Abstract
Background
The observed heterogeneity in rubella-specific immune response phenotypes post-MMR vaccination is thought to be explained, in part, by inter-individual genetic variation.
Methods
In this study, single nucleotide polymorphisms (SNPs) and multiple haplotypes in several candidate genes were analyzed for associations with more than one rubella-specific immune response outcome, including secreted IFN-γ, secreted IL-6, and neutralizing antibody titers.
Results
Overall, we identified 23 SNPs in 10 different genes that were significantly associated with at least two rubella-specific immune responses. Of these SNPs, we detected eight in the PVRL3 gene, five in the PVRL1 gene, one in the TRIM22 gene, two in the IL10RB gene, two in the TLR4 gene, and five in other genes (PVR, ADAR, ZFP57, MX1, and BTN2A1/BTN3A3). The PVRL3 gene haplotype GACGGGGGCAGCAAAAAGAAGAGGAAAGAACAA was significantly associated with both higher IFN-γ secretion (t-statistic: 4.43, p<0.0001) and higher neutralizing antibody titers (t-statistic: 3.14, p=0.002).
Conclusions
Our results suggest that there is evidence of multigenic associations among identified gene SNPs, and that polymorphisms in these candidate genes contribute to the overall observed differences between individuals in response to live rubella virus vaccine. These results will aid our understanding of mechanisms behind rubella-specific immune response to MMR vaccine and influence the development of vaccines in the future.
Keywords: Rubella vaccine, single nucleotide polymorphisms (SNPs), genetic association, neutralizing antibodies, cytokines
MeSH Keywords: Rubella, Rubella Vaccine, Rubella virus, Measles-Mumps-Rubella Vaccine, Polymorphism, Single Nucleotide, Genetic Association Studies, Antibodies, Neutralizing, Cytokines
1. Introduction
The morbidity associated with rubella virus (RV) infection remains of great concern. Although typical childhood or adulthood infection can be benign (signified by rash, fever, lymphadenopathy, and malaise) (2011), rubella infection is particularly dangerous in pregnant women, resulting in congenital defects of the fetus (Plotkin 2001) or, in severe cases, perinatal death (Sydnor and Perl 2014). The most effective way to prevent rubella infection and reduce the morbidity associated with congenital rubella syndrome is maternal immunization with the measles-mumps-rubella (MMR) virus vaccine, which has successfully reduced rubella infection by > 99% since its introduction in 1971 in the U.S. (Lievano et al. 2012).
While many individuals develop protective immunity against rubella after MMR vaccination (~ 95%) (2011), others remain susceptible to infection for several reasons, including waning rubella-specific immune memory and primary vaccine non-response to RV (Sydnor and Perl 2014), both of which are influenced by genetic factors. Our previous study aimed to identify genetic influences on response to rubella vaccine (Ovsyannikova et al. 2007; Ovsyannikova et al. 2009a; Ovsyannikova et al. 2014a). Twenty-seven genes, including genes in the HLA-A and HLA-B loci, were found to differ between low and high antibody responders after stimulation with RV (Haralambieva et al. 2013), suggesting that genetics play a large role in one’s ability to develop protective immunity against rubella after immunization. In this study, we identified several single-nucleotide polymorphisms (SNPs)/haplotypes in candidate immune response genes that are significantly associated with multiple rubella vaccine-induced immune response outcomes after MMR immunization.
To our knowledge, this study is the first study of its kind to identify polymorphisms in several candidate genes that are significantly associated with more than one rubella-specific immune response (i.e., secreted IFN-γ, IL-6, and neutralizing antibody titers) post-MMR vaccination. Developing an understanding of the function of genetic variability on immune response to rubella immunization is critical for designing more effective vaccines in the future.
2. Materials and Methods
The methods described in this study are similar to those published for our previous studies (Haralambieva et al. 2014; Haralambieva et al. 2011a; Lambert et al. 2013; Lambert et al. 2014; Ovsyannikova et al. 2011a; Ovsyannikova et al. 2011b).
2.1 Study Subjects and Immunization
Subjects from a previously described cohort were utilized for this study (Haralambieva et al. 2010; Ovsyannikova et al. 2010a; Ovsyannikova et al. 2011b; Ovsyannikova et al. 2004; Ovsyannikova et al. 2005). The study cohort comprised a combined sample of 1,052 subjects from three independent cohorts of healthy children in Rochester, MN. Specifically, 368 healthy children, ranging in age from 12 to 18 years, were recruited from Rochester, MN, between the years 2001–2002. In 2006–2007, we enrolled an additional 396 healthy children (age 11–19 years) as part of our original MMR vaccine studies. In 2008–2009, additional subjects, ranging in age from 11 to 22 years, were added to this cohort, resulting in a cohort of 1,052 subjects. Prior to participation in these studies, subjects provided documentation of receiving two doses of rubella-containing vaccine. After excluding subjects without genotyping data, 1,039 subjects remained for analysis. Each subject provided a written record of receiving two age-appropriate doses of MMR vaccine. Permission to conduct this study was granted by the institutional review board of Mayo Clinic.
2.2 Antibody Measurement
Rubella-specific neutralizing antibody (NA) titers were quantified for each subject using a method that has been previously published (Lambert et al. 2014). In brief, a modified soluble immunocolorimetric (ICA)-based neutralization assay (sICNA) was optimized for high-throughput measurement and analysis. Measurements were reported as the highest dilution at which there was a 50% reduction in viral activity (NT50).
2.3 Secreted Cytokine Measurement
Secreted rubella-specific IFN-γ and IL-6 were measured by conducting enzyme-linked immunosorbent assays (ELISAs). The complete protocol for this methodology has been previously published (Dhiman et al. 2010; Lambert et al. 2013; Ovsyannikova et al. 2009b). To summarize, cryopreserved peripheral blood mononuclear cells (PBMCs) from each subject were cultured, in triplicate (2×105 cells per well in 96-well plates), with either media (control wells) or the W-Therien strain of RV (MOI=5). PHA (5 µg/ml) was used as a positive control. Cell cultures were incubated based upon previous optimization results: 48 h for maximal IFN-γ secretion, 24 h for maximal IL-6 secretion. ELISAs were performed using the manufacturer’s recommendations (BD Pharmingen), and plates were read at 450 nm on a microplate reader (Molecular Devices Corporation, Sunnyvale, CA).
2.4 Candidate gene SNP genotyping
The description of the tagging SNP selection strategies and genotyping methods has been previously described (Haralambieva et al. 2014; Haralambieva et al. 2011a; Ovsyannikova et al. 2011a; Ovsyannikova et al. 2011b). SNPs within candidate genes, 5 kb upstream and downstream for each candidate gene, were chosen based on the linkage disequilibrium (LD) tagSNP selection algorithm (Yen et al. 2006) from the Hapmap Phase II (http://www.hapmap.org), Seattle SNPs (http://pga.mbt.washington.edu/), and NIEHS SNPs (http://egp.gs.washington.edu/), with SNP minor allele frequencies ≥ 0.05, LD threshold of r2 ≥ 0.90. Overall, 768 SNPs in 92 candidate genes were analyzed as part of this study (Haralambieva et al. 2014; Pankratz et al. 2010). The 768 SNPs were genotyped using a custom-designed 768-plex Illumina GoldenGate™ assay (Illumina Inc., San Diego, CA) following the manufacturer’s instructions. The BeadStudio 2 software was used to call genotypes.
2.5 Statistical Analysis
Our goal was to determine whether there were genetic variants that shared associations with multiple immune response phenotypes for rubella. We have reported many of these associations previously, and the analytical methods used to assess the associations are outlined in those publications (Haralambieva et al. 2011a; Ovsyannikova et al. 2010b; Ovsyannikova et al. 2012; Ovsyannikova et al. 2011b). Briefly, to assess the additive genetic association with the logarithmic transformation of neutralizing antibody NT50 titer, simple linear regression models were employed. All other phenotypes had multiple observations per subject and were evaluated with repeated measures approaches, including an unstructured within-person variance-covariance matrix to account for within-subject correlations. A test of the ordinal genotype by stimulation status interaction assessed the effect of genotype on the average difference between stimulated and unstimulated PBMC samples. Our primary tests of association for each of these phenotypes were adjusted for a number of potentially important covariates, including age at enrollment, ages at first and second vaccinations, sex, time between most recent vaccination and study participation, batch/run number of assay and the first 3 population stratification eigenvectors. The q-values were then computed. The p-values from these primary tests of association were compared on a SNP-by-SNP basis to identify polymorphisms significant (p < 0.05) for more than one phenotype. Six-hundred and five SNPs were used in these comparisons. Summaries of the measures of virus-specific immune responses were obtained for these SNPs, broken by allelic category, as noted in our previously published work (Haralambieva et al. 2011a; Ovsyannikova et al. 2010a; Ovsyannikova et al. 2010b; Ovsyannikova et al. 2011b).
Further exploration of these data then commenced via pos-hoc haplotype estimation and analysis. In the absence of pedigree data and known linkage phase, there may be multiple possible haplotypes for an observed genotype. To address this complication, the expectation-maximum (EM) algorithm, as per Schaid et al. (Schaid et al. 2002), was used to establish the conditional posterior probabilities of a given haplotype with an observed genotype. From these probabilities, a design matrix of expected haplotype counts was constructed and used to investigate associations with phenotype. The above techniques were applied utilizing the Haplo.Stats package with the default settings for batch size, maximum number of iterations and convergence criteria. Haplotypes with less than a 1% frequency were eliminated to help reduce the error associated with estimation.
3. Results
3.1 Demographics
In total, 1,039 subjects participated in this study. The median age at the time of enrollment was 15.1 years with a range of 11–22 years. Slightly over half (55.2%) of the cohort consisted of male subjects while the remaining 44.8% were female. Caucasian-Americans accounted for 85.0% of the cohort, and 8.1% of subjects identified as either Black or African-American. Quantification of NA titers, IL-6 secretion, and IFN-γ secretion within the cohort yielded median values of 57.4 (range 17.0–2391.2), 3595.7 ng/µl (range −957.3–5831.8 ng/µl), and 6.1 ng/µl (range −239.0–579.4 ng/µl), respectively. See Table 1 for a summary of subject demographics.
Table 1.
Subject demographics and immune outcomes summary.
| Subject Demographics and Immune Outcomes Summary | ||
|---|---|---|
| Total (N=1,039) |
P Value | |
| Age | <0.0001 | |
| N | 1039 | |
| Mean (SD) | 15.1 (2.2) | |
| Median | 15.0 | |
| Q1, Q3 | 13.0, 17.0 | |
| Range | (11.0–22.0) | |
| Age at First Vaccination (Months) | 0.0002 | |
| N | 1039 | |
| Mean (SD) | 20.1 (20.9) | |
| Median | 15.0 | |
| Q1, Q3 | 15.0, 16.0 | |
| Range | (11.0–185.0) | |
| Age at Second Vaccination (Years) | <0.0001 | |
| N | 1039 | |
| Mean (SD) | 8.4 (3.5) | |
| Median | 9.0 | |
| Q1, Q3 | 5.0, 12.0 | |
| Range | (1.0–17.0) | |
|
Time from Second Vaccination to Enrollment (Years) |
<0.0001 | |
| N | 1039 | |
| Mean (SD) | 6.7 (2.9) | |
| Median | 6.4 | |
| Q1, Q3 | 4.6, 8.6 | |
| Range | (0.4–16.8) | |
| Gender | 0.6255 | |
| Male | 574 (55.2%) | |
| Female | 465 (44.8%) | |
| Race | <0.0001 | |
| American Indian, Alaska Native | 4 (0.4%) | |
| Asian, Hawaiian, Pacific Islander | 27 (2.6%) | |
| Black or African American | 84 (8.1%) | |
| Caucasian-American | 883 (85.0%) | |
| Multiple | 28 (2.7%) | |
| Other | 7 (0.7%) | |
| Unknown | 6 (0.6%) | |
| Ethnicity | 0.0089 | |
| Not Hispanic or Latino | 1012 (97.4%) | |
| Hispanic or Latino | 20 (1.9%) | |
| Don't Know | 7 (0.7%) | |
| Neutralizing Antibody (NT50) | 0.0034 | |
| N | 1029 | |
| Mean (SD) | 81.6 (123.5) | |
| Median | 57.4 | |
| Q1, Q3 | 34.9, 95.5 | |
| Range | (17.0–2391.2) | |
| IL-6 (ng/µl) | <0.0001 | |
| N | 988 | |
| Mean (SD) | 3435.4 (908.0) | |
| Median | 3595.7 | |
| Q1, Q3 | 3027.1, 4005.1 | |
| Range | (−957.3–5831.8) | |
| IFN-γ (ng/µl) | <0.0001 | |
| N | 969 | |
| Mean (SD) | 24.4 (70.0) | |
| Median | 6.1 | |
| Q1, Q3 | 1.5, 20.0 | |
| Range | (−239.0–579.4) | |
Abbreviations: IL-interleukin; IFN-interferon; SD-standard deviation; Q1-first quartile; Q3-third quartile; NT50-neutralizing titer
Negative cytokine values indicate that the unstimulated secretion levels were, on average, higher than the rubella virus-stimulated secretion levels.
3.2 Associations between SNPs and Immune Measures
Since the study subjects were racially diverse, genotype-phenotype data were analyzed for a combined cohort of 1,039 subjects (Table 2), and, in addition, separately for 883 Caucasian subjects (Supplementary Table 1)
Table 2.
SNPs associated with more than one rubella-specific immune response outcome after MMR vaccination.
| Cohort | SNP ID | Chromosome / Position |
Gene | Location | Immune Measure |
N | Genotype | No. | Immune Response Outcome (IQR) |
P Value | Immune Measure |
N | Genotype | No. | Immune Response Outcome (IQR) |
P Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rubella Vaccine Study |
rs4682233 | 3/ 1.11×108 | PVRL3 | Intron | Secreted IL-6 (ng/µl) |
969 | AA AG GG |
953 16 0 |
3609.7 (3049.2, 4006.3) 3372.2 (1952.7, 4094.1) |
0.000675 | Secreted IFN-γ (ng/µl) |
969 | AA AG GG |
953 16 0 |
6.2 (1.5, 20.2) 3.7 (−5.8, 10.7) |
0.000498 |
| Rubella Vaccine Study |
rs72935984 | 3/ 1.11×108 | PVRL3 | Intron | Secreted IL-6 (ng/µl) |
962 | CC CA AA |
944 18 0 |
3609.8 (3048.0, 4007.3) 3608.4 (2134.9, 4123.8) |
0.001363 | Secreted IFN-γ (ng/µl) |
962 | CC CA AA |
944 18 0 |
6.2 (1.5, 20.0) 5.4 (−0.4, 27.3) |
0.004264 |
| Rubella Vaccine Study |
rs75054607 +NA |
3/ 1.11×108 | PVRL3 | Intron | Secreted IFN-γ (ng/µl) |
969 | AA AG GG |
946 22 1 |
6.1 (1.5, 19.7) 11.6 (3.9, 33.6) 508.8 (508.8, 508.8) |
0.000028 | NA (NT50) |
1029 | AA AG GG |
1004 23 2 |
56.6 (34.6, 93.9) 112.5 (65.5, 159.4) 192.1 (31.2, 353.0) |
0.001575 |
| Rubella Vaccine Study |
rs76464851 +NA |
3/ 1.11×108 | PVRL3 | Intron | Secreted IFN-γ (ng/µl) |
969 | AA AG GG |
946 22 1 |
6.1 (1.5, 19.7) 11.6 (3.9, 33.6) 508.8 (508.8, 508.8) |
0.000028 | NA (NT50) |
1029 | AA AG GG |
1004 23 2 |
56.6 (34.6, 93.9) 112.5 (65.5, 159.4) 192.1 (31.2, 353.0) |
0.001575 |
| Rubella Vaccine Study |
rs78157313 +NA |
3/ 1.11×108 | PVRL3 | Intron | Secreted IFN-γ (ng/µl) |
967 | AA AG GG |
944 22 1 |
6.1 (1.5, 19.8) 11.6 (3.9, 33.6) 508.8 (508.8, 508.8) |
0.000029 | NA (NT50) |
1027 | AA AG GG |
1002 23 2 |
56.6 (34.6, 93.8) 112.5 (65.5, 159.4) 192.1 (31.2, 353.0) |
0.001575 |
| Rubella Vaccine Study |
rs72937914 +IFN-γ |
3/1.11 ×108 | PVRL3 | Intron | Secreted IL-6 (ng/µl) |
969 | AA AC CC |
942 27 0 |
3612.3 (3056.4, 4008.4) 2917.9 (2076.4, 4064.3) |
0.000386 | Secreted IFN-γ (ng/µl) |
969 | AA AC CC |
942 27 0 |
6.1 (1.5, 19.9) 6.1 (−0.4, 27.3) |
0.040073 |
| Rubella Vaccine Study |
rs10433385 +IFN-γ |
3/1.11 ×108 | PVRL3 | Intergenic | Secreted IL-6 (ng/µl) |
968 | AA AG GG |
929 36 3 |
3613.2 (3056.4, 4020.6) 3398.5 (2754.5, 3870.5) 2717.6 (2617.3, 4306.3) |
0.008086 | Secreted IFN-γ (ng/µl) |
968 | AA AG GG |
929 36 3 |
6.3 (1.6, 20.8) 3.5 (0.1, 7.7) 3.4 (2.6, 16.6) |
0.012882 |
| Rubella Vaccine Study |
rs78545860 +IFN-γ |
3/1.11×108 | PVRL3 | Intergenic | Secreted IL-6 (ng/µl) |
969 | AA AG GG |
930 36 3 |
3614.0 (3056.4, 4020.6) 3398.5 (2754.5, 3870.5) 2717.6 (2617.3, 4306.3) |
0.008063 | Secreted IFN-γ (ng/µl) |
969 | AA AG GG |
930 36 3 |
6.3 (1.6, 20.8) 3.5 (0.1, 7.7) 3.4 (2.6, 16.6) |
0.012874 |
| Rubella Vaccine Study |
rs11820364 +NA |
11/1.2×108 | PVRL1 | Intron | Secreted IL-6 (ng/µl) |
969 | GG GA AA |
953 15 1 |
3595.6 (3036.5, 4000.4) 4328.9 (3759.0, 4494.6) 2271.6 (2271.6, 2271.6) |
0.000102 | NA (NT50) |
1029 | GG GA AA |
1010 18 1 |
57.4 (34.9, 95.3) 67.3 (32.5, 126.5) 75.8 (75.8, 75.8) |
0.00822 |
| Rubella Vaccine Study |
rs61247604 | 11/1.2×108 | PVRL1 | Intron | Secreted IL-6 (ng/µl) |
968 | GG GA AA |
956 12 0 |
3596.0 (3037.7, 4003.1) 4178.1 (3605.9, 4384.3) |
0.000332 | NA (NT50) |
1028 | GG GA AA |
1015 13 0 |
57.4 (34.8, 95.8) 63.1 (36.2, 74.5) |
0.000477 |
| Rubella Vaccine Study |
rs73571285* +IFN-γ |
11/1.2×108 | PVRL1 | Intron | Secreted IL-6 (ng/µl) |
969 | AA AG GG |
953 14 2 |
3600.2 (3049.7, 4003.7) 4011.0 (2630.3, 4439.8) −450.2 (−957.3, 56.9) |
0.000144 | Secreted IFN-γ (ng/µl) |
969 | AA AG GG |
953 14 2 |
6.2 (1.5, 20.0) 4.8 (−0.9, 62.3) −51.9 (−60.5, −43.3) |
0.022627 |
| Rubella Vaccine Study |
rs73571287* +IFN-γ |
11/1.2×108 | PVRL1 | Intron | Secreted IL-6 (ng/µl) |
968 | GG GA AA |
959 8 1 |
3598.9 (3043.4, 4003.9) 4011.0 (1079.8, 4310.4) 56.9 (56.9, 56.9) |
9×10−7 | Secreted IFN-γ (ng/µl) |
968 | GG GA AA |
959 8 1 |
6.2 (1.5, 20.2) 3.7 (−1.1, 38.4) −43.3 (−43.3, −43.3) |
0.02229 |
| Rubella Vaccine Study |
rs79849521 ++ |
11/1.2×108 | PVRL1 | Intron | Secreted IL-6 (ng/µl) |
969 | GG GA AA |
959 10 0 |
3598.9 (3036.5, 4003.9) 4099.9 (3356.6, 4328.9) |
0.026416 | NA (NT50) |
1029 | GG GA AA |
1018 11 0 |
57.4 (34.9, 95.7) 63.1 (33.4, 75.8) |
0.004395 |
| Rubella Vaccine Study |
rs4936489 ++ |
11/1.2×108 | PVRL1 | Intergenic | Secreted IL-6 (ng/µl) |
968 | GG GA AA |
936 31 1 |
3605.0 (3041.1, 4005.1) 3514.8 (2803.7, 4126.2) 3696.9 (3696.9, 3696.9) |
0.042337 | NA (NT50) |
1028 | GG GA AA |
992 35 1 |
57.4 (34.7, 95.2) 65.5 (41.0, 101.3) 77.3 (77.3, 77.3) |
0.03046 |
| Rubella Vaccine Study |
rs73578845+ NA |
11/1.19×108 | PVRL1 | Intergenic | Secreted IL-6 (ng/µl) |
969 | GG GC CC |
956 11 2 |
3596.0 (3041.1, 4003.1) 4078.1 (2299.1, 4439.8) 4099.9 (3970.0, 4229.9) |
0.000315 | NA (NT50) |
1029 | GG GC CC |
1015 12 2 |
57.4 (34.6, 95.4) 62.5 (52.7, 112.3) 116.5 (74.5, 158.6) |
0.037341 |
| Rubella Vaccine Study |
rs2291842 +NA |
11/5719667 | TRIM2 2 |
Coding | Secreted IFN-γ (ng/µl) |
969 | AA AG GG |
639 285 45 |
5.9 (1.5, 19.9) 6.1 (1.9, 18.0) 11.5 (2.7, 42.5) |
0.014516 | NA (NT50) |
1029 | AA AG GG |
683 299 47 |
56.6 (34.6, 95.7) 58.9 (35.1, 94.8) 61.0 (34.9, 97.4) |
0.029181 |
| Rubella Vaccine Study |
rs2291842 +NA |
11/5719667 | TRIM2 2 |
Coding | Secreted IL-6 (ng/µl) |
969 | AA AG GG |
639 285 45 |
3619.8 (3056.4, 4002.4) 3595.8 (3024.7, 4038.5) 3350.8 (3025.7, 3868.0) |
0.002321 | NA (NT50) |
1029 | AA AG GG |
683 299 47 |
56.6 (34.6, 95.7) 58.9 (35.1, 94.8) 61.0 (34.9, 97.4) |
0.029181 |
| Rubella Vaccine Study |
rs2291842 +IFN-γ |
11/5719667 | TRIM2 2 |
Coding | Secreted IL-6 (ng/µl) |
969 | AA AG GG |
639 285 45 |
3619.8 (3056.4, 4002.4) 3595.8 (3024.7, 4038.5) 3350.8 (3025.7, 3868.0) |
0.002321 | Secreted IFN-γ (ng/µl) |
969 | AA AG GG |
639 285 45 |
5.9 (1.5, 19.9) 6.1 (1.3, 18.0) 11.5 (2.7, 42.5) |
0.014516 |
| Rubella Vaccine Study |
rs962859~ +IFN-γ |
21/34648123 | IL10RB | Intron | Secreted IL-6 (ng/µl) |
954 | AA AC CC |
323 483 148 |
3660.3 (3064.3, 4061.3) 3569.1 (3023.7, 4000.9) 3533.8 (3066.0, 3900.4) |
0.008451 | Secreted IFN-γ (ng/µl) |
954 | AA AC CC |
323 483 148 |
6.9 (1.8, 22.8) 4.9 (1.2, 18.2) 7.5 (2.5, 21.4) |
0.027823 |
| Rubella Vaccine Study |
rs2284552~ +IFN-γ |
21/34644082 | IL10RB | Intron | Secreted IL-6 (ng/µl) |
968 | CC CA AA |
600 321 47 |
3535.6 (2995.2, 3975.5) 3668.8 (3160.0, 4044.1) 3666.1 (3064.3, 4150.4) |
7.89×10−5 | Secreted IFN-γ (ng/µl) |
968 | CC CA AA |
600 321 47 |
5.9 (1.2, 19.2) 6.2 (1.9, 21.7) 7.9 (1.6, 30.7) |
0.018961 |
| Rubella Vaccine Study |
rs5030728 ++ |
9/1.2×108 | TLR4 | Intron | Secreted IL-6 (ng/µl) |
965 | GG GA AA |
489 398 78 |
3619.4 (3066.3, 4016.0) 3597.3 (3015.3, 3984.0) 3569.5 (2943.2, 4033.6) |
0.018059 | Secreted IFN-γ (ng/µl) |
965 | GG GA AA |
489 398 78 |
5.5 (0.8, 19.7) 6.4 (1.9, 20.0) 6.5 (1.4, 25.2) |
0.037292 |
| Rubella Vaccine Study |
rs2770150 +IFN-γ |
9/1.2×108 | TLR4 | Intergenic | Secreted IL-6 (ng/µl) |
968 | AA AG GG |
516 389 63 |
3615.4 (3064.5, 4005.1) 3600.2 (3018.3, 4008.4) 3542.9 (2943.2, 4085.3) |
0.01256 | Secreted IFN-γ (ng/µl) |
968 | AA AG GG |
516 389 63 |
5.9 (1.1, 20.0) 6.3 (1.7, 19.2) 6.6 (1.1, 27.5) |
0.017476 |
| Rubella Vaccine Study |
seq- rs112723153 +IFN-γ |
19/45178636 | PVR | Intron | Secreted IL-6 (ng/µl) |
969 | GG GA AA |
961 8 0 |
3609.8 (3049.2, 4008.4) 2936.2 (2438.8, 3855.6) |
0.011401 | Secreted IFN-γ (ng/µl) |
969 | GG GA AA |
961 8 0 |
6.1 (1.5, 20.0) 10.4 (0.3, 34.5) |
0.041896 |
| Rubella Vaccine Study |
rs2229857~ +IFN-γ |
1/1.55 ×108 | ADAR | Coding | Secreted IL-6 (ng/µl) |
968 | GG GA AA |
505 374 89 |
3595.6 (3058.5, 4006.3) 3594.7 (3036.5, 4033.3) 3732.8 (3023.6, 3969.3) |
0.000435 | Secreted IFN-γ (ng/µl) |
968 | GG GA AA |
505 374 89 |
6.0 (1.4, 20.0) 5.9 (1.5, 19.2) 8.4 (2.3, 22.1) |
0.01358 |
| Rubella Vaccine Study |
rs3870968 +IFN-γ |
6/29647149 | ZFP57 | Intron | Secreted IL-6 (ng/µl) |
969 | CC CA AA |
866 102 1 |
3619.6 (3038.9, 4024.9) 3523.0 (3032.0, 3900.6) 3643.5 (3643.5, 3643.5) |
0.006571 | Secreted IFN-γ (ng/µl) |
969 | CC CA AA |
866 102 1 |
5.9 (1.3, 19.2) 9.0 (3.2, 38.2) 16.1 (16.1, 16.1) |
0.018177 |
| Rubella Vaccine Study |
rs469483~ +IFN-γ |
21/42818515 | MX1 | Intron | Secreted IL-6 (ng/µl) |
969 | AA AG GG |
308 485 176 |
3528.1 (2975.4, 3968.9) 3641.8 (3064.3, 4080.6) 3598.0 (3096.5, 3975.8) |
0.002902 | Secreted IFN-γ (ng/µl) |
969 | AA AG GG |
308 485 176 |
5.0 (0.9, 18.5) 6.6 (1.8, 21.8) 6.4 (1.6, 19.5) |
0.019999 |
| Rubella Vaccine Study |
rs2393657 ++ |
6/26457688 | BTN2A 1/BTN3 A3 |
Intergenic | Secreted IL-6 (ng/µl) |
961 | CC CA AA | 854 107 0 |
3619.9 (3064.3, 4023.2) 3398.9 (2750.9, 3832.4) |
0.031523 | Secreted IFN-γ (ng/µl) |
961 | CC CA AA |
854 107 0 |
6.2 (1.6, 20.8) 6.1 (0.4, 15.2) |
0.034371 |
Abbreviations: NA-neutralizing antibody; SNP ID-single nucleotide polymorphism identification; IL-interleukin; IFN-interferon; IQR-interquartile range; NT50-neutralizing titer; A-Adenine; C-Cytosine; G-Guanine; No.-number
Negative values indicate that the unstimulated secretion levels were higher than the rubella virus-stimulated secretion levels.
Loss of significant association when homozygous minor allele genotype (observed only in few subjects) was excluded from analysis
+One phenotype failed to remain significantly associated with genotype as determined by having a q-value greater than 0.1.
++Two phenotypes failed to remain significantly associated with genotype as determined by having a q-value greater than 0.1.
~IL-6 remained significantly associated with genotype as determined by having a q-value greater than 0.1 in the Caucasian subset.
PVRL3 (Nectin-3) Gene Associations
Overall, we identified 23 SNPs in 10 genes that were significantly associated with more than one rubella-specific immune phenotype post-MMR vaccination, including eight SNPs in the PVRL3 gene. Of these eight SNPs, five were significantly associated with rubella-specific IL-6 and IFN-γ secretion: rs4682233 (p<0.0007), rs72935984 (p<0.004), rs72937914 (p<0.04), rs10433385 (p<0.01), and rs78545860 (p<0.01). Interestingly, the major alleles of these SNPs were all associated with an increase in IL-6 secretion, and enhanced IFN-γ secretion was observed in four of the five. The remaining three SNPs identified in the PVRL3 gene (rs75054607, rs76464851, and rs78157313) were significantly associated with IFN-γ secretion and NA titers (p<0.002, p<0.002, p<0.002, respectively). Though relatively rare (only one homozygous minor allele GG genotype was observed), increasing copies of the minor alleles in all three of these SNPs resulted in a greater than 80-fold increase in IFN-γ secretion from baseline and a greater than three-fold increase in NA titers from baseline (Table 2). These associations remained statistically significant when excluding the homozygous minor allele genotype observed only in one subject (IFN-γ, p=0.004; NT50, p=0.0007).
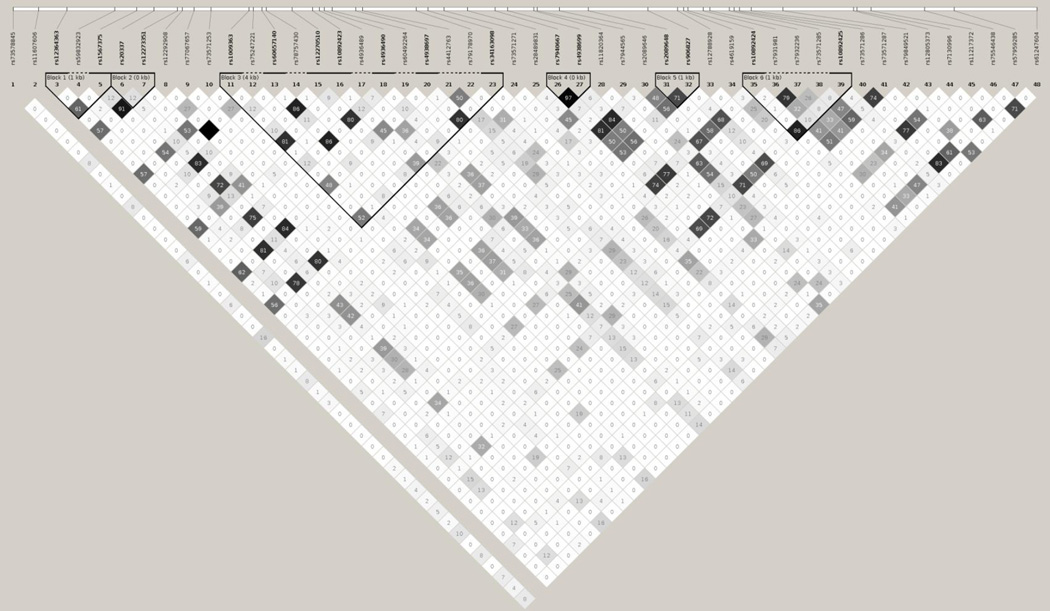
Several of the SNPs in the PVRL3 gene are in high linkage disequilibrium (LD) and are likely to be inherited together. SNPs in high LD (r2≥0.92) include rs72935984 and rs4682233; rs75054607, rs78157313 and rs76464851; rs10433385 and rs78545860 (Figure 1).
Figure 1.
Haplotype block structure of the PVRL3 genetic variants, analyzed using Haploview software, version 4.2 (all SNPs presented were genotyped and utilized in the construction of haplotypes). The r2 color scheme is: white (r2=0), shades of grey (0<r2<1), black (r2=1). The numbers report the r2 value multiplied by 100.
PVRL1 (Nectin-1) Gene Associations
Five SNPs in the PVRL1 gene were also found to be significantly associated with multiple rubella-specific immune response outcomes. The homozygous major alleles of the rs11820364 (p<0.008; one homozygous minor allele genotype was observed) and rs61247604 (p<0.0005; no homozygous minor allele genotype was found) SNPs were associated with a decrease in both IL-6 secretion and NA titers. Both associations remained significant when excluding the homozygous minor allele genotype from analysis (rs11820364, IL-6, p=0.002; NT50, p=0.02; rs61247604, IL-6, p=0.0003; NT50, p=0.0005). An increase in the count of major alleles of the rs73571285 (p<0.02) and rs73571287 (p<0.02) SNPs were associated with an increase in IL-6 secretion and a decrease in secreted IFN-γ; however, rs73571285 demonstrated no association with neither IL-6 nor IFN-γ secretion, and rs73571287 displayed no association with IFN-γ secretion when excluding the homozygous minor allele genotypes from statistical analysis (data not shown). The three remaining SNPs in the PVRL1 gene (rs79849521, p<0.03; rs4936489, p<0.03; and rs73578845, p<0.04) were significantly associated with both IL-6 secretion and NA titers. Likewise, when examining associations without the homozygous minor genotypes that have few (0–2) subjects in that category, IL-6 association with the PVRL1 rs73578845 becomes more significant (p<0.0001) but the NA titer association becomes less significant (p=0.104). Decreasing the count of the major alleles of the rs79849521 resulted in an increase from baseline in IL-6 secretion, as well as an increase in NA titers. Conversely, the major allele of rs4936489 tended to correlate with enhanced IL-6 (p=0.04) secretion and decreased NA titers (p=0.03). These associations remained statistically significant when excluding the homozygous minor allele genotype observed only in one individual (IL-6, p=0.009; NT50, p=0.04) (Table 2).
Several intronic SNPs in the PVRL1 gene have a moderate likelihood of being inherited together. SNPs with an LD of r2≥0.33 include: rs11820364 with rs79849521 and rs61247604, rs79849521 with rs61247604, and rs73571285 with rs73571287 (Figure 2).
Figure 2.
Haplotype block structure of the PVRL1 genetic variants, analyzed using Haploview software, version 4.2 (all SNPs presented were genotyped and utilized in the construction of haplotypes). The r2 color scheme is: white (r2=0), shades of grey (0<r2<1), black (r2=1). The numbers report the r2 value multiplied by 100. Blocks are missing when the minor allele frequency for at least one SNP is 0.
TRIM22 (Tripartite Motif Containing 22) Gene Associations
One coding SNP in the TRIM22 gene, rs2291842 (p<0.01), was significantly associated with all three rubella-specific immune response outcomes in this study (IFN-γ, IL-6, and NA titers). For this SNP, increasing numbers of the minor allele were associated with an increase in NA titers (1.1-fold) and IFN-γ secretion (2.0-fold); however, the major allele seemed to correlate with enhanced secretion of IL-6 (1.1-fold) (Table 2).
Other Gene Associations
We identified nine other associations between multiple rubella-specific immune phenotypes and SNPs from the following genes: IL10RB (interleukin 10 receptor beta; 2 SNPs); TLR4 (toll-like receptor 4; 2 SNPs); PVR (poliovirus receptor; 1 SNP); ADAR (adenosine deaminase; 1 non-synonymous SNP); ZFP57 (zinc finger protein 57; 1 SNP); MX1 (MX dynamin-like GTPase 1; 1 SNP); and BTN2A1/BTN3A3 (butyrophilin, subfamily 2/3, member A1/3; 1 SNP). The two SNPs in the TLR4 gene (rs5030728, p<0.04; and rs2770150, p<0.02) are both significantly associated with both IL-6 secretion and IFN-γ secretion. The major alleles in both SNPs are associated with increased secretion of IL-6; however, subjects with minor alleles of these SNPs tended to secrete increased levels of IFN-γ (Table 2).
Associations between PVRL1 and PVRL3 Haplotypes and Rubella Immune Response Outcomes
The Haploview output for the PVRL3 and PVRL1 gene SNPs that were genotyped and were significant in the study is shown in Figures 1 and 2. Among the seven PVRL1 haplotypes with frequencies ≥1% in the study cohort, the global statistical tests revealed a significant association (global p=0.017) between lower IFN-γ secretion and the PVRL1 haplotype GAAGCGCGGCGGCGGAGGGAGGGGGCCGGAGCAGCGCAGGGGTCCGGG (t-statistic: −2.49, p=0.01) (Table 3). This specific PVRL1 haplotype was not associated with rubella-specific IL-6 secretion or NA titers. In addition, we identified six haplotypes (with frequencies ≥1%) in the PVRL 3 gene in our study cohort (Table 3). The global tests demonstrated highly significant associations between IFN-γ secretion (global p=0.0001) and NA titers (global p=0.037) and the PVRL3 haplotype. Specifically, the PVRL3 haplotype GACGGGGGCAGCAAAAAGAAGAGGAAAGAACAA was significantly associated with both higher IFN-γ production (t-statistic: 4.43, p<0.0001) and higher NA titers (t-statistic: 3.14, p=0.0018). PVRL1 and PVRL3 gene haplotype associations with rubella-specific immune response outcomes in the Caucasian cohort are shown in Supplementary Table 2.
Table 3.
PVRL1 and PVRL3 gene haplotype associations with rubella-specific immune response outcomes in the study cohort.
| PVRL1 (Nectin-1) Gene Allelea | Immune Outcome |
Frequency | Test Statistic |
Allele P Value |
Global P Value |
|---|---|---|---|---|---|
| 0.0174 | |||||
| GAGGCACGGCAGAGAGGAGAGGGGGCCGGAGCGGCGCAGGGGTCCGGG | IFN-γ | 0.0377 | 0.7327 | 0.4640 | |
| GAGGAGAGGCAGCGGGGGGGGGAGGCCGGAGCGGCGCAGGGGTCCGGG | IFN-γ | 0.3452 | −0.8671 | 0.3861 | |
| GAGGAGAGGCAGCGGAGGGGGGAGGCCGGAGCGGCGCAGGGGTCCGGG | IFN-γ | 0.0168 | −0.4096 | 0.6822 | |
| GAAGCGCGGCGGCGGAGGGAGGGGGCCGGAGCGGCGCAGGGGTCCGGG | IFN-γ | 0.2571 | −1.6004 | 0.1099 | |
| GAAGCGCGGCGGCGGAGGGAGGGGGCCGGAGCGGCGCAGGGGTCGGGG | IFN-γ | 0.0682 | 0.3797 | 0.7042 | |
| GAAGCGCGGCGGCGGAGGGAGGGGGCCGGAGCAGCGCAGGGGTCCGGG | IFN-γ | 0.0662 | −2.4912 | 0.0129 | |
| GAAGCACGGCAGAGAGGAGAGGGGGAGGGGAAGGAGCAAGGGTCCGGG | IFN-γ | 0.0498 | 1.3947 | 0.1635 | |
| 0.2050 | |||||
| GAGGCACGGCAGAGAGGAGAGGGGGCCGGAGCGGCGCAGGGGTCCGGG | IL-6 | 0.0377 | 1.6917 | 0.0911 | |
| GAGGAGAGGCAGCGGGGGGGGGAGGCCGGAGCGGCGCAGGGGTCCGGG | IL-6 | 0.3452 | 1.3917 | 0.1643 | |
| GAGGAGAGGCAGCGGAGGGGGGAGGCCGGAGCGGCGCAGGGGTCCGGG | IL-6 | 0.0168 | 1.7540 | 0.0798 | |
| GAAGCGCGGCGGCGGAGGGAGGGGGCCGGAGCGGCGCAGGGGTCCGGG | IL-6 | 0.2571 | 1.1243 | 0.2612 | |
| GAAGCGCGGCGGCGGAGGGAGGGGGCCGGAGCGGCGCAGGGGTCGGGG | IL-6 | 0.0682 | 1.2159 | 0.2243 | |
| GAAGCGCGGCGGCGGAGGGAGGGGGCCGGAGCAGCGCAGGGGTCCGGG | IL-6 | 0.0662 | −0.8107 | 0.4178 | |
| GAAGCACGGCAGAGAGGAGAGGGGGAGGGGAAGGAGCAAGGGTCCGGG | IL-6 | 0.0498 | −0.0479 | 0.9618 | |
| 0.9084 | |||||
| GAGGCACGGCAGAGAGGAGAGGGGGCCGGAGCGGCGCAGGGGTCCGGG | NA | 0.0377 | 0.4669 | 0.6407 | |
| GAGGAGAGGCAGCGGGGGGGGGAGGCCGGAGCGGCGCAGGGGTCCGGG | NA | 0.3452 | 0.2172 | 0.8281 | |
| GAGGAGAGGCAGCGGAGGGGGGAGGCCGGAGCGGCGCAGGGGTCCGGG | NA | 0.0168 | −0.2991 | 0.7649 | |
| GAAGCGCGGCGGCGGAGGGAGGGGGCCGGAGCGGCGCAGGGGTCCGGG | NA | 0.2571 | 0.8906 | 0.3734 | |
| GAAGCGCGGCGGCGGAGGGAGGGGGCCGGAGCGGCGCAGGGGTCGGGG | NA | 0.0682 | 1.0419 | 0.2977 | |
| GAAGCGCGGCGGCGGAGGGAGGGGGCCGGAGCAGCGCAGGGGTCCGGG | NA | 0.0662 | 0.7988 | 0.4246 | |
| GAAGCACGGCAGAGAGGAGAGGGGGAGGGGAAGGAGCAAGGGTCCGGG | NA | 0.0498 | 0.7432 | 0.4575 | |
| PVRL3 (Nectin-3) Gene Alleleb | |||||
| 0.0001 | |||||
| CACGGGAGAAGCAAGCACAAAAACGGAAAACAA | IFN-γ | 0.7719 | 1.4267 | 0.1540 | |
| CACGGGAGAAGCAAGCAGAAAAACGGAAAAAAA | IFN-γ | 0.0939 | 1.4610 | 0.1444 | |
| CACAGGAGCAGCAAGAAGGAAAACAAAAAACAA | IFN-γ | 0.0160 | −0.0068 | 0.9946 | |
| GACGGGGGCAGCAAAAAGAAGAGGAAAGAACAA | IFN-γ | 0.0119 | 4.4292 | <0.0001 | |
| GACGAAAGCAACCAAAAGAAAAGGAAAAAACAA | IFN-γ | 0.0294 | 1.5462 | 0.1224 | |
| GACAGGAGCAGCAAGAAGGAAAACAAAAAACAA | IFN-γ | 0.0186 | −1.2745 | 0.2028 | |
| 0.6698 | |||||
| CACGGGAGAAGCAAGCACAAAAACGGAAAACAA | IL-6 | 0.7719 | −0.7554 | 0.4502 | |
| CACGGGAGAAGCAAGCAGAAAAACGGAAAAAAA | IL-6 | 0.0939 | −0.1292 | 0.8973 | |
| CACAGGAGCAGCAAGAAGGAAAACAAAAAACAA | IL-6 | 0.0160 | −0.2601 | 0.7949 | |
| GACGGGGGCAGCAAAAAGAAGAGGAAAGAACAA | IL-6 | 0.0119 | −1.3743 | 0.1697 | |
| GACGAAAGCAACCAAAAGAAAAGGAAAAAACAA | IL-6 | 0.0294 | −0.9995 | 0.3178 | |
| GACAGGAGCAGCAAGAAGGAAAACAAAAAACAA | IL-6 | 0.0186 | −1.2313 | 0.2185 | |
| 0.0372 | |||||
| CACGGGAGAAGCAAGCACAAAAACGGAAAACAA | NA | 0.7719 | −0.2135 | 0.8310 | |
| CACGGGAGAAGCAAGCAGAAAAACGGAAAAAAA | NA | 0.0939 | −0.3169 | 0.7514 | |
| CACAGGAGCAGCAAGAAGGAAAACAAAAAACAA | NA | 0.0160 | −0.3080 | 0.7581 | |
| GACGGGGGCAGCAAAAAGAAGAGGAAAGAACAA | NA | 0.0119 | 3.1359 | 0.0018 | |
| GACGAAAGCAACCAAAAGAAAAGGAAAAAACAA | NA | 0.0294 | −0.5452 | 0.5858 | |
| GACAGGAGCAGCAAGAAGGAAAACAAAAAACAA | NA | 0.0186 | −0.7710 | 0.4409 |
NOTE
PVRL1 genetic variants from left to right: rs73578845, rs11607606, rs12364363, rs59832923, rs1567375, rs20337, rs12273351, rs12292908, rs77067657, rs73571253, rs1009363, rs75247221, rs60657140, rs78757430, rs12270510, rs10892423, rs4936489, rs4936490, rs60492264, rs4938697, rs4412763, rs79178970, rs34163098, rs73571271, rs28489831, rs7940667, rs4938699, rs11820364, rs7944565, rs2089646, rs2089648, rs906827, rs12788928, rs4619159, rs10892424, rs7931981, rs7932236, rs73571285, rs10892425, rs73571286, rs73571287 rs79849521, rs12805373, rs7130996, rs11217372, rs75546438, rs57959285, rs61247604
PVRL3 genetic variants from left to right: rs1871026, rs9859451, rs7645248, rs7642664, rs873132, rs873133, rs75054607, rs16857598, rs1547518, rs6765133, rs7433877, rs72935984, rs72935989, rs717160, rs9288918, rs1477848, rs72937908, rs1477844, rs7616280, rs75354183, rs78157313, rs72937914, rs72937915, rs9882945, rs1351049, rs56668317, rs4682233, rs76464851, rs60379798, rs10433385, rs73229194, rs78545860, rs1351048
P-values calculated using linear (for antibodies) and linear mixed models (secreted cytokines) while adjusting for the potentially confounding variables of sex, age at enrollment, vaccination history (age at first immunization, age at second immunization, and time from last immunization to blood draw), batch/run number of immune assay used to measure immune outcome, and differences in population genetics (eigenvalues). Allelic P values compare individual haplotypes to all other haplotypes combined. Statistically significant P values (P<0.05) are in boldface type.
Discussion
Previous studies have identified a broad collection of SNPs/haplotypes identified from HLA and non-HLA genes significantly associated with humoral and cellular immune responses to rubella vaccine (Haralambieva et al. 2010; Haralambieva et al. 2014; Ovsyannikova et al. 2010a; Ovsyannikova et al. 2010b; Ovsyannikova et al. 2004; Ovsyannikova et al. 2005; Ovsyannikova et al. 2009b; Pankratz et al. 2010). The purpose of this exploratory study was to identify SNPs in several candidate genes that were significantly associated with more than one rubella-specific immune response post-MMR vaccination. To our knowledge, this is the first study that has identified genes associated with multiple immune response outcomes. Because the identified genotypes are significantly associated with more than one RV-specific outcome, it may be suggested that they have a greater impact on overall immune response to vaccination (Ovsyannikova et al. 2014b). Overall, we identified 23 SNPs in 10 genes that were associated with at least two rubella-specific immune response outcomes, including secreted IL-6, secreted IFN-γ, or neutralizing antibody titers, suggesting joint effects of various genes/genetic variants in the control of the vaccine-induced immune response.
Our statistical analysis results demonstrated evidence for the role of multiple SNPs (some in LD) in the poliovirus receptor (PVR), poliovirus receptor-related 1(PVRL1), and poliovirus receptor-related 3 (PVRL3) genes for being immunologically relevant to the development of both antibody and cytokine immune responses to rubella vaccine. Little is known about the role of genetic variants within these genes in the genetic control of immune response to rubella vaccination; however, It has been demonstrated that the PVR (CD155) gene encodes a transmembrane glycoprotein that belongs to the immunoglobulin (Ig) superfamily and serves as a cellular entry receptor for poliovirus that mediates cell-cell adhesion and cell migration (He et al. 2000). While evidence for PVR’s contribution to RV vaccine-induced immunity is not strong, CD155/PVR was demonstrated to play a role in regulation of Th2 phenotype polarization, NK (natural killer) cell activation, secretion of lytic granules and IFN-γ, and modulation of antigen-specific IgG antibodies in response to TLR agonists (Fuchs et al. 2004; Kamran et al. 2013).
We detected eight SNPs in the PVRL3 (Nectin-3, CD113) gene that were significantly associated with more than one rubella-specific immune response post-MMR vaccination. The effects of these SNPs were also detected at the haplotype level, where haplotype analysis showed a significant association between the PVRL3 GACGGGGGCAGCAAAAAGAAGAGGAAAGAACAA haplotype and both higher rubella-specific NA titers and IFN-γ production. This increases our confidence that the PVRL3 gene locus/allelic variants play a role in the development of RV-induced humoral and cellular immune response. The Nectin family is considered a member of the immunoglobulin super family due to structural similarities (Takai et al. 2008). Nectin-3 has been shown to play a critical role in the control of junctions between endothelial cells, which is important for the transmigration of immune cells during infection (Devilard et al. 2013). Once expressed on the surface of T lymphocytes, Nectin-3 binds to Nectin-2 expressed on endothelial cells. Results from previous studies suggest that this trans-interaction induces the opening of endothelial cell junctions and is required for efficient and effective extravasation of lymphocytes from the blood to sites of infection (Devilard et al. 2013).
Additionally, we identified several SNPs in the PVRL1 (Nectin-1, CD111) gene that were significantly associated with multiple rubella-specific immune response phenotypes. As another member of the Nectin family, Nectin-1 has been shown to influence viral infection. A study conducted by Geraghty et al. identified Nectin-1 (referred to as HveC) as the primary receptor allowing for initial mucosal infection of herpes simplex virus 1 (HSV-1) and HSV-2 and subsequent entry into epithelial and neuronal cells (Geraghty et al. 1998; Satoh-Horikawa et al. 2000). It is unknown how the PVRL1 gene and Nectin gene family relate specifically to immune response to RV although speculations can be made.
Based on the functional role of Nectins in lymphocyte transmigration, HSV infection (Geraghty et al. 1998; Satoh-Horikawa et al. 2000) , and measles virus infection (Muhlebach et al. 2011), it might be speculated that they also play a role in the propagation and/or elimination of RV. Since RV replicates in mucosal cells of the nasopharynx, lymphocyte transmigration is essential for terminating viral replication. Therefore, polymorphisms in the Nectin-3 gene could significantly impact the ability to evade rubella infection. Likewise, Nectin-1 has been shown to mediate cellular infection of HSV and pseudorabies virus (PRV) as well suggesting that a polymorphism in the Nectin-1 gene may enhance or decrease one’s susceptibility to rubella viral infection via downstream effects in viral receptor-mediated entry into host cells and control following immune response. Future studies should, therefore, be aimed at determining if such a relationship between Nectin genes and live RV vaccine exists.
In addition, we identified a coding SNP, rs2291842, in the TRIM22 gene coding region that was significantly associated with all three immune response outcomes. TRIM22 is a member of the tripartite motif family, which is involved in a various array of cellular processes, including differentiation, regulation, and apoptosis (Reymond et al. 2001). For example, rs2291841 in the TRIM22 gene was previously associated with a higher IFN-γ ELISPOT response to measles virus vaccine, while TRIM22 rs885002 was associated with a diminished IL-10 and TNF-α measles virus-specific response (Ovsyannikova et al. 2013). Previous studies also identify TRIM22 as a key regulator of signaling pathways in the innate immune system, especially the antiviral response (McNab et al. 2011). These studies also suggest that IFN-γ induces TRIM22 expression; this leads to an increase in the transcription factor nuclear factor-κB, which has been shown to stimulate pro-inflammatory cytokine secretion, including IL-6 secretion (Yu et al. 2011). The minor allele variant (GG) of the coding rs2291842 identified in this study was associated with an increase from baseline in both rubella-specific IFN-γ secretion and rubella-specific NA titers. Conversely, the major allele variant (AA) of this SNP was associated with secreted IL-6. We speculate that this coding polymorphism may affect TRIM22 protein structure and hence antiviral function and IFN-γ, IL-6 and antibody production by NK/T cells, T cells/macrophages and B cells, respectively. Our earlier studies on live RV vaccine identified associations between SNPs in the TRIM22 gene and rubella-specific IFN-γ, IL-2, and IL-6 secretion levels (Ovsyannikova et al. 2010a), as well as humoral immunity after rubella vaccination (Ovsyannikova et al. 2010b). Therefore, a polymorphism in the TRIM22 gene may impact the response to live RV vaccine due to its significance in innate antiviral immune response.
Two SNPs in the IL10RB gene, rs962859 and rs2284552, were found to be significantly associated with both rubella-specific IFN-γ and IL-6 secretion. Expression of IL10RB is essential for signal transduction induced by IL-10. The interaction between IL10RB, IL10RA, and IL-10 has been shown to impede the secretion of many cytokines, including IL-6 (Dokter et al. 1996). Interestingly, in this study, the major allele variant of the rs962859 SNP and the minor allele variant of the rs2284552 SNP were associated with a significant increase in secreted IL-6 levels, suggesting that polymorphisms in this gene alter this sensitive IL-10 pathway.
Finally, we identified two SNPs in the TLR4 gene that were significantly associated with rubella-specific IL-6 and IFN-γ secretion. Toll-like receptor protein 4 is encoded by the TLR4 gene, and it is important for pathogen recognition and activation of innate immune response pathways (Kopp and Medzhitov 1999). The major allele variants of the rs5030728 and rs2770150 SNPs of the TLR4 gene were found to be significantly associated with increases in rubella-specific IL-6 secretion; however, they were also associated with decreases in rubella-specific IFN-γ secretion. Cytokines IL-6 and IFN-γ are known to have functionally distinct roles and regulate many biological processes, including antiviral immune response. However, data suggest that IL-6 and IFN-γ induce overlapping sets of genes and both signal through a common regulatory JAK/STAT signaling pathway (Qi et al. 2013; Yuan et al. 1994). The role of TLR4 in the immune response to viral pathogens (i.e., viral envelope glycoprotein) has been widely studied (Barton 2007; Ovsyannikova et al. 2011b; Puthothu et al. 2006; Zhou et al. 2011), and our results support the importance of this gene in the immune response to live RV vaccine. We also identified one SNP in each of the following genes that were significantly associated with more than one rubella-specific immune response post-MMR vaccination: PVR, ZFP57, BTN2A1/BTN3A3 and IFN-γ-induced antiviral MX1 and ADAR. For example, a non-synonymous rs2229857 (Lys384/Arg) in the antiviral RNA-specific ADAR gene, known to be involved in RNA editing and gene regulation, demonstrated an allele dose-related increase in IL-6 and IFN-γ secretion with the representation of a minor allele. Consistent with the current results, our earlier vaccine study with measles demonstrated an association between ADAR SNP rs2229857 and measles virus-specific IFN-γ ELISPOT responses (Haralambieva et al. 2011b). We speculate that this genetic variant is likely to be involved in regulation of virus-induced cellular immune mechanisms. This study is strengthened by several factors. The recruitment and utilization of a relatively large sample population (1,039 subjects, 85% Caucasian) with documented vaccine coverage and no circulating wild-type RV enhances our confidence that the immune outcomes measured reflected rubella immunization alone and not disease. Additionally, the relatively small range of age at time of enrollment limits additional factors, such as immunosenescence and waning immune response. The chief limitation of this study is the sole use of candidate genes for statistical analysis and the possibility of detecting potential false-positive associations. This study could be improved upon by having a more genetically diverse population, and future studies will explore whether these associations are observed in other genetically distinct populations. The associations we report herein cannot be generalized to other ethnic groups not examined in this study. We observed very small or no representation of a minor allelic variant for some SNPs in the PVRL1, PVRL3, PVR and other candidate genes that may have skewed the IL-6, IFN-γ and NA immune responses. Multiple statistical tests were completed for this rubella vaccine study; thus, it is possible that a number of false-positive associations with immune response outcomes have been found. It is important to note that after controlling for multiple testing via q-value, some of the SNP-specific tests failed to remain significant at the p<0.1 level. These SNPs have been noted in Table 2. These associations require confirmation in a separate cohort to understand their functional significance. Future replication studies are necessary to validate all of these results.
In conclusion, this is the first study that analyzed and identified SNPs/haplotypes significantly associated with more than one rubella-specific immune response post-MMR vaccination. Our results provided additional insights into multigenic and haplotypic associations between candidate gene SNPs and rubella vaccine-specific NA titers and cytokine production. The results from this study suggest that polymorphisms in these genes contribute to the overall heterogeneity in rubella-specific immune response phenotypes in individuals after being immunized with the MMR vaccine. In the future, these results could aid the prediction of immune response phenotypes in patients pre-vaccination, as well as influence the design of better vaccines through generation of new knowledge and the identification of targets and biomarkers for vaccine response (Poland et al. 2011a; Poland et al. 2009; Poland et al. 2011b).
Supplementary Material
Acknowledgements
We thank the Mayo Clinic Vaccine Research Group staff and subjects who participated in our studies. We thank Caroline L. Vitse for her editorial assistance with this manuscript. We thank Nathaniel D. Warner for his assistance with this study. Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under award numbers R37AI48793 (which recently received a MERIT Award) and R01AI33144. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Presented in part: The 18th Annual Conference on Vaccine Research, Bethesda, MD, April 13-15, 2015 (Abstract S1).
Conflict of interest
Dr. Poland is the chair of a Safety Evaluation Committee for novel non-rubella investigational vaccine trials being conducted by Merck Research Laboratories. Dr. Poland offers consultative advice on vaccine development to Merck & Co. Inc., CSL Biotherapies, Avianax, Sanofi Pasteur, Dynavax, Novartis Vaccines and Therapeutics, PAXVAX Inc, Emergent Biosolutions, Adjuvance, and Vaxess. Drs. Poland and Ovsyannikova hold two patents related to measles and vaccinia peptide research. These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
References
- Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2011;60:1–45. [PubMed] [Google Scholar]
- Barton GM. Viral recognition by Toll-like receptors. Seminars in Immunology. 2007;19:33–40. doi: 10.1016/j.smim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Devilard E, Xerri L, Dubreuil P, Lopez M, Reymond N. Nectin-3 (CD113) interacts with Nectin-2 (CD112) to promote lymphocyte transendothelial migration. PLos ONE. 2013;8:e77424. doi: 10.1371/journal.pone.0077424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman N, Haralambieva IH, Vierkant RA, Pankratz VS, Ryan E, Jacobson RM, Ovsyannikova IG, Poland GA. Predominant inflammatory cytokine secretion pattern in response to two doses of live rubella vaccine in health vaccinees. Cytokine. 2010;50:24–29. doi: 10.1016/j.cyto.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokter WH, Koopmans SB, Vellenga E. Effects of IL-10 and IL-4 on LPS-induced transcription factors (AP-1, NF-IL6 and NF-kappa B) which are involved in IL-6 regulation. Leukemia. 1996;10:1308–1316. [PubMed] [Google Scholar]
- Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) Journal of Immunology. 2004;172:3994–3998. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- Haralambieva IH, Dhiman N, Ovsyannikova IG, Vierkant RA, Pankratz VS, Jacobson RM, Poland GA. 2'-5'-Oligoadenylate synthetase single-nucleotide polymorphisms and haplotypes are associated with variations in immune responses to rubella vaccine. Hum.Immunol. 2010;71:383–391. doi: 10.1016/j.humimm.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralambieva IH, Lambert ND, Ovsyannikova IG, Kennedy RB, Larrabee BR, Pankrantz VS, Poland GA. Associations between single nucleotide polymorphisms in cellular viral receptors and attachment factor-related genes and humoral immunity to rubella vaccination. PLos ONE. 2014;9:e99997. doi: 10.1371/journal.pone.0099997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralambieva IH, Oberg AL, Ovsyannikova IG, Kennedy RB, Grill DE, Middha S, Bot BM, Wang VW, Smith DI, Jacobson RM, Poland GA. Genome-wide characterization of transcriptional patterns in high and low antibody responders to rubella vaccination. PLos ONE. 2013;8:e62149. doi: 10.1371/journal.pone.0062149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralambieva IH, Ovsyannikova IG, Kennedy RB, Vierkant RA, Pankratz SV, Jacobson RM, Poland GA. Associations between single nucleotide polymorphisms and haplotypes in cytokine and cytokine receptor genes and immunity to measles vaccination. Vaccine. 2011a;29:7883–7895. doi: 10.1016/j.vaccine.2011.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralambieva IH, Ovsyannikova IG, Umlauf BJ, Vierkant RA, Pankratz SV, Jacobson RM, Poland GA. Genetic polymorphisms in host antiviral genes: associations with humoral and cellular immunity to measles vaccine. Vaccine. 2011b;29:8988–8997. doi: 10.1016/j.vaccine.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Bowman VD, Mueller S, Bator CM, Bella J, Peng X, Baker TS, Wimmer E, Kuhn RJ, Rossmann MG. Interaction of the poliovirus receptor with poliovirus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:79–84. doi: 10.1073/pnas.97.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamran N, Takai Y, Miyoshi J, Biswas SK, Wong JS, Gasser S. Toll-like receptor ligands induce expression of the costimulatory molecule CD155 on antigen-presenting cells. PLos ONE. 2013;8:e54406. doi: 10.1371/journal.pone.0054406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Current Opinion in Immunology. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- Lambert ND, Haralambieva IH, Ovsyannikova IG, Larrabee BR, Pankratz VS, Poland GA. Characterization of humoral and cellular immunity to rubella vaccine in four distinct cohorts. Immunologic Research. 2013;58:1–8. doi: 10.1007/s12026-013-8475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert ND, Pankratz VS, Larrabee BR, Ogee-Nwankwo A, Chen MH, Icenogle JP, Poland GA. High-throughput Assay Optimization and Statistical Interpolation of Rubella-Specific Neutralizing Antibody Titers. Clinical and Vaccine Immunology. 2014;21:340–346. doi: 10.1128/CVI.00681-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievano F, Galea SA, Thornton M, Wiedmann RT, Manoff SB, Tran TN, Amin MA, Seminack MM, Vagie KA, Dana A, Plotkin SA. Measles, mumps, and rubella virus vaccine (M-M-RII): a review of 32 years of clinical and postmarketing experience. Vaccine. 2012;30:6918–6926. doi: 10.1016/j.vaccine.2012.08.057. [DOI] [PubMed] [Google Scholar]
- McNab FW, Rajsbaum R, Stoye JP, O'Garra A. Tripartite-motif proteins and innate immune regulation. Current Opinion in Immunology. 2011;23:46–56. doi: 10.1016/j.coi.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Muhlebach MD, Mateo M, Sinn PL, Prufer S, Uhlig KM, Leonard VH, Navaratnarajah CK, Frenzke M, Wong XX, Sawatsky B, Ramachandran S, McCray PB, Jr, Cichutek K, von MV, Lopez M, Cattaneo R. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480:530–533. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Dhiman N, Haralambieva IH, Vierkant RA, O'Byrne MM, Jacobson RM, Poland GA. Rubella vaccine-induced cellular immunity: evidence of associations with polymorphisms in the Toll-like, vitamin A and D receptors, and innate immune response genes. Human Genetics. 2010a;127:207–221. doi: 10.1007/s00439-009-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Haralambieva IH, Dhiman N, O'Byrne MM, Pankratz VS, Jacobson RM, Poland GA. Polymorphisms in the vitamin A receptor and innate immunity genes influence the antibody response to rubella vaccination. J Infect Dis. 2010b;201:207–213. doi: 10.1086/649588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Haralambieva IH, Vierkant RA, O'Byrne MM, Jacobson RM, Poland GA. The association of CD46, SLAM, and CD209 cellular receptor gene SNPs with variations in measles vaccine-induced immune responses--a replication study and examination of novel polymorphisms. Human Heredity. 2011a;72:206–223. doi: 10.1159/000331585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Haralambieva IH, Vierkant RA, O'Byrne MM, Jacobson RM, Poland GA. Effects of vitamin A and D receptor gene polymophisms/haplotypes on immune responses to measles vaccine. Pharmacogenetics and Genomics. 2012;22:20–31. doi: 10.1097/FPC.0b013e32834df186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Haralambieva IH, Vierkant RA, O'Byrne MM, Poland GA. Associations between polymorphisms in the antiviral TRIM genes and measles vaccine immunity. Human Immunology. 2013;74:768–774. doi: 10.1016/j.humimm.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Haralambieva IH, Vierkant RA, Pankratz VS, Poland GA. The role of polymorphisms in toll-like receptors and their associated intracellular signaling genes in measles vaccine immunity. Human Genetics. 2011b;130:547–561. doi: 10.1007/s00439-011-0977-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Jacobson RM, Ryan JE, Dhiman N, Vierkant RA, Poland GA. Relationship between HLA polymorphisms and gamma interferon and interleukin-10 cytokine production in healthy individuals after rubella vaccination. Clin Vaccine Immunol. 2007;14:115–122. doi: 10.1128/CVI.00247-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Jacobson RM, Vierkant RA, Jacobsen SJ, Pankratz VS, Poland GA. The contribution of HLA class I antigens in immune status following two doses of rubella vaccination. Human Immunology. 2004;65:1506–1515. doi: 10.1016/j.humimm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Ovsyannikova IG, Jacobson RM, Vierkant RA, Jacobsen SJ, Pankratz VS, Poland GA. Human leukocyte antigen class II alleles and rubella-specific humoral and cell-mediated immunity following measles-mumps-rubella-II vaccination. Journal of Infectious Diseases. 2005;191:515–519. doi: 10.1086/427558. [DOI] [PubMed] [Google Scholar]
- Ovsyannikova IG, Jacobson RM, Vierkant RA, O'Byrne MM, Poland GA. Replication of rubella vaccine population genetic studies: validation of HLA genotype and humoral response associations. Vaccine. 2009a;27:6926–6931. doi: 10.1016/j.vaccine.2009.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Pankratz VS, Larrabee BR, Jacobson RM, Poland GA. HLA genotypes and rubella vaccine immune response: additional evidence. Vaccine. 2014a;32:4206–4213. doi: 10.1016/j.vaccine.2014.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Ryan JE, Vierkant RA, O'Byrne MM, Jacobson RM, Poland GA. Influence of host genetic variation on rubella-specific T cell cytokine responses following rubella vaccination. Vaccine. 2009b;27:3359–3366. doi: 10.1016/j.vaccine.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Salk HM, Larrabee BR, Pankratz VS, Poland GA. Single-nucleotide polymorphism associations in common with immune responses to measles and rubella vaccines. Immunogenetics. 2014b;66:663–669. doi: 10.1007/s00251-014-0796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratz VS, Vierkant RA, O'Byrne MM, Ovsyannikova IG, Poland GA. Associations between SNPs in candidate immune-relevant genes and rubella antibody levels: a multigenic assessment. BMC Immunol. 2010;11:48. doi: 10.1186/1471-2172-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA. Rubella eradication. Vaccine. 2001;19:3311–3319. doi: 10.1016/s0264-410x(01)00073-1. [DOI] [PubMed] [Google Scholar]
- Poland GA, Kennedy RB, Ovsyannikova IG. Vaccinomics and personalized vaccinology: Is science leading us toward a new path of directed vaccine development and discovery? PLoS Pathogens. 2011a;7:e1002344. doi: 10.1371/journal.ppat.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland GA, Ovsyannikova IG, Jacobson RM. Application of pharmacogenomics to vaccines. Pharmacogenomics. 2009;10:837–852. doi: 10.2217/PGS.09.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland GA, Ovsyannikova IG, Kennedy RB, Haralambieva IH, Jacobson RM. Vaccinomics and a new paradigm for the development of preventive vaccines against viral infections. Omics. 2011b;15:625–636. doi: 10.1089/omi.2011.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthothu B, Forster J, Heinzmann A, Krueger M. TLR-4 and CD14 polymorphisms in respiratory syncytial virus associated disease. Dis Markers. 2006;22:303–308. doi: 10.1155/2006/865890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi YF, Huang YX, Wang HY, Zhang Y, Bao YL, Sun LG, Wu Y, Yu CL, Song ZB, Zheng LH, Sun Y, Wang GN, Li YX. Elucidating the crosstalk mechanism between IFN-gamma and IL-6 via mathematical modelling. BMC Bioinformatics. 2013;14:41. doi: 10.1186/1471-2105-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A. The tripartite motif family identifies cell compartments. The EMBO journal. 2001;20:2140–2151. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. The Journal of Biological Chemistry. 2000;275:10291–10299. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. American Journal of Human Genetics. 2002;70:425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydnor E, Perl TM. Healthcare providers as sources of vaccine-preventable diseases. Vaccine. 2014;32:4814–4822. doi: 10.1016/j.vaccine.2014.03.097. [DOI] [PubMed] [Google Scholar]
- Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nature reviews. Molecular cell biology. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. The Journal of clinical investigation. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Gao B, Duan Z, Xu W, Xiong S. Identification of tripartite motif-containing 22 (TRIM22) as a novel NF-kappaB activator. Biochemical and Biophysical Research Communications. 2011;410:247–251. doi: 10.1016/j.bbrc.2011.05.124. [DOI] [PubMed] [Google Scholar]
- Yuan J, Wegenka UM, Lutticken C, Buschmann J, Decker T, Schindler C, Heinrich PC, Horn F. The signalling pathways of interleukin-6 and gamma interferon converge by the activation of different transcription factors which bind to common responsive DNA elements. Molecular and cellular biology. 1994;14:1657–1668. doi: 10.1128/mcb.14.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Wei B, Xing C, Xie H, Yu X, Wu L, Zheng S. Polymorphism in 3'-untranslated region of toll-like receptor 4 gene is associated with protection from hepatitis B virus recurrence after liver transplantation. Transplant infectious disease : an official journal of the Transplantation Society. 2011;13:250–258. doi: 10.1111/j.1399-3062.2010.00574.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.