asthma Education Program in a Rural, Latino/a Population. J Asthma. 2011;48(2):139–146. doi: 10.3109/02770903.2010.529221. [DOI] [PubMed] [Google Scholar]
- 21.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong JL, Fitzpatrick CF, Loftus CT, Yost MG, Tchong-French M, Karr CJ. Development of a unique multi-contaminant air sampling device for a childhood asthma cohort in an agricultural environment. Environ Sci Process Impacts. 2013;15(9):1760–7. doi: 10.1039/c3em00330b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diggle Peter J, Heagerty Patrick, Liang Kung-Yee, Zeger Scott L. Analysis of Longitudinal Data. Oxford: Oxford University press; 2002. [Google Scholar]
- 24.Donham KJ, Lee JA, Thu K, Reynolds SJ. Assessment of air quality at neighbor residences in the vicinity of swine production facilities. J Agromedicine. 2006;11(3/4):15–24. doi: 10.1300/J096v11n03_03. [DOI] [PubMed] [Google Scholar]
- 25.Buijsman E, Maas HFM, Asman WAH. Anthropogenic NH3 emissions in Europe. Atmos Environ. 1987;21(5):1009–1022. [Google Scholar]
- 26.Hristov AN, Hanigan M, Cole A, et al. Review: Ammonia emissions from dairy farms and beef feedlots. Can J Anim Sci. 2011;91(1):1–35. [Google Scholar]
- 27.Gomzi M, Saric M. Respiratory impairment among children living in the vicinity of a fertilizer plant. Int Arch Occup Environ Health. 1997;70(5):314–320. doi: 10.1007/s004200050224. [DOI] [PubMed] [Google Scholar]
- 28.Blanes-Vidal V, Bælum J, Schwartz J, Løfstrøm P, Christensen LP. Respiratory and sensory irritation symptoms among residents exposed to low-to-moderate air pollution from biodegradable wastes. J Expo Sci Environ Epidemiol. 2014 Apr 16; doi: 10.1038/jes.2014.20. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz DA, Thorne PS, Jagielo PJ, White GE, Bleuer SA, Frees KL. Endotoxin responsiveness and grain dust-induced inflammation in the lower respiratory tract. Am J Physiol. 1994;267(5 Pt 1):L609–17. doi: 10.1152/ajplung.1994.267.5.L609. [DOI] [PubMed] [Google Scholar]
- 30.Campagna D, Kathman SJ, Pierson R, et al. Ambient hydrogen sulfide, total reduced sulfur, and hospital visits for respiratory diseases in northeast Nebraska, 1998-2000. J Expo Anal Environ Epidemiol. 2004;14(2):180–7. doi: 10.1038/sj.jea.7500313. [DOI] [PubMed] [Google Scholar]
- 31.Heederik D, Sigsgaard T, Thorne PS, et al. Health effects of airborne exposures from concentrated animal feeding operations. Environ Health Perspect. 2007;115(2):298–302. doi: 10.1289/ehp.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds SJ, Donham KJ, Whitten P, Merchant JA, Burmeister LF, Popendorf WJ. Longitudinal evaluation of dose-response relationships for environmental exposures and pulmonary function in swine production workers. Am J Ind Med. 1996;29:33–40. doi: 10.1002/(SICI)1097-0274(199601)29:1<33::AID-AJIM5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Shenker M, Christiani D, Cormier Y, et al. Respiratory health hazards in agriculture. Am J Respir Crit Care Med Suppl. 1998;158(pt 2):S1–S76. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- 34.Von Essen S, Moore G, Gibbs S, et al. Respiratory issues in beef and pork production: recommendations from an expert panel. J Agromedicine. 2010;15:216–25. doi: 10.1080/1059924X.2010.486283. [DOI] [PubMed] [Google Scholar]
- 35.Iowa State University and The University of Iowa. Iowa Concentrated Animal Feeding Operations Air Quality Study. Iowa City, IA: The University of Iowa College of Public Health; 2002. [Accessed May 23, 2014]. Available https://www.public-health.uiowa.edu/ehsrc/CAFOstudy.htm. [Google Scholar]
- 36.O'Connor AM, Auvermann B, Bickett-Weddle D, et al. The association between proximity to animal feeding operations and community health: a systematic review. PLoS One. 2010;5(3):e9530. doi: 10.1371/journal.pone.0009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.May S, Romberger DJ, Poole JA. Respiratory health effects of large animal farming environments. J Toxicol Environ Health B Crit Rev. 2012;15(8):524–541. doi: 10.1080/10937404.2012.744288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wing S, Cole D, Grant G. Environmental injustice in North Carolina's hog industry. Environ Health Perspect. 2000;108:225–231. doi: 10.1289/ehp.00108225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Mutius E, Radon K. Living on a farm: Impact on asthma induction and clinical course. Immunol Allergy Clin North Am. 2008;28(3):631–647. doi: 10.1016/j.iac.2008.03.010. [DOI] [PubMed] [Google Scholar]