Abstract
The pathogenesis of alcoholic liver disease (ALD) is multifactorial and characterized by steatosis, steatohepatitis and cirrhosis. Several signaling pathways in different liver cell types that contribute to the development and progression of alcoholic liver injury have been identified. Among these, immune cells and signaling pathways are the most prominent and central to ALD. Both innate and adaptive immune responses contribute to ALD. The key features of inflammatory pathways in ALD including liver innate and adaptive immune cell types, signaling receptors/pathways, and pro- and antiinflammatory/protective responses are summarized here.
Keywords: ALD, ASH, Hepatic inflammation, PRRs, Cytokines, Immunity
Introduction
Alcohol is the most commonly abused drug and the second most common cause of liver disease to HCV infection, contributing to 20–25 % of the total cases in the US [1]. Alcoholic liver disease (ALD) is characterized by a spectrum of pathological conditions such as fatty liver disease or steatosis and steatohepatitis, which can progress to fibrosis, cirrhosis and hepatocellular carcinoma. Although chronic alcohol consumption leads to fatty liver, only 30 %of heavy drinkers develop advanced liver injury. The progression of liver injury caused by alcohol involves parenchymal and non-parenchymal cells in the liver, including innate and adaptive immune cells as well as infiltrating cells, adding to the damage and inflammation. Despite a number of studies using in vitro and in vivo approaches, the pathophysiological mechanisms associated with alcoholic liver injury are still unclear, limiting effective therapies. Understanding the importance of innate and adaptive immune signaling in ALD could provide insights into its pathogenesis and uncover new potential targets for therapeutic intervention. Here we summarize innate and adaptive immune cells involved in liver inflammatory responses, the signaling pathway molecules involved and mediators including cytokines and chemokines that propagate alcoholic injury in the liver (Fig. 1).
Fig. 1.
Immune cells and signaling intermediates in ALD. Alcohol stimulates innate and adaptive immune cells in liver via PRRs, TLRs and interleukin receptors activating downstream intracellular adapters/kinases and transcription factors to produce pro- and antiinflammatory cytokine mediators
Innate and adaptive immunity in liver
The innate immune system is a universal and ancient form of host defense against infections. Cells of the innate immune system include monocytes, macrophages, dendritic cells (DC), neutrophils, NK cells and NKT cells, which are responsible for orchestrating the initial immune response. Cytokines, chemokines and other mediators released by innate immune cells as well as cell-cell interactions lead to adaptive immune responses important in inflammation and immunity. Adaptive immunity refers to lymphocyte-mediated immune responses tailored to specific pathogen or neoplastic cells. It is classified as humoral or cell mediated, principally executed by B and T lymphocytes, respectively. In the liver, adaptive responses primarily include antigen-specific proliferation of T cells, deletion of activated T cells, induction of tolerance to ingested and self-antigens, and removal of signaling and effector molecules following inflammation.
The liver is a predominant organ of innate immune activation and is crucial to the recognition of invading pathogens and toxins. In the liver, parenchymal hepatocytes occupy almost 80 % of the total liver volume and perform the majority of numerous liver functions. On the other hand, non-parenchymal liver cells, which contribute only 6.5 % to the liver volume, but 40 % to the total number of liver cells, are localized in the sinusoidal compartment of the tissue. The walls of the hepatic sinusoid are lined by different innate immune cell types: sinusoidal endothelial cells (SEC), Kupffer cells (KC) and hepatic stellate cells. Additionally, intrahepatic lymphocytes (IHL), including liver-specific natural killer (NK) cells, are often present in the sinusoidal lumen. Liver innate immune cells including KCs, DC, NK and natural killer-T (NKT) cells are dysfunctional in ALD. In addition, recruitment and infiltration of bone marrow-derived cells (BMDC) and neutrophils further contribute to the progression of alcoholic liver injury. Emerging evidence suggests that inflammation is the driving force in the development of ALD, and activation of innate immune cells is the hallmark of the alcohol-mediated inflammatory cascade in the liver [2]. Several signaling pathways driven by pattern recognition receptors (PRRs) on innate immune cells and downstream adapters and kinases contribute to inflammatory cell activation [3]. On the other hand, the understanding of the effect of alcohol on adaptive immune T and B lymphocytes in the liver is still emerging. Involvement of Th1 cells in liver injury as well as circulating IgG in alcoholic hepatitis patients provides evidence for the importance of adaptive immunity in ALD.
Innate immune cell activation in ALD
The diverse pathogenesis of ALD involves direct or indirect activation of not only resident KCs/macrophages, but also infiltrating innate immune cells such as bone marrow-derived cells (BMDCs), DCs, neutrophils, NK and NKT cells that migrate to the liver, adding to the inflammatory response and propagation of liver injury.
Kupffer cells and bone marrow-derived cells (BMDC)
KCs or resident macrophages, located in liver sinusoids, play an important role in alcoholic liver injury. Dr. Thurman et al. [4] provided the first evidence of a “two-hit” model of early alcoholic liver injury where recognition of gut-derived endotoxin by the KCs is the crucial step. PRRs including Toll-like receptors (TLRs) and NOD-like receptors (NLRs) are primarily expressed on KCs and drive inflammatory responses in the liver [5]. Activation of KCs leads to upregulation of the surface markers, CD68 and CD163, produces immune mediators such as IL-1, TNFα and IL-6, chemokines including IL-8, MCP-1, MIP-1 and RANTES, as well as reactive oxygen species (ROS) [5]. Circulating endotoxin during alcohol exposure is recognized by CD14/TLR4 on KCs and activates downstream IRAK, IKK and transcription factor NFκB, resulting in induction of proinflammatory cytokines [3]. Studies showing the importance of TLR4 and proinflammatory responses in early alcoholic liver injury point to a critical role not only for KCs, but also for bone marrow-derived myeloid cells [6, 7]. While regular KCs are able to eliminate pathogens, drugs and toxins, alcohol-exposed KCs exhibit increased sensitivity to lipopolysaccharides (LPSs), attributed to ROS production [8]. It is perceivable that alcohol-induced ROS and increased sensitization of KCs are major players in ALD. Recent studies show that in a rodentmodel of ALD, chronic alcohol feeding causes a shift of KCs to classical M1 macrophages [9]. Further, recent studies emphasize the M1 macrophage function of KCs in ALD [10]. Although resident KCs are important, infiltration of BMDCs contribute to ALD. While BMDCs affect restoration of liver function by hepatocyte regeneration, in the context of disease, these cells can also play an important role in liver injury based on the inflammatory milieu to which they are recruited. Alcohol liver injury mobilizes CD34+ stem cells into the circulation and recruits them into the liver. Alcoholic hepatitis patients show increased CD34+ cell counts in liver tissue and in blood as compared to controls [11].
Dendritic cells
Dendritic cells (DCs) are classical antigen-presenting cells in the liver found in the portal triad and the central veins; they efficiently capture and process antigens in the liver. Owing to their efficient antigen-presenting function, hepatic DCs can readily tip the balance between an active immune response and immune tolerance [12]. DC maturation is associated with upregulation of surface MHC class II, CD80 and CD86. Two major DC subtypes present in the liver are myeloid and plasmacytoid [13]. Myeloid DCs (mDCs) are CD14−, BDCA-1+, CD11c+, CD83+, CD33+ and HLA-DRbright cells, and they produce cytokines including IL-12 and IL-10 and little IFNα in response to pathogens. Plasmacytoid DCs (pDCs), predominant viral sensors, are potent producers of type-I IFNs and HLA-DRbright, BDCA-2+, BDCA-4+ and CD123bright cells, but lack the myeloid markers CD11c and CD33. Both mDCs and pDCs are present in normal liver, but can also be recruited in response to infectious stimulation [13]. Recent studies show that chronic alcoholic liver injury is associated with inhibition of myeloid dendritic cell function [14]. However, whether alcohol affects the status of pDCs in ALD remains to be investigated.
Neutrophils
Recruitment of neutrophils in the intralobular region of the liver is a hallmark of alcoholic hepatitis [15]. Inflammatory mediators, particularly chemokines such as IL-8 and IL-17, play an essential role in neutrophil infiltration in ALD [15, 16]. These mediators not only recruit neutrophils, but also prime neutrophils for increased ROS production, creating an oxidative environment in the liver, leading to mitochondrial injury and hepatocyte damage. Increased myeloperoxidase activity, activation of NADPH oxidase and iNOS in recruited neutrophils further contribute to tissue damage [15]. Furthermore, mature neutrophils reside in the bone marrow and are rapidly mobilized during an inflammatory episode such as in alcoholic hepatitis patients [17]. Alcoholic cirrhosis patients exhibit lower phagocytic activity and dysfunction despite inflammation in peripheral tissue [18]. Increased expression of TLR4, 2 and 9 influenced neutrophil dysfunction without any effect on phagocytic activity [19]. Recent studies show that neutrophil infiltration is mediated by E-selectin during alcoholic liver injury [20]. How defective neutrophils contribute to ALD requires further investigation.
NK and NK-T cells
The role of natural killer (NK) and NK-like T cells (NK-T) in alcoholic liver injury is poorly understood. NK cells are regulated by cytokines derived from KCs such as IL-12 and IL-18, produce large amounts of antiviral interferon gamma (IFNγ) and modulate T cell responses in the liver. The antifibrotic role of NK cells via direct NKG2D and TRAIL-dependent killing of hepatic stellate cells or increased IFNγ was reported [21]. In a murine ALD model, alcohol decreased NK cell cytotoxicity against hepatic stellate cells by decreasing expression of TRAIL, NKG2D and IFNγ [21] and reducing NK cell release from bone marrow as well as enhancing splenic NK cell apoptosis [22]. On the other hand, NK-T cell’s capability to steer the immune system to either inflammation or tolerance [23] was based on the different types: type I invariant or type II is associated with hepatosteatosis. NK-T cells are abundant in the liver and recognize lipid antigens via presentation by the non-classical MHC class I-like molecule CD1. The change in NKT cell numbers in animal models of alcohol-related hepatosteatosis is associated with a disruption of cytokine homeostasis, resulting in a more pronounced release of proinflammatory cytokines, which render the steatotic liver highly susceptible to secondary insults [24]. Liver NKT cells increase in ALD, and further activation by alpha-galactosylceramide causes lethal liver injury [24]. Alcohol-fed NK-T cell-deficient mice exhibit a delay in alcohol-induced liver injury [24]. In general, based on the tissue microenvironment, reduced function of NK and increased numbers as well as function of NK-T cells can accelerate early liver injury by producing proinflammatory cytokines and killing hepatocytes in an oxidative milieu. Future investigation of the precise role of NK and NK-T cells in the pathogenesis of ALD is warranted.
Adaptive immunity in ALD
The contribution of adaptive immune cells to alcohol-induced liver inflammation has received little or no attention. Lymphocyte infiltration in the liver is commonly observed in patients with alcoholic hepatitis. Malondialdehyde-acetaldehyde (MAA) adducts induced in alcoholic liver are immunogenic and can induce adaptive responses [25]. While the contribution of MAA adducts to the pathogenesis of ALD is unclear, recent data suggest that MAA-modified cytosolic proteins can induce liver damage and increase proinflammatory cytokines and profibrotic factors [26]. T cell proliferation and IgG antibodies were also detected in the livers after immunization with MAA adducts, suggesting a role for adaptive responses in disease pathophysiology [26, 27].
Th17 cells
Interleukin-17 (IL-17)-producing CD4+ T cells (Th17) are important “players” in inflammation-associated diseases. In patients with alcoholic hepatitis, a recent study showed increased IL-17 levels in human ALD [28]. CD4+ T cells producing IL-17 (Th17) cells were identified in peripheral blood of alcoholic cirrhosis patients and liver inflammatory infiltrates [28]. Further IL-17+ cells correlated with the stage of the disease, and IL-17 receptors were identified on hepatic stellate cells. These observations suggest that increased Th17 cell recruitment and activation in the liver are associated with ALD and likely promote inflammation and fibrogenesis. The importance of Th-17-associated mechanisms and their participation in ALD needs further investigation.
Immune signaling pathways in ALD
Innate immune signaling pathways including PRRs, adapters and downstream kinases as well as transcription factors have been the major focus in ALD research. A number of studies have pointed to their potential applicability as likely therapeutic targets. Immune signaling mechanisms in adaptive T and B cells remain to be investigated.
Pattern recognition receptors, adapters and kinases in ALD
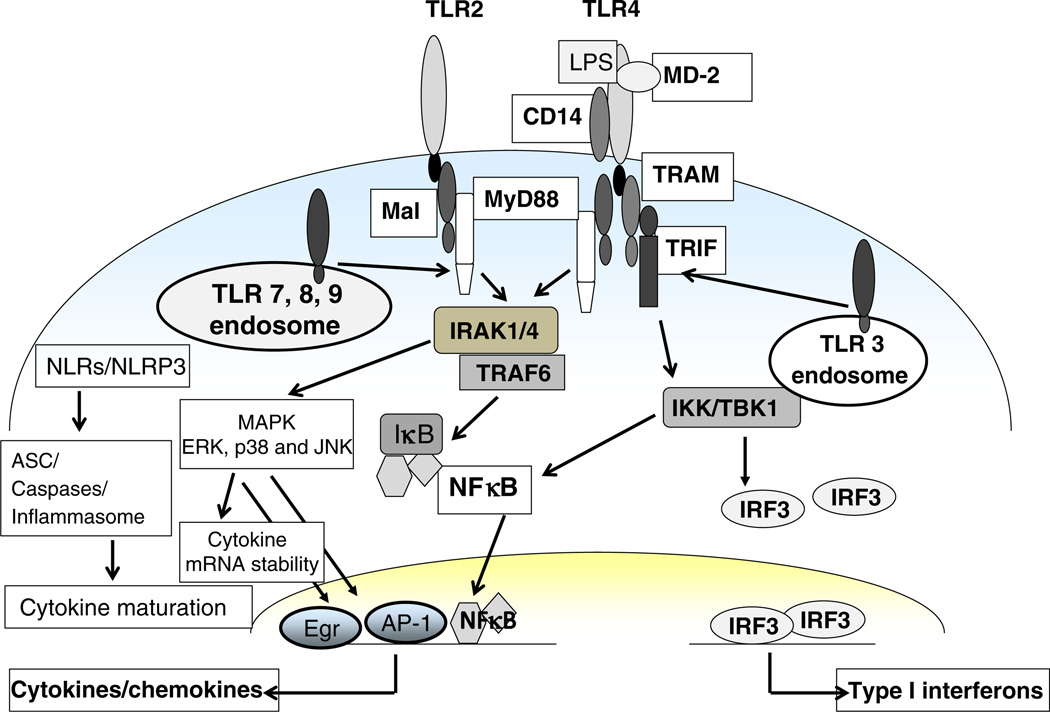
PRRs are membrane-associated, endosomal and cytoplasmic and recognize pathogens or danger-associated molecular patterns (DAMPs) [3]. The different groups of PRRs comprising the TLRs, cytoplasmic NLRs and RIG-I-like receptors (RLRs) bind to distinct DAMPs and enable downstream signaling [3]. Increasing evidence suggests that various PRRs and signaling components play an important role in the pathogenesis of ALD (Fig. 2). The role of TLRs and particularly TLR4 has been investigated in alcoholic liver injury [6, 7, 29]. In ALD, recognition of gut endotoxin/LPS by the CD14/TLR4 complex activates downstream signaling in liver macrophages [2]. The contribution of oxidative stress due to ROS generation largely adds to alcohol-induced sensitization of liver macrophages and inflammatory responses [30]. A pivotal role for ROS-mediated activation of NADPH oxidase, increased TLR expression [31] and stress proteins hsp70 and hsp90 [32] in alcoholic macrophage activation is reported. The role of DAMPs in ALD is not well understood. Among the DAMPs, necrotic or apoptotic bodies generated because of alcohol-induced oxidative stress could be recognized by PRRs and play an important role in liver injury [33]. While research until now has focused on TLRs, recent studies show alcohol-mediated activation of the NOD-like receptor, NLRP3, in alcoholic liver [34]. On the other hand, TLR3 activation induced protection from alcoholic liver injury because of IL-10 production by KC and stellate cells [35]. Thus, it is increasingly evident that besides TLR4, oxidative stress and intracellular PRRs are also major players in ALD.
Fig. 2.
Pattern recognition receptor (PRR) signaling in ALD. PRRs in ALD activate downstream signaling adaptors, kinases and transcription factors to induce proinflammatory cytokines and chemokines
Engagement of TLR4 by endotoxin leads to its activation and recruitment of IRAK-1 to the TLR4 complex via interaction with MyD88 and IRAK-4. The importance of the common TLR4 adaptor molecule, MyD88, in a mouse model of alcoholic liver injury is shown [6]. MyD88 knockout mice were highly susceptible to alcohol-induced fatty liver, likely due to increased oxidative stress [6]. TLR4-induced MyD88-dependent and -independent pathways led to the IKK kinase activation crucial in proinflammatory gene expression [3]. Chronic alcohol exposure induces LPS-mediated IKK kinase activation in murine macrophages [32]. Stress-mediated molecular chaperone, hsp90, plays an important role in maintaining IKK activity in chronic alcohol-exposed macrophages. Inhibition of hsp90 decreases IKK activity and prevents alcohol-mediated proinflammatory cytokine expression [32]. Downstream from PRRs, members of the mitogen-activated protein kinase (MAPK) family including extracellular receptor-activated kinases 1/2 (ERK1/2), p38 and c-jun-N-terminal kinase (JNK) are activated. Chronic alcohol enhances LPS-induced ERK1/2 activation and transcription of Egr-1, contributing to murine hepatic macrophage activation [36, 37]. KCs exposed to chronic alcohol in vivo exhibit increased LPS-induced p38 activity and decreased JNK activity [38]. Thus, alcohol regulates PRR-associated adapter molecules and intracellular kinases, resulting in cytokine/chemokine alterations in ALD.
Transcription factors in ALD
In innate immune cells, TLR-induced MyD88-dependent and -independent signaling leads to activation of NFκB and/or interferon regulatory factor 3 (IRF3), respectively, resulting in induction of proinflammatory cytokines or type I IFN. In ALD, LPS/TLR4-mediated NFκB activation in monocytes and macrophages contributes to production of proinflammatory cytokines such as TNFα [39]. On the other hand, LPS activated IRF3 also binds to the TNFα promoter and promotes macrophage activation by chronic alcohol exposure [40]. Whether NFκB and IRF3 act in concert with each other to increase proinflammatory cytokines and liver injury is not yet clear. Another transcription factor, AP-1 induced by TLR4 and JNK, leads to phosphorylation of c-jun and binds to the TNFα promoter. Oxidative stress-mediated transcription factor HSF-1 is activated in chronic alcohol-exposed macrophages [32]. However, target genes hsp70 and hsp90 are differentially regulated with selective induction of hsp90 in chronic alcohol-exposed macrophages. It is likely that chronic alcohol exposure induces hsp90, selectively exploiting its chaperone function of kinases such as IKK, crucial in the induction of inflammatory responses in the liver [32]. Transcription factors such as SREBP and PPARα also play a pivotal role in early alcoholic liver injury [41]. Decreased PPARα activity in hepatocytes promoted oxidative stress in the alcoholic liver and increased sensitization of TNFα-induced liver injury [41]. The role of STAT3, another transcription factor in alcoholic liver injury, was extensively studied by Gao and colleagues [42]. KCs from alcohol-fed hepatocyte-specific STAT3KO mice produced similar amounts of ROS and proinflammatory cytokines compared to control mice. On the other hand, KCs from myeloid-specific-STAT3KO mice produced higher ROS and TNFα compared with wild-type controls. These results suggest that STAT3 in hepatocytes promotes oxidative stress and inflammation, whereas Kupffer cell STAT3 reduces hepatic inflammation during alcoholic liver injury [42]. Endothelial STAT3 seems to play an important dual role of attenuating hepatic inflammation and sinusoidal endothelial cell death during alcoholic liver injury [43]. Thus, based on the cell type involved, transcription factors can exert distinct downstream gene expression. Further exploration of novel transcription factors and related epigenetic events at the chromatin level in immune cells will advance our understanding of their role in the pathogenesis of ALD.
Mediators in ALD: cytokines and chemokines
Proinflammatory cytokines in ALD
Major proinflammatory cytokines including TNFα, IL-1β and IL-6 are increased in ALD and have been extensively studied. Liver immune cells produce TNFα, which is important in liver homeostasis and can activate both pro-and antiapoptotic signaling in the liver. TNFα production was increased in serum or PBMCs and shown to correlate with disease severity in alcoholic hepatitis. Hence, targeting TNFα as a potential therapeutic strategy was tested in clinical trials using anti-TNFα antibodies in patients with acute alcoholic steatohepatitis but failed because of severe side effects including infections [44]. The role of IL-1β in the pathogenesis of ALD was recently uncovered by Petrasek et al. [34], who showed caspase-dependent upregulation of IL-1β and signaling of IL-1R1, crucial in the pathogenesis of ALD. IL-6 in the liver induces an acute-phase response and has important hepatoprotective effects. Another critical proinflammatory cytokine, IL-8, is increased in alcoholic hepatitis patients and is linked to neutrophil infiltration [15]. The importance of the balance of Th1 and Th2 cytokines in the liver microenvironment has been proposed. Increased expression of the Th1 cytokines IFNγ and IL-12 was reported in alcohol-fed rats and contributes to alcoholic steatosis [45]. IL-17, another proinflammatory cytokine secreted by T cells, correlated with infiltrating immune cells and the fibrosis score in alcoholic hepatitis patients, suggesting its role in the pathogenesis of ALD [28].
Chemokines are chemotactic cytokines that recruit leukocytes to the sites of injury and inflammation. Resident hepatic cell types secrete CXC chemokines such as IL-8, MIP-2 and CINC-1, resulting in migration of NK, NKT, CD4+ T cells and neutrophils in ALD. The role of the CC chemokine, MCP-1, in ALD was recently shown [46]. On the other hand, deficiency of the MCP-1 receptor, CCR2, exhibits alcohol-induced liver injury, suggesting that CCR2-mediated immune cell infiltration does not play a role in the pathogenesis of ALD [46]. Recent studies indicate an important role for macrophage migration inhibitor factor (MIF) in immune cell infiltration during ALD [47]. Using selective blockade of chemokines or chemokine receptors to understand the distinct effects on recruitment of immune cells and their possible development as therapeutic strategies in ALD is needed.
Antiinflammatory mediators in ALD
Antiinflammatory or protective cytokines curb the inflammatory response during ALD and are pivotal to the development and progression of liver injury. Various antiinflammatory mediators such as IL-6, IL-22, IL-10, prostaglandins and TGF-β render protection toward the inflammatory response and alleviate ALD. In addition, intracellular signaling molecules such as IRAK-M [39] contribute to the antiinflammatory effects in ALD. Chronic alcohol exposure did not significantly affect IL-10 in wild-type mice [48], but recent studies show that IL-6/STAT3-mediated mechanisms contribute to amelioration of alcoholic liver injury in IL-10-deficient mice [43]. Further, decreased IL-10 in alcohol-exposed type-I IFN receptor-deficient mice further supports a protective role for IL-10 and type I interferons in ALD [49]. Another antiinflammatory mediator, adiponectin, was decreased after chronic alcohol feeding, and treatment of mice with adiponectin prevents alcohol-induced liver injury [9]. Recent studies provide evidence for a hepatoprotective role for cytokine IL-22 via activation of STAT3 and its potential use as a therapeutic target in ALD [50]. Thus, understanding the antiinflammatory mechanisms in ALD is crucial not only for the pathogenesis but also for the development of therapy.
Summary and perspectives
ALD is multifactorial, involving various cell types and intracellular signaling pathways. While various mechanisms of ALD have been emerging, the importance of inflammatory signaling pathways in liver-resident immune cells in the pathogenesis of ALD has been identified. How infiltrating innate and adaptive immune cells participate in disease progression needs further investigation. The reason for only 35 % of heavy drinkers developing ALD remains unanswered. Until now, key signaling cascades in innate immune cells such as PRRs, adapters and cellular kinases and transcription factors resulting in cytokine/chemokine production have been investigated in alcoholic liver injury. Future approaches to study cellular and molecular interactions of innate and adaptive immune cells in ALD could offer a powerful tool to understand the diagnosis, prognosis and treatment of ALD.
Acknowledgements
This work was supported by PHS Grant No. AA017986 from the National Institute of Alcohol Abuse and Alcoholism (NIAAA), and PR100284 and PR120783 from the Department of Defense (DoD). Its contents are the sole responsibility of the authors and do not necessarily represent the views of the NIAAA or DoD.
Footnotes
Compliance with ethical requirements This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of interest Pranoti Mandrekar and Aditya Ambade declare that they do not have any conflicts of interest.
Contributor Information
Pranoti Mandrekar, Email: pranoti.mandrekar@umassmed.edu, Department of Medicine, University of Massachusetts Medical School, LRB 221, 364 Plantation Street, Worcester, MA 01605, USA.
Aditya Ambade, Department of Medicine, University of Massachusetts Medical School, LRB 270i, 364 Plantation Street, Worcester, MA 01605, USA.
References
- 1.Singal AK, Anand BS. Recent trends in the epidemiology of alcoholic liver disease. Clin Liver Dis. 2013;2:53–56. doi: 10.1002/cld.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 4.Thurman RG, Bradford BU, Iimuro Y, Frankenberg MV, Knecht KT, Connor HD, et al. Mechanisms of alcohol-induced hepatotoxicity: studies in rats. Front Biosci. 1999;4:e42–e46. doi: 10.2741/A478. [DOI] [PubMed] [Google Scholar]
- 5.Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: a contemporary view on liver diseases. Hepatology. 2006;44:287–298. doi: 10.1002/hep.21308. [DOI] [PubMed] [Google Scholar]
- 6.Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, et al. The critical role of Toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509–1518. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348–1356. doi: 10.1189/jlb.1005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem. 2011;286:13460–13469. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and non-alcoholic fatty liver disease. Hepatology. 2013;59:130–142. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]
- 11.Dalakas E, Newsome PN, Boyle S, Brown R, Pryde A, McCall S, et al. Bone marrow stem cells contribute to alcohol liver fibrosis in humans. Stem Cells Dev. 2010;19:1417–1425. doi: 10.1089/scd.2009.0387. [DOI] [PubMed] [Google Scholar]
- 12.Bosma BM, Metselaar HJ, Mancham S, Boor PP, Kusters JG, Kazemier G, et al. Characterization of human liver dendritic cells in liver grafts and perfusates. Liver Transplant. 2006;12:384–393. doi: 10.1002/lt.20659. [DOI] [PubMed] [Google Scholar]
- 13.Yoneyama H, Ichida T. Recruitment of dendritic cells to pathological niches in inflamed liver. Med Mol Morphol. 2005;38:136–141. doi: 10.1007/s00795-005-0289-0. [DOI] [PubMed] [Google Scholar]
- 14.Feng D, Eken A, Ortiz V, Wands JR. Chronic alcohol-induced liver disease inhibits dendritic cell function. Liver Int. 2011;31:950–963. doi: 10.1111/j.1478-3231.2011.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaeschke H. Neutrophil-mediated tissue injury in alcoholic hepatitis. Alcohol. 2002;27:23–27. doi: 10.1016/s0741-8329(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol. 2003;170:4665–4672. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- 17.Ramaiah SK, Jaeschke H. Hepatic neutrophil infiltration in the pathogenesis of alcohol-induced liver injury. Toxicol Mech Methods. 2007;17:431–440. doi: 10.1080/00952990701407702. [DOI] [PubMed] [Google Scholar]
- 18.Tritto G, Bechlis Z, Stadlbauer V, Davies N, Frances R, Shah N, et al. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol. 2011;55:574–581. doi: 10.1016/j.jhep.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 19.Stadlbauer V, Mookerjee RP, Wright GA, Davies NA, Jurgens G, Hallstrom S, et al. Role of Toll-like receptors 2, 4, and 9 in mediating neutrophil dysfunction in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G15–G22. doi: 10.1152/ajpgi.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury: A critical role for E-selectin. Hepatology. 2013;9(9):1132–1140. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Meadows GG. Exogenous IL-15 in combination with IL-15R alpha rescues natural killer cells from apoptosis induced by chronic alcohol consumption. Alcohol Clin Exp Res. 2009;33:419–427. doi: 10.1111/j.1530-0277.2008.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minagawa M, Deng Q, Liu ZX, Tsukamoto H, Dennert G. Activated natural killer T cells induce liver injury by Fas and tumor necrosis factor-alpha during alcohol consumption. Gastroenterology. 2004;126:1387–1399. doi: 10.1053/j.gastro.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Thiele GM, Freeman TL, Klassen LW. Immunologic mechanisms of alcoholic liver injury. Semin Liver Dis. 2004;24:273–287. doi: 10.1055/s-2004-832940. [DOI] [PubMed] [Google Scholar]
- 26.Thiele GM, Duryee MJ, Willis MS, Tuma DJ, Radio SJ Hunter CD, et al. Autoimmune hepatitis induced by syngeneic liver cytosolic proteins biotransformed by alcohol metabolites. Alcohol Clin Exp Res. 2010;34:2126–2136. doi: 10.1111/j.1530-0277.2010.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolla R, Vay D, Mottaran E, Parodi M, Traverso N, Arico S, et al. Detection of circulating antibodies against malondialdehyde–acetaldehyde adducts in patients with alcohol-induced liver disease. Hepatology. 2000;31:878–884. doi: 10.1053/he.2000.5373. [DOI] [PubMed] [Google Scholar]
- 28.Lemmers A, Moreno C, Gustot T, Marechal R, Degre D, Demetter P, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–657. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 29.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 30.Muralidharan S, Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol. 2013;94(6):1167–1184. doi: 10.1189/jlb.0313153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, et al. Differential liver sensitization to Toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 32.Mandrekar P, Catalano D, Jeliazkova V, Kodys K. Alcohol exposure regulates heat shock transcription factor binding and heat shock proteins 70 and 90 in monocytes and macrophages: implication for TNF-alpha regulation. J Leukoc Biol. 2008;84:1335–1345. doi: 10.1189/jlb.0407256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McVicker BL, Tuma DJ, Kharbanda KK, Kubik JL, Casey CA. Effect of chronic ethanol administration on the in vitro production of proinflammatory cytokines by rat Kupffer cells in the presence of apoptotic cells. Alcohol Clin Exp Res. 2007;31:122–129. doi: 10.1111/j.1530-0277.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 34.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byun JS, Suh YG, Yi HS, Lee YS, Jeong WI. Activation of Toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice. J Hepatol. 2013;58:342–349. doi: 10.1016/j.jhep.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2002;282:G6–G15. doi: 10.1152/ajpgi.00328.2001. [DOI] [PubMed] [Google Scholar]
- 37.Shi L, Kishore R, McMullen MR, Nagy LE. Chronic ethanol increases lipopolysaccharide-stimulated Egr-1 expression in RAW 264.7 macrophages: contribution to enhanced tumor necrosis factor alpha production. J Biol Chem. 2002;277:14777–14785. doi: 10.1074/jbc.M108967200. [DOI] [PubMed] [Google Scholar]
- 38.Kishore R, McMullen MR, Nagy LE. Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: role of A+U-rich elements and p38 mitogen-activated protein kinase signaling pathway. J Biol Chem. 2001;276:41930–41937. doi: 10.1074/jbc.M107181200. [DOI] [PubMed] [Google Scholar]
- 39.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320–1227. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, et al. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crabb DW, Galli A, Fischer M, You M. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha. Alcohol. 2004;34:35–38. doi: 10.1016/j.alcohol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–1158. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller AM, Wang H, Park O, Horiguchi N, Lafdil F, Mukhopadhyay P, et al. Anti-inflammatory and anti-apoptotic roles of endothelial cell STAT3 in alcoholic liver injury. Alcohol Clin Exp Res. 2010;34:719–725. doi: 10.1111/j.1530-0277.2009.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menon KV, Stadheim L, Kamath PS, Wiesner RH, Gores GJ, Peine CJ, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol. 2004;99:255–260. doi: 10.1111/j.1572-0241.2004.04034.x. [DOI] [PubMed] [Google Scholar]
- 45.Olleros ML, Martin ML, Vesin D, Fotio AL, Santiago-Raber ML, Rubbia-Brandt L, et al. Fat diet and alcohol-induced steatohepatitis after LPS challenge in mice: role of bioactive TNF and Th1 type cytokines. Cytokine. 2008;44:118–125. doi: 10.1016/j.cyto.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185–2197. doi: 10.1002/hep.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnes MA, McMullen MR, Roychowdhury S, Pisano SG, Liu X, Stavitsky AB, et al. Macrophage migration inhibitory factor contributes to ethanol-induced liver injury by mediating cell injury, steatohepatitis, and steatosis. Hepatology. 2013;57:1980–1991. doi: 10.1002/hep.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill DB, D’Souza NB, Lee EY, Burikhanov R, Deaciuc IV, de Villiers WJ. A role for interleukin-10 in alcohol-induced liver sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res. 2002;26:74–82. [PubMed] [Google Scholar]
- 49.Petrasek J, Dolganiuc A, Csak T, Nath B, Hritz I, Kodys K, et al. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology. 2011;53:649–660. doi: 10.1002/hep.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong X, Feng D, Mathews S, Gao B. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol. 2013;28(Suppl 1):56–60. doi: 10.1111/jgh.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]