Abstract
Vitamin D has endocrine function as a key regulator of calcium absorption and bone homeostasis and also has intracrine function as an immunomodulator. Vitamin D deficiency before hematopoietic stem cell transplantation (HSCT) has been variably associated with higher risks of graft-versus-host disease (GVHD) and mortality. Children are at particular risk of growth impairment and bony abnormalities in the face of prolonged deficiency. There are few longitudinal studies of vitamin D deficient children receiving HSCT, and the prevalence and consequences of vitamin D deficiency 100 days after transplant has been poorly studied. Serum samples from 134 consecutive HSCT patients prospectively enrolled into an HSCT sample repository were tested for 25-hydroxy (25 OH) vitamin D levels before starting HSCT (baseline) and at 100 days after transplantation. Ninety-four of 134 patients (70%) had a vitamin D level < 30 ng/mL before HSCT, despite supplemental therapy in 16% of subjects. Post-transplant samples were available in 129 patients who survived to day 100 post-transplant. Vitamin D deficiency persisted in 66 of 87 patients (76%) who were already deficient before HSCT. Moreover, 24 patients with normal vitamin D levels before HSCT were vitamin D deficient by day 100. Overall, 68% of patients were vitamin D deficient (<30 ng/mL) at day 100, and one third of these cases had severe vitamin D deficiency (<20 ng/mL). Low vitamin D levels before HSCT were not associated with subsequent acute or chronic GVHD, contrary to some prior reports. However, severe vitamin D deficiency (<20 ng/mL) at 100 days post-HSCT was associated with decreased overall survival after transplantation (P = .044, 1-year rate of overall survival: 70% versus 84.1%). We conclude that all pediatric transplant recipients should be screened for vitamin D deficiency before HSCT and at day 100 post-transplant and that aggressive supplementation is needed to maintain sufficient levels.
Keywords: Pediatric HSCT, Vitamin D deficiency, GVHD, Mortality
INTRODUCTION
Vitamin D has important endocrine functions, such as modifying absorption of dietary calcium and regulating bone homeostasis. Severe dietary vitamin D deficiency manifests as rickets in children. In children, the bone matrix is poorly mineralized, and abnormal chondrocyte maturation leads to the classical bowing of the legs described in rachitic children, together with widened epiphyseal plates at the end of long bones and costochondral junctions, frontal bossing of the skull, and delayed tooth eruption [1]. In contrast, adults have sufficient mineral in the long bones and epiphyseal plates are closed, so there are no visible skeletal abnormalities but rather manifestations of osteomalacia. The unmineralized matrix beneath the periosteal membrane, which is heavily innervated with sensory fibers, is hydrated and pushed upward, often causing throbbing, aching bone pain. These data support possible important differences in clinical manifestations of vitamin D deficiency between children and adults.
Studies have indicated widespread vitamin D deficiency or insufficiency in the US population, perhaps because of inadequate dietary intake, sedentary lifestyles, and reduced sun exposure [1,2]. Understanding the true prevalence of clinically significant vitamin D deficiency is complicated by changing views on optimal vitamin D levels. There is broad agreement that levels less than 20 ng/mL indicate deficiency, and many consider levels below 30 ng/mL insufficient [1,3,4]. However, some other authors argue that levels above 50 or 75 ng/mL are optimal, based at least in part on the serum vitamin D level above which no further suppression of parathyroid hormone occurs [2,4,5].
Vitamin D is stored in the body as 25-(OH)-vitamin D, an inactive form that is activated enzymatically to 1,25 (OH) vitamin D in the kidneys, an activity that is impaired in significant renal dysfunction. The same enzyme (25-hydroxyvitamin D-1-hydroxylase) activity that activates vitamin D in the kidney is present in other tissues, including breast, prostate, brain, activated T cells, and antigen-presenting cells such as activated macrophages. The binding of 1,25 (OH) vitamin D to the nuclear vitamin D receptor in macrophages, dendritic cells, and T cells increases expression of cathelicidin, promoting innate immune responses and destruction of infective agents [6,7]. There is also likely secretion of 1,25 (OH) vitamin D, acting locally on T and B lymphocytes. Vitamin D receptor stimulation leads to modification of cytokine secretion, through direct interaction with vitamin D responsive promoter elements and perhaps indirectly through interaction with other transcription factors [8]. These data support potential important effects of vitamin D deficiency on immune recovery and perhaps on graft-versus-host disease (GVHD) after hematopoietic stem cell transplantation (HSCT).
Previous studies of vitamin D deficiency after HSCT have produced conflicting results, with some indicating reduced survival and increased risk of acute or chronic GVHD and some finding no impact on some or all of these endpoints. Few studies have studied vitamin D levels patients longitudinally, and most studies examined adults and not children. Our center focuses on children with genetic disorders who generally have not had prior chemotherapy, so we hypothesized that the frequency of vitamin D deficiency might be lower in our patient population. Moreover, approximately half of our children receive reduced-intensity conditioning regimens that we hypothesized would be less likely to be associated with vitamin D deficiency. In contrast to these hypotheses, we found a very high frequency of vitamin D deficiency before transplant and found that many children remain or become vitamin D deficient during the first 100 days after transplant.
METHODS
Patients and Transplant Procedures
Patient and transplant characteristics are summarized in Table 1. Children in this study were enrolled in 1 of 2 prospective cohort studies, the first a biomarkers study for thrombotic microangiopathy and the second the Cincinnati Children's Hospital and Medical Center BMT repository. All consecutive participants in these 2 studies were eligible to participate and had signed consent for sample collection for biological studies of complications of transplantation. The institutional review board of Cincinnati Children's Hospital Medical Center approved the study.
Table 1.
Patient (N = 134) and Transplant Demographics
| Characteristic | Value |
|---|---|
| Male/female | 87/47 |
| Mean age, yr (range) | 7.1 (2.7-14.9) |
| Diagnosis | |
| Bone marrow failure | 36 (27%) |
| Immune deficiency | 52 (38.8%) |
| Malignancy | 38 (28.2%) |
| Genetic/metabolic | 8 (6%) |
| Race | |
| White | 116 (86.6%) |
| Nonwhite | 18 (13.4%) |
| Conditioning regimen | |
| Myeloablative | 73 (54.4%) |
| Reduced intensity | 61 (45.5%) |
| Donor type | |
| Related | 40 (29.8%) |
| Unrelated | 94 (70.2%) |
| Stem cell source | |
| Bone marrow | 108 (80.6%) |
| PBSC | 16 (11.9%) |
| Cord blood | 10 (7.5%) |
| HLA matching | |
| 8/8 Allele matched | 108 (80.6%) |
| Mismatched | 26 (19.4%) |
PBSC indicates peripheral blood stem cell.
Median patient age was 7.1 years, and most patients were transplanted for a nonmalignant disease. Most recipients were white, and almost half received a reduced-intensity preparative regimen. Most donors were unrelated adult donors, and most grafts were bone marrow. Clinical data were abstracted from the transplant database and verified by chart review if needed. Registered dieticians with experience in HSCT oversaw dietary issues in all children, but routine measurements of vitamin D were not made without clinical indications. If a clinical indication arose and vitamin D deficiency or insufficiency were identified, supplementation with recommended daily doses were prescribed.
Dietary Management and Supportive Care during HSCT
A registered dietician assessed and followed all patients undergoing HSCT. Patients were allowed to eat normally while following the dietary restrictions of the low bacteria diet. If unable to consume adequate calories, patients were started on enteral feeding using an age-appropriate formula. Parenteral nutrition was provided to children unable to tolerate enteral nutrition. Vitamin and mineral supplementation were provided on an as-needed basis. Antifungal prophylaxis was primarily voriconazole, with pharmacogenetically assigned drug dosing, adjusted pharmacokinetically to maintain therapeutic levels. Children with contraindication or intolerance to voriconazole were treated with micafungin.
Vitamin D Analysis by Ultra-High-Performance Liquid Chromatography Coupled to Electrospray Tandem Mass Spectrometry
Serum samples were collected prospectively on consenting HSCT recipients less than 18 years old receiving their first HSCT, and samples were stored at −°C until analyzed. Human serum concentrations of vitamin D (vitamin D2 and D3) and 25-hydroxyvitamin D (25-OH D2 and 25-OH-D3) were determined by ultra-high-performance liquid chromatography coupled to electrospray tandem mass spectrometry (Waters, Milford, MA) [9]. Serum samples were extracted by liquideliquid extraction with methyl tert-butyl ether/ethyl acetate/hexane (5:4:1). Combined extracts were dried and derived by 4-phenyl-1,2,4-thriazoline-3,5-dione before transfer to sample vials. Quantification was conducted with multiple reaction monitoring and with a stable isotope dilution ultra-high-performance liquid chromatography coupled to electrospray tandem mass spectrometry method on a Supelcosil LC-18-DB column (33 × 3 mm, 3 μm; Sigma, St. Louis, MO). A vitamin D level < 30 ng/mL was defined as vitamin D insufficiency, and a vitamin D level < 20 ng/mL was defined as vitamin D deficiency.
Statistical Analysis
Continuous and categorical variables were compared using Wilcoxon rank sum test and Fisher's exact test, respectively. Survival and cumulative incidence curves, percentages, and standard errors were computed using the Kaplan-Meier method. Survival between groups was assessed using log rank tests. For other time to event data, Gray's method for competing risks was used. Death and relapse (for GVHD) were treated as competing risks. Cox proportional hazards was used for the multivariable survival models. A backward selection model was used, including all variables mentioned in the demographic table, but only significant variables were retained in the final model. All statistical computations were performed using R software (version 3.0.1; www.r-project.org). Statistical significance was determined at the .05 level.
RESULTS
Vitamin D Deficiency
Seventy percent of children (94/134) were vitamin D insufficient (<30 ng/mL) and 33% (45/134) were vitamin D deficient (<20 ng/mL) before start of transplant (Table 2). There was no significant difference in mean vitamin D level according to latitude of residence (P = 1.0) or cystatin C–estimated glomerular filtration rate. Median cystatin C–estimated glomerular filtration rate was 99.9 mL/min/1.73 m2 (range, 90.6 to 109.5) in those with vitamin D > 20 ng/mL before HSCT and was 100.6 mL/min/1.73 m2 (range, 88.5 to 117.2) in those with vitamin D < 20 ng/mL (P =.54). Similarly, median cystatin C–estimated glomerular filtration rate was 80.0 mL/min/1.73 m2 (range, 64.7 to 91.3) in those with vitamin D > 20 ng/mL 100 days post-HSCT and was 73.9 mL/min/1.73 m2 (range, 63.8 to 91) in those with vitamin D < 20 ng/mL (P = .52). Moreover, vitamin D deficiency was not more frequent in nonwhite children (P = .3), although the number of nonwhite children was small (n = 18), limiting the ability to see all but a large difference. Some seasonal difference was seen, with 50% of children transplanted in the winter quarter having a pre-HSCT vitamin D level less than 20 ng/mL compared with 13% transplanted in spring, 33% in summer, and 37% in fall (P = .025). Twenty-eight children (21%) were receiving vitamin D supplements before transplant; despite this, 13 of these children (46%) were vitamin D insufficient (30 ng/mL) and 5 had severe vitamin D deficiency (20 ng/mL).
Table 2.
Vitamin D Levels before HSCT
| Vitamin D Insufficiency (<30 ng/mL) | Vitamin D Deficiency (<20 ng/mL) | |
|---|---|---|
| All patients (N = 134) | 70% (94/134) | 33% (45/134) |
| Patients on vitamin D supplementation (n = 28) | 46% (13/28) | 18% (5/28) |
| Patients not on vitamin D supplementation (n = 106) | 76% (81/106) | 38% (40/106) |
At day 100, 68% of children (88/129) were vitamin D insufficient (<30 ng/mL) and 31% vitamin D deficient (<20 ng/mL) (Table 3). Twenty-nine children (21%) were receiving vitamin D at day 100; despite this, 11 of these children (38%) were still vitamin D insufficient (30 ng/mL) and 11 had severe vitamin D deficiency (20 ng/mL). Seventy-six percent of children who were already deficient at baseline remained deficient or insufficient until 100 days. Fifty-seven percent of children with normal vitamin D levels before transplant-developed deficiency or insufficiency by 100 days after transplantation.
Table 3.
Vitamin D Levels at Day 100
| Vitamin D Insufficiency (<30 ng/mL) | Vitamin D Deficiency (<20 ng/mL) | |
|---|---|---|
| All patients (N = 129) | 68% (88/129) | 31% (40/129) |
| Patients on vitamin D supplementation (n = 29) | 38% (11/29) | 38% (11/29) |
| Patients not on vitamin D supplementation (n = 100) | 77% (77/100) | 29% (29/100) |
Engraftment and Acute and Chronic GVHD
No differences were detected in time to engraftment between vitamin D insufficient and deficient children and children with adequate vitamin D measured before HSCT. The cumulative incidence of acute GVHD was similar in children with vitamin D insufficiency and those without (P =.49, 100-day acute GVHD incidence 32% versus 40%) and in children with vitamin D deficiency and those without (P = .73, 100-day acute GVHD incidence 33% versus 35%), measured before HSCT. Similarly, the cumulative incidence of chronic GVHD was similar in children with vitamin D insufficiency and those without (P = .24, 1-year cGVHD incidence 4% versus 7%) and in children with vitamin D deficiency and those without (P = .86, 1-year cGVHD incidence 3% versus 13%), measured before HSCT.
Survival
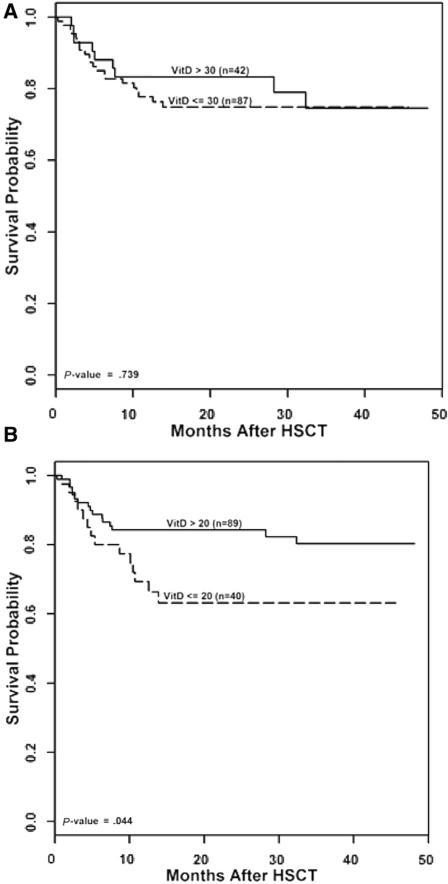
Overall survival was similar in children with vitamin D insufficiency at baseline and those without (78% versus 75%, P =.65) and in children with vitamin D deficiency at baseline and those without (75% versus 78%, P = .54). There was no difference in survival between children with vitamin D levels above and below 30 ng/mL at day 100 (vitamin D insufficiency; 78% versus 83%, P = .77). In contrast, survival was significantly reduced in children with severe vitamin D deficiency (20 ng/mL) at day 100 (Figure 1). A multivariable model, adjusting survival for occurrence of GVHD, showed an increased mortality associated with GVHD (odds ratio, 2.67; P < .01) and a nonstatistically significant reduction in mortality in children with a vitamin D level > 20 ng/mL (odds ratio, .56; P = .11), raising the possibility that increased mortality associated with vitamin D deficiency may be due in part to an association of vitamin D deficiency with GVHD and steroid use.
Figure 1.
Overall survival after HSCT in children with a day 100 vitamin D level < 30 ng/mL (A) and <20 ng/mL (B).
Infections
The frequency of viral infections was not increased in children with vitamin D levels above and below 20 ng/mL before HSCT (42/89 [47%] versus 23/45 [51%], P = .36). In addition, the frequency of bacterial infections was similar in those with vitamin D levels above and below 20 ng/mL before HSCT (20/89 [22%] versus 9/45 [20%], P = 1.0). Examination of causes of death showed no difference in the number of deaths from sepsis among children with vitamin D levels below and above 20 ng/mL at day 100 (5/40 versus 9/88, not significant).
DISCUSSION
This study reports the frequency and impact of vitamin D deficiency in a cohort of children receiving transplant mostly for nonmalignant diagnoses. The data show a high frequency of vitamin D deficiency. Importantly, some vitamin D–sufficient children become vitamin D deficient in the first 100 days after transplant, and those who were vitamin D deficient before transplant in general remained insufficient, despite supervision by a registered dietician supplying recommended supplementation, suggesting that higher doses of supplements may be needed for this population. Our data also showed an association between very low (<20 ng/mL) levels of vitamin D and reduced survival. The significance of this difference was lost after adjustment for GVHD, and we propose that the effect of vitamin D deficiency on survival may be indirect and a consequence of GVHD rather than vitamin D deficiency per se.
Our data show that most children were vitamin D insufficient or deficient before the start of transplant. These data should be considered in the light of reports of frequent low vitamin D levels in healthy US children. Weng et al. [2] measured 25(OH) vitamin D levels in 382 normal children ages 6 to 21 in the United States and found a median concentration of 28 ng/mL, with 55% of subjects having levels below 30 ng/mL. We found levels below 30 ng/mL in 70% of children before bone marrow transplantation (BMT), a number perhaps not greatly different from the normal population. Previous reports of pediatric transplant have generally found similar low levels before HSCT [10-12]. In contrast, Hansson et al. [13] reported insufficiency in only 30% of a population of 123 Swedish children, perhaps reflecting better population vitamin D status in Sweden.
A previous study of 53 adult patients reported a significant association between low levels of 25(OH) vitamin D before BMT and subsequent risk of chronic GVHD [14]. We did not find an association in our study, in agreement with the report of Hansson et al. These differing findings may reflect biological differences between children and adults or perhaps lower frequency of chronic GVHD in pediatric populations, limiting power to detect a modest effect.
Our data indicate reduced survival in children who were deficient in vitamin D at day 100 post-HSCT. Although most studies addressing vitamin D deficiency and HSCT do not report impact on survival, Hansson et al. [13] did report improved survival in children with malignancies who were vitamin D sufficient compared with those who were vitamin D insufficient. Previous work has suggested an association between acute GVHD and vitamin D insufficiency, perhaps indicating an association between the steroids used to treat GVHD and vitamin D insufficiency rather than a causative role for vitamin D deficiency and GVHD. Sproat et al. [15] reported that 89% of 58 adults, mostly tested because of the presence of GVHD, were vitamin D insufficient. In our own study, we found that the frequencies of grades II to IV and III to IV acute GVHD were not significantly higher in vitamin D–deficient children. However, adjustment of survival for occurrence of acute GVHD reduced the significance of the survival difference seen in vitamin D–deficient children, suggesting that at least part of the increased mortality might be due to the occurrence of GVHD and the associated steroid use contributed to the vitamin D insufficiency. We recognize that our data do not prove a causal or noncausal relationship between vitamin D insufficiency and increased mortality, and other explanations are also possible. Vitamin D insufficiency has been linked with pulmonary disease such as chronic obstructive pulmonary disease and acute respiratory distress syndrome, and increased susceptibility to infection might influence survival in our children [16-18].
Our data include measurements made before BMT and at day 100 and showed that most children who were vitamin D insufficient at the outset remained vitamin D insufficient at day 100. Moreover, more than half the children who were vitamin D sufficient before HSCT became insufficient by day 100 with standard dietary supervision. These data indicate the need for measurement of vitamin D levels in all HSCT recipients and aggressive vitamin D replacement, with follow-up to ensure replete levels. Impaired hepatic and renal function post-HSCT may increase the level of vitamin D needed for optimal health, and compliance with therapy may not be optimal; therefore, continued surveillance is important. Vitamin D replacement may require higher daily dosing than the recommended daily dietary requirement for healthy persons, a value that is controversial and may be lower than is needed for optimal health in light of lower population sun exposure and poor intake of vitamin D from diet [19]. The American Academy of Pediatrics increased the recommended daily intake of vitamin D from 200 to 400 IU/day for children and adolescents [20]. In addition, some investigators suggest that much higher levels (75 to 100 ng/mL), selected because these are levels at which suppression of parathyroid hormone has plateaued, should be regarded as optimal, as opposed to levels > 30 ng/mL [2-4]. Higher targets, if shown to be optimal, will require much more aggressive replacement strategies.
All transplant recipients at our center now have a level measured at baseline and monthly thereafter until day 100. Insufficient children (<30 ng/mL) receive replacement until replete, using 2000 IU daily for at least 6 weeks or 50,000 IU weekly for at least 6 weeks to achieve a level > 30 mg/dL. Children with levels less than 10 ng/mL receive 50,000 IU weekly for at least 6 weeks. Once replete, children continue on maintenance therapy of 600 to 1000 IU/day. We recognize that requirements may be higher in children on steroids and antifungal therapy, so children with GVHD on steroids may need maintenance dosing of 1200 to 3000 IU/day. All HSCT recipients are evaluated 1 year after transplant by a pediatric endocrinologist when, among other things, vitamin D status is assessed and bone density measured if clinically indicated.
Footnotes
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
REFERENCES
- 1.Wacker M, Holick MF. Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111–148. doi: 10.3390/nu5010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weng FL, Shults J, Leonard MB, et al. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007;86:150–158. doi: 10.1093/ajcn/86.1.150. [DOI] [PubMed] [Google Scholar]
- 3.Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011;14:938–939. doi: 10.1017/S1368980011000565. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 5.Lips P. Relative value of 25(OH)D and 1,25(OH)2D measurements. J Bone Miner Res. 2007;22:1668–1671. doi: 10.1359/jbmr.070716. [DOI] [PubMed] [Google Scholar]
- 6.Chun RF, Adams JS, Hewison M. Back to the future: a new look at “old” vitamin D. J Endocrinol. 2008;198:261–269. doi: 10.1677/JOE-08-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Ding S, Schoenmakers I, Jones K, et al. Quantitative determination of vitamin D metabolites in plasma using UHPLC-MS/MS. Anal Bioanal Chem. 2010;398:779–789. doi: 10.1007/s00216-010-3993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bechard LJ, Gordon C, Feldman HA, et al. Bone loss and vitamin D deficiency in children undergoing hematopoietic cell transplantation. Pediatr Blood Cancer. 2015;62:687–692. doi: 10.1002/pbc.25370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan CN, Vrooman L, Apfelbaum EM, et al. 25-Hydroxy vitamin D deficiency following pediatric hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2011;17:749–753. doi: 10.1016/j.bbmt.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Campos DJ, Boguszewski CL, Funke VA, et al. Bone mineral density, vitamin D, and nutritional status of children submitted to hematopoietic stem cell transplantation. Nutrition. 2014;30:654–659. doi: 10.1016/j.nut.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Hansson ME, Norlin AC, Omazic B, et al. Vitamin d levels affect outcome in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1537–1543. doi: 10.1016/j.bbmt.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 14.Glotzbecker B, Ho VT, Aldridge J, et al. Low levels of 25-hydroxyvitamin D before allogeneic hematopoietic SCT correlate with the development of chronic GVHD. Bone Marrow Transplant. 2013;48:593–597. doi: 10.1038/bmt.2012.177. [DOI] [PubMed] [Google Scholar]
- 15.Sproat L, Bolwell B, Rybicki L, et al. Vitamin D level after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2011;17:1079–1083. doi: 10.1016/j.bbmt.2010.12.704. [DOI] [PubMed] [Google Scholar]
- 16.Martineau AR, James WY, Hooper RL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2015;3:120–130. doi: 10.1016/S2213-2600(14)70255-3. [DOI] [PubMed] [Google Scholar]
- 17.Dancer RCA, Parekh D, Lax S, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015;70:617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant CC, Kaur S, Waymouth E, et al. Reduced primary care respiratory infection visits following pregnancy and infancy vitamin D supplementation: a randomised controlled trial. Acta Paediatr. 2015;104:396–404. doi: 10.1111/apa.12819. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 20.Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding. American Academy of Pediatrics Committee on Nutrition Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]