Salt treatment of maize increased the sugar sensing metabolite trehalose-6-phosphate, as well as sucrose and hexose sugars, leading to reductions in spikelet growth, silk growth and kernel set.
Abstract
Little is known about how salt impacts primary metabolic pathways of C4 plants, particularly related to kernel development and seed set. Osmotic stress was applied to maize (Zea mays) B73 by irrigation with increasing concentrations of NaCl from the initiation of floral organs until 3 d after pollination. At silking, photosynthesis was reduced to only 2% of control plants. Salt treatment was found to reduce spikelet growth, silk growth, and kernel set. Osmotic stress resulted in higher concentrations of sucrose (Suc) and hexose sugars in leaf, cob, and kernels at silking, pollination, and 3 d after pollination. Citric acid cycle intermediates were lower in salt-treated tissues, indicating that these sugars were unavailable for use in respiration. The sugar-signaling metabolite trehalose-6-phosphate was elevated in leaf, cob, and kernels at silking as a consequence of salt treatment but decreased thereafter even as Suc levels continued to rise. Interestingly, the transcripts of trehalose pathway genes were most affected by salt treatment in leaf tissue. On the other hand, transcripts of the SUCROSE NONFERMENTING-RELATED KINASE1 (SnRK1) marker genes were most affected in reproductive tissue. Overall, both source and sink strength are reduced by salt, and the data indicate that trehalose-6-phosphate and SnRK1 may have different roles in source and sink tissues. Kernel abortion resulting from osmotic stress is not from a lack of carbohydrate reserves but from the inability to utilize these energy reserves.
Crop plants face a broad range of environmental stresses. Among them, salt stress is highly problematic, since most cultivated crops are sensitive to it (Hasanuzzaman et al., 2013). Each year, soil salinity increases worldwide, and it is predicted that it will cause a loss of up to 50% of the cultivatable land by 2050 (Mahajan and Tuteja, 2005). Salt mainly affects two developmental processes: establishment and reproduction in crops (Samineni et al., 2011; Hasanuzzaman et al., 2013; Hassanzadehdelouei et al., 2013). Salt can reduce plant height, branching, branch length, leaf number, root/shoot weight, and early vegetative development (for review, see Hasanuzzaman et al., 2013). The effect of salt on plant establishment was assessed in maize (Zea mays; Cha-Um and Kirdmanee, 2009), sorghum (Sorghum bicolor; El Naim et al., 2012), rice (Oryza sativa; Hakim et al., 2010), wheat (Triticum aestivum; Khayatnezhad et al., 2010), and soybean (Glycine max; Rastegar et al., 2011). Despite the severe effects of salt on plants, minimal data are available on this topic for plants at reproductive stage, especially for maize.
Abiotic stresses such as salinity and drought have a common but not exclusive osmotic component. Salinity generates an immediate osmotic stress followed by a later ion toxicity after continued exposure (Jain and Selvaraj, 1997; Carillo et al., 2011). Osmotic stress resulting from drought or salinized soils can be disastrous for crops such as maize, primarily because of its impact on early kernel development (Westgate and Boyer, 1985). When applied during ear formation, osmotic stress impairs ovule formation and decreases their number (Bruce et al., 2002), while stress applied around pollination will impair fertilization by delaying silk emergence and/or reducing silk receptivity or causing kernel abortion (Westgate and Boyer, 1986; Otegui et al., 1995; Khorasani et al., 2011). In Arabidopsis (Arabidopsis thaliana), osmotic stress (200 mm NaCl or 400 mm mannitol) induces extensive ovule abortion (approximately 90%) within 12 h of treatment. In this case, most ovules (75%) abort prior to fertilization and very few embryos develop to mature seed (Sun et al., 2004). Stress-induced embryo abortion at early stages has been reported in soybean, chickpea (Cicer arietinum), and wheat (Setter et al., 2011). In maize, most studies related to osmotic stress have focused on the effect of water deficit on early kernel development. Maize plants are most susceptible during a period of 2 weeks around the time of silking, and kernel abortion is the limiting factor for yield (Westgate and Boyer, 1985; Boyer, 2010). Recent studies identify events as early as 1 d after pollination (DAP) to be critical for determining whether the embryo will abort (Wobus and Weber, 1999; Kakumanu et al., 2012). Regardless, few data are available on the effect of salt stress on early kernel development and abortion.
Kernel abortion is primarily due to the deleterious effect of osmotic stress on carbohydrate metabolism, ultimately leading to starvation of the developing embryo (Zinselmeier et al., 1999). Osmotic stress has an impact on both the source of carbohydrate (photosynthesis) and the mobilization/utilization of carbohydrate reserves (sink strength). In maize, kernel abortion induced by osmotic stress correlates with reduced evapotranspiration and photosynthesis (Westgate and Boyer, 1985; Otegui et al., 1995; Setter et al., 2001). Along with impaired photosynthesis in source leaves comes a reduction of seed sink strength. Abortion caused by osmotic stress correlates with depleted Suc and reduced sugar levels, reduced Suc-degrading enzyme activity and transcript levels, and depletion of starch in the kernels. These events occur in a short period of time around pollination and can be partially prevented by stem Suc feeding (Zinselmeier et al., 1995, 1999; Andersen et al., 2002; McLaughlin and Boyer, 2004; Boyer, 2010). As a result of impaired photosynthesis and sink strength, sugar allocation to the reproductive organs is disrupted and the young embryo rapidly starves and aborts.
Sugars not only serve as a source of carbon units and metabolic energy but also function as signaling molecules reporting carbon status within the cell (León and Sheen, 2003; Ruan et al., 2010). Trehalose is a nonreducing disaccharide found in archebacteria, eubacteria, fungi, plants, and invertebrates. In the majority of flowering plants, trehalose and its precursor, trehalose-6-phosphate (T6P), both accumulate to very low levels (less than 10 µmol g−1 fresh weight and 10 nmol g−1 fresh weight, respectively). T6P correlates with diurnal changes in Suc levels and in the response to exogenous Suc supply, suggesting a role as a Suc signal (Lunn et al., 2006, 2014; Nunes et al., 2013; Yadav et al., 2014). The trehalose synthesis pathway is highly conserved throughout the plant kingdom (including in maize), where trehalose is formed in two steps (Wingler, 2002; Henry et al., 2014). First, UDP-Glc and Glc-6-P are condensed by the enzyme TREHALOSE-6-PHOSPHATE SYNTHASE (TPS) to produce T6P. T6P is subsequently dephosphorylated by TREHALOSE-6-PHOSPHATE PHOSPHATASE (TPP) to form trehalose. Overexpression or mutation of certain TPS/TPP genes has dramatic effects on plant metabolism and development (Eastmond et al., 2002; Ponnu et al., 2011).
The trehalose pathway has a long association with abiotic stress tolerance (Garg et al., 2002; Iordachescu and Imai, 2008; Fernandez et al., 2010) and has come to the fore recently as a mechanism involved in the regulation of sugar metabolism (Lunn et al., 2006; Martínez-Barajas et al., 2011; Carillo et al., 2013; Yadav et al., 2014). In Arabidopsis, there are four class I TPS genes, of which AtTPS1, AtTPS2, and AtTPS4 have been shown to encode functional enzymes (Delorge et al., 2015). AtTPS1 was shown to be essential for early seed development, since its mutation is embryo lethal in Arabidopsis (Eastmond et al., 2002). Attps1 mutants stop developing after embryo morphogenesis occurs (between 7 and 15 d after flowering), mainly due to perturbations of cell wall synthesis, cell division, and sugar and lipid metabolism (Gómez et al., 2006). Consistently, during early stages of wheat grain development, T6P content peaks between 1 and 7 d after anthesis, then drops dramatically and remains lower until seed maturity. In this instance, T6P level correlates with Suc content (Martínez-Barajas et al., 2011). A mutation in the RAMOSA3 TPP gene in maize results in extensive branching of inflorescences; however, as of yet, no changes in T6P levels have been observed (Satoh-Nagasawa et al., 2006; Carillo et al., 2013). It has been suggested that differences in trehalose or the ratio between trehalose and T6P may trigger the developmental changes in ramosa3 mutant inflorescences (Carillo et al., 2013).
The trehalose pathway has also been implicated in stress tolerance and recovery (for review, see Iordachescu and Imai 2008; Fernandez et al., 2010). T6P and trehalose levels, as well as TPS/TPP gene expression, are affected by stress (Pramanik and Imai, 2005; Iordachescu and Imai, 2008; Fernandez et al., 2010; Nunes et al., 2013). Either sugar starvation or extended darkness in Arabidopsis leaves resulted in lower T6P levels. Lunn et al. (2006) reported that T6P content was 6 times lower in carbon-starved Arabidopsis seedlings. Readdition of Suc (15 mm) resulted in an increase in T6P within 30 min. TPS activity is induced by osmotic stress (water deficit) in roots and shoots of stress-sensitive rice lines but only in shoots of tolerant ones (Elbashiti et al., 2005). Along with this, transgenic plants expressing certain TPS/TPP genes from bacterial, fungal, or plant origin show improved tolerance to various abiotic stresses, sometimes associated with an increase in trehalose levels (Garg et al., 2002; Jang et al., 2003; Cortina and Culianez-Macia, 2005; Karim et al., 2007; Miranda et al., 2007; Ge et al., 2008). In maize, exogenous application of trehalose induces water deficit tolerance (Ali and Ashraf, 2011). Most recently, it was demonstrated that the targeted overexpression of a TPP to developing maize ears improves the allocation of carbon to the ear during water deficit stress (Nuccio et al., 2015). Altogether, these data strongly suggest a central role for the trehalose pathway in the integration of stress, metabolism, and seed development. However, little is known about its role in controlling early seed development under osmotic stress.
In addition to its role in sensing cytosolic Suc levels, T6P was recently shown to inhibit in vitro SUCROSE NONFERMENTING-RELATED KINASE1 (SnRK1) activity (Zhang et al., 2009). SnRK1 is a central integrator of stress and energy status (for review, see Halford and Hey 2009; Baena-González, 2010; Smeekens et al., 2010; Schluepmann et al., 2012). Inhibition of SnRK1 by T6P potentially provides an explanation for some of the effects of T6P in plants. T6P and SnRK1 signaling is involved in seed development as well as stress response and recovery (Martinez-Barajas et al., 2011; Nunes et al., 2013), likely through the regulation of genes involved in the utilization of Suc in growth and development and the synthesis of end products. Clearly, SnRK1-independent mechanisms are possible and likely at least in mature leaf tissue (Lunn et al., 2006; Zhang et al., 2009). Lunn et al. (2014) proposed that T6P regulates Suc homeostasis based on evidence from transgenic Arabidopsis plants with perturbed T6P levels. Recently, Nuccio et al. (2015) showed that decreased levels of T6P in transgenic maize resulted in higher levels of Suc in young maize ears.
Little is known of the trehalose pathway, its gene regulation, and its role in central metabolism for an agriculturally important crop such as maize. Since the development of modern hybrids, yield in maize has been improved primarily through sustaining photosynthetic output throughout the grain-filling stage and increased dry matter accumulation and partitioning of photosynthate to the ear during the critical kernel determination period (silking; Tollenaar and Lee, 2006). Surprisingly, yield potential on an individual plant basis has not actually been improved significantly through plant breeding (Echarte et al., 2013). Breeders have rather focused their attention not on increasing primary productivity but on improving stress tolerance, thus permitting greater plant populations and dependable productivity in a variety of environments (Duvick, 2005). Here, to our knowledge for the first time, we report evidence indicating a role for the trehalose pathway in regulating reproductive development, particularly during osmotic stress. We investigated the effect of 75 mm NaCl applied incrementally from the initiation of floral organs. Our data show differential regulation of both the trehalose pathway and SnRK1 in leaves compared with reproductive tissue, revealing future strategies for the improvement of maize performance under saline conditions.
RESULTS
Salt Impairs Plant Growth and Reproduction
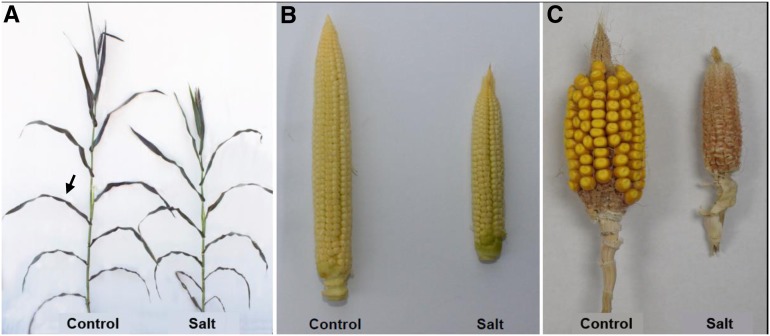
We first assessed the effect of long-term salt treatment on maize growth and development. Greenhouse-grown B73 maize plants were subjected to a gradual increase of salt concentration from 12.5 to 75 mm starting at stage V7 (early formation of floral organs) until 3 DAP. Control plants received nutrient solution only. We chose a maximal NaCl concentration of 75 mm from previous published studies on salt stress in maize (Fortmeier and Schubert, 1995; Yang and Lu, 2005; Niu et al., 2012) and through our empirical data. At 75 mm NaCl, there was a small but significant reduction in vegetative growth and a more significant impact on kernel set. For yield studies, plants were manually pollinated at full silking stage with fresh pollen from control plants. Phenotypic data regarding maize plant growth and development are presented in Figure 1 and Table I. The growth of plants under salt treatment was significantly delayed. This had two main consequences: plants were shorter (Fig. 1A; Table I) and floral development was delayed and impaired (Fig. 1, B and C; Table I). Salt delayed floral development through an increase of the interval from anthesis to silking and from first to full silking (Table I). Ear length was also reduced by salt, mainly because of the malformation of kernels at the tip of the ear (Fig. 1B). In contrast, developed kernels were bigger in salt-treated plants than in control plants at pollination and at 3 DAP (Table I). Salt induced partial and complete kernel abortion when applied until 3 DAP and full maturity, respectively (Fig. 1C; Table I).
Figure 1.
Phenotypes of salt-stressed maize plants. Images represent plants at silking (A), ears at the pollination stage (B), and ears at maturity (C). Salt stress was applied at the V7 to V8 stage by gradually increasing the salts (12.5, 25, 50, and 75 mm NaCl:CaCl2, 5.7:1 ratio) added to the nutrient solution every 3 d. Control plants were supplied with nutrient solution only. Representative plants were used for these images.
Table I. Phenotypes of control and salt-stressed maize plants.
Plants were treated as described previously, and their height was measured at the reproductive stage. For ear length and young kernel fresh weight, developmental stage is precise. Yield was measured on plants exposed to stress until maturity. Values in boldface are significantly different from the control (P < 0.001). Silking interval represents days of pollen shed. Values are means ± se.
| Treatment | Stage | Plant Height | Anthesis Silking Interval | Silking Interval | Ear Length | Young Kernel Fresh Weight | Yield (No. of Mature Seeds per Ear) |
|---|---|---|---|---|---|---|---|
| m | d | cm | mg | ||||
| Control | Silking | 2.33 ± 0.22 | 2.1 ± 1.36 | 1.4 ± 0.55 | 7.91 ± 0.49 | 6.21 ± 1.49 | 157.89 ± 58.37 |
| Pollination | 9.5 ± 0.45 | 10.44 ± 1.6 | |||||
| 3 DAP | 11.1 ± 0.66 | 20.47 ± 4.46 | |||||
| n | 54 | 41 | 35 | 6–10 | 6–10 | 9 | |
| Salt | Silking | 1.85 ± 0.16 | 4.28 ± 2.04 | 2.03 ± 0.81 | 7.33 ± 0.75 | 8.6 ± 1.33 | 2 ± 5.08 |
| Pollination | 8.08 ± 0.8 | 17.83 ± 2.97 | |||||
| 3 DAP | 8.5 ± 0.66 | 23.33 ± 5.72 | |||||
| n | 54 | 36 | 30 | 6–10 | 6–10 | 18 | |
Salt Represses Photosynthesis Yet Results in an Increase in Sugar Levels
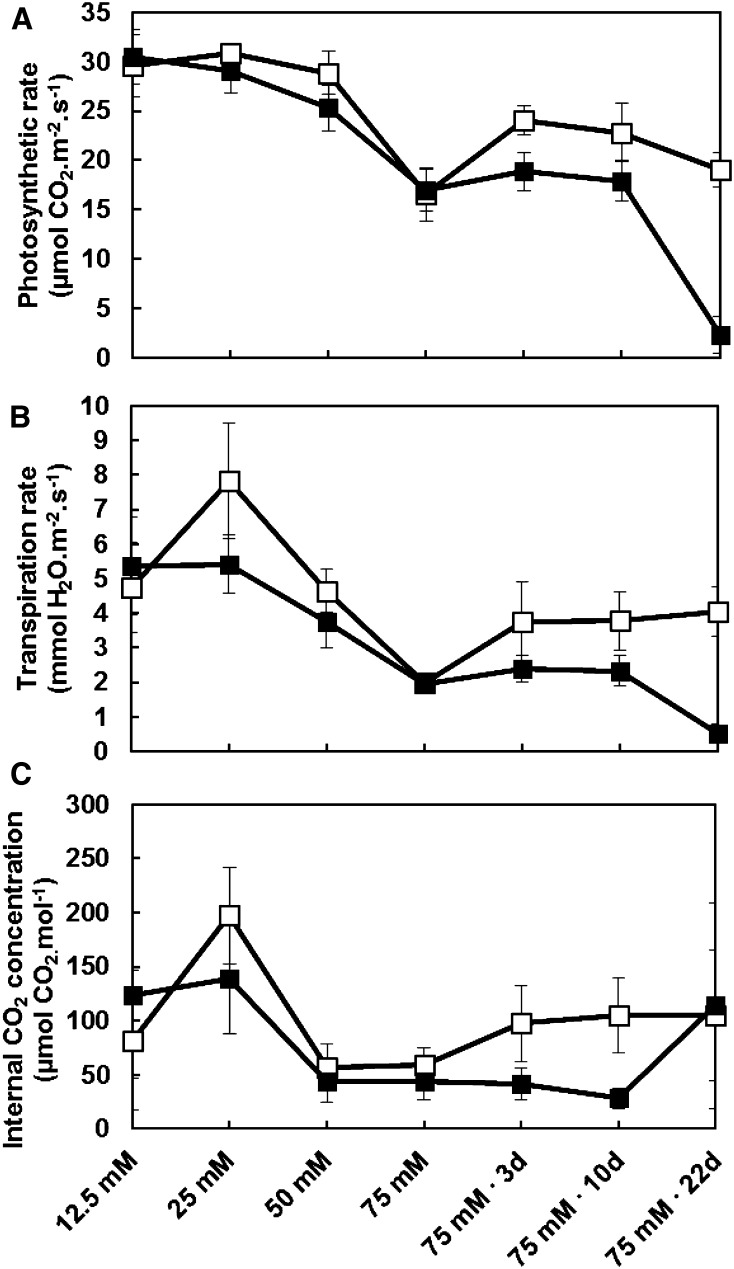
To estimate the impact of salt on maize sugar metabolism, we first measured photosynthetic rate, transpiration rate, and internal CO2 concentration of the ear leaf during the period of treatment (Fig. 2). Photosynthesis was reduced in salt-treated plants compared with control plants (Fig. 2A). This change began after plants were switched to 75 mm salt for 3 d and became highly significant after they reached the reproductive stage (22 d at 75 mm). At this stage, photosynthesis was somewhat lower in control plants and reduced to nearly zero in salt-treated ones. Dense cloud cover on the day that salt-treated plants were switched from 50 to 75 mm salt resulted in an unexpected decrease in photosynthetic rate (Fig. 2A). Evapotranspiration rates and internal CO2 concentration were highly correlated and very similar to photosynthetic rates (Fig. 2, B and C).
Figure 2.
Photosynthetic data from control and salt-stressed plants. Greenhouse-grown maize plants (B73) were assessed for photosynthetic rate (A), transpiration rate (B), and internal CO2 concentration (C). Measurements were taken at the initiation of salt treatment (12.5 mm NaCl), with each increase in salt concentration, and after 3, 10, and 22 d on 75 mm NaCl. Photosynthetic rate was lower than expected when NaCl was increased to 75 mm. This was a consequence of cloud cover. On such days, C4 plants take a long time to become photosynthetically active, despite the external light source provided in the chamber. After plants are in low light for some time, a sudden increase in photon flux (from the Li-Cor apparatus) will not result in an instantaneous increase in photosynthesis. An induction period of up to 60 min is due to a buildup of ribulose 1,5-bisphosphate, activation of Rubisco, and opening of stomata (Sims and Pearcy, 1994). White squares are control plants, and black squares are salt-treated plants. Values are means ± sd (n = 3).
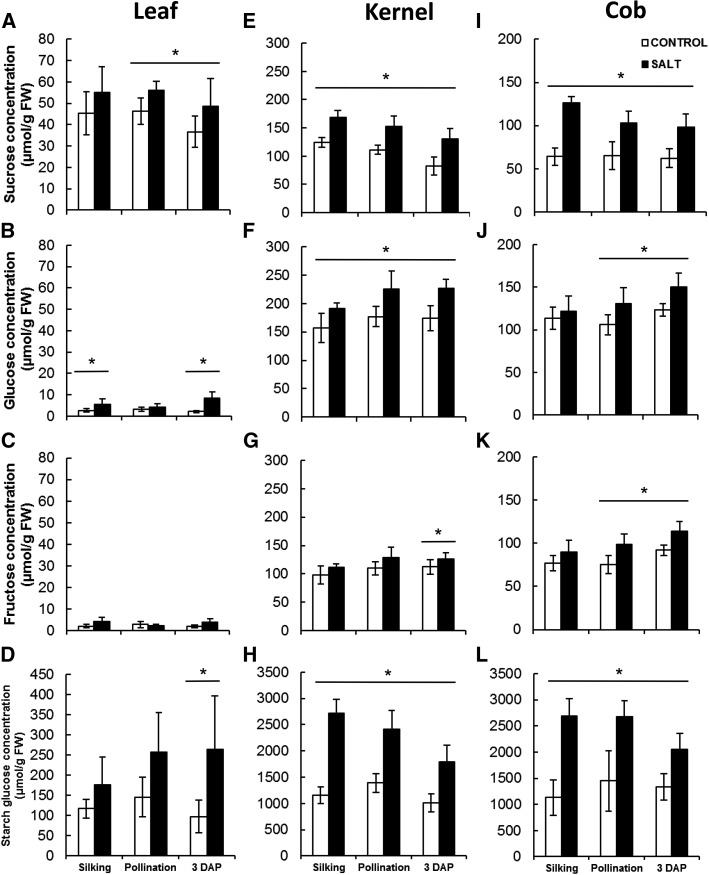
Soluble sugars and starch were measured in control and salt-treated leaf, kernel, and sustaining cob tissue at silking (first silk), pollination (full silking), and 3 DAP using capillary high-pressure ion chromatography (HPIC; Fig. 3). In all three tissues, starch, Suc, Glc, and Fru were the main forms of carbohydrate detected. Both sugar and starch levels were lower in leaf than in kernel and sustaining cob tissue. In the leaf, starch and Suc were the primary carbohydrates, with a higher level in salt-treated plants than in controls (Fig. 3, A–D). Differences were significant for Suc at pollination and for Suc and starch at 3 DAP. No significant changes were observed between the different developmental stages. Kernels displayed about the same amount of Suc and hexose sugars. Kernel sugar and starch content were significantly higher in salt-treated plants than in control ones at all stages, except for Fru at silking and pollination (Fig. 3, E–H). A very similar trend was observed in the sustaining cob tissues (Fig. 3, I–L). In these two tissues, starch and Suc contents decreased slightly with developmental stage, while hexose content increased slightly (Fig. 3, E–L).
Figure 3.
Sugar and starch contents of leaf (A–D), kernel (E–H), and cob (I–L) tissue from control (white bars) and salt-stressed (black bars) plants at silking, pollination, and 3 DAP. Plants were grown under control or salt stress conditions as described in “Materials and Methods.” Kernel refers to the ovule (at silking and pollination) and the embryo (at 3 DAP). Suc (A, E, and I), Glc (B, F, and J), Fru (C, G, and K), and starch (D, H, and L) contents were analyzed using capillary HPIC. Student’s t test was used to determine significant changes. Asterisks indicate significant differences from the controls (P < 0.05; n = 6–10). Values are means ± sd. FW, Fresh weight.
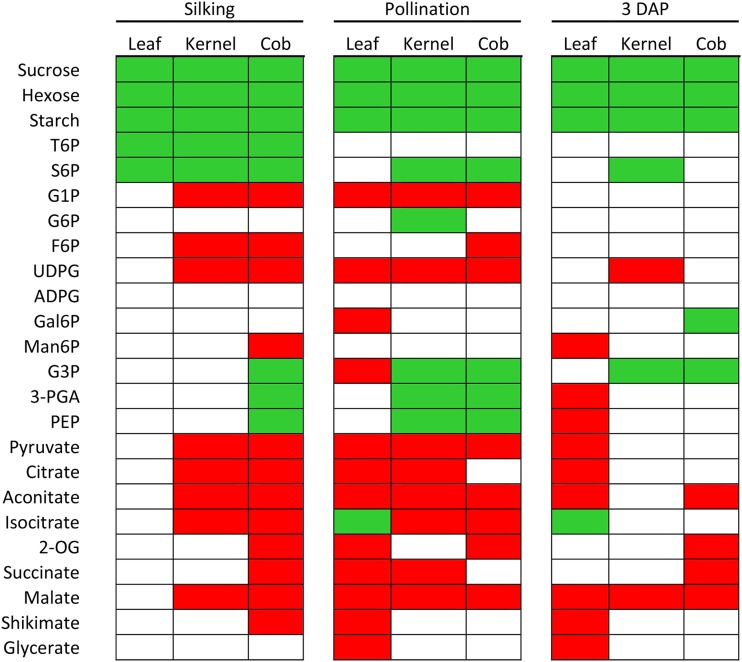
Primary metabolites from glycolysis, the citric acid cycle, and the Calvin-Benson cycle were quantified on samples from control and salt-stressed tissue using HPIC coupled to tandem mass spectrometry (Supplemental Fig. S1). Metabolite levels fluctuated in salt-treated plants compared with controls. These changes are shown in the heat map displayed in Figure 4. While intermediates of Suc synthesis, soluble sugars, and starch increased under salt, most of the other metabolites tended to decrease, especially in leaf. Exceptions to these general trends were isocitrate in leaf and Glc-3-P, 3-phosphoglyceraldehyde, and phosphoenolpyruvate in kernel and cob, where these metabolites increased.
Figure 4.
Effects of salt treatment on metabolite levels (on a fresh weight basis) in leaf, kernel, and cob tissue at silking, pollination, and 3 DAP. All metabolites were measured as described by Lunn et al. (2006). The data used for the analysis and an explanation of metabolite abbreviations are presented in Supplemental Figure S1. Student’s t tests was performed on four to six independent biological replicates using the Microsoft Excel statistical package. For each time point, significant differences (P < 0.05) between control and salt-treated plants are indicated in green (increase) and red (decrease), whereas nonsignificant differences are indicated in white.
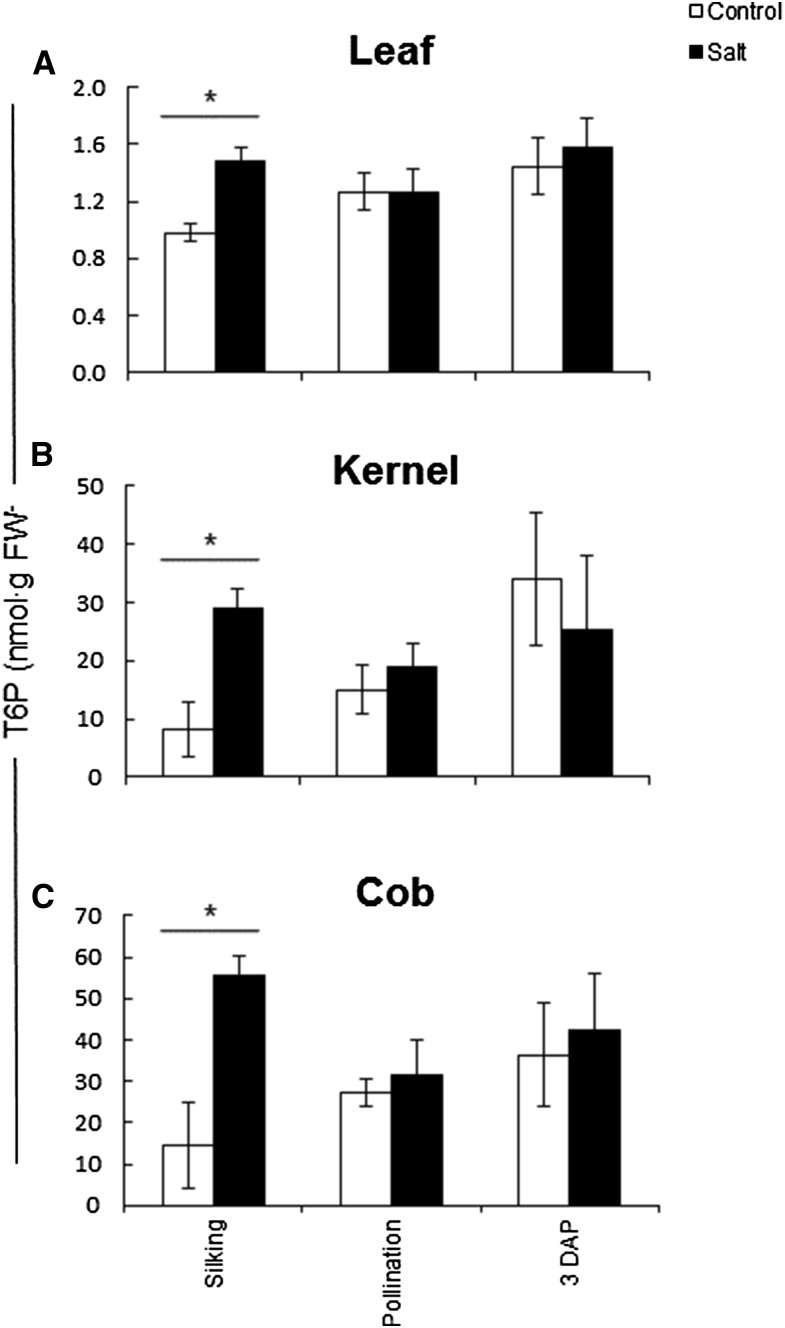
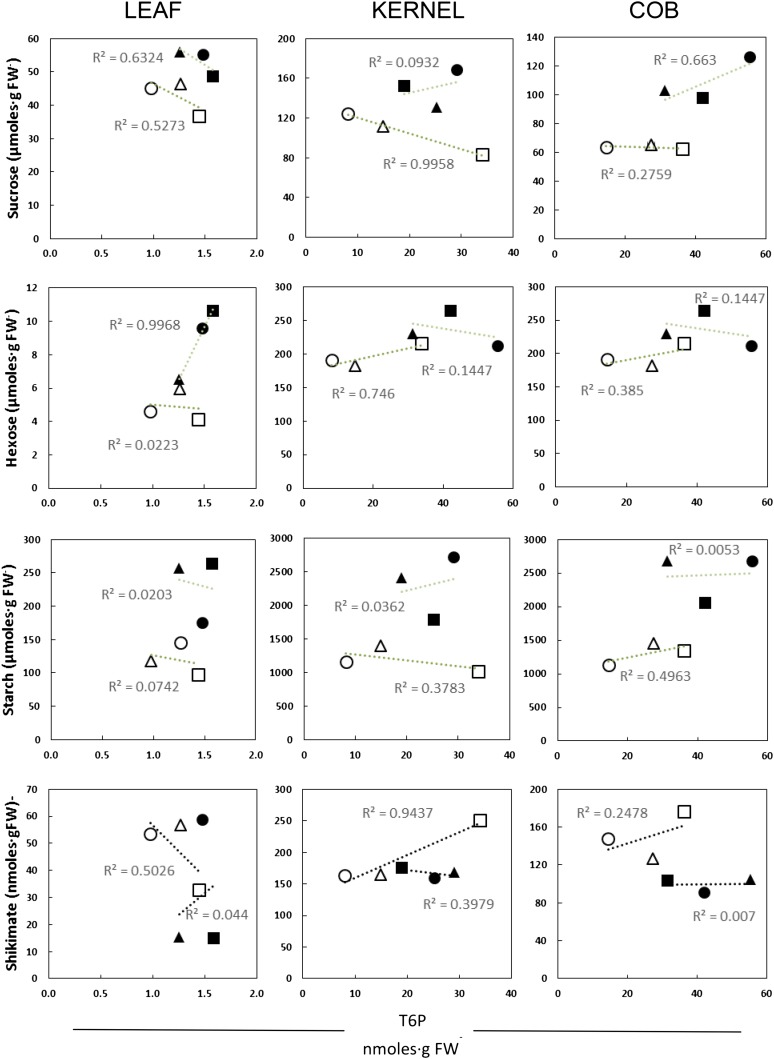
We measured T6P levels in leaf, kernel, and cob tissue and assessed the effect of salt treatment (Fig. 5). T6P levels were lower in leaf (1–1.6 nmol g−1 fresh weight) than in kernel (8–35 nmol g−1 fresh weight) and cob (15–55 nmol g−1 fresh weight) tissue. In all tissues, T6P levels were significantly higher in salt-treated plants compared with control plants, but only at the silking stage. The observed increase in T6P was 50% in leaf, 200% in kernel, and 250% in cob. We looked for correlations between T6P levels and other key metabolites: Suc, hexoses, starch, and shikimate (Fig. 6). A significant negative correlation (r2 > 0.9) between T6P and Suc was observed in control kernel tissue, while a significant positive correlation was found between T6P and shikimate (an indicator of anabolic growth). Also, a significant positive correlation was observed for T6P and hexose sugars in leaf tissue from salt-treated plants.
Figure 5.
T6P contents in control and salt-stressed plants. T6P content was determined in leaf (A), kernel (B), and cob (C) tissue from control (white bars) and salt-stressed (black bars) maize plants using anion-exchange chromatography in tandem with mass spectrometry. Asterisks indicate significant differences from the control (P < 0.05; n = 6–10) as determined by Student’s t test. Values are means ± sd. FW, Fresh weight.
Figure 6.
Correlations between T6P and key metabolites. Linear regression analysis was performed to compare T6P levels (x axis) with the key metabolites Suc, hexoses, starch, and shikimate (y axis). Plant stages are indicated as follows: circles, silking; triangles, pollination; and squares, 3 DAP. Control (white markers) and salt-treated (black markers) plants are represented. Moderate correlation (r2 > 0.5) and high correlation (r2 > 0.9) were found. FW, Fresh weight.
Previous research indicated a close correlation between T6P and Suc levels within a given tissue over the short term, for example, during the diurnal cycle in leaves or after exogenous addition of sugars to seedlings. In fact, the T6P-Suc ratio is a critical parameter indicative of Suc homeostasis in plant cells and varies between tissues, species, and in response to environmental changes (Carillo et al., 2013; Yadav et al., 2014). In Table II, the ratios of T6P to Suc, Glc, and Fru were determined before and after pollination for leaf, kernel, and cob tissue in control and salt-treated plants. Student’s t test was performed to look for significant interactions between T6P and these other metabolites. The asterisks in Table II indicate a high level of significance (P > 0.05). Notably, in control plants, the T6P-Suc ratio increased significantly in leaf, kernel, and cob tissue after pollination. Similarly the T6P-Glc and T6P-Fru ratios increased in leaf and kernel. Salt treatment had no effect on the T6P-Suc ratio in prepollination plants; however, in postpollination kernels, osmotic stress resulted in a significant decrease in the T6P-Suc ratio. Little change is seen in the T6P-sugar ratio between prepollination and postpollination in salt-stressed plants.
Table II. Sugar ratios in maize kernels.
The metabolite ratio for each sample was derived by simple division. Student’s t test was performed between the series of derived ratios based on treatment: prepollination versus postpollination and typical watering versus salt treated. n = 5 to 7.
| Sugar | Developmental Stage | Control |
Salt Treatment |
||
|---|---|---|---|---|---|
| Prepollination | Postpollination | Prepollination | Postpollination | ||
| T6P:Suc | Silking | 0.03 | 0.04a | 0.03 | 0.04a |
| Pollination | 0.10 | 0.42a | 0.14 | 0.19b | |
| 3 DAP | 0.30 | 0.54a | 0.32 | 0.47 | |
| T6P:Glc | Silking | 0.41 | 0.69a | 0.34 | 0.19b |
| Pollination | 0.06 | 0.19a | 0.10 | 0.11 | |
| 3 DAP | 0.19 | 0.27 | 0.25 | 0.30 | |
| T6P:Fru | Silking | 0.48 | 0.69a | 0.75 | 0.42b |
| Pollination | 0.10 | 0.29a | 0.17 | 0.20 | |
| 3 DAP | 0.27 | 0.36 | 0.35 | 0.27 | |
Significant difference (P > 0.05) between prepollination and postpollination. bSignificant difference (P > 0.05) between control and salt treated.
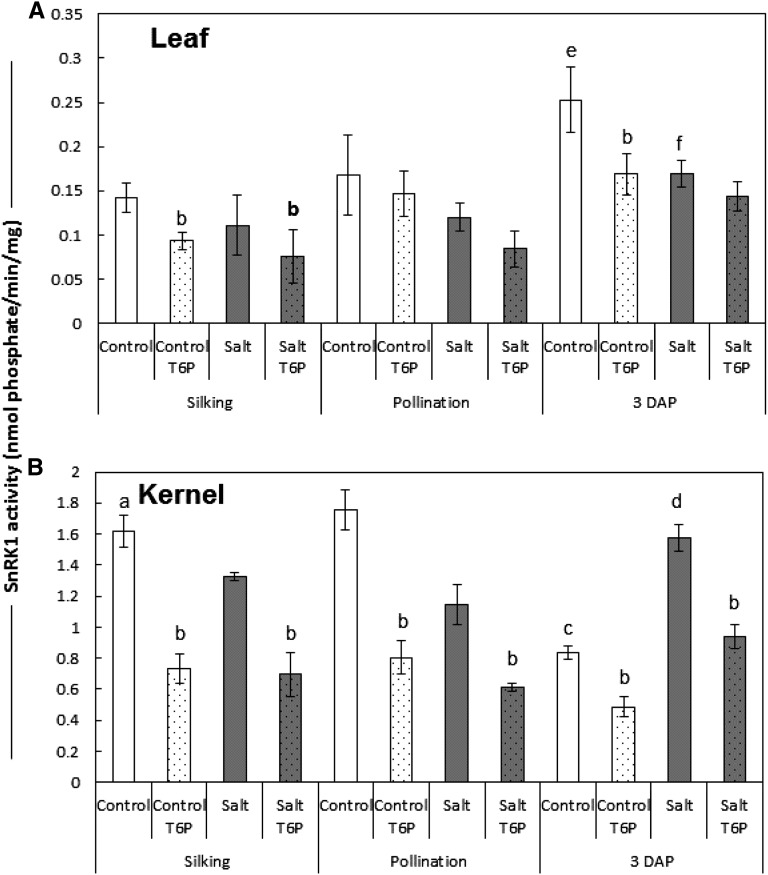
In vitro SnRK1 activity was determined in control and salt-treated leaf and kernel tissue. SnRK1 activity was significantly higher (approximately 5- to 10-fold) in kernel than in leaf tissue. It was also found that the SnRK1 activity in kernel extracts was more sensitive to inhibition by the addition of 1 mm T6P than in leaf tissues (Fig. 7). Kernels from control plants show a large drop in SnRK1 activity after pollination; however, in salt-treated plants, SnRK1 activity was significantly lower at pollination and increased after pollination. In control leaves, SnRK1 activity increased from silking to 3 DAP. Salt stress resulted in a significant reduction in leaf SnRK1 activity at 3 DAP.
Figure 7.
In vitro SnRK1 activity for control and salt-treated leaf and kernel tissue. SnRK1 activity was determined in tissue extracts from control and salt-stressed plants at silking, pollination, and 3 DAP as described by Zhang et al. (2009). Exogenous T6P was added to some samples to determine the fraction of SnRK1 activity sensitive to T6P inhibition (1 mm). Lowercase letters indicate significant difference from controls (P < 0.05; n = 3) as follows: SnRK1 activity higher in kernel than leaf (a); exogenous T6P inhibits SnRK1 activity to a greater extent in kernel tissue (b); SnRK1 activity drops significantly in control kernels after pollination (c); kernel SnRK1 activity increases after pollination in salt-treated plants (d); SnRK1 activity increases significantly in control leaves after pollination (e); and salt treatment results in a significant decrease in SnRK1 activity at 3 DAP (f). Values are means ± sd.
Salt Effect on TPS/TPP, Sugar Metabolism, and SRK1 Target Gene Expression
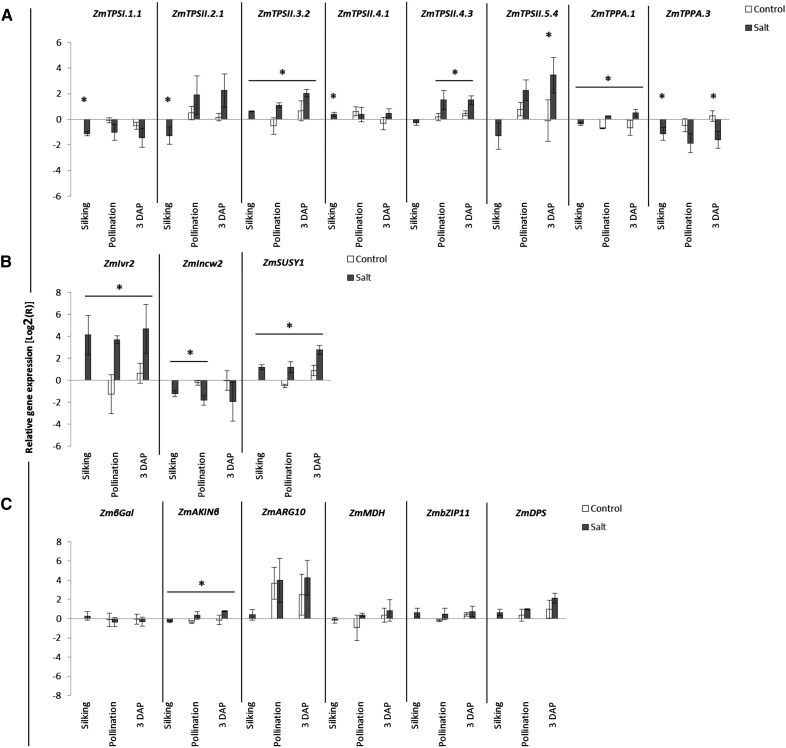
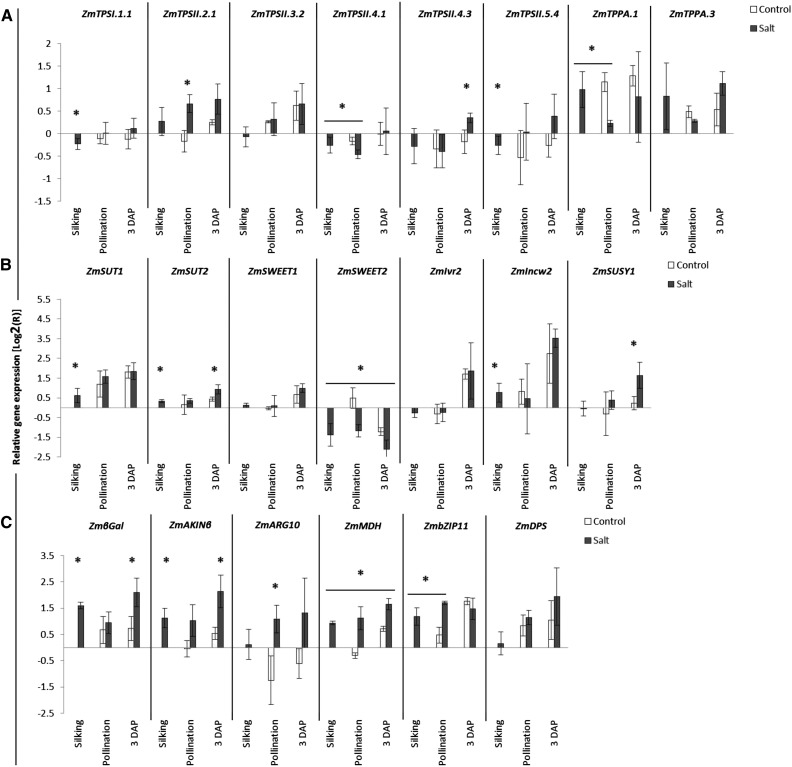
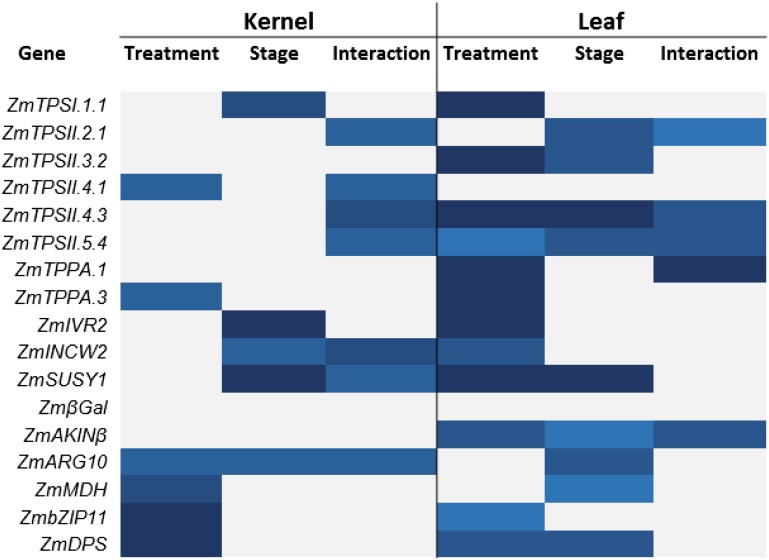
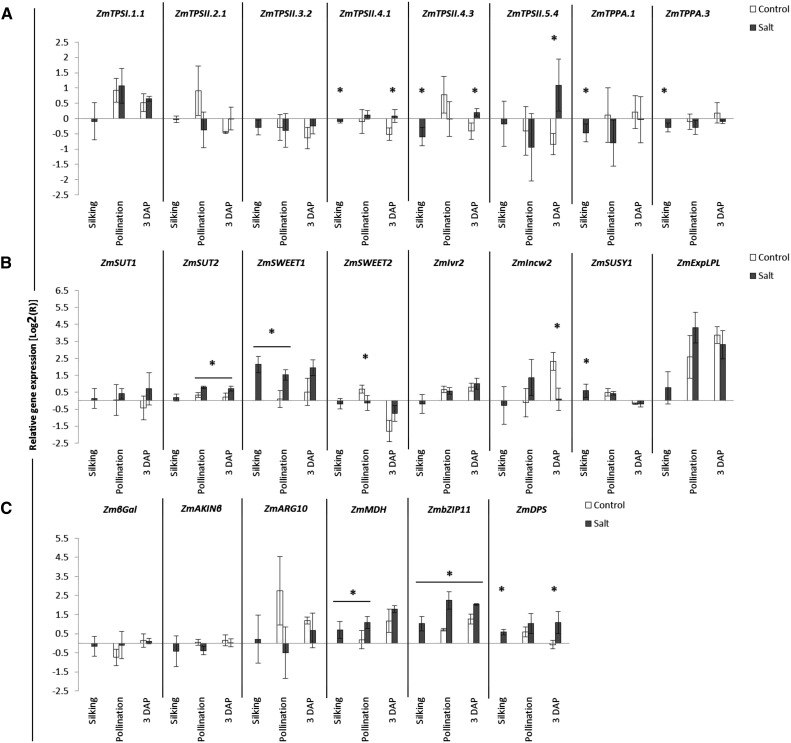
To assess the effect of osmotic stress on carbohydrate metabolism and the trehalose pathway, as well as the role of trehalose metabolism in kernel abortion, we measured transcript levels for several key genes involved in carbohydrate metabolism in control and salt-treated plants using quantitative reverse transcription (qRT)-PCR (Figs. 8–10). In leaf, transcripts for all TPS/TPP genes tested were affected by salt (Fig. 8A). ZmTPSI.1.1, ZmTPSII.2.1, and ZmTPPA.3 transcripts were repressed under osmotic stress. In contrast, the other TPS and TPP genes were induced by salt. Overall, ANOVA tests indicate that salt significantly affects most TPS genes tested in leaf, with the exception of ZmTPSII.2.1 and ZmTPSII.4.1 (Fig. 11). Developmental stage also significantly affected a number of these genes (mostly class II TPS).
Figure 8.
Relative mRNA levels in leaf tissue for trehalose biosynthetic genes (A), carbohydrate metabolism genes (B), and sugar transport genes (C). Transcript levels were determined by qRT-PCR in control and salt-stressed plants at silking, pollination, and 3 DAP. Plants were grown as described in “Materials and Methods.” Results are expressed relative to the average levels of three stably expressed genes (PROTEIN PHOSPHATASE 2A COMPLEX A-2 SUBUNIT [PP2AA-2], GLYCERALDEHYDE PHOSPHATE DEHYDROGENASE1 [GAPDH1], and Fbox3). Asterisks indicate that salt treatment had a significant effect over controls (P < 0.05; n = 3).
Figure 10.
Relative mRNA levels in cob tissue for trehalose biosynthetic genes (A), carbohydrate metabolism genes (B), and sugar transport genes (C). Transcript levels were determined by qRT-PCR in control and salt-stressed plants at silking, pollination, and 3 DAP. Plants were grown as described in “Materials and Methods.” Results are expressed relative to the average levels of three stably expressed genes (PP2AA-2, GAPDH1, and Fbox3). Asterisks indicate that salt treatment had a significant effect over controls (P < 0.05; n = 3).
Figure 11.
Heat map indicating the overall effect of salt treatment and developmental stage on transcript levels in kernel and leaf tissues. ANOVA was performed on three independent biological replicates using Microsoft Excel. Significant differences are indicated by color: P < 0.05, light blue; P < 0.01, medium blue; and P < 0.001, dark blue. Nonsignificant differences are indicated with white bars.
Among those sugar transporters tested in leaf, only the ZmSWEET1 transcript was induced by salt (Fig. 8B). Transcripts of Suc-degrading enzymes were strongly affected by osmotic stress in leaf tissue. Both ZmIVR2 (vacuolar) and ZmSUSY1 (cytoplasmic) Suc-degrading enzyme transcript levels were strongly induced by salt. On the other hand, transcript levels of ZmINCW2 (cell wall) were strongly repressed by osmotic stress. Overall, salt has a strong effect on most of the transcripts tested in leaf (Fig. 11).
qRT-PCR primers were designed for the maize homologs of several Arabidopsis SnRK1 target genes. βGALACTOSIDASE, AMP-ACTIVATED PROTEIN KINASE β (AKINβ), and ARGININE10 (ARG10) are target genes induced by SnRK1, while MALATE DEHYDROGENASE (MDH), IRON REGULATED TRANSPORTER (β-ZIP11), and DEHYDRODOLICHYL DIPHOSPHATE SYNTHASE1 (DPS) are repressed by SnRK1 (Baena-González et al., 2007). In leaf, only AKINβ was significantly affected by salt for each time point, with a slight repression followed by a small induction (Fig. 8C). Overall, for leaf, salt slightly affected AKINβ, bZIP1, and DPS, and developmental stage had a small effect on AKINβ, ARG10, MDH, and DPS (Fig. 11).
In kernels, transcript levels of ZmTPSI.1.1 were unaffected by salt, while its expression changed throughout development, with a peak at pollination (Fig. 9A). Among the other TPS/TPP genes expressed in the kernel, both ZmTPSII.2.1 and ZmTPSII.3.2 were stably expressed. ZmTPSII.4.1, ZmTPSII.4.3, and ZmTPSII.5.3 showed similar patterns, with a difference in magnitude. They were all slightly repressed under salt treatment at silking and then induced 3 DAP by salt compared with the control. The main TPP isoforms, ZmTPPA.1 and ZmTPPA.3, were both slightly repressed by salt at silking. ANOVA revealed that, overall, salt significantly affected only ZmTPSII.4.1 and ZmTPPA.3, while stage affected only ZmTPSI.1.1 (Fig. 11).
Figure 9.
Relative mRNA levels in kernel tissue for trehalose biosynthetic genes (A), carbohydrate metabolism genes (B), and sugar transport genes (C). Transcript levels were determined by qRT-PCR in control and salt-stressed plants at silking, pollination, and 3 DAP. Plants were grown as described in “Materials and Methods.” Results are expressed relative to the average levels of three stably expressed genes (PP2AA-2, GAPDH1, and Fbox3). Asterisks indicate that salt treatment had a significant effect over controls (P < 0.05; n = 3).
Transcript levels of the sugar transporters ZmSUT2 and SWEET1 were induced by salt stress, while ZmSWEET2 was slightly repressed by salt (Fig. 9B). Transcript levels of the Suc-degrading enzymes ZmIVR2, ZmINCW2 (both involved in early kernel abortion), and ZmSUSY1 (SHRUNKEN1; involved in starch biosynthesis), as well as a marker of early kernel development (ZmExpLPL; ExpLPL is for lipoprotein lipase), were also measured (Fig. 9B). ANOVA showed that most of them were affected by stage but not salt. ZmIVR2, ZmINCW2, and ZmExpLPL were generally induced over time, while ZmSUSY1 was repressed slightly (Fig. 11). However, ZmINCW2 was significantly repressed by salt 3 DAP, while ZmSUSY1 was induced at the silking stage (Fig. 9B). Among SnRK1 target genes, only ARG10, MDH, bZIP11, and DPS transcripts were affected by salt stress, with ARG10 being repressed and the others being induced by osmotic stress. ZmMDH and ZmbZIP11 transcripts were stage sensitive with a general induction over time (Fig. 8C).
In cob tissue, some of the TPS/TPP genes were significantly affected by salt (Fig. 10A). Some were induced (ZmTPSII.2.1 and ZmTPSII.4.3), while others were repressed (ZmTPSI.1.1, ZmTPSII.4.1, and ZmTPSII.5.4.). ZmTPPA.1 was induced by salt at silking and then repressed at pollination (Fig. 10A). ANOVA revealed that, overall, salt only affected ZmTPSII.2.1 and ZmTPPA.1 (Fig. 11). Stage only had an effect on ZmTPSII.2.1 and ZmTPSII.3.2. Among sugar transporters, ZmSUT1 and ZmSUT2 were slightly induced at various stages, while ZmSWEET2 was strongly repressed by salt (Fig. 10B). Transcript levels of the Suc-degrading enzymes ZmINCW2 and ZmSUSY1 were induced by salt at silking and 3 DAP, respectively. Developmental stage strongly affected all genes as well. Almost all SnRK1-induced and -repressed targets were strongly and collectively induced by salt, with the exception of DPS, which was only increased across developmental stages (Fig. 10C). Stage also had a strong effect on all genes but ZmARG10.
DISCUSSION
Abiotic stress such as that caused by drought or salinized soils has a severe impact on plant growth, reproduction, and crop yield. Most important is the sensitivity of cereal crops such as maize to abiotic stress around the time of flowering, often leading to a dramatic drop in grain production. Here, we showed that salinity stress imposed on flowering maize plants resulted in reduced yield as a consequence of kernel abortion. The observed kernel abortion is correlated to a reduction in source strength in the leaf and a reduction in sink strength in the kernel. We showed that salt-induced osmotic stress impacts photosynthesis, cellular respiration, and growth despite the presence of sufficient sugars in the source and sink tissues. We demonstrated strong effects on the expression of the trehalose pathway genes and T6P, with large differences between source and sink tissues. Differential effects on SnRK1 marker genes were also observed between source and sink tissues, possibly indicating unrelated functions.
Salt Stress Inhibits Photosynthesis, Respiration, and Growth
Source strength in leaf is ultimately impacted by the reduction in photosynthesis as a consequence of salt treatment. As long as the vacuole is capable of storing the excess NaCl, the salt treatment only provides an osmotic stress by reducing soil water potential (phase 1), and leaves quickly senesce after the capacity of the vacuole is exceeded (phase 2; Munns, 1993). By 3 DAP, we observed some senescence in the oldest leaves; however, most leaves were green and maintaining turgor (Fig. 1). At silking, source tissue (leaf) from salt-treated plants had overall metabolite levels indistinguishable from control plants, with the exception of Suc and hexoses, although photosynthesis was inhibited by as much as 98% (Fig. 2). This is consistent with results from other species, including maize, sorghum (Niu et al., 2012), wheat (Yousfi et al., 2010; Mguis et al., 2013), rice (Sultana et al., 1999), and Arabidopsis (Stepien and Johnson, 2009). Regardless of this extreme decrease in photosynthesis, sugar and starch concentrations increased in source (leaf), transfer (cob), as well as sink (kernel) tissues (Fig. 3). The higher sugar levels in leaf cells possibly resulted in the observed inhibition of photosynthesis. This was previously observed in maize shoots (De Costa et al., 2007), rice shoots (Amirjani, 2011), and tomato fruits (Yin et al., 2010). However, 3 d later at pollination, we observed a significant decrease in metabolites involved in Suc synthesis, glycolysis, the citric acid cycle, and amino acid synthesis, resulting in a suppression of cellular respiration and growth. This observation reflects the cumulative effects of osmotic and ionic stress on the leaf.
Salt Reduces Source and Sink Strength
Our results indicate that salt treatment represses metabolism in both source and sink tissues in maize. Source and sink strength are primarily dictated by the concentration gradient established between carbohydrate-containing cells and actively growing tissues. It is counterintuitive that higher Suc concentrations found in leaf tissue from salt-stressed plants would result in weaker source strength. However, in leaf tissues, the higher concentration of soluble sugars observed in osmotically stressed plants is a result of osmotic adjustments in response to salt stress, hence inhibiting photosynthesis, as observed previously in chickpea (Flowers et al., 2010). Lower demand in sink tissues results in higher Suc levels in photosynthetic tissues, followed by feedback inhibition of the Calvin-Benson cycle, increased hydrolysis of Suc, and osmotic adjustment using hexoses. We observed that the transcripts for the vacuolar invertase (ZmIVR2) and the cytoplasmic Suc synthase (ZmSUSY1) were induced by salt treatment in the leaf tissue (Fig. 8B). Increased Suc hydrolysis resulted in a reduction of the ratio of Suc to hexose from 9:1 to 4:1 as calculated from the data in Figure 3. In light of reduced growth and respiration, hexose sugars are likely contributing to osmotic adjustment. This is supported by the observation that an exogenous application of Suc enhances the tolerance of Arabidopsis seedlings to salt (Qiu et al., 2014).
Sink strength is driven by low levels of Suc as a result of high levels of consumption via respiration or anabolic synthesis. Here, we show that salt-stressed plants accumulate higher intracellular levels of Suc in sink and transfer tissues. Pollination initiates rapid growth in the ear; however, the significantly reduced levels of glycolysis and citric acid cycle intermediates in the ears from salt-stressed plants indicate that the energy status is insufficient to sustain rapid growth. Consistently lower levels of shikimate in sink tissues from salt-treated plants indicate that these cells are not investing their energy reserves (starch and sugars) in growth (amino acid synthesis). These metabolic results are similar to those observed in salt-treated Arabidopsis, Lotus japonicus, and rice (Sanchez et al., 2008). Interestingly, in kernels, we did not observe a shift from Suc to hexose sugars in response to salt-induced osmotic stress. The ratio of Suc to hexose in kernels at 3 DAP goes up from 1:3.6 in controls to 1:2.7 in salt-treated plants. Andersen et al. (2002) observed a 2-fold increase in ear Suc levels in response to drought stress, while hexose sugars decreased by as much as 50%. At 3 DAP, they observed that the Suc-to-hexose ratio went from 1:6.6 in controls to 1:1 in drought-stressed plants. Hütsch et al. (2014) concluded that the inhibition of soluble acid invertase by salt stress was a key factor in reduced kernel setting in maize yet was not a factor in explaining genotypic differences in salt tolerance. Salt stress also induced the putative Suc facilitator ZmSWEET1 in kernel tissue (Fig. 9B; Chen et al., 2012). Presumably, this Suc efflux protein is being made as a consequence of the elevated Suc levels in the kernel. In transfer tissues (cob), salt treatment strongly repressed the expression of the putative Suc facilitator ZmSWEET2, the only Suc transporter highly specific to cob tissue (based on microarray gene expression patterns) and presumed to function in phloem unloading. This suggests that phloem unloading is being strongly repressed under the conditions of the salt stress, thus decreasing the available photosynthate for the developing kernel.
The T6P-Suc Ratio in Maize Varies between Tissues and Stages of Development
There is overwhelming evidence that the trehalose metabolic pathway is essential for plant growth and reproduction (O’Hara et al., 2013; Lunn et al., 2014). In vascular plants, trehalose metabolism has assumed a role in Suc signaling and as a regulator of carbohydrate metabolism. The foundation of the T6P-Suc nexus lies in the strongly positive correlation between Suc and T6P levels observed during the diurnal cycle in leaves and in response to exogenous sugars (Lunn et al., 2006; Yadav et al., 2014). In Arabidopsis and wheat, high Suc and T6P levels usually correlate with active growth (Martínez-Barajas et al., 2011; Schluepmann et al., 2012; Lunn et al., 2014). At a given tissue and developmental stage, T6P levels usually mirror Suc levels and, therefore, are a good indicator of sugar availability in these species (Martínez-Barajas et al., 2011; Lunn et al., 2014). In control plants, kernel tissue had 300% more Suc and 1,000% more T6P than in mature leaf tissue (Figs. 2 and 3). This supports the strong linkage between Suc and T6P reported previously in Arabidopsis (Carillo et al., 2013; Yadav et al., 2014). We did not observe a strong positive correlation for the T6P-Suc ratio in developing maize kernels from silking to 3 DAP (Table II; Fig. 6). We interpret this as reflecting the changes in metabolism occurring in the developing ovule as it readies for fertilization and begins development of the embryo. We see this as a change in the set point for the T6P-Suc relationship, allowing the regulation of intracellular Suc levels by T6P within different ranges that are appropriate for the developmental stage of the kernel. This is not unlike the differences observed in the T6P-Suc ratio between Arabidopsis seedlings, rosettes, and shoot apices (Lunn et al., 2014).
A Role for the T6P/SnRK1 Signaling Pathway in Salt-Stressed Kernels
The T6P/SnRK1 sugar-sensing mechanism is known for its central role in the stress response (Zhang et al., 2009). Stresses that result in starvation, such as leaf shading, lead to low sugar and low T6P, thus promoting SnRK1 activation. SnRK1 in turn initiates metabolic reprograming to switch between anabolism and catabolism (Baena-González et al., 2007; Henry et al., 2014). This enables the plant to regulate metabolism and growth responses in relation to carbon status. Other stresses, such as cold and nutrient depletion, result in sugar and T6P accumulation (Nunes et al., 2013). Upon prolonged salt treatment, we observed a 136% increase in Suc and a 333% increase in T6P in sink tissues, although only at the silking stage (Fig. 5). SnRK1 activities were 283% lower at silking when compared with controls. T6P was shown previously to inhibit SnRK1 activity (Zhang et al., 2009), specifically in sink tissues (Nunes et al., 2013). Our data agree with previous findings showing that SnRK1 activity is higher and more sensitive to T6P inhibition in sink than in source tissues (Fig. 7). Accordingly, we observed that SnRK1-repressible genes, MDH, bZIP11, and DPS, were derepressed upon salt stress (Fig. 9C), suggesting that T6P is inactivating SnRK1 in immature kernels. No significant correlations were observed between Suc, T6P, and SnRK1 in kernels from osmotically stressed plants. These data are consistent with the results obtained when Arabidopsis seedlings were treated with short-term cold or nitrogen deprivation (Nunes et al., 2013). Altogether, these results indicate that the T6P and SnRK1 signaling pathway might be used differently depending on the stress considered and its effect on various metabolite levels.
A Role for the Trehalose Pathway Independent from SnRK1 in Source Tissues Subjected to Salt Stress
Among the trehalose pathway transcripts affected by salt in source tissues are several class II TPS genes. Although the function of this group of TPS genes is unknown, in maize, these genes were shown previously to be closely regulated at the transcript level. Indeed, they were strongly induced in young leaf by prolonged darkness and then strongly repressed upon the return to light (Henry et al., 2014). These proteins have a similar structure to class I TPS but lack certain amino acids essential for substrate recognition and generally do not possess catalytic activity (Yang et al., 2012; Henry et al., 2014). In rice, some of them were shown to form complexes with class I TPS and are suspected to have a regulatory function (Zang et al., 2011). Therefore, they could have a similar regulatory function in mature maize leaf tissue. Salt treatment of flowering plants increases class II TPS mRNAs in leaf, similar to the way extended darkness increased class II mRNAs in immature leaf (Henry et al., 2014). The only difference is that SnRK1 target genes are not activated in the mature leaf, as SnRK1 is far less active in mature leaves. Class II TPS gene induction is independent of SnRK1 activity, indicating that transcriptional activation of the class II genes bypasses the T6P and SnRK1 signal transduction routes. Salt treatment also lowers ZmTPS1.1 and ZmTPPA.3 mRNA levels in mature leaf, similar to what was observed during the recovery from extended shading (Henry et al., 2014). Here, we show that, in maize, source tissue T6P and the trehalose pathway can function independently of SnRK1 signaling. First, we observed a significant increase of T6P at silking, when SnRK1 activity is very low and mostly insensitive to T6P (Fig. 4). Second, we observed large changes in the expression of the trehalose pathway genes specific to source tissues. Taken together, these results indicate that, in source tissues, the trehalose pathway is affected by salt stress and has the potential to act independently from the SnRK1 pathway. In maize leaf, SnRK1 activity is very low and insensitive to T6P inhibition (Fig. 7). In vitro SnRK1 activity was 15 times lower in leaf as compared with kernel and cob. Accordingly, SnRK1 target genes showed no change during development or as a consequence of salt treatment, in spite of the increase in T6P levels in this tissue (Fig. 7A). No significant correlations were observed between Suc, T6P, and SnRK1 in kernels from osmotically stressed plants, which indicates that the SnRK1 signaling pathway is mainly inactive in source tissues. Therefore, if T6P has any signaling function in source tissues, it must be through yet uncharacterized pathways. In fungus, where the trehalose pathway is highly similar to the one in plants (Avonce et al., 2006), T6P can be sensed through the TPS1 enzyme itself (Wilson et al., 2007). Indeed, in Magnaporthe oryzae, TPS1 has a dual enzymatic/sensing function, and both these functions seem to be independent (Wilson et al., 2007; Fernandez and Wilson, 2011). Such a dual function could be conserved in plant TPS. By analogy, Hexokinase was initially characterized for its enzymatic properties but later found to function as the primary hexose sugar sensor in plants (Cho et al., 2006, 2010; Granot et al., 2013). A dual role for TPS would enable the plant to sense changes in Glc-6-P, UDP-Glc, and/or T6P levels independently from the SnRK1 pathway and trigger an independent physiological response in source tissues. Separating the enzymatic from the sensing functions of a plant protein can be challenging, especially as in the case of TPS, when there are multiple isoforms. Therefore, we suggest that this hypothesis should be investigated in the near future.
CONCLUSION
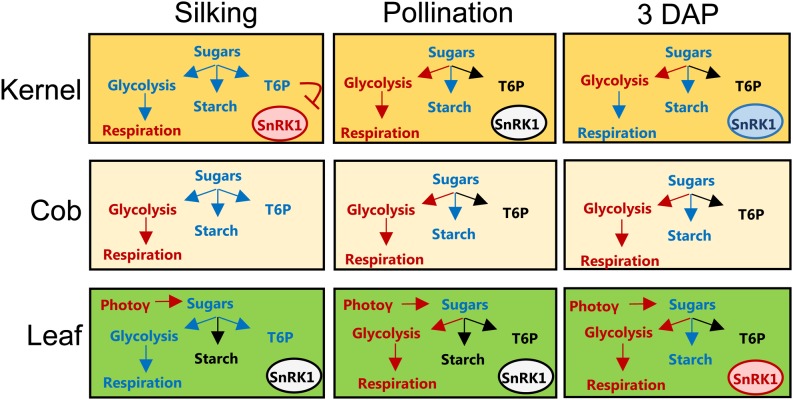
A profound shift in metabolism was observed in well-watered maize kernels after pollination. This switch is diagrammed in a model depicting the metabolic effects of salt treatment on flowering maize plants (Fig. 12). During the 6-d period from silking to 3 DAP, there was an increase in T6P and a decrease in Suc, citric acid cycle intermediates, and SnRK1 activity. This period also saw an increase in invertase gene expression (ZmIVR2 and ZmINCW2) and shikimate, an indicator of amino acid synthesis. All of this indicates a transition from catabolism to anabolism occurring at the time of pollination. It remains unclear if T6P initiates this switch or is induced in response to the switchover. Salt treatment of plants results in much higher kernel T6P levels at silking and, thus, a high rate of kernel abortion. T6P levels are responsive to reduced Suc uptake in sink tissue as a consequence of reduced photosynthesis and activity of sugar transporters. This suggests that T6P levels are a critical component of stress-induced kernel abortion. Understanding the role of T6P and the trehalose pathway in the events leading up to kernel abortion will enable us to improve stress tolerance in grain crops.
Figure 12.
Model depicting the metabolic effects of salt treatment on flowering maize plants at silking, pollination, and 3 DAP. Tissues represented are kernel, cob, and leaf. Watering with 150 mm NaCl results in either no effect (black), decreased levels (red), or increased levels (blue). Photoγ, Photosynthesis.
MATERIALS AND METHODS
Plant Growth, Treatment, and Harvest
Inbred B73 maize (Zea mays) plants were used. Seeds were sterilized for 15 min with 15% (v/v) bleach, thoroughly rinsed with sterile water, incubated for 1 min in 70% (v/v) ethanol, rinsed again, and soaked for 5 min. Seeds were then rolled in germinating paper and germinated for 4 d in the presence of 1 mm CaSO4 solution in a growth chamber (24°C, 220 ± 30 µmol m−2 s−1, and 16-/8-h day/night cycle). Seedlings were planted into germinating trays containing potting mix (34% [w/w] peat, 31% [w/w] perlite, 31% [w/w] vermiculite, and 4% [w/w] soil) and grown under the same conditions as described previously (Henry et al., 2014). At 14 DAG, plants were transferred to pots (8.5 × 8.5 × 7 inches) containing calcinated clay. They were subsequently grown in twin rows (pots aligned in both directions and touching each other) with a constant supply of nutrient solution (Peters 20:20:20, nitrogen:phosphorus:potassium, 200 ppm) from underneath in a greenhouse (14-/10-h day/night cycle, 24°C ± 2°C and 20°C ± 2°C for day and night, respectively) until they reached the V7 to V8 stage (ear and tassel initiation stage). Salt stress was then progressively applied by adding 25, 50, and 75 mm NaCl:CaCl2 (5.7:1 ratio) to the nutrient solution every 3 d. Salt-stressed plants were then kept under 75 mm salt solution until harvesting, and the solution was changed every 3 to 4 d. Control plants were supplied with nutrient solution only during the whole experiment. At the reproductive stage, ears were bagged with paper bags before silking and hand pollinated with fresh pollen from control plants at the pollination stage.
Harvesting was performed from 9 am to 12 pm on plants at the following stages: 1, silking, first silk emergence; 2, pollination, full silking; and 3, 3 DAP. Using a hole punch, tissues from the blade of the leaf neighboring the upper ear were collected. The ear was then dissected, and silks were removed to proceed with the harvesting of kernel and sustaining cob tissues (from the middle one-third longitudinal section of the ear) using a razor blade. For simplicity’s sake, the term kernel is used throughout to refer to the ovule (at silking and pollination) and the embryo (at 3 DAP). Collected tissues (approximately 100–200 mg) were instantly frozen in liquid nitrogen and stored at −80°C until use. Some pollinated plants were also grown until the mature stage to determine yield. In that case, salt treatment was stopped right after pollination.
Morphological Measurements
Plant vegetative growth was assessed by measuring plant height (from crown to flag leaf node) at the pollination stage using a graduated stick. Reproductive growth was determined by following anthesis (1) and anthesis-silking (2) intervals (time from first to full pollen-shedding stages [1] or first pollen-shading to first silk-emergence stages [2]). Ear length was also measured at the harvesting stage. Yield was then reflected by counting and weighing dry seeds.
Photosynthesis Measurements
Leaf photosynthesis and stomatal conductance were determined using the LI-6400 open gas-exchange system coupled to the 6400-02B light-emitting diode light source (Li-Cor). For each treatment, three measurements were taken on the leaf axil to the upper ear of six different plants between 1 and 3 pm. The following settings were used: leaf temperature of 25°C ± 1°C, light intensity of 1,200 µmol m−2 s−1, and air containing 350 µmol mol−1 CO2.
Carbohydrate and Metabolite Analysis
Frozen tissues (20–100 mg) were weighed and ground for 30 to 60 s while frozen using a Tissue Lyser II (Qiagen). Sugars (Suc, Fru, and Glc) were then extracted following the method of Lunn et al. (2006) using lactose as an internal standard. Starch was extracted from the pellet generated during the extraction of soluble sugars and quantified by the analysis of Glc resulting from hydrolysis. Samples were analyzed with a high-pressure capillary ion chromatograph system (ICS-5000, PA-20 column; Thermo Scientific Dionex) using a 1-μL injection volume and 45 mm KOH as eluent. Sugar peaks were identified in comparison with known sugars and an internal standard (lactose), and data were analyzed using the formula described previously (Henry et al., 2014) with lactose/Glc ratio of 1:10 for leaf and 1:100 for kernel and cob. The method of Lunn et al. (2006) using anion-exchange liquid chromatography linked to tandem mass spectrometry was used to quantify T6P and other phosphorylated metabolites.
In Vitro SnRK1 Activity
Total soluble protein was extracted from 200 mg of tissue ground under liquid nitrogen in a mortar and pestle in 600 µL of ice-cold homogenization buffer of 100 mm Tricine-NaOH, pH 8, 25 mm NaF, 5 mm dithiothreitol, 2 mm tetrasodium pyrophosphate, 0.5 mm EDTA, 0.5 mm EGTA, 1 mm benzamidine, 1 mm phenylmethylsulfonyl fluoride, 1 mm protease inhibitor cocktail (Sigma; P9599), phosphatase inhibitors (PhosStop; Roche), and insoluble polyvinylpyrrolidone to 2% (w/v). Homogenate was centrifuged at 13,000g at 4°C. Supernatant (250 µL) was desalted in Illustra NAP-5 columns (GE Healthcare) preequilibrated with homogenization buffer. Eluent was supplemented with protease inhibitor cocktail and okadaic acid to 2.5 mm before freezing in liquid nitrogen. The SnRK1 activity of three replicates for each time point was determined as described (Zhang et al., 2009) in a final volume of 25 µL in microtiter plate wells at 30°C. Assay medium was 40 mm HEPES-NaOH, pH 7.5, 5 mm MgCl2, 200 mm ATP containing 12.5 kBq of [γ-33P]ATP (PerkinElmer), 200 µm AMARA peptide (Enzo Life Sciences), 5 mm dithiothreitol, 1 µM okadaic acid, and 1 mm protease inhibitor cocktail (Sigma; P9599). For inhibition assays, a final concentration of 1 mm T6P was added to the mix. Assays were started with 5 µL of extract and stopped after 6 min by transferring 15 µL to 4-cm2 squares of Whatman P81 phosphocellulose paper immersed immediately in 1% phosphoric acid. These were then washed with four 800-mL volumes of 1% phosphoric acid, immersed in acetone for 15 min, air dried, and transferred to vials with 3.5 mL of scintillation cocktail (Ultima Gold).
mRNA Analysis by qRT-PCR
RNA isolation from maize tissue and qRT-PCR were performed as described by Henry et al. (2014). Reverse transcription quality and absence of genomic DNA contamination were then checked by semiquantitative PCR on 5 µL of complementary DNA (1:100 dilution) in a final volume of 25 µL, using ZmEF1-1 alpha primers (forward, 5′-AGACTCACATCAACATTGTGGTCAT-3′; reverse, 5′-GTTGTCACCTTCAAAACCAGAGATT-3′) designed around an intronic region and GoTaq DNA Polymerase (Promega) as recommended by the supplier. For each time point and biological replicate, qRT-PCR was repeated three times. Experiments were performed on three biological replicates. Three out of eight reference genes (Supplemental Table S1) were selected for the normalization of gene expression using the Genorm software (Vandesompele et al., 2002). Relative gene expression was calculated using the method of Hellemans et al. (2007). Primer efficiency was determined using the method described by Pfaffl et al. (2001) with the following dilutions of 14-d-old B73 seedling complementary DNA: 1:75, 1:100, 1:250, 1:500, and 1:1,000.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Raw metabolite data.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Regina Feil, John E. Lunn, and Mark Stitt for performing the measurements of T6P and other phosphorylated metabolites; the Harkamal Walia, George Graef, and Brian Waters laboratories for technical support concerning RNA and sugar extraction, qRT-PCR, and protein activity assays, respectively; and the greenhouse facility workers for daily maintenance of the plants.
Glossary
- DAP
days after pollination
- T6P
trehalose-6-phosphate
- HPIC
high-pressure ion chromatography
- qRT
quantitative reverse transcription
Footnotes
Articles can be viewed without a subscription.
References
- Ali Q, Ashraf M (2011) Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defence mechanism. J Agron Crop Sci 197: 258–271 [Google Scholar]
- Amirjani MR. (2011) Effect of salinity stress on growth, sugar content, pigments and enzyme activity of rice. Int J Bot 7: 73–81 [Google Scholar]
- Andersen MN, Asch F, Wu Y, Jensen CR, Naested H, Mogensen VO, Koch KE (2002) Soluble invertase expression is an early target of drought stress during the critical, abortion-sensitive phase of young ovary development in maize. Plant Physiol 130: 591–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avonce N, Mendoza-Vargas A, Morett E, Iturriaga G (2006) Insights on the evolution of trehalose biosynthesis. BMC Evol Biol 6: 109–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-González E. (2010) Energy signaling in the regulation of gene expression during stress. Mol Plant 3: 300–313 [DOI] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Boyer JS. (2010) Drought decision-making. J Exp Bot 61: 3493–3497 [DOI] [PubMed] [Google Scholar]
- Bruce WB, Edmeades GO, Barker TC (2002) Molecular and physiological approaches to maize improvement for drought tolerance. J Exp Bot 53: 13–25 [PubMed] [Google Scholar]
- Carillo P, Annunziata MG, Pontecorvo G, Fuggi A, Woodrow P (2011). Salinity stress and salt tolerance. In Shanker A, ed, Abiotic Stress in Plants: Mechanisms and Adaptations. InTech, doi/10.5772/22331 [Google Scholar]
- Carillo P, Feil R, Gibon Y, Satoh-Nagasawa N, Jackson D, Bläsing OE, Stitt M, Lunn JE (2013) A fluorometric assay for trehalose in the picomole range. Plant Methods 9: 21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha-Um S, Kirdmanee C (2009) Effect of salt stress on proline accumulation, photosynthetic ability and growth characters in two maize cultivars. Pak J Bot 41: 87–98 [Google Scholar]
- Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, Fernie AR, Frommer WB (2012) Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335: 207–211 [DOI] [PubMed] [Google Scholar]
- Cho YH, Sheen J, Yoo SD (2010) Low glucose uncouples hexokinase1-dependent sugar signaling from stress and defense hormone abscisic acid and C2H4 responses in Arabidopsis. Plant Physiol 152: 1180–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YH, Yoo SD, Sheen J (2006) Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell 127: 579–589 [DOI] [PubMed] [Google Scholar]
- Cortina C, Culianez-Macia FA (2005) Tomato abiotic stress enhanced tolerance by trehalose biosynthesis. Plant Sci 169: 75–82 [Google Scholar]
- De Costa W, Zörb C, Hartung W, Schubert S (2007) Salt resistance is determined by osmotic adjustment and abscisic acid in newly developed maize hybrids in the first phase of salt stress. Physiol Plant 131: 311–321 [DOI] [PubMed] [Google Scholar]
- Delorge I, Figueroa CM, Feil R, Lunn JE, Van Dijck P (2015) Trehalose-6-phosphate synthase 1 is not the only active TPS in Arabidopsis thaliana. Biochem J 466: 283–290 [DOI] [PubMed] [Google Scholar]
- Duvick DN. (2005) Genetic progress in yield of United States maize (Zea mays L.). Maydica 50: 193–202 [Google Scholar]
- Eastmond PJ, van Dijken AJH, Spielman M, Kerr A, Tissier AF, Dickinson HG, Jones JDG, Smeekens SC, Graham IA (2002) Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J 29: 225–235 [DOI] [PubMed] [Google Scholar]
- Echarte L, Nagore JL, Matteo JD, Cambareri M, Robles M, Maggiora AD (2013) Grain yield determination and resource use efficiency in maize hybrids released in different decades. In Stoytcheva M, Zlatev R, eds, Agricultural Chemistry. Pleiades Publishing, Inc., Moscow, pp 19–36 [Google Scholar]
- Elbashiti T, Hamamci H, Oktem H, Yucel M, El-Bashiti T, Öktem HA, Yücel M (2005) Biochemical analysis of trehalose and its metabolizing enzymes in wheat under abiotic stress conditions. Plant Sci 169: 47–54 [Google Scholar]
- El Naim AM, Mohammed KE, Ibrahim EA, Suleiman NN (2012) Impact of salinity on seed germination and early seedling growth of three sorghum (Sorghum bicolor L. Moench) cultivars. Sci Technol 2: 16–20 [Google Scholar]
- Fernandez J, Wilson R (2011) The sugar sensor, trehalose-6-phosphate synthase (Tps1), regulates primary and secondary metabolism during infection by the rice blast fungus: Will Magnaporthe oryzae's “sweet tooth” become its “Achilles’ heel”? Mycology 2: 46–53 [Google Scholar]
- Fernandez O, Béthencourt L, Quero A, Sangwan RS, Clément C (2010) Trehalose and plant stress responses: friend or foe? Trends Plant Sci 15: 409–417 [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Gaur PM, Gowda CLL, Krishnamurthy L, Samineni S, Siddique KHM, Turner NC, Vadez V, Varshney RK, Colmer TD (2010) Salt sensitivity in chickpea. Plant Cell Environ 33: 490–509 [DOI] [PubMed] [Google Scholar]
- Fortmeier R, Schubert S (1995) Salt tolerance of maize (Zea mays L.): the role of sodium exclusion. Plant Cell Environ 18: 1041–1047 [Google Scholar]
- Garg AK, Kim JK, Owens TG, Ranwala AP, Choi YD, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99: 15898–15903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge LF, Chao DY, Shi M, Zhu MZ, Gao JP, Lin HX (2008) Overexpression of the trehalose-6-phosphate phosphatase gene OsTPP1 confers stress tolerance in rice and results in the activation of stress responsive genes. Planta 228: 191–201 [DOI] [PubMed] [Google Scholar]
- Gómez LD, Baud S, Gilday A, Li Y, Graham IA (2006) Delayed embryo development in the ARABIDOPSIS TREHALOSE-6-PHOSPHATE SYNTHASE 1 mutant is associated with altered cell wall structure, decreased cell division and starch accumulation. Plant J 46: 69–84 [DOI] [PubMed] [Google Scholar]
- Granot D, David-Schwartz R, Kelly G (2013) Hexose kinases and their role in sugar-sensing and plant development. Front Plant Sci 4: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim MA, Juraimi AS, Begum M, Hanafi MM, Ismail MR, Selamat A (2010) Effect of salt stress on germination and early seedling growth of rice (Oryza sativa L.). Afr J Biotechnol 9: 1911–1918 [Google Scholar]
- Halford NG, Hey SJ (2009) Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem J 419: 247–259 [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Fujita M (2013) Ecophysiology and responses of plants under salt stress. In Ahmad P, Azooz MM, Prasad MNV, eds, Ecophysiology and Responses of Plants under Salt Stress. Springer, New York, pp 25–87 [Google Scholar]
- Hassanzadehdelouei M, Vazin F, Nadaf J (2013) Effect of salt stress in different stages of growth on qualitative and quantitative characteristics of cumin (Cuminum cyminum L.). Cercet Agron Mold 46: 89–97 [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, Bledsoe SW, Siekman A, Kollman A, Waters BM, Feil R, Stitt M, Lagrimini LM (2014) The trehalose pathway in maize: conservation and gene regulation in response to the diurnal cycle and extended darkness. J Exp Bot 65: 5959–5973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütsch BW, Saqib M, Osthushenrich T, Schubert S (2014) Invertase activity limits grain yield of maize under salt stress. J Plant Nutr Soil Sci 177: 278–286 [Google Scholar]
- Iordachescu M, Imai R (2008) Trehalose biosynthesis in response to abiotic stresses. J Integr Plant Biol 50: 1223–1229 [DOI] [PubMed] [Google Scholar]
- Jain RK, Selvaraj G (1997) Molecular genetic improvement of salt tolerance in plants. Biotechnol Annu Rev 3: 245–267 [Google Scholar]
- Jang IC, Oh SJ, Seo JS, Choi WB, Song SI, Kim CH, Kim YS, Seo HS, Choi YD, Nahm BH, et al. (2003) Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol 131: 516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakumanu A, Ambavaram MMR, Klumas C, Krishnan A, Batlang U, Myers E, Grene R, Pereira A (2012) Effects of drought on gene expression in maize reproductive and leaf meristem tissue revealed by RNA-Seq. Plant Physiol 160: 846–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Aronsson H, Ericson H, Pirhonen M, Leyman B, Welin B, Mäntylä E, Palva ET, Van Dijck P, Holmström KO (2007) Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Mol Biol 64: 371–386 [DOI] [PubMed] [Google Scholar]
- Khayatnezhad M, Gholamin R, Club YR, Branch IAU (2010) Study of NaCl salinity effect on wheat (Triticum aestivum L.) cultivars at germination stage. Am Eurasian J Agric Environ Sci 9: 128–132 [Google Scholar]
- Khorasani S, Mostafavi K, Heidarian A (2011) Response of maize (Zea mays L.) hybrids and inbred lines to salinity stress under field condition. Tech J Eng Appl Sci 2: 1–7 [Google Scholar]
- León P, Sheen J (2003) Sugar and hormone connections. Trends Plant Sci 8: 110–116 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Delorge I, Figueroa CM, Van Dijck P, Stitt M (2014) Trehalose metabolism in plants. Plant J 79: 544–567 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444: 139–158 [DOI] [PubMed] [Google Scholar]
- Martínez-Barajas E, Delatte T, Schluepmann H, de Jong GJ, Somsen GW, Nunes C, Primavesi LF, Coello P, Mitchell RA, Paul MJ (2011) Wheat grain development is characterized by remarkable trehalose 6-phosphate accumulation pregrain filling: tissue distribution and relationship to SNF1-related protein kinase1 activity. Plant Physiol 156: 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JE, Boyer JS (2004) Sugar-responsive gene expression, invertase activity, and senescence in aborting maize ovaries at low water potentials. Ann Bot (Lond) 94: 675–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mguis K, Albouchi A, Abassi M, Khadhri A, Ykoubi-Tej M, Mahjoub A, Ben Brahim N, Ouerghi Z (2013) Responses of leaf growth and gas exchanges to salt stress during reproductive stage in wild wheat relative Aegilops geniculata Roth. and wheat (Triticum durum Desf.). Acta Physiol Plant 35: 1453–1461 [Google Scholar]
- Miranda JA, Avonce N, Suárez R, Thevelein JM, Van Dijck P, Iturriaga G (2007) A bifunctional TPS-TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis. Planta 226: 1411–1421 [DOI] [PubMed] [Google Scholar]
- Munns R. (1993) Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ 16: 15–24 [Google Scholar]
- Niu G, Xu W, Rodriguez D, Sun Y (2012) Growth and physiological responses of maize and sorghum genotypes to salt stress. ISRN Agron 2012: 1–12 [Google Scholar]
- Nuccio ML, Wu J, Mowers R, Zhou H-P, Meghji M, Primavesi LF, Paul MJ, Chen X, Gao Y, Haque E, Basu SS, Lagrimini LM (2015) Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat Biotechnol 33: 862–869 [DOI] [PubMed] [Google Scholar]
- Nunes C, O’Hara LE, Primavesi LF, Delatte TL, Schluepmann H, Somsen GW, Silva AB, Fevereiro PS, Wingler A, Paul MJ (2013) The trehalose 6-phosphate/SnRK1 signaling pathway primes growth recovery following relief of sink limitation. Plant Physiol 162: 1720–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara LE, Paul MJ, Wingler A (2013) How do sugars regulate plant growth and development? New insight into the role of trehalose-6-phosphate. Mol Plant 6: 261–274 [DOI] [PubMed] [Google Scholar]
- Otegui ME, Andrade FH, Suero EE (1995) Growth, water use, and kernel abortion of maize subjected to drought at silking. Front Crop Res 40: 87–94 [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnu J, Wahl V, Schmid M (2011) Trehalose-6-phosphate: connecting plant metabolism and development. Front Plant Sci 2: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik MHR, Imai R (2005) Functional identification of a trehalose 6-phosphate phosphatase gene that is involved in transient induction of trehalose biosynthesis during chilling stress in rice. Plant Mol Biol 58: 751–762 [DOI] [PubMed] [Google Scholar]
- Qiu Z, Wang Y, Zhu A, Peng F, Wang L (2014) Exogenous sucrose can enhance tolerance of Arabidopsis thaliana seedlings to salt stress. Biol Plant 58: 611–617 [Google Scholar]
- Rastegar Z, Aghighi M, Kandi S (2011) The effect of salinity and seed size on seed reserve utilization and seedling growth of soybean. Int J Agron Plant Prod 2: 1–4 [Google Scholar]
- Ruan YL, Jin Y, Yang YJ, Li GJ, Boyer JS (2010) Sugar input, metabolism, and signaling mediated by invertase: roles in development, yield potential, and response to drought and heat. Mol Plant 3: 942–955 [DOI] [PubMed] [Google Scholar]
- Samineni S, Siddique KHM, Gaur PM, Colmer TD (2011) Salt sensitivity of the vegetative and reproductive stages in chickpea (Cicer arietinum L.): podding is a particularly sensitive stage. Environ Exp Bot 71: 260–268 [Google Scholar]
- Sanchez DH, Siahpoosh MR, Roessner U, Udvardi M, Kopka J (2008) Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiol Plant 132: 209–219 [DOI] [PubMed] [Google Scholar]
- Satoh-Nagasawa N, Nagasawa N, Malcomber S, Sakai H, Jackson D (2006) A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 441: 227–230 [DOI] [PubMed] [Google Scholar]
- Schluepmann H, Berke L, Sanchez-Perez GF (2012) Metabolism control over growth: a case for trehalose-6-phosphate in plants. J Exp Bot 63: 3379–3390 [DOI] [PubMed] [Google Scholar]
- Setter TL, Flannigan BA, Melkonian J (2001) Loss of kernel set due to water deficit and shade in maize. Crop Sci 41: 1530–1540 [Google Scholar]
- Setter TL, Yan J, Warburton M, Ribaut JM, Xu Y, Sawkins M, Buckler ES, Zhang Z, Gore MA (2011) Genetic association mapping identifies single nucleotide polymorphisms in genes that affect abscisic acid levels in maize floral tissues during drought. J Exp Bot 62: 701–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims DA, Pearcy RW (1994) Scaling sun and shade photosynthetic acclimation of Alocasia macrorrhiza to whole-plant performance. I. Carbon balance and allocation at different daily photon flux densities. Plant Cell Environ 17: 881–887 [Google Scholar]
- Smeekens S, Ma J, Hanson J, Rolland F (2010) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13: 274–279 [DOI] [PubMed] [Google Scholar]
- Stepien P, Johnson GN (2009) Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol 149: 1154–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana N, Ikeda T, Itoh R (1999) Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ Exp Bot 42: 211–220 [Google Scholar]
- Sun K, Hunt K, Hauser BA (2004) Ovule abortion in Arabidopsis triggered by stress. Plant Physiol 135: 2358–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollenaar M, Lee EA (2006) Dissection of physiological processes underlying grain yield in maize by examining genetic improvement and heterosis. Crop Sci 51: 399–408 [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: research0034.1–research0034.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgate ME, Boyer JS (1985) Carbohydrate reserves and reproductive development at low leaf water potentials in maize. Crop Sci 25: 762–769 [Google Scholar]
- Westgate ME, Boyer JS (1986) Reproduction at low and pollen water potentials in maize. Crop Sci 26: 951–956 [Google Scholar]
- Wilson RA, Jenkinson JM, Gibson RP, Littlechild JA, Wang Z-Y, Talbot NJ (2007) Tps1 regulates the pentose phosphate pathway, nitrogen metabolism and fungal virulence. EMBO J 26: 3673–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A. (2002) The function of trehalose biosynthesis in plants. Phytochemistry 60: 437–440 [DOI] [PubMed] [Google Scholar]
- Wobus U, Weber H (1999) Sugars as signal molecules in plant seed development. Biol Chem 380: 937–944 [DOI] [PubMed] [Google Scholar]
- Yadav UP, Ivakov A, Feil R, Duan GY, Walther D, Giavalisco P, Piques M, Carillo P, Hubberten HM, Stitt M, et al. (2014) The sucrose-trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. J Exp Bot 65: 1051–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HL, Liu YJ, Wang CL, Zeng QY (2012) Molecular evolution of trehalose-6-phosphate synthase (TPS) gene family in Populus, Arabidopsis and rice. PLoS ONE 7: e42438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Lu C (2005) Photosynthesis is improved by exogenous glycinebetaine in salt-stressed maize plants. Physiol Plant 124: 343–352 [Google Scholar]
- Yin YG, Kobayashi Y, Sanuki A, Kondo S, Fukuda N, Ezura H, Sugaya S, Matsukura C (2010) Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) fruits in an ABA- and osmotic stress-independent manner. J Exp Bot 61: 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousfi S, Serret MD, Voltas J, Araus JL (2010) Effect of salinity and water stress during the reproductive stage on growth, ion concentrations, Delta 13C, and Delta 15N of durum wheat and related amphiploids. J Exp Bot 61: 3529–3542 [DOI] [PubMed] [Google Scholar]
- Zang B, Li H, Li W, Deng XW, Wang X (2011) Analysis of trehalose-6-phosphate synthase (TPS) gene family suggests the formation of TPS complexes in rice. Plant Mol Biol 76: 507–522 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ (2009) Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol 149: 1860–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinselmeier C, Jeong BR, Boyer JS (1999) Starch and the control of kernel number in maize at low water potentials. Plant Physiol 121: 25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinselmeier C, Westgate ME, Schussler JR, Jones RJ (1995) Low water potential disrupts carbohydrate metabolism in maize (Zea mays L.) ovaries. Plant Physiol 107: 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.