Abstract
BACKGROUND
We reviewed the published literature to assess the association between maternal periconceptional physical activity and the risk for major, non-chromosomal, birth defects and whether this varies by pre-pregnancy obesity.
METHODS
We conducted a systematic literature search of MEDLINE, EMBASE, and CINAHL databases. Data were abstracted from all articles that met our inclusion criteria and included information on physical activity intensity (mild, moderate, and vigorous) and modality (i.e., standing, lifting, other). We assessed occupational and recreational physical activity separately. The quality of included articles was assessed using the Newcastle–Ottawa Scale.
RESULTS
Of 3316 screened articles, 11 were included in this review. Of the four studies that assessed prolonged standing, two reported a positive association with risk for some birth defects; null associations were observed in the other two studies. Associations between heavy lifting or other occupational physical activity exposures and risk for birth defects were inconsistent. A protective association between leisure-time physical activity (i.e., active sports, swimming) and some birth defects (e.g., neural tube defects), was suggested by the results of two studies. Only one study reported assessment of possible effect modification by maternal body mass index (BMI).
DISCUSSION
Our review suggests that there may be some associations between occupational and leisure-time physical activities and some, major non-chromosomal, birth defects, but relatively limited published research exists on these associations. Further research in this area should include differentiation of birth defects phenotypes, valid assessments of all domains of physical activity, including household and transportation activity, and account for the potential influence of pre-pregnancy BMI.
Keywords: physical activity, birth defects, systematic review
INTRODUCTION
Birth defects are a major contributor to infant mortality and lifelong morbidity. Two modifiable risk factors of importance today in terms of the spectrum of birth defects affected and risk factor prevalence are maternal pre-pregnancy diabetes and obesity (Correa et al., 2008; Reece, 2008). Pre-pregnancy diabetes has been associated with increased risk for isolated and multiple defects involving most organ systems (Correa et al., 2008). Pre-pregnancy obesity has been associated with several types of defects including neural tube defects, cleft lip (with and without cleft palate), and some cardiovascular defects (Waller et al., 2007; Stothard et al., 2009). The mechanisms underlying these associations are unclear but are hypothesized to be associated with fetal exposure to metabolic disturbances common to both diabetes and obesity. In 2005 to 2006, approximately 3% of U.S. childbearing-aged women had diabetes which was a larger prevalence than that in 1988 to 1994 (Cowie et al., 2009). In 2007 to 2008, 34% of women ages 20 to 39 were considered obese (body mass index [BMI] >30 kg/m2; Flegal et al., 2010). Given the high prevalence of obesity and increased prevalence of diabetes, interventions to prevent and manage these conditions may help prevent birth defects.
In light of its effectiveness in reducing visceral adiposity and preserving insulin function (Kitabchi et al., 2005; Lee et al., 2005; Hordern et al., 2008; Hordern et al., 2012), physical activity has been recommended for the prevention and management of both obesity and diabetes (U.S. Department of Health and Human Services, 2008). In 2005, approximately 50% of women of childbearing age met the U.S. Department of Health and Human Services recommendation of at least 30 minutes a day of moderate intensity activity five or more days a week or at least 20 minutes a day of vigorous intensity activity three or more days a week (Centers for Disease Control and Prevention, 2007). These data were self-reported and collected by the Behavioral Risk Factor Surveillance System. In 1999 to 2006, only about 23% of U.S. pregnant women met the 2008 Department of Health and Human Services (2008) recommendation of at least 150 minutes per week of moderate intensity aerobic activity (Evenson and Wen, 2010). An increase in physical activity in these populations may reduce the risk of birth defects by altering diabetes and obesity prevalences among these women.
Although promotion of physical activity may in principle represent an important strategy to prevent birth defects, the association between periconceptional physical activity and birth defects is unclear. Previous systematic reviews have suggested that maternal physical activity may reduce the risk of adverse pregnancy outcomes often associated with diabetes and obesity, such as preterm delivery, stillbirth, and perinatal mortality (Domingues et al., 2009; Schlüssel et al., 2008; Takito et al., 2009). To our knowledge, there is no published systematic review of the effect of physical activity on birth defects. The objective of this review of the published literature was to assess how different types of physical activity (i.e., occupational, transportation, housework, and/or leisure-time) during the periconceptional period may influence the risk of major birth defects in offspring and the extent to which this influence might vary by maternal pre-pregnancy obesity.
MATERIALS AND METHODS
Study Selection and Data Abstraction
We conducted a systematic literature search of MEDLINE, EMBASE, and CINAHL from the start of each database (1954, 1988, and 1989, respectively) through February 2011 with no language restrictions. We used combinations of the search terms ‘physical activity’, ‘pregnancy/periconception’, and ‘birth defects’ in addition to specific types of exercise and specific defect groups. The complete search strategy is provided in (supporting online information) Appendix 1. We searched for original research studies of case-control, cohort, clinical trial, and cross-sectional design. The search strategy was developed by three authors (JT, MEC, and AC) with the assistance of a medical librarian. All major birth defects were included in the review except for the following: chromosomal disorders (due to the genetic causes of these disorders), the category of multiple anomalies that includes syndromes, other recognizable syndromes, and defects that are exceedingly rare or are poorly ascertained/classified. Studies that included chromosomal anomalies in addition to other major structural birth defects were included but only the eligible defects were considered as part of the review. Additional articles for inclusion were identified by screening the references of relevant articles.
Titles and abstracts of all articles were screened by at least two authors. Articles were excluded from further review if the abstract clearly indicated it did not meet our criteria (original studies that examined the association of physical activity during pregnancy and subsequent birth defects). Editorials, letters, commentaries, reviews, and animal studies were excluded. Full articles were reviewed for any manuscript whose title and abstract suggested it may meet our inclusion criteria. Articles were included if they provided a measure of the association (odds ratios, relative risks, prevalence difference) between levels of physical activity exposure and one or more major birth defect of interest, or provided data that could be used to calculate such a measure. Any periconceptional or prenatal physical activity (e.g., standing, sitting, heavy lifting, walking) from any domain (occupation, transportation, leisure-time, or housework) was included. Information including type of physical activity exposure, study design, and controlled covariates was abstracted from included articles by one author (JT or ALF) and confirmed by a second author (MEC).
Physical activity exposures were classified into three intensity categories: mild, moderate, and vigorous. If a given article did not describe the physical activity intensity, a classification was made by reviewers (JT and MEC) on the basis of the description and metabolic equivalent (MET) values in Ainsworth et al. (2000) for physical activity.
Quality Assessment
The quality of each included article was assessed independently by two authors using an adapted version of the Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses (Wells et al., 2010). Any discrepancies between the two independent quality assessments were discussed to reach an agreement on the NOS score for each article. The adapted NOS scale was tailored for the subject of this review and is presented in (supporting online information) Appendices 2 and 3.
RESULTS
Article Screening and Inclusion
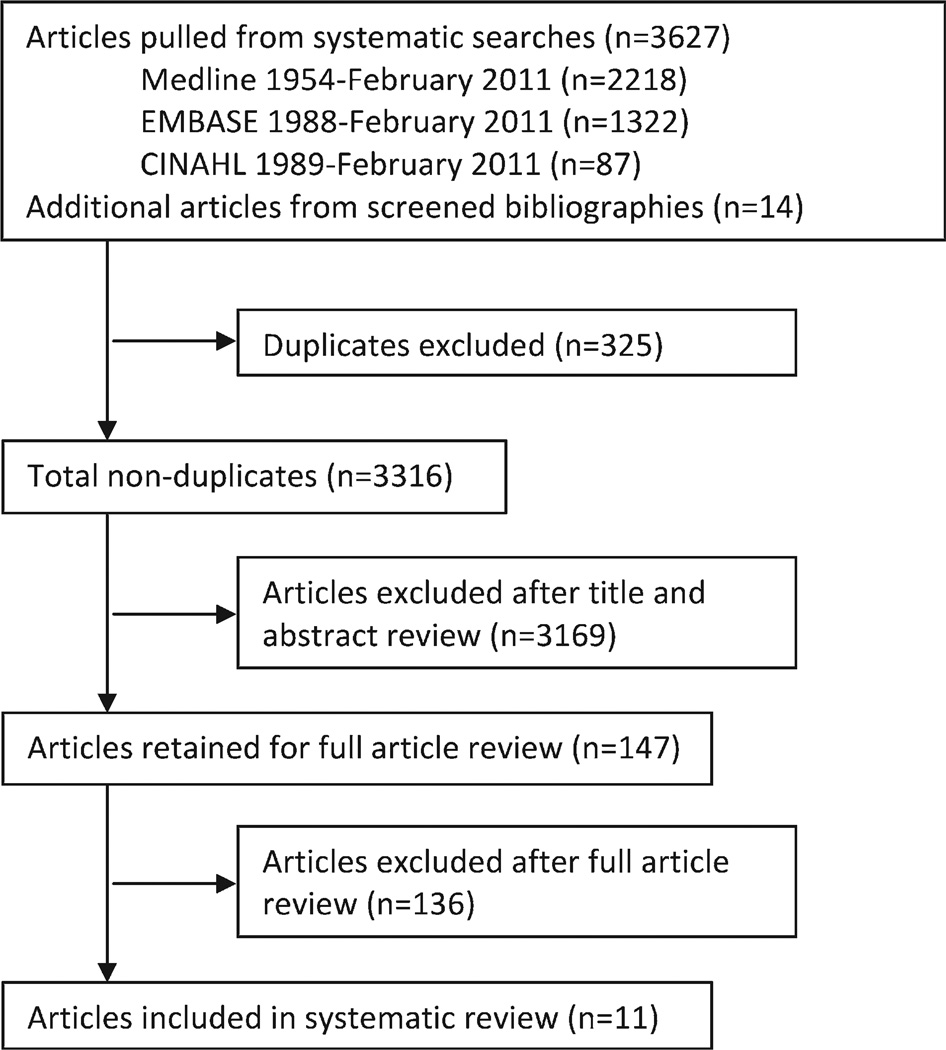
Of the 3316 articles screened for inclusion in this review, 3169 were excluded after examination of the title and abstract (Fig. 1). We screened 147 full articles ultimately yielding 11 included articles. Common reasons for exclusion were lack of birth defects as an outcome and lack of information on physical activity as an exposure.
Figure 1.
Flowchart of article selection.
The included articles were composed of eight case control studies (Kyyrönen et al., 1989; Nurminen et al., 1989; Taskinen et al., 1990; Lin et al., 1998; Lerman et al., 2001; Carmichael et al., 2002; Judge et al., 2004; Iszatt et al., 2011) and three cohort studies (McDonald et al., 1988; Clapp, 1989; Juhl et al., 2010; Tables 1A, B). Six of the 11 included articles assessed occupational physical activity (i.e., prolonged standing, heavy lifting), four assessed leisure-time physical activity (i.e., swimming, bicycling, active sports), and one article did not collect information on the setting of physical activity (Judge et al., 2004). No articles explicitly assessed household or transportation activities. Specific birth defects examined included neural tube defects, orofacial clefts, hypospadias, and cardiovascular malformations. Some articles and analyses did not differentiate between birth defects phenotypes, but rather examined “all cases” or “all congenital malformations.”
Table 1.
| A | ||||||
|---|---|---|---|---|---|---|
| Characteristics of Included Case-Control Studies Examining Physical Activity and Birth Defects | ||||||
| First author/ year, location (enrollment period) |
Physical activity assessment | |||||
| Controls n | Cases, n (type, sample size) |
Case ascertainment and classification | Domain (intensity of activity) type |
Weeks gestation | NOS score |
|
| Carmichael/2002, USA, California (1989–1991) | 539 | Any neural tube defect, 538 |
|
Leisure-time (vigorous)a
Leisure-time (mild)a
|
3 mo before conception through conception | 6 |
| Iszatt/2011, Southeast England (2000–2003) | 490 | Hypospadias, 471 |
|
Leisure-time (moderate/vigorous)
|
First trimester | 5 |
| Judge/2004, USA, New York (1988–1991) | 1066 | Congenital cardiovascular defects, 502 |
|
Any setting (moderate)b
|
Pregnancy recognition to the end of the first trimester | 6 |
| Kyyrönen/1989, Finland (1973–1983) | 93 | Any congenital malformation, 24 |
|
Occupationalc
|
First trimester | 6 |
| Lerman/2001, Israel (not reported) | 633 | Any congenital malformation, 45 |
|
Occupational (physiotherapists) (moderate)b | Entire pregnancy | 4 |
|
|
|||||
| Lin/1998, USA, New York (1983–1986) | 1154 | All cases, 528
|
|
Occupational (mild)b |
Between 1 month before and 3 months after the last normal menstrual period | 7 |
| Nurminen/1989, Finland (not reported) | 928 | All cases, 906
|
|
Occupational(mild to moderate)d
|
First trimester | 6 |
| Taskinen/1990, Finland (1973–1982) | 187 | Any congenital malformation, 46 |
|
Occupational (physiotherapists) (moderate)b | First trimester | 4 |
|
|
|||||
| B | ||||||
|---|---|---|---|---|---|---|
| Characteristics of Included Cohort Studies Examining Physical Activity and Birth Defects | ||||||
| First author/ year, location (enrollment period) |
Sample size at follow-up no. (% of baseline) |
Physical activity assessment | ||||
| Birth defects, n | Case ascertainment and classification |
Domain (intensity of activity) type |
Weeks gestation | NOS score |
||
| Clapp/1989, USA (not reported) | 94 (81.7) | Any birth defect, 2e
|
|
Leisure-time (vigorous)f
|
2 weeks before conception through 8 weeks of gestation | 5 |
| Juhl/2010, Denmark (1996–2002) | 48,781 (66.6) | Any birth defect, 3740
|
|
Leisure-time (mild to vigorous)
|
Before 31 weeks | 7 |
| McDonald/1988, Canada, Montreal (1982–1984) | 47,913 (85.5) | Musculoskeletal, 597g
|
|
Occupational (mild to moderate)h
|
Before 20 weeks; 20–27 weeks; 28–31 weeks | 3 |
NOS, Newcastle–Ottawa Scale; ICD, International Classification of Diseases.
The authors constructed a physical activity scoring index using the process developed in the Health Habits and History Questionnaire (Block G, et al. Heath habits and history questionnaire: Diet history and other risk factors. Personal Computer System Packet. Washington, DC: National Cancer Institute, 1989). The index took into account physical activity type and frequency.
-
–Heavy lifting was categorized as a 4.0; 11615 “lifting items continually, with limited walking or resting” or 11630, “standing; moderate/heavy [lifting more than 50 lbs, masonry, painting, paper hanging].”
-
–Prolonged standing was categorized as 2.0–4.0; 11610, “standing; light/moderate,…, patient care” or 11620, “standing; moderate (assembling at fast rate, intermittent, lifting 50 lbs, hitch/twisting ropes).”
-
–Sitting ≥75% was categorized as 1.5–2.5; 11580, “sitting-light office work, general (chemistry lab work,…, light assembly/repair), sitting, reading, driving at work” or 11585, “sitting-meetings, general, and/or with talking involved, eating at a business meeting.”
-
–Standing ≥75% was categorized as 2.0–4.0; 11610, “standing; light/moderate,…, patient care” or 11620, “standing; moderate (assembling at fast rate, intermittent, lifting 50 lbs, hitch/twisting ropes).”
-
–Mixed sitting and standing, 25 to 75% was categorized as 1.5–3.0; 11580 or 11585 to standing light moderate 11610.
-
–Very active or strenuous work (including lifting >25%) was categorized as 8.0 or higher; 11140 (e.g., “Farming, bailing hay, cleaning barn, poultry work, vigorous effort).”
-
–Swimming was categorized as 4.0–10; Swimming 18230–8350.
The authors assigned each participant a heavy lifting score based on daily heavy lifting and weight per lift (10–19 kg or 5–9 kg). The scores were then used to classify individuals into those who performed heavy lifting frequently and those who seldom performed heavy lifting.
The authors used a weighted activity score to classify mothers into each of these activity groups. The reference group consisted of women with sedentary work activities. Only two mothers were classified into a category of high mean physical load in the first trimester. These mothers were included in the moderate group for analyses.
NOS, Newcastle–Ottawa Scale; ICD, International Classification of Diseases.
Three infants in this cohort were born with birth defects, only two of which met our inclusion criteria. The third infant was born with trisomy 21.
All runners and aerobic dancers exercised at least 3 times a week for a minimum of 20 minutes at each session at a density above 50% of their maximum capacity. During each session the average intensity ranged from 52 to 83% of maximum capacity for runners and 20 to 40% for aerobic dancers.
The analysis splits musculoskeletal defects into the groups shown below, but does not report the number of infants in each of the groups.
-
–Heavy lifting was categorized as a 4.0; 11615 “lifting items continually, with limited walking or resting” or 11630, “standing; moderate/heavy [lifting more than 50 lbs, masonry, painting, paper hanging].”
-
–Standing ≥8.0 hours per day was categorized as 3.0–3.5; 11610, “standing; light/moderate,…, patient care” or 11620, “standing; moderate (assembling at fast rate, intermittent, lifting 50 lbs, hitch/twisting ropes).”
-
–Sitting was classified as 1.5; 11580, “sitting – meetings, general, and/or talking involved, eating at a business meeting.”
Study quality scores from the NOS assessment ranged from four to seven for case control studies (of nine points maximum) and from three to seven for cohort studies (of eight points maximum). Overall, studies used high quality methods of outcome assessment with some differentiating between birth defect phenotypes. According to our assessment, key limitations in the majority of studies were potential confounding and measurement error in the assessment of physical activity exposure. One covariate not assessed by most studies was pre-pregnancy BMI. Of our 11 included studies, the study by Carmichael et al. (2002) was the only one to include pre-pregnancy BMI as a potential confounder in statistical analyses. This study also assessed whether there was interaction between this variable and the exposure of interest, periconceptional physical activity. Two additional articles collected information on participant BMI, but did not control for it in statistical analyses (Judge et al., 2004; Juhl et al., 2010). The remaining eight articles did not collect this information. Below, we have summarized results relevant to occupational and leisure-time physical activity.
Occupational Physical Activity
Six studies assessed the association between occupational physical activity and one or more major, non-chromosomal, birth defect phenotypes or unspecified congenital malformations. Occupational physical activities assessed included heavy lifting, prolonged standing, and any occupational physical activity with at least a moderate load. In this section, we also present the results of an additional study by Judge et al. (2004) that assessed exposures to heavy lifting and prolonged standing both in and outside of an occupational setting.
Heavy Lifting
Five articles (four case-control studies and one cohort study) examined the potential association between heavy lifting and birth defects, most of which focused on exposure during the first trimester (Table 2A). Data from the four case-control studies showed no significant associations between heavy lifting during pregnancy and the birth defects examined. Unspecified congenital malformations were the outcome of interest in three of these studies, while the fourth focused on congenital cardiovascular malformations. The definition of “heavy lifting” varied considerably, both in weight and frequency, between studies. For example, in Judge et al. (2004), the weight load had to be at least 50 pounds to count as “heavy lifting” but could occur at any frequency during pregnancy. Alternatively, Lerman et al. (2001) did not define the weight of a “heavy” load, but specified that the lifting activity needed to occur at least five times a week to be classified in the exposed group.
Table 2.
| A | |||
|---|---|---|---|
| Association between Occupational Exposure to Heavy Lifting and Birth Defects | |||
| Reference | Outcome | Exposure index | Results OR (95% CI) |
| Judge 2004 | Congenital cardiovascular malformations | No heavy lifting | 1.00 (reference) |
| Any heavy lifting | 0.80 (0.57–1.11) Adjusteda | ||
| <10 hours/week | 0.87 (0.58–1.30) Adjusteda | ||
| ≥10 hours/week | 0.68 (0.40–1.16) Adjusteda | ||
| Kyyrönen 1989 | Congenital malformations unspecified | No heavy lifting | 1.00 (reference) |
| Any heavy lifting | 0.66 (0.24–1.83)b | ||
| Lerman 2001 | Congenital malformations unspecified | No heavy lifting | 1.00 (reference) |
| Any heavy lifting | 0.98 (0.60–2.07) | ||
| 5–25 times/week | 1.06 (0.65–2.46) | ||
| >25 times/week | 0.82 (0.32–2.11) | ||
| Taskinen 1990 | Congenital malformations unspecified | Heavy lifting (>10 kg) or patient transfers 5–49 times/week | 0.9 (0.5–1.8) |
| Heavy lifting (>10 kg) or patient transfers ≥50 times/week | 2.3 (0.4–12.9) | ||
| Results: Ratios of observed to expected counts | |||
| McDonald 1988 | Club foot | At any time | 1.15 |
| Before 20 weeks | 1.31 | ||
| 20–27 weeks | 0.96 | ||
| 28–31 weeks | 1.04 | ||
| Other musculoskeletal defects | At any time | 0.73 | |
| Before 20 weeks | 0.75 | ||
| 20–27 weeks | 1.29 | ||
| 28–31 weeks | 0.44 | ||
| Hernias | At any time | 1.46* | |
| Before 20 weeks | 1.73* | ||
| 20–27 weeks | 0.67 | ||
| 28–31 weeks | 1.53 | ||
| B | |||
|---|---|---|---|
| Association between Standing in an Occupational Setting and Birth Defects | |||
| Reference | Outcome | Exposure index | Results OR (95% CI) |
| Judge 2004 | Congenital cardiovascular malformations | No prolonged standing | 1.00 (reference) |
| Any prolonged standing | 1.03 (0.82–1.28) Adjusteda | ||
| <25 hours/week | 0.87 (0.63–1.18) Adjusteda | ||
| ≥25 hours/week | 1.14 (0.88–1.49) Adjusteda | ||
| Lin 1998 | Neural tube defects | Sitting and standing | 1.0 (reference) |
| Standing ≥75% | 1.04 (0.57–1.89) | ||
| Oral cleft defects | Sitting and standing | 1.0 (reference) | |
| Standing ≥75% | 1.75 (1.07–2.88)* | ||
| Nurminen 1989 | Central nervous system defects | Sedentary work | 1.0 (reference) |
| Standing work | 1.7 (1.2–2.5)* Adjustedb | ||
| Orofacial clefts | Sedentary work | 1.0 (reference) | |
| Standing work | 1.0 (0.8–1.4) Adjustedb | ||
| Skeletal defects | Sedentary work | 1.0 (reference) | |
| Standing work | 0.9 (0.6–1.3) Adjustedb | ||
| Cardiovascular defects | Sedentary work | 1.0 (reference) | |
| Standing work | 1.5 (0.9–2.4) Adjustedb | ||
| Results: Ratios of observed to expected counts | |||
| McDonald 1988 | Club foot | At any time | 1.13 |
| Before 20 weeks | 1.28 | ||
| 20–27 weeks | 1.18 | ||
| 28–31 weeks | 0.88 | ||
| Other musculoskeletal defects | At any time | 0.93 | |
| Before 20 weeks | 0.43 | ||
| 20–27 weeks | 1.36 | ||
| 28–31 weeks | 1.49 | ||
| Hernias | At any time | 0.98 | |
| Before 20 weeks | 1.07 | ||
| 20–27 weeks | 0.90 | ||
| 28–31 weeks | 0.87 | ||
| C | |||
|---|---|---|---|
| Association between Exposure to Occupational Physical Activity other than Standing and Heavy Lifting and Birth Defects | |||
| Reference | Outcome | Exposure Index | Results OR (95% CI) |
| Lin 1998 | Neural tube defects | Mixed sitting and standing | 1.00 (reference) |
| Active/strenuous work including lifting | 0.92 (0.47–1.78) | ||
| Oral cleft defects | Mixed sitting and standing | 1.00 (reference) | |
| Active/strenuous work including lifting | 1.32 (0.76–2.28) | ||
| Nurminen 1989 | Central nervous system defects | Sedentary work | 1.0 (reference) |
| Work with moderate physical load | 3.0 (1.6–5.5)* Adjusteda | ||
| Work involving walking | 1.4 (0.8–2.5) Adjusteda | ||
| Orofacial clefts | Sedentary work | 1.0 (reference) | |
| Work with moderate physical load | 1.8 (1.1–3.0)* Adjusteda | ||
| Work involving walking | 1.3 (0.8–2.1) Adjusteda | ||
| Skeletal defects | Sedentary work | 1.0 (reference) | |
| Work with moderate physical load | 0.9 (0.5–1.8) Adjusteda | ||
| Work involving walking | 0.7 (0.4–1.3) Adjusteda | ||
| Cardiovascular defects | Sedentary work | 1.0 (reference) | |
| Work with moderate physical load | 1.7 (0.7–4.0) Adjusteda | ||
| Work involving walking | 2.0 (1.0–3.8) Adjusteda | ||
| Results: Ratios of observed to expected counts | |||
| McDonald 1988 | Club foot | At any time | 1.22 |
| Before 20 weeks | 1.54* | ||
| 20–27 weeks | 1.81 | ||
| 28–31 weeks | 0.54 | ||
| Other musculoskeletal defects | At any time | 0.72 | |
| Before 20 weeks | 0.43 | ||
| 20–27 weeks | 1.22 | ||
| 28–31 weeks | 0.87 | ||
| Hernias | At any time | 1.51 | |
| Before 20 weeks | 1.80 | ||
| 20–27 weeks | 1.34 | ||
| 28–31 weeks | 1.24 | ||
| D | |||
|---|---|---|---|
| Association between Leisure-Time Physical Activity and Birth Defects | |||
| Reference | Outcome | Exposure index | Results OR (95% CI) |
| Carmichael 2002 | Neural tube defects | No active sports | 1.0 (reference) |
| Active sports | 0.65 (0.48–0.90)* | ||
| No physical exercises | 1.0 (reference) | ||
| Physical exercises | 0.72 (0.55–0.96)* | ||
| No jogging or running | 1.0 (reference) | ||
| Jogging or running | 0.93 (0.64–1.33) | ||
| No swimming or long walks | 1.0 (reference) | ||
| Swimming or long walks | 0.77 (0.58–1.01) | ||
| No gardening, fishing, or hunting | 1.0 (reference) | ||
| Gardening, fishing, or hunting | 0.66 (0.48–0.92)* | ||
| No other physical activity | 1.0 (reference) | ||
| Any other physical activity | 0.95 (0.68–1.33) | ||
| No frequent vigorous activity | 1.0 (reference) | ||
| Any frequent vigorous activitya | 0.64 (0.48–0.87)* | ||
| 1-unit change in index scoreb | 0.97 (0.94–0.99)* | ||
| 5-unit change in index scoreb | 0.84 (0.74–0.94)* | ||
| 10-unit change in index scoreb | 0.70 (0.55–0.89)* | ||
| Iszatt 2011 | Hypospadias | No swimming | 1.00 (reference) |
| Any swimming | 0.74 (0.54–1.00) Adjustedc | ||
| Juhl 2010 | Any congenital malformations | No exercise | 1.00 (reference) |
| Swimming | 0.89 (0.80–0.98)* Adjustedd | ||
| Bicycling (no swimming) | 0.94 (0.84–1.04) Adjustedd | ||
| Circulatory system defects | No exercise | 1.00 (reference) | |
| Swimming | 1.01 (0.82–1.25) Adjustedd | ||
| Bicycling (no swimming) | 0.93 (0.73–1.19) Adjustedd | ||
| Respiratory system defects | No exercise | 1.00 (reference) | |
| Swimming | 0.59 (0.29–1.17) Adjustedd | ||
| Bicycling (no swimming) | 0.61 (0.30–1.27) Adjustedd | ||
| Cleft lip/palate | No exercise | 1.00 (reference) | |
| Swimming | 0.63 (0.35–1.13) Adjustedd | ||
| Bicycling (no swimming) | 1.17 (0.72–1.92) Adjustedd | ||
| Clapp 1989 | Two cases of congenital abnormalities were identified in this sample of aerobic dancers (n = 32), runners (n = 41), and physically active controls (n = 21): an infant with subcoronal hypospadias born to an aerobic dancer, and an infant with digital webbing or partial syndactyly born to a runner. | ||
Adjusted for maternal chronic diabetes, fever during pregnancy, binge drinking during early pregnancy, family history of congenital cardiovascular malformations, infant gender, caffeine consumption during early pregnancy, and maternal chronic asthma.
Odds ratio and confidence interval calculated from reported counts using: Bland and Altman. The odds ratio. BMJ 2000;320:1468.
p value < 0.05.
OR, odds ratio; CI, confidence interval.
Adjusted for maternal chronic diabetes, fever during pregnancy, binge drinking during early pregnancy, family history of congenital cardiovascular malformations, and infant gender.
Adjusted for work characteristics, maternal age of ≥35 years, birth order higher than three, two or more induced abortions, previous miscarriage, previous malformed child, previous stillbirth, regular smoking, alcohol consumption, intake of drugs in the first trimester, and common cold or fever in the first trimester.
p value < 0.05.
OR, odds ratio; CI, confidence interval.
Adjusted for work characteristics, maternal age of ≥35 years, birth order higher than three, two or more induced abortions, previous miscarriage, previous malformed child, previous stillbirth, regular smoking, alcohol consumption, intake of drugs in the first trimester, and common cold or fever in the first trimester.
p value < 0.05.
OR, odds ratio; CI, confidence interval.
Any frequent vigorous activity includes active sports, physical exercises, jogging or running, or swimming or long walks, which were engaged in “a few times a month” or more.
A continuous physical activity index was created to quantify total physical activity by combining data on exertion and frequency of each physical activity type.
Adjusted for low birth weight, folate-supplement use during pregnancy, maternal smoking during weeks 6 through 18 of pregnancy, maternal occupational exposure to phthalates, and family income.
Adjusted for alcohol consumption and sex of the offspring.
p value < 0.05.
OR, odds ratio; CI, confidence interval.
McDonald et al. (1988), the only cohort study that examined this association, observed significantly more infants with congenital hernias than expected who were born to mothers exposed to heavy lifting before 20 weeks of gestation (ratio of observed to expected: 1.73, p value < 0.05; hernia location was unspecified). This reported association was unadjusted for potential confounders and it was unclear whether exposure information was obtained using a validated instrument.
Standing
In four studies, investigators examined the association between standing during the periconceptional period and specific birth defects (Table 2B). Lin et al. (1998) observed a significant increase in the odds of oral cleft defects associated with a woman spending more than 75% of her working hours standing (odds ratio [OR], 1.75; 95% confidence interval [CI], 1.07–2.88), but they did not observe an association between neural tube defects (type not specified) and the same exposure. Nurminen et al. (1989) observed a significantly elevated odds ratio for the association between standing work (when compared to sedentary work) and central nervous system defects (OR, 1.7; 95% CI, 1.2–2.5), but did not observe significant associations with orofacial clefts, skeletal defects, or cardiovascular defects. In the other two studies (Judge et al., 2004 and McDonald et al., 1988), standing during pregnancy was not significantly associated with congenital cardiovascular defects nor musculoskeletal birth defects, respectively.
In studies that examined the association between standing and birth defects, investigators used different exposure definitions and reference groups. Some studies defined standing exposure during pregnancy by hours per week whereas others defined it as percent of work time a woman spent standing. Similarly, some studies used no prolonged standing as their reference group, whereas others used mixed sitting and standing.
Other Occupational Physical Activity
In addition to heavy lifting and standing, three studies also examined the following occupational exposures during pregnancy: active/strenuous work, work with a moderate physical load, work involving walking, and overall physical effort (Table 2C). Nurminen et al. (1989) observed significantly elevated odds ratios for the associations of work with a moderate physical load during pregnancy with central nervous system defects and orofacial clefts (OR, 3.0; 95% CI, 1.6–5.5 and OR, 1.8; 95% CI, 1.1–3.0, respectively), but not with skeletal defects or cardiovascular defects. All of the associations presented in their article controlled for some potential confounding factors, including older maternal age and regular smoking. The association between work with a moderate physical load and central nervous system defects was the only significant adjusted result reported in the included studies on birth defects and this category of physical activity (occupational exposures other than lifting or standing).
McDonald et al. (1988) observed a significant association between physical effort before 20 weeks and club foot. This association was not controlled for potential confounders and was also not seen with physical effort in other gestational periods. All other estimated measures of association were consistent with the null and/or crude estimates.
Leisure-Time Physical Activity
Leisure-time physical activity is a broad category including activities such as jogging, gardening, swimming, and bicycling. While in most of the studies in which these activities were examined the results were suggestive of a protective association between these exposures and some birth defects, only two studies had significant associations, only one of which was adjusted for potential confounders (Table 2D).
Carmichael et al. (2002) examined the association between seven categories of leisure-time physical activity and neural tube defects. All results suggested a protective association between physical activity during pregnancy and neural tube defects with odds ratios of less than one, and four of these associations were statistically significant (active sports, physical exercises, gardening, fishing or hunting, and frequent vigorous activity). In addition to examining different types of leisure-time physical activity, these authors created an index of total leisure-time physical activity. An increase in overall physical activity was significantly associated with a decrease in the odds of having a child with a neural tube defect, but only among women who did not take a multivitamin or mineral supplement during pregnancy (OR, 5 unit change in activity 0.72; 95% CI, 0.56–0.94). There was no suggestion from their results that the relationship between physical activity and neural tube defects was modified by pre-pregnancy obesity status (p value for the product term > 0.10; joint effect of exposures not reported).
Juhl et al. (2010) observed a significant protective association between swimming during pregnancy and having a child with “any congenital malformations” when controlling for alcohol consumption and offspring sex (OR, 0.89; 95% CI, 0.80–0.98). This association was not statistically significant when examining separate birth defect phenotypes (OR, circulatory system defects 1.01; 95% CI, 0.82–1.25; OR, respiratory system defects 0.59; 95% CI, 0.29–1.17; OR, cleft lip/palate 0.63; 95% CI, 0.35–1.13), although power to detect an association in these separate phenotypes was low (number of affected infants 108, 9, and 13, respectively). No associations were observed between bicycling during pregnancy and the birth defects studied.
DISCUSSION
It is unclear from this systematic review whether there is an association between maternal occupational physical activity and major, non-chromosomal, birth defects. Our results did not suggest that there is an association between the birth defects examined and maternal occupational heavy lifting, but did suggest some associations between specific birth defects and prolonged standing and other occupational physical activity. Outside of the occupational domain, our results suggest that there may be a protective association between periconceptional leisure-time physical activity and some birth defect phenotypes. These initial findings merit further research among more diverse populations and phenotypes with better characterization of physical activity.
Our review identified gaps that need to be filled to have a full understanding of the roles of different types of physical activity and how they contribute to or decrease birth defects risk. As identified by our quality assessment, strengths of many completed studies on this topic are the separate examination of different physical activity domains, the ability to differentiate between birth defect phenotypes, and defect classification from medical records. An additional strength of completed research on this topic is the frequent use of a case-control study design which allows studies to detect modest associations with the relatively rare outcome of birth defects.
Limitations of published research on this topic include inadequate control for potential confounders, the use of limited and inconsistent exposure ascertainment methods, and in some studies, the inability to differentiate between potentially etiologically different phenotypes. When specific birth defect phenotypes were assessed, associations with physical activity were observed for some phenotypes, but not for others, which highlights the importance of continuing to differentiate between birth defects phenotypes in future research. In some studies, limitations in the assessment of physical activity may be the result of the focus on a main exposure other than physical activity. In future studies, potential confounders should be chosen based on previous findings and be specific to different birth defect categories. Physical activity exposure should be ascertained using biologic measures or questionnaires validated against better measurements, such as physical activity records, accelerometers, or biologic measures (e.g., the National Cancer Institute, 2010, summarizes findings from validation studies for physical activity questionnaires). As with other studies of physical activity during pregnancy, assessment should include physical activity from all domains (i.e., occupational, leisure-time, transportation, and housework) to achieve a comprehensive assessment of exposure (Chasan–Taber et al., 2007) as well as standardized measures of level of intensity of physical activity.
The heterogeneity of physical activity domains and intensities presents an important challenge in conducting these types of studies and drawing conclusions from their results. The details and setting of the physical activity may both be important in determining its potential effects. For example, for heavy lifting exposure, it is important to not only measure whether or not an individual completes any heavy lifting, but also the weight of the load, the frequency of the lifting, and the time period during pregnancy when the lifting activity occurs. Physical activity may be acting as a surrogate for other periconceptional exposures. If this is the case, the same physical activity in an occupational setting and in a leisure setting may show different associations with birth defects. For example, heavy lifting in an occupational setting may be an indicator of a job that involves manual labor. In this scenario, a measure of heavy lifting could be a surrogate for a poor work atmosphere, stress caused by an environment out of one’s control, or low socioeconomic status. The same exposure in a leisure setting may not be associated with any of these conditions.
Previous research suggests that pre-pregnancy BMI modifies the relationship between gestational diabetes mellitus and birth defects (Correa et al., 2008). Given the complex relationship between obesity, diabetes, and physical activity, pre-pregnancy BMI may also modify the association between physical activity and birth defects. This association may depend on individual characteristics such as diet, lifestyle choices, and health conditions other than diabetes. Future research on this topic should assess the potential influence of pre-pregnancy BMI and diabetes on these associations. A full list of recommendations for future research is included in Table 3.
Table 3.
Recommendations for Future Research on Prenatal Physical Activity and Birth Defects
| 1. | Differentiate between birth defect phenotypes. |
| 2. | Treat different physical activity domains (occupational, leisure time, household, and transportation) as separate exposures. |
| 3. | Ascertain physical activity exposure using biologic measures or questionnaires validated against better measurements (e.g., physical activity records or accelerometers). |
| 4. | Choose potential confounders based on the results of previous studies that are specific to each birth defect examined. |
| 5. | Assess the potential influence of pre-pregnancy body mass index and diabetes on the associations between physical activity and birth defects. |
Understanding whether or not there is a relationship between physical activity and birth defects is important for the prevention of these outcomes. Obesity and diabetes are both occurring at increasing rates in the United States. Physical activity can help prevent or manage both conditions. Although physical activity has this beneficial influence on obesity and diabetes, we do not yet understand its influence on birth defects. We also need to understand the detrimental association of physical activity and birth defects that has been observed in some studies to make recommendations to pregnant women. Currently, the U.S. Department of Health and Human Services (2008) and other organizations recommend pregnant women engage in moderate aerobic activity during pregnancy (ACOG, 2002; Kaiser and Allen, 2008). Future research on the possible association between physical activity and birth defects will help us better guide pregnant women to make healthy lifestyle choices before and during pregnancy while minimizing risks to their infant.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Rebecca Satterthwaite for the development and completion of the systematic searches for this project.
This research was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and Centers for Disease Control and Prevention.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- ACOG Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99:171–173. doi: 10.1016/s0029-7844(01)01749-5. [DOI] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9) Suppl:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Neri E, et al. Physical activity and risk of neural tube defects. Matern Child Health J. 2002;6:151–157. doi: 10.1023/a:1019722011688. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Prevalence of regular physical activity among adults-United States, 2001 and 2005. MMWR Morb Mortal Wkly Rep. 2007;56:1209–1212. [PubMed] [Google Scholar]
- Chasan–Taber L, Evenson KR, Sternfeld B, Kengeri S. Assessment of recreational physical activity during pregnancy in epidemiologic studies of birthweight and length of gestation: methodologic aspects. Women Health. 2007;45:85–107. doi: 10.1300/J013v45n04_05. [DOI] [PubMed] [Google Scholar]
- Clapp JF., 3rd The effects of maternal exercise on early pregnancy outcome. Am J Obstet Gynecol. 1989;161(6 Pt 1):1453–1457. doi: 10.1016/0002-9378(89)90903-4. [DOI] [PubMed] [Google Scholar]
- Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199:237.e231–237.e239. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues MR, Matijasevich A, Barros AJ. Physical activity and preterm birth: a literature review. Sports Med. 2009;39:961–975. doi: 10.2165/11317900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Evenson KR, Wen F. National trends in self-reported physical activity and sedentary behaviors among pregnant women: NHANES 1999–2006. Prev Med. 2010;50:123–128. doi: 10.1016/j.ypmed.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Hordern MD, Cooney LM, Beller EM, et al. Determinants of changes in blood glucose response to short-term exercise training in patients with Type 2 diabetes. Clin Sci (Lond) 2008;115:273–281. doi: 10.1042/CS20070422. [DOI] [PubMed] [Google Scholar]
- Hordern MD, Dunstan DW, Prins JB, et al. Exercise prescription for patients with type 2 diabetes and pre-diabetes: a position statement from exercise and sport science Australia. J Sci Med Sport. 2012;15:25–31. doi: 10.1016/j.jsams.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Iszatt N, Nieuwenhuijsen MJ, Nelson P, et al. Water consumption and use, trihalomethane exposure, and the risk of hypospadias. Pediatrics. 2011;127:e389–e397. doi: 10.1542/peds.2009-3356. [DOI] [PubMed] [Google Scholar]
- Judge CM, Chasan–Taber L, Gensburg L, et al. Physical exposures during pregnancy and congenital cardiovascular malformations. Paediatr Perinat Epidemiol. 2004;18:352–360. doi: 10.1111/j.1365-3016.2004.00586.x. [DOI] [PubMed] [Google Scholar]
- Juhl M, Kogevinas M, Andersen PK, et al. Is swimming during pregnancy a safe exercise? Epidemiology. 2010;21:253–258. doi: 10.1097/EDE.0b013e3181cb6267. [DOI] [PubMed] [Google Scholar]
- Kaiser L, Allen LH American Dietetic Association. Position of the American Dietetic Association: nutrition and lifestyle for a healthy pregnancy outcome. J Am Diet Assoc. 2008;108:553–561. doi: 10.1016/j.jada.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Kitabchi AE, Temprosa M, Knowler WC, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyyrönen P, Taskinen H, Lindbohm ML, et al. Spontaneous abortions and congenital malformations among women exposed to tetra-chloroethylene in dry cleaning. J Epidemiol Community Health. 1989;43:346–351. doi: 10.1136/jech.43.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kuk JL, Davidson LE, et al. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J Appl Physiol. 2005;99:1220–1225. doi: 10.1152/japplphysiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- Lerman Y, Jacubovich R, Green MS. Pregnancy outcome following exposure to shortwaves among female physiotherapists in Israel. Am J Ind Med. 2001;39:499–504. doi: 10.1002/ajim.1043. [DOI] [PubMed] [Google Scholar]
- Lin S, Gensburg L, Marshall EG, et al. Effects of maternal work activity during pregnancy on infant malformations. J Occup Environ Med. 1998;40:829–834. doi: 10.1097/00043764-199809000-00013. [DOI] [PubMed] [Google Scholar]
- McDonald AD, McDonald JC, Armstrong B, et al. Congenital defects and work in pregnancy. Br J Ind Med. 1988;45:581–588. doi: 10.1136/oem.45.9.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. Physical Activity Questionnaires (PAQ) Validation Studies. U.S. National Institutes of Health. 2010 Available at: http://appliedresearch.cancer.gov/tools/paq/validation.html.
- Nurminen T, Lusa S, Ilmarinen J, Kurppa K. Physical work load, fetal development and course of pregnancy. Scand J Work Environ Health. 1989;15:404–414. doi: 10.5271/sjweh.1832. [DOI] [PubMed] [Google Scholar]
- Reece EA. Obesity, diabetes, and links to congenital defects: a review of the evidence and recommendations for intervention. J Matern Fetal Neonatal Med. 2008;21:173–180. doi: 10.1080/14767050801929885. [DOI] [PubMed] [Google Scholar]
- Schlüssel MM, Souza EB, Reichenheim ME, Kac G. Physical activity during pregnancy and maternal-child health outcomes: a systematic literature review. Cad Saude Publica. 2008;24(Suppl 4):s531–s544. doi: 10.1590/s0102-311x2008001600006. [DOI] [PubMed] [Google Scholar]
- Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301:636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- Takito MY, Benício MH, Neri Lde C. Physical activity by pregnant women and outcomes for newborns: a systematic review. Rev Saude Publica. 2009;43:1059–1069. doi: 10.1590/s0034-89102009005000074. [DOI] [PubMed] [Google Scholar]
- Taskinen H, Kyyröonen P, Hemminki K. Effects of ultrasound, shortwaves, and physical exertion on pregnancy outcome in physiotherapists. J Epidemiol Community Health. 1990;44:196–201. doi: 10.1136/jech.44.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Washington D.C.: 2008. 2008 Physical Activity Guidelines for Americans. [Google Scholar]
- Waller DK, Shaw GM, Rasmussen SA, et al. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. 2007;161:745–750. doi: 10.1001/archpedi.161.8.745. [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Health Research Institute; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.