Abstract
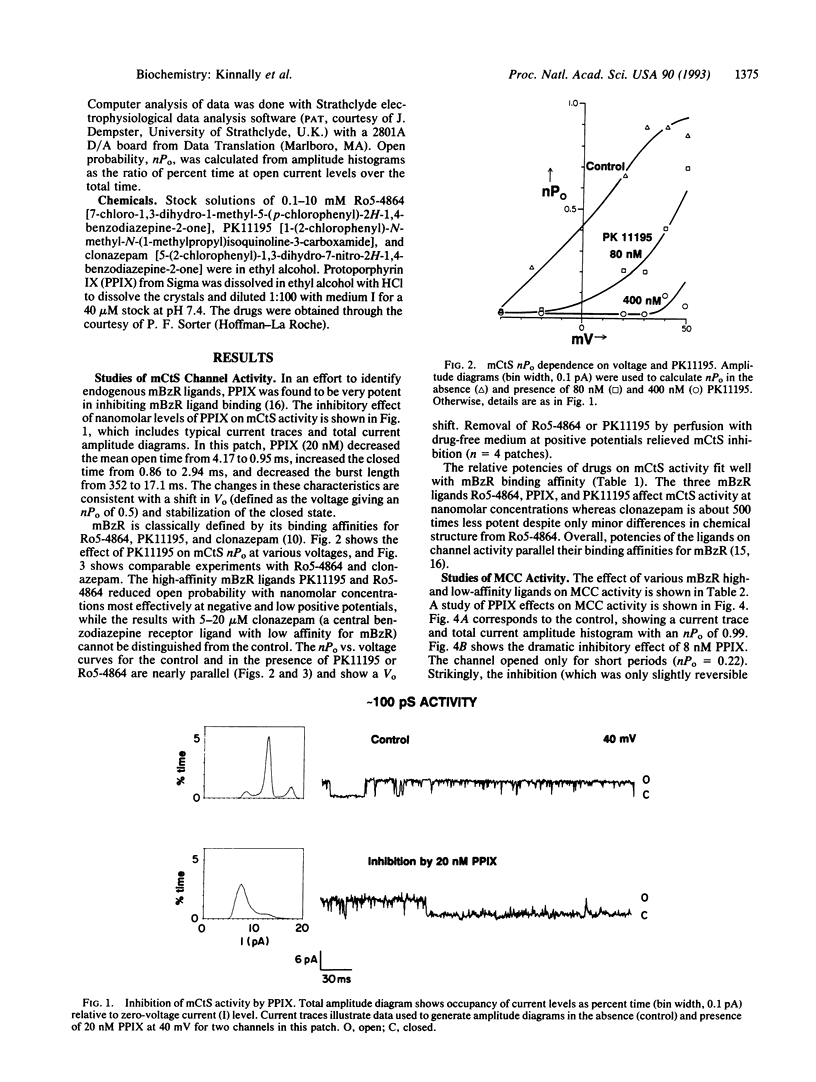
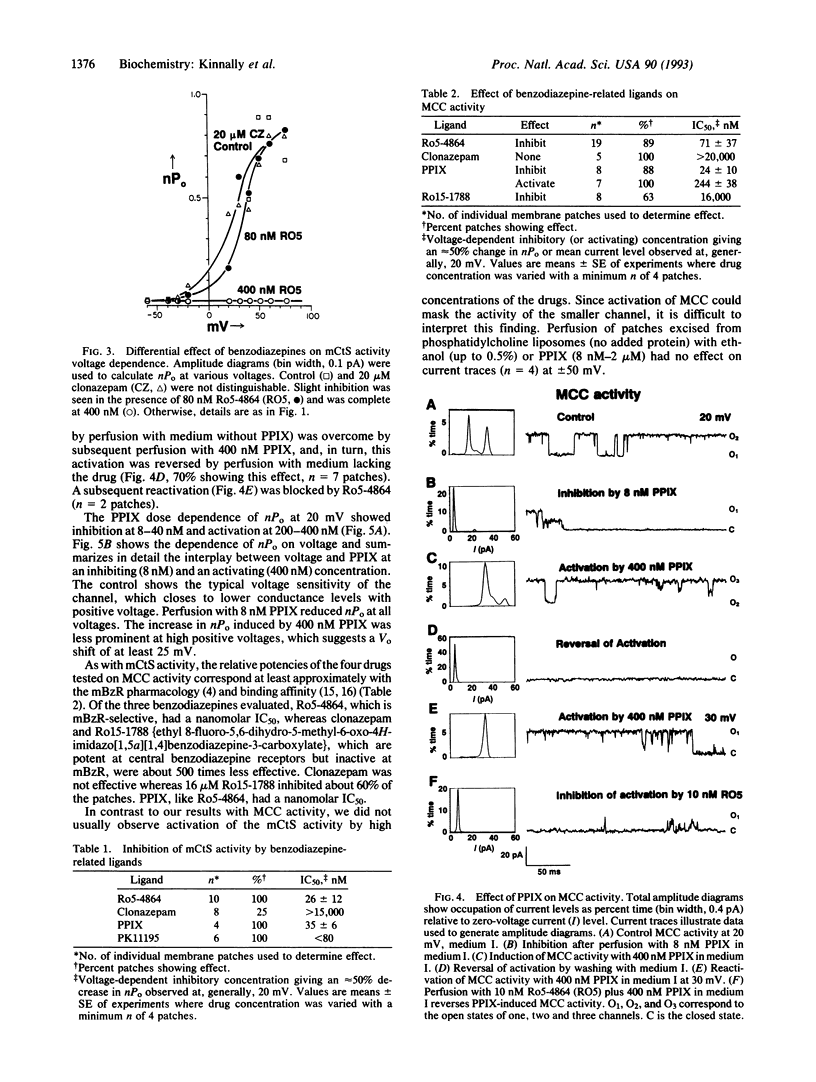
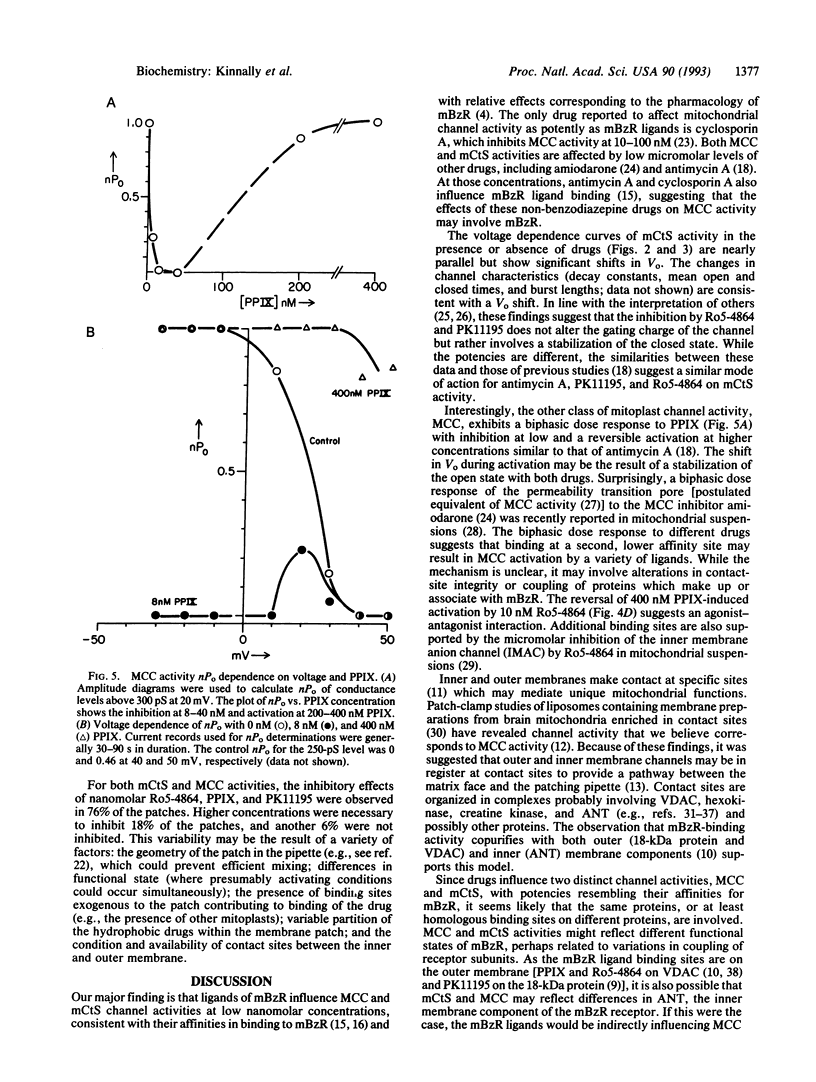
The mitochrondrial benzodiazepine receptor (mBzR) binds a subset of benzodiazepines and isoquinoline carboxamides with nanomolar affinity and consists of the voltage-dependent anion channel, the adenine nucleotide translocator, and an 18-kDa protein. The effect of ligands of the mBzR on two inner mitochondrial membrane channel activities was determined with patch-clamp techniques. The relative inhibitory potencies of the drugs resemble their binding affinities for the mBzR. Ro5-4864 and protoporphyrin IX inhibit activity of the multiple conductance channel (MCC) and the mitochondrial centum-picosiemen (mCtS) channel activities at nanomolar concentrations. PK11195 inhibits mCtS activity at similar levels. Higher concentrations of protoporphyrin IX induce MCC but possibly not mCtS activity. Clonazepam, which has low affinity for mBzR, is at least 500 times less potent at both channel activities. Ro15-1788, which also has a low mBzR affinity, inhibits MCC at very high concentrations (16 microM). The findings indicate an association of these two channel activities with the proteins forming the mBzR complex and are consistent with an interaction of inner and outer membrane channels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonenko Y. N., Kinnally K. W., Perini S., Tedeschi H. Selective effect of inhibitors on inner mitochondrial membrane channels. FEBS Lett. 1991 Jul 8;285(1):89–93. doi: 10.1016/0014-5793(91)80731-h. [DOI] [PubMed] [Google Scholar]
- Arora K. K., Pedersen P. L. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem. 1988 Nov 25;263(33):17422–17428. [PubMed] [Google Scholar]
- Basile A. S., Bolger G. T., Lueddens H. W., Skolnick P. Electrophysiological actions of Ro5-4864 on cerebellar Purkinje neurons: evidence for "peripheral" benzodiazepine receptor-mediated depression. J Pharmacol Exp Ther. 1989 Jan;248(1):463–469. [PubMed] [Google Scholar]
- Beavis A. D. Properties of the inner membrane anion channel in intact mitochondria. J Bioenerg Biomembr. 1992 Feb;24(1):77–90. doi: 10.1007/BF00769534. [DOI] [PubMed] [Google Scholar]
- BeltrandelRio H., Wilson J. E. Hexokinase of rat brain mitochondria: relative importance of adenylate kinase and oxidative phosphorylation as sources of substrate ATP, and interaction with intramitochondrial compartments of ATP and ADP. Arch Biochem Biophys. 1991 Apr;286(1):183–194. doi: 10.1016/0003-9861(91)90026-f. [DOI] [PubMed] [Google Scholar]
- Benz R., Brdiczka D. The cation-selective substate of the mitochondrial outer membrane pore: single-channel conductance and influence on intermembrane and peripheral kinases. J Bioenerg Biomembr. 1992 Feb;24(1):33–39. doi: 10.1007/BF00769528. [DOI] [PubMed] [Google Scholar]
- Campo M. L., Kinnally K. W., Tedeschi H. The effect of antimycin A on mouse liver inner mitochondrial membrane channel activity. J Biol Chem. 1992 Apr 25;267(12):8123–8127. [PubMed] [Google Scholar]
- Costa G., Kinnally K. W., Diwan J. J. Patch clamp analysis of a partially purified ion channel from rat liver mitochondria. Biochem Biophys Res Commun. 1991 Feb 28;175(1):305–310. doi: 10.1016/s0006-291x(05)81235-5. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science. 1985 Sep 20;229(4719):1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- Decker G. L., Greenawalt J. W. Ultrastructural and biochemical studies of mitoplasts and outer membranes derived from French-pressed mitochondria. Advances in mitochondrial subfractionation. J Ultrastruct Res. 1977 Apr;59(1):44–56. doi: 10.1016/s0022-5320(77)80027-0. [DOI] [PubMed] [Google Scholar]
- Hackenbrock C. R. Chemical and physical fixation of isolated mitochondria in low-energy and high-energy states. Proc Natl Acad Sci U S A. 1968 Oct;61(2):598–605. doi: 10.1073/pnas.61.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. D., Beyer C. F., Malkowitz L., Beer B., Blume A. J. Mitochondrial benzodiazepine receptors mediate inhibition of mitochondrial respiratory control. Mol Pharmacol. 1989 Jan;35(1):157–163. [PubMed] [Google Scholar]
- Hirsch J. D., Beyer C. F., Malkowitz L., Loullis C. C., Blume A. J. Characterization of ligand binding to mitochondrial benzodiazepine receptors. Mol Pharmacol. 1989 Jan;35(1):164–172. [PubMed] [Google Scholar]
- Holden M. J., Colombini M. The mitochondrial outer membrane channel, VDAC, is modulated by a soluble protein. FEBS Lett. 1988 Dec 5;241(1-2):105–109. doi: 10.1016/0014-5793(88)81040-8. [DOI] [PubMed] [Google Scholar]
- Kinnally K. W., Antonenko Y. N., Zorov D. B. Modulation of inner mitochondrial membrane channel activity. J Bioenerg Biomembr. 1992 Feb;24(1):99–110. doi: 10.1007/BF00769536. [DOI] [PubMed] [Google Scholar]
- Krause J., Hay R., Kowollik C., Brdiczka D. Cross-linking analysis of yeast mitochondrial outer membrane. Biochim Biophys Acta. 1986 Sep 11;860(3):690–698. doi: 10.1016/0005-2736(86)90568-7. [DOI] [PubMed] [Google Scholar]
- Krueger K. E., Papadopoulos V. Mitochondrial benzodiazepine receptors and the regulation of steroid biosynthesis. Annu Rev Pharmacol Toxicol. 1992;32:211–237. doi: 10.1146/annurev.pa.32.040192.001235. [DOI] [PubMed] [Google Scholar]
- Krueger K. E., Papadopoulos V. Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J Biol Chem. 1990 Sep 5;265(25):15015–15022. [PubMed] [Google Scholar]
- McEnery M. W., Snowman A. M., Trifiletti R. R., Snyder S. H. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEnery M. W. The mitochondrial benzodiazepine receptor: evidence for association with the voltage-dependent anion channel (VDAC). J Bioenerg Biomembr. 1992 Feb;24(1):63–69. doi: 10.1007/BF00769532. [DOI] [PubMed] [Google Scholar]
- Mestre M., Carriot T., Belin C., Uzan A., Renault C., Dubroeucq M. C., Guérémy C., Le Fur G. Electrophysiological and pharmacological characterization of peripheral benzodiazepine receptors in a guinea pig heart preparation. Life Sci. 1984 Aug 27;35(9):953–962. doi: 10.1016/0024-3205(84)90661-1. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Latorre R. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers. Evidence for two voltage-dependent Ca2+ binding reactions. J Gen Physiol. 1983 Oct;82(4):511–542. doi: 10.1085/jgp.82.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran O., Sandri G., Panfili E., Stühmer W., Sorgato M. C. Electrophysiological characterization of contact sites in brain mitochondria. J Biol Chem. 1990 Jan 15;265(2):908–913. [PubMed] [Google Scholar]
- Moreno-Sánchez R., Hogue B. A., Bravo C., Newman A. H., Basile A. S., Chiang P. K. Inhibition of substrate oxidation in mitochondria by the peripheral-type benzodiazepine receptor ligand AHN 086. Biochem Pharmacol. 1991 May 15;41(10):1479–1484. doi: 10.1016/0006-2952(91)90564-l. [DOI] [PubMed] [Google Scholar]
- Nakashima R. A., Mangan P. S., Colombini M., Pedersen P. L. Hexokinase receptor complex in hepatoma mitochondria: evidence from N,N'-dicyclohexylcarbodiimide-labeling studies for the involvement of the pore-forming protein VDAC. Biochemistry. 1986 Mar 11;25(5):1015–1021. doi: 10.1021/bi00353a010. [DOI] [PubMed] [Google Scholar]
- Okabe T., Fujisawa M., Takaku F. Long-term cultivation and differentiation of human erythroleukemia cells in a protein-free chemically defined medium. Proc Natl Acad Sci U S A. 1984 Jan;81(2):453–455. doi: 10.1073/pnas.81.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V., Mukhin A. G., Costa E., Krueger K. E. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J Biol Chem. 1990 Mar 5;265(7):3772–3779. [PubMed] [Google Scholar]
- Ruknudin A., Song M. J., Sachs F. The ultrastructure of patch-clamped membranes: a study using high voltage electron microscopy. J Cell Biol. 1991 Jan;112(1):125–134. doi: 10.1083/jcb.112.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri G., Siagri M., Panfili E. Influence of Ca2+ on the isolation from rat brain mitochondria of a fraction enriched of boundary membrane contact sites. Cell Calcium. 1988 Aug;9(4):159–165. doi: 10.1016/0143-4160(88)90020-6. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Verma A., Trifiletti R. R. The peripheral-type benzodiazepine receptor: a protein of mitochondrial outer membranes utilizing porphyrins as endogenous ligands. FASEB J. 1987 Oct;1(4):282–288. doi: 10.1096/fasebj.1.4.2820823. [DOI] [PubMed] [Google Scholar]
- Sorgato M. C., Keller B. U., Stühmer W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature. 1987 Dec 3;330(6147):498–500. doi: 10.1038/330498a0. [DOI] [PubMed] [Google Scholar]
- Sprengel R., Werner P., Seeburg P. H., Mukhin A. G., Santi M. R., Grayson D. R., Guidotti A., Krueger K. E. Molecular cloning and expression of cDNA encoding a peripheral-type benzodiazepine receptor. J Biol Chem. 1989 Dec 5;264(34):20415–20421. [PubMed] [Google Scholar]
- Szabó I., Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J Biol Chem. 1991 Feb 25;266(6):3376–3379. [PubMed] [Google Scholar]
- Tallman J. F., Paul S. M., Skolnick P., Gallager D. W. Receptors for the age of anxiety: pharmacology of the benzodiazepines. Science. 1980 Jan 18;207(4428):274–281. doi: 10.1126/science.6101294. [DOI] [PubMed] [Google Scholar]
- Verma A., Snyder S. H. Characterization of porphyrin interactions with peripheral type benzodiazepine receptors. Mol Pharmacol. 1988 Dec;34(6):800–805. [PubMed] [Google Scholar]
- Vorobjev I. A., Zorov D. B. Diazepam inhibits cell respiration and induces fragmentation of mitochondrial reticulum. FEBS Lett. 1983 Nov 14;163(2):311–314. doi: 10.1016/0014-5793(83)80842-4. [DOI] [PubMed] [Google Scholar]
- Weiler U., Riesinger I., Knoll G., Brdiczka D. The regulation of mitochondrial-bound hexokinases in the liver. Biochem Med. 1985 Apr;33(2):223–235. doi: 10.1016/0006-2944(85)90031-6. [DOI] [PubMed] [Google Scholar]
- Zorov D. B., Kinnally K. W., Perini S., Tedeschi H. Multiple conductance levels in rat heart inner mitochondrial membranes studied by patch clamping. Biochim Biophys Acta. 1992 Apr 13;1105(2):263–270. doi: 10.1016/0005-2736(92)90203-x. [DOI] [PubMed] [Google Scholar]



