Abstract
The substantial merit of Cordyceps s.l. spp. in terms of medicinal benefits is largely appreciated. Nevertheless, only few studies have characterized and examined the clinical complications of the use of health tonics containing these species. Here, we epitypified C. formosana isolates that were collected and characterized as Ophiocordyceps formosana based on morphological characteristics, molecular phylogenetic analyses, and metabolite profiling. Thus, we renamed and transferred C. formosana to the new protologue Ophiocordyceps formosana (Kobayasi & Shimizu) Wang, Tsai, Tzean & Shen comb. nov. Additionally, the pharmacological potential of O. formosana was evaluated based on the hot-water extract from its mycelium. The relative amounts of the known bioactive ingredients that are unique to Cordyceps s.l. species in O. formosana were found to be similar to the amounts in O. sinensis and C. militaris, indicating the potential applicability of O. formosana for pharmacological uses. Additionally, we found that O. formosana exhibited antioxidation activities in vitro and in vivo that were similar to those of O. sinensis and C. militaris. Furthermore, O. formosana also displayed conspicuously effective antitumor activity compared with the tested Cordyceps s.l. species. Intrinsically, O. formosana exhibited less toxicity than the other Cordyceps species. Together, our data suggest that the metabolites of O. formosana may play active roles in complementary medicine.
1. Introduction
The entomopathogenic fungus Cordyceps s.l. (sensu lato) species, including Ophiocordyceps sinensis and Cordyceps militaris, are among the prestigious traditional Chinese medicines and have a long history of use as tonics and folk medicines that can be applied to cancer and diabetes treatments and used as antioxidants among other uses [1]. Rather than the safety of the use of Cordyceps s.l. (sensu lato) species, much attention has been focused on their high value due to their pharmacological and therapeutic benefits in the treatment of respiratory and cerebrovascular diseases [2], enhancement of immunoregulatory function [3], regulation of liver metabolism [4], and treatment of cancer [5]. In this regard, in recent years, much effort has been devoted to discovering the bioactive ingredients, pharmacological efficacies, and mechanisms of action of the secondary metabolites that are derived from Cordyceps s.l. spp. For instance, Cordyceps s.l. spp. have been well characterized as possessing abundant bioactive compounds, such as cordycepin, D-mannitol (cordycepic acid), adenosine, polysaccharides, vitamins, and enzymes. Among these compounds, cordycepin (3-deoxyadenosine) is uniquely produced in Cordyceps spp., is a key active constituent of medicines, and confers broad-spectrum biological activity due to the steroidogenic [6] and antitumor proliferation and antimetastasis activities [7, 8] that are mediated by its antioxidation effects [9]. Additionally, reactive oxygen species (ROS) have been found to contribute to cellular necrosis and a variety of pathological conditions, including cancer [10], degenerative diseases in neurons [11], hepatopathies [12], atherosclerosis [13], and even aging [14]. Therefore, Cordyceps s.l. species have been repeatedly reported as antioxidants [15] due to their free radical scavenging abilities.
Cordyceps s.l. is a large paraphyletic group in Clavicipitaceae s.l. that comprises species with a wide range of hosts that span from insects and spiders to Ophiobolus and truffle-like fungi [16, 17]. Initially, the classification was based on the color and shape of the stromata, the perithecia, the sizes and shapes of asci and ascospores, and the host species. According to these characteristics, Kobayasi classified the genus into three subgenera, Cordyceps, Ophiocordyceps, and Neocordyceps [17]. Later, two extra subgenera were introduced, Racemella and Cryptocordyceps, by Mains [18]. Since then, the definitions of several original subgenera have experienced drastic changes. Specifically, for rapid and accurate identification and phylogenetic studies, molecular markers, such as internal transcribed spacers (ITSs), RNA polymerase II (RPB), and elongation factor (EF), have been widely used [19, 20]. In 2007, Sung et al. revised the classification based on phylogenetic analyses of multiple genes. Consequently, Clavicipitaceae s.l. was divided into three families, Cordycipitaceae, Clavicipitaceae s.s., and Ophiocordycipitaceae. Among these families, Cordyceps s.l. was separated into several genera that included Cordyceps s.s., Elaphocordyceps, Metacordyceps, and Ophiocordyceps. Indeed, the connections between the morphological characteristics and the molecular phylogeny provide invaluable information about Cordyceps s.l. that can be used to identify species and genera based on molecular evidence [21].
Ophiocordyceps formosana was first reported in 1981 by Kobayasi [22], and the etymology referred to its original discovery as an indigenous Cordyceps s.l. fungus in Taiwan. However, the characteristics of O. formosana were poorly documented with respect to the morphological description. Physiological and biochemical analyses and descriptions of its pharmacological applications were completely absent. Accordingly, the growth habits and morphological features of the known herbal Cordyceps s.l. species attracted our attention for their potential medicinal applications. Nevertheless, until now, only few and scattered findings regarding O. formosana have been reported in Taiwan and China, and sufficient specimens and samples for further characterization and investigation are lacking.
The safety and medicinal efficacy of Cordyceps s.l. and related products have been the focus of the development of complementary and traditional medicine. However, due to its limited distribution, high price, overexploitation, and difficulty related to artificial culture, O. sinensis has become an endangered species. Here, we further established an epitypified O. formosana that is phylogenetically related to O. sinensis. Additionally, we evaluated its effective pharmacological potentials in terms of antitumor and antioxidation bioactivities to elucidate the medicinal merit of O. formosana as a substitute for O. sinensis and C. militaris.
2. Materials and Methods
2.1. Sample Collection and Maintenance
Ophiocordyceps formosana specimens were collected from mummified darkling beetle bodies residing in decayed wood in Lala Shan, Taoyuan County, Taiwan (latitude: 24°42′1.20′′N, longitude: 121°25′49.20′′E) in the summer of 2013. The collected samples were immediately photographed and brought back to the Applied Mycology Laboratory of the National Taiwan University in Taipei for spore and mycelium isolation according to a standard decontamination procedure. Several isolates, including O. formosana MUCHO 815 and O. formosana NTU00035, were successfully obtained and cultivated on PDA and S-DAY plates at 25°C. Every 3-4 weeks, the colonies were harvested. The harvested colonies were first desiccated at 55°C overnight and then stored in a desiccator for future use. To maintain and preserve the O. formosana samples, small cubic mycelia-containing agars were routinely transferred to new PDA or S-DAY plates for maintenance. Cordyceps militaris mycelia and fruiting bodies were obtained from Mucho Biotechnology Inc. as described previously [23]. The mycelia and fruiting bodies of O. sinensis were purchased from Tongrentang Co., Ltd., Beijing, China.
2.2. Micromorphological Characteristic Examination
Microscopic examinations were performed via cryosectioning and examination under a compound light microscope (Olympus, Japan). The samples were fixed with 4% formaldehyde in PBS for one day with one change of solution. After the samples were mounted in optimal cutting temperature (OCT) compound, cryosectioning was performed with a LEICA CM3050-S cryostat (Leica Biosystems, Germany) to obtain sections ranging in thickness from 5 μm to 10 μm. The sections were examined and photographed under an Olympus BH2 light microscope (Olympus, Japan) equipped with a Canon-ds126 camera.
2.3. Genomic DNA Extraction from Cordyceps spp
The genomic DNA extraction from Cordyceps spp. was performed according to a modified version of the protocol of Doyle [24]. Briefly, either the fungal samples were directly cut down from the stroma and stalks of Cordyceps or mycelia were harvested from culture plates. The collected samples were lyophilized and ground to powder with a pestle with 500 μL of hot CTAB buffer (2% CTAB, 1.4 M NaCl, 20 mM EDTA, 100 mM Tris at pH 8.0, and 2% PVP-40) in combination with 3 μL of fresh β-mercaptoethanol. After incubation at 60°C for 20 minutes, 500 μL of chloroform : isoamyl alcohol (CI; 24 : 1) was added and mixed gently for 10 minutes prior to centrifugation at 13,200 g for 2 minutes. The supernatant was carefully transferred to a new Eppendorf tube following the administration of a 0.6x volume of isopropanol. Next, the mixture was allowed to incubate at −20°C for 30 minutes to precipitate the DNA. The DNA was harvested by centrifugation at 13,200 g for 30 minutes along with a single 75% cold ethanol wash, drying with a vacuum (LABCONCO, USA), and resuspension in 50 μL of ddH2O.
2.4. DNA Amplification and Sequencing
PCR was employed for the amplification of specific DNA fragments as described below. In general, the PCR reaction mixtures contained 2.5 μL of 10x reaction buffer, 1 μL of 10 μM of each specific primer (see Supporting Table 1 in Supplementary Material available online at http://dx.doi.org/10.1155/2015/189891), 0.5 μL of 10 mM dNTPs, 2 μL of template DNA, and 0.3 μL of 1 U Taq polymerase in a total volume of 25 μL. The RPB1 sequences were amplified using the forward primer cRPB1 and the reverse primer RPB1c-r [25] according to the following steps: denaturation at 95°C for 5 minutes; 40 cycles of 95°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute; and an additional extension step at 72°C for 10 minutes. The RPB2 sequences were amplified with the forward primer fRPB2-5f and the reverse primer fRPB2-7cr [26] according to the following steps: denaturation at 95°C for 5 minutes; 40 cycles of 95°C for 50 seconds, 55°C for 1 minute, and 72°C for 90 seconds; and an additional extension step at 72°C for 10 minutes. The EF1-α sequences were amplified using the forward primer EF1-2218R and the reverse primer EF1-983F [27] according to the following steps: denaturation at 94°C for 5 minutes; 40 cycles of 94°C for 1 minute, 46°C for 30 seconds, and 72°C for 2 minutes; and an additional extension step at 72°C for 7 minutes. The PCR products were electrophoresed and eluted for sequencing using an ABI 3730XL system (Applied Biosystems, MA, USA). The obtained sequences were then assembled and trimmed using a ContigExpress of vector NTI.
2.5. Phylogenetic Analysis
The nucleotide sequences of three genes, that is, the largest and second largest subunits of RNA polymerase II (RPB1, RPB2) and translation elongation factor 1α (EF1-α), were either obtained from our sequenced PCR products of the O. formosana isolates, retrieved from the NCBI database following the criteria described by Sung et al. [21], or acquired by whole genome ortholog BlastNs of C. militaris CM01. A total of 51 species/isolates were included in this study (Supporting Table 3). The nucleotide sequences of these three genes were concatenated, aligned, and tested in models using MEGA 6.0 [28]. The best model according to the AIC and BIC values was the GTR+I+G model. The aligned sequences were analyzed using CIPRES portal-based Raxml blackbox (RAxML 7.2.7) [29] for maximum likelihood (ML) tree searching with the default settings, an estimation of the gamma distribution, invariable sites, and Bayesian inference of the phylogeny with MrBayes 3.2.3 with the parameters obtained from the model test. The resulting phylogenetic trees were saved and plotted using Adobe Illustrator CS3.
2.6. Cordyceps Extract Preparation and HPLC Analysis
All of the desiccated Cordyceps samples that were subjected to HPLC (HITACHI, D-2000 HSM, Japan) analyses for de novo secondary metabolites were pulverized with a homogenizer at 1,300 rpm and subsequently stored in a humidity-controlled desiccator at room temperature. The preparation of the Cordyceps extracts was performed according to the standard operation procedure described below. Briefly, 1 g of Cordyceps powder was dissolved with deionized water at a solid-to-solvent ratio of 1 : 40 by vortexing for 30 seconds in a 50 mL falcon tube (BD Biosciences, USA). Next, the extraction was performed with 50°C hot water reflux for 2 hours (including the first 30 minutes of ultrasonication). After 20 min of centrifugation at 4,000 rpm (approximately 3,200 g), the supernatant was collected and filtered with a 0.22 μm filter. The resulting extracts were directly subjected to HPLC analyses or stored at −20°C.
High-performance liquid chromatography (HPLC) was performed on RP-18 column (150252 Purospher STAR RP-18 endcapped (5 μm) LiChroCART 250-4, Merck) at a flow rate of 1 mL/min. The composition of the mobile phase was 20% methanol/H2O. Three of the indicated components, D-mannitol, adenosine, and cordycepin, were detected at 260 nm using a DAD detector and the corresponding retention times of the individual standard compounds in the same HPLC conditions. Quantitation was preformed according to the established standard curve of each compound.
2.7. Cell Culture and MTT Assay
The MTT assay was used to measure the abilities of the Cordyceps extracts to inhibit human cancer cell proliferation. Approximately 5 × 103 cells were seeded onto each well of a 96-well plate containing 200 μL of culture medium (per well) 24 hours before treatment. After exposure to various concentrations of the indicated extracts for 96 hours, the cells were incubated with 20 μL of MTT (Thiazolyl Blue Tetrazolium Bromide, Sigma, USA) solution for 4 hours. Cell proliferation was measured by the absorption at 570 nm using a spectrophotometer (SpectraMax 190 UV-Vis Microplate, Molecular Devices, USA). The measurements were performed in triplicate. The percentages of viable cells were calculated as (sample OD570 nm/Mock OD570 nm) × 100 (%) [30].
2.8. DPPH Antioxidation Assay
The scavenging of DPPH radicals was estimated by vigorously mixing equal amounts of the Cordyceps extract and DPPH solution (0.1 mM in methanol). The mixture was then incubated at room temperature for 50 minutes before measurement at 517 nm using a spectrophotometer (SpectraMax 190 UV-Vis Microplate, Molecular Devices, USA). The scavenging activity was determined by comparing the blank (100%) that contained only DPPH and solvent. The scavenging efficiency percentages were calculated as (sample OD517 nm/Mock OD517 nm) × 100 (%) [31].
2.9. Cell-Based ROS Scavenging Assay
Approximately 5 × 104 CHO-K1 cells were seeded in each well of a 12-well plate and exposed to the indicated Cordyceps extracts for 1 hour after 16 hours of serum starvation. Here, the 10 mM N-acetylcysteine treatment served as a positive control. After H2O2 (1800 μM) challenge for 5 minutes, the cells were incubated with fluorescent probes (CM-H2DCFDA, Sigma, USA) for 30 minutes in a 37°C incubator (light was excluded). Finally, the cells were trypsinized and subjected to ROS quantification via the detection of the DCF levels, which corresponded to the relative ROS amounts at 525 nm using a flow cytometer (BD FACSCanto, USA).
2.10. Tumor Xenografts
An in vivo tumorigenesis assay was conducted as previously described [32]. Briefly, 5 × 106 MDA-MB-231 breast cancer cells were inoculated into the left thighs of 10-week-old nude mice (BALB/cAnN.Cg-Foxn1 nu/CrlNarl) via subcutaneous injections. The oral gavage treatments with O. formosana extract began daily immediately after 10 days when the tumor growth was palpable. The mice were divided into three groups: two groups were treated with O. formosana water extracts at concentrations of 1x (25 mg/mL, 100 μL/day) and 5x (125 mg/mL, 100 μL/day), and a control group was treated with PBS. The tumor sizes were measured every four days and calculated with the formula tumor size = 0.4 × length × width2 according to previous reports [33]. Statistical strategy was based on Ku's study [34]. After 26 days of O. formosana water extract treatment, the tumors in the mice were photographed.
2.11. Statistical Analyses
All data are expressed as the means ± the SEMs based on at least three independent experiments. The statistical analyses were performed using one-way analyses of variance (ANOVA) followed by Tukey's tests, and significance was measured as indicated.
3. Results
3.1. Collection and Morphological Features of Ophiocordyceps formosana
We collected Cordyceps spp. from varied potential niches in Taiwan to assess their potential medicinal values and applications. In Taoyuan County of Taiwan (GPS: altitude 1600 m, latitude: 24°42′1.20′′N, longitude: 121°25′49.20′′E; collectors: Y.-W. Wang, S.-H. Tsai, T.-W. Hong, J.-Y. Lai, T.-L. Shen, and others; Figure 1(h)), two Ophiocordyceps formosana samples were found growing on darkling beetle larvae (Tenebrionoidea) that resided on fallen decayed wood in August of 2013. Based on examination of the macroscopic and microscopic details of the newly collected O. formosana, the morphological features were described and documented as shown in Figure 1.
Figure 1.
Morphological and anatomical features of the Ophiocordyceps formosana collected from Taoyuan County, Taiwan. (a) The fungal ascostromata arise from the host Tenebrionoidea insect corpses with cylindrical stalks. Bar = 10 mm. (b) The orange stromatal head exhibits an oblong shape. Bar = 3 mm. (c) An ovoid perithecium embedded in a stroma is shown in cross section. The asci reside within the ovoid perithecium and obliquely align with the perithecium. Bar = 100 μm. (d) The ostiole of a perithecium showing a narrow opening in the neck surrounded by a thick perithecial wall. Bar = 25 μm. (e) Cylindrical partspores derived from fragmented ascospores. Bar = 10 μm. (f) The colonies were velutinous or floccose with exudating droplets and orange to white in color on a PDA culture plate. (g) A Hirsutella anamorph of O. formosana; the branched conidiophore is curl-shaped and bears baciloid-shaped conidia. Bar = 10 μm. (h) The documented habitats of O. formosana specimens in Taiwan [22, 35] (source: Taiwan; 23°46′50.90′′N and 120°57′50.54′′E from Google Earth).
Obviously, the ascostromata arose from the heads or abdomens of the infected insect hosts (here, darkling beetle larva of the Tenebrionoidea superfamily), and the stalks exhibited long-cylindrical shapes of 10–30 mm × 0.5–2 mm in size that were orange (6A7) in color and bore short hairs but lacked membranous sheaths. The oblong ascostromata heads were 4–6 mm × 1–4 mm in size and largely consisted of pseudoparenchymatous epidermal tissues. Moreover, the perithecia were embedded within the stromata and were brownish orange (7C8) and ovoid shaped (360–480 × 240–320 μm) with short necks, with ostioles of 60 μm in width. The perithecial wall was 20 μm thick underneath with approximately 40 μm-thick epidermal tissue. Furthermore, the asci displayed long-cylindrical shapes with attenuated bases (6.5–7.9 μm × 160–240 μm) and thick dome-shaped apices (3.9–5.3 × 3.2–4.6 μm) along with narrow slits. The asci contained eight ascospores and hyaline, were cylindrical and filamentous, and were often fragmented into more than 10–20 cylindrical and truncated partspores (2.6–3.0 × 6.5–7.3 μm) at maturity. Colonies grown on potato dextrose agar (PDA) were orange (5A7) to white in color and pulvinate to umbonate in shape and exhibited velutinous to floccose mycelia that were whitish light-orange to pink in color and exudation droplets. The anamorphic state was similar to Hirsutella type, and the conidiogenous cells were monophialidic, hyaline, and elongated ampuliform-shaped (1.5–2.3 × 8.6–17.0 μm). The conidia were hyaline and cylindrical (1.6–2.3 × 3.6–6.9 μm in size).
3.2. Molecular Identification of Ophiocordyceps formosana by RPB1, RPB2, and EF1-α
In addition to the morphological features, we amplified the 790-, 1062-, and 1068-base pair (bp) nucleotide sequences of the RPB1, RPB2, and EF1-α genes from the isolates of O. formosana, respectively, by polymerase chain reaction (PCR) with the corresponding specific primers listed in Supporting Table 1. The obtained and sequenced genes were subjected to BLAST in the NCBI database (http://www.ncbi.nlm.nih.gov/). The top hits of the 3 sequences were Cordyceps formosana TNM F13893, Ophiocordyceps coenomyia NBRC 106964, and Cordyceps formosana TNM F13893 with identities of 100% (635/635), 90% (956/1065), and 99% (847/849), respectively. Although the top hit for RPB2 was not Cordyceps formosana, the sequence shared 100% identity with the Cordyceps formosana TNM F13893 with 47% coverage (Supporting Table 2). The ITS sequences were not utilized due to time and reagent limitations. However, the multigene analysis results of the three genes were sufficient to indicate that our collected specimens were identical to Cordyceps formosana TNM F13893 as verified by the molecular markers.
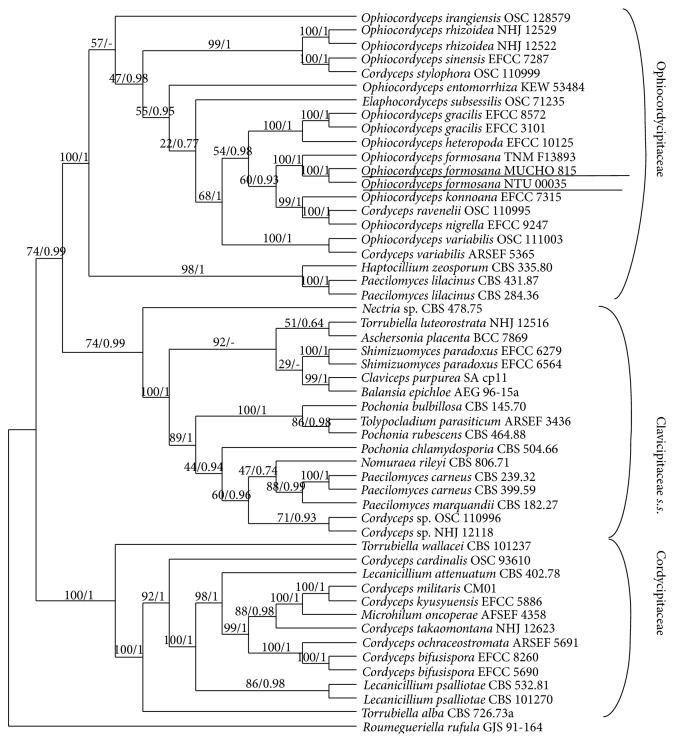
Indeed, the phylogenetic trees based on the maximum likelihood method and Bayesian principles resulted in two identical trees with different support strengths. A maximum likelihood bootstrap tree is shown in Figure 2, and the posterior probability ML bootstrap support values are denoted after the slashes. The topologies of Cordyceps s.l. in the three newly created families, Ophiocordycipitaceae, Cordycipitaceae, and Clavicipitaceae s.s., were largely congruent with the results of Sung et al. [21]. O. formosana resided within the Ophiocordycipitaceae clade with a strong bootstrap support value (BS = 10/PP = 1) rather than in the other allied taxa of Cordycipitaceae and Clavicipitaceae s.s. Phylogenetically, O. formosana obviously exhibited a closer relatedness to O. sinensis than to C. militaris. However, in contrast, C. militaris was placed in the Cordycipitaceae clade, which was distantly related to O. sinensis and O. formosana. Intriguingly, the O. formosana collected in Taiwan from the varied geographic regions in this study were in the clade of O. formosana isolated by another group. Based on the unambiguous morphological traits and molecular phylogenetic study, we recombined the previously erected Cordyceps formosana as Ophiocordyceps formosana, and the epitypic specimen was designated as O. formosana MUCHO 815.
Figure 2.
Phylogenetic tree of Cordyceps s.l. Phylogenetic cladogram of Cordyceps s.l. showing the three newly created families, Clavicipitaceae s.s., Cordycipitaceae, and Ophiocordycipitaceae, as inferred by the maximum likelihood (ML) method and Bayesian inference of the phylogeny using three concatenated genes (RPB1, RPB2, and EF1-α). The bootstrap support values are denoted on the branches in front of the posterior probabilities. The underlined Ophiocordyceps formosana NTU 00035 and MUCHO 815 samples were collected from Taiwan in this study.
3.3. Chemical Profiles of the Ophiocordyceps formosana Water Extracts
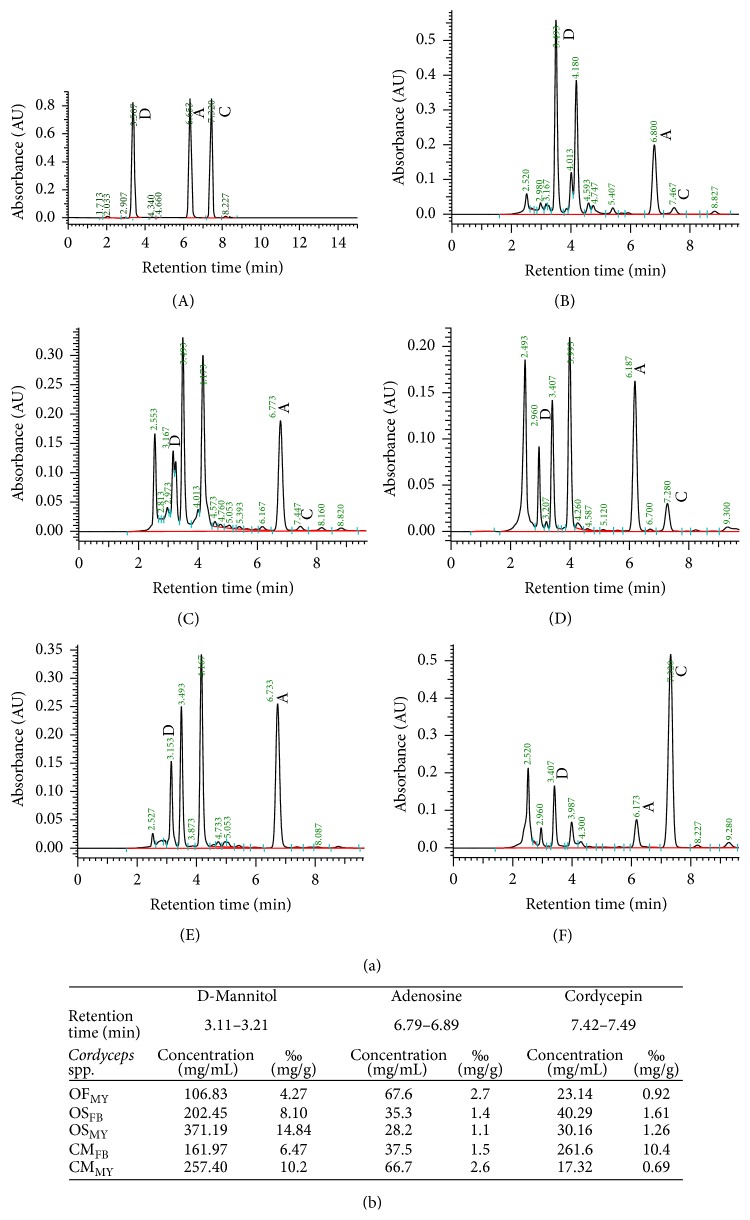
The chemical profiles of the extracts from the mycelia and fruiting bodies of the various Cordyceps species, including O. sinensis and C. militaris, were compared with O. formosana. To perform the investigations, the hot water extracts of the tested Cordyceps samples were subjected to HPLC analyses. The HPLC profile of O. formosana MUCHO 815 (Figure 3(a)) was reminiscent of those of O. sinensis and C. militaris, which indicated that the biochemical properties of O. formosana were similar to those of other well-known Cordyceps species. Additionally, we compared three predominate metabolites that were unique in Cordyceps spp., cordycepin, adenosine, and D-mannitol. As shown in Figure 3(b), O. formosana MUCHO 815 possesses amounts of these three major bioactive compounds in terms of quantities and percentiles that were similar to other Cordyceps species. Collectively, we epitypified O. formosana based on detailed studies of its morphological, molecular, and biochemical features.
Figure 3.
HPLC profiles and analyses of extracts from various Cordyceps species. (a) The HPLC retention times are shown for D-mannitol (designated as “D”), adenosine (designated as “A”), and cordycepin (designated as “C”) (A). The HPLC profiles extracted from the O. formosana mycelia (B, OFMY), the O. sinensis mycelis (C, OSMY) and fruiting bodies (D, OSFB), and the C. militaris mycelia (E, CMMY) and fruiting bodies (F, CMFB). (b) The quantitative results for the indicated compounds were calculated according to the area under the peak that corresponded to the standard calibration; r 2 > 0.999.
3.4. The Ophiocordyceps formosana Water Extract Displayed Antioxidation Activity
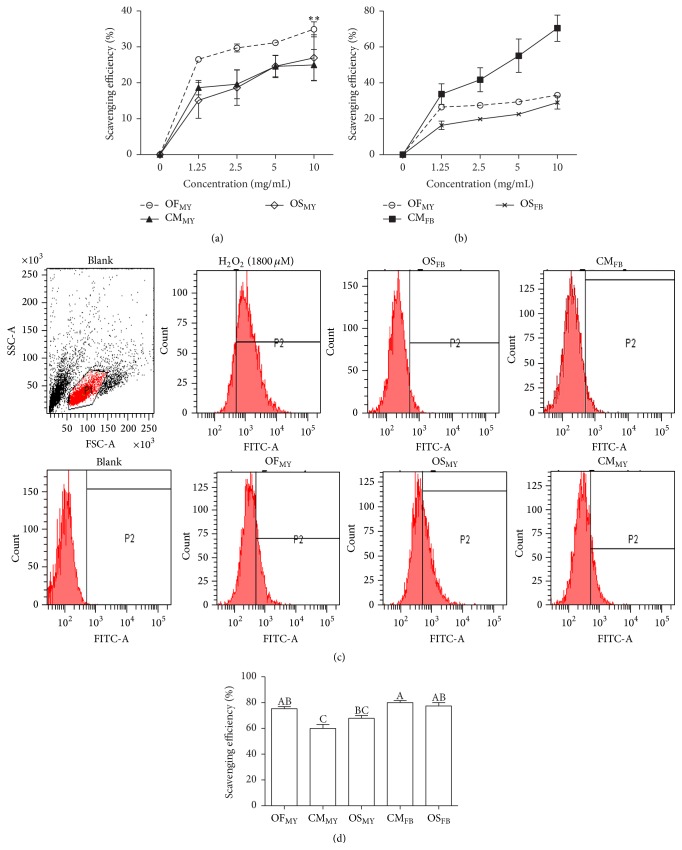
Ophiocordyceps formosana displayed biochemical features that were analogous to those of other valuable medicinal Cordyceps spp. s.l. We furthermore examined its biological and pharmacological properties. The nature of the antioxidant activity of the hot water extract from O. formosana was evaluated based on DPPH assay and cell-based ROS scavenging bioactivity. As shown in Figures 4(a) and 4(b), the hot water extract of O. formosana MUCHO 815 apparently exhibited a strong antioxidant capability compared with the extracts from the mycelia of O. sinensis and C. militaris (Figure 4(a)) that was even comparable with that of the extract derived from the O. sinensis fruiting body (Figure 4(b)). Coincidently, an in vivo cell-based ROS scavenging assay using CHO-K1 cells revealed that O. formosana exhibited a nonstatistically significantly greater antioxidant effect than the mycelial extracts (Figure 4(c)). Notably, the extract from the O. formosana mycelia was also comparable to the extracts derived from the O. sinensis and C. militaris fruiting bodies (Figure 4(d)). Our data imply that O. formosana may serve as an alternative and/or substitute for tonic and medicinal uses of O. sinensis and C. militaris.
Figure 4.
The in vitro and in vivo antioxidation activities of the extracts from various Cordyceps species. (a) The in vitro DPPH antioxidant activities of the water extracts of O. formosana, C. militaris, and O. sinensis mycelia (designated as OFMY, CMMY, and OSMY, resp.). (b) The DPPH antioxidant activity of the O. formosana mycelial water extract compared to those of the water extracts of the C. militaris and O. sinensis fruiting bodies (designated as CMFB and OSFB, resp.). (c, d) The in vivo cell-based ROS scavenging effect as measured by flow cytometry (c) and the quantification results (d). ABCBar graphs without the same letter are significantly different from one another in each group (p < 0.05).
3.5. The Ophiocordyceps formosana Water Extract Exhibits Anticancer Activity
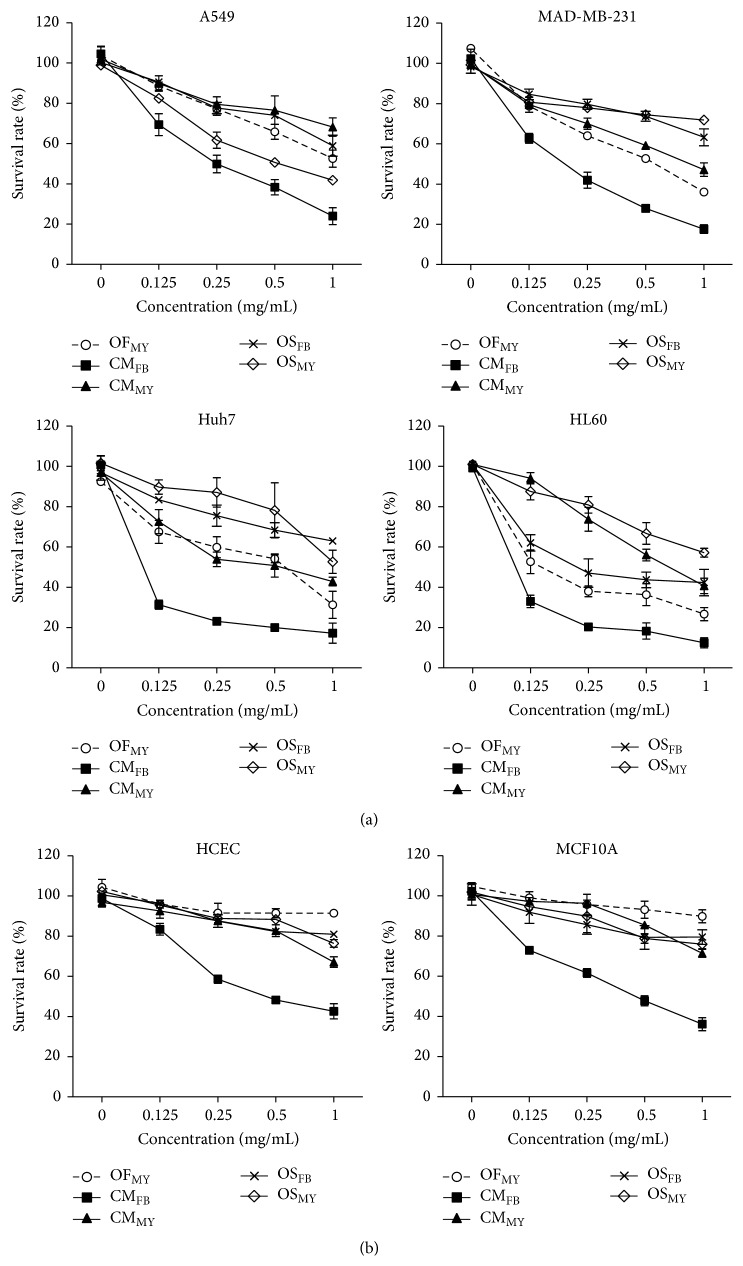
To determine the anticancer potential of O. formosana, the hot water extracts from the mycelia and fruiting bodies of O. formosana, O. sinensis, and C. militaris were tested against various human cancer cell lines, including A549 lung cancer, MDA-MB-231 breast cancer, Huh7 liver cancer, and HL-60 leukemia cells, at different dosages. As shown in Figure 5(a), the O. formosana extract exerted anticancer activities IC50s of 1.0 mg/mL for the A549 cells, 0.53 mg/mL for the MDA-MB-231 cells, 0.44 mg/mL for the Huh7 cells, and 0.19 mg/mL for the HL-60 cells. Apparently, the O. formosana extract exhibited more effective cancer cell suppression activities than the extracts from the O. sinensis and C. militaris mycelia that were even comparable to those of the extract derived from the O. sinensis fruiting bodies in all tested cancer cell lines (Table 1). Strikingly, the O. formosana extract yielded the lowest toxicities (maintaining a survival rate greater than 90%) to the tested normal cell lines, which included HCEC normal human corneal epithelial cells and MCF10A normal human breast epithelial cells, among all of the other Cordyceps extracts as shown in Figure 5(b).
Figure 5.
Antitumor activities of the extracts from various Cordyceps species. (a) Survival rates of various cancer cell lines, including the human lung cancer A549, human breast cancer MDA-MB-231, human liver cancer Huh7, and human leukemia HL60 cell lines exposed to the water extracts of O. formosana (OFMY), C. militaris (CMMY and CMFB), or O. sinensis (OSMY and OSFB). (b) The survival rates of human normal cell lines, human corneal epithelial HCEC cells, and human lung epithelial MCF10A cells that were exposed to the water extracts of O. formosana, C. militaris, or O. sinensis.
Table 1.
The IC50 values for each of the extracts of the various Cordyceps spp. with antitumor activities.
| Cell line (IC50) | OFMY | CMFB | CMMY | OSFB | OSMY |
|---|---|---|---|---|---|
| A549 | 1.00 ± 1.09 | 0.28 ± 0.06 | 1.70 ± 0.21 | 1.31 ± 0.62 | 0.54 ± 0.57 |
| MDA-MB-231 | 0.53 ± 0.15 | 0.20 ± 0.09 | 0.85 ± 0.08 | 2.75 ± 0.11 | 6.86 ± 0.06 |
| Huh7 | 0.44 ± 0.16 | 0.01 ± 0.01 | 0.44 ± 0.13 | 2.68 ± 0.11 | 1.16 ± 0.43 |
| HL-60 | 0.19 ± 0.13 | 0.07 ± 0.01 | 0.72 ± 0.17 | 0.32 ± 0.15 | 1.14 ± 0.15 |
OFMY: O. formosana mycelium; CMFB: C. militaris fruiting body; CMMY: C. militaris mycelium; OSFB: O. sinensis fruiting body; OSMY: O. sinensis mycelium.
3.6. In Vivo Anticancer Activity of the Ophiocordyceps formosana Water Extracts
To further validate the antitumor activities of the extracts from O. formosana, we conducted an in vivo antitumorigenesis assay using tumor xenograft mouse models. Briefly, nude mice were first implemented with the human MDA-MB-231 breast cancer cells and then treated with the water extracts of O. formosana at low (2.5 mg/day) and high doses (12.5 mg/day) daily by oral gavages (Figure 6). The results showed that the treated groups exhibited decreases in tumor size compared to the nontreated group in a dose-dependent manner. Our study highlights the potential future applications of O. formosana in tonics and medicines.
Figure 6.
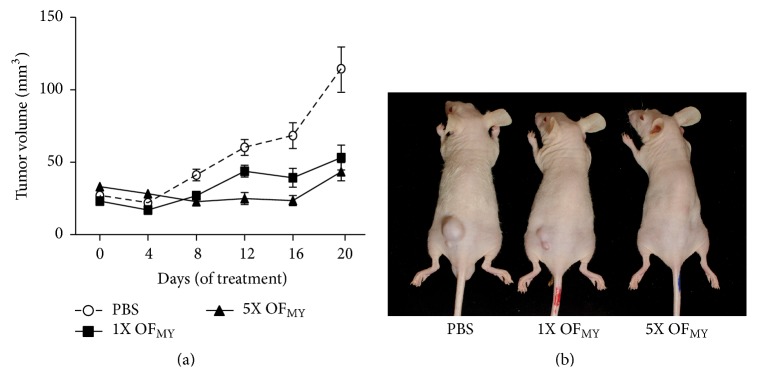
In vivo antitumor activities of the extracts from O. formosana. (a) Mice were xenografted with human MDA-MB231 breast cancer cells and then orally treated with the water extracts of O. formosana (OFMY) with 1x (2.5 mg, n = 3), 5x (12.5 mg, n = 3), or PBS only daily. The tumor sizes were recorded every four days and showed decreases in tumor volumes in accordance with the dosage of treated OFMY water extracts. Note that bar is shown as SEM. (b) Photos of tumors in the mice with or without treatments on day 20.
4. Discussion
Ophiocordyceps formosana was originally collected from Xitao, Nantou County, Taiwan, and described and named Cordyceps formosana in the subgenus Eucordyceps by Kobayasi [17, 22]. Nevertheless, Ophiocordyceps formosana did not receive much attention until a revisit based on a new collection found in the Guanwu National Forest Recreation Area of Hsinchu County, Taiwan [35]. Consistent with our discovery, the indigenous Ophiocordyceps formosana seemed to prefer to inhabit Tenebrionoidea larva in areas of temperate broadleaf forests at approximately 1500–1800 m in altitude (Figure 1(h)). More recently, O. formosana was also found in Huangshan, Anhui Providence of China, by Li et al., which extended the distribution of O. formosana beyond Taiwan [36].
Morphologically, our collected samples were nearly almost identical to the holotype of Cordyceps formosana described by Kobayasi [22] and Li et al. [36], which therefore justified the identification of the entomopathogen discovered in this study as C. formosana (Figure 1). However, the morphology and the genotype of C. formosana had not been clearly defined and linked. To address this issue, the nucleotide sequences of the RPB1, RPB2, and EF1-α housekeeping genes that were obtained and sequenced from our fungal samples were aligned and compared with the documented allied sequences of the reported C. formosana TNM F13893 to further validate the identities of both fungal specimens. The reason that we used RPB1, RPB2, and EF1-α to infer the species phylogeny was based on a hypothesis that essential genes are more likely to be subjected to the same selection forces, and convergent evolution in amino acid sequences is less likely to occur; this hypothesis has also been supported in several previous studies of the phylogenies of the Cordyceps relatives [20, 21]. Other approaches involving the use of a neutral locus, such ITS, should be appropriate for inferring species phylogeny; however, ITS sequences change too fast and are therefore unsuitable for inferring the phylogenies of distantly related species. Indeed, we failed to obtain the ITS sequence of C. formosana using the “so-called” conserved primer sequences. Therefore, in this study, we were dealing with distantly related species, so these three genes were selected to infer the species phylogeny. Interestingly, the phylogenetic tree-based maximum likelihood and Bayesian inference phylogenetic methods both revealed the same topology, although, due to restrictions in computer capacity, we were unable to rule out the possibility that the trees were trapped in local optima. However, we replicated two runs of the BI analyses, and the results were identical, which supports our hypothesis that Ophiocordyceps formosana resides in Ophiocordycipitaceae. Due to the clear evidence from not only the morphological characteristics but also the molecular linkages, we epitypified C. formosana to O. formosana. Moreover, the anamorphic state of Hirsutella type in culture was also consistent with the description from Li et al. [36]. According to the definition of Ophiocordyceps proposed by Kobayasi, the anamorphs of this genus consist of four genera: Hirsutella, Hymenostilbe, Paraisaria, and Syngliocladium types [22]. The results of the phylogenetic investigation further support our suggestion for combining and transferring C. formosana to the new protologue Ophiocordyceps formosana (Kobayasi & Shimizu) Wang, Tsai, Tzean & Shen comb. nov. (see Supporting Text 1 for details), although the comparisons of several phylogenetically related Ophiocordyceps spp. indicated that O. formosana apparently displays a brighter yellow color of its stromata that distinguishes it from other species in this clade (Figure 1). For example, O. ravenelii exhibits superficially distributed perithecia and dark brown-colored clavate stromata [37]. The stromata of O. nigrella are also dark brown and clavate, but the perithecia are embedded in mycelial tissues [38], whereas O. konnoana exhibits yellow, clavate stromata and superficial perithecia [39]. However, O. heteropoda and O. gracilis exhibit embedded perithecia and capitate stromata [40, 41], which indicates that these two species may be comparatively less related to O. formosana. Interestingly, the clade that was phylogenetically allied to O. formosana and included O. ravenelii, O. nigrella, and O. konnoana appears to exhibit a similar host preference, that is, Coleoptera larvae. In contrast, O. heteropoda and O. gracilis parasitize Auchenorrhyncha (cicada) nymphs and Lepidopteran larvae, respectively. Indeed, regarding phytopathogens, linkages between host ranges and evolution have been suggested [42]. Although it is currently recognized as belonging to Cordyceps s.l., the strict host specificities of some of the clades, such as Elaphocordyceps, may be due to recent host jumping [21, 43] that might have given rise to strong driving forces for an evolutionarily genetic drift. Moreover, this clade also shares morphological features of the stromata; however, this seems to be only a symplesiomorphic character within Ophiocordyceps spp. [43].
The adverse effects of oxidative stress on human health have attracted considerable interest. Indeed, the exposure of organisms or cells to excessive amounts of reactive oxygen species (ROS) results in striking homeostatic imbalances [44]. Eventually, high levels of ROS are capable of inducing cellular and DNA damage, triggering cell apoptosis, and contributing to deleterious complications, such as aging, high blood pressure, inflammatory responses, and even cancer. Two Cordyceps spp., that is, O. sinensis and C. militaris, are commonly used to ameliorate conditions associated with aging [45] and several malignant complications, including lung disorders [46], diabetes [47], kidney failure [48], and cancer [23]. It is believed that the antioxidation activities derived from different preparations or fractions of Cordyceps are effective in therapeutics [49]. Using the in vitro DPPH assay and the cell-based anti-ROS scavenging assay (Figure 4), the extract from the mycelia of O. formosana was found to exhibit prominent antioxidative activities that exceeded those of the mycelia of C. militaris and O. sinensis and were even comparable to those of the fruiting bodies of O. sinensis. Hence, our evidence-based results suggest the value of the complementary and alternative medicine O. formosana for oxidation-related complications.
In recent years, increasing amounts of evidence have indicated that Cordyceps spp. are competent as alternative antitumor medicines and adjuvants for chemo- and radiotherapy in the treatment of various cancers [50]. Notably, the hot water extract from the mycelia of O. formosana exhibits superior antitumor activities against various cancer cell lines via induction of cancer apoptosis (unpublished data), including breast, lung, and liver cancers and leukemia, compared to extracts from the mycelia of O. sinensis and C. militaris (Figure 5(a)). Moreover, the O. formosana mycelial extract even exhibited an anticancer efficacy that was comparable to that of the extract from the O. sinensis fruiting bodies (Table 1); therefore, benefits are available for many markets because a massive supply is achievable. Intriguingly, the relatively lower toxicity of O. formosana to normal cells renders O. formosana as more competent as an alternative medicine for the treatment of cancer (Figure 5(b)). In support of this idea, we have observed no apparent deleterious effects on any of the dissected tissues/organs or in the in vivo biochemistries of mice that have been orally treated with O. formosana for 14 successive days at concentrations of up to 2 g/kg (unpublished data).
5. Conclusions
In conclusion, we established an evidence-based alternative indigenous medicinal Cordyceps s.l. named Ophiocordyceps formosana that exhibits pharmacological potential as an anticancer and antioxidative stress-related complication agent. With the support of the HPLC analyses, the chemical profile indicated that key bioactive ingredients, including D-mannitol, adenosine, and cordycepin, were present in Ophiocordyceps formosana at amounts that were comparable or even exceeded those in other known traditional medicinal Cordyceps species. In addition to its high level of pharmacological efficacy, O. formosana also exhibited lower toxicity than the documented valuable Cordyceps species O. sinensis and C. militaris. Together, these findings support the notion that this epitypified Ophiocordyceps formosana (Cordyceps s.l.) can serve as an alternative medicinal fungus.
Supplementary Material
Degenerated primer sequences (Supporting Table 1) derived from the largest subunit of RNA polymerase II (RPB1), the second large subunit of RNA polymerase II (RPB2) and translation elongation factor-1α (EF1-α) genes were synthesized according to previous reports (30, 31, 32) and used for the molecular identifications of Cordyceps spp, (s. l.), including Ophiocordycpes formosana. The amplified 790-, 1062-, and 1068-base pair (bp) nucleotides, respectively, from our collected samples were sequenced and subjected to BLAST in the NCBI database (). The closest species and strains matched, NCBI accession number, and nucleotide identify of the 3 sequences were listed (Supporting Table 2).
A list of nucleotide sequences of the 3 genes (i.e. RPB1, RPB2, and EF-1a) from 51 species/isolates was retrieved from the NCBI database following the criteria described by Sung et al or obtained from our sequenced PCR products of the O. formosana isolates (Supporting Table 3).
Acknowledgments
The authors gratefully thank Drs. Bo-Chi Chen, Jun-Yang Liou, Lee-Yen Sheen, and Shang-Ming Chou for their critical reading and comments on this paper.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Ling C.-Q., Yue X.-Q., Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. Journal of Integrative Medicine. 2014;12(4):331–335. doi: 10.1016/S2095-4964(14)60038-8. [DOI] [PubMed] [Google Scholar]
- 2.Wang J., Liu Y.-M., Cao W., Yao K.-W., Liu Z.-Q., Guo J.-Y. Anti-inflammation and antioxidant effect of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metabolic Brain Disease. 2012;27(2):159–165. doi: 10.1007/s11011-012-9282-1. [DOI] [PubMed] [Google Scholar]
- 3.Shi B., Wang Z., Jin H., Chen Y. W., Wang Q., Qian Y. Immunoregulatory Cordyceps sinensis increases regulatory T cells to Th17 cell ratio and delays diabetes in NOD mice. International Immunopharmacology. 2009;9(5):582–586. doi: 10.1016/j.intimp.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Manabe N., Azuma Y., Sugimoto M., et al. Effects of the mycelial extract of cultured Cordyceps sinensis on in vivo hepatic energy metabolism and blood flow in dietary hypoferric anaemic mice. British Journal of Nutrition. 2000;83(2):197–204. doi: 10.1017/s0007114500000258. [DOI] [PubMed] [Google Scholar]
- 5.Jayakumar T., Chiu C.-C., Wang S.-H., Chou D.-S., Huang Y.-K., Sheu J.-R. Anti-cancer effects of CME-1, a novel polysaccharide, purified from the mycelia of Cordyceps sinensis against B16-F10 melanoma cells. Journal of Cancer Research and Therapeutics. 2014;10(1):43–49. doi: 10.4103/0973-1482.131365. [DOI] [PubMed] [Google Scholar]
- 6.Pao H.-Y., Pan B.-S., Leu S.-F., Huang B.-M. Cordycepin stimulated steroidogenesis in MA-10 mouse Leydig tumor cells through the protein kinase C pathway. Journal of Agricultural and Food Chemistry. 2012;60(19):4905–4913. doi: 10.1021/jf205091b. [DOI] [PubMed] [Google Scholar]
- 7.Sato A., Yoshikawa N., Kubo E., et al. Inhibitory effect of cordycepin on experimental hepatic metastasis of B16-F0 mouse melanoma cells. In Vivo. 2013;27(6):729–732. [PubMed] [Google Scholar]
- 8.Wu J. Y., Zhang Q. X., Leung P. H. Inhibitory effects of ethyl acetate extract of Cordyceps sinensis mycelium on various cancer cells in culture and B16 melanoma in C57BL/6 mice. Phytomedicine. 2007;14(1):43–49. doi: 10.1016/j.phymed.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Ramesh T., Yoo S.-K., Kim S.-W., et al. Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Experimental Gerontology. 2012;47(12):979–987. doi: 10.1016/j.exger.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Faisal F. A., Sundi D., Cooper J. L., et al. Racial disparities in oncologic outcomes after radical prostatectomy: long-term follow-up. Urology. 2014;84(6):1434–1441. doi: 10.1016/j.urology.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiurenkov I. N., Volotova E. V., Kurkin D. V., Bakulin D. A., Logvinov I. O., Antipova T. A. Neuroprotective effect of neuroglutam under conditions of activated free radical oxidation. Eksperimental'naia i Klinicheskaia Farmakologiia. 2014;77(8):16–19. [PubMed] [Google Scholar]
- 12.Matsui K., Kawaguchi Y., Ozaki T., et al. Effect of active hexose correlated compound on the production of nitric oxide in hepatocytes. Journal of Parenteral and Enteral Nutrition. 2007;31(5):373–380. doi: 10.1177/0148607107031005373. [DOI] [PubMed] [Google Scholar]
- 13.Park E.-S., Kang D.-H., Yang M.-K., et al. Cordycepin, 3′-deoxyadenosine, prevents rat hearts from ischemia/reperfusion injury via activation of Akt/GSK-3β/p70S6K signaling pathway and HO-1 expression. Cardiovascular Toxicology. 2014;14(1):1–9. doi: 10.1007/s12012-013-9232-0. [DOI] [PubMed] [Google Scholar]
- 14.Ji D.-B., Ye J., Li C.-L., Wang Y.-H., Zhao J., Cai S.-Q. Antiaging effect of Cordyceps sinensis extract. Phytotherapy Research. 2009;23(1):116–122. doi: 10.1002/ptr.2576. [DOI] [PubMed] [Google Scholar]
- 15.Li S. P., Zhao K. J., Ji Z. N., et al. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sciences. 2003;73(19):2503–2513. doi: 10.1016/s0024-3205(03)00652-0. [DOI] [PubMed] [Google Scholar]
- 16.Kobayasi Y. The genus Cordyceps and its allies. Science Reports of the Tokyo Bunrika Daigaku Section B. 1941;5(84):53–260. [Google Scholar]
- 17.Kobayasi Y. Keys to the taxa of the genera Cordyceps and Torrubiella . Transaction of the Mycological Society of Japan. 1982;23:329–364. [Google Scholar]
- 18.Mains E. B. North American entomogenous species of Cordyceps. Mycologia. 1958;50(2):169–222. doi: 10.2307/3756193. [DOI] [Google Scholar]
- 19.Schoch C. L., Seifert K. A., Huhndorf S., et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(16):6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanabe Y., Saikawa M., Watanabe M. M., Sugiyama J. Molecular phylogeny of Zygomycota based on EF-1α and RPB1 sequences: limitations and utility of alternative markers to rDNA. Molecular Phylogenetics and Evolution. 2004;30(2):438–449. doi: 10.1016/s1055-7903(03)00185-4. [DOI] [PubMed] [Google Scholar]
- 21.Sung G.-H., Hywel-Jones N. L., Sung J.-M., Luangsa-ard J. J., Shrestha B., Spatafora J. W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in Mycology. 2007;57:5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayasi Y. The genus Cordyceps and its allies from Taiwan (Formosa) Bulletin of the National Science Museum. Series B (Tokyo) 1981;7(4):113–122. [Google Scholar]
- 23.Chou S.-M., Lai W.-J., Hong T.-W., et al. Synergistic property of cordycepin in cultivated Cordyceps militaris-mediated apoptosis in human leukemia cells. Phytomedicine. 2014;21(12):1516–1524. doi: 10.1016/j.phymed.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Doyle J. J. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- 25.Castlebury L. A., Rossman A. Y., Sung G.-H., Hyten A. S., Spatafora J. W. Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycological Research. 2004;108(8):864–872. doi: 10.1017/s0953756204000607. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y. J., Whelen S., Hall B. D. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology and Evolution. 1999;16(12):1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- 27.Rehner S. A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97(1):84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- 28.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 30.Mondal J., Bishayee K., Panigrahi A. K., Khuda-Bukhsh A. R. Low doses of ethanolic extract of Boldo (Peumus boldus) can ameliorate toxicity generated by cisplatin in normal liver cells of mice in vivo and in WRL-68 cells in vitro, but not in cancer cells in vivo or in vitro. Journal of Integrative Medicine. 2014;12(5):425–438. doi: 10.1016/s2095-4964(14)60045-5. [DOI] [PubMed] [Google Scholar]
- 31.Saha S., Hossain F., Anisuzzman M., Islam M. K. Pharmacological evaluation of Musa seminifera Lour. fruit. Journal of Integrative Medicine. 2013;11(4):253–261. doi: 10.3736/jintegrmed2013025. [DOI] [PubMed] [Google Scholar]
- 32.Chu P.-Y., Li T.-K., Ding S.-T., Lai I.-R., Shen T.-L. EGF-induced Grb7 recruits and promotes ras activity essential for the tumorigenicity of Sk-Br3 breast cancer cells. The Journal of Biological Chemistry. 2010;285(38):29279–29285. doi: 10.1074/jbc.c110.114124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu K.-H., Chang Y.-F., Yin P.-H., et al. In vitro and in vivo apoptosis-inducing antileukemic effects of Mucuna macrocarpa stem extract on HL-60 human leukemia cells. Integrative Cancer Therapies. 2010;9(3):298–308. doi: 10.1177/1534735410378661. [DOI] [PubMed] [Google Scholar]
- 34.Ku H. H. Notes on the use of propagation of error formulas. Journal of Research of the National Bureau of Standards. 1966;70(4):263–273. [Google Scholar]
- 35.Wang Y.-Z., Chou W.-N. Investigation of the Environmental and Ecological Indicators—The Research Report for Guanwu Mushroom. Taichung, Taiwan: Shei-Pa National Park, Construction and Planning Agency Ministry of the Interior; 2004. [Google Scholar]
- 36.Li C.-R., Xia C.-R., Lin Y.-R., Fan M.-Z., Li Z.-Z. Hirsutella huangshanensis sp. nov. The anamorph of Cordyceps formosana and its infection on Tenebrio molitor . Mycosystema. 1985;24(3):349–355. [Google Scholar]
- 37.Mains E. B. Cordyceps Stylophora and Cordyceps ravenelii . Mycologia. 1941;33(6):611–617. doi: 10.2307/3754779. [DOI] [Google Scholar]
- 38.Kobayasi Y. Cordyceps species from Japan 6. Bulletin of the National Science Museum, Series B (Tokyo) 1983;9(1):1–21. [Google Scholar]
- 39.Kobayasi Y., Shimizu D. Cordyceps species from Japan 2. Bulletin of the National Science Museum Tokyo Series B. 1980;6(3):77–96. [Google Scholar]
- 40.Gorbunova I. A., Kryukov V. Y., Zibzeev E. G. First records of the entomopathogenic fungus Ophiocordyceps gracilis (Ascomycota, Hypocreales) from Siberia. Euroasian Entomological Journal. 2011;10(1):17–18. [Google Scholar]
- 41.Kobayasi Y. On the genus Cordyceps and its allies on cicadae from Japan. Bulletin of the Biogeographical Society of Japan. 1939;9:145–176. [Google Scholar]
- 42.Schulze-Lefert P., Panstruga R. A molecular evolutionary concept connecting nonhost resistance, pathogen host range, and pathogen speciation. Trends in Plant Science. 2011;16(3):117–125. doi: 10.1016/j.tplants.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Nikoh N., Fukatsu T. Interkingdom host jumping underground: phylogenetic analysis of entomoparasitic fungi of the genus Cordyceps . Molecular Biology and Evolution. 2000;17(4):629–638. doi: 10.1093/oxfordjournals.molbev.a026341. [DOI] [PubMed] [Google Scholar]
- 44.Gutteridge J. M. C., Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Annals of the New York Academy of Sciences. 2000;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 45.Li X.-T., Li H.-C., Li C.-B., Dou D.-Q., Gao M.-B. Protective effects on mitochondria and anti-aging activity of polysaccharides from cultivated fruiting bodies of Cordyceps militaris . American Journal of Chinese Medicine. 2010;38(6):1093–1106. doi: 10.1142/s0192415x10008494. [DOI] [PubMed] [Google Scholar]
- 46.Chen M., Cheung F. W. K., Chan M. H., et al. Protective roles of on Cordyceps lung fibrosis in cellular and rat models. Journal of Ethnopharmacology. 2012;143(2):448–454. doi: 10.1016/j.jep.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong Y., Jing T., Meng Q., et al. Studies on the antidiabetic activities of cordyceps militaris extract in diet-streptozotocin-induced diabetic sprague-dawley rats. BioMed Research International. 2014;2014:11. doi: 10.1155/2014/160980.160980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H.-P., Liu C.-W., Chang H.-W., Tsai J.-W., Sung Y.-Z., Chang L.-C. Cordyceps sinensis protects against renal ischemia/reperfusion injury in rats. Molecular Biology Reports. 2013;40(3):2347–2355. doi: 10.1007/s11033-012-2316-2. [DOI] [PubMed] [Google Scholar]
- 49.Wang B.-J., Won S.-J., Yu Z.-R., Su C.-L. Free radical scavenging and apoptotic effects of Cordyceps sinensis fractionated by supercritical carbon dioxide. Food and Chemical Toxicology. 2005;43(4):543–552. doi: 10.1016/j.fct.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Wu W.-D., Hu Z.-M., Shang M.-J., et al. Cordycepin down-regulates multiple drug resistant (MDR)/HIF-1alpha through regulating AMPK/mTORC1 signaling in GBC-SD gallbladder cancer cells. International Journal of Molecular Sciences. 2014;15(7):12778–12790. doi: 10.3390/ijms150712778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Degenerated primer sequences (Supporting Table 1) derived from the largest subunit of RNA polymerase II (RPB1), the second large subunit of RNA polymerase II (RPB2) and translation elongation factor-1α (EF1-α) genes were synthesized according to previous reports (30, 31, 32) and used for the molecular identifications of Cordyceps spp, (s. l.), including Ophiocordycpes formosana. The amplified 790-, 1062-, and 1068-base pair (bp) nucleotides, respectively, from our collected samples were sequenced and subjected to BLAST in the NCBI database (). The closest species and strains matched, NCBI accession number, and nucleotide identify of the 3 sequences were listed (Supporting Table 2).
A list of nucleotide sequences of the 3 genes (i.e. RPB1, RPB2, and EF-1a) from 51 species/isolates was retrieved from the NCBI database following the criteria described by Sung et al or obtained from our sequenced PCR products of the O. formosana isolates (Supporting Table 3).