Intracellular guard cell carbonic anhydrases have CO2 roles in stomatal movements.
Abstract
Elevated carbon dioxide (CO2) in leaves closes stomatal apertures. Research has shown key functions of the β-carbonic anhydrases (βCA1 and βCA4) in rapid CO2-induced stomatal movements by catalytic transmission of the CO2 signal in guard cells. However, the underlying mechanisms remain unclear, because initial studies indicate that these Arabidopsis (Arabidopsis thaliana) βCAs are targeted to distinct intracellular compartments upon expression in tobacco (Nicotiana benthamiana) cells. Which cellular location of these enzymes plays a key role in native guard cells in CO2-regulated stomatal movements remains unknown. Here, we express fluorescently tagged CAs in guard cells of ca1ca4 double-mutant plants and show that the specific locations of βCA4 at the plasma membrane and βCA1 in native guard cell chloroplasts each can mediate rapid CO2 control of stomatal movements. Localization and complementation analyses using a mammalian αCAII-yellow fluorescent protein in guard cells further show that cytoplasmic localization is also sufficient to restore CO2 regulation of stomatal conductance. Mathematical modeling of cellular CO2 catalysis suggests that the dynamics of the intracellular HCO3− concentration change in guard cells can be driven by plasma membrane and cytoplasmic localizations of CAs but not as clearly by chloroplast targeting. Moreover, modeling supports the notion that the intracellular HCO3− concentration dynamics in guard cells are a key mechanism in mediating CO2-regulated stomatal movements but that an additional chloroplast role of CAs exists that has yet to be identified.
Diverse roles of carbonic anhydrases (CAs) in a broad range of biochemical processes have been investigated such as carboxylation or decarboxylation reactions, including photosynthesis and respiration. CAs are some of the most rapid enzymes known that facilitate the catalysis of CO2 and water to bicarbonate and protons. CA proteins can be grouped into several major distinct classes (α, β, and γ; Hewett-Emmett and Tashian, 1996; Tripp et al., 2001) and also, δ- and ε-classes (Lane and Morel, 2000; So et al., 2004). To date, all CAs identified in animal systems belong to α-class, whereas in plants and algae, known CAs are more diverse, belonging to the α-, β-, γ-, and δ-classes.
In algae, a key function of CAs is in the CO2-concentrating mechanism, which concentrates inorganic carbon for efficient photosynthetic activity (Badger and Price, 2003; Spalding, 2008; Moroney et al., 2011; Wang et al., 2011; Ludwig, 2012). In the microalga Chlamydomonas reinhardtii, two periplasmic αCAs function in supplying inorganic carbon for photosynthesis (Fukuzawa et al., 1990), and mitochondria-localized βCAs are involved in converting CO2 produced in mitochondria into HCO3− (Raven, 2001; Giordano et al., 2003). In C4 plants, CAs provide HCO3− to phosphoenolpyruate carboxylase to produce the C4 acid oxaloacetic acid. The cytosolic βCA in Flaveria bidentis was identified to function in, but is not rate limiting for, C4 photosynthesis as found in antisense suppression analyses (von Caemmerer et al., 2004). It has been suggested that CA activity in C4 plants is near rate limiting for photosynthesis (Hatch and Burnell, 1990; Cousins et al., 2008). A recent study in maize (Zea mays) showed that CAs are not limiting for C4 photosynthesis, at least at current ambient CO2 conditions, but become limiting at low CO2 concentrations in leaves (partial pressure of CO2 inside leaf [Ci]; Studer et al., 2014). Photosynthetic rates in maize ca1ca2 double-mutant plants were not reduced under current and elevated CO2 partial pressures but were impaired at low partial pressures (Studer et al., 2014). In C3 plants, the roles of CAs in limiting photosynthesis are less clear, but γCAs do have biological roles in mitochondrial physiology (Perales et al., 2005) and function in male sterility (Villarreal et al., 2009).
In addition to the roles of CAs in biochemical processes, recent research has shown that CAs function in animal CO2 signaling pathways. An αCA has been identified in mice as a CO2 perception mechanism controlling the olfactory response of guanylyl cyclase D neurons (GC-D+) to CO2 that triggers an avoidance behavior (Hu et al., 2007). An inhibitor of CAs reduces the ability of rodents to detect CO2, providing pharmacological evidence that CAs function as olfactory CO2 receptors (Ferris et al., 2007; Hu et al., 2007). It was found that CO2-induced action potentials occur in nerves that connect to taste receptors (sour sensing) cells in the mouse tongue (Chandrashekar et al., 2009). When taste receptor cells in which the carbonic anhydrase4 (Car4) is expressed at the surface were ablated in the mouse tongue, the response to CO2 disappeared, indicating that CA is an essential component of the CO2 response linked with sour taste (Chandrashekar et al., 2009).
In plants, CO2 signaling mechanisms control stomatal movements. Stomata in the epidermis of aerial tissues enable CO2 influx for photosynthesis (Cardon et al., 1994; DeLucia et al., 1999; Medlyn et al., 2001; Hetherington and Woodward, 2003). Guard cells that form stomatal pores have mechanisms to respond to an increase in the CO2 concentration in leaves (Ci), resulting in closing of stomatal pores. However, how elevated CO2 is transduced in guard cells remains less understood. Two βCAs, CA1 and CA4, were identified that function in the CO2 response (Hu et al., 2010). Arabidopsis (Arabidopsis thaliana) plants lacking these two enzymes displayed slowed stomatal responses to CO2 changes (Hu et al., 2010), and these impaired CO2 responses can be restored by expression of either βCA1, βCA4, or an unrelated human αCAII in guard cells using a strong guard cell promoter (Hu et al., 2010). These findings support the hypotheses that the enzymatic activities of βCA1 and βCA4 mediate the stomatal CO2 response and that these βCAs do not function as noncatalytic CO2 receptors. Additional electrophysiological analyses showed that intracellular HCO3− ions in guard cells lead to enhanced activation of guard cell anion channels and together with the above analyses, suggest that this stomatal CO2 response mediated by βCA1/βCA4 can be perceived directly by guard cells (Xue et al., 2011).
Additional findings identified GROWTH CONTROL BY ABSCISIC ACID2 (GCA2), OPEN STOMATA1 (OST1), and SLOW ANION CHANNEL-ASSOCIATED1 (SLAC1) as positive regulators (Young et al., 2006; Negi et al., 2008; Vahisalu et al., 2008; Xue et al., 2011) and the HIGH LEAF TEMPERATURE1 (HT1) kinase and a malate uptake transporter ATP-BINDING CASSETTE B14 (AtABCB14) as negative regulators (Hashimoto et al., 2006; Lee et al., 2008) in the high CO2-induced stomatal closing signaling pathway. A current molecular genetic and physiological model of this pathway was proposed: elevated CO2 accelerates the conversion by βCA1 and βCA4 into bicarbonate; elevated bicarbonate together with CO2 act as an intracellular messenger to activate S-type anion channels through OST1, thus triggering closure of stomata (Xue et al., 2011). Recently, the RESISTANT TO HIGH CARBON DIOXIDE1 (RHC1) MULTIDRUG AND TOXIC COMPOUND EXTRUSION (MATE) transmembrane protein was reported to function as an HCO3− sensing protein, interact with HT1, and release OST1 to phosphorylate SLAC1 channels (Tian et al., 2015). Beyond the function of βCA1 and βCA4 in CO2 control of stomatal movements, βCA1 and βCA4 were also identified as functioning in CO2 regulation of stomatal development together with the secreted EPIDERMAL PATTERNING FACTOR2 (EPF2) signaling peptide and a secreted protease CARBON DIOXIDE RESPONSE SECRETED PROTEASE (CRSP) in a distinct OST1-independent pathway (Engineer et al., 2014). Leaf epidermes of ca1ca4 plants exhibit an enhancement in stomatal density and index upon CO2 elevation compared with low CO2-grown plants, in contrast to wild-type plants.
Previous research has suggested that the enzymatic activity of βCA1 and βCA4 functions in CO2 control of stomatal movements (Hu et al., 2010; Xue et al., 2011). However, βCA1 and βCA4 were found at different cellular locations when expressed in tobacco (Nicotiana benthamiana) cells (Fabre et al., 2007; Hu et al., 2010), and which cellular location of these enzymes plays a key role in CO2-regulated stomatal movements and thus, the underlying mechanisms has remained unclear. Previous research did not use fluorescent protein fusion constructs to localize individual CAs in their native guard cells in complementation lines; therefore, the required cellular localizations of CAs for mutant complementation have remained unknown, and the relevance of these cellular locations remains unknown (Hu et al., 2010). It seems likely that overexpression in guard cells could have resulted in overlapping cellular localizations, and this might explain the complementation of stomatal conductance phenotypes by either CA alone.
In this study, we used yellow fluorescent protein (YFP) or GFP protein fusions to cellularly localize the individual CAs, βCA1 or βCA4, while in parallel, determining their effects on CO2-regulated stomatal responses. We report here that the βCA4 at the plasma membrane and βCA1 in chloroplasts each function in CO2 control of stomatal conductance changes. Together with combined targeting and complementation analyses with a nucleo/cytoplasmically localized mammalian αCAII expressed in guard cells and mathematical modeling of CO2 fluxes and catalysis by CAs in guard cells, the presented data suggest that the intracellular rate of bicarbonate concentration changes in guard cells is a key mechanism for CO2 control of stomatal movements.
RESULTS
Expression of YFP-Tagged βCA1 in Chloroplasts Rescues the Insensitive Stomatal Responses of ca1ca4 Plants to CO2 Changes
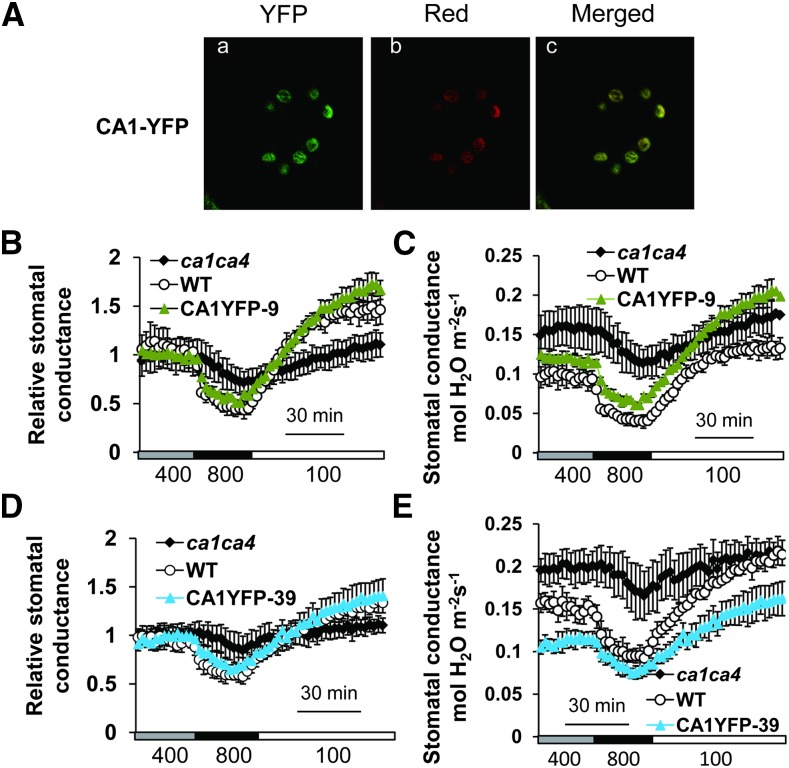
To characterize the cellular locations of βCA1 and βCA4 and gain insight into the sites of CO2 responsiveness in guard cells, we generated transgenic ca1ca4 plants expressing YFP fused to the C terminus of βCA1 or βCA4. These constructs were targeted to mature guard cells using the guard cell promoter pGC1 (Yang et al., 2008). Analyses of nonguard cells in leaves, including epidermal pavement cells and mesophyll cells, showed no clear YFP fluorescence for the YFP fusion lines used for further analyses. Confocal microscopy analyses showed that YFP-tagged CA1 was targeted to the chloroplasts of guard cells (Fig. 1A), but no clear signal was detected in other compartments and locations in guard cells in leaves from more than 200 plants.
Figure 1.
Chloroplast targeting of βCA1 restores CO2 responsiveness when expressed in ca1ca4 mutant guard cells. A, Confocal images showing CA1-YFP localization in chloroplasts of ca1ca4 guard cells of line CA1YFP-9. Aa, CA1-YFP fusion proteins. Ab, Red chloroplast autofluorescence. Ac, Merged images. B to E, Time-resolved stomatal conductance responses to [CO2] changes in two independent CA1-YFP-expressing lines in the ca1ca4 background together with the wild type (WT) and ca1ca4 mutant controls. Ambient CO2 concentrations are shown below traces in microliters per liter in all gas exchange figure panels. Absolute stomatal conductance data are shown in C and E, and their corresponding relative stomatal conductance data are shown in B and D. n = 4 for each genotype. Data represent mean ± sem.
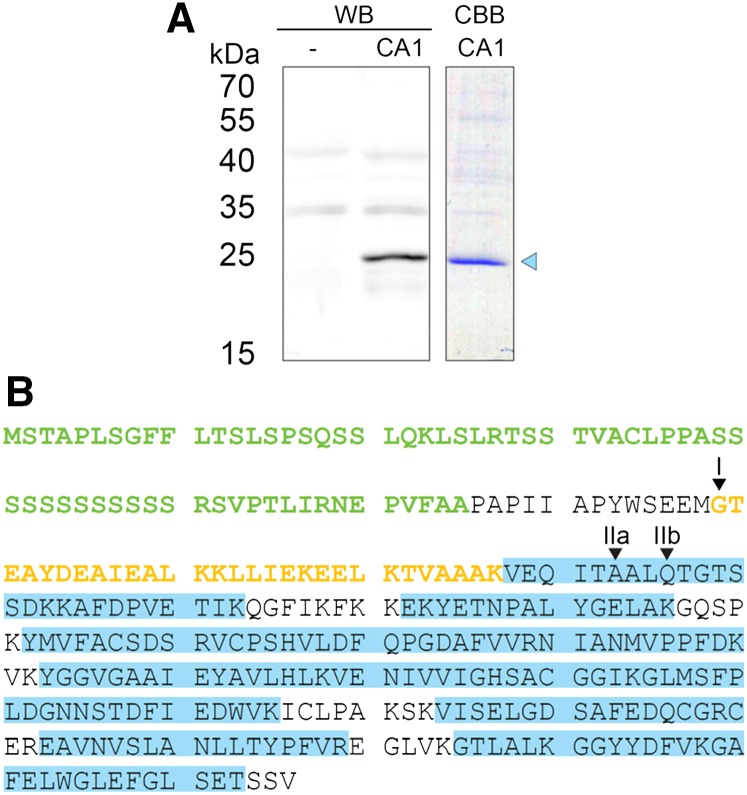
Previous research showed that βCA1 targeted mainly to chloroplasts upon expression in tobacco leaves (Fabre et al., 2007); however, a fraction of βCA1 expressed in tobacco leaves was also found at the plasma membrane (Hu et al., 2010). To further investigate this difference, we expressed different portions of βCA1 fused to YFPs in tobacco leaves. Biochemical studies of pea (Pisum sativum) CA had previously identified cleavage of the chloroplast targeting sequence at two different sites, resulting in two different protein lengths (Johansson and Forsman, 1992). The respective predicted cleavage sites are located before and after a highly charged region of the protein corresponding to amino acid 107 in βCA1 (Johansson and Forsman, 1992). We analyzed the protein sequences of βCA1 expressed in tobacco leaves by western blot and identified a prominent protein band with an apparent Mr of 25 kD (Fig. 2A). Additional analysis by tandem mass spectroscopy identified peptides starting from approximately amino acid 108. This indicates cleavage of βCA1 approximately upstream of amino acid 108 in a highly charged region consisting of seven acidic and five basic residues, which was similarly described for pea CA (Fig. 2B).
Figure 2.
Western-blot (WB) and tandem mass spectrometry analyses of βCA1 expressed in tobacco. A, WB of total protein extract from tobacco expressing p19-silencing inhibitor (lane 1) or p19-silencing inhibitor plus βCA1-StrepII (lane 2). βCA1-StrepII was purified from this extract and analyzed by Coomassie Brilliant Blue (CBB) staining (lane 3). The band representing cleaved βCA1-StrepII (blue triangle) was further analyzed by tandem mass spectrometry. B, Tandem mass spectrometry analysis of βCA1. The highly charged region at the N terminus is marked in yellow, and the chloroplast transit peptide is in green. Homologous sites to the cleavage site in pea CA are marked as I and II, with two different sites identified for II (Johansson and Forsman, 1992). Gels in A were run in separate experiments.
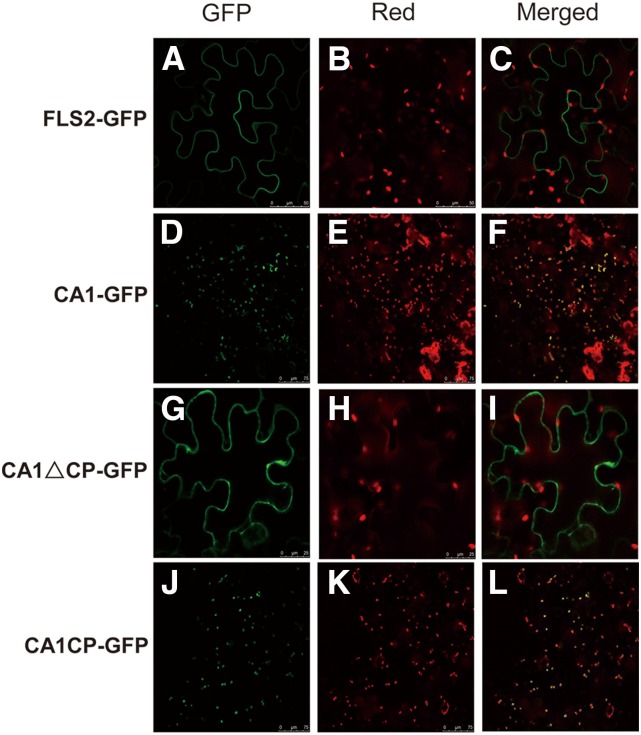
Microscopic analysis of YFP-tagged βCA1 showed that the N terminus spanning the first 65 amino acids of βCA1 containing the chloroplast peptide signal was targeted to chloroplasts, whereas expression of a truncated βCA1 without the first 65 amino acids (CA166–330) was localized in the vicinity of the plasma membrane or cytoplasm (Fig. 3). These data suggest that, in tobacco plants, βCA1 could be cleaved, which results in the partial cytoplasm/plasma membrane localization of βCA1 in this system (Hu et al., 2010). Expression in tobacco may thus lead to mistargeting of some of the cleaved βCA1 protein.
Figure 3.
Subcellular localization of GFP fused to different deletions of βCA1 in transiently transformed tobacco epidermes. CA1-GFP is the full-length βCA1 protein fused to GFP that localizes to chloroplasts. CA1ΔCP-GFP is the GFP-fused βCA1 protein with the first 65 amino acids truncated, which includes a sequence from the chloroplast signal peptide. CA1CP-GFP represents the first 65 amino acids only containing chloroplast signal peptide sequence of βCA1 fused to GFP that expresses in chloroplasts. FLAGELLIN-SENSING2 (FLS2) was used as a control plasma membrane protein. A, D, G, and J, GFP. B, E, H, and K, Chloroplast autofluorescence. C, F, I, and L, Merged images.
We investigated whether the YFP-tagged βCA1 protein expressed in native guard cell chloroplasts in ca1ca4 plants can complement the reduced CO2 response phenotype of ca1ca4 double-mutant plants. Gas exchange analyses in three independent transgenic lines showed complementation of the ca1ca4 double mutant (Fig. 1, B–E).
Expression of YFP-Tagged βCA4 at the Plasma Membrane Rescues the Insensitive Stomatal Responses of ca1ca4 to CO2 Changes
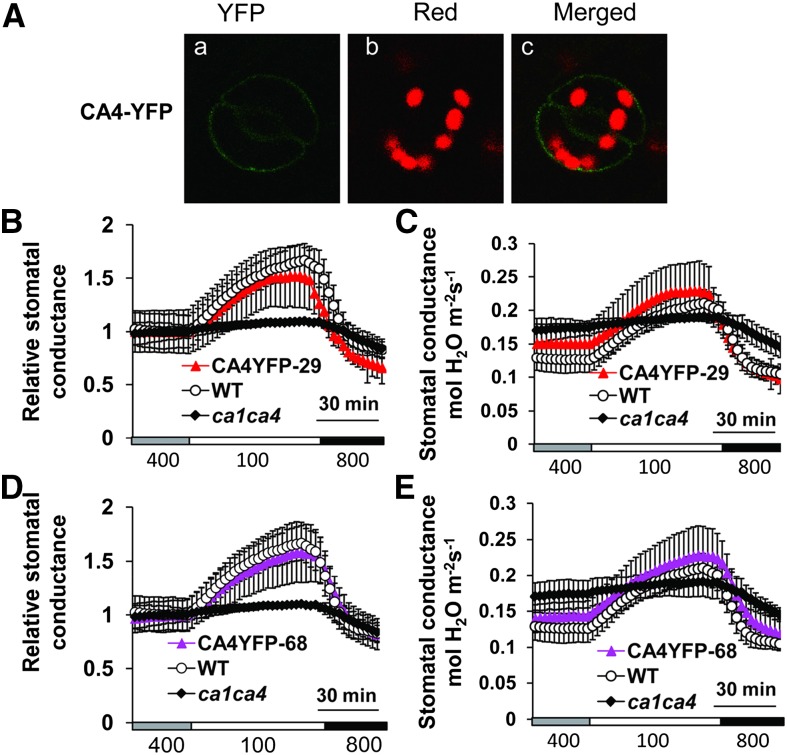
In contrast to CA1-YFP, YFP-tagged βCA4 was detected at the plasma membrane of ca1ca4 guard cells (Fig. 4A), consistent with transient expression in tobacco cells (Fabre et al., 2007; Hu et al., 2010). The CO2 responses of βCA4-YFP-expressing transgenic lines were also complemented by the guard cell plasma membrane-localized βCA4-YFP in three independent lines (Fig. 4, B–E). Interestingly, these findings suggest that the βCA1-YFP and βCA4-YFP fusion constructs are functional, although these βCAs have distinct cellular sites of activity. These results show that the localization of βCA4 at the plasma membrane is sufficient to mediate CO2-induced stomatal closing.
Figure 4.
Plasma membrane targeting of βCA4 restores CO2 responsiveness when expressed in ca1ca4 mutant guard cells. A, Confocal images showing CA4-YFP localization at the plasma membrane of ca1ca4 guard cells of line CA4YFP-68. Aa, YFP; Ab, red chlorophyll autofluorescence; Ac, merged image. B to E, Time-resolved stomatal conductance in two independent CA4-YFP-expressing ca1ca4 lines and control plants. Absolute stomatal conductance data are shown in B and D, and their corresponding relative stomatal conductance data are shown in C and E. n = 4 for each genotype. Data represent mean ± sem. WT, Wild type.
The above findings show that βCA1 and βCA4 localize to different cellular compartments in guard cells but that their expression regulates CO2-induced stomatal movements. We therefore investigated whether mistargeting of βCA4 to chloroplasts would result in functional CO2-regulated stomatal movements in ca1ca4 guard cells. For mistargeting βCA4 to chloroplasts, the chloroplast transit peptide comprising the 55 N-terminal residues of the CHLOROPLAST-LOCALIZED IRON-SULFUR CLUSTER ASSEMBLY-LIKE PROTEIN (CpIscA) protein (Abdel-Ghany et al., 2005) was fused to βCA4-GFP, and the construct was driven by the guard cell promoter pGC1 (Yang et al., 2008; Supplemental Fig. S1A). Confocal imaging analyses confirmed that βCA4-GFP was expressed in guard cell chloroplasts (Supplemental Fig. S1B). Three randomly selected independent transgenic ΔCplscA-CA4-GFP-expressing lines mistargeting βCA4 to chloroplasts in the ca1ca4 background were chosen for analyses of CO2 regulation of stomatal conductance. Notably, none of the investigated lines exhibited complete wild-type CO2 responses (Supplemental Fig. S1, C–H). An apparent putative average weak recovery of the CO2 response was observed in the ΔCplscA-CA4-GFP-12 line. However, this CO2 response was not significant for the first 30 min after CO2 was shifted to 100 μL L−1 (Supplemental Fig. S1G; P = 0.06 compared with the wild type and P = 0.88 compared with ca1ca4 plants).
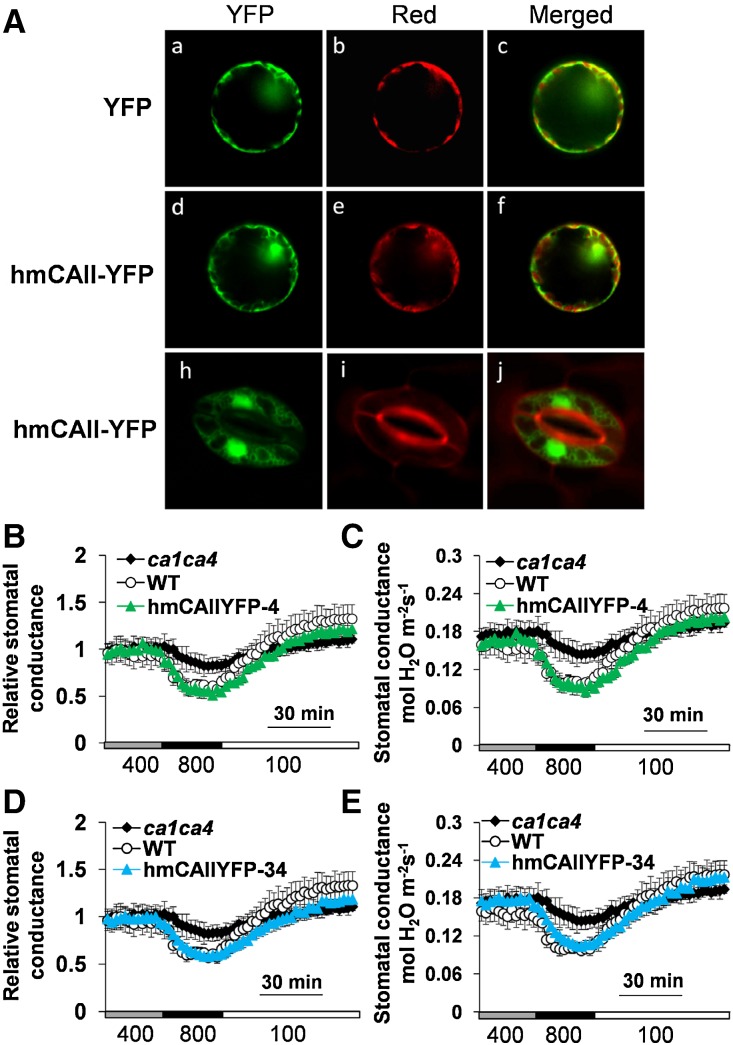
The Phenotype of ca1ca4 Plants Was Recovered by YFP-Tagged Human αCAII Expression in Cytosol and Nuclei
Previous data showed that a structurally unrelated human αCAII without YFP fusion also restored the stomatal response of ca1ca4 to [CO2] shifts (Hu et al., 2010). However, the cellular targeting of this unrelated αCAII was not resolved. We investigated the cellular targeting and in parallel, stomatal CO2 responses in plants expressing YFP-tagged human αCAII. First, we transiently expressed the human CAII-YFP fusion construct in tobacco epidermal cell protoplasts. YFP signals were detected in both cytoplasm and the nuclear region and did not overlap with chlorophyll fluorescence of chloroplasts (Fig. 5, Ad–Af). Second, the YFP-tagged human αCAII was expressed in ca1ca4 guard cells using the pGC1 promoter. The YFP-tagged human αCAII was detected in the cytoplasm and nucleus of guard cells (Fig. 5, Ah–Aj), similar to its expression in tobacco epidermal cells.
Figure 5.
YFP-tagged human αCAII in guard cell cytoplasm and nucleus rescues the CO2 responsiveness of the ca1ca4 double mutant. A, Subcellular localization analyses of human αCAII-YFP in transiently transformed tobacco epidermal protoplasts and stably transformed guard cells of ca1ca4 plants. Aa to Af, Confocal image analyses in tobacco epidermal protoplasts. Ah to Aj, Confocal images of human αCAII-YFP-expressing transgenic ca1ca4 guard cells stained with FM4-64 ([N-{3-triethylammoniumpropyl}-4-{6-(4-[diethylamino]phenyl)hexatrienyl}pyridinium dibromide]). Aa, Ad, and Ah, YFP. Ab and Ae, Red chloroplast autofluorescence. Ai, Red fluorescence by FM4-64. Ac, Af, and Aj, Merged images. B to E, Time-resolved relative stomatal conductance in two independent human CAII-YFP-expressing ca1ca4 lines and control plants. Absolute stomatal conductance data are shown in C and E, and their corresponding relative stomatal conductance responses are shown in B and F. n = 4 for each genotype. Data represent mean ± sem. WT, Wild type.
Stomatal conductance changes in response to CO2 changes were determined in randomly selected human αCAII-YFP-expressing ca1ca4 transgenic lines. Notably, human αCAII-YFP expression also rescued the stomatal responses to CO2 changes of ca1ca4 mutant plants (Fig. 5, B–E). These data suggest that the activities of human αCAII-YFP fusion proteins in the cytoplasm and nucleus are sufficient for regulation of stomatal CO2 responses. Remarkably, together, all of these findings show that the investigated CAs are targeted to distinct cellular sites but retain, each one separately, the ability to complement the ca1ca4 double mutant.
Compartmental Model for CO2 Influx in Guard Cells Supports the Function of CAs in CO2 Regulation of Stomatal Movements
To explore the above findings and the potential signaling components in CO2 regulation of the stomatal closing pathway, we constructed a simplified model for the catalytic activity of CAs targeted to different compartments. We developed a compartmental mathematical model using parameters from a recent study on CO2 influx into Xenopus laevis oocytes (Somersalo et al., 2012), which was modified to accommodate the much smaller cell size of guard cells. This model describes the interconversion of CO2, HCO3−, and protons. Initially, we modeled the guard cell as a single compartment with spatially uniform concentration of reactants. This simplification was chosen given the small size of guard cells (approximately 10 μm) together with the large diffusion constants of the reactants CO2 and HCO3−. We incorporated CA-like activity by multiplying the rate constants k1 and k−1 (equations in “Materials and Methods”) by an acceleration factor A as described in the work by Somersalo et al. (2012). The starting condition for the simulation was at a CO2 concentration (Ci) of 200 μL L−1 for both the leaf and the intracellular fluid (ICF), consistent with directly measured Ci values in leaves during the light period (Hanstein et al., 2001). A change of Ci to 800 μL L−1, similar to leaves exposed to darkness (Hanstein et al., 2001), results in an influx of CO2 into the cell, such that the internal CO2 concentration rapidly approaches the external value. The simulated intracellular HCO3− concentration was plotted as a function of time in the presence and absence of CAs (Somersalo et al., 2012). In the presence of CAs, an acceleration factor of A = 5 was assumed for CO2 dissociation in the cell (Somersalo et al., 2012; Fig. 6A, black curve; A = 5, with CA activity). In the absence of CA activity (A = 1), the predicted increase in the intracellular bicarbonate concentration occurred on a substantially slowed timescale (Fig. 6A, red curve; A = 1, without CA). Clearly, the presence of CAs (A > 1) changes the time course of the predicted bicarbonate concentration increase (Fig. 6A). Because elevated intracellular bicarbonate elevation causes stomatal closure and activates S-type anion channels in guard cells (Hu et al., 2010; Xue et al., 2011), the predicted time courses of [bicarbonate] change correlate with the slowed stomatal response to [CO2] shifts in ca1ca4 mutant plants. Note also that the steady-state value of the system is independent of the acceleration factor and thus, eventually, stomata may be predicted to be close to a similar level in ca1ca4 mutant plants, albeit at a slower rate. This result can be explained by noting that, in a theoretical system that behaves as a closed system to all solutes other than CO2, the CO2/HCO3− buffering power in the steady state does not depend on the presence of CA. Thus, the results of our simulations could potentially explain the phenotype of stomatal movements observed in ca1ca4 guard cells (Hu et al., 2010).
Figure 6.
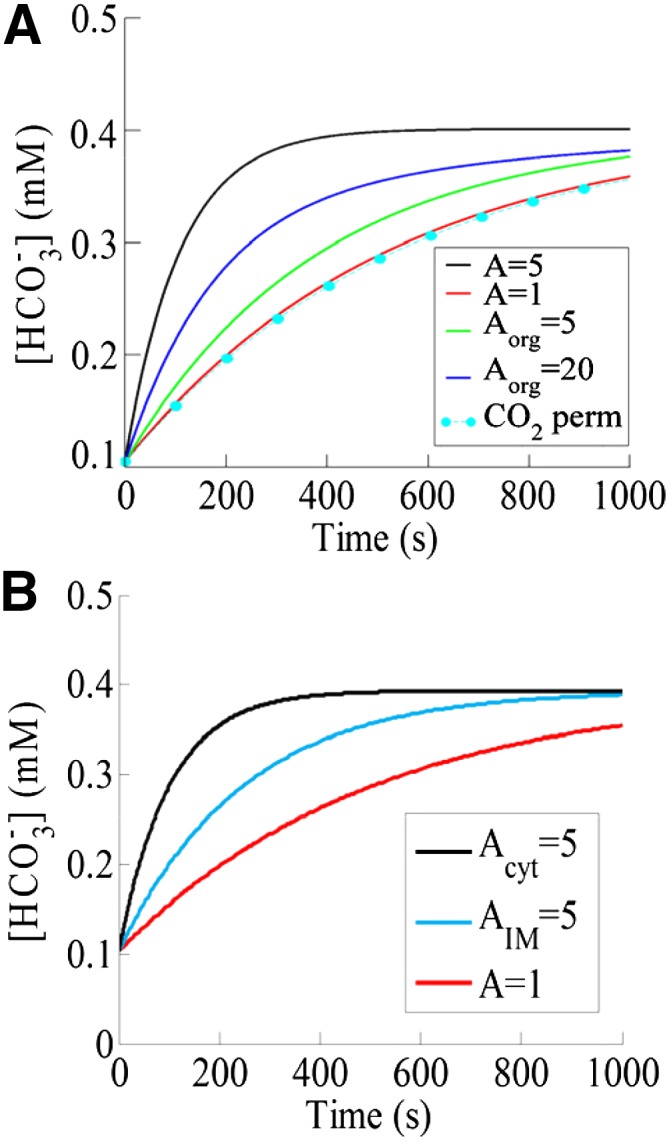
Computer simulations of HCO3− dynamics. A, Simulated intracellular HCO3− concentration as a function of time with CA activity (black curve) and without CA activity (red curve) in a compartmental model of a single guard cell. The increase in external CO2 from 200 to 800 μL L−1 at time zero and the prior equilibrated internal (200 μL L−1) CO2 concentration at time zero lead to an influx of CO2 into the cell. The presence of CAs results in faster dynamics of intracellular HCO3− concentration changes. Also shown is the simulated HCO3− concentration in the cytosol in a two-compartment model that includes an organelle (i.e. chloroplast) with two different CA-like activities (green curve, Aorg = 5; and blue curve, Aorg = 20) that is permeable to all components. The HCO3− dynamics for an organellar membrane (Aorg = 20 and A = 1) that is only permeable to CO2 are shown as cyan symbols (CO2 perm). B, Simulated HCO3− concentration as a function of time using a spatial reaction-diffusion model of a cell. Shown are the dynamics with CA-like activity (A = 5) implemented everywhere in the cytosol (black curve), at the inner leaflet of the plasma membrane (cyan curve), and without any CA activity (red curve; A = 1).
To determine the effects of CA localization for driving intracellular bicarbonate concentration changes, we have also simulated a small internal compartment representing an organelle, such as the chloroplast, within a larger compartment that corresponds to the guard cell. The acceleration factor in the guard cell’s cytosol of ca1ca4 mutant plants was taken to be one, whereas within the organelle, Aorg was varied. Our simulations revealed that, for a chloroplast with an inner envelope membrane that does not permit substantial HCO3− release from the organelle back into the cytoplasm, the time course of cytosolic HCO3− concentration is largely unaffected (not accelerated) by the presence of organellar CA (Fig. 6A, cyan). The slight reduction in cytosolic HCO3− compared with A = 1 (Fig. 6A) can be understood by realizing that, because of the organellar CA, the chloroplast acts as a small sink for cytosolic CO2, resulting in a marginally smaller cytosolic CO2 and thus, a slightly slower production of cytosolic HCO3−.
Even assuming a chloroplast inner envelope membrane that is permeable to all components, including HCO3− flux from the chloroplast into the cytoplasm and choosing Aorg = 5 resulted in cytosolic HCO3− dynamics that are considerably slower than those in our single-compartment model (compare the black and green curves in Fig. 6A). However, increasing the value of the organellar acceleration factor Aorg is equivalent to choosing a CA with a larger activity in the organelle and will speed up this dynamics. We have verified this through explicit simulations that the predicted time course of HCO3− can be made similar to the one obtained using a single compartment with A = 5, if the organellar CA has a larger activity (blue curve in Fig. 6A; Aorg = 20). These predictions may explain our experimental findings that the mistargeting of βCA4 to chloroplasts cannot complement the insensitive stomatal responses to CO2 changes of the ca1ca4 mutant (Supplemental Fig. S1), whereas at the plasma membrane, the βCA4 activity is sufficient. Furthermore, because it seems unlikely that the chloroplast membrane permits large rates of HCO3− or H2CO3 efflux from the chloroplast back to the cytoplasm, it also suggests that the role of organellar βCA1 might be more complex than simulated in our simple compartmental model (Fig. 6A; Aorg = 5 or 20). This view is also supported by our additional simplified simulation assuming no efflux of HCO3− from chloroplasts to the cytoplasm (Fig. 6A, cyan) and that chloroplasts have an important, yet to be identified contribution to CO2 regulation of stomatal movements (see “Discussion”).
Reaction-Diffusion Model of CO2 Influx into Guard Cells Can Explain the Effect of CA Localization for Regulation of Stomatal Movements
To investigate further the effect of CA localization for driving intracellular HCO3− concentration changes, we used a distinct full spatial reaction-diffusion mathematical model, as reported by Somersalo et al. (2012), appropriately modified to represent a single cell of guard cell dimension surrounded by the bulk extracellular cell wall space (for details, see “Materials and Methods”). Unlike the simplified compartmental model described above, this model accounts for spatial variability and allows for the implementation of CA-like activity at the inner leaflet of the plasma membrane. As before, we allowed the system to reach equilibrium at a low Ci and then increased the CO2 concentration as described in “Materials and Methods.” Figure 6B illustrates the predicted intracellular HCO3− concentration as a function of time for a simulation in which CA activity (Acyt = 5) is implemented everywhere inside the cell (Fig. 6B, black curve; cytoplasm) or only at the plasma membrane (Fig. 6B, cyan curve; AIM = 5). As a control, we have also plotted the time course of the HCO3− concentration for a simulation in which no CA-like activity is implemented (A = 1; red curve in Fig. 6B). Note that each curve corresponds to the concentration at a depth of approximately 5 µm into the cell. We have verified that this spatial model predicts virtually identical dynamics of HCO3− concentration changes at any depth into the cell. This can be explained because of the small size of guard cells and the large diffusion rates of the reactants inside the cell (see above).
Consistent with the single-compartment model of a guard cell (Fig. 6A), in the absence of CA-like activity, intracellular HCO3− concentration rises very slowly, whereas in the presence of CA-like activity, the production of HCO3− occurs at a faster rate and therefore, results in faster dynamics of HCO3− concentration changes. When CA-like activity is implemented everywhere in the cytosol (Acyt = 5; black curve in Fig. 6B), the rise in HCO3− concentration is faster than when CA-like activity is implemented only at the inner leaflet of the plasma membrane (Fig. 6B, cyan curve; AIM = 5). This difference in rates of HCO3− concentration changes predicted in this simplified model occurs, because when CA-like activity is implemented everywhere in the cell, there is a rapid consumption of incoming CO2 at every location within the cell, whereas when CA-like activity is implemented only at the inner leaflet of the plasma membrane, this conversion occurs only near the plasma membrane. Nevertheless, the HCO3− concentration in the presence of membrane-bound CAs increases faster than in the absence of any CA-like activity. Moreover, this increase could be even faster by assuming an even larger CA activity at the membrane.
One prediction of the above model, assuming that HCO3− is the intracellular signal that transmits the CO2 response (Xue et al., 2011; Tian et al., 2015), is that longer exposures to elevated CO2 should enable stomata of ca1ca4 mutant plants to close to similar levels as wild-type plants but at a slowed rate. Because the rate of stomatal closing was found previously to be slowed in ca1ca4 mutant plants (Hu et al., 2010), we conducted experiments in which prolonged elevated CO2 concentrations were applied. The stomatal conductance of ca1ca4 mutant plants was reduced to a similar level compared with wild-type plants when CO2 was increased from 400 to 800 μL L−1 for more than 90 min under the imposed conditions (Fig. 7). These findings also point to secondary compensatory feedback mechanisms that may strengthen the response to CO2 shifts in ca1ca4 plants due to the enhanced stomatal conductance (Hu et al., 2010; Engineer et al., 2014). These experiments suggest that, if the starting CO2 concentration is 400 μL L−1 and the CO2 concentration is rapidly increased to 800 μL L−1 for prolonged exposures, stomata eventually can close to similar or even lower levels than wild-type plants.
Figure 7.
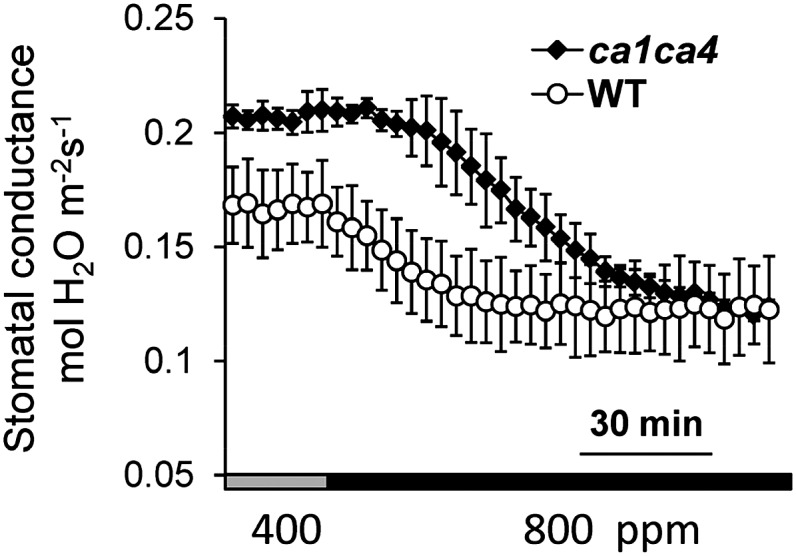
Stomatal conductance of ca1ca4 plants to a stepped increase and long exposure to high CO2. Stomatal conductance analyses of ca1ca4 and wild-type (WT) plants in response to a step to high CO2 for a long exposure (120 min). n = 4 leaves for each genotype.
DISCUSSION
This study investigates the cellular localizations of CAs in guard cells and the roles that these distinct CA enzymes play in regulating rapid stomatal conductance changes in response to CO2 concentration changes. Interestingly, use of fluorescently tagged CAs here shows that targeting of either native βCA4 to the plasma membrane or βCA1 to the chloroplast is sufficient for recovering CO2 responses (Fig. 1). However, when ΔCplscA-CA4-GFP was experimentally targeted to chloroplasts, the stomatal CO2 response was not restored (Supplemental Fig. S1). It is conceivable that the precise targeting within chloroplast compartments of CAs is essential or that the CA activity of this fusion protein was diminished. Consistent with these results, previous membrane proteomic studies in Arabidopsis (Froehlich et al., 2003; Kawamura and Uemura, 2003) and transient expression in tobacco (Fabre et al., 2007; Hu et al., 2010) indicated that βCA1 is expressed in chloroplasts, with a small fraction of cleaved βCA1 protein detected at the plasma membrane or in the cytoplasm upon transient overexpression in tobacco leaves (Figs. 2 and 3), and that βCA4 localizes at the plasma membrane. The mechanism mediating βCA4 localization at the plasma membrane will require further research. Here, we show that both of these distinct cellular locations are sufficient to restore CO2 signal transduction.
The findings that ca1ca4 double-mutant leaves produce a considerably slowed CO2 response in rapid CO2-controlled stomatal movements together with complementation of this phenotype for either of the differential native localizations of the βCA1 and βCA4 (Figs. 1 and 4) suggest relevant and unique roles for βCA1 and βCA4 in the CO2 response. The two mathematical models applied here may explain these unexpected findings. Active CAs modeled in a small cell of guard cell dimensions are predicted to accelerate the bicarbonate dynamics in the cytosol and accelerate CO2 flux into guard cells (Fig. 6), thus inducing stomatal movement responses. Furthermore, modeling of CA activity at the plasma membrane or in the cytosol suggests that either activity should be able to accelerate increases in HCO3− concentration in guard cells (Fig. 6B). It is interesting that plasma membrane and chloroplast overexpression localizations both complement the mutant. The simplified models thus predict that the intermediate rates of HCO3− production at the plasma membrane relative to a bulk cytoplasmic location are sufficient for the biological response. The prediction that HCO3− increases are not highly sensitive to the precise location of CAs, at the plasma membrane or in the cytoplasm, is consistent with the small dimensions of guard cells and the rapid diffusion rates of bicarbonate in solution. Any spatial separation of reactants at the inner leaflet of the plasma membrane or in the bulk cytosol will thus equilibrate rapidly. These predictions correlate with the complementation data found here for distinct cellular localizations of CAs at the plasma membrane (Fig. 4) or in the cytoplasm (Fig. 5) and would support a model in which the intracellular bicarbonate concentration is a mediator of stomatal closing.
Mathematical modeling also predicted that, after long CO2 exposures, wild-type and CA mutant stomata should reach similar HCO3− concentrations and thus, possibly stomatal apertures, albeit at slower rates in CA mutant leaves. When steps to 800 μL L−1 were applied for 120 min, stomatal conductance of both genotypes reached similar levels (Fig. 7). This was unexpected, because ca1ca4 leaves have a higher stomatal density and index than wild-type leaves, including in mature leaves (Engineer et al., 2014) used here for gas exchange analyses. Thus, findings from long CO2 pulses further indicate that the response to CO2 steps maybe partially up-regulated in ca1ca4 mutants as a secondary compensatory feedback mechanism in ca1ca4 mutant plants. A compensatory response of stomatal conductance was recently identified in slac1 mutants (Laanemets et al., 2013). Additional research will be needed to investigate possible secondary compensatory feedback response mechanisms in stomatal movement mutants.
Additional mathematical modeling suggests that CAs confined to small intracellular organelles, such as chloroplasts, are not as effective in accelerating the dynamics of cytoplasmic [HCO3−] as those localized either at the inner leaflet of the plasma membrane or in the cytosol (Fig. 6A). This principle may partially explain why βCA4 expressed in chloroplasts could not complement the CO2-insensitive stomatal responses in ca1ca4 mutant leaves, whereas βCA4 at the plasma membrane could. Chloroplastic βCA1 activity may be larger than mistargeted chloroplast βCA4 activity. Analysis of biochemical CA activities in the wild type, ca1 and ca4 single-mutant plants, and ca1ca4 double-mutant plants has previously shown that βCA1 accounts for 80% of the catalytic CA activity in planta (Hu et al., 2010). In contrast, βCA4 mutation had a limited effect on the overall catalytic CA activity in planta (Hu et al., 2010). Thus, the complementation by the chloroplast-targeted βCA1 is presumably because of some combination of a higher catalytic activity or a higher protein density compared with βCA4. Note also that we may have mistargeted βCA4 to the improper chloroplast compartment.
The simplified intracellular compartment modeling pursued here indicates that the function of βCA1 in chloroplasts may not simply be to accelerate the rate of cytosolic [HCO3−] increase at elevated CO2. Furthermore, chloroplasts are expected not to easily permit substantial rates of release of bicarbonate from the chloroplast stroma back into the cytoplasm in the presence of a highly active CA, such as βCA1. This is exemplified in the extreme (boundary condition) model assuming that the inner chloroplast envelope membrane does not permit HCO3− release (Fig. 6A, cyan). Thus, an additional yet to be identified chloroplast function of βCA1 is predicted to be required in the rapid CO2 control of stomatal movements.
This study does not exclude an additional important function of βCA1 in chloroplasts in addition to a possible contribution to mediating acceleration of the dynamics of intracellular HCO3− concentration changes in guard cells. Chloroplast reactions and a chloroplast HCO3−-CO2 sink may contribute to the CO2 responses by enhancing the plasma membrane cellular influx of CO2-HCO3− into guard cells, and additional research will be needed to investigate putative chloroplast contributions to the CO2 response. Note that guard cell chloroplast functions during CO2 control of stomatal movements may be independent of Rubisco (von Caemmerer et al., 2004) and are not dependent on guard cell photosynthesis (Azoulay-Shemer et al., 2015). Guard cell chloroplasts could function in malate-starch interconversion during stomatal movements (Outlaw and Manchester, 1979).
Recent findings in maize show that ZmCA1 and ZmCA2 are not limiting for photosynthesis at current ambient CO2 concentrations, but these cytoplasm-targeted CAs function in CO2 flux and maintenance of high rates of photosynthesis when CO2 availability is limiting (Studer et al., 2014). Targeting of the highly active human αCAII (Kern et al., 1995; Hu et al., 2010) is sufficient to restore CO2 control of stomatal conductance, despite being targeted to the guard cell cytoplasm (Fig. 5). These findings are consistent with the CO2-HCO3− model, because the cytoplasmic CA activity would be predicted to cause an accelerated increase in the HCO3− concentration in the cytoplasm (Fig. 6), which in turn, is known to be required for high CO2-activated S-type anion channels in guard cells (Xue et al., 2011; Tian et al., 2015). Furthermore, a large cytoplasmic human αCAII activity may also enhance the flux of CO2-HCO3− across the plasma membrane to chloroplasts and other possible intracellular CO2-HCO3− sinks.
In summary, this study shows that the cellular location of three distinct CA proteins differs in guard cells, at the plasma membrane, in the cytoplasm, and in chloroplasts, but interestingly, these three locations are sufficient for mediating CO2 control of gas exchange in plants. Mathematical modeling suggests that CA-induced acceleration of changes in the intracellular bicarbonate concentration rather than cellular CO2-HCO3− flux alone is an important mechanism for CO2 control of stomatal movements and that this mechanism can be achieved by distinct cellular locations of CAs. An additional mathematical model indicates that guard cell chloroplasts have an additional yet to be identified function in CO2 control of stomatal movements that will require further investigation.
MATERIALS AND METHODS
Plant Growth and Genotypes
Arabidopsis (Arabidopsis thaliana) plants used in this study were Columbia-0 ecotype and grown in a Conviron Growth Chamber at 21°C with 60% to 80% humidity and a 16-h-light/8-h-dark photoperiod regime at approximately 75 µmol m−2 s−1. The isolation of the βCA ca1ca4 double mutant was as described in the work by Hu et al. (2010).
YFP-Tagged CA Constructs and Expression in ca1ca4
For YFP-tagged expression of βCA1, βCA4, and human CAII, complementary DNAs in guard cells of ca1ca4, βCA1, βCA4, and human CAII were amplified and recombined into the binary vector pXC27G-YFP, which was derived from pXCSG-YFP (Feys et al., 2005). The 35S promoter was replaced by the mature guard cell-preferential promoter pGC1 (Yang et al., 2008). βCA1 was amplified by primers CA1F (5′-ggggacaagtttgtacaaaaaagcaggctatagtagcttccataagagtc-3′) and CA1R (5′-ggggaccactttgtacaagaaagctgggtcaagtccccaaagctcaaa-3′). βCA4 was amplified by primers CA4F (5′-ggggacaagtttgtacaaaaaagcaggctaatggctcctgcattcgg-3′) and CA4R (5′-ggggaccactttgtacaagaaagctgggtaggcaaaagcaggagtg-3′). Human CAII was amplified by primers CAIIF (5′-ggggacaagtttgtacaaaaaagcaggctcgaccatgtcccatcact-3′) and CAIIR (5′-ggggaccactttgtacaagaaagctgggttttgcctgttcttcagtg-3′) from the complementary DNA clone SC107902 (OriGENE; Hu et al., 2010). For targeting CA4 to guard cell chloroplasts in ca1ca4 mutant plants, the construct ΔCpIscA-CA4-GFP in a modified pGreen vector driven by the mature guard cell-preferential promoter pGC1 (Yang et al., 2008) was created. CA4 was amplified by primers CA4F2 (5′-aactgcagaatggctcctgcattcgg-3′; with PstI adapter) and CA4R2 (5′-aaggatccaggcaaaagcaggagtg-3′; with BamHI adapter) and fused to GFP in the vector pGreen-pGC1-GFP at the sites of PstI and BamHI to create the construct pGreen-pGC1-CA4-GFP. ΔCpIscA, the 55 N-terminal amino acids of the chloroplast-localized CpIscA protein (Abdel-Ghany et al., 2005), was amplified by ΔCpIscAF (5′-aaccatggcgcatttgaatcagcaga-3′; with NcoI adapter) and ΔCpIscAR (5′-aactgcaggaagcggatcgaacagaaa-3′; with PstI adapter) and fused to CA4-GFP at the sites of NcoI and PstI in the construct. Constructs generated were transformed into ca1ca4 plants by the floral dipping method (Clough and Bent, 1998).
Subcellular Localization Analyses of YFP-Tagged CA Proteins
To analyze the localization of different portions of βCA1 in the tobacco (Nicotiana benthamiana) epidermis, the full length of βCA1 was amplified by the primers CA1-F (5′-ggggacaagtttgtacaaaaaagcaggctatgtcgaccgctcctctctc-3′) and CA1-R (5′-ggggaccactttgtacaagaaagctgggtacagcttccaatgtagtatggtagcc-3′) and fused to GFP in the vector barII_pUBQ10_GFPc-GWB (provided by Jorg Kudla) as CA1-GFP. CA1ΔCP-GFP is the βCA1 protein with the first 65 amino acids deleted. It is amplified by CA1 (NDel)-F (5′-ggggacaagtttgtacaaaaaagcaggctcctgctcctatcattgcccc-3′) and CA1-R and fused to GFP; CA1CP-GFP represents the first 65 amino acids of βCA1 amplified by CA1-F and CA1-R(Del) (5′-ggggaccactttgtacaagaaagctgggtaagcggcaaaaactggctcgtt-3′) fused to GFP. To transiently express human CAII-YFP in the tobacco epidermis, the human CAII amplified by primers CAIIF2 and CAIIR2 was fused to YFP in the vector construct pXCSG-YFP (Feys et al., 2005). The pH35YG vector containing 35S::YFP was used as a positive control for cytoplasmic and nuclear localizations (Kubo et al., 2005). FLAGELLIN-SENSING2 is used as a control of membrane protein. Protoplasts were isolated from infiltrated leaves as described previously (Hu et al., 2010). For the localization of YFP-tagged CA1 and CA4 in ca1ca4 guard cells, the leaf epidermal layers were directly imaged for YFP fluorescence using confocal microscopy. For YFP-tagged human CAII in ca1ca4 guard cells, the leaf epidermal layers were first stained with FM4-64 for 10 min. Fluorescence imaging was acquired by spinning-disc confocal microscopy with an electron-multiplying CCD camera (CascadeII: 512; Photometrics) using Metamorph software (Universal Imaging).
Tandem Mass Spectrometry Analysis of βCA1
Transient transformation of 4-week-old tobacco plants and purification of βCA1-StrepII were performed as described (Witte et al., 2004). For SDS-PAGE, one volume of washed Macroprep beads were resuspended in one volume of 2× SDS-gel loading buffer and heated to 90°C for 6 min. Proteins were separated by SDS-PAGE and analyzed by Strep Tactin-alkaline phosphatase conjugate (IBA) as described (Witte et al., 2004). Tryptic digestion of StrepII-purified proteins was performed in gel. Proteins were reduced with 10 mm dithiothreitol at 56°C for 1 h and alkylated with 55 mm iodoacetamide at room temperature for 45 min. Proteins were digested using 0.4 µg of Trypsin Singles (Sigma Aldrich) at room temperature overnight. Digests were desalted for electrospray mass spectrometry with a C18 Reverse-Phase ZipTip Resin (Millipore). A QSTAR Elite Quadrupole-TOF Mass Spectrometer equipped with a Nano-Spray Source and nanoLC System (AB Sciex) was used for analysis. Resulting wiff files were converted into mzXML format and analyzed using PEAKS Studio 7.0. Database search was performed against The Arabidopsis Information Resource Database (TAIR10_pep_20101214) with a 0.1% false discovery rate cutoff. Carbamidomethylation was set as a fixed modification, and phosphorylation and Met oxidation were selected as variable modifications. Additional variable modifications were identified by PEAKS PTM software (Bioinformatics Solutions Inc.). Fragment ion tolerance was set to 0.1 D, and variable modifications per peptide were limited to three.
Time-Resolved Intact Leaf Gas Exchange Analyses
Gas exchange analyses were performed on rosette leaves from 5-week-old plants in response to the imposed leaf [CO2] changes at 150-mmol m−2 s−1 light (Parabolic Aluminum Reflector) fluence rate using an Li6400XT Gas Exchange Analyzer fitted with a fluorimeter chamber (Li-Cor Inc.) as described in Hu et al., 2010. Stomatal conductance was first stabilized at ambient [CO2] (400 μL L−1) for 30 min; then, unless otherwise noted in the figures, [CO2] was shifted to a high concentration (800 μL L−1) for 30, 60, or 90 min and again changed to a low concentration (100 μL L−1) for at least 30 min. Alternately, [CO2] was held at the ambient CO2 concentration for at least 30 min, shifted to a low concentration (100 μL L−1), and then changed to a high concentration for indicated times noted in the figures. The data presented are means of at least three leaves per genotype per treatment ± sem. Relative stomatal conductance values were determined by normalization relative to the average of 10 data points preceding the [CO2] transitions (400–800 μL L−1 or 400–100 μL L−1).
Compartmental Model for CO2 Influx in Guard Cells
A simplified model in guard cells for CO2 catalysis was constructed by modification of a model examining CO2 influx in oocytes (Somersalo et al., 2012). The model describes the formation and dissociation of carbonic acid as
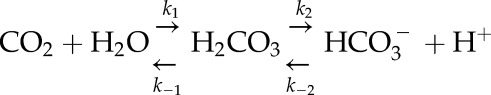 |
along with a reaction describing a non-CO2/HCO3− buffer
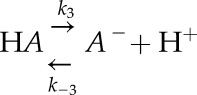 |
where k1, k−1, k2, k−2, k3, and k−3 are rate constants. We incorporated CA-like activity by multiplying the rate constants k1 and k−1 by an acceleration factor A (Somersalo et al., 2012). We assumed that the membrane is permeable to CO2 with a permeability constant Pm. The rate equations corresponding to the above reactions can be easily obtained. For example, for the CO2 concentration, we have
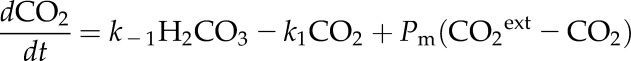 |
where CO2ext is the external CO2 concentration. The resulting set of equations was solved using an algorithm suitable for stiff ordinary differential equations using parameter values in Somersalo et al., 2012. As initial conditions, the initial internal and external CO2 concentration were set to 200 μL L−1, similar to CO2 conditions measured in leaves exposed to light (Hanstein et al., 2001), whereas the internal and external pH levels were taken to be 7.4 and 6, respectively. Finally, the acceleration factor caused by CA activity was chosen to be five (Somersalo et al., 2012). At the start of the simulation, the external CO2 concentration was set to 800 μL L−1. For determining the effects of CA localization, a small internal compartment representing an organelle, such as the chloroplast taking up 10% of the cell volume within a larger compartment that corresponds to the guard cell, was simulated. The organelle membrane was modeled as being permeable to either all components or exclusively CO2 using the same permeability constants as for the cell membrane. The acceleration factor within the compartment representing the ca1ca4 mutant guard cell’s cytosol was taken to be one, whereas this acceleration within the organelle, Aorg, was varied.
Reaction-Diffusion Model of CO2 Influx into Guard Cells
As a starting point, we used the above-mentioned reaction-diffusion model of CO2 influx into a Xenopus laevis oocyte developed by Somersalo et al. (2012). This model assumes that the cell is a perfectly symmetric sphere surrounded by a thin layer of extracellular unconvected fluid (EUF), which in turn, is surrounded by the bulk extracellular fluid (BECF). In both EUF and ICF, the model accounts for the CO2/HCO3− equilibrium, including the slow conversion of CO2 into H2CO3 and vice versa, and a multitude of non-CO2/HCO3− buffers. Solutes can diffuse within the EUF and ICF. The plasma membrane is infinitely thin and permeable to CO2. The EUF, ICF, and the plasma membrane have properties of pure water. Mathematically, the changes of solute concentrations in time and space are described by a coupled system of partial differential equations, which are solved assuming spherical radial symmetry (i.e. solute concentrations depend only on the radial distance from the center of the cell). Numerically, the system of partial differential equations is solved using the method of lines with appropriate spatial discretization schemes, and the resulting system of ordinary differential equations is solved using the MATLAB stiff ode solver, ode15s. The details of the numerical implementation are provided in Somersalo et al., 2012. Here, we explain how we modified this model to account for guard cell dimensions and the experimental conditions in this study. We assumed that the guard cell is a sphere with a radius of 10 µm surrounded by a 5-µm-thick EUF. The EUF represents the aqueous solution in the space in the cell walls surrounding the guard cell. We assumed that the EUF contains only the CO2/HCO3− buffer system but that the ICF contains both a CO2/HCO3− buffer system and a single non-CO2/HCO3− buffer. For diffusion constants, rate constants, and the properties of the intracellular non-CO2/HCO3− buffer system, we used the values reported in Somersalo et al., 2012. As done in the work by Somersalo et al. (2012), we implemented CA-like activity by multiplying the rate constants k+1 and k−1 by the same acceleration factor A.
We performed each of the three simulations illustrated in Figure 6B in two steps. First, we exposed the guard cell ([CO2]ICF = 0, pHICF 7.40) to a BECF-containing equilibrated CO2/HCO3− ([CO2]BECF = 200 μL L−1, pHBECF 6.00) and waited for the system to achieve equilibrium (i.e. [CO2]ICF = [CO2]BECF = 200 μL L−1). We ran this first simulation for 10,000 s (data not shown). Second, we used the final state of this first simulation to define the intracellular composition of the guard cell and ran a second simulation, in which we shifted [CO2]BECF from 200 to 800 μL L−1 and pHBECF to 6.00. Figure 6B shows the second of these simulations.
Statistical Analyses
Data are represented as mean ± sem unless otherwise noted. Comparisons between continuous variables were performed using the Student’s t test with a two-tailed distribution and two-sample equal variance.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AT3G01500 for CA1 and AT1G70410 for CA4.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Chloroplast mistargeted ΔCpIscA-CA4-GFP does not complement CO2 responsiveness of the ca1ca4 double mutant.
Supplementary Material
Glossary
- BECF
bulk extracellular fluid
- Ci
partial pressure of CO2 inside leaf
- EUF
extracellular unconvected fluid
- ICF
intracellular fluid
Footnotes
This work was supported by the National Science Foundation (grant no. MCB1414339 to J.I.S. and W.J.R.). Chloroplast targeting analyses of CAs were funded by the Division of Chemical, Geo, and Biosciences, Office of Basic Energy Sciences, U.S. Department of Energy (grant no. DE–FG02–03ER15449 to J.I.S.) and the National Institutes of Health (grant no. GM060396 to J.I.S.). Research at Huazhong Agricultural University was funded by the China National Natural Science Foundation (grant no. 31271515), the Program for New Century Excellent Talents in University (NCET–12–0861), and the Fundamental Research Funds for the Central Universities (projects 2662013PY019 and 2662013JQ002 to H.H.). Research of R.O. and W.F.B. was funded by the Office of Naval Research (grant no. N00014–11–1–0889, N00014–14–1–0716), National Institutes of Health (grant no. U01–GM111251), and the Meyer/Scarpa Chair to W.F.B. C.E. was supported in part by the San Diego Center for Systems Biology (Systems Biology seed grant no. GM085764).
Articles can be viewed without a subscription.
References
- Abdel-Ghany SE, Ye H, Garifullina GF, Zhang L, Pilon-Smits EA, Pilon M (2005) Iron-sulfur cluster biogenesis in chloroplasts: involvement of the scaffold protein CpIscA. Plant Physiol 138: 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay-Shemer T, Palomares A, Bagheri A, Israelsson-Nordstrom M, Engineer CB, Bargmann BOR, Stephan AB, Schroeder JI (2015) Guard cell photosynthesis is critical for stomatal turgor production, yet does not directly mediate CO2- and ABA-induced stomatal closing. Plant J 83: 567–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Price GD (2003) CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J Exp Bot 54: 609–622 [DOI] [PubMed] [Google Scholar]
- Cardon ZG, Berry JA, Woodrow IE (1994) Dependence of the extent and direction of average stomatal response in Zea mays L. and Phaseolus vulgaris L. on the frequency of fluctuations in environmental stimuli. Plant Physiol 105: 1007–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS (2009) The taste of carbonation. Science 326: 443–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S (2008) C4 photosynthetic isotope exchange in NAD-ME- and NADP-ME-type grasses. J Exp Bot 59: 1695–1703 [DOI] [PubMed] [Google Scholar]
- DeLucia EH, Hamilton JG, Naidu SL, Thomas RB, Andrews JA, Finzi A, Lavine M, Matamala R, Mohan JE, Hendrey GR, et al. (1999) Net primary production of a forest ecosystem with experimental CO2 enrichment. Science 284: 1177–1179 [DOI] [PubMed] [Google Scholar]
- Engineer CB, Ghassemian M, Anderson JC, Peck SC, Hu H, Schroeder JI (2014) Carbonic anhydrases, EPF2 and a novel protease mediate CO2 control of stomatal development. Nature 513: 246–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre N, Reiter IM, Becuwe-Linka N, Genty B, Rumeau D (2007) Characterization and expression analysis of genes encoding alpha and beta carbonic anhydrases in Arabidopsis. Plant Cell Environ 30: 617–629 [DOI] [PubMed] [Google Scholar]
- Ferris KE, Clark RD, Coates EL (2007) Topical inhibition of nasal carbonic anhydrase affects the CO2 detection threshold in rats. Chem Senses 32: 263–271 [DOI] [PubMed] [Google Scholar]
- Feys BJ, Wiermer M, Bhat RA, Moisan LJ, Medina-Escobar N, Neu C, Cabral A, Parker JE (2005) Arabidopsis SENESCENCE-ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell 17: 2601–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JE, Wilkerson CG, Ray WK, McAndrew RS, Osteryoung KW, Gage DA, Phinney BS (2003) Proteomic study of the Arabidopsis thaliana chloroplastic envelope membrane utilizing alternatives to traditional two-dimensional electrophoresis. J Proteome Res 2: 413–425 [DOI] [PubMed] [Google Scholar]
- Fukuzawa H, Fujiwara S, Yamamoto Y, Dionisio-Sese ML, Miyachi S (1990) cDNA cloning, sequence, and expression of carbonic anhydrase in Chlamydomonas reinhardtii: regulation by environmental CO2 concentration. Proc Natl Acad Sci USA 87: 4383–4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M, Norici A, Forssen M, Eriksson M, Raven JA (2003) An anaplerotic role for mitochondrial carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol 132: 2126–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstein S, de Beer D, Fellea HH (2001) Miniaturised carbon dioxide sensor designed for measurements within plant leaves. Sens Actuators B Chem 81: 107–114 [Google Scholar]
- Hashimoto M, Negi J, Young J, Israelsson M, Schroeder JI, Iba K (2006) Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat Cell Biol 8: 391–397 [DOI] [PubMed] [Google Scholar]
- Hatch MD, Burnell JN (1990) Carbonic anhydrase activity in leaves and its role in the first step of C4 photosynthesis. Plant Physiol 93: 825–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424: 901–908 [DOI] [PubMed] [Google Scholar]
- Hewett-Emmett D, Tashian RE (1996) Functional diversity, conservation, and convergence in the evolution of the alpha-, beta-, and gamma-carbonic anhydrase gene families. Mol Phylogenet Evol 5: 50–77 [DOI] [PubMed] [Google Scholar]
- Hu H, Boisson-Dernier A, Israelsson-Nordström M, Böhmer M, Xue S, Ries A, Godoski J, Kuhn JM, Schroeder JI (2010) Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat Cell Biol 12: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M (2007) Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science 317: 953–957 [DOI] [PubMed] [Google Scholar]
- Johansson IM, Forsman C (1992) Processing of the chloroplast transit peptide of pea carbonic anhydrase in chloroplasts and in Escherichia coli: identification of two cleavage sites. FEBS Lett 314: 232–236 [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Uemura M (2003) Mass spectrometric approach for identifying putative plasma membrane proteins of Arabidopsis leaves associated with cold acclimation. Plant J 36: 141–154 [DOI] [PubMed] [Google Scholar]
- Kern G, Kern D, Schmid FX, Fischer G (1995) A kinetic analysis of the folding of human carbonic anhydrase II and its catalysis by cyclophilin. J Biol Chem 270: 740–745 [DOI] [PubMed] [Google Scholar]
- Kubo M, Udagawa M, Nishikubo N, Horiguchi G, Yamaguchi M, Ito J, Mimura T, Fukuda H, Demura T (2005) Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev 19: 1855–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanemets K, Wang YF, Lindgren O, Wu J, Nishimura N, Lee S, Caddell D, Merilo E, Brosche M, Kilk K, et al. (2013) Mutations in the SLAC1 anion channel slow stomatal opening and severely reduce K+ uptake channel activity via enhanced cytosolic [Ca2+] and increased Ca2+ sensitivity of K+ uptake channels. New Phytol 197: 88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TW, Morel FM (2000) A biological function for cadmium in marine diatoms. Proc Natl Acad Sci USA 97: 4627–4631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Choi Y, Burla B, Kim YY, Jeon B, Maeshima M, Yoo JY, Martinoia E, Lee Y (2008) The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat Cell Biol 10: 1217–1223 [DOI] [PubMed] [Google Scholar]
- Ludwig M. (2012) Carbonic anhydrase and the molecular evolution of C4 photosynthesis. Plant Cell Environ 35: 22–37 [DOI] [PubMed] [Google Scholar]
- Medlyn BE, Barton CVM, Broadmeadow MSJ, Ceulemans R, De Angelis P, Forstreuter M, Freeman M, Jackson SB, Kellomaki S, Laitat E, et al. (2001) Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol 149: 247–264 [DOI] [PubMed] [Google Scholar]
- Moroney JV, Ma Y, Frey WD, Fusilier KA, Pham TT, Simms TA, DiMario RJ, Yang J, Mukherjee B (2011) The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: intracellular location, expression, and physiological roles. Photosynth Res 109: 133–149 [DOI] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486 [DOI] [PubMed] [Google Scholar]
- Outlaw WH, Manchester J (1979) Guard cell starch concentration quantitatively related to stomatal aperture. Plant Physiol 64: 79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales M, Eubel H, Heinemeyer J, Colaneri A, Zabaleta E, Braun HP (2005) Disruption of a nuclear gene encoding a mitochondrial gamma carbonic anhydrase reduces complex I and supercomplex I + III2 levels and alters mitochondrial physiology in Arabidopsis. J Mol Biol 350: 263–277 [DOI] [PubMed] [Google Scholar]
- Raven JA. (2001) An aquatic perspective on the concepts of Ingestad relating plant nutrition to plant growth. Physiol Plant 113: 301–307 [DOI] [PubMed] [Google Scholar]
- So AK, Espie GS, Williams EB, Shively JM, Heinhorst S, Cannon GC (2004) A novel evolutionary lineage of carbonic anhydrase (epsilon class) is a component of the carboxysome shell. J Bacteriol 186: 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somersalo E, Occhipinti R, Boron WF, Calvetti D (2012) A reaction-diffusion model of CO2 influx into an oocyte. J Theor Biol 309: 185–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding MH. (2008) Microalgal carbon-dioxide-concentrating mechanisms: chlamydomonas inorganic carbon transporters. J Exp Bot 59: 1463–1473 [DOI] [PubMed] [Google Scholar]
- Studer AJ, Gandin A, Kolbe AR, Wang L, Cousins AB, Brutnell TP (2014) A limited role for carbonic anhydrase in C4 photosynthesis as revealed by a ca1ca2 double mutant in maize. Plant Physiol 165: 608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Hou C, Ren Z, Pan Y, Jia J, Zhang H, Bai F, Zhang P, Zhu H, He Y, et al. (2015) A molecular pathway for CO2 response in Arabidopsis guard cells. Nat Commun 6: 6057. [DOI] [PubMed] [Google Scholar]
- Tripp BC, Smith K, Ferry JG (2001) Carbonic anhydrase: new insights for an ancient enzyme. J Biol Chem 276: 48615–48618 [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, et al. (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal F, Martín V, Colaneri A, González-Schain N, Perales M, Martín M, Lombardo C, Braun HP, Bartoli C, Zabaleta E (2009) Ectopic expression of mitochondrial gamma carbonic anhydrase 2 causes male sterility by anther indehiscence. Plant Mol Biol 70: 471–485 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Lawson T, Oxborough K, Baker NR, Andrews TJ, Raines CA (2004) Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J Exp Bot 55: 1157–1166 [DOI] [PubMed] [Google Scholar]
- Wang Y, Duanmu D, Spalding MH (2011) Carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii: inorganic carbon transport and CO2 recapture. Photosynth Res 109: 115–122 [DOI] [PubMed] [Google Scholar]
- Witte CP, Noël LD, Gielbert J, Parker JE, Romeis T (2004) Rapid one-step protein purification from plant material using the eight-amino acid StrepII epitope. Plant Mol Biol 55: 135–147 [DOI] [PubMed] [Google Scholar]
- Xue S, Hu H, Ries A, Merilo E, Kollist H, Schroeder JI (2011) Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J 30: 1645–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI (2008) Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI (2006) CO(2) signaling in guard cells: calcium sensitivity response modulation, a Ca(2+)-independent phase, and CO(2) insensitivity of the gca2 mutant. Proc Natl Acad Sci USA 103: 7506–7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.