Summary
Background
Ponatinib has shown efficacy in patients with refractory chronic myeloid leukaemia (CML) and in those with CML with a Thr315Ile mutation. We aimed to investigate the activity and safety of ponatinib as first-line treatment for patients with chronic-phase CML.
Methods
We did a single-arm, phase 2 trial at MD Anderson Cancer Center in Houston, TX, USA. Between May 3, 2012, and Sept 24, 2013, we enrolled patients with early (<6 months) chronic-phase CML and treated them with oral ponatinib once a day. Patients enrolled before July 25, 2013, were given a starting dose of 45 mg per day; we lowered this due to tolerability issues and patients enrolled after this date were given a starting dose of 30 mg per day. After a warning by the US Food and Drug Administration (FDA) in Oct 6, 2013, for vascular complications with ponatinib, we started all patients on aspirin 81 mg daily and reduced the dose of ponatinib to 30 mg or 15 mg per day for all patients. The primary endpoint was the proportion of patients who achieved complete cytogenetic response by 6 months in the per-protocol population. This trial is registered with ClinicalTrials.gov, number NCT01570868.
Findings
We enrolled 51 patients. Median follow-up was 20.9 months (IQR 14.9–25.2). 43 patients were started on 45 mg ponatinib every day; eight patients were started on 30 mg per day. 43 (94%) of 46 evaluable patients achieved complete cytogenetic response at 6 months. Most frequent toxicities included skin-related effects (n=35; 69%) and elevated lipase (n=32; 63%). Cardiovascular events (mainly hypertension) occurred in 25 (49%) patients. Grade 3–4 myelosuppression occurred in 15 (29%) patients. Five (10%) patients developed cerebrovascular or vaso-occlusive disease. 43 (85%) patients needed treatment interruptions at some time and 45 (88%) needed dose reductions. The study was terminated June 18, 2014, at the recommendation of the FDA due to concern about the increased risk of thromboembolism with ponatinib.
Interpretation
Patients with newly diagnosed CML in chronic phase respond well to treatment with ponatinib, with most achieving a complete cytogenetic response. Dose adjustment, extensive monitoring, and counselling of the patients for thromboembolic events is needed for patients on ponatinib therapy. However, due to the risk of vascular thrombotic events and the availability of alternative options for these patients, other drugs should be considered first in the frontline setting.
Funding
MD Anderson Cancer Center, National Cancer Institute, ARIAD Pharmaceutical.
Introduction
Imatinib1–3 and second-generation tyrosine kinase inhibitors (TKIs) such as dasatinib4,5 or nilotinib6,7 result in many patients responding to therapy and have excellent long-term outcomes as first-line treatment in patients with chronic-phase chronic myeloid leukaemia (CML). However, up to 25–30% of patients are resistant to first-line TKI therapy8,9 through various mechanisms, most often mutations of ABL kinase domain.10,11 Thus, outcomes could be improved by treatments that circumvent or prevent TKI resistance.
Ponatinib is a third-generation TKI that is highly active in patients with CML with resistance to multiple TKIs or with a Thr315Ile mutation.12–16 In mutagenesis assays, ponatinib also prevents the emergence of resistant clones.12 Ponatinib is a multikinase inhibitor that inhibits kinases other than non-BCR-ABL1 kinase such as FLT3, FGFR, PDGFR, KIT, RET, SRC, and VEGFR.17–19 In a phase 2 trial of patients with CML resistant to multiple TKIs,14 a major cytogenetic response (MCyR) occurred in 60% of 267 patients treated. Complete cytogenetic response (CCyR) occurred in 53%, major molecular response (MMR) in 59%, and MR4.5 in 20% of patients. 2 year progression-free survival was 67% and 2 year overall survival was 86%.20
Based on these results, in May, 2012, we started a phase 2 trial to assess whether ponatinib was safe and active as initial therapy for patients with CML in chronic phase.
Methods
Study design and participants
We did a single-arm, phase 2 study. The protocol is available in the appendix. Between May 3, 2012, and Sept 24, 2013, we enrolled patients with CML in early chronic phase from MD Anderson Cancer Center, Houston, TX, USA. Eligibility criteria for patients included diagnosis of chronic-phase CML within 6 months; no previous therapy for CML other than hydroxycarbamide or 1 month or less of therapy with approved TKIs; age 18 years and older; Eastern Cooperative Oncology Group performance status 0–2; and adequate organ function (total bilirubin <1.5 upper limit of normal [ULN], alanine amino transferase <2.5 × ULN, and creatinine clearance ≥30 mL/min [by Cockroft and Gault formula; 0.501 mL/s per m2]). Patients with clonal evolution at the time of diagnosis were eligible. We excluded patients with cardiac disorders, including New York Heart Association cardiac class 3–4 heart disease, active cardiac symptoms or history of unstable angina or myocardial infarction within 3 months; peripheral arterial disease; venous thromboembolism; stroke; congenital prolonged QTc syndrome or pretreatment QTc of longer than 470 ms; uncontrolled hypertension; and history of clinically significant ventricular arrhythmias. We also excluded patients with history of pancreatitis, those who were pregnant or breastfeeding, and those with uncontrolled psychiatry disorders.
The study was approved by the institutional review board (IRB) of MD Anderson Cancer Center, and all patients signed an IRB-approved informed consent form.
Procedures
We started patients on oral ponatinib 45 mg every day. This was amended on July 25, 2013, to a starting dose of 30 mg every day because of the high frequency of dose-reduction requirements among patients treated with 45 mg. Dose reductions to 30 mg per day, 15 mg per day, or 15 mg every other day were indicated for safety or tolerability. After a warning from the US Food and Drug Administration (FDA) on Oct 6, 2013, about vascular complications with ponatinib, we started all patients on aspirin 81 mg daily and the dose of ponatinib was reduced to 30 mg or 15 mg every day for all patients. Details about treatment after ponatinib were not collected on this clinical trial and therefore not analysed here.
Patients had complete blood count and blood chemistry (including liver function tests, amylase, and lipase) taken every 1–2 weeks for the first 3 months and then every 6 weeks thereafter. Metabolic alterations (ie, lipid profile) and coagulation studies were not routinely collected in this study. We had not noted lymphocytosis by standard differential count in patients taking ponatinib, and therefore we did not do a formal analysis for lymphocyte subpopulations in this study. We did bone marrow aspiration, cytogenetic analysis, and peripheral-blood quantitative reverse transcription (RT)-PCR for BCR-ABL at baseline, every 3 months for 1 year, and every 6 months thereafter.
Outcomes
The primary endpoint was the proportion of patients to achieve CCyR by 6 months. Secondary endpoints were cytogenetic and molecular response, time to progression, and toxicity profile. Response criteria were as previously defined.21 Cyto genetic response was based on G-banded karyotypes with at least 20 metaphases analysed and categorised as complete (0% Philadelphia [Ph]-positive metaphases), partial (1–35% Ph-positive metaphases), major (≤35% Ph-positive metaphases), or minor (36–95% Ph-positive metaphases). MMR was defined as a BCR-ABL/ABL transcript ratio of 0.1% or lower (international scale); MR4.5 as a ratio of 0.0032% or lower.
Statistical analysis
On the basis of other TKI trials with a probability that CCyR will follow non-informative β (0.65, 0.34), we calculated that a sample size of 80 people was needed to give 95% posterior credible interval for CCyR at 6 months. However, we ended enrolment after reaching 51 patients at the recom mendation of the FDA on Oct 6, 2013, because of safety concerns related to the increased cumulative incidence of serious arterial thrombotic events in ponatinib trials.
Response data were assessed in the intention-to-treat population, which included all enrolled patients with available data. Event-free survival was measured from the start of treatment to the date of any of the following events while on therapy:22 loss of complete haematological remission, loss of major cytogenetic response, progression to accelerated or blast phase, or death from any cause while on study. Overall survival was measured from the time that treatment was started to the date of death from any cause at any time or date of last follow-up. Transformation-free survival was measured from the start of therapy to the date of transformation to accelerated or blast phase while on therapy or to the date of last follow-up. We estimated survival probabilities by the Kaplan-Meier method and compared with the log-rank test. We did statistical analysis with Stata/SE version 13.1. This trial is registered with ClinicalTrials.gov, number NCT01570868. The registration profile was changed from chronic phase CML to accelerated phase CML on Aug 5, 2014; other study numbers are MDACC study number 2012-0074 and NCI-2012-00572.
Role of the funding source
The study was sponsored by MD Anderson Cancer Center. ARIAD Pharmaceuticals provided drug and partial financial support for the study from the ponatinib investigator sponsored trial programme. JC and HK designed the trial. ARIAD Pharmaceuticals reviewed and provided comments on the design of the study but did not collect, analyse, or interpret data, nor did they participate in the writing of the manuscript other than acknowledgment of the final version of the manuscript. The corresponding author had full access to all of the data and the final responsibility to submit for publication. All authors had access to the raw data.
Results
We enrolled 51 patients with a median age of 48 years (range 21–75) and median time from diagnosis to treatment of 0.7 months (0–2; table 1, figure 1). Sokal risk score was low for most patients (table 1) and EUTOS score was low risk in 41 (91%) of 45 patients evaluated and high risk in four (9%). Median follow-up was 20.9 months (IQR 14.9–25.2). Only one of six patients who had previously received a TKI had achieved complete haematological response (receiving dasatinib for 30 days) and 73% Ph-positive metaphases at the time of initial presentation. Among cardiovascular risk factors, median body-mass index (BMI) was 28.2 kg/m2 (range 18.6–48.4; 20% ≥35 kg/m2), and 17 (34%) patients had a history of hypertension, 14 (28%) were active or past smokers, and seven (14%) had a history of hyperlipidaemia.
Table 1.
Baseline patient and disease characteristics
| Patients (n=51) | |
|---|---|
| Median age (years) | 48 (21–74) |
|
| |
| Men | 29 (57%) |
|
| |
| Women | 22 (43%) |
|
| |
| Sokal risk score | |
| Low | 35 (69%) |
| Intermediate | 11 (22%) |
| High | 5 (10%) |
|
| |
| White blood cell count (×109 cells per L) | 27.6 (2.5–193.7) |
|
| |
| Haemoglobin (g/L) | 117.3 (86.1–167.4) |
|
| |
| Peripheral blood blasts (%) | 0 (0–4) |
|
| |
| Platelets (×109 per L) | 321 (104–2000) |
|
| |
| Serum lactate dehydrogenase (μkat/L) | 15.60 (6.93–58.73) |
|
| |
| Splenomegaly (spleen size >10 cm) | 1 (2%) |
|
| |
| Previous imatinib (<1 month) | 4 (8%) |
|
| |
| Previous dasatinib (<1 month) | 2 (4%) |
|
| |
| Clonal evolution | 1 (2%) |
|
| |
| der(9) deletion | 7 (14%) |
|
| |
| Median follow-up (months) | 22.7 (9.8–31) |
|
| |
| Number of patients followed for at least 12 months | 37 (73%) |
Data are n (%) or median (range). No variables had missing values.
Figure 1. Study profile.
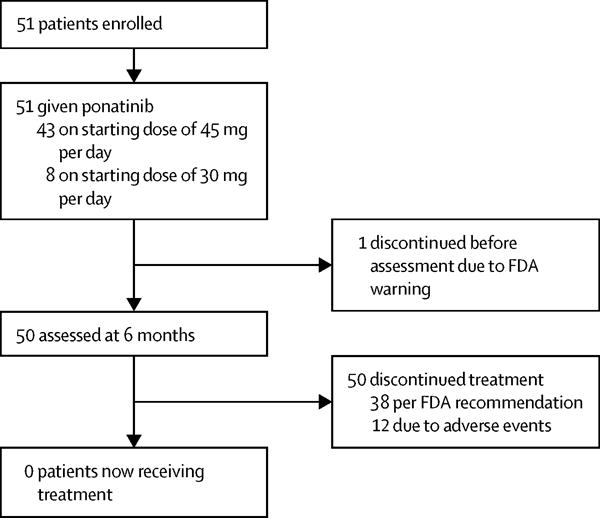
FDA=Food and Drug Administration.
43 patients were given a starting dose of 45 mg per day of ponatinib (figure 1). Due to tolerability issues, patients enrolled after July 25, 2013 (n=8), were given a starting dose of 30 mg. One patient discontinued therapy before completing 3 months of treatment with ponatinib due to the FDA warning and did not have cytogenetic or molecular response evaluation during the study (per protocol, first evaluation planned at 3 months) and was therefore considered not evaluable for response analysis. All other patients (n=50) achieved a complete haematological response. The cumulative proportion of patients to achieve CCyR was 96% (48 of 50), MMR was 80% (40 of 50), and MR4.5 was 55% (28 of 50). The median time to achieve complete haematological response was 0.6 months (IQR 0.46–0.91; range 0.2–2.8), whereas median time to achieve CCyR was 2.89 months (2.75–2.98; 2.5–8.5), median time to MMR was 2.9 months (2.82–5.71; 2.6–11.7), and median time to MR4.5 was 5.9 months (5.74–6.6; 2.8–18.04).
Table 2 provides a summary of cytogenetic and molecular responses by time during therapy for patients still evaluable in the study. At 3 months, 90% (45 of 50 patients) had achieved CCyR, 52% (26) had achieved MMR, and 4% (two) had achieved MR4.5 (table 2). At 6 months (the primary endpoint), the proportion of patients who had achieved CCyR was 94% (43 of 46; table 2). 38 (83%) patients had achieved MMR and 23 (50%) had achieved MR4.5 (table 2). The median BCR-ABL transcript level at 3 months was 0.096 (IQR 0.021–0.350) and at 6 months was 0.005 (0.0035–0.0315). No patients had undetectable BCR-ABL1 at 3 months, whereas 11 (22%) had undetectable levels at 6 months. Furthermore, 47 (94%) patients achieved 10% or lower BCR-ABL/ABL at 3 months (figure 2). 96% (44 of 46 patients) achieved levels of 1% or lower at 6 months.
Table 2.
Cytogenetic and molecular response to frontline ponatinib over time
| 3 months | 6 months | 9 months | 12 months | 18 months | |
|---|---|---|---|---|---|
|
Cytogenetic response
| |||||
| CCyR (%) | 45/50 (90%) | 43/46 (94%) | 37/40 (93%) | 26/27 (96%) | 21/20 (96%) |
| PCyR (%) | 3/50 (6%) | 1/46 (2%) | 3/40 (7%) | 1/27 (4%) | 1/22 (5%) |
| No CG (%) | 2/50 (4%) | 2/46 (4%) | 0 | 0 | 0 |
| Not evaluable | 1* (1) | 5* (3) | 11* (5) | 24* (16) | 29* (20) |
|
| |||||
|
Molecular response
| |||||
| MMR (%) | 26/50 (52%) | 38/46 (83%) | 33/40 (83%) | 22/27 (82%) | 20/22 (91%) |
| MR4.5 (%) | 2/50 (4%) | 23/46 (50%) | 51/40 (53%) | 15/27 (56%) | 16/22 (73%) |
| No MMR (%) | 24/50 (48%) | 8/46 (17%) | 7/40 (17%) | 5/27 (19%) | 2/22 (9%) |
| Not evaluable | 1* (1) | 5* (3) | 11* (5) | 24* (16) | 29* (20) |
|
| |||||
|
Responses according to European Leukemia Net (ELN) categories23
| |||||
| Optimal | 48/50 (96%) | 43/46 (96%) | .. | 22/27 (82%) | .. |
| Warning | 48/50 (4%) | 1/46 (2%) | .. | 4/27 (15%) | .. |
| Failure | .. | 1/46 (2%) | .. | 1/27 (3%) | .. |
CCyR=complete cytogenetic response. PCyR=partial cytogenetic response. CG=cytogenetic response. MMR=major molecular response. MR4.5=deep molecular response.
All patients who were not evaluable came off study due to US Food and Drug Administration warning (indicated in parentheses) or adverse events.
Figure 2.
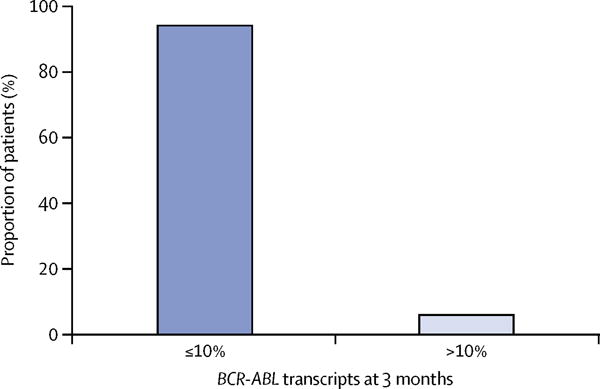
BCR-ABL transcript levels at 3 months (≤10% vs >10%)
None of the 50 patients progressed, including no transformations to accelerated or blast phase throughout the observation period, and all patients were alive at the time of last follow up. Estimated event-free survival, transformation-free survival, and overall survival at 24 months are 100% (figure 3; appendix). On June 18, 2014, all patients were switched to other TKIs and the trial was stopped as per US FDA recommendations.
Figure 3.
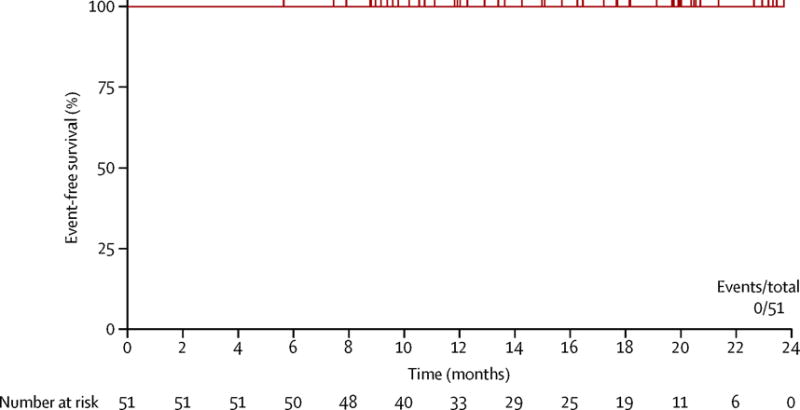
Event-free survival
43 (85%) patients needed treatment interruptions at some time during the course of their treatment (appendix). The median duration of treatment interruptions was 9 days (range 1–48). 45 (88%) patients needed dose reductions: 33 (66%) due to adverse events and 12 (24%) as per FDA recommendations. 11 (22%) patients needed more than one dose reduction. The median dose for all patients was 30 mg per day (range 15–45). During the first 3 months of therapy, 27 of the 43 patients who started on a dose of 45 mg per day had treatment interruptions and 18 had dose reductions to 30 mg or 15 mg a day, all due to toxicities (16 due to elevated lipase and pancreatitis, one for atypical chest pain, and one for non-neutropenic fever). Four of eight patients who started on a dose of 30 mg per day had dose interruptions and six had dose reduction to 15 mg per day (two due to pancreatitis and four due to FDA warning). All patients have now discontinued ponatinib: 38 per FDA recommendation following concern about increased risk of thrombo embolism and 13 due to adverse events. Of these 13 patients who withdrew, three had vaso-occlusive disease grade 3 (peripheral arterial disease, femoral artery thrombosis, carotid arterial disease), one had acute coronary syndrome and one had myocardial infarction (grade 3), two patients had cerebrovascular events grade 2 (two transient ischaemic attack and one stroke; of note one patient had both stroke and transient ischaemic attack), two patients had multiple toxicities, one with grade 3 hypertension, one with atypical chest pain and grade 2 arthralgia, one grade 3 skin xerosis, and one grade 4 myelosuppression.
We include all enrolled patients in calculations for toxicity. The most frequent non-haematological adverse events (any grade) were dermatological: 35 (69%) patients had rash (two patients grade 3–4), 22 (43%) had dry skin (all grade 1–2), 15 (30%) had rash with dry skin, and 14 (28%) had alopecia (table 3). 32 (63%) patients had elevated serum lipase, with grade 3–4 elevation in 23 (45%) patients. 23 (45%) patients had pancreatitis, with symptomatic grade 3 pancreatitis in ten (20%; nine of these with radiological findings of pancreatitis). Constipation was reported by 26 (51%) patients, all grade 1–2, and abdominal pain reported by 21 (41%), of which ten had pancreatitis; the others were due to constipation or were non-specific. Other non-haematological adverse events included headache, elevated serum amylase, elevated alanine aminotransferase, arthralgias, and fatigue (table 3). Six (12%) patients reported memory impairment (all grade 1–2; table 3).
Table 3.
Adverse events
| Grade 1–2 | Grade 3 | Grade 4 | |
|---|---|---|---|
|
Non-haematological
| |||
| Rash* | 33 (65%) | 2 (4%) | 0 |
| Elevated serum lipase | 9 (18%) | 19 (37%) | 4 (8%) |
| Constipation | 26 (51%) | 0 | 0 |
| Pancreatitis† | 13 (25%) | 10 (20%) | 0 |
| Dry skin or xerosis | 22 (43%) | 0 | 0 |
| Abdominal pain‡ | 17 (33%) | 4 (8%) | 0 |
| Headache | 17 (33%) | 0 | 0 |
| Amylase | 13 (25%) | 4 (8%) | 0 |
| Elevated ALT | 14 (27%) | 3 (6%) | 0 |
| Arthralgia | 16 (31%) | 0 | 0 |
| Fatigue | 15 (29%) | 1 (2%) | 0 |
| Alopecia | 14 (28%) | 0 | 0 |
| Infections (non-neutropenic)§ | 11 (22%) | 3 (6%) | 0 |
| Influenza-like syndrome | 11 (22%) | 0 | 0 |
| Elevated AST | 9 (18%) | 1 (2%) | 0 |
| Memory impairment | 6 (12%) | 0 | 0 |
|
| |||
|
Haematological
| |||
| Thrombocytopenia | 10 (33%) | 5 (10%) | 2 (4%) |
| Neutropenia | 1 (2%) | 2 (4%) | 4 (8%) |
| Anaemia | 0 | 0 | 2 (4%) |
|
| |||
|
Cardiovascular
| |||
| Hypertension¶ | 8 (16%) | 7 (14%) | 0 |
| Chest pain‖ | 8 (16%) | 0 | 0 |
| Raynaud’s syndrome | 2 (4%) | 0 | 0 |
| Cerebrovascular events** | 2 (4%) | 0 | 0 |
| Vaso-occlusive disease | 0 | 3 (6%) | 0 |
| Acute coronary syndrome | 0 | 1 (2%) | 0 |
| Myocardial infarction | 0 | 1 (2%) | 0 |
| Palpitations | 1 (2%) | 0 | 0 |
| Prolonged QTc interval | 1 (2%) | 0 | 0 |
| Pericarditis | 1 (2%) | 0 | 0 |
Data are n (%). No patients had grade 5 adverse events. ALT=alanine aminotransferase. AST=aspartate aminotransferase.
15 patients had both rash and dry skin.
Ten of 23 patients had symptomatic grade 3 pancreatitis (nine with CT or ultrasound findings of pancreatitis) and 13 of 23 patients had only chemical pancreatitis; two patients had a repeated episode of pancreatitis.
11 of 21 patients had pancreatitis and the other cases were due to constipation or non-specific causes.
Six of 14 patients had more than one episode of infections.
Six patients had new-onset hypertension, and nine had pre-existing hypertension (six had worsening, three were stable); five patients with grade 3 hypertension were on 45 mg and two on 30 mg doses.
One patient had pain due to grade 2 pericarditis and seven had negative ECG and cardiac enzymes.
One patient had transient ischaemic attack and stroke; another had transient ischaemic attack (same patient also developed carotid arterial disease).
Half of all patients (25 [49%]) had cardiac and vascular events; 11 (22%) had more than one (table 3). Hypertension was observed in 15 (29%) patients (seven grade 3–4). Six patients had new-onset hypertension and nine had pre-existing hypertension (six had worsening of hypertension, three had stable hypertension). Five of seven patients with grade 3–4 hypertension were given a starting ponatinib dose of 45 mg and two were started on 30 mg per day. Among the six patients with new-onset hypertension, two had a past history of smoking, median BMI was 29.7 kg/m2 (range 22.4–37.9) and four had a past history of hyperlipidaemia. Hypertension was well controlled and reversible after ponatinib dose reduction or discontinuation and dose adjustments of anti-hypertensive drugs. None of the patients who discontinued ponatinib had persistent uncontrolled hypertension.
Chest pain occurred in eight patients (seven were found to have negative cardiac enzymes and ECG, and one had pericarditis grade 2). Acute coronary syndrome was seen in one patient and myocardial infarction in one patient (both grade 3). Of the two patients with acute coronary syndrome and myocardial infarction, BMI was 41.6 kg/m2 and 22.4 kg/m2, respectively, none had a past history of smoking, one had pre-existing hyper tension, one had past history of coronary artery disease, and both patients had a past history of hyperlipidaemia. The patient with acute coronary syndrome had a coronary artery bypass surgery and symptoms resolved. The patient with myocardial infarction had a coronary stent placed with no further symptoms of coronary artery disease. The same patient also developed peripheral arterial disease that needed a stent within 6 months of discontinuing ponatinib. Vaso-occlusive disease was recorded in three patients (one carotid arterial disease and two peripheral arterial disease, all grade 3); median BMI was 28.4 kg/m2 (21.6–36.6), two had a past history of smoking, two had hypertension, one had a past history of hyperlipidaemia, and one had diabetes. The three patients were treated with antiplatelet drugs and peripheral arterial stenting; one of them had a toe amputation after discontinuing ponatinib. All others had no further symptoms. Cerebrovascular events occurred in two patients (one patient had grade 2 stroke and grade 2 transient ischaemic attack and another patient had grade 2 transient ischaemic attack). Among the two patients with cerebrovascular events, median BMI was 32.8 kg/m2 (29.1 and 36.6), neither patient had a past history of smoking and both had pre-existing hypertension and a past history of hyper lipidaemia. The patient with carotid arterial disease had another episode of transient ischaemic attack with persistent carotid arterial disease documented by four-vessel cerebral and carotid arteriogram within 6 months of discontinuing ponatinib. The patient with both stroke and transient ischaemic attack developed another stroke within a month after discontinuing ponatinib. Other cardiac or vascular adverse events did not recur after the discontinuation of ponatinib. Raynaud’s phenomenon was observed in two patients (grade 1 and grade 2; both patients had toe cramps with tingling), and palpitations and prolonged QTc interval was seen in one patient each. One patient who did not have cardiac or vascular events on ponatinib developed pulmonary hypertension within 1 month of discontinuing ponatinib. Haematological toxicity was noted in 26 (51%) patients in total and included thrombocytopenia in 17 (33%) patients, neutropenia in seven (14%), and anaemia in two (4%; table 3).
Discussion
We show that ponatinib induces deep and early responses when used as first-line treatment in patients with chronic-phase CML. Use of ponatinib in this setting was associated with frequent elevation of pancreatic enzymes and the occurrence of some arteriothrombotic events.
Imatinib has considerably improved outcomes for patients with chronic-phase CML. Dasatinib and nilotinib further improved the rate, depth, and time to response when used as a first-line therapy. However, second-generation TKIs have not led to an improvement in overall or event-free survival compared with patients treated with imatinib. Different studies show the clinical significance for attainment of early and deep cytogenetic and molecular responses from TKI therapy on patient outcomes.24–26 ENESTnd6,27 and DASISION4 reported that higher proportions of patients treated with the nilotinib and dasatinib, respectively, achieve deeper responses at earlier timepoints compared with those given standard-dose imatinib. In a series of more than 400 patients treated at MD Anderson Cancer Center, we recently reported that early responses at 3 months were commonly observed with imatinib 800 mg or a second-generation TKI.26 Still, about 15% of patients treated with newer TKIs do not reach this milestone, and up to 30% of patients discontinue therapy by 3 to 4 years. Additionally, resistance mutations in the ABL1 kinase domain might develop with dasatinib or nilotinib.28 Thus, despite the considerable improvement in outcome for most patients, improved treatment options are needed that can not only circumvent and prevent TKI resistance and ABL mutations, but can also provide very deep and durable responses at early timepoints for all patients. Ponatinib is a third-generation TKI that prevents emergence of resistant clones in preclinical studies and showed considerable clinical benefit in patients with refractory CML (all phases) or Ph-positive acute lymphoblastic leukaemia.13,14
We report here that ponatinib treatment led to very rapid and deep responses, with complete cytogenetic response rates of 90% at 3 months. This compares favourably to those reported with imatinib or second-generation TKIs.26 Similarly, MMR at 6 months and 12 months were 83%, which also compares favourably to those reported for other TKIs. Furthermore, at 3 months, 94% of patients achieved a BCR-ABL/ABL ratio of 10% or less, which is now considered an optimal response according to the European Leukaemia Network recommendations. With imatinib, this level of response is achieved by about 65% of patients, and with dasatinib, nilotinib, or bosutinib in 85–90%.27,29,30 Although we acknowledge the short duration of follow-up because of the early termination of the trial, no patients developed any events or transformed to accelerated or blast phase and all patients were alive at the end of 2 years of follow-up. Thus, ponatinib might be able to improve further the responses and long-term outcome of patients with CML when used for therapy at the time of diagnosis. Although we did not design this study to explore superiority to other treatment options available or to explore the possible reasons for a superiority, ponatinib’s unique structure with a carbon-carbon triple bond, which allows it to circumvent the steric hindrance in the Thr315Ile mutant, might also provide an advantage in potency in previously untreated CML. Ponatinib inhibits native and mutate ABL kinases with higher affinity than other TKIs.16 Furthermore, the atomic structure of ponatinib studied with molecular dynamics and free-energy calculations showed that ponatinib binding to native and mutant ABL kinase is more preferential and stronger than with other TKIs.31 Whether off-target effects of ponatinib such as FLT3 inhibition can also contribute to higher efficacy is unclear.32
Thus despite the activity reported here, adverse events occurred in a substantial proportion of patients. As in previous studies of ponatinib in heavily treated patients, dermatological and pancreatic toxicity were the most common adverse events. This led to treatment interruptions in more than 80% of patients and dose reductions in nearly two-thirds. Also, these events led to a protocol amendment to use 30 mg daily as the starting dose. Because of the early termination of the study, it is impossible to assess whether this strategy could be of benefit in this setting or, particularly, in others in which ponatinib is used and approved. Pooled data from 683 patients who received ponatinib in phase 1, 2, or first-line trials suggested that dose intensity might be associated with the frequency of adverse events, including cardiovascular events.33 This hypothesis needs to be tested prospectively to improve the safety profile of a drug with substantial clinical efficacy. Although not as frequent as dermatological or pancreatic events, the major safety concern of ponatinib is the development of arterial thrombotic events. These events have been noted with all TKIs, albeit with different frequencies. Estimation of the relative frequencies of these events with different drugs is difficult because of the difference in reporting among different trials, but it seems that these events are more common with ponatinib. Ponatinib and, to some extent nilotinib, are associated with development of not only cardiovascular and cerebrovascular events (also seen with other drugs), but also peripheral arterial occlusive disease.34
Since ponatinib is a multikinase inhibitor, it is possible that inhibition of certain VEGF, FGFR, or PDGFR can promote endothelial dysfunction, inhibit the survival of human endothelial cell lines, and predispose to thromboembolic events.35–37 However, other mechanisms might have a role as other drugs (eg, nilotinib, dasatinib) have no known VEGFR-inhibitory effect and yet are associated with occurrence of these events. Patients with pre-existing risk factors for atherogenesis or thromboembolic risk are particularly prone to vascular events with ponatinib. Since ABL1 has a key role in cardiomyocyte development and FGFR inhibition can suppress cardiomyocyte proliferation and development, it is possible that ponatinib might also be toxic to cardiomyocyte due to its inhibitory effect on c-ABL and FGFR1.38–40 Such an effect (at least through inhibition of c-ABL) has also been described for imatinib.39 The adverse events profile in our study was similar to that for ponatinib in the EPIC trial.41
In summary, our study shows that ponatinib is a very potent TKI with high clinical activity in the first-line treatment of patients with chronic-phase CML. However, at the doses currently used in other settings, the safety profile might not be appropriate for treatment of this patient population who have other treatment options with high efficacy. Additional studies are needed to test whether interventions such as lowering of the starting dose, patient selection, aggressive management of hypertension, and use of aspirin prophylaxis might decrease the incidence of undesirable events. For now, ponatinib should be used only for patients who have failed other therapies.
Supplementary Material
Research in context.
Evidence before this study
We searched Medline and PubMed for research articles published in English before March 30, 2015, about first-line treatment for patients with chronic-phase chronic myeloid leukaemia (CML) with different tyrosine-kinase inhibitors (TKI) such as imatinib, dasatinib, nilotinib, and bosutinib. We used the following keywords: “ponatinib”, “chronic myeloid leukemia in chronic phase”, “CML”, “CML-CP”, and “tyrosine kinase inhibitors (TKI)”. We identified ten relevant research articles that showed the superiority of second-generation TKIs such as dasatinib and nilotinib in randomised clinical trials in terms of response, tolerability, and different side-effect profile from imatinib. Since ponatinib is a third-generation TKI that has shown significant efficacy in resistant CML (even resistant to multiple second-generation TKI) and can prevent the emergence of resistance in vitro, we wanted to assess whether ponatinib could further improve the depth of responses and favourable toxicities to improve front-line results.
Added value of this study
We aimed to assess the effect on responses, outcome, and side-effects when ponatinib, a novel third-generation TKI, is used in the first-line setting. Responses were deep and early, but the adverse event profile was severe and the trial was terminated.
Implications of all the available evidence
Ponatinib is highly active in the first-line treatment of patients with chronic-phase CML. However, at the doses currently used in other settings, the safety profile might not be appropriate for treatment of this patient population and other treatment options have high efficacy.
Acknowledgments
This study was supported in part by the MD Anderson Cancer Center Support Grant CA016672 and Award Number P01 CA049639 from the National Cancer Institute (National Institutes of Health). ARIAD Pharmaceuticals provided free drug and financial support for the study from the ponatinib investigator sponsored trial programme.
Footnotes
Declaration of interests
HK declares research grants from Novartis, ARIAD Pharmaceuticals, Bristol-Myers Squibb (BMS), and Pfizer. EJ declares consultancy for BMS, Pfizer, and ARIAD, and research grants from Teva. JC declares research support from ARIAD, BMS, Novartis, Pfizer, and Teva and was a consultant for ARIAD, BMS, Pfizer, and Teva. All other authors declare no competing interests.
Contributors
HK and JC designed the study. PJ, HK, GNG, and JC analysed results. PJ and JC wrote the paper. PJ, HK, and JC did clinical correlation. HK, SO’B, FR, EJ, NP, ND, AF, GB, and JC contributed patient samples. All authors reviewed and gave the final approval for the paper.
References
- 1.Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with Imatinib. Blood. 2009;114:1126. [Google Scholar]
- 2.Baccarani M, Rosti G, Castagnetti F, et al. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: a European LeukemiaNet Study. Blood. 2009;113:4497–504. doi: 10.1182/blood-2008-12-191254. [DOI] [PubMed] [Google Scholar]
- 3.Cortes JE, Baccarani M, Guilhot F, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol. 2010;28:424–30. doi: 10.1200/JCO.2009.25.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119:1123–29. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes JE, Jones D, O’Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28:398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2302. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 7.Cortes JE, Jones D, O’Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28:392–97. doi: 10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah NP, Skaggs BJ, Branford S, et al. Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. J Clin Invest. 2007;117:2562–69. doi: 10.1172/JCI30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radich J. Structure, function, and resistance in chronic myeloid leukemia. Cancer Cell. 2014;26:305–06. doi: 10.1016/j.ccr.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118:1208–15. doi: 10.1182/blood-2010-12-326405. [DOI] [PubMed] [Google Scholar]
- 11.Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8:1018–29. doi: 10.1016/S1470-2045(07)70342-X. [DOI] [PubMed] [Google Scholar]
- 12.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–12. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367:2075–88. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–96. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zabriskie MS, Eide CA, Tantravahi SK, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. 2014;26:428–442. doi: 10.1016/j.ccr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou T, Commodore L, Huang WS, et al. Structural mechanism of the Pan-BCR-ABL inhibitor ponatinib (AP24534): lessons for overcoming kinase inhibitor resistance. Chem Biol Drug Des. 2011;77:1–11. doi: 10.1111/j.1747-0285.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 17.De Falco V, Buonocore P, Muthu M, et al. Ponatinib (AP24534) is a novel potent inhibitor of oncogenic RET mutants associated with thyroid cancer. J Clin Endocrinol Metab. 2013;98:E811–19. doi: 10.1210/jc.2012-2672. [DOI] [PubMed] [Google Scholar]
- 18.Ren M, Qin H, Ren R, Cowell JK. Ponatinib suppresses the development of myeloid and lymphoid malignancies associated with FGFR1 abnormalities. Leukemia. 2013;27:32–40. doi: 10.1038/leu.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garner AP, Gozgit JM, Anjum R, et al. Ponatinib inhibits polyclonal drug-resistant KIT oncoproteins and shows therapeutic potential in heavily pretreated gastrointestinal stromal tumor (GIST) patients. Clin Cancer Res. 2014;20:5745–55. doi: 10.1158/1078-0432.CCR-14-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Long-term follow-up of ponatinib efficacy and safety in the phase 2 PACE trial. Blood. 2014;124:3135. doi: 10.1182/blood-2016-09-739086. (abstr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–51. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronicphase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 23.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118:4541–46. doi: 10.1182/blood-2011-04-348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marin D, Hedgley C, Clark RE, et al. Predictive value of early molecular response in patients with chronic myeloid leukemia treated with first-line dasatinib. Blood. 2012;120:291–94. doi: 10.1182/blood-2012-01-407486. [DOI] [PubMed] [Google Scholar]
- 26.Jain P, Kantarjian H, Nazha A, et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: results with four tyrosine kinase inhibitor modalities. Blood. 2013;121:4867–74. doi: 10.1182/blood-2013-03-490128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2013;123:1353–60. doi: 10.1182/blood-2013-06-510396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes J, Jabbour E, Kantarjian H, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood. 2007;110:4005–11. doi: 10.1182/blood-2007-03-080838. [DOI] [PubMed] [Google Scholar]
- 29.Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014;123:494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486–92. doi: 10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanneeru K, Guruprasad L. Ponatinib is a pan-BCR-ABL kinase inhibitor: MD simulations and SIE study. PLoS One. 2013;8:e78556. doi: 10.1371/journal.pone.0078556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gozgit JM, Wong MJ, Wardwell S, et al. Potent activity of ponatinib (AP24534) in models of FLT3-driven acute myeloid leukemia and other hematologic malignancies. Mol Cancer Ther. 2011;10:1028–35. doi: 10.1158/1535-7163.MCT-10-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knickerbocker R, Dorer DJ, Haluska FG, et al. Impact of dose intensity of ponatinib on selected adverse events: multivariate analyses from a pooled population of clinical trial patients. Blood. 2014;124:4546. doi: 10.1016/j.leukres.2016.07.007. (abstr) [DOI] [PubMed] [Google Scholar]
- 34.Valent P, Hadzijusufovic E, Schernthaner G, Wolf D, Rea D, le Coutre P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood. 2014;125:901–06. doi: 10.1182/blood-2014-09-594432. [DOI] [PubMed] [Google Scholar]
- 35.Rivera VM, P J, Gonzalvez F, Baker F, Gozgit JM, Hodgson G. Comparative TKI profiling analyses to explore potential mechanisms of ponatinib-associated arterial thrombotic events. Blood. 2014;124:1783. (abstr) [Google Scholar]
- 36.Emir H, Albrecht-Schgoer K, Huber K, et al. Nilotinib exerts direct pro-atherogenic and anti-angiogenic effects on vascular endothelial cells: a potential explanation for drug-iduced vasculopathy in CML. Blood. 2013;122:257. (abstr) [Google Scholar]
- 37.Talbert DR, Doherty KR, Trusk PB, Moran DM, Shell SA, Bacus S. A multi-parameter in vitro screen in human stem cell-derived cardiomyocytes identifies ponatinib-induced structural and functional cardiac toxicity. Toxicol Sci. 2015;143:147–55. doi: 10.1093/toxsci/kfu215. [DOI] [PubMed] [Google Scholar]
- 38.Qiu Z, Cang Y, Goff SP. c-Abl tyrosine kinase regulates cardiac growth and development. Proc Natl Acad Sci USA. 2010;107:1136–41. doi: 10.1073/pnas.0913131107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerkela R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–16. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 40.Seyed M, Dimario JX. Fibroblast growth factor receptor 1 gene expression is required for cardiomyocyte proliferation and is repressed by Sp3. J Mol Cell Cardiol. 2008;44:510–19. doi: 10.1016/j.yjmcc.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Lipton JH, Chuah C, Bresler AG, et al. Epic: a phase 3 trial of ponatinib compared with imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CP-CML) Blood. 2014;124:519. (abstr) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


