Abstract
Despite extensive research on thoracolumbar fractures, controversy still exists about which approach is the most appropriate. Lack of evidence-based practice may result in patients being treated inappropriately. The objective of study was to perform a systematic review of the effectiveness of the anterior and posterior approaches in the treatment of thoracolumbar fractures. We conducted searches of PubMed and the Cochrane Library, searching for relevant trials up to August 2013 that compared anterior and posterior for the treatment of thoracolumbar fractures. The key words “anterior,” “posterior,” “thoracolumbar fracture,” “CCT,” and “RCT” were used. We assessed all included literature by using the Cochrane handbook (version 5.1). The results were expressed as the mean difference for continuous outcomes and risk difference for dichotomous outcomes, with a 95% confidence interval, using RevMan version 5.2. There were 3 randomized controlled trials and 11 clinical controlled trials included. The meta-analysis showed no significant difference between groups regarding Cobb angle, the Frankel scale, ASIA/JOA motor score, complications, and number of patients returning to work. Compared with the anterior approach, the posterior approach demonstrated superior canal decompression. In the burst fracture subgroup, operative times were significantly shorter and perioperative blood loss was less in the posterior approach group. The posterior approach is more effective for canal decompression, operative times, and perioperative blood loss. However, because of the lack of randomized controlled trials, and because of large sample size studies, heterogeneity was significant between reports. The optimal treatment for thoracolumbar fractures requires further study.
Key words: Anterior, Posterior, Thoracolumbar fracture, Systematic review
Approximately 90% of all spinal fractures occur at the thoracolumbar junction,1–3 including burst fractures, osteoporotic thoracolumbar vertebral collapse, and chronic thoracolumbar fractures as the primary etiologies. The main treatment modality for thoracolumbar fractures is open reduction and internal fixation.4 There are two main surgical approaches: anterior and posterior. However, the best approach remains controversial. We aimed to apply the methodology of systematic review and meta-analysis to thoracolumbar fractures in order to evaluate the effectiveness of the anterior versus posterior approach in their treatment.
Methods
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) patients with T10~L2 thoracolumbar fractures, with or without neurologic deficit, and a minimum age of 18 years; (2) anterior versus posterior approach was the mode of intervention; (3) more than 10 patients were studied, with a minimum follow-up period of 12 months; (4) article type included randomized controlled trials (RCTs) and clinical controlled trials (CCTs); and (5) evaluation included imaging, neurologic examination, and function index (at least 1 item).
Exclusion criteria were as follows: (1) severe open fracture; (2) cancer, infectious disease, hematologic system diseases, or other severe disease; (3) no need for surgical treatment; and (4) fractures higher than T10 or lower than L2.5,6
Measurement index
Measurement indices used included: (1) Cobb angle (kyphosis) of the spinal column; (2) ASIA/JOA motor score; (3) average canal decompression; (4) Frankel scale for the recovery of neurologic function; (5) postoperative complications; (6) number of patients returning to work; (7) average blood loss (mL); and (8) average operative time (minutes).
Literature search
Searches of PubMed and the Cochrane Library databases for relevant trials up to August 2013 that compared anterior and posterior for the treatment of thoracolumbar fractures were performed for RCTs and CCTs using the key words “anterior,” “posterior,” and “thoracolumbar fracture.” The literature search was done by two authors independently.
Assessment of risk of bias in included studies
We assessed risk of bias using the Cochrane Collaboration's tool for assessing risk of bias as described in section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions. The following domains were assessed as “low risk of bias,” “unclear” (uncertain risk of bias), or “high risk of bias”7:
-
1.
sequence generation;
-
2.
allocation concealment;
-
3.
blinding of participants and personnel;
-
4.
blinding of outcomes assessment;
-
5.
incomplete outcome data;
-
6.
selective outcome reporting;
-
7.
other bias.
We also categorized and reported the overall risk of bias of the included studies according to the following:
-
1.
Low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria were met;
-
2.
Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more criteria were assessed as unclear;
-
3.
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria were not met.
Data synthesis
We used Microsoft Excel to record and check all data, and we calculated the standard deviation by the following equation:
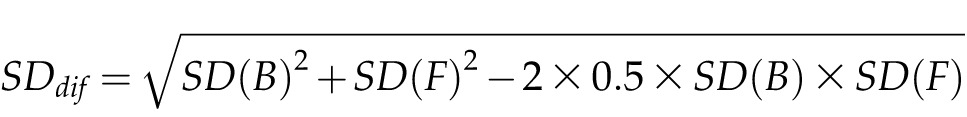 |
Data synthesis was carried out using RevMan software, version 5.2. The χ2 test (statistical heterogeneity was considered significant at a P value greater than 0.1) and the I2 statistic were used to assess statistical heterogeneity in each study.8 Substantial heterogeneity was indicated when there was an I2 value of 50% or higher. When heterogeneity existed, pooled data were meta-analyzed using a random-effects model. Otherwise, a fixed-effects model was used for analysis.9 The risk difference (RD) and 95% confidence interval (CI) were calculated for dichotomous outcomes, whereas the mean difference (MD) and 95% CI were used for continuous outcomes.
Results
Results of literature search
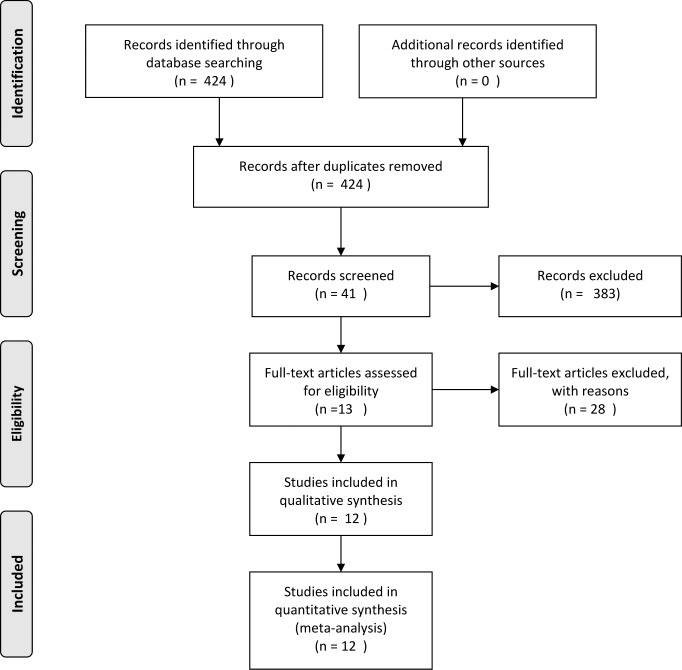
The detailed selection process is shown in Fig. 1.
Fig. 1.
Process of study review.
Methodologic quality assessment and characteristics
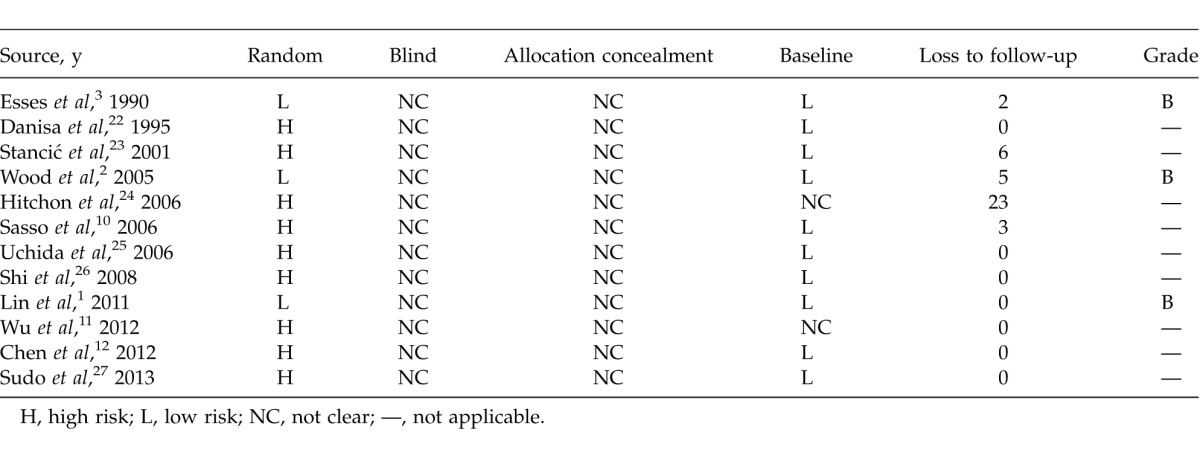
For the 12 included studies, 3 RCTs were graded B. The others were CCTs. Characteristics are shown in Table 1 and results of assessment in Table 2.
Table 1.
Basic characteristics of anterior versus posterior approaches for the treatment of thoracolumbar fractures
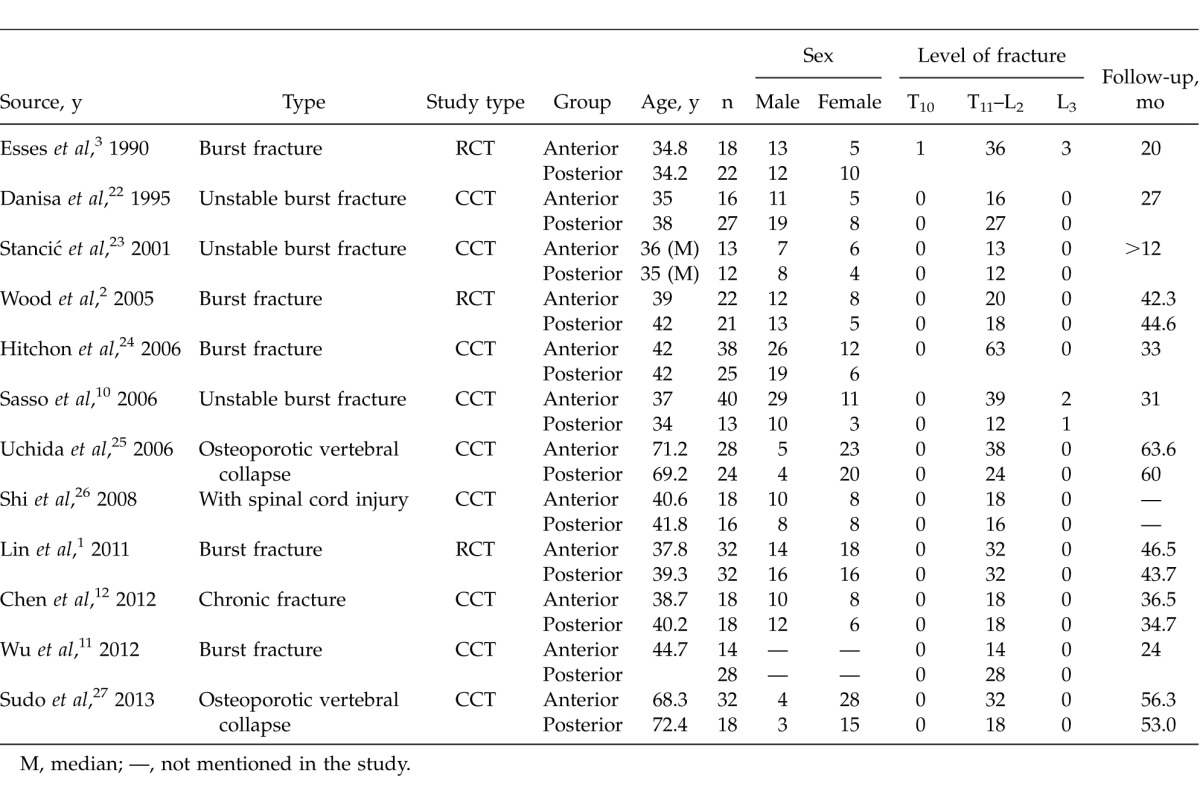
Table 2.
Methodologic quality of anterior versus posterior for treatment of thoracolumbar fractures
Results of meta-analysis
Analysis of the kyphosis in the short term
In 8 studies, the kyphosis change between preoperative and postoperative was compared in adult thoracolumbar fractures. Among 370 patients, there were 180 patients in the anterior group and 190 patients in the posterior group. Kyphosis in the short term was similar between groups [P > 0.1, WMD = −1.91 (−6.14, 2.33); heterogeneity: χ2 = 6.04, df = 7, P < 0.00001; I2 = 64.3%, random-effects model; Fig. 2]. The burst fractures subgroup also showed no difference between groups [P > 0.1, WMD = 0.51 (−0.85, 1.86); heterogeneity: χ2 = 0.8, df = 4, P = 0.94; I2 = 0%; Fig. 2].
Fig. 2.
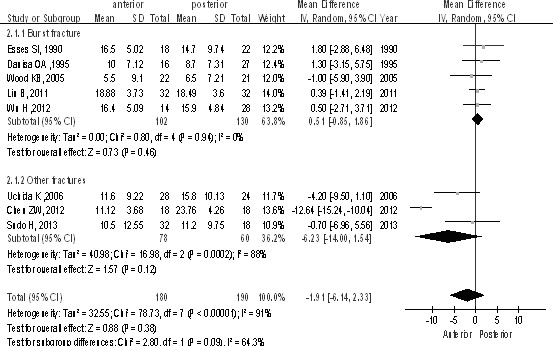
Kyphosis in the short term between anterior and posterior approaches in the treatment of thoracolumbar fractures.
Analysis of the kyphosis in long-term effect
The studies (n = 467 patients; 236 in the anterior surgery group and 231 in the posterior surgery group) reported kyphosis as a long-term effect. There was no statistically significant difference in kyphosis in the long term between anterior and posterior approaches [P > 0.1, WMD = −1.25 (−4.65, 2.15); heterogeneity: χ2 = 84.71, df = 9, P < 0.00001; I2 = 89%, random-effects model; Fig. 3]. The burst fractures subgroup also showed no difference between groups [P > 0.1, WMD = 0.98 (−0.28, 2.24); heterogeneity: χ2 = 1.79, df = 5, P = 0.88; I2 = 0%; Fig. 3].
Fig. 3.
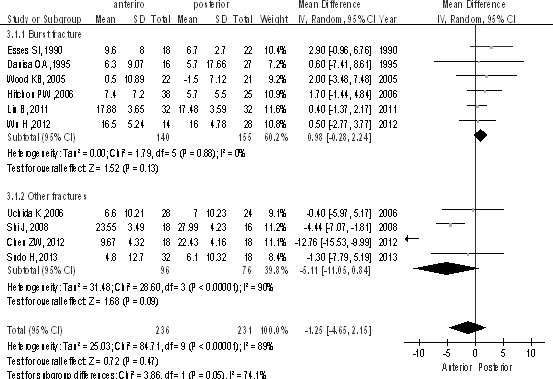
Kyphosis as a long-term effect between anterior and posterior approaches in the treatment of thoracolumbar fractures.
Analysis of ASIA/JOA motor score
All 236 patients (128 for the anterior approach and 108 for the posterior approach) were included, from which 2 studies used the ASIA motor score and 3 studies used the JOA motor score. There was no statistically significant difference both in the ASIA subgroup [P > 0.1, WMD = 0.00 (−4.32, 4.32); heterogeneity: χ2 = 0, df = 1, P = 1; I2 = 0%; Fig. 4] and in JOA subgroup [P > 0.1, WMD = −0.09 (−1.48, 1.29); heterogeneity: χ2 = 13.84, df = 2, P = 0.001; I2 = 86%; Fig. 4].
Fig. 4.
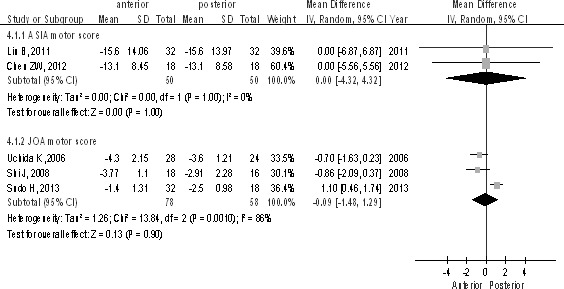
The ASIA/JOA motor score between anterior and posterior approaches in the treatment of thoracolumbar fractures.
Analysis of canal decompression
Only 2 studies recorded canal decompression at 2-year follow-up. Canal decompression was significantly better in the posterior group compared with the anterior group [P < 0.1, WMD = 16.29 (−2.33, 34.90); heterogeneity: χ2 = 11.81, df = 2, P = 0.0006; I2 = 92%; Fig. 5].
Fig. 5.
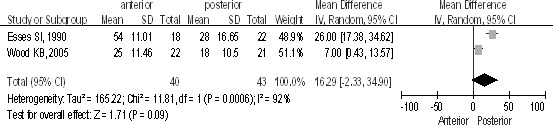
Canal decompression at 2-year follow-up between the anterior and posterior approaches in the treatment of thoracolumbar fractures.
Analysis of the Frankel scale for the recovery of neurologic function
There were 6 studies (n = 297 patients; 160 for the anterior group and 137 for the posterior group) comparing the Frankel scale, preoperatively and at the 2-year follow-up point, showing no difference between groups [P > 0.1, RR = 1.09 (0.88, 1.34); heterogeneity: χ2 = 18.04, df = 5, P = 0.003; I2 = 72%; Fig. 6].
Fig. 6.
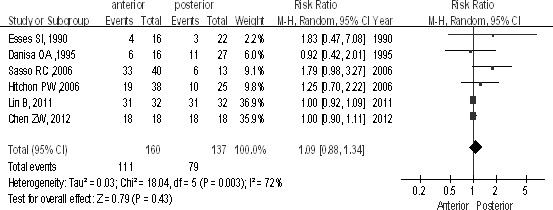
The Frankel scale for the recovery of neurologic function between the anterior and posterior approaches in the treatment of thoracolumbar fractures.
Analysis of complications after surgery
In 8 studies, 364 patients were included, in which 201 patients underwent the anterior approach and 163 underwent the posterior approach. No difference was seen between groups [P > 0.1, RR = 0.86 (0.37, 2.05); heterogeneity: χ2 = 17.87, df = 7, P = 0.01; I2 = 61%; Fig. 7] and in the burst fractures subgroup [P > 0.1, RR = 0.61 (0.23, 1.62); heterogeneity: χ2 = 11.22, df = 5, P = 0.05; I2 = 55%; Fig. 7].
Fig. 7.
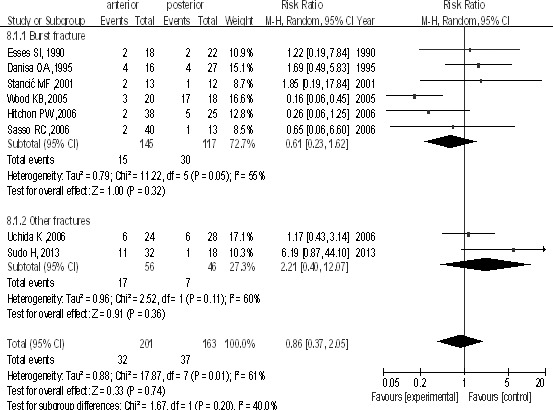
Complications after surgery between the anterior and posterior approaches in the treatment of thoracolumbar fractures.
Analysis of patients returning to work
There were 4 studies (n = 140 patients; 68 for the anterior approach and 72 for the posterior approach). The study showed no difference between groups [P > 0.1, RR = 1.05 (0.86, 1.27); heterogeneity: χ2 = 1.10, df = 3, P = 0.78; I2 = 0%; Fig. 8].
Fig. 8.
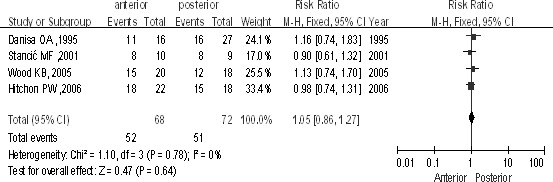
Patients return to work between the anterior approach and the posterior approach in the treatment of thoracolumbar fractures.
Analysis of average blood loss (milliliters)
Of the 337 patients from 8 studies, 165 patients underwent the anterior approach and 172 the posterior approach. The posterior group had less intraoperative blood loss than the anterior group in total [P < 0.1, WMD = 252.72 (102.40, 403.04); heterogeneity: χ2 = 126.55, df = 7, P < 0.00001; I2 = 94%; Fig. 9] and in the burst fractures subgroup [P < 0.1, WMD = 335.17 (118.37, 551.96); heterogeneity: χ2 = 110.38, df = 4, P < 0.00001; I2 = 96%; Fig. 9].
Fig. 9.
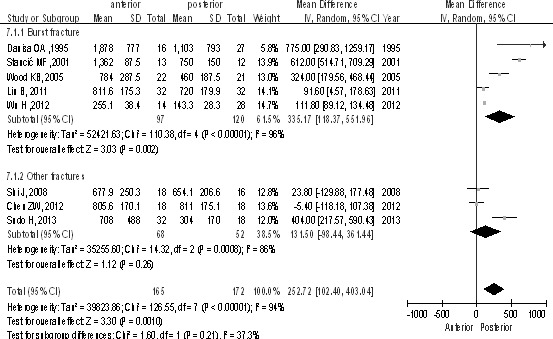
Average intraoperative blood loss (milliliters) between the anterior and posterior approaches in the treatment of thoracolumbar fractures.
Analysis of average length of operative time (minutes)
There were 9 studies (n = 400 patients; 203 for the anterior approach and 197 for the posterior approach). The posterior group had shorter operative time than the anterior group in total [P < 0.1, WMD = 42.72 (12.82, 72.63); heterogeneity: χ2 = 155.81, df = 8, P < 0.00001; I2 = 95%; Fig. 10] and in the burst fractures subgroup [P < 0.1, WMD = 63.10 (24.20, 102.00); heterogeneity: χ2 = 120.34, df = 5, P < 0.00001; I2 = 96%; Fig. 10].
Fig. 10.
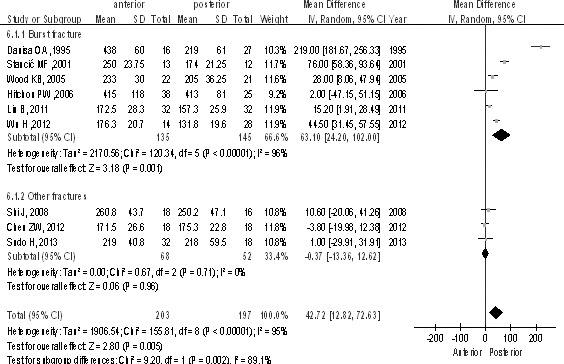
Length of operative time (minutes) between the anterior and posterior approaches in the treatment of thoracolumbar fractures.
Discussion
The question of how thoracolumbar fractures should be approached and stabilized (anteriorly or posteriorly) has remained a controversial subject.1,10–12 Although there are several reports of thoracolumbar fractures, most studies are based on only one surgical method or on internal fixation.13,14 There is a paucity of evidence-based literature on the subject.5,15
Kyphosis (Cobb angle)
The middle column of the spine is the key determinant of spinal stability, and its involvement will be a sufficient criterion for instability.16 Titanium mesh cages filled with autologous bone graft have been used to reconstruct defects created by decompressive corpectomy for thoracolumbar burst fractures, allowing for restoration of height and correction of kyphosis.17–20 The first prospective randomized study2 of not only radiographic and perioperative clinical aspects, but also patient-reported functional outcomes of anterior and posterior surgery, found no significant difference between the two groups when evaluating fracture kyphosis on admission, at discharge, or at final follow-up. Similarly, Esses et al3 performed an RCT in 1990, in which no significant difference was found when comparing anterior and posterior approaches. The above two studies support our study, in which no significant difference of the short- and long-term effects of kyphosis (Cobb angle) was found in the treatment of thoracolumbar fractures. The anterior approach can provide excellent exposure for reconstruction with titanium mesh cages filled with autologous bone graft in the case of fracture with loss of support of the anterior and middle columns. Meanwhile, Lin et al1 suggest that titanium mesh cages filled with autologous bone graft can be inserted into the decompressive space in the posterior approach. Laminectomy may destabilize the spine with progression of deformity, but posterior pedicle screw segmental instrumentation could allow for more rigid fixation by the fixation of 3 columns.
Postoperative complications
Postoperative complications include implant loosening, translocation, and screw breakage; reoperation after internal fixation and bone grafting failure; pulmonary complications; urinary system infection; and pseudoarthrosis. Our study showed no significant difference in postoperative complications between the anterior and posterior approaches, or in the burst fractures subgroup. Wood et al2 reported 17 complications in 18 individuals treated with posterior surgery, whereas of the 20 patients treated with the anterior approach, there were only 3 complications but no infections, with no instrumentation-related problems. Complications included late removal of painful posterior instrumentation, instrumentation breaks, and failure of posterior instrumentation. However, Lin et al1 reported fewer complications of hemopneumothorax, abdominal distension, and constipation in the posterior group, because the posterior approach avoids violating the pulmonary, visceral, and vascular structures, and is less technically demanding.
Canal decompression and the Frankel scale
The midsagittal diameter of the spinal canal at the level of the injury was measured on computed tomography and is to be used as an index to describe canal decompression. Our study included 2 RCTs in the random-effect model, showing that the posterior approach has a better canal decompression effect. Meanwhile, Ayberk et al21 reported the posterior approach to be the better choice in the short term and in follow-up, with the following advantages: (1) the posterior approach is simpler, with less trauma, blood loss, cost, and better recovery of neurologic function; (2) anterior decompression could be completed through the inside of the pedicle; and (3) through internal fixation, the posterior longitudinal ligament pulls the bone block of the anterior canal back, achieving decompression indirectly. Some authors1,21 consider that the neurologic deficit is always caused by impact and compression to the ventral surface of the spinal cord in most patients with thoracolumbar fractures, and that the anterior approach can provide optimal direct exposure for visualization of the ventral aspect of the dura during decompression. Because of the lack of studies on this subject, reliability of this result is low.
Blood loss (milliliters) and operative time (minutes)
Our study found no significant difference between groups regarding blood loss and operative times. However, in the burst fractures subgroup, the posterior group recorded less blood loss and operative time than the anterior surgical group. Tian et al5 achieved the same result in a systematic review of anterior versus posterior surgical treatment of thoracolumbar fractures, claiming that the anterior approach had more injuries and blood loss secondary to bleeding of the venous plexus on the cancellous bone surface and dural sac during decompression. After decompression, bone block, and internal fixation, bleeding was controlled. Operative time is related to the surgeon's experience. As a traditional surgical approach, the posterior approach is more familiar to surgeons in general.
Other aspects
In 2 studies, hospital stay and operative cost were reported, considering that the posterior approach was preferred. Danisa et al22 reported the anterior, posterior, and anterior-posterior combined approaches. Meanwhile, Wu et al11 reported the anterior, posterior, and paraspinal approaches. There were few studies involving the anterior-posterior combined approach and paraspinal approach, which require further study.
In summary, literature involving the anterior and posterior approaches had a large degree of heterogeneity, lack of RCTs, difficulty in blinding, small sample sizes, long follow-up periods, and large numbers of patients lost to follow-up, among other things. There was a significant difference in canal decompression, intraoperative blood loss, and operative times. Large–sample size, multicenter RCTs are necessary.
Acknowledgments
Qicong Zhu and Fengchao Shi contributed equally to the present study.
References
- 1.Lin B, Chen ZW, Guo ZM, Liu H, Yi ZK. Anterior approach versus posterior approach with subtotal corpectomy, decompression, and reconstruction of spine in the treatment of thoracolumbar burst fractures: a prospective randomized controlled study. J Spinal Disord Tech. (in press) doi: 10.1097/BSD.0b013e3182204c53. [DOI] [PubMed] [Google Scholar]
- 2.Wood KB, Bohn D, Mehbod A. Anterior versus posterior treatment of stable thoracolumbar burst fractures without neurologic deficit: a prospective, randomized study. J Spinal Disord Tech. 2005;18(suppl):S15–S23. doi: 10.1097/01.bsd.0000132287.65702.8a. [DOI] [PubMed] [Google Scholar]
- 3.Esses SI, Botsford DJ, Kostuik JP. Evaluation of surgical treatment for burst fractures. Spine. 1990;15(7):667–673. doi: 10.1097/00007632-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Reinhold M, Knop C, Beisse R, Audige L, Kandziora F, Pizanis A. Operative treatment of traumatic fractures of the thoracic and lumbar spinal column: part III: follow up data [in German] Der Unfallchirurg. 2009;112(3):294–316. doi: 10.1007/s00113-008-1539-0. [DOI] [PubMed] [Google Scholar]
- 5.Tian H, Song YC, Chen JT, Ma N, Wang C, Xu Q. Systematic review of anterior versus posterior surgical treatments of thoracolumbar fractures [in Chinese] Zhonghua wai ke za zhi. 2008;46(20):1562–1567. [PubMed] [Google Scholar]
- 6.Thomas KC, Bailey CS, Dvorak MF, Kwon B, Fisher C. Comparison of operative and nonoperative treatment for thoracolumbar burst fractures in patients without neurological deficit: a systematic review. J Neurosurg Spine. 2006;4(5):351–358. doi: 10.3171/spi.2006.4.5.351. [DOI] [PubMed] [Google Scholar]
- 7.Al-Asfoor A, Fedorowicz Z, Lodge M. Resection versus no intervention or other surgical interventions for colorectal cancer liver metastases. Cochrane Database Syst Rev. 2008;(2):CD006039 doi: 10.1002/14651858.CD006039.pub4. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 10.Sasso RC, Renkens K, Hanson D, Reilly T, McGuire RA, Jr, Best NM. Unstable thoracolumbar burst fractures: anterior-only versus short-segment posterior fixation. J Spinal Disord Tech. 2006;19(4):242–248. doi: 10.1097/01.bsd.0000211298.59884.24. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Fu C, Yu W, Wang J. The options of the three different surgical approaches for the treatment of Denis type A and B thoracolumbar burst fracture. Eur J Orthop Surg Traumatol. 2014;24(1):29–35. doi: 10.1007/s00590-012-1152-3. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZW, Ding ZQ, Zhai WL, Lian KJ, Kang LQ, Guo LX. Anterior versus posterior approach in the treatment of chronic thoracolumbar fractures. Orthopedics. 2012;35(2):e219–e224. doi: 10.3928/01477447-20120123-05. [DOI] [PubMed] [Google Scholar]
- 13.Tezeren G, Kuru I. Posterior fixation of thoracolumbar burst fracture: short-segment pedicle fixation versus long-segment instrumentation. J Spinal Disord Tech. 2005;18(6):485–488. doi: 10.1097/01.bsd.0000149874.61397.38. [DOI] [PubMed] [Google Scholar]
- 14.Verlaan JJ, Diekerhof CH, Buskens E, van der Tweel I, Verbout AJ, Dhert WJ. Surgical treatment of traumatic fractures of the thoracic and lumbar spine: a systematic review of the literature on techniques, complications, and outcome. Spine. 2004;29(7):803–814. doi: 10.1097/01.brs.0000116990.31984.a9. [DOI] [PubMed] [Google Scholar]
- 15.Vaccaro AR, Lim MR, Hurlbert RJ, Lehman RA, Jr, Harrop J, Fisher DC. Surgical decision making for unstable thoracolumbar spine injuries: results of a consensus panel review by the Spine Trauma Study Group. J Spinal Disord Tech. 2006;19(1):1–10. doi: 10.1097/01.bsd.0000180080.59559.45. [DOI] [PubMed] [Google Scholar]
- 16.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8(8):817–831. doi: 10.1097/00007632-198311000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Wiggins GC, Rauzzino MJ, Shaffrey CI, Nockels RP, Whitehill R, Alden TD. A new technique for the surgical management of unstable thoracolumbar burst fractures: a modification of the anterior approach and an outcome comparison to traditional methods. Neurosurg Focus. 1999;7(1):e3. doi: 10.3171/foc.1999.7.1.5. [DOI] [PubMed] [Google Scholar]
- 18.Dvorak MF, Kwon BK, Fisher CG, Eiserloh HL, III, Boyd M, Wing PC. Effectiveness of titanium mesh cylindrical cages in anterior column reconstruction after thoracic and lumbar vertebral body resection. Spine. 2003;28(9):902–908. doi: 10.1097/01.BRS.0000058712.88053.13. [DOI] [PubMed] [Google Scholar]
- 19.Robertson PA, Rawlinson HJ, Hadlow AT. Radiologic stability of titanium mesh cages for anterior spinal reconstruction following thoracolumbar corpectomy. J Spinal Disord Tech. 2004;17(1):44–52. doi: 10.1097/00024720-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Payer M. Unstable burst fractures of the thoraco-lumbar junction: treatment by posterior bisegmental correction/fixation and staged anterior corpectomy and titanium cage implantation. Acta Neurochir (Wien) 2006;148(3):299–306. doi: 10.1007/s00701-005-0681-5. discussion 306. [DOI] [PubMed] [Google Scholar]
- 21.Ayberk G, Ozveren MF, Altundal N, Tosun H, Seckin Z, Kilicarslan K. Three column stabilization through posterior approach alone: transpedicular placement of distractable cage with transpedicular screw fixation. Neurol Med Chir (Tokyo) 2008;48(1):8–14. doi: 10.2176/nmc.48.8. discussion 14. [DOI] [PubMed] [Google Scholar]
- 22.Danisa OA, Shaffrey CI, Jane JA, Whitehill R, Wang GJ, Szabo TA. Surgical approaches for the correction of unstable thoracolumbar burst fractures: a retrospective analysis of treatment outcomes. J Neurosurg. 1995;83(6):977–983. doi: 10.3171/jns.1995.83.6.0977. [DOI] [PubMed] [Google Scholar]
- 23.Stancić MF, Gregorović E, Nozica E, Penezić L. Anterior decompression and fixation versus posterior reposition and semirigid fixation in the treatment of unstable burst thoracolumbar fracture: prospective clinical trial. Croat Med J. 2001;42(1):49–53. [PubMed] [Google Scholar]
- 24.Hitchon PW, Torner J, Eichholz KM, Beeler SN. Comparison of anterolateral and posterior approaches in the management of thoracolumbar burst fractures. J Neurosurg Spine. 2006;5(2):117–125. doi: 10.3171/spi.2006.5.2.117. [DOI] [PubMed] [Google Scholar]
- 25.Uchida K, Kobayashi S, Matsuzaki M, Nakajima H, Shimada S, Yayama T. Anterior versus posterior surgery for osteoporotic vertebral collapse with neurological deficit in the thoracolumbar spine. Eur Spine J. 2006;15(12):1759–1767. doi: 10.1007/s00586-006-0106-z. [DOI] [PubMed] [Google Scholar]
- 26.Shi J, Bai L, Ding H, Zhao H, Wang Z. Comparative study of different operating methods in treating old thoracolumbar fractures with spinal cord injury [in Chinese] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008;22(11):1327–1329. [PubMed] [Google Scholar]
- 27.Sudo H, Ito M, Kaneda K, Abumi K, Kotani Y, Nagahama K. Anterior decompression and strut graft versus posterior decompression and pedicle screw fixation with vertebroplasty for osteoporotic thoracolumbar vertebral collapse with neurologic deficits. Spine J. 2013;13(12):1726–1732. doi: 10.1016/j.spinee.2013.05.041. [DOI] [PubMed] [Google Scholar]