In this issue, de Kort and colleagues report a thorough analysis of acute antibody-mediated rejection (acute AMR) in pancreas allografts. The study is an important practical reference for those working in the field of pancreas transplantation, and adds considerably to the number of published cases. The first message is that all grafts lost to rejection had C4d deposition, confirming the importance of antibody-mediated rejection in pancreas, as documented in the kidney. The second message is that the combination of C4d and donor HLA-specific antibody (DSA) has greater prognostic significance than either alone. Perhaps this is due in part to the vagaries of interpretation of C4d in paraffin-embedded tissue (in fact one of their cases had C4d in the pretransplant biopsy, presumably an artifact). None of the Leiden cases were treated specifically for antibody-mediated rejection, however, the paper does not separate outcome data by treatment.
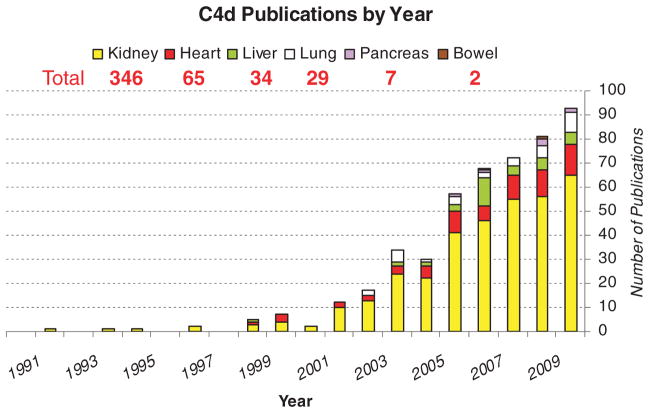
The paper raises the issue of the extension of AMR to other organs beyond the kidney, where is it is well established. Many types of vascularized organ allografts, if not all, are likely to be affected by donor reactive HLA antibodies binding to the graft endothelium. Four types of antibody effects have been established in kidney allografts: three types of antibody-mediated rejection (aka humoral rejection): hyperacute, acute, and chronic, and one type of smoldering, interaction without overt rejection, sometimes termed ‘accommodation’ (1). Significant effort to extend these observations to other organs is evident in the publications on C4d over the decade (Figure 1).
Figure 1.
Publication by year of clinical C4d studies in organ allografts. Data from PubMed searches on ‘C4d’, ‘transplantation’ and each organ.
Consensus agreement on the definition of acute AMR, and sometimes even its existence has not been achieved in any organ except the kidney, and possibly the pancreas and heart (Table 1). Pancreas has a working proposal (2) and this paper helps solidify those recommendations. Elizabeth Hammond drew attention to the possibility of acute AMR in cardiac allografts many years ago, and just in the last few years progress has been made in an effort to reach consensus, although agreement has not been achieved (3). A consensus agreement, however imperfect, is vital step forward that allows comparison studies, refinement of criteria and ultimately diagnostic accuracy. The liver has a checkered literature, with many different C4d patterns described for acute AMR. However, only the sinusoidal and periportal capillary C4d pattern are convincing to this writer (4,5). Rare lung transplants have conspicuous C4d deposition along pulmonary capillaries (personal observations), but the patchy distribution of C4d, autofluorescent elastin and artifacts in formalin fixed immunohistochemistry have created difficulties in interpretation. Small bowel transplants and composite grafts have yet to display clear evidence of antibody-mediated rejection.
Table 1.
Accepted organ specific criteria for antibody effects on allografts
| Kidney | Heart | Pancreas | Lung | Liver | Bowel | |
|---|---|---|---|---|---|---|
| Hyperacute rejection | + | + | + | + | + | |
| Acute humoral rejection | + | ± | ± | |||
| Chronic humoral rejection | 1 | |||||
| Accommodation1 | + | ± |
+, consensus established; ±, consensus in process: blank, no consensus.
The Banff classification uses the term ‘C4d deposition without morphological evidence of active rejection’ to indicate a state in which antidonor antibody reacts with the graft endothelium without causing overt injury.
Most important, in no transplanted organs other than the kidney have criteria been developed for chronic AMR, a condition that has been increasingly identified as a major cause of late kidney graft failure (6). This should be applicable to the heart, because ample studies in experimental animals have shown that chronic cardiac allograft vasculopathy (CAV) can be triggered by DSA. Some (7), but not all (8), studies of CAV in human heart transplants show an association with C4d deposition in myocardial capillaries. Limited studies in the liver have raised the possibility of C4d patterns that are associated with chronic graft injury and deserve further validation (4–5).
Investigators clearly need to explore and evaluate new dimensions of antibody-mediated endothelial injury. Banu Sis and colleagues have published evidence that endothelial gene expression can be increased in association with DSA in the absence of diagnostic levels of C4d deposition, especially in late graft biopsies, and when detected has a worse outcome than DSA alone (9). Measuring changes in the protein levels encoded by these genes is a challenge, because some baseline expression is present. An alternative strategy taken by Elaine Reed’s group is to detect altered endothelial signaling in tissue sections by staining for phosphorylated signaling proteins (e.g. pAKT p70S6 kinase). Complement fixation is not necessary for some responses of endothelial cells to DSA, because it can beelicited with F(ab)2 fragments (10). Indeed, in mice DSA promotes CAV even without complement fixation (e.g. C3 knockout mice) or C4d deposition (11). Particularly in the chronic setting, we must seek pathologic markers of antibody effects that are complement independent.
Finally, a consistent refrain has emerged from many different studies with C4d and antibody-mediated rejection, namely that the worst outcome is when a combination of features is present, whether it is C4d+DSA (as in the de Koot paper or the DeKAF study) (Arthur Matas, personal communication), or C4d and evidence of pathologic injury such as transplant glomerulopathy (12). Single markers such as C4d, pathology or DSA alone are relatively poor predictors Adding more dimensions to our evaluation such as gene expression and endothelial activation proteins, will likely enhance our diagnostic acumen and someday help guide therapy.
References
- 1.Colvin RB. Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046–1056. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 2.Drachenberg CB, Odorico J, Demetris AJ, et al. Banff schema for grading pancreas allograft rejection: Working proposal by a multi-disciplinary international consensus panel. Am J Transplant. 2008;8:1237–1249. doi: 10.1111/j.1600-6143.2008.02212.x. [DOI] [PubMed] [Google Scholar]
- 3.Tan CD, Baldwin WM, 3rd, Rodriguez ER. Update on cardiac transplantation pathology. Arch Pathol Lab Med. 2007;131:1169–1191. doi: 10.5858/2007-131-1169-UOCTP. [DOI] [PubMed] [Google Scholar]
- 4.Troxell ML, Higgins JP, Kambham N. Evaluation of C4d staining in liver and small intestine allografts. Arch Pathol Lab Med. 2006;130:1489–1496. doi: 10.5858/2006-130-1489-EOCSIL. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy COC, Herriot MM, Harrison DJ, Bathgate AJ. C4d immunopositivity is uncommon in ABO compatible liver allografts, but correlates partially with lymphocytotoxic antibodies. Histopathology. 2007;50:739–749. doi: 10.1111/j.1365-2559.2007.02677.x. [DOI] [PubMed] [Google Scholar]
- 6.Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520–2531. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez ER, Skojec DV, Tan CD, et al. Antibody-mediated rejection in human cardiac allografts: evaluation of immunoglobulins and complement activation products C4d and C3d as markers. Am J Transplant. 2005;5:2778–2785. doi: 10.1111/j.1600-6143.2005.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith RN, Brousaides N, Grazette L, et al. C4d deposition in cardiac allografts correlates with alloantibody. J Heart Lung Transplant. 2005;24:1202–1210. doi: 10.1016/j.healun.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9:2312–2323. doi: 10.1111/j.1600-6143.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 10.Jindra PT, Jin YP, Rozengurt E, Reed EF. HLA class I antibody-mediated endothelial cell proliferation via the mTOR pathway. J Immunol. 2008;180:2357–2366. doi: 10.4049/jimmunol.180.4.2357. [DOI] [PubMed] [Google Scholar]
- 11.Hirohashi T, Uehara S, Chase CM, et al. Complement independent antibody-mediated endarteritis and transplant arteriopathy in mice. Am J Transplant. 2010;9:1–8. doi: 10.1111/j.1600-6143.2009.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kieran N, Wang X, Perkins J, et al. Combination of peritubular c4d and transplant glomerulopathy predicts late renal allograft failure. J Am Soc Nephrol. 2009;20:2260–2268. doi: 10.1681/ASN.2009020199. [DOI] [PMC free article] [PubMed] [Google Scholar]