Blue light responses of stomata occur in a wide lineage of plants except the fern species of Polypodiopsida.
Abstract
Light is a major environmental factor required for stomatal opening. Blue light (BL) induces stomatal opening in higher plants as a signal under the photosynthetic active radiation. The stomatal BL response is not present in the fern species of Polypodiopsida. The acquisition of a stomatal BL response might provide competitive advantages in both the uptake of CO2 and prevention of water loss with the ability to rapidly open and close stomata. We surveyed the stomatal opening in response to strong red light (RL) and weak BL under the RL with gas exchange technique in a diverse selection of plant species from euphyllophytes, including spermatophytes and monilophytes, to lycophytes. We showed the presence of RL-induced stomatal opening in most of these species and found that the BL responses operated in all euphyllophytes except Polypodiopsida. We also confirmed that the stomatal opening in lycophytes, the early vascular plants, is driven by plasma membrane proton-translocating adenosine triphosphatase and K+ accumulation in guard cells, which is the same mechanism operating in stomata of angiosperms. These results suggest that the early vascular plants respond to both RL and BL and actively regulate stomatal aperture. We also found three plant species that absolutely require BL for both stomatal opening and photosynthetic CO2 fixation, including a gymnosperm, C. revoluta, and the ferns Equisetum hyemale and Psilotum nudum.
Stomata regulate gas exchange between plants and the atmosphere (Zeiger, 1983; Assmann, 1993; Roelfsema and Hedrich, 2005; Shimazaki et al., 2007; Kim et al., 2010). Acquisition of stomata was a key step in the evolution of terrestrial plants by allowing uptake of CO2 from the atmosphere and accelerating the provision of nutrients via the transpiration stream within the plant (Hetherington and Woodward, 2003; McAdam and Brodribb, 2013). Stomatal aperture is regulated by changes in the turgor of guard cells, which are induced by environmental factors and endogenous phytohormones. Light is a major factor in the promotion of stomatal opening, and the opening is mediated via two distinct light-regulated pathways that are known as photosynthesis- and blue light (BL)-dependent responses under photosynthetic active radiation (PAR; Vavasseur and Raghavendra, 2005; Shimazaki et al., 2007; Lawson et al., 2014).
The photosynthesis-dependent stomatal opening is induced by a continuous high intensity of light, and the action spectrum for the stomatal opening resembles that of photosynthetic pigments in leaves (Willmer and Fricker, 1996). Both mesophyll and guard cells possess photosynthetically active chloroplasts, and their photosynthesis has been suggested to contribute to stomatal opening in leaves. The decrease in the concentration of intercellular CO2 (Ci) caused by photosynthetic CO2 fixation or some unidentified mediators and metabolites from mesophyll cells is supposed to elicit stomatal opening, although the exact nature of the events is unclear (Wong et al., 1979; Vavasseur and Raghavendra, 2005; Roelfsema et al., 2006; Mott et al., 2008; Lawson et al., 2014).
BL-dependent stomatal opening requires a strong intensity of PAR as a background: weak BL solely scarcely elicits stomatal opening, but the same intensity of BL induces the fast and large stomatal opening in the presence of strong red light (RL; Ogawa et al., 1978; Shimazaki et al., 2007). Since such stomatal opening requires BL under the RL or PAR, we call the opening reaction a BL-dependent response of stomata. BL-dependent stomatal response takes place and proceeds in natural environments because the sunlight contains both BL and RL and facilitates photosynthetic CO2 fixation (Assmann, 1988; Takemiya et al., 2013a). In this stomatal response, BL and PAR (BL, RL, and other wavelengths of light) seem to act as a signal and an energy source, respectively.
The BL-dependent stomatal opening is initiated by the absorption of BL by phototropin1 and phototropin2 (Kinoshita et al., 2001), the plant-specific BL receptors, in guard cells followed by activation of the plasma membrane proton-translocating adenosine triphosphatase (H+-ATPase; Kinoshita and Shimazaki, 1999). Two newly identified proteins, protein phosphatase1 and blue light signaling1 (BLUS1), mediate the signaling between phototropins and H+-ATPase (Takemiya et al., 2006, 2013a, 2013b). The activated H+-ATPase evokes a plasma membrane hyperpolarization, which drives K+ uptake through the voltage-gated, inward-rectifying K+ channels (Assmann, 1993; Shimazaki et al., 2007; Kim et al., 2010; Kollist et al., 2014). The accumulation of K+ causes water uptake and increases turgor pressure of guard cells, and finally results in stomatal opening. The BL-dependent opening is enhanced by RL, and BL at low intensity is effective in the presence of RL (Ogawa et al., 1978; Iino et al., 1985; Shimazaki et al., 2007; Suetsugu et al., 2014). These stomatal responses by RL and BL are commonly observed in a number of seed plants so far examined.
Fine control of stomatal aperture to various environmental factors has been characterized in many angiosperms. Although morphological and mechanical diversity of stomata is widely documented, little is known about the functional diversity (Willmer and Fricker, 1996; Hetherington and Woodward, 2003). Our previous study indicated that BL-dependent stomatal response is absent in the major fern species of Polypodiopsida, including Adiantum capillus-veneris, Pteris cretica, Asplenium scolopendrium, and Nephrolepis auriculata, but the stomata of these species open by PAR including RL (Doi et al., 2006). When the epidermal peels isolated from A. capillus-veneris are treated with photosynthetic electron transport inhibitor 3-(3,4-dichlorophenyl)-1,1dimethylurea (Doi and Shimazaki, 2008), the response is completely inhibited, but the responses in the seed plants of Vicia faba and Commelina communis are relatively insensitive to 3-(3,4-dichlorophenyl)-1,1dimethylurea (Schwartz and Zeiger, 1984). These findings suggest that there is functional diversity in light-dependent stomatal response in different lineages of land plants. In accord with this notion, the different sensitivities of stomatal response to abscisic acid and CO2 have been reported among the plant species of angiosperm, gymnosperm, ferns, and lycophytes (Mansfield and Willmer, 1969; Brodribb and McAdam, 2011), although the exact responsiveness to abscisic acid and CO2 is still debated (Chater et al., 2011, 2013; Ruszala et al., 2011; McAdam and Brodribb, 2013).
To address the origin and distribution of stomatal light responses, we investigated the presence of a stomatal response using a gas exchange method and various lineages of vascular plants, including euphyllophytes and lycophytes. Unexpectedly, all plant lineages except Polypodiopsida in monilophytes exhibited a stomatal response to BL in the presence of RL, suggesting that the response was present in the early evolutionary stage of vascular plants. We also report the stomatal opening in response to RL in these plant species.
RESULTS
Typical BL Responses of Stomata in Angiosperms and Ferns
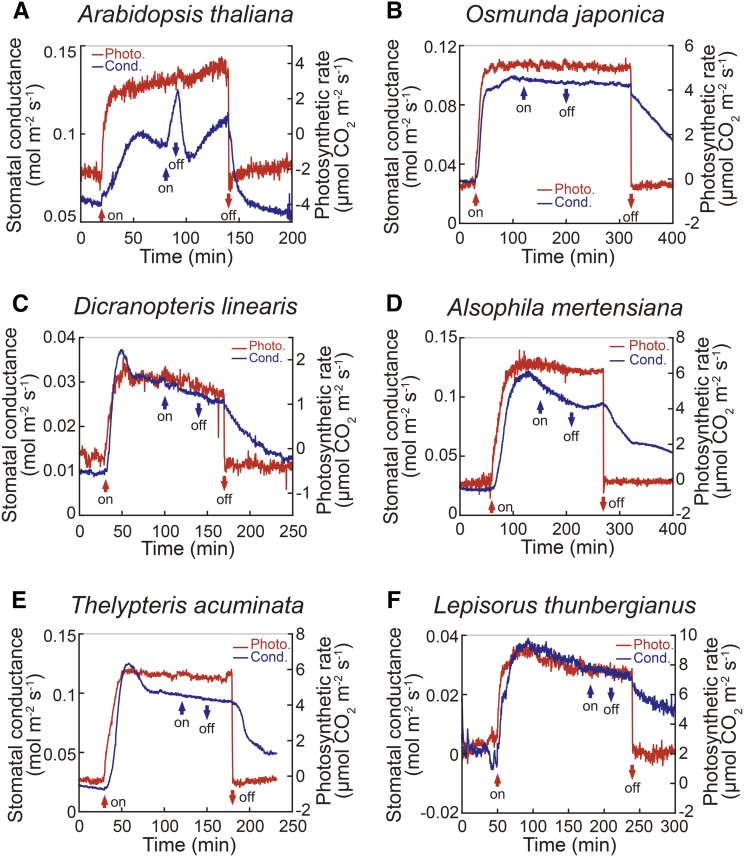
To distinguish the stomatal response specific to BL from that of RL, we measured stomatal conductance by gas exchange technique using a dual-beam protocol (Ogawa et al., 1978; Assmann et al., 1985; Iino et al., 1985; Shimazaki et al., 1986). The method allows for discrimination of the photosynthesis- and BL-dependent stomatal opening. All of the plants tested were kept in darkness for 12 h before measurements. When the dark-adapted Arabidopsis (Arabidopsis thaliana) leaf was irradiated with a high intensity of RL at 600 µmol m−2 s−1, a gradual increase in stomatal conductance occurred and reached maximum within 30 min with an immediate photosynthetic CO2 uptake at maximum within 10 min (Fig. 1A). Such CO2 uptake by RL is often found in Arabidopsis and is due to the significant stomatal opening in the dark, which allows sufficient provision of CO2 for photosynthesis without further opening (Lascève et al., 1997; Caird et al., 2007). When a low intensity of BL at 5 µmol m−2 s−1 was superimposed on RL, stomatal conductance increased rapidly and reached a peak, followed by a fast decline after turning off the BL. No increase in the conductance occurred when RL at 5 µmol m−2 s−1 was superimposed on the RL instead of BL (not shown). We call this response BL-dependent stomatal opening, but this response occurs under the natural environment because the sunlight consists of both RL and BL.
Figure 1.
Stomatal conductance (cond.; blue line) and photosynthetic (photo.) CO2 uptake (red line) in response to light in leaves of Arabidopsis (A) and Polypodiopsida ferns (B–F). The plant leaves were irradiated with continuous RL at 600 µmol m−2 s−1 and BL at 5 µmol m−2 s−1 at the position of the upward red and blue arrows, respectively. BL was superimposed on the RL. Red and blue downward arrows indicate the termination of RL and BL, respectively. The experiments for stomatal conductance in each plant species were done at least three times (typically five times), and typical data were presented.
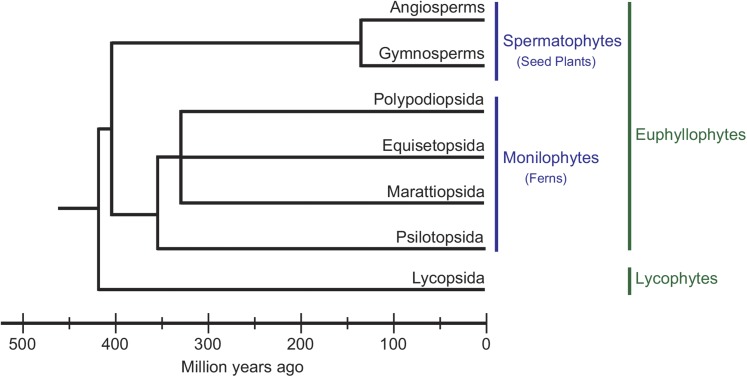
In recent phylogenetic analyses, the vascular plants are divided into euphyllophytes and lycophytes (Pryer et al., 2001; Smith et al., 2006). The euphyllophytes consist of spermatophytes and monilophytes; the monilophytes comprise four fern species: Polypodiopsida, Equisetopsida, Marattiopsida, and Psilotopsida (Fig. 2). Polypodiopsida, which diversified after the advent of angiosperms as modern fern species (Schneider et al., 2004), is the largest fern lineage and consists of seven major groups: Osumundales, Hymenophyllales, Gleicheniales, Schizaeales, Salviniales, Cyatheales, and Polypodiales (Smith et al., 2006; Wolf et al., 2011). Hymenophyllales ferns, which reside in the water, do not have stomata. We have reported that five fern species within Schizaeales and Polypodiales lack the stomatal responses to BL (Doi et al., 2006). To clarify whether the absence of stomatal BL response is a common feature among these species, we investigated stomatal responses in four lineages, including Osumundales, Gleicheniales, Cyatheales, and Polypodiales. Light responses of stomata and photosynthetic CO2 uptake in Osmunda japonica (Osumundales), Dicranopteris linearis (Gleicheniales), Alsophila mertensiana (Cyatheales), Thelypteris acuminata (Polypodiales), and Lepisorus thunbergianus (Polypodiales) were presented (Fig. 1, B–F). When the strong RL (600 µmol m−2 s−1) that saturates photosynthesis was applied to the upper surface of the leaf, photosynthetic CO2 uptake increased rapidly to the maximum value. Stomatal conductance showed almost the same time course as that of the CO2 uptake in response to RL, and reached the maximum about 20 to 60 min after the onset of RL. The stomatal responses to RL in Polypodiopsida ferns were faster than that of Arabidopsis in general (Fig. 1), as has been reported previously (Doi et al., 2006). Prolonged exposure to RL caused a slight and gradual decrease in the conductance in D. linearis, A. mertensiana, T. acuminata, and L. thunbergianus. A weak BL (5 µmol m−2 s−1) superimposed on RL did not increase stomatal conductance in these Polypodiopsida ferns. The results indicate that all of these stomata examined here open by RL, whereas they do not exhibit BL-dependent stomatal opening. The lack of BL-dependent stomatal response was confirmed in five evolutionary diverse fern species of Polypodiopsida (Doi et al., 2006).
Figure 2.
Phylogenetic tree showing relationships between major groups of extant vascular plants, based on previously published phylogenetic studies (Pryer et al., 2001; Wikström and Kenrick, 2001; Schneider et al., 2004; Smith et al., 2006).
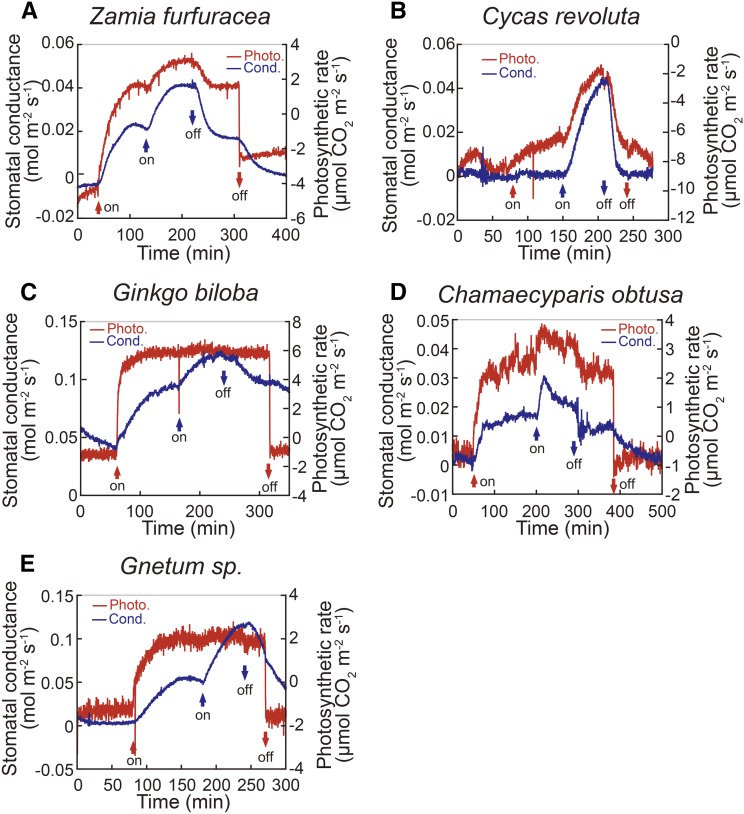
Stomatal Response to Light in Gymnosperms
Given the lack of BL response of stomata in Polypodiopsida ferns, it is interesting to know whether other plant lineages, including gymnosperms and lycophytes, show a response (Fig. 2). Gymnosperms comprise four clades, including Cycadopsida, Ginkgoopsida, Coniferopsida, and Gnetopsida. Figure 3 shows typical light responses of stomatal conductance and photosynthesis in a representative species of each clade: Z. furfuracea (Cycadopsida), Cycas revoluta (Cycadopsida), G. biloba (Ginkgoopsida), C. obtusa (Coniferopsida), and Gnetum spp. (Gnetopsida). Irradiation of leaves with RL at 600 µmol m−2 s−1 increased stomatal conductance in these species, except for C. revoluta. When weak BL at 5 µmol m−2 s−1 was applied to the leaf superimposed on the RL, stomatal conductance and the CO2 uptake increased in all of these species. Stomata of C. revoluta did not respond to RL and only a slight CO2 uptake occurred, but a large increase in conductance was elicited by BL with a simultaneous large CO2 uptake (Fig. 3B; Supplemental Fig. S1). From these results, we conclude that the gymnosperms possess BL response of stomata. Furthermore, a sole irradiation of C. revoluta leaf with weak BL did not cause an increase in stomatal conductance, but a subsequent RL induced large stomatal opening and CO2 uptake (Supplemental Fig. S1), suggesting that RL is required for BL-dependent stomatal opening in this plant species.
Figure 3.
Stomatal conductance (cond.; blue line) and photosynthetic (photo.) CO2 uptake (red line) in response to light in leaves of Zamia furfuracea (A), Cycas revoluta (B), Ginkgo biloba (C), Chamaecyparis obtusa (D), and Gunetum spp. (E). Plants were treated with light as shown in Figure 1.
Stomatal Responses in Fern Species Other Than Polypodiopsida
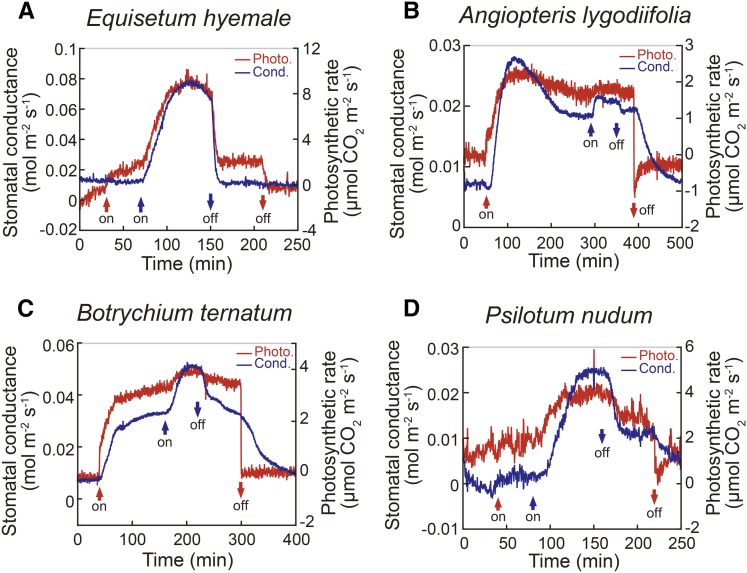
We hypothesized that BL response of stomata was acquired in plants after the evolution of seed plants because both gymnosperms and angiosperms possess the response but the extant major fern Polypodiopsida does not. We therefore investigated whether the stomata of fern species that evolved earlier than Polypodiopsida were able to open stomata in response to BL.
Unexpectedly, as shown in Figure 4, three fern classes, Equisetopsida, Marattiopsida, and Psilotopsida (Fig. 2), showed stomatal opening in responses to BL, except Polypodiopsida (Fig. 1). When these fern species were irradiated with strong RL, stomata of A. lygodiifolia (Marattiopsida) and B. ternatum (Psilotopsida) opened and photosynthetic CO2 uptake proceeded, but stomata of E. hyemale (Equisetopsida) and P. nudum (Psilotopsida) did not open and no CO2 uptake occurred. Interestingly, stomata of P. nudum and E. hyemale showed a large stomatal opening by BL, and photosynthetic CO2 uptake occurred in parallel with an increase in stomatal conductance. The results indicated that the ferns of Equisetopsida, Marattiopsida, and Psilotopsida have BL response of stomata, and the BL responses of Equisetopsida and Psilotopsida are essential for photosynthetic CO2 fixation in these plant species by eliminating the barrier against CO2 entry into the leaf.
Figure 4.
Stomatal conductance (cond.; blue line) and photosynthetic (photo.) CO2 uptake (red line) in response to light in leaves of Equisetum hyemale (A), Angiopteris lygodiifolia (B), Botrychium ternatum (C), and Psilotum nudum (D). Plants were treated with light as shown in Figure 1.
Stomatal Responses in Lycophytes
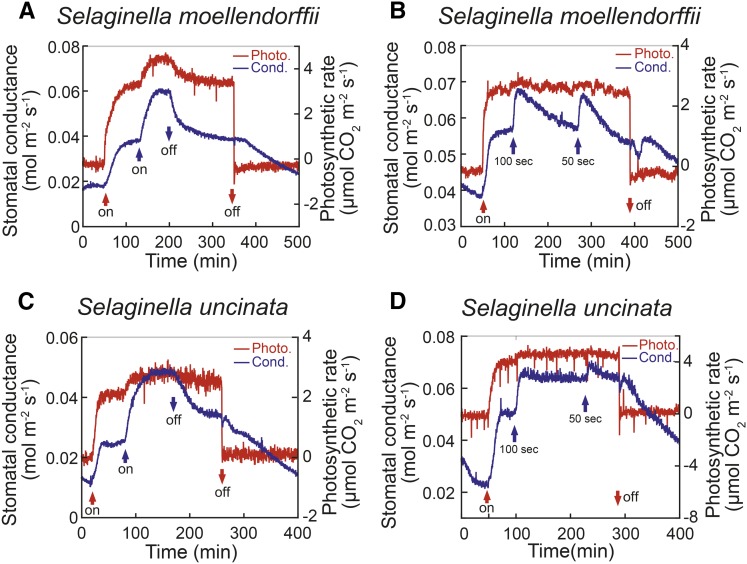
As described above, stomatal BL response operates in fern species older than Polypodiopsida, and this observation prompted us to investigate the responses in lycophytes, which are the most primitive extant vascular plants that appeared about 420 million years ago. We used Selaginella moellendorffii and Selaginella uncinata, a sister group to the ferns and the seed plants, as representatives of lycophytes (Fig. 2). Selaginella spp. have fronds that consist of microphylls and stems, and their stomata are in line with the central part of the microphyll (Soni et al., 2012). Stomatal conductance and photosynthetic CO2 uptake were increased by strong RL (Fig. 5). After conductance reached a steady state, weak BL on the RL further increased both stomatal conductance and the CO2 uptake in S. moellendorffii and S. uncinata (Fig. 5, A and C). Since a short pulse of blue light is able to elicit stomatal opening in Arabidopsis and other seed plants (Assmann et al., 1985; Iino et al., 1985; Shimazaki et al., 2007), we tested the effect of a pulse on the microphylls. A pulse of BL superimposed on RL also induced fast stomatal opening in these plants (Fig. 5, B and D), and stomata in the plants closed more slowly after the pulse than those in Arabidopsis. From these results, we conclude that BL-dependent stomatal response operates in lycophytes, although the detailed mechanism has yet to be clarified.
Figure 5.
Stomatal conductance (cond.; blue line) and photosynthetic (photo.) CO2 uptake (red line) in response to light in leaves of S. moellendorffii (A and B) and S. uncinata (C and D). Plants were treated with light as shown in Figure 1. Upward arrows of blue in B and D indicate the application of BL pulse at 150 µmol m−2 s−1.
H+-ATPase and K+ Accumulation in Guard Cells Are Responsible for Stomatal Opening in Lycophytes
We have shown that BL-dependent stomatal response operates in vascular plants, except Polypodiopsida ferns. It is unclear whether the stomatal responses in these diverse lineages of plants have the same mechanisms as those in angiosperms like Arabidopsis. To test this, we investigated the involvement of H+-ATPase and K+ accumulation in the opening response using the earliest evolved vascular plants of S. uncinata. Recent investigation reported light-induced stomatal opening in this same species (Ruszala et al., 2011) with concomitant K+ accumulation in guard cells. Furthermore, fusicoccin, an activator of the plasma membrane H+-ATPase, induced stomatal opening, as has been reported in several plant species of angiosperms (Shimazaki et al., 2007). We performed essentially the same experimental work. When the detached microphylls from S. uncinata were irradiated by both RL (600 µmol m−2 s−1) and BL (5 µmol m−2 s−1) for 2 h, stomata opened (Supplemental Fig. S2) and accumulated a substantial amount of K+ in guard cells (Supplemental Fig. S2). When fusicoccin was administered to the epidermal peels from this plant in the dark, stomata opened but the apertures of stomata were not as large as those under the light (Supplemental Fig. S2). The results suggest that the H+-ATPase drives K+ uptake in guard cells, and the accumulated K+ is responsible for stomatal opening, at least partially, in S. uncinata. Quantitative relationships among stomatal aperture, H+-ATPase activity, and the accumulation of K+ in guard cells could not be obtained because of technical difficulty.
DISCUSSION
BL Response of Stomata Is Present in Early Vascular Plants
Stomatal responses to light have been characterized in angiosperms such as Arabidopsis, C. communis, and V. faba (Zeiger, 1983; Assmann, 1993; Willmer and Fricker, 1996; Roelfsema et al., 2006; Shimazaki et al., 2007). In these plants, stomatal opening is mediated by two light signaling cascades, BL-dependent and photosynthesis-induced responses (Shimazaki et al., 2007; Kollist et al., 2014; Lawson et al., 2014). Both responses are observed in angiosperms, but no comprehensive analysis has been done on the evolution of stomatal response across phylogenetically divergent species of vascular plants. We have shown that the stomata of Polypodiopsida ferns lack the BL-dependent stomatal response in intact plants, and that RL-induced stomatal opening is mediated by guard cell chloroplasts using the isolated epidermal peels from the fern (Doi et al., 2006; Doi and Shimazaki, 2008). We therefore suspected that the stomatal responses to light have diverged across species of vascular plants. To examine functional diversity of stomata, we investigated stomatal responses to strong RL (photosynthesis induced) and weak BL superimposed on RL in three large lineages of the vascular plants, including gymnosperms, ferns, and lycophytes.
We confirmed the lack of stomatal BL response in four major lineages of modern fern Polypodiopsida (Schneider et al., 2004). Stomata in all Polypodiopsida tested in this study did not open in response to BL in the presence of RL, although RL induced stomatal opening (Figs. 1 and 2). We also found that most of the plant species in euphyllophytes and lycophytes (Fig. 2) showed stomatal opening in response to RL except two species of fern, E. hyemale and P. nudum (Fig. 4), and gymnosperms of C. revoluta (Fig. 3). However, all of these vascular plant species, including fern species (except Polypodiopsida), exhibited the typical BL response of stomata: weak BL required strong RL as a background to induce stomatal opening. We note here that plant species of E. hyemale, P. nudum, C. revoluta (Supplemental Fig. S1), S. uncinata, and S. moellendorffii did not respond to weak BL in the absence of RL. From these results, we concluded that the common ancestor of vascular plants of euphyllophytes and lycophytes emerged about 420 million years ago and acquired BL-dependent stomatal response, although the associated molecular mechanisms in these plants have yet to be determined. The modern ferns of Polypodiopsida likely lost the ability to respond to BL when they evolved under the canopy of higher plants or grew in environments that reduced sensitivity to BL (Schneider et al., 2004). Instead, it appears that Polypodiopsida acquired a chimera of the red/far-red light receptor phytochrome and phototropin (NEOCHROME), and showed extremely high sensitivity to white light in chloroplast accumulation and phototropic bending, which might enhance fitness of these plants when growing under a canopy (Kawai et al., 2003; Suetsugu et al., 2005).
Recent studies indicated that the stomatal responses to CO2 and abscisic acid are absent in Polypodiopsida and lycophytes, and the stomatal aperture of these plants is regulated by passive control of guard cell turgor by leaf water status (McAdam and Brodribb, 2013), differing from angiosperms (Doi and Shimazaki, 2008; Brodribb and McAdam, 2011; McAdam and Brodribb, 2012). By contrast, other groups reported that the stomata of mosses and lycophytes are sensitive to both CO2 and abscisic acid (Chater et al., 2011; Ruszala et al., 2011), and that even the stomata in sporophytes of moss Physcomitrella patens, the most basal land plant group, exhibited light and H+-ATPase-driven stomatal opening (Chater et al., 2011). Our studies on the light-induced stomatal opening from euphyllophytes to lycophytes indicated that stomatal movement is regulated by both photosynthesis- and BL-dependent ion transport. Although it was not determined whether phototropins function as BL receptors in this plant species, it is clear that the BL-dependent stomatal opening is present in the initial stage of land plant evolution.
Molecular Mechanisms of Selaginella spp. BL Response of Stomata
The signaling pathway regulating the BL-dependent stomatal response is known to contain BL receptor phototropins, a protein kinase BLUS1, a type1 protein phosphatase, the plasma membrane H+-ATPase, and inward-rectifying K+ (K+in) channels in Arabidopsis (Kinoshita et al., 2001; Shimazaki et al., 2007; Zhao et al., 2012; Takemiya et al., 2013a; Kollist et al., 2014). Although all of the older land plants, except Polypodiopsida, show BL-dependent stomatal response, the signaling components responsible for these processes are largely unknown among the diverse species. DNA sequence databases of S. moellendorffii indicate the presence of homologs of phototropins and those of H+-ATPase (Banks et al., 2011), but homologs of BLUS1 and K+in channels have not been identified in the lycophytes (Gomez-Porras et al., 2012). However, both S. moellendorffii and S. uncinata exhibited clear BL-specific stomatal opening (Fig. 5). This agrees with previous observations that a specific activator of the H+-ATPase, fusicoccin, promotes stomatal opening in epidermal peels from S. uncinata in dark with concomitant accumulation of K+ in the guard cells (Ruszala et al., 2011). These results suggest that the H+-ATPase drives K+ uptake in guard cells, and the K+ functions as osmotica in stomatal opening in a manner consistent with angiosperms, although Selaginella spp. does not have homologs of K+ channel in Arabidopsis1 (KAT1), KAT2 and Arabidopsis K+ transporter1. Big K+-like channels found in Selaginella spp. might compensate for the absence of Shaker-like K+in channels during K+ uptake in guard cells (Gomez-Porras et al., 2012). We note here that the primitive nonvascular land plant of P. patens opens stomata in response to fusicoccin (Chater et al., 2011) and possesses four genes coding K+in-like channels (Gomez-Porras et al., 2012).
Light (RL plus BL) enhances stomatal opening in Selaginella spp. epidermis, and the stomatal aperture was larger under light than in the presence of fusicoccin in dark. This is opposite to the response of stomata in the epidermis of angiosperms; in this latter case, the stomatal aperture exposed to fusicoccin was larger than stomata exposed to light in Arabidopsis and other higher plants (Shimazaki et al., 1993, 2007; Takemiya et al., 2013a). These observations may suggest differences in the mechanism of stomatal opening between angiosperms and lycophytes. It is possible that stomatal opening is enhanced by inhibition of anion channels by BL in lycophytes, as has been suggested in Arabidopsis and V. faba (Marten et al., 2007), and this response is greater in lycophytes than angiosperms. Inhibition of anion channels would not occur with the application of fusicoccin.
The Role of BL Response of Stomata
We found that the stomata in some species of ferns and gymnosperms (Figs. 3 and 4) did not respond to RL, but responded to the superimposed weak BL with simultaneous CO2 uptake. These plant species must experience a drastic reduction of Ci under strong RL because of photosynthetic CO2 fixation in the leaf and closed stomata. These observations indicate that the decreased Ci with RL is not sufficient for stomatal opening in these plant species and further implicates the functional roles of BL-dependent stomatal opening. The BL response of stomata depends on Ci in the leaf, and therefore, one role of this response is to meet the demand of CO2 for photosynthesis by opening stomata (Assmann, 1988). This notion is supported by a recent study showing that a mutant variant lacking a stomatal BL response was less sensitive to decreased CO2 than wild-type plants (Takemiya et al., 2013a). The other proposed role is that the BL response can indirectly monitor the magnitude of photosynthetic CO2 fixation through the absorption of PAR in guard cell chloroplasts (Suetsugu et al., 2014). The responses described above also suggest the presence of fine control of stomatal aperture in ferns and gymnosperms through light quality and intensity.
MATERIALS AND METHODS
Plants and Growth Conditions
Zamia furfuracea, Dicranopteris linearis, Angiopteris lygodiifolia, Botrychium ternatum, Equisetum hyemale, Psilotum nudum, Selaginella moellendorffii, and Selaginella uncinata were purchased from local nurseries. Chamaecyparis obtusa was kindly provided by Dr. Eiji Gotoh (Faculty of Agriculture, Kyushu University). Lepisorus thunbergianus, Thelypteris acuminata, Cycas revoluta, and Ginkgo biloba were transplanted from the Hakozaki campus (Kyushu University). Osmunda japonica was transplanted from Karatsu in the northern part of the Kyushu islands. C. obtusa was grown outside. L. thunbergianus, T. acuminata, D. linearis, B. ternatum, and P. nudum were grown in a growth room under a white fluorescent lamp (50 µmol m−2 s−1) on a 14-h-light/10-h-dark cycle at 24°C. S. moellendorffii and S. uncinata were grown in a growth room maintained at more than 90% relative humidity under a white fluorescent lamp (20 µmol m−2 s−1) on a 14-h-light/10-h-dark cycle at 24°C. Z. furfuracea, O. japonica, A. lygodiifolia, and E. hyemale were grown in the greenhouse under natural light conditions.
Gas Exchange Measurements
Measurement of gas exchange of intact plants was carried out using an open path gas exchange system (LI-6400; LI-COR Inc.) equipped with a normal chamber (LI-COR Inc.). Plants were kept in the dark overnight before the measurements to induce stomatal closing. The leaves and/or fronds were clamped with a gas-tight normal chamber. The leaf temperature was maintained at 24°C. Measurements were conducted under a constant CO2 concentration of 365 µL L−1 and a relative humidity of 50% to 60%. RL and BL were provided by a light-emitting diode (ISL-150×150; CCS Inc.). Data were recorded at 20-s intervals and processed with a KaleidaGraph (Synergy Software). Photon fluence rates were determined with a LI-2500 light meter equipped with an LI190SA quantum sensor (LI-COR Inc.).
Determination of Stomatal Apertures
Selaginella spp. plants were kept in the dark overnight before measurements. Lateral microphylls were obtained from the plants with tweezers under a safety light. These microphylls were immersed in a solution containing 10 mm MES, 30 mm KCl, and 0.1 mm CaCl2, pH 6.05, in petri dishes and kept in the dark for 2 h. The microphylls were irradiated by RL together with BL for 2 h. Fusicoccin (10 μm) was added to the solution in which the microphylls were immersed, and they were incubated in the dark for 2 h. Micrographs of over 50 stomata were obtained using a Nikon fluorescence microscope equipped with a CCD camera. The stomatal aperture was defined as the width-to-length ratio of the stomatal pores.
Cytochemical Detection of K+ in Guard Cells
Lateral microphylls were obtained and immersed in the same solution as described above. After 2-h incubation under light, accumulation of K+ by guard cells was quantified by staining with sodium hexanitrocobaltate (III) as described previously (Green et al., 1990; Willmer and Fricker, 1996).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Absolute requirement of BL in stomatal opening of C. revoluta.
Supplemental Figure S2. Stomatal opening and K+ accumulation in guard cells of Selaginella spp.
Supplementary Material
Glossary
- BL
blue light
- PAR
photosynthetic active radiation
- Ci
concentration of intercellular CO2
- RL
red light
- H+-ATPase
proton-translocating adenosine triphosphatase
- K+in
inward-rectifying K+
Footnotes
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Sports, Science, Culture, and Technology of Japan (grant nos. 21227001 and 26251032 to K.S.).
Articles can be viewed without a subscription.
References
- Assmann SM. (1988) Enhancement of the stomatal response to blue light by red light, reduced intercellular concentrations of CO2, and low vapor pressure differences. Plant Physiol 87: 226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM. (1993) Signal transduction in guard cells. Annu Rev Cell Biol 9: 345–375 [DOI] [PubMed] [Google Scholar]
- Assmann SM, Simoncini L, Schroeder JI (1985) Blue light activates electrogenic proton pumping in guard cell protoplasts of Vicia faba L. Nature 318: 285–287 [Google Scholar]
- Banks JA, Nishiyama T, Hasebe M, Bowman JL, Gribskov M, dePamphilis C, Albert VA, Aono N, Aoyama T, Ambrose BA, et al. (2011) The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science 332: 960–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SA (2011) Passive origins of stomatal control in vascular plants. Science 331: 582–585 [DOI] [PubMed] [Google Scholar]
- Caird MA, Richards JH, Donovan LA (2007) Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol 143: 4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater C, Gray JE, Beerling DJ (2013) Early evolutionary acquisition of stomatal control and development gene signalling networks. Curr Opin Plant Biol 16: 638–646 [DOI] [PubMed] [Google Scholar]
- Chater C, Kamisugi Y, Movahedi M, Fleming A, Cuming AC, Gray JE, Beerling DJ (2011) Regulatory mechanism controlling stomatal behavior conserved across 400 million years of land plant evolution. Curr Biol 21: 1025–1029 [DOI] [PubMed] [Google Scholar]
- Doi M, Shimazaki K (2008) The stomata of the fern Adiantum capillus-veneris do not respond to CO2 in the dark and open by photosynthesis in guard cells. Plant Physiol 147: 922–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Wada M, Shimazaki K (2006) The fern Adiantum capillus-veneris lacks stomatal responses to blue light. Plant Cell Physiol 47: 748–755 [DOI] [PubMed] [Google Scholar]
- Gomez-Porras JL, Riaño-Pachón DM, Benito B, Haro R, Sklodowski K, Rodríguez-Navarro A, Dreyer I (2012) Phylogenetic analysis of k(+) transporters in bryophytes, lycophytes, and flowering plants indicates a specialization of vascular plants. Front Plant Sci 3: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DB, Dodge SM, Lee JR, Tallman G (1990) Effect of sodium hexanitrocobaltate (III) decomposition on its staining of intracellular potassium ions. Stain Technol 65: 15–24 [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424: 901–908 [DOI] [PubMed] [Google Scholar]
- Iino M, Ogawa T, Zeiger E (1985) Kinetic properties of the blue-light response of stomata. Proc Natl Acad Sci USA 82: 8019–8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H, Kanegae T, Christensen S, Kiyosue T, Sato Y, Imaizumi T, Kadota A, Wada M (2003) Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421: 287–290 [DOI] [PubMed] [Google Scholar]
- Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki Ki (1999) Blue light activates the plasma membrane H(+)-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18: 5548–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Nuhkat M, Roelfsema MRG (2014) Closing gaps: linking elements that control stomatal movement. New Phytol 203: 44–62 [DOI] [PubMed] [Google Scholar]
- Lascève G, Leymarie J, Vavasseur A (1997) Alterations in light-induced stomatal opening in a starch-deficient mutant of Arabidopsis thaliana L. deficient in chloroplast phosphoglucomutase activity. Plant Cell Environ 20: 350–358 [Google Scholar]
- Lawson T, Simkin AJ, Kelly G, Granot D (2014) Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol 203: 1064–1081 [DOI] [PubMed] [Google Scholar]
- Mansfield TA, Willmer CM (1969) Stomatal responses to light and carbon dioxide in the hart’s-tongue fern, Phyllitis scolopendrium Newm. New Phytol 68: 63–66 [Google Scholar]
- Marten H, Hedrich R, Roelfsema MRG (2007) Blue light inhibits guard cell plasma membrane anion channels in a phototropin-dependent manner. Plant J 50: 29–39 [DOI] [PubMed] [Google Scholar]
- McAdam SA, Brodribb TJ (2012) Fern and lycophyte guard cells do not respond to endogenous abscisic acid. Plant Cell 24: 1510–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SA, Brodribb TJ (2013) Ancestral stomatal control results in a canalization of fern and lycophyte adaptation to drought. New Phytol 198: 429–441 [DOI] [PubMed] [Google Scholar]
- Mott KA, Sibbernsen ED, Shope JC (2008) The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ 31: 1299–1306 [DOI] [PubMed] [Google Scholar]
- Ogawa T, Ishikawa H, Shimada K, Shibata K (1978) Synergistic action of red and blue light and action spectra for malate formation in guard cells of Vicia faba L. Planta 142: 61–65 [DOI] [PubMed] [Google Scholar]
- Pryer KM, Schneider H, Smith AR, Cranfill R, Wolf PG, Hunt JS, Sipes SD (2001) Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature 409: 618–622 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R (2005) In the light of stomatal opening: new insights into ‘the Watergate’. New Phytol 167: 665–691 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Konrad KR, Marten H, Psaras GK, Hartung W, Hedrich R (2006) Guard cells in albino leaf patches do not respond to photosynthetically active radiation, but are sensitive to blue light, CO2 and abscisic acid. Plant Cell Environ 29: 1595–1605 [DOI] [PubMed] [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, Chater C, Casson SA, Gray JE, Hetherington AM (2011) Land plants acquired active stomatal control early in their evolutionary history. Curr Biol 21: 1030–1035 [DOI] [PubMed] [Google Scholar]
- Schneider H, Schuettpelz E, Pryer KM, Cranfill R, Magallón S, Lupia R (2004) Ferns diversified in the shadow of angiosperms. Nature 428: 553–557 [DOI] [PubMed] [Google Scholar]
- Schwartz A, Zeiger E (1984) Metabolic energy for stomatal opening. Roles of photophosphorylation and oxidative phosphorylation. Planta 161: 129–136 [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Iino M, Zeiger E (1986) Blue light-dependent proton extrusion by guard-cell protoplasts of Vicia faba. Nature 319: 324–326 [Google Scholar]
- Shimazaki K, Omasa K, Kinoshita T, Nishimura M (1993) Properties of the signal transduction pathway in the blue light response of stomatal guard cells of Vicia faba and Commelina benghalensis. Plant Cell Physiol 34: 1321–1327 [Google Scholar]
- Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG (2006) A classification for extant ferns. Taxon 55: 705–731 [Google Scholar]
- Soni DK, Ranjan S, Singh R, Khare PB, Pathre UV, Shirke PA (2012) Photosynthetic characteristics and the response of stomata to environmental determinants and ABA in Selaginella bryopteris, a resurrection spike moss species. Plant Sci 191-192: 43–52 [DOI] [PubMed] [Google Scholar]
- Suetsugu N, Mittmann F, Wagner G, Hughes J, Wada M (2005) A chimeric photoreceptor gene, NEOCHROME, has arisen twice during plant evolution. Proc Natl Acad Sci USA 102: 13705–13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N, Takami T, Ebisu Y, Watanabe H, Iiboshi C, Doi M, Shimazaki K (2014) Guard cell chloroplasts are essential for blue light-dependent stomatal opening in Arabidopsis. PLoS One 9: e108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemiya A, Kinoshita T, Asanuma M, Shimazaki K (2006) Protein phosphatase 1 positively regulates stomatal opening in response to blue light in Vicia faba. Proc Natl Acad Sci USA 103: 13549–13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemiya A, Sugiyama N, Fujimoto H, Tsutsumi T, Yamauchi S, Hiyama A, Tada Y, Christie JM, Shimazaki K (2013a) Phosphorylation of BLUS1 kinase by phototropins is a primary step in stomatal opening. Nat Commun 4: 2094 [DOI] [PubMed] [Google Scholar]
- Takemiya A, Yamauchi S, Yano T, Ariyoshi C, Shimazaki K (2013b) Identification of a regulatory subunit of protein phosphatase 1 which mediates blue light signaling for stomatal opening. Plant Cell Physiol 54: 24–35 [DOI] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra AS (2005) Guard cell metabolism and CO2 sensing. New Phytol 165: 665–682 [DOI] [PubMed] [Google Scholar]
- Wikström N, Kenrick P (2001) Evolution of Lycopodiaceae (Lycopsida): estimating divergence times from rbcL gene sequences by use of nonparametric rate smoothing. Mol Phylogenet Evol 19: 177–186 [DOI] [PubMed] [Google Scholar]
- Willmer CM, Fricker MD (1996) Stomata. Ed 2 Chapman & Hall, London [Google Scholar]
- Wolf PG, Der JP, Duffy AM, Davidson JB, Grusz AL, Pryer KM (2011) The evolution of chloroplast genes and genomes in ferns. Plant Mol Biol 76: 251–261 [DOI] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282: 424–426 [Google Scholar]
- Zeiger E. (1983) The biology of stomatal guard cells. Annu Rev Plant Physiol 34: 441–474 [Google Scholar]
- Zhao X, Qiao XR, Yuan J, Ma XF, Zhang X (2012) Nitric oxide inhibits blue light-induced stomatal opening by regulating the K+ influx in guard cells. Plant Sci 184: 29–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.