A protein phosphatase negatively regulates Arabidopsis leaf senescence through dephosphorylating a senescence-promoting receptor-like kinase.
Abstract
Reversible protein phosphorylation mediated by protein kinases and phosphatases plays an important role in the regulation of leaf senescence. We previously reported that the leucine-rich repeat receptor-like kinase SENESCENCE-ASSOCIATED RECEPTOR-LIKE KINASE (AtSARK) positively regulates leaf senescence in Arabidopsis (Arabidopsis thaliana). Here, we report the involvement of a protein serine/threonine phosphatase 2C-type protein phosphatase, SENESCENCE-SUPPRESSED PROTEIN PHOSPHATASE (SSPP), in the negative regulation of Arabidopsis leaf senescence. SSPP transcript levels decreased greatly during both natural senescence and SARK-induced precocious senescence. Overexpression of SSPP significantly delayed leaf senescence in Arabidopsis. Protein pull-down and bimolecular fluorescence complementation assays demonstrated that the cytosol-localized SSPP could interact with the cytoplasmic domain of the plasma membrane-localized AtSARK. In vitro assays showed that SSPP has protein phosphatase function and can dephosphorylate the cytosolic domain of AtSARK. Consistent with these observations, overexpression of SSPP effectively rescued AtSARK-induced precocious leaf senescence and changes in hormonal responses. All our results suggested that SSPP functions in sustaining proper leaf longevity and preventing early senescence by suppressing or perturbing SARK-mediated senescence signal transduction.
As the final stage of leaf development, senescence occurs in an age-dependent manner and in response to the interplay of multiple internal and external signals (Gan and Amasino, 1995; Miller et al., 1999; Guo and Gan, 2005; Zhang and Zhou, 2013). Leaf senescence also plays important roles in plant fitness by recycling nutrients to vigorously growing organs (Lohman et al., 1994). Modifications of this process directly affect the agricultural traits of crop plants (Zhang et al., 1987; Rivero et al., 2007). Substantial progress has been made in addressing the underlying molecular mechanisms of senescence (Lim et al., 2007; Thomas, 2013), but the distinct pathways that transduce different signals to control the initiation and progression of leaf senescence remain unclear.
Reversible protein phosphorylation, catalyzed by protein kinases and phosphatases, plays a critical role in cellular signaling. The involvement of specific protein kinases in the regulation of leaf senescence has also been suggested. For example, the membrane-bound receptor protein kinase RPK1 affects Arabidopsis (Arabidopsis thaliana) leaf senescence induced by abscisic acid (ABA); loss-of-function mutants of RPK1 exhibit delayed symptoms in both age-dependent and ABA-induced senescence (Lee et al., 2011). ARABIDOPSIS HISTIDINE KINASE3 functions as the major cytokinin receptor kinase that mediates the antisenescence effect of cytokinins through the specific phosphorylation of ARABIDOPSIS RESPONSE REGULATOR2 (Kim et al., 2006). ENHANCED DISEASE RESISTANCE1, a CONSTITUTIVE TRIPLE RESPONSE1-like kinase, functions as a negative regulator of ethylene-induced senescence in an ETHYLENE INSENSITIVE2 (EIN2)-dependent manner (Frye et al., 2001; Tang et al., 2005). In addition, a member of the ACTIVITY OF BC1 COMPLEX (ABC1) protein kinase family, OsABC1-2, confers enhanced tolerance to dark-induced senescence in rice (Oryza sativa; Gao et al., 2012). A mitogen-activated protein kinase cascade involving MAP KINASE KINASE9 and its downstream target MITOGEN-ACTIVATED PROTEIN KINASE6 (MPK6) plays a positive role in regulating leaf senescence (Zhou et al., 2009). Also, SUCROSE-NONFERMENTATION1-RELATED PROTEIN KINASE1 (SnRK1), an energy sensor kinase, plays a negative role in the regulation of leaf senescence (Cho et al., 2012).
Dephosphorylation by protein phosphatases functions as a balancing switch to reverse the effects of phosphorylation by protein kinases. Removal of phosphates often renders protein kinases inactive, effectively halting their cellular functions. Some examples include KINASE-ASSOCIATED PROTEIN PHOSPHATASE, which functions as a negative regulator of the CLAVATA1 signal transduction pathway, and group A PROTEIN SERINE/THREONINE PHOSPHATASE 2C (PP2C), which efficiently inactivates SnRK2s (subclass III SnRK2) in ABA signaling (Stone et al., 1998; Schweighofer et al., 2004; Umezawa et al., 2009). In recent years, evidence has emerged that protein phosphatases also have pivotal functions in the regulation of leaf senescence. For example, the PP2C family protein phosphatase SENESCENCE-ASSOCIATED GENE113 (SAG113), which serves as a negative regulator of ABA signal transduction, is involved in the control of water loss during leaf senescence in Arabidopsis (Zhang et al., 2012). Silencing of the protein phosphatase MITOGEN-ACTIVATED PROTEIN KINASE PHOSPHATASE2, which positively regulates oxidative stress tolerance and inactivates the MPK3 and MPK6 mitogen-activated protein kinases in Arabidopsis, promotes early senescence (Lee and Ellis, 2007; Li et al., 2012). However, few key protein phosphatases that interact with a known senescence-associated protein kinase and function in the regulation of leaf senescence have been characterized.
We previously reported that a soybean (Glycine max) dual-specificity kinase, GmSARK, and its Arabidopsis homolog, AtSARK, regulate leaf senescence through the synergistic actions of auxin and ethylene (Xu et al., 2011). In this study, we cloned and identified a PP2C-type protein phosphatase, SENESCENCE-SUPPRESSED PROTEIN PHOSPHATASE (SSPP), which negatively regulates leaf senescence in Arabidopsis. The transcript level of SSPP was greatly reduced during both natural senescence and SARK-induced precocious senescence in Arabidopsis. Overexpression of SSPP significantly delayed leaf senescence in transgenic Arabidopsis. Protein pull-down and bimolecular fluorescence complementation (BiFC) assays demonstrated that the cytosol-localized SSPP could interact with the plasma membrane-localized AtSARK both in vitro and in vivo. SSPP also dephosphorylated the cytoplasmic domain of AtSARK in vitro. Overexpression of SSPP effectively restored SARK-induced precocious leaf senescence. All results suggested that SSPP negatively regulates leaf senescence through suppressing or perturbing SARK-mediated senescence signal transduction by directly dephosphorylating AtSARK.
RESULTS
The Expression of SSPP Is Suppressed during Leaf Senescence
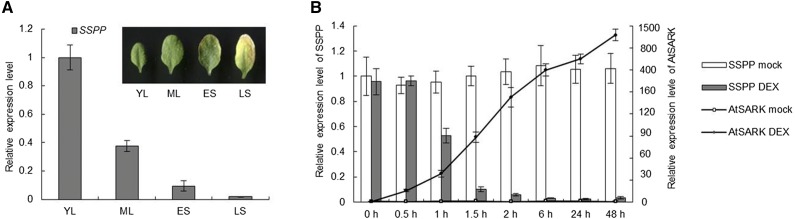
We previously performed a microarray analysis to detect changes in the transcriptome of the SARK-induced early-senescent Arabidopsis seedlings and identified one gene (GenBank accession no. At5g02760) with substantially decreased transcript levels (F. Xu and N.N. Wang, unpublished data). Here, based on our subsequent observations, we call this gene SSPP. To confirm the involvement of SSPP in leaf senescence, we used quantitative reverse transcription (RT)-PCR to measure its transcript levels in both natural leaf senescence and SARK-induced early senescence processes. The SSPP transcript level was high in young leaves and decreased gradually as the leaves developed from nonsenescent to late senescence stages (Fig. 1A). For SARK-induced senescence, we used the dexamethasone (DEX)-inducible construct GVG:AtSARK; as shown in our previous report (Xu et al., 2011), DEX treatment resulted in a continuous increase in the transcript level of AtSARK and an early senescence phenotype in the vertically grown 4-d-old GVG:AtSARK transgenic seedlings (Fig. 1B). In these seedlings, a rapid decrease in the SSPP transcript levels was found after 1 h of DEX treatment. Upon 2 h of DEX treatment, the SSPP mRNA level dropped to 10% of its untreated level. No accumulation of AtSARK transcript was detected in the mock-treated GVG:AtSARK seedlings, in which the transcription level of SSPP was also not affected (Fig. 1B).
Figure 1.
The expression of SSPP is suppressed during leaf senescence. A, Quantitative RT-PCR analysis of the expression level of SSPP in senescing leaves. YL, Young leaves; ML, mature leaves; ES, early senescence stage leaves; LS, late senescence stage leaves. The TIP41-like gene was used as an internal control in RT-PCR, and the data are displayed as relative expression to YL. Three biological replicates with at least three technical repeats were done. Error bars represent sd. B, Comparative analyses of the time-course expression profiles of AtSARK and SSPP in a typical GVG:AtSARK line, AtS20, upon mock and DEX treatments. Four-day-old transgenic Arabidopsis seedlings were incubated on a 10 μm DEX-containing plate for 0, 0.5, 1, 1.5, 2, 6, 24, or 48 h. Data are normalized to the mock-treated control at 0 h. Three biological replicates with at least three technical repeats were done. Error bars represent sd.
SSPP Encodes a PP2C-Type Protein Phosphatase
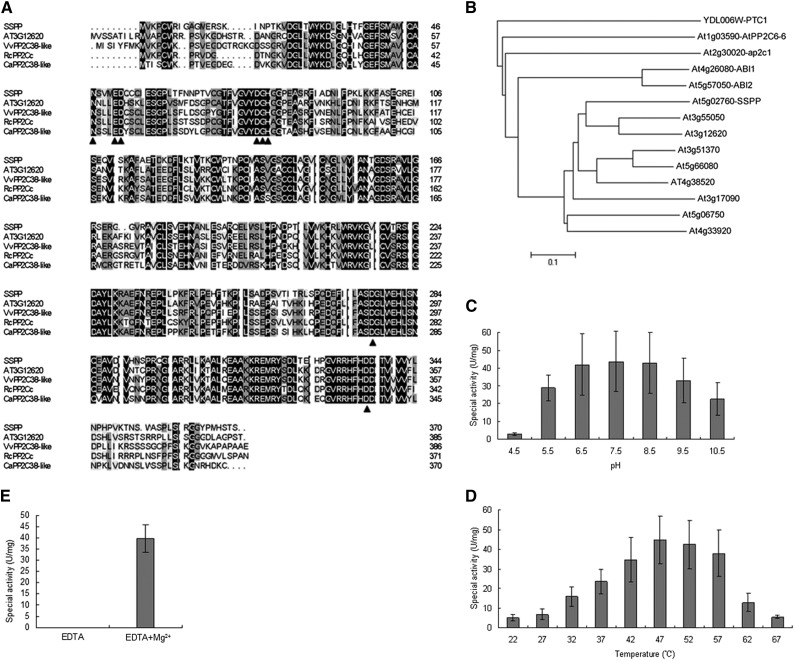
The complete sequence of SSPP consists of 1,543 bp of complementary DNA (cDNA) and encodes a protein of 370 amino acids. The predicted SSPP protein shows high sequence similarity to a Thellungiella halophila putative PP2C family protein (GenBank accession no. BAJ33929), with 91% amino acid sequence identity. RefSeq Protein database searches (http://blast.ncbi.nlm.nih.gov) revealed that SSPP exhibits 66% amino acid identity with Vitis vinifera PP2C38-like and Cicer arietinum PP2C38-like. In Arabidopsis, SSPP is most similar to the predicted PP2C family protein At3g12620, with 58% identity on the amino acid level (Fig. 2A). SSPP contains the amino acids that form the catalytic sites in the channel of the β-sandwich of PP2C homologs (Das et al., 1996; Fig. 2A). Consistent with a previous report (Schweighofer et al., 2004), a phylogenetic tree also shows that SSPP has high similarity to the PP2C group D subfamily proteins (Fig. 2B).
Figure 2.
Amino acid sequence analysis of SSPP and biochemical characterization of SSPP phosphatase activity. A, Alignment of the predicted amino acid sequence of SSPP with its homologs from different plant species. Comparison of the protein sequences of SSPP and its homologs from Arabidopsis (At3g12620), Vitis vinifera (VvPP2C38-like; XP_002276631.1), Ricinus communis (RcPP2C; XP_002525208.1), and Cicer arietinum (CaPP2C38-like; XP_004493923.1) was performed by ClustalX and displayed by DNAMAN software. Black arrowheads indicate the conserved active sites that feature PP2C homologs. B, Phylogenetic tree of PP2Cs related to SSPP. A neighbor-joining tree was built on the full-length protein sequences of PP2C subfamily D members. The scale bar is an indicator of genetic distance based on branch length. C and D, pH (C) and temperature (D) optima for SSPP activity with pNPP as the substrate. E, The phosphatase activity of SSPP was determined in the presence or absence of Mg2+. Reaction conditions are described in “Materials and Methods.” Three independent replicates were done to give the average results shown here. Error bars represent sd. U, Units.
To determine if SSPP can function as a protein phosphatase, the recombinant SSPP protein with a GLUTATHIONE S-TRANSFERASE (GST) tag was expressed in Escherichia coli and purified by affinity chromatography using a Glutathione-Sepharose 4B column. After treating GST-SSPP with the site-specific PreScission protease to remove the GST tag, we tested the purified SSPP in a phosphatase assay using p-nitrophenyl phosphate (pNPP) as a substrate (An and Carmichael, 1994). SSPP exhibited obvious phosphatase activity, successfully cleaving the phosphate from pNPP and generating a yellow nitrophenol product that was quantitated by A405. As shown in Figure 2, SSPP functions at an optimum pH between 6.5 and 8.5 (Fig. 2C) and an optimum temperature between 42°C and 57°C (Fig. 2D). In addition, SSPP exhibited an absolute requirement for Mg2+, indicating that SSPP is an Mg2+-dependent phosphatase enzyme (Fig. 2E).
Overexpression of SSPP Significantly Delays Leaf Senescence
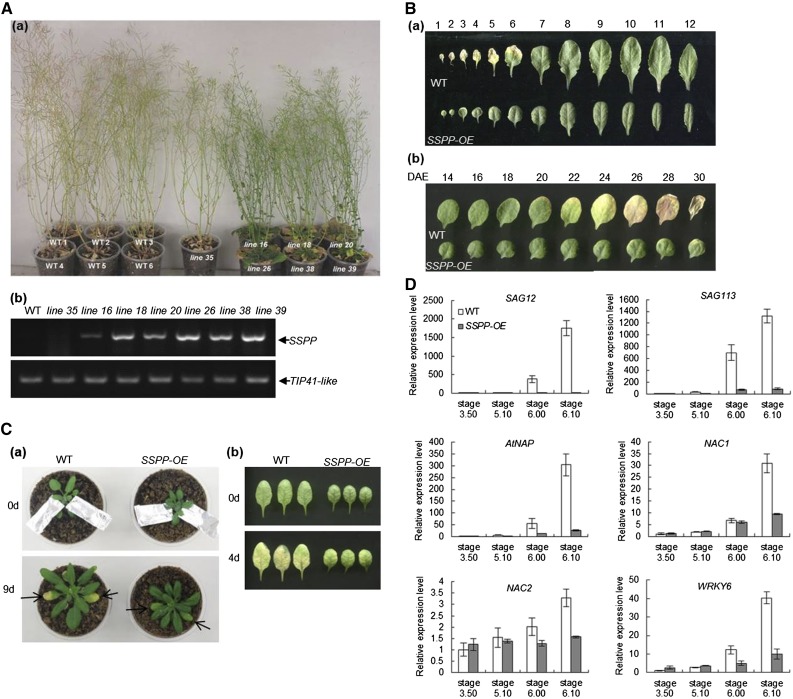
To further examine the biological functions of SSPP, we tested the effects of SSPP overexpression. Multiple independent lines of 35S:SSPP transgenic Arabidopsis were generated by the Agrobacterium tumefaciens-mediated floral dip method (Clough and Bent, 1998). Except for line 35, in which the expression of SSPP was silenced, the other six lines exhibited a significant delay in senescence (Fig. 3A), and we selected line 38 as a typical line for further study. To gain a better view of the function of SSPP in leaf senescence, the rosette leaves of 33-d-old 35S:SSPP plants and the developing fifth leaves of 35S:SSPP plants were compared with their corresponding wild-type controls. As shown in Figure 3B, the 35S:SSPP transgenic plants showed an obvious delay in natural leaf senescence. We also tested dark-induced senescence using either attached or detached fifth and sixth leaves at the mature stage (from plants at Boyes growth stage 5.10; Supplemental Fig. S1). It was found that both the attached 35S:SSPP leaves individually covered by aluminum foil for 9 d (Fig. 3C, a) and the detached 35S:SSPP leaves incubated in darkness for 4 d (Fig. 3C, b) exhibited much delayed senescence when compared with their corresponding wild-type controls (Fig. 3C).
Figure 3.
Overexpression of SSPP delays leaf senescence. A, a, Seven independent 35S:SSPP transgenic lines (lines 16, 18, 20, 26, 38, 35, and 39) and their wild-type (WT) controls were cultivated under long-day photoperiod conditions for up to 57 d. b, Determination of SSPP transcript levels in the above 35S:SSPP transgenic lines by semiquantitative RT-PCR. The sixth leaves of 33-d-old plants were sampled. The TIP41-like gene was used as an internal control. Three biological replicates were done to give the typical results shown here. B, a, Leaves from 33-d-old wild-type and 35S:SSPP plants were laid out in order of emergence. b, Age-dependent senescence phenotype of the fifth rosette leaves of wild-type and 35S:SSPP plants. DAE, Days after emergence. C, Dark-induced senescence was delayed in 35S:SSPP transgenic Arabidopsis. a, The fifth and sixth leaves of wild-type and 35S:SSPP transgenic plants at stage 5.10 under normal growth conditions were covered by aluminum foil for up to 9 d. b, The fifth leaves of wild-type and 35S:SSPP transgenic plants at stage 5.10 were detached and incubated in darkness for 4 d. D, Overexpression of SSPP reduced the transcript levels of several senescence-related marker genes in Arabidopsis plants. The sixth leaves of the wild type and 35S:SSPP transgenic plants at four different developmental stages were sampled. The transcript levels of the marker genes were determined by quantitative RT-PCR, with the expression of TIP41-like as an internal control. Values are normalized relative to the expression in the wild-type control at stage 3.50. Three biological replicates with at least three technical repeats were done for each gene. Error bars represent sd.
Based on the Boyes growth stage ontology (Boyes et al., 2001), the sixth leaves of the wild-type and 35S:SSPP transgenic plants at four different developmental stages, stage 3.50, stage 5.10, stage 6.00, and stage 6.10 (Supplemental Fig. S1), were sampled to assay the transcript levels of known senescence-associated genes. The tested genes included the age-related senescence marker gene SAG12 (Gan and Amasino, 1995), the PP2C family protein phosphatase gene SAG113 (Zhang et al., 2012), and four critical senescence-related transcription factors, NAC1 (Kim et al., 2009), NAC2 (Kim et al., 2009), WRKY6 (Robatzek and Somssich, 2002), and AtNAP (Guo and Gan, 2006). Quantitative RT-PCR analysis revealed a remarkable increase in the transcript levels of these genes when the wild-type plants developed from stage 5.10 to stage 6.00 and stage 6.10; however, the increase in the expression of these senescence-associated genes was significantly inhibited in the 35S:SSPP plants (Fig. 3D). Besides significantly delayed leaf senescence, overexpression of SSPP in Arabidopsis also resulted in shorter roots, smaller rosette leaves with a curved surface, and shorter plant height (Supplemental Fig. S2, A–F). The 35S:SSPP plants also exhibited a delay of 5 d in bolting time.
To further reveal the function of SSPP in senescence, we next examined the SSPP loss-of-function phenotype. We obtained a Salk transfer DNA (T-DNA) insertion line, SALK_099356C, which has a T-DNA insertion in the last (fourth) exon, and named it sspp-1. However, except for the weak advance in dark-induced senescence, no significant difference in growth and development between the sspp-1 mutant and wild-type plants was observed (Supplemental Fig. S3), indicating that redundant genes may regulate leaf senescence in Arabidopsis.
Overexpression of SSPP Sustains Chloroplast Structure and Function
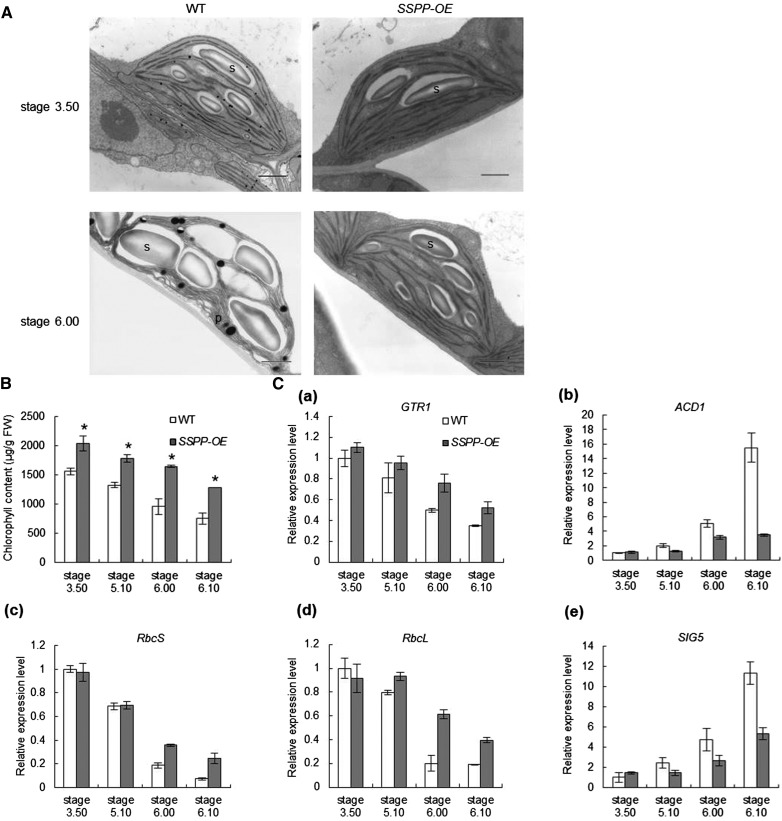
To investigate the cellular events caused by the overexpression of SSPP, mesophyll cells from the sixth leaves of 35S:SSPP transgenic plants at stages 3.50 and 6.00 were examined by electron microscopy. As shown in Figure 4A, the color of the osmium-fixed chloroplasts of 35S:SSPP transgenic plants was darker than that of the wild-type plants, suggesting that the 35S:SSPP chloroplasts were more active at both stages. In the younger sixth leaves from plants at stage 3.50, both the SSPP-overexpressing and wild-type chloroplasts exhibited similar inner membrane systems (Fig. 4A). However, when the plants developed from stage 3.50 to stage 6.00, the sixth leaves of the wild-type plant displayed early senescence symptoms accompanied by many huge starch grains accumulated in their chloroplasts; oppositely, the SSPP-overexpressing chloroplasts exhibited a much better organized inner membrane system (Fig. 4A). The chlorophyll contents in the sixth leaves at all four tested developmental stages, 3.50, 5.10, 6.00, and 6.10, were significantly increased in the 35S:SSPP transgenic plants (Fig. 4B).
Figure 4.
Overexpression of SSPP sustains the structure and function of chloroplasts. A, Ultrastructural morphology of chloroplasts in mesophyll cells from the sixth leaves of wild-type (WT) and 35S:SSPP transgenic plants at stages 3.50 and 6.00. P, Plastoglobuli; S, starch. Bars = 1 μm. B, Comparisons of chlorophyll contents of the sixth leaves of wild-type and 35S:SSPP transgenic plants at four different developmental stages. Data are means ± sd of three experiments. Significant differences between wild-type and 35S:SSPP transgenic plants are indicated by asterisks on the error bars (Student’s t test, P < 0.05). FW, Fresh weight. C, Overexpression of SSPP changes the expression levels of genes involved in chlorophyll metabolism and chloroplast functions in Arabidopsis plants. The sixth leaves of wild-type and 35S:SSPP transgenic plants at four developmental stages were sampled. The transcript levels of the marker genes were determined by quantitative RT-PCR, with the expression of TIP41-like as an internal control. Values are normalized relative to the expression in the wild-type control at stage 3.50. Three biological replicates with at least three technical repeats were done for each gene. Error bars represent sd.
Quantitative RT-PCR was used to measure the expression of genes encoding key enzymes involved in chlorophyll metabolism and chloroplast functions in the 35S:SSPP transgenic plants at the above-mentioned four developmental stages. It was found that when developing from stage 3.50 to stage 6.10, the transcript levels of GTR1, which encodes the chlorophyll biosynthesis enzyme glutamyl tRNA reductase (McCormac et al., 2001), and two photosynthetic genes, RUBISCO LARGE SUBUNIT (RbcL) and RUBISCO SMALL SUBUNIT (RbcS) (Krebbers et al., 1988; Isono et al., 1997), were gradually decreased in the sixth leaves of wild-type plants; however, the decrease in the transcription of these genes was significantly retarded in the corresponding 35S:SSPP transgenic leaves (Fig. 4C). On the contrary, the age-induced increase in the transcript levels of ACCELERATED CELL DEATH1 (ACD1), which encodes the chlorophyll breakdown enzyme pheide α-oxygenase (Pruzinská et al., 2003), and a plastid sigma factor, SIG5, which is induced under adverse conditions to protect plants from stresses by enhancing the repair of the PSII reaction center (Nagashima et al., 2004), was greatly suppressed in the SSPP-overexpressing plants (Fig. 4C). These results, consistent with the ultrastructural morphology analysis of the transgenic chloroplasts (Fig. 4A), suggested that overexpression of SSPP sustains the structure and function of chloroplasts.
Effects of SSPP Overexpression on Cytokinin, Auxin, and Ethylene Responses
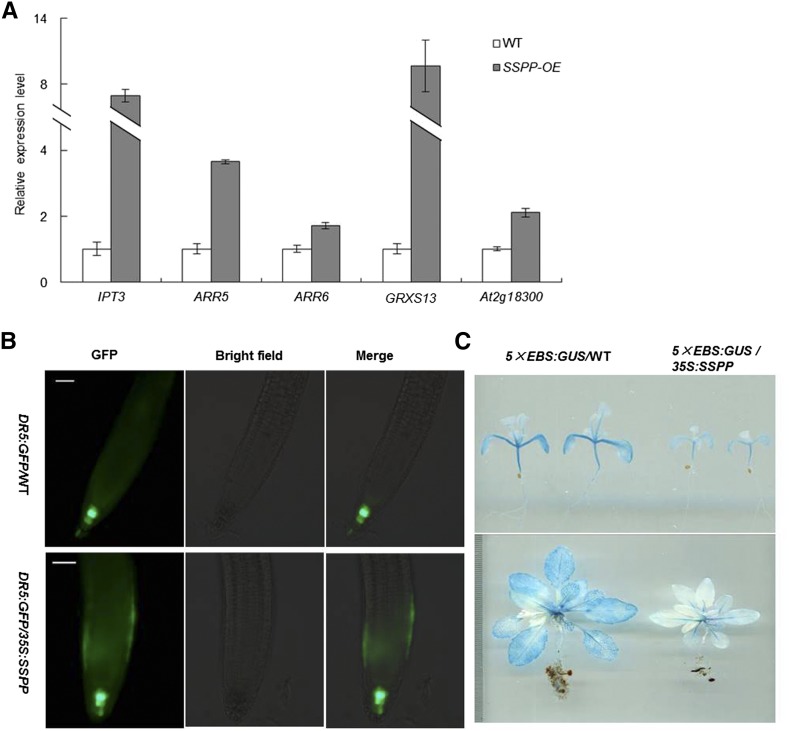
Cytokinin is well known as a senescence-delaying hormone. Increases in cytokinin levels lead to delayed senescence in many plant species, including rice (Kudo et al., 2012), maize (Zea mays; Pineda Rodo et al., 2008), and iris (Iris × hollandica ‘Blue Magic’; van Doorn et al., 2013). To examine the effect of SSPP overexpression on cytokinin pathways, quantitative RT-PCR was used to measure transcript levels of several cytokinin-responsive marker genes, including ISOPENTENYL TRANSFERASE3 (IPT3), which encodes the key enzyme of cytokinin biosynthesis, the type A ARABIDOPSIS RESPONSE REGULATORs (ARRs) ARR5 and ARR6, which have been commonly used as cytokinin-inducible markers (Cui et al., 2010), GRXS13, a cytokinin up-regulated gene encoding for two CC-type GLUTAREDOXIN (GRX) isoforms (Nemhauser et al., 2006; Laporte et al., 2012), and At2g18300, which encodes a basic helix-loop-helix cytokinin-responsive transcription factor (Brenner et al., 2005). Quantitative RT-PCR analysis revealed that the expression of these five genes in leaves at the mature stage was significantly higher in the SSPP overexpression plants (Fig. 5A). These results indicated that SSPP overexpression enhanced cytokinin responses in the transgenic Arabidopsis plants.
Figure 5.
Overexpression of SSPP alters multiple hormone responses in Arabidopsis. A, Changes in the expression of cytokinin-related marker genes in 35S:SSPP plants. The sixth leaves of wild-type (WT) and SSPP-overexpressing plants at stage 5.10 were sampled. The transcript levels of the marker genes were determined by quantitative RT-PCR, with the expression of TIP41-like as an internal control. The data are shown relative to the wild-type control. Three biological replicates with at least three technical repeats were done. Error bars represent sd. B, Expression of DR5:GFP in the roots of 7-d-old wild-type (DR5:GFP/WT) and SSPP-overexpressing (DR5:GFP/35S:SSPP) seedlings. Bars = 50 μm. C, Expression of 5×EBS:GUS in 2-week-old (top) and 4-week-old (bottom) wild-type (5×EBS:GUS/WT) and SSPP-overexpressing (5×EBS:GUS/35S:SSPP) plants.
DIRECT REPEAT5 (DR5) is a synthetic promoter consisting of seven tandem repeats of an auxin-responsive TGTCTC element and a minimal 35S cauliflower mosaic virus promoter (Ulmasov et al., 1997). The DR5 promoter fused to a reporter gene has been widely used as a good tool to monitor auxin response in planta (Sabatini et al., 1999; Wang et al., 2005). To examine auxin pathways, we used DR5:GFP to detect auxin accumulation and distribution in the SSPP-overexpressing seedlings. In the wild-type background, the fluorescence of DR5:GFP was mainly detected in the quiescent center and columella cells of roots (Fig. 5B). In the SSPP-overexpressing background, besides in the quiescent center cells, DR5:GFP signals also occurred in the epidermis of the root apical meristem (Fig. 5B). We observed no significant difference in the intensity of the DR5:GFP signal between the DR5:GFP/35S:SSPP and DR5:GFP/wild-type roots (Fig. 5B).
Ethylene plays a critical role in the regulation of leaf senescence. To examine ethylene pathways in SSPP-overexpressing plants, the expression levels of another reporter construct, 5×EBS:GUS, were examined. 5×EBS is also a synthetic promoter that consists of five tandem repeats of the EIN3-binding site (EBS) followed by the minimal 35S promoter (Stepanova et al., 2007). EIN3 is a transcription factor that acts as a positive regulator of the ethylene signal transduction pathway (Chao et al., 1997; Solano et al., 1998); thus, 5×EBS:GUS has been used previously to monitor the primary ethylene response in planta (Vandenbussche et al., 2010). The 5×EBS:GUS/35S:SSPP plants were obtained by crossing 5×EBS:GUS with 35S:SSPP transgenic plants. Histochemical GUS staining revealed a dramatic decrease in the activity of 5×EBS:GUS in SSPP-overexpressing plants (Fig. 5C). We also used gas chromatography to measure ethylene levels and found that 3-d-old etiolated seedlings of 35S:SSPP plants showed a slight but significant reduction of ethylene emission compared with the wild-type control (Supplemental Fig. S4A, a). Moreover, when treated with 1 μm 1-aminocyclopropane-1-carboxylic acid, the ratio of exaggerated apical hook formation in the 35S:SSPP etiolated seedlings was dramatically decreased (Supplemental Fig. S4A, b and c). Consistent with the GUS staining results in 5×EBS:GUS/35S:SSPP plants, the transcript levels of several ethylene response markers (the plant defensin gene PDF1.2, BASIC CHITINASE [CHI-B], PATHOGENESIS-RELATED PROTEIN4 [PR4], and ETHYLENE RESPONSE FACTOR11 [ERF11]) were all greatly decreased by the overexpression of SSPP (Supplemental Fig. S4B). These results indicated that the overexpression of SSPP not only suppressed the biosynthesis of ethylene but also reduced the responses to ethylene in Arabidopsis.
Subcellular Localization of AtSARK and SSPP
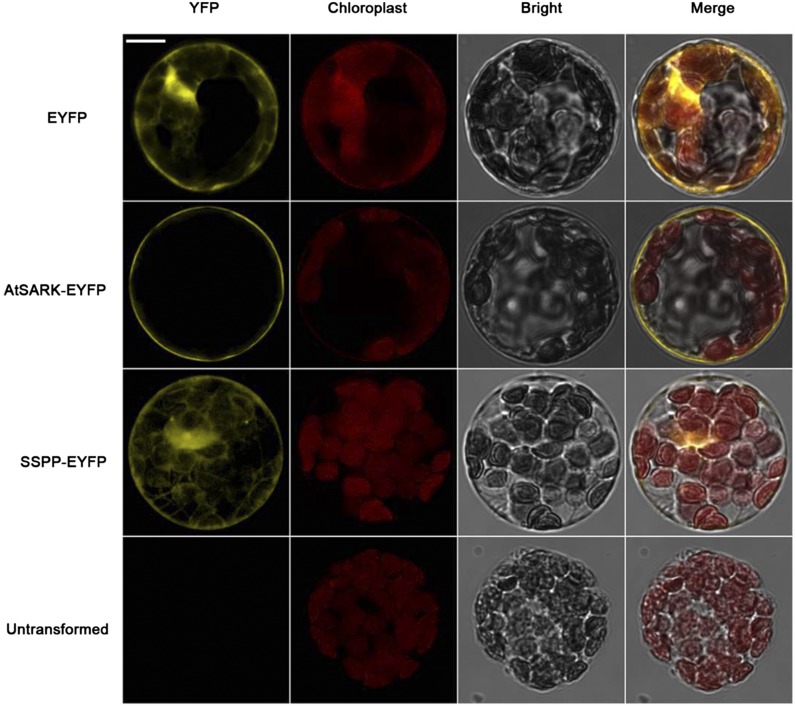
To determine the subcellular localization of the AtSARK and SSPP proteins, enhanced yellow fluorescent protein (EYFP) fused C terminally to AtSARK and SSPP were transiently expressed in Arabidopsis mesophyll protoplasts under the control of the cauliflower mosaic virus 35S promoter. In the EYFP control, yellow fluorescence was observed in the cytoplasm. The yellow fluorescence of AtSARK-EYFP was detected as a fine ring at the cell periphery, external to the chloroplasts, indicating that AtSARK localizes to the plasma membrane (Fig. 6). SSPP-EYFP expression was detected in the cytoplasm (Fig. 6), indicating that SSPP localizes in the cytoplasm.
Figure 6.
Subcellular localizations of AtSARK and SSPP in Arabidopsis protoplasts. Confocal laser scanning microscopy images of Arabidopsis protoplasts transiently expressing EYFP, AtSARK-EYFP, and SSPP-EYFP are shown. Bar = 20 μm.
SSPP Partially Dephosphorylates the Autophosphorylated Cytoplasmic Domain of AtSARK
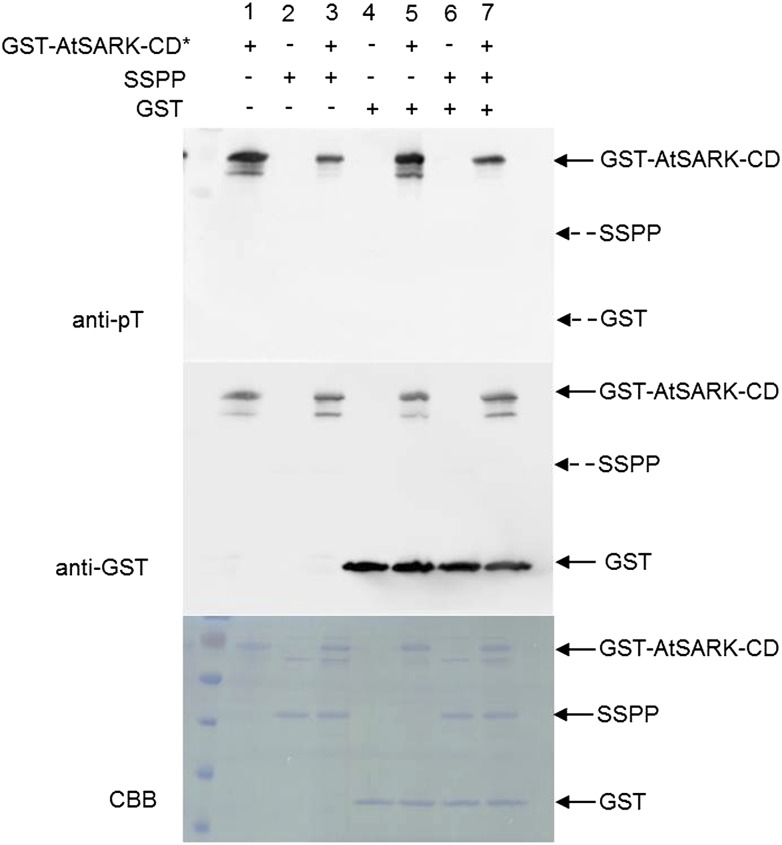
To determine whether AtSARK and SSPP can serve as substrates for each other, we performed in vitro phosphorylation and dephosphorylation assays. SSPP and the cytoplasmic domain of AtSARK (AtSARK-CD) were fused with GST and expressed in E. coli. The GST fusion proteins were purified by affinity chromatography using a Glutathione-Sepharose 4B column, and the GST tag was removed from SSPP using the site-specific PreScission protease. Purified GST-AtSARK-CD was autophosphorylated for 10 min before incubating with SSPP or GST for further analysis. The in vitro protein phosphorylations were detected by immunoblot using anti-phospho-Thr antibody. As shown in Figure 7, when the autophosphorylated GST-AtSARK-CD (lane 1) or the autophosphorylated GST-AtSARK-CD together with purified GST (lane 5) were incubated in the ATP-containing protein phosphorylation reaction mixture, only the GST-AtSARK-CD band was revealed by immunoblot analysis, confirming that the active AtSARK-CD protein exhibited autophosphorylation and the GST tag had no effect on the ability of AtSARK-CD to autophosphorylate. Purified SSPP (lane 2), GST (lane 4), or both combined together (lane 6) displayed no signal of protein phosphorylation, indicating that neither SSPP nor GST protein has autophosphorylation activity. When the autophosphorylated GST-AtSARK-CD was incubated with purified SSPP in the reaction mixture, no matter whether GST was added, no band corresponding to SSPP was detected by anti-phospho-Thr antibody. However, the intensity of the autophosphorylated GST-AtSARK-CD bands decreased significantly (Fig. 7, lanes 3 and 7). These results indicated that AtSARK does not phosphorylate SSPP but that SSPP can dephosphorylate the autophosphorylated AtSARK-CD. Also, the SSPP-mediated dephosphorylation of AtSARK-CD was independent of the GST tag.
Figure 7.
SSPP partially dephosphorylates the autophosphorylated cytoplasmic domain of AtSARK. Equal amounts (0.2 μg) of autophosphorylated GST-AtSARK-CD, SSPP, or GST were incubated in reaction buffer for 20 min. The reaction products were separated by 12% (w/v) SDS-PAGE. Autophosphorylation of AtSARK-CD was detected by anti-phospho-Thr antibody (anti-pT). The polyvinylidene difluoride membrane was stripped and reprobed with anti-GST antibody to ensure equivalent protein loading. A solid arrow indicates the migration of each protein, and dashed arrows indicate the predicted positions of SSPP and GST. Three independent replicates were done to give the typical results shown here. CBB, Coomassie Brilliant Blue.
SSPP Interacts with AtSARK in Vitro and in Vivo
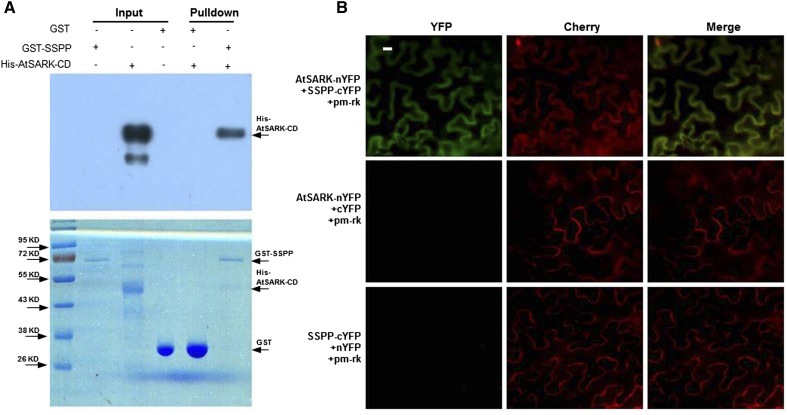
SSPP dephosphorylates the cytoplasmic domain of AtSARK (AtSARK-CD); therefore, we tested whether SSPP and AtSARK interact physically. We first tested for this interaction using an in vitro pull-down assay. We expressed AtSARK-CD fused to His and SSPP fused to GST in E. coli and purified the fusion proteins. GST-SSPP or GST was bound to a Glutathione-Sepharose column and incubated with His-AtSARK-CD, and then the eluted proteins were separated by SDS-PAGE and subjected to immunoblot analysis with His antibody (Fig. 8A). Immunoblotting detected a single band corresponding to His-AtSARK-CD in the proteins eluted from the GST-SSPP column (Fig. 8A, lane 5) but detected no signal in the proteins eluted from the GST column (Fig. 8A, lane 4), indicating that SSPP interacts physically with AtSARK-CD in vitro.
Figure 8.
SSPP interacts with AtSARK in vitro and in vivo. A, Pull-down assay showing the interaction between SSPP and AtSARK-CD. His-AtSARK-CD proteins were incubated with GST-SSPP or GST bound columns. Proteins bound to GST or GST-SSPP columns were pelleted, subjected to 12% (w/v) SDS-PAGE, stained with Coomassie Blue (bottom), or detected by immunoblot analysis using anti-His antibody (top). A solid arrow indicates the migration of each protein. Three independent replicates were done to give the typical results shown here. B, BiFC analysis of the interaction between AtSARK and SSPP in N. benthamiana leaves. Fluorescence images of N. benthamiana epidermal cells agroinfiltrated with a mixture of A. tumefaciens strains harboring constructs encoding the indicated fusion proteins and the plasma membrane marker pm-rk are shown. Each image is representative of at least three experiments. Bar = 20 μm.
To confirm the interaction between AtSARK and SSPP in plant cells, we used a BiFC assay based on split yellow fluorescent protein (YFP; Bracha-Drori et al., 2004). The different combinations of the N- and C-terminal ends of YFP fused to AtSARK or SSPP were transiently coexpressed, together with the plasma membrane marker pm-rk-CD3-1007 (Nelson et al., 2007), in Nicotiana benthamiana leaves. As shown in Figure 8B, an obvious fluorescent signal was detected in the plasma membrane for the AtSARK-nYFP +SSPP-cYFP combination, but no significant signals were detected in controls lacking either AtSARK or SSPP. The fluorescent signal perfectly overlapped with the red fluorescence of the coexpressed plasma membrane marker pm-rk. The BiFC assay thus confirmed that SSPP interacts with AtSARK in the plasma membrane of cells.
Additionally, we found that the kinase domain of GmSARK (GmSARK-KD), a soybean homolog of AtSARK, interacts with SSPP in our yeast two-hybrid system (Supplemental Fig. S5), suggesting that the interaction between SARK and SSPP may be conserved.
Overexpression of SSPP Rescues SARK-Induced Premature Leaf Senescence in Arabidopsis
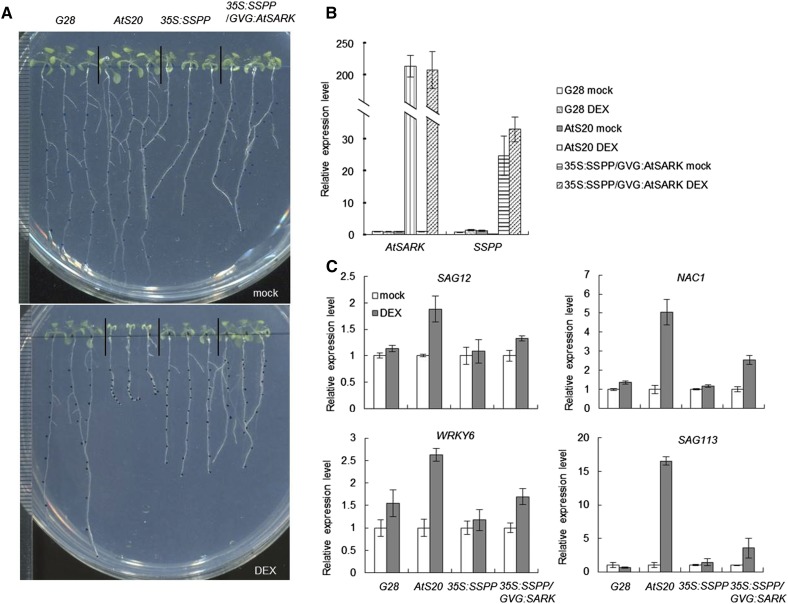
To investigate whether SSPP affects AtSARK function, we tested whether the overexpression of SSPP could alter the induction of senescence by AtSARK. To this end, we obtained 35S:SSPP/GVG:AtSARK plants by transferring SSPP into GVG:AtSARK transgenic plants. When grown on DEX-containing plates, 7-d-old transgenic seedlings of G28, a homozygous GVG:GUS control line (Xu et al., 2011), displayed normal growth and development (Fig. 9A). By contrast, the seedlings of AtS20, a typical GVG:AtSARK line, displayed an early senescence phenotype (Fig. 9A). However, the 35S:SSPP/GVG:AtSARK seedlings showed no early senescence (Fig. 9A). The DEX-induced AtSARK overexpression and 35S promoter-derived SSPP overexpression in the transgenic plants were confirmed by quantitative RT-PCR (Fig. 9B). In addition to suppressing the visible senescence phenotype, overexpression of SSPP in the 35S:SSPP/GVG:AtSARK double transgenic seedlings also suppressed the AtSARK-induced increases in expression of several marker genes for senescence, such as SAG12, NAC1, WRKY6, and SAG113 (Fig. 9C). All of these results suggested that SSPP functions as a negative regulator of AtSARK during leaf senescence.
Figure 9.
Overexpression of SSPP rescues the SARK-induced precocious leaf senescence in Arabidopsis. A, Four-day-old GVG:GUS (G28), GVG:AtSARK (AtS20), 35S:SSPP, and 35S:SSPP/GVG:AtSARK transgenic plants were grown on vertical plates containing either 10 μm DEX or its mock solution for an additional 7 d. Results from one out of three biological replicates are shown. B, Determination of AtSARK and SSPP transcript levels by quantitative RT-PCR. Four-day-old GVG:GUS, GVG:AtSARK, and GVG:AtSARK/35S:SSPP were incubated with either 10 μm DEX or its mock solution for 24 h. The data are displayed as relative expression to the mock-treated control. Three biological replicates with at least three technical repeats were done. Error bars represent sd. C, Overexpression of SSPP effectively reduces the AtSARK-induced expression of senescence-related marker genes in Arabidopsis. Quantitative RT-PCR analysis was used to determine the expression levels of SAG12, NAC1, WRKY6, and SAG113 in 4-d-old GVG:GUS, GVG:AtSARK, 35S:SSPP, and 35S:SSPP/GVG:AtSARK transgenic seedlings treated with either 10 μm DEX or its mock solution for 24 h. TIP41-like was used as an internal control. In all cases, data are shown relative to the mock-treated control. Three biological replicates with at least three technical repeats were done.
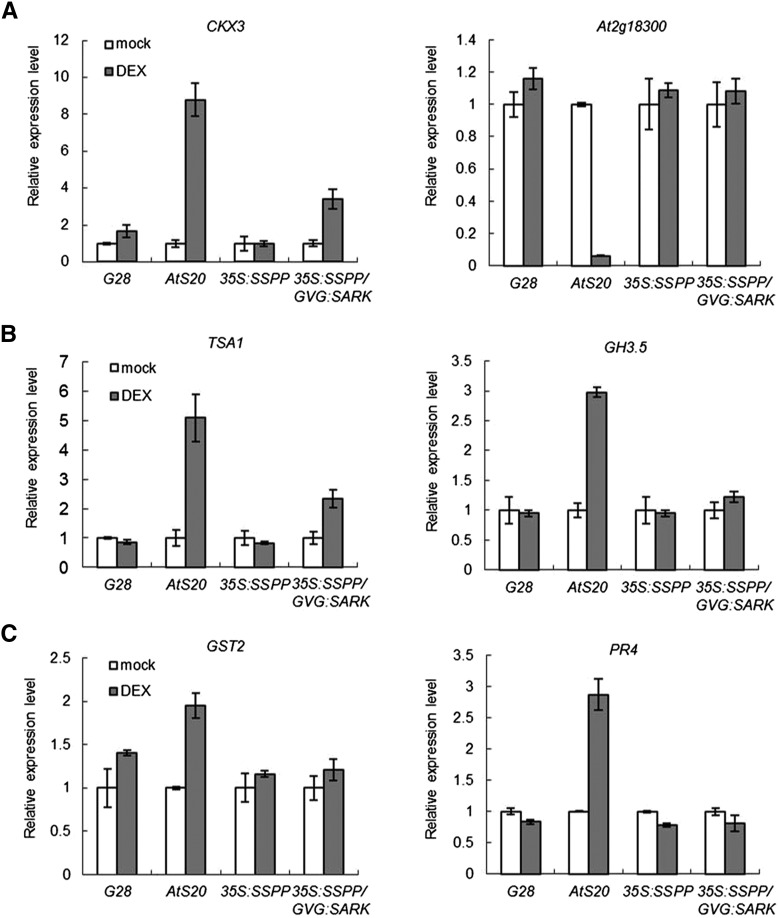
AtSARK was suggested previously to regulate leaf senescence through auxin and ethylene (Xu et al., 2011). Quantitative RT-PCR revealed that overexpression of SSPP also suppressed the AtSARK-induced increases in transcript levels of CKX3, a gene encoding cytokinin oxidase, which degrades cytokinin; TRYPTOPHAN SYNTHASE α CHAIN1 (TSA1), an auxin synthesis-related gene; GH3.5, a member of the early auxin-responsive gene family GRETCHEN HAGEN3 (GH3); and two ethylene-responsive marker genes, GST2 and PR4. The AtSARK-induced decreases in the expression of At2g18300, a cytokinin-responsive marker gene, was also recovered in the 35S:SSPP/GVG:AtSARK double transgenic seedlings (Fig. 10).
Figure 10.
AtSARK-induced changes in the expression of cytokinin-related (A), auxin-related (B), and ethylene-related (C) genes were suppressed by the overexpression of SSPP in Arabidopsis. The transcript levels of the hormone-related genes were determined by quantitative RT-PCR. Four-day-old GVG:GUS (G28), GVG:AtSARK (AtS20), 35S:SSPP, and 35S:SSPP/GVG:AtSARK transgenic seedlings were treated with either 10 μm DEX or its mock solution for 24 h. TIP41-like was used as an internal control. In all cases, data are shown relative to the mock-treated control. Three biological replicates with at least three technical repeats were done.
Comparison of the Spatial and Temporal Expression of AtSARK and SSPP during Leaf Development
To further reveal the molecular mechanism underlying the interactions between SSPP and AtSARK, the promoter-GUS reporter system was used to compare the spatial and temporal expression of SSPP and AtSARK during Arabidopsis leaf development. Twenty-eight-day-old SSPP:GUS and AtSARK:GUS transgenic Arabidopsis plants with the first and second leaves beginning to yellow were sampled for histochemical GUS staining. The SSPP promoter showed strong activity in juvenile leaves, and this activity gradually decreased with increasing leaf age (Fig. 11). By contrast, the AtSARK promoter showed weak activity in juvenile leaves, but this activity gradually increased with increasing leaf age (Fig. 11). The expression of SSPP and AtSARK partially overlapped from the second leaf to the eighth leaf in 28-d-old plants, corresponding to the mature leaf to early senescent leaf stages, implying that during these stages, SSPP might suppress the functions of AtSARK.
Figure 11.
Comparison of the spatial and temporal expression of SSPP and AtSARK during leaf development. Twenty-eight-day-old SSPP:GUS (top) and AtSARK:GUS (bottom) transgenic Arabidopsis plants were sampled for histochemical GUS staining. Leaves from the transgenic plants were laid out in order of leaf emergence as indicated by the numbers. Results from one out of three biological replicates are shown.
DISCUSSION
Protein kinase- and phosphatase-mediated reversible protein phosphorylation plays a critical role in cellular signaling. Many reports have revealed the involvement of protein kinases in the regulation of leaf senescence (Zhou et al., 2009; Xu et al., 2011; Cho et al., 2012); however, the reports on protein phosphatase are relatively few. In this study, we found that an SSPP functions in the regulation of leaf senescence. Overexpression of SSPP effectively attenuated age-dependent natural leaf senescence and SARK-induced early leaf senescence in Arabidopsis (Fig. 1). SSPP overexpression prevented a multitude of changes induced by leaf senescence, including the increased expression of senescence-associated marker genes, enhanced ethylene responses, and down-regulated cytokinin functions (Figs. 9C and 10). All of these results suggested that SSPP functions as a negative regulator of leaf senescence.
We reported previously that AtSARK functions as a key positive regulator of Arabidopsis leaf senescence (Xu et al., 2011). AtSARK localizes to the plasma membrane and SSPP localizes to the cytoplasm (Fig. 6); thus, it was interesting to find that SSPP can interact directly with the cytoplasmic domain of AtSARK (AtSARK-CD), as shown by our in vitro pull-down assay (Fig. 8A) and BiFC (Fig. 8B). Also, the autophosphorylated cytoplasmic domain of AtSARK could be dephosphorylated by SSPP (Fig. 7), implying that SSPP might exert its negative regulatory function on leaf senescence by keeping the dephosphorylation status of AtSARK. Kinases often autocatalytically phosphorylate key amino acid residues to relieve autoinhibition or enhance catalytic efficiency (Zenke et al., 1999; Canova et al., 2008). Our preliminary results suggested that SARK function requires the autophosphorylation of key residues (data not shown). Thus, it is not surprising to find that overexpression of SSPP effectively rescued the AtSARK-induced premature leaf senescence in Arabidopsis (Fig. 9A). Overexpression of SSPP also effectively suppressed the AtSARK-mediated induction of the senescence marker genes SAG12, NAC1, WRKY6, and SAG113 (Fig. 9C) and the AtSARK-induced changes in expression of the phytohormone-related marker genes CKX3, At2g18300, TSA1, GH3.5, PR4, and GST2 (Fig. 10). All of these observations support our model that SSPP functions by dephosphorylating AtSARK to attenuate SARK-mediated senescence signaling.
It is notable that SSPP could interact directly with the cytoplasmic domain of GmSARK, a soybean homolog of AtSARK, in a yeast two-hybrid system (Supplemental Fig. S5). This result suggested that the regulation of leaf senescence by SSPP and SARK might be a conserved mechanism (Supplemental Fig. S5).
Leaves are the major organs for photosynthesis. The leaf longevity directly affects the lifetime carbon fixation of plants and, accordingly, affects plant growth and development (Kikuzawa and Ackerly, 1999). Different plant species have different leaf longevities (Kikuzawa, 1991), and the normal growth and development of each plant species requires proper leaf longevity (Jonasson, 1989). Comparison of the spatial and temporal expression patterns of SSPP and AtSARK revealed that SSPP was highly expressed in young leaves and mature leaves, where the promoter activity of AtSARK was quite low (Figs. 1A and 11). Additionally, the accumulation of SSPP protein would, as mentioned above, inhibit the function of AtSARK by direct interaction. Taken together, these results indicated that SSPP repressed the function of AtSARK to sustain leaf function and prevent early leaf senescence. As the leaves developed from mature to early senescence stages, SSPP promoter activity gradually decreased (Figs. 1A and 11). Because of the reduction of accumulation of SSPP, the inhibitory effects on AtSARK were relieved, thereby promoting leaf senescence.
Cytokinin is best known as a senescence-delaying hormone. Many plant species show a decline in levels of foliar cytokinin during leaf senescence (Singh et al., 1992). Accordingly, sustaining the proper cytokinin level in plants can effectively prolong leaf longevity and delay senescence (Hwang et al., 2012). Although exogenous application of cytokinin had no obvious effect on the activity of the SSPP promoter (data not shown), the transcription levels of genes involved in cytokinin biosynthesis and responses all increased in 35S:SSPP plants (Fig. 5A). These results implied that, besides functioning as a negative regulator of AtSARK, SSPP also played a role in sustaining or enhancing cytokinin responses in plants.
Ethylene functions as an endogenous modulator of plant ageing and as a strong promoter of senescence (Jing et al., 2005; Kim et al., 2009; Li et al., 2013). SARK-induced leaf senescence requires increases in ethylene biosynthesis and responses. Exogenous application of aminoethoxyvinylglycine, an inhibitor of ethylene biosynthesis, or mutation in EIN2, a critical component of ethylene signal transduction, could effectively suppress the SARK-induced early leaf senescence symptoms (Xu et al., 2011). Overexpression of SSPP significantly reduced ethylene responses both in the developing rosettes and in the SARK-overexpressing senescent leaves (Figs. 5C and 10C; Supplemental Fig. S4). Because of the low transcription level of AtSARK in young seedlings, the above results suggested that SSPP might exert its negative effects on the onset and progression of leaf senescence through two independent pathways: directly inhibiting the functions of AtSARK and negatively regulating the biosynthesis and functions of ethylene. The characterization and identification of more components involved in SSPP-mediated ethylene repression will help elucidate the mechanisms controlling leaf longevity.
In conclusion, we postulate that SSPP functions in sustaining normal leaf development from young leaf to mature leaf stages, maintaining functions of the mature leaf, and specifying proper leaf longevity by preventing early senescence. The significant decrease in expression of SSPP during natural leaf senescence (Fig. 1A) suggested that leaf age or other developmental signals negatively regulate SSPP. Moreover, the observation that AtSARK also suppressed SSPP expression (Fig. 1A) implied that, once leaf senescence began, the increase in AtSARK expression or the senescence process itself exerted a negative feedback effect on the functions of SSPP, to further facilitate the progression of senescence. Further work on the mechanisms underlying the regulation of SSPP expression and isolation of the upstream regulators of SSPP will help to improve our understanding of the roles that SSPP plays in leaf senescence.
The sspp-1 mutants, which have a T-DNA knockout allele of SSPP, developed similar to the wild-type plants (Supplemental Fig. S3), suggesting the existence of other functionally redundant genes of SSPP. The identification of more interaction partners for AtSARK and SSPP will help to elucidate the molecular mechanisms underlying the SSPP- and SARK-mediated regulation of leaf development in higher plants.
Auxin triggers a plethora of developmental events (Löfke et al., 2013). Many if not all of auxin’s actions, such as embryonic axis formation, postembryonic organ formation, tropic growth responses, and vascular tissue development, rely on its differential distribution within plant tissues as manifested by local auxin maxima and minima, also referred to as auxin gradients (Benková et al., 2003; Friml et al., 2003; Scarpella et al., 2006; Tanaka et al., 2006; Kleine-Vehn et al., 2010). The role of auxin in regulating senescence is not clear. Several studies support the idea that auxin negatively regulates leaf senescence (Shoji et al., 1951; Lim et al., 2010; Kim et al., 2011), but other lines of evidence indicate that auxin positively regulates leaf senescence. We have previously reported that SARK-mediated leaf senescence requires an increase in the biosynthesis of and responses to auxin (Xu et al., 2011). Most recently, a gene known to be strongly induced by auxin, SMALL AUXIN UPREGULATED RNA36, was shown to promote leaf senescence (Hou et al., 2013). In summary, the role of auxin in leaf senescence appears to be very complex and may not be simply explained by variation in the auxin level during leaf senescence. Although exerting opposite effects on leaf senescence, both SSPP and AtSARK could alter the expression pattern of the auxin reporter DR5:GUS (Xu et al., 2011). These results implied that the changes in auxin distribution, but not the variations in auxin level, play a major role in the regulation of leaf senescence. Further studies are needed to elucidate the mechanisms of auxin function in senescence.
MATERIALS AND METHODS
Amino Acid Sequence Alignment and Phylogenetic Analyses
The full-length PP2C protein sequences were retrieved from GenBank. Amino acid sequences were aligned using ClustalX2 (http://www.clustal.org/; Thompson et al., 1994). The phylogenetic tree was generated in ClustalX2 by the neighbor-joining method using 1,000 replicates and displayed using MEGA5 (http://www.megasoftware.net/; Kumar et al., 1994).
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 was used in this study. Seeds were surface sterilized in 10% (v/v) sodium hypochlorite for 2 min, washed 10 times with sterilized water, germinated, and grown on vertical plates (one-half-strength Murashige and Skoog [MS] medium containing 0.8% [w/v] agar, pH 5.7, 1% [w/v] Suc, supplemented with or without antibiotics and chemical reagents) at 20°C ± 1°C with cycles of 16 h of light and 8 h of darkness under 100 to 150 μmol m−2 s−1 light intensity. The 10-d-old seedlings were transferred to soil and grown under the same conditions for further experiments and seed production.
For phenotypic analyses of the etiolated seedlings of 35S:SSPP transgenic Arabidopsis, seeds were germinated and grown on vertical plates (one-half-strength MS medium containing 0.8% [w/v] agar, pH 5.7, 1% [w/v] Suc, supplemented with 1 μm 1-aminocyclopropane-1-carboxylic acid). Three-day-old etiolated seedlings were imaged with a scanner.
The dark-induced senescence analysis was performed using both attached and detached fifth and sixth leaves at the mature stage (from plants at Boyes growth stage 5.10). The attached leaves under normal growth conditions were individually covered by aluminum foil for 9 d, and the detached leaves on wet filter paper were incubated in darkness for 4 d before the effects of dark treatments were recorded using a camera (Canon PowerShot G10) or scanner (Epson 1260).
Measurements of Ethylene Emission
Ethylene emission of the wild type and 35S:SSPP was determined in 3-d-old etiolated seedlings by gas chromatography (Agilent 6890N) as described by Li et al. (2009).
Constructs, Plant Transformation, and Crossing
To construct the 35S:SSPP fusion gene, the full-length cDNA of SSPP was amplified from Arabidopsis cDNA by RT-PCR. The pair of primers used in the PCR was SSPP-35S-TA-1 and SSPP-35S-TA-2. The DNA fragment was inserted into the binary vector pBI121 to create the recombinant transcription unit 35S:SSPP.
To construct the SSPP:GUS fusion gene, a 1,516-bp DNA fragment covering the 5′ flanking region of the SSPP gene was amplified from Arabidopsis genomic DNA by PCR. The pair of primers used in the PCR was cP-SSPP-T-1 and cP-SSPP-T-2. The DNA fragment was inserted into the binary vector pCAMBIA1301 to create the recombinant transcription unit SSPP:GUS. The transcript was terminated with a NOPALINE SYNTHASE terminator.
For constructing the GST-SSPP recombinant transcription unit, the SSPP coding region was amplified with SSPP cDNA as the template using SSPP-EcoRI-F and SSPP-XhoI-R. The DNA fragment was inserted into the vector pGEX-6P-1 to create the recombinant transcription unit GST-SSPP.
For the construction of the His-AtSARK-CD and GST-AtSARK-CD recombinant transcription units, the DNA fragment covering the intracellular domain of AtSARK (residues 432–1,515; 1,137 bp) was amplified by PCR from the corresponding full-length cDNA using AtSARK-CD-EcoRI-F and AtSARK-CD-SalI-R. The fragment was inserted into the vector pET28a or pGEX-6P-1 to create the fusion gene His-AtSARK-CD or GST-AtSARK-CD, respectively.
For the construction of the AtSARK-EYFP, SSPP-EYFP, AtSARK-nYFP, and SSPP-cYFP recombinant transcription units, the SSPP and AtSARK coding regions were amplified from the corresponding full-length cDNA using the following primer pairs: AtSARK-EcoRI-F and AtSARK-SalI-R for AtSARK-EYFP, AtSARK-XbaI-F and AtSARK-BamHI-R for AtSARK-nYFP, SSPP-EcoRI-F and SSPP-SalI-R for SSPP-EYFP, and SSPP-XbaI-F and SSPP-SalI-R for SSPP-cYFP. The corresponding fragment of AtSARK and SSPP was inserted into the vector pSAT6-EYFP-N1 to create the constructs AtSARK-EYFP and SSPP-EYFP. The fragment of AtSARK was inserted into the vector pSPYNE-35S to create the construct AtSARK-nYFP, and the fragment of SSPP was inserted into pSPYCE-35S to create the construct SSPP-cYFP.
For the construction of the BD-GmSARK-KD recombinant transcription unit, a DNA fragment covering the kinase domain of GmSARK (residues 660–940; 840 bp) was amplified by PCR from soybean (Glycine max) cDNA by RT-PCR. The pair of primers used in the PCR was GmKD-1 and GmMKD-2. The fragment was inserted into the vector pGBKT7 to create the fusion gene BD-GmSARK-KD.
For the construction of the AD-SSPP recombinant transcription unit, the full-length cDNA of SSPP was amplified from Arabidopsis cDNA by RT-PCR. The pair of primers used in the PCR was SSPP-EcoRI-F and SSPP-XhoI-R. The fragment was inserted into the vector pGBKT7 to create the fusion gene AD-SSPP.
The recombinant plasmids were introduced into Agrobacterium tumefaciens strain GV3101 and transformed into wild-type Columbia-0 Arabidopsis plants using the floral dip method (Clough and Bent, 1998). Transformants were screened on one-half-strength MS medium containing 30 mg L−1 hygromycin or 30 mg L−1 kanamycin, and the resistant seedlings were transferred to soil and verified by semiquantitative RT-PCR. The PCR primers used to confirm the recombinant transgenes in transgenic plants are listed in Supplemental Table S1. Homozygous T3 plants were used for all experiments.
The 5×EBS:GUS/35S:SSPP plants were obtained by crossing the 35S:SSPP transgenic line with 5×EBS:GUS plants. The A. tumefaciens strain GV3101 carrying the DR5:GFP construct was used to transform the homozygous 35S:SSPP line to produce DR5:GFP/35S:SSPP plants. The A. tumefaciens strain GV3101 carrying the 35S:SSPP construct was used to transform the homozygous GVG:AtSARK line to produce 35S:SSPP/GVG:AtSARK plants. Homozygous plants were identified by segregation analysis, comparison with the parental phenotypes, and PCR-based genotyping in the F3 progeny.
RNA Isolation and RT-PCR Analysis of Gene Expression
RNA extraction, cDNA synthesis, and RT-PCR analysis were done as described previously (Liu et al., 2010). Real-time RT-PCR analysis was performed using SYBR Green Perfect mix (TaKaRa) on an iQ5 (Bio-Rad), following the manufacturer’s instructions. Three independent repeats were done to give the typical results shown here. All primers used in RT-PCR analysis are listed in Supplemental Table S1.
Histochemical GUS Staining, Chlorophyll Content Determination, and Transmission Electron Microscopy
Histochemical GUS staining and transmission electron microscopy were done as described previously (Liu et al., 2010). Chlorophyll content was spectrophotometrically measured as described (Arnon, 1949). At least three independent samples were examined to give the typical results shown in this article.
Protoplast Transfection, BiFC Assay, and Fluorescence Microscopy Analyses
Protoplasts were prepared and transformed according to the protocols of Yoo et al. (2007). The plasmids of SSPP-EYFP, AtSARK-EYFP, and the EYFP control were prepared with the Vigorous Plasmid Maxprep Kit (Vigorous Biotechnology). The transformed protoplasts were assayed for fluorescence 12 to 18 h after transfection.
For infiltration of Nicotiana benthamiana, the A. tumefaciens strain GV3101 was infiltrated into the abaxial air space of 2- to 4-week-old plants as described (Voinnet et al., 2003). The p19 protein of Tomato bushy stunt virus was used to suppress gene silencing. Coinfiltration of A. tumefaciens strains containing the BiFC constructs and the p19 silencing plasmid was carried out at an optical density at 600 nm of 0.6:0.6:0.3. Epidermal cell layers of tobacco (Nicotiana tabacum) leaves were assayed for fluorescence 2 d after infiltration.
The fluorescence signal was collected with a laser scanning confocal microscope (Leica TCS-SP5). The image data were processed using Adobe Photoshop (www.adobe.com).
Protein Expression, Purification, and Pull-Down Assay
The GST-SSPP, GST-AtSARK-CD, and His-AtSARK-CD fusion proteins were expressed in Escherichia coli Rosetta 2 (DE3) plysS and purified by affinity chromatography using Glutathione-Sepharose 4B columns (GE Healthcare) and nickel-nitrilotriacetic acid agarose columns (GE Healthcare) according to the manufacturer’s recommendations. The GST tag of GST-SSPP was removed by PreScission protease (GE Healthcare) according to the manufacturer’s recommendations.
The in vitro pull-down assay was performed as described previously (Zhang et al., 2013). Briefly, the columns or beads bound with GST-SSPP and GST were washed with phosphate-buffered saline (0.14 m NaCl, 2.7 mm KCl, 10.1 mm Na2HPO4, and 1.8 mm KH2PO4) for GST pull-down assays. Each reaction contained approximately 30 μg of His-tagged fusion protein. After being added to Glutathione-Sepharose 4B columns carrying a GST fusion protein, reaction mixtures were incubated for at least 1 h at 4°C under gentle rotation. After being washed five times with phosphate-buffered saline, proteins were eluted and boiled for 10 min. The proteins were separated by 12% (w/v) SDS-PAGE for Coomassie Brilliant Blue staining and immunodetection with anti-His antiserum at a 1:2,000 dilution.
Phosphatase Activity Assay
The optimal activity conditions of SSPP were determined as described previously (Wu et al., 2011) with some modifications. Briefly, to determine the optimal pH for SSPP activity, phosphatase activity assays were performed in 100 mm buffers, including sodium acetate buffer (pH 4.5 and 5.5), Tris/HCl buffer (pH 6.5–8.5), or Gly/NaOH buffer (pH 9.5 and 10.5), with 1 μg of purified SSPP protein and 7.5 mm pNPP (New England Biolabs), and the reactions were initiated by adding purified SSPP and incubated for 30 min at 47°C. To determine the optimal temperature for SSPP activity, phosphatase activity was assayed by incubating 1 μg of the protein and 7.5 mm pNPP in 100 mm Tris-HCl buffer (pH 7.5). The Mg2+-dependent phosphatase activity of SSPP was assayed by incubating 1 μg of SSPP and 7.5 mm pNPP in 100 mm Tris-HCl buffer (pH 7.5) containing 1 mm EDTA with or without 50 mm Mg2+ and incubated for 30 min at 47°C.
In Vitro Kinase Assay
The in vitro protein phosphorylation experiments were performed essentially as reported previously (Shalitin et al., 2003) with minor modifications. A total of 0.2 μg of GST-AtSARK-CD protein was incubated in the phosphorylation buffer (25 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 10 mm MnCl2, 1 mm dithiothreitol, and 1 μg μL−1 ATP) at 28°C for 10 min to autophosphorylate before an equal amount of SSPP was added in the mixture and incubated together with GST-AtSARK-CD for a further 20 min. The phosphorylation reactions were stopped by adding 2× SDS-PAGE sample buffers and boiling for 10 min. The proteins were separated on 12% (w/v) SDS-PAGE gels. Gels were stained with Coomassie Blue. Immunoblot analysis was performed to check the in vitro phosphorylation states of the purified proteins by anti-phospho-Thr antibody (Cell Signaling Technology). Protein bands were visualized using the ECL Western Blotting Reagent Pack (GE Healthcare) following the manufacturer’s instructions. The images were recorded by a chemiluminescence imaging system (Tanon 5500). The polyvinylidene difluoride membrane was stripped and reprobed with anti-GST antibody (Cell Signaling Technology) to ensure equivalent protein loading. Data shown are representative of at least three independent experiments.
Yeast Two-Hybrid Assays
Yeast two-hybrid analysis was performed using the MATCHMAKER GAL4 system (Clontech) as described in the Yeast Protocols Handbook (Clontech). The bait plasmids containing sequences for GmSARK-KD and prey plasmids containing SSPP were sequentially transformed into the yeast (Saccharomyces cerevisiae) strain AH109 (Clontech). Confirmation of the presence of both vectors was performed by growing the yeast on medium lacking Trp and Leu. Experimental protein-protein interaction was determined by growth on synthetic dextrose/-Leu-Trp-His with 1 mm 3-amino-l-trosme medium or synthetic dextrose/-Trp/5-bromo-4-chloro-3-indoly-α-d-galactopyranoside plates. For control experiments, yeast strains were generated with the pGADT7-T plasmid and either the pGBKT7-53 or pGBKT7-Lam vector for positive and negative controls, respectively. Yeast was transformed with the BD-GmSARK-KD plasmid for self-activation assay.
Sequence data from this article can be found in The Arabidopsis Information Resource or GenBank/EMBL database under the following accession numbers: SSPP (At5g02760), AtSARK (At4g30520), VvPP2C38-like (XM_002276595), RcPP2Cc (XM_002525162), CaPP2C38-like (XM_004493866), SAG12 (At5g45890), SAG113 (At5g59220), AtNAP (At1g69490), WRKY6 (At1g62300), NAC1 (At1g56010), NAC2 (At5g04410), GTR1 (At1g58290), ACD1 (At3g44880), RbcL (AtCg00490.1), RbcS (At1g67090), SIG5 (At5g24120), IPT3 (At3g63110), ARR5 (At3g48100), ARR6 (At5g62920), GRXS13 (At1g03850), At2g18300, CKX3 (At5g56970), TSA1 (At3g54640), GH3.5 (At4g27260), GST2 (At4g02520), PR4 (At3g04720), PDF1.2 (At5g44420), CHI-B (At3g12500), ERF11 (At1g28370), GmSARK (AY687391), and TIP41-like (At4g34270).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Photograph of the wild-type and 35S:SSPP transgenic plants at four developmental stages (3.50, 5.10, 6.00, and 6.10).
Supplemental Figure S2. Morphology of SSPP-overexpressing plants.
Supplemental Figure S3. Morphology of the SSPP T-DNA insertion line sspp-1.
Supplemental Figure S4. Effects of SSPP overexpression on the functions of and responses to ethylene in Arabidopsis.
Supplemental Figure S5. SSPP interacts with the kinase domain of GmSARK (GmSARK-KD) in the yeast two-hybrid system.
Supplemental Table S1. Gene-specific primers used in this study.
Supplementary Material
Acknowledgments
We thank Dr. Nam-Hai Chua (The Rockefeller University) for providing the pTA7002 vector, Dr. Hongwei Guo (Peking University) for the homozygous 5×EBS:GUS seeds, Dr. Shuhua Yang (China Agricultural University) for guidance on the BiFC assay of the SSPP and AtSARK interaction, Dr. Qingqiu Gong (College of Life Sciences, Nankai University) for providing the plasma membrane marker pm-rk, and Dr. Yuanyuan Mei (College of Life Sciences, Nankai University) for critical reading of the article.
Glossary
- ABA
abscisic acid
- BiFC
bimolecular fluorescence complementation
- RT
reverse transcription
- DEX
dexamethasone
- cDNA
complementary DNA
- pNPP
p-nitrophenyl phosphate
- T-DNA
transfer DNA
- MS
Murashige and Skoog
Footnotes
This work was supported by the National Natural Science Foundation of China (grant no. 31170261), the Key Grant Project of the Chinese Ministry of Education (grant no. 313032), the Specialized Research Fund for the Doctoral Program of Higher Education (grant no. 20130031130003), and the Key Project on the Breeding of New Genetically Modified Species (grant nos. 2014ZX08004–005–004 and 2014ZX08009–030B–002).
Articles can be viewed without a subscription.
References
- An J, Carmichael WW (1994) Use of a colorimetric protein phosphatase inhibition assay and enzyme linked immunosorbent assay for the study of microcystins and nodularins. Toxicon 32: 1495–1507 [DOI] [PubMed] [Google Scholar]
- Arnon DI. (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha-Drori K, Shichrur K, Katz A, Oliva M, Angelovici R, Yalovsky S, Ohad N (2004) Detection of protein-protein interactions in plants using bimolecular fluorescence complementation. Plant J 40: 419–427 [DOI] [PubMed] [Google Scholar]
- Brenner WG, Romanov GA, Köllmer I, Bürkle L, Schmülling T (2005) Immediate-early and delayed cytokinin response genes of Arabidopsis thaliana identified by genome-wide expression profiling reveal novel cytokinin-sensitive processes and suggest cytokinin action through transcriptional cascades. Plant J 44: 314–333 [DOI] [PubMed] [Google Scholar]
- Canova MJ, Veyron-Churlet R, Zanella-Cleon I, Cohen-Gonsaud M, Cozzone AJ, Becchi M, Kremer L, Molle V (2008) The Mycobacterium tuberculosis serine/threonine kinase PknL phosphorylates Rv2175c: mass spectrometric profiling of the activation loop phosphorylation sites and their role in the recruitment of Rv2175c. Proteomics 8: 521–533 [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Cho YH, Hong JW, Kim EC, Yoo SD (2012) Regulatory functions of SnRK1 in stress-responsive gene expression and in plant growth and development. Plant Physiol 158: 1955–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cui X, Ge C, Wang R, Wang H, Chen W, Fu Z, Jiang X, Li J, Wang Y (2010) The BUD2 mutation affects plant architecture through altering cytokinin and auxin responses in Arabidopsis. Cell Res 20: 576–586 [DOI] [PubMed] [Google Scholar]
- Das AK, Helps NR, Cohen PT, Barford D (1996) Crystal structure of the protein serine/threonine phosphatase 2C at 2.0 A resolution. EMBO J 15: 6798–6809 [PMC free article] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Frye CA, Tang D, Innes RW (2001) Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc Natl Acad Sci USA 98: 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S, Amasino RM (1995) Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270: 1986–1988 [DOI] [PubMed] [Google Scholar]
- Gao Q, Yang Z, Zhou Y, Yin Z, Qiu J, Liang G, Xu C (2012) Characterization of an Abc1 kinase family gene OsABC1-2 conferring enhanced tolerance to dark-induced stress in rice. Gene 498: 155–163 [DOI] [PubMed] [Google Scholar]
- Guo Y, Gan S (2005) Leaf senescence: signals, execution, and regulation. Curr Top Dev Biol 71: 83–112 [DOI] [PubMed] [Google Scholar]
- Guo Y, Gan S (2006) AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J 46: 601–612 [DOI] [PubMed] [Google Scholar]
- Hou K, Wu W, Gan SS (2013) SAUR36, a small auxin up RNA gene, is involved in the promotion of leaf senescence in Arabidopsis. Plant Physiol 161: 1002–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Sheen J, Müller B (2012) Cytokinin signaling networks. Annu Rev Plant Biol 63: 353–380 [DOI] [PubMed] [Google Scholar]
- Isono K, Niwa Y, Satoh K, Kobayashi H (1997) Evidence for transcriptional regulation of plastid photosynthesis genes in Arabidopsis thaliana roots. Plant Physiol 114: 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing HC, Schippers JH, Hille J, Dijkwel PP (2005) Ethylene-induced leaf senescence depends on age-related changes and OLD genes in Arabidopsis. J Exp Bot 56: 2915–2923 [DOI] [PubMed] [Google Scholar]
- Jonasson S. (1989) Implications of leaf longevity, leaf nutrient re-absorption and translocation for the resource economy of five evergreen plant species. Oikos 56: 121–131 [Google Scholar]
- Kikuzawa K. (1991) A cost-benefit analysis of leaf habit and leaf longevity of trees and their geographical pattern. Am Nat 138: 1250–1263 [Google Scholar]
- Kikuzawa K, Ackerly D (1999) Significance of leaf longevity in plants. Plant Species Biol 14: 39–45 [Google Scholar]
- Kim HJ, Ryu H, Hong SH, Woo HR, Lim PO, Lee IC, Sheen J, Nam HG, Hwang I (2006) Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc Natl Acad Sci USA 103: 814–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Woo HR, Kim J, Lim PO, Lee IC, Choi SH, Hwang D, Nam HG (2009) Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 323: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Kim JI, Murphy AS, Baek D, Lee SW, Yun DJ, Bressan RA, Narasimhan ML (2011) YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana. J Exp Bot 62: 3981–3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Ding Z, Jones AR, Tasaka M, Morita MT, Friml J (2010) Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc Natl Acad Sci USA 107: 22344–22349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebbers E, Seurinck J, Herdies L, Cashmore ARP, Timko MP (1988) Four genes in two diverged subfamilies encode the ribulose-1,5-bisphosphate carboxylase small subunit polypeptides of Arabidopsis thaliana. Plant Mol Biol 11: 745–759 [DOI] [PubMed] [Google Scholar]
- Kudo T, Makita N, Kojima M, Tokunaga H, Sakakibara H (2012) Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-zeatin-O-glucosyltransferase in rice. Plant Physiol 160: 319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M (1994) MEGA: Molecular Evolutionary Genetics Analysis software for microcomputers. Comput Appl Biosci 10: 189–191 [DOI] [PubMed] [Google Scholar]
- Laporte D, Olate E, Salinas P, Salazar M, Jordana X, Holuigue L (2012) Glutaredoxin GRXS13 plays a key role in protection against photooxidative stress in Arabidopsis. J Exp Bot 63: 503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IC, Hong SW, Whang SS, Lim PO, Nam HG, Koo JC (2011) Age-dependent action of an ABA-inducible receptor kinase, RPK1, as a positive regulator of senescence in Arabidopsis leaves. Plant Cell Physiol 52: 651–662 [DOI] [PubMed] [Google Scholar]
- Lee JS, Ellis BE (2007) Arabidopsis MAPK phosphatase 2 (MKP2) positively regulates oxidative stress tolerance and inactivates the MPK3 and MPK6 MAPKs. J Biol Chem 282: 25020–25029 [DOI] [PubMed] [Google Scholar]
- Li H, Wong WS, Zhu L, Guo HW, Ecker J, Li N (2009) Phosphoproteomic analysis of ethylene-regulated protein phosphorylation in etiolated seedlings of Arabidopsis mutant ein2 using two-dimensional separations coupled with a hybrid quadrupole time-of-flight mass spectrometer. Proteomics 9: 1646–1661 [DOI] [PubMed] [Google Scholar]
- Li Z, Peng J, Wen X, Guo H (2012) Gene network analysis and functional studies of senescence-associated genes reveal novel regulators of Arabidopsis leaf senescence. J Integr Plant Biol 54: 526–539 [DOI] [PubMed] [Google Scholar]
- Li Z, Peng J, Wen X, Guo H (2013) ETHYLENE-INSENSITIVE3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 25: 3311–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58: 115–136 [DOI] [PubMed] [Google Scholar]
- Lim PO, Lee IC, Kim J, Kim HJ, Ryu JS, Woo HR, Nam HG (2010) Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity. J Exp Bot 61: 1419–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Gong Q, Ma Y, Li P, Li J, Yang S, Yuan L, Yu Y, Pan D, Xu F, et al. (2010) cpSecA, a thylakoid protein translocase subunit, is essential for photosynthetic development in Arabidopsis. J Exp Bot 61: 1655–1669 [DOI] [PubMed] [Google Scholar]
- Löfke C, Luschnig C, Kleine-Vehn J (2013) Posttranslational modification and trafficking of PIN auxin efflux carriers. Mech Dev 130: 82–94 [DOI] [PubMed] [Google Scholar]
- Lohman KN, Gan S, John MC, Amasino RM (1994) Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiol Plant 92: 322–328 [Google Scholar]
- McCormac AC, Fischer A, Kumar AM, Söll D, Terry MJ (2001) Regulation of HEMA1 expression by phytochrome and a plastid signal during de-etiolation in Arabidopsis thaliana. Plant J 25: 549–561 [DOI] [PubMed] [Google Scholar]
- Miller JD, Arteca RN, Pell EJ (1999) Senescence-associated gene expression during ozone-induced leaf senescence in Arabidopsis. Plant Physiol 120: 1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima A, Hanaoka M, Shikanai T, Fujiwara M, Kanamaru K, Takahashi H, Tanaka K (2004) The multiple-stress responsive plastid sigma factor, SIG5, directs activation of the psbD blue light-responsive promoter (BLRP) in Arabidopsis thaliana. Plant Cell Physiol 45: 357–368 [DOI] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Nemhauser JL, Hong F, Chory J (2006) Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126: 467–475 [DOI] [PubMed] [Google Scholar]
- Pineda Rodo A, Brugière N, Vankova R, Malbeck J, Olson JM, Haines SC, Martin RC, Habben JE, Mok DW, Mok MC (2008) Over-expression of a zeatin O-glucosylation gene in maize leads to growth retardation and tasselseed formation. J Exp Bot 59: 2673–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzinská A, Tanner G, Anders I, Roca M, Hörtensteiner S (2003) Chlorophyll breakdown: pheophorbide a oxygenase is a Rieske-type iron-sulfur protein, encoded by the accelerated cell death 1 gene. Proc Natl Acad Sci USA 100: 15259–15264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E (2007) Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci USA 104: 19631–19636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S, Somssich IE (2002) Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev 16: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al. (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T (2006) Control of leaf vascular patterning by polar auxin transport. Genes Dev 20: 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer A, Hirt H, Meskiene I (2004) Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci 9: 236–243 [DOI] [PubMed] [Google Scholar]
- Shalitin D, Yu X, Maymon M, Mockler T, Lin C (2003) Blue light-dependent in vivo and in vitro phosphorylation of Arabidopsis cryptochrome 1. Plant Cell 15: 2421–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji K, Addicott FT, Swets WA (1951) Auxin in relation to leaf blade abscission. Plant Physiol 26: 189–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Letham DS, Palni LMS (1992) Cytokinin biochemistry in relation to leaf senescence. VII. Endogenous cytokinin levels and exogenous applications of cytokinins in relation to sequential leaf senescence of tobacco. Physiol Plant 86: 388–397 [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19: 2169–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Trotochaud AE, Walker JC, Clark SE (1998) Control of meristem development by CLAVATA1 receptor kinase and kinase-associated protein phosphatase interactions. Plant Physiol 117: 1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Dhonukshe P, Brewer PB, Friml J (2006) Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell Mol Life Sci 63: 2738–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Christiansen KM, Innes RW (2005) Regulation of plant disease resistance, stress responses, cell death, and ethylene signaling in Arabidopsis by the EDR1 protein kinase. Plant Physiol 138: 1018–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. (2013) Senescence, ageing and death of the whole plant. New Phytol 197: 696–711 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA 106: 17588–17593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Petrásek J, Zádníková P, Hoyerová K, Pesek B, Raz V, Swarup R, Bennett M, Zazímalová E, Benková E, et al. (2010) The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 137: 597–606 [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Celikel FG, Park C, Harkema H (2013) Delay of Iris flower senescence by cytokinins and jasmonates. Physiol Plant 148: 105–120 [DOI] [PubMed] [Google Scholar]
- Voinnet O, Rivas S, Mestre P, Baulcombe D (2003) An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 33: 949–956 [DOI] [PubMed] [Google Scholar]
- Wang JW, Wang LJ, Mao YB, Cai WJ, Xue HW, Chen XY (2005) Control of root cap formation by microRNA-targeted auxin response factors in Arabidopsis. Plant Cell 17: 2204–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Liu MS, Lin TP, Cheng YS (2011) Structural and functional assays of AtTLP18.3 identify its novel acid phosphatase activity in thylakoid lumen. Plant Physiol 157: 1015–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Meng T, Li P, Yu Y, Cui Y, Wang Y, Gong Q, Wang NN (2011) A soybean dual-specificity kinase, GmSARK, and its Arabidopsis homolog, AtSARK, regulate leaf senescence through synergistic actions of auxin and ethylene. Plant Physiol 157: 2131–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zenke FT, King CC, Bohl BP, Bokoch GM (1999) Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J Biol Chem 274: 32565–32573 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou C (2013) Signal transduction in leaf senescence. Plant Mol Biol 82: 539–545 [DOI] [PubMed] [Google Scholar]
- Zhang K, Xia X, Zhang Y, Gan SS (2012) An ABA-regulated and Golgi-localized protein phosphatase controls water loss during leaf senescence in Arabidopsis. Plant J 69: 667–678 [DOI] [PubMed] [Google Scholar]
- Zhang R, Letham DS, Parker CW, Schroeder H, Higgins TJV (1987) Retardation of soybean leaf senescence and associated effects on seed composition. J Plant Growth Regul 6: 15–21 [Google Scholar]
- Zhang Y, Li B, Xu Y, Li H, Li S, Zhang D, Mao Z, Guo S, Yang C, Weng Y, et al. (2013) The cyclophilin CYP20-2 modulates the conformation of BRASSINAZOLE-RESISTANT1, which binds the promoter of FLOWERING LOCUS D to regulate flowering in Arabidopsis. Plant Cell 25: 2504–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Cai Z, Guo Y, Gan S (2009) An Arabidopsis mitogen-activated protein kinase cascade, MKK9-MPK6, plays a role in leaf senescence. Plant Physiol 150: 167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.