Abstract
The objective of this paper was to evaluate whether delaying surgery following long-course chemoradiotherapy for rectal cancer correlates with pathologic complete response. Pre-operative chemoradiotherapy (CRT) is standard practice in the UK for the management of locally advanced rectal cancer. Optimal timing of surgery following CRT is still not clearly defined. All patients with a diagnosis of rectal cancer who had undergone long-course CRT prior to surgery between January 2008 and December 2011 were included. Statistical analysis was performed using Stata 11. Fifty-nine patients received long-course CRT prior to surgery in the selected period. Twenty-seven percent (16/59) of patients showed a complete histopathologic response and 59.3% (35/59) of patients had tumor down-staging from radiologically-assessed node positive to histologically-proven node negative disease. There was no statistically significant delay to surgery after completion of CRT in the 16 patients with complete response (CR) compared with the rest of the group [IR: incomplete response; CR group median: 74.5 days (IQR: 70–87.5) and IR group median: 72 days (IQR: 57–83), P = 0.470]. Although no statistically significant predictors of either complete response or tumor nodal status down-staging were identified in logistic regression analyses, a trend toward complete response was seen with longer delay to surgery following completion of long-course CRT.
Key words: Interval to surgery, Rectal cancer, Long-course chemoradiotherapy
In the multimodal management of rectal cancer, surgical resection remains the mainstay of treatment. Total mesorectal excision (TME) has become the standard operative technique resulting in reduced rates of local recurrence compared with previous conventional surgery.1,2 Apart from surgery, neoadjuvant radiotherapy is employed in resectable rectal cancer to reduce the risk of local recurrence, and in locally-advanced rectal cancer, to downsize the tumor and facilitate subsequent successful R0 resection or sphincter-preserving surgery.3,4 Two meta-analyses have reported that preoperative radiotherapy plus surgery when compared with surgery alone significantly reduced the 5-year overall mortality rate, cancer-related mortality rate, and local recurrence rates in resectable rectal cancer.5,6
Preoperative radiotherapy is usually given either as a short- or long-course treatment schedule. Short-course radiotherapy typically involves 25 Gy in 5 fractions given in 1 week,7 whereas long-course treatment consists of 45 Gy given in 25 fractions over 5 weeks as standard8 with concomitant chemotherapy as a radiosensitizer. The Swedish Rectal Cancer Trial showed statistically significant reduction in the local recurrence rates and increase in the overall survival rates at a median follow-up of 13 years in the group receiving short-course preoperative radiotherapy compared with surgery alone.7 The Dutch trial also confirmed that short-course radiotherapy reduced the risk of local recurrence in patients who underwent a standardized TME.9 Although no chemotherapy was considered in the above studies, the EORTC Radiotherapy Group trial concluded that long-course preoperative radiotherapy with chemotherapy given either preoperatively or postoperatively conferred significant benefit in terms of local control, but did not improve survival.8 Finally, the German Rectal Cancer Study Group showed that preoperative chemoradiotherapy (CRT) compared with postoperative CRT improved local recurrence rates and was associated with reduced toxicity.10
A 6 to 8 week interval to surgery from completion of neoadjuvant CRT has become standard practice since the results of the Lyons R90-01 study were published.11 In this trial, a longer interval of 6 weeks when compared to 2 weeks post-CRT was associated with increased tumor down-staging.11 However, it is not clear whether a yet longer delay before surgery might result in further tumor down-staging or in higher rates of pathologic complete response. The aim of our retrospective study was to evaluate whether a longer interval between completion of long-course CRT and surgery for locally-advanced rectal cancer might maximize the effectiveness of CRT in achieving complete response.
Materials and Methods
Study population
A total of 219 consecutive patients, who were diagnosed with rectal cancer between January 2008 and December 2011 at Peterborough City Hospital (PCH), were identified from a prospectively assembled colorectal cancer database. Fifty-nine out of 219 patients received long-course CRT prior to surgery. In accordance with local guidelines, long-course CRT was considered in patients that had a rectal tumor <3 mm from mesorectum on MRI, which corresponds to a histologic distance of less than 1 mm. All cases were discussed at the colorectal multidisciplinary team (MDT) meeting.
Treatment
Long-course preoperative CRT protocol
The chemotherapy regime consisted of oral capecitabine 825 mg/m2 twice daily for the duration of the radiotherapy including weekends.12 Radiotherapy was administered at Cambridge University Hospitals NHS Trust. Rectal radiotherapy was conformally planned. The patients were positioned prone with an anal marker in place for their CT planning scan. Volumes were outlined on the Prosoma contouring system and planned using the ARPS system. The treated volume included the whole of the rectum, the associated rectal fascia and the draining lymph nodes. The radiotherapy course was 45 Gy in 25 fractions over 35 days prescribed to the ICRU reference point.
Any deviation from the standard protocol of long-course CRT was accounted for. A boost dose of radiotherapy was prescribed in 6 patients either because they could not have chemotherapy due to renal impairment (1 patient only) or could not complete chemotherapy due to toxicity. Eleven patients with high stage tumors also received neoadjuvant chemotherapy with 4 cycles of oxaliplatin and capecitabine prior to commencing long course CRT. This course was comprised of three weekly cycles of oxaliplatin 130 mg/m2 dL and capecitabine 1000 mg/m2 d1–15.
Surgery
All 59 patients underwent surgery after completion of long-course CRT. The operations performed were either anterior resection or abdominoperineal excision done laparoscopically or as open procedures. Total mesorectal excision (TME) was performed in all cases. Five different surgeons carried out the 59 operations. Surgery was performed within 5 to 14 weeks after completion of CRT. The timing of surgery was not determined by any tumor features or by possible tumor response to the long course CRT. Each surgeon had a standard delay from CRT to surgery. Four out of 5 surgeons tended to operate at 6 to 8 weeks, whereas one surgeon used to operate at around 10 weeks post completion of CRT. However, further delays to surgery occurred in all surgeons due to timetables and waiting lists exigencies.
Pathology
A dedicated team of pathologists that attended the colorectal MDT meeting reviewed the histology of the resected specimens. Histopathologic complete response was defined as the absence of residual cancer and fibrosis extending through the rectal wall.13
Data analyses and statistics
Data were extracted from the prospectively maintained colorectal cancer database, patient case notes and the computer reporting systems at PCH. Radiotherapy records were accessed from Cambridge University Hospitals NHS Trust. All statistical analysis was performed using Excel and Stata 11. Median values of group characteristics were compared with χ2 test. A P value of <0.05 was considered statistically significant. Logistic regression analyses were performed to predict the odds of complete response or tumor down-staging to the delay of surgery.
Study approval
This study was registered with the PCH Audit Department.
Results
Fifty-nine patients (41 men, 18 women) received long-course CRT after being diagnosed with locally advanced rectal cancer between January 2008 and December 2011 at PCH. After definitive surgery, 16 patients (27%) showed histopathologic complete response. The study population was divided in two groups; a group of 16 patients that showed complete response (CR) and another group of 43 patients that had incomplete response (IR) after neo-adjuvant CRT. The demographic characteristics, preoperative staging and additional CRT treatment to the long course are shown in Table 1 for the CR and IR groups. Our results showed that there was no statistically significant difference with regards to timing of surgery following completion of long course CRT between the CR and IR groups (CR group median: 74.5 days [IQR, 70–87.5] and IR group median: 72 days [IQR, 57–83], P = 0.470 x2 test).
Table 1.
Comparison of delay from completion of neo-adjuvant chemoradiotherapy to surgery between the CR and IR groups
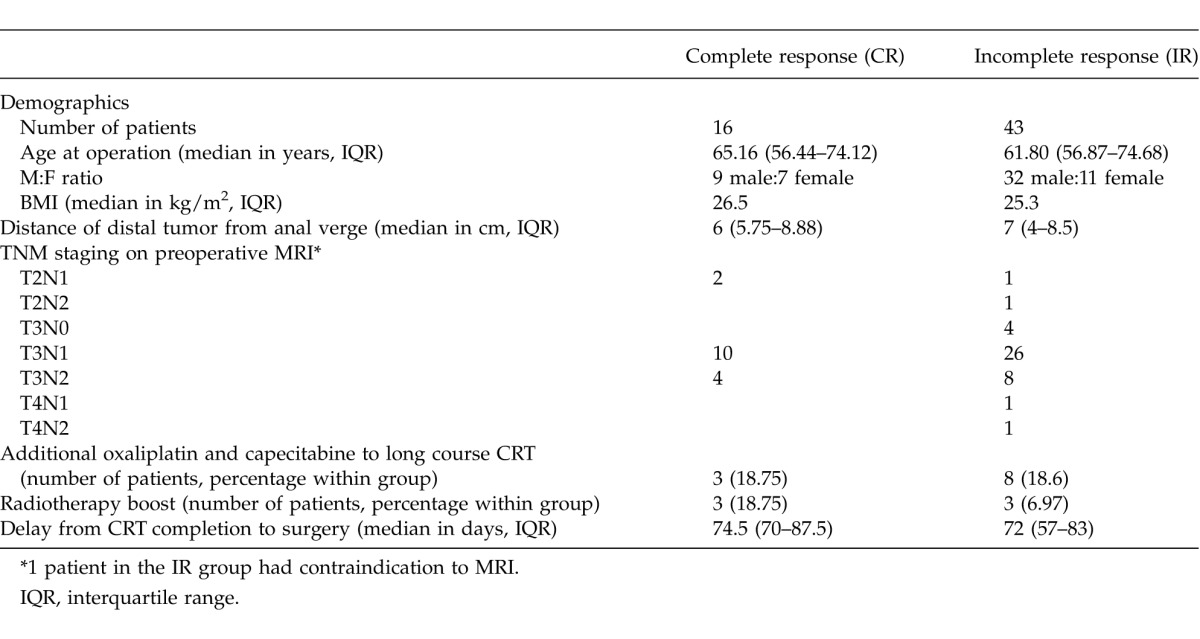
Subsequently, a logistic regression analysis was performed in this patient cohort in order to identify variables predicting complete response. The variables included in the regression model were: age, gender, body mass index (BMI), anemia, duration of radiotherapy, radiotherapy boost, completion of chemotherapy, additional chemotherapy course with oxaliplatin and capecitabine, and days from completion of long-course CRT to surgery. There was no statistically significant predictor of complete response (Table 2). However, when the predictive probability of complete response was plotted against the timing of surgery in days postcompletion of long-course CRT both in a univariate or multivariate analysis, an upward trend was seen (Fig. 1).
Table 2.
Logistic regression analysis predicting the outcome of complete response
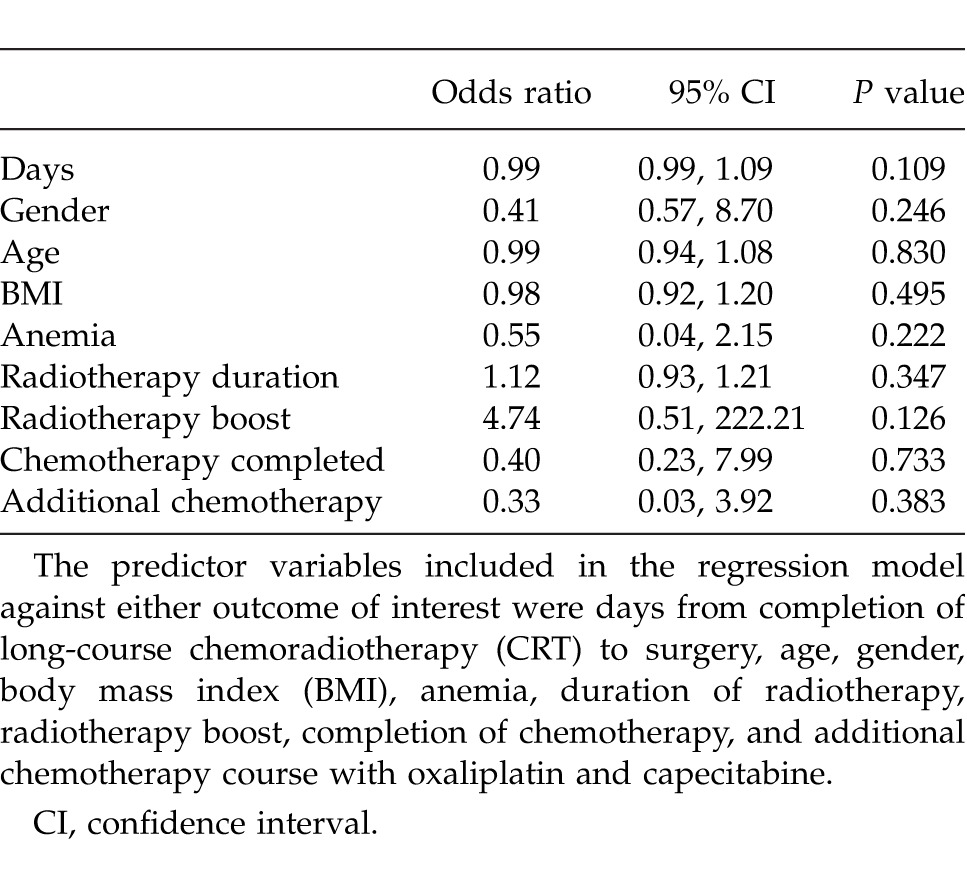
Fig. 1.
The predictive probability of complete response occurring with the delay in surgery from completion of long-course chemoradiotherapy (CRT). The probability of the outcome occurring was adjusted against age, gender, body mass index (BMI), anemia, duration of radiotherapy, radiotherapy boost, completion of chemotherapy, and additional chemotherapy course with oxaliplatin and capecitabine.
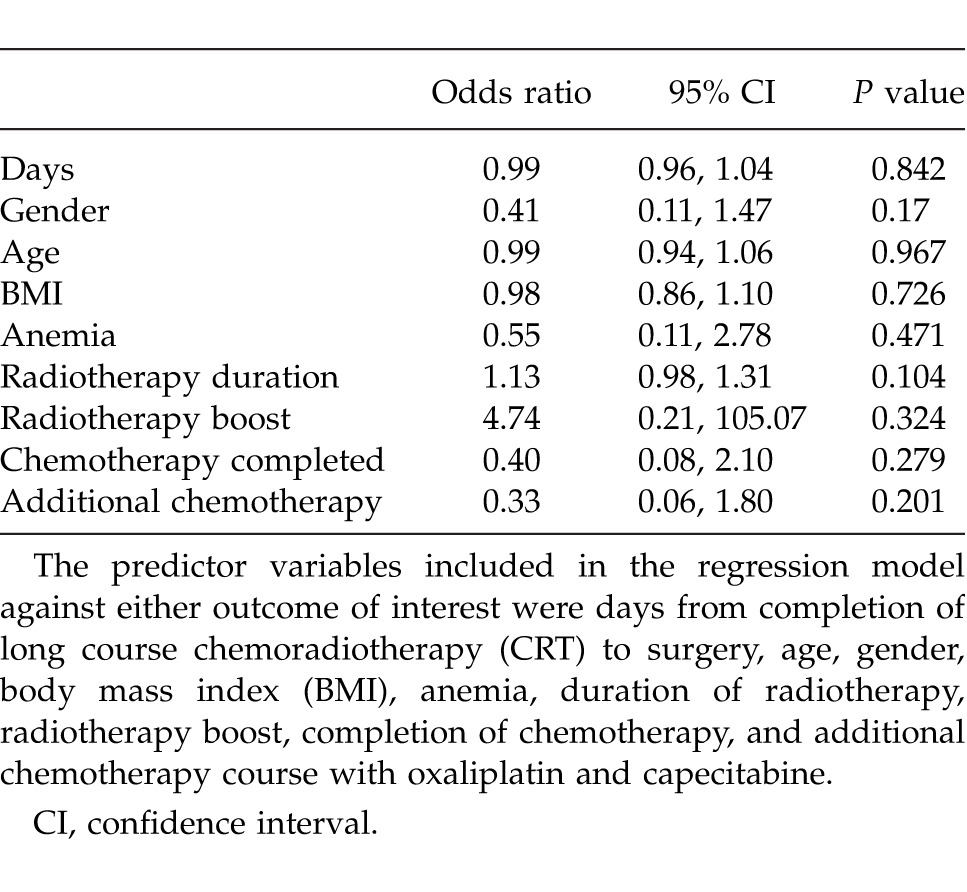
Finally, the outcome of tumor nodal status down-staging from radiologically assessed node positive to histologically proven node negative disease was studied. 35 out of 59 patients (59.3%) were down-staged from radiologically-assessed node positive to histologically node negative disease following chemoradiotherapy. A logistic regression analysis including the same predictor variables used in the previous regression model showed no significant predictors of tumor nodal status down-staging (Table 3).
Table 3.
Logistic regression analysis predicting the outcome of nodal status down-staging
Discussion
Although the benefits of neoadjuvant CRT in local disease control, patient survival, and sphincter-preserving surgery have been reported,3,10 the optimal timing of surgery following completion of CRT while achieving pathologic complete response remains uncertain. The Lyon R90-01 trial showed that a longer interval of 6 to 8 weeks, compared with 2 weeks, between preoperative irradiation and surgery increased tumor down-staging (26% versus 10%, respectively, P = 0.005),11 but had no effect on 5-year survival.14 A retrospective study by Tulchinsky et al showed a statistically significant increased rate of complete response and near complete response in patients operated after an interval of >7 weeks from completion of neoadjuvant CRT compared with those operated on at <7 weeks.15 The same study also showed significantly better disease-free survival rates in patients operated on >7 weeks from completion of CRT.15 Contrary to the above, a preliminary study of 33 patients, by Stein et al,16 compared the outcome of complete response between a 4- to 8-week interval group and a 10- to 14-week CRT-to-surgery interval group and reported no statistically significant difference.
In our retrospective study, a 27% complete response rate was seen among a cohort of 59 consecutive patients who received long course CRT for locally advanced rectal cancer within a period of 4 years. This is comparable with the 28% complete response rate reported by Tulchinsky et al.15 A preliminary analysis of our data involving the first 33 patients from this cohort suggested a correlation between a longer delay to surgery after the completion of CRT and achieving complete response.17 When our analysis was extended to involve the larger cohort of patients spanning 4 consecutive years, no statistical significance with regard to the timing of surgery and achieving complete response was reached but still a trend toward complete response with a longer delay to surgery was seen. In a multivariate logistic regression analysis, no significant predictors of complete response were identified. Our results are in agreement with the findings by Stein et al who showed no statistically significant difference with regard to timing of surgery postcompletion of CRT and achieving complete response.16
The second part of the analysis investigated whether the timing of surgery following completion of neoadjuvant CRT had any correlation with tumor down-staging from pre-CRT node positive disease (assessed pre-CRT MRI imaging) to post-CRT node negative disease (reported on histology). A study by Yeo et al showed that, even after total regression of the primary rectal tumor, achieving node negative disease after preoperative CRT had significantly favorable long-term outcomes in terms of 5-year disease free survival and overall survival (88.5% and 94.8%) compared with positive node status post neoadjuvant CRT (45.2% and 73.8%).18 In our multivariate logistic regression analysis, no significant predictors of tumor down-staging from MRI proven pre-CRT node positive disease to histology reported post-CRT node negative disease were identified.
We recognize that the limitations of this study are its retrospective nature and the small number of patients involved. However, robust statistical methods were employed and multivariate regression analyses did not show any significant predictors of complete response or tumor nodal status down-staging. As the current study only showed a trend toward complete response with delay in surgery following long-course CRT, prospective randomized trials are required to ascertain the optimal timing to surgery after completion of long-course CRT.
Acknowledgments
No funding was received for this study. There are no conflicts of interest to declare.
References
- 1.Heald RJ, Ryall RDH. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1(8496):1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 2.Enker WE. Total mesorectal excision: the new golden standard of surgery for rectal cancer. Ann Med. 1997;29(2):127–133. doi: 10.3109/07853899709113698. [DOI] [PubMed] [Google Scholar]
- 3.Lim CS, Mehigan BJ, Hartley JE, Monson JRT. Neoadjuvant therapy in the treatment of high risk rectal carcinoma. Surg Oncol. 1999;8(1):1–11. doi: 10.1016/s0960-7404(99)00017-1. [DOI] [PubMed] [Google Scholar]
- 4.Marks G, Mohiuddin M, Masoni L, Montori A. High-dose preoperative radiation therapy as the key to extending sphincter-preservation surgery for cancer of the distal rectum. Surg Oncol Clin North Am. 1992;1:71–86. [Google Scholar]
- 5.Camma C, Giunta M, Fiorica F, Pagliaro L, Craxi A, Cottone M. Preoperative radiotherapy for resectable rectal cancer. A meta-analysis. JAMA. 2000;284(8):1008–1015. doi: 10.1001/jama.284.8.1008. [DOI] [PubMed] [Google Scholar]
- 6.Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001;358(9290):1291–304. doi: 10.1016/S0140-6736(01)06409-1. [DOI] [PubMed] [Google Scholar]
- 7.Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Onc. 2005;23(24):5644–5650. doi: 10.1200/JCO.2005.08.144. [DOI] [PubMed] [Google Scholar]
- 8.Bosset J, Collette L, Calais G, Mineur L, Maingon P. Radosevic-Jelic L. for EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. NEJM. 2006;355(11):1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 9.Kapiteijn E, Marijnen CAM, Nastegaal ID, Putter H, Steup WH, Wiggers T, for the Dutch Colorectal Cancer Group Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. NEJM. 2001;345(9):638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 10.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, for the German Rectal Cancer Study Group Preoperative versus postoperative chemoradiotherapy for rectal cancer. NEJM. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 11.Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R- 90-01 randomized trial. J Clin Oncol. 1999;17(8):2396–2402. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 12.Dunst J, Reese T, Sutter T, Zuhlke H, Hinke A, Kolling-Schlebusch K. Phase I trial evaluating the concurrent combination of radiotherapy and capecitabine in rectal cancer. J Clin Oncol. 2002;20(19):3983–3991. doi: 10.1200/JCO.2002.02.049. [DOI] [PubMed] [Google Scholar]
- 13.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF. Pathological assessment of tumour regression after preoperative chemoradiotherapy for oesophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Glehen O, Chapet O, Adham M, Nemoz JC, Gerard JP, Lyons Oncology Group Long-term results of the Lyons R90-01 randomized trial of preoperative radiotherapy with delayed surgery and its effect on sphincter- saving surgery in rectal cancer. Br J Surg. 2003;90(8):996–998. doi: 10.1002/bjs.4162. [DOI] [PubMed] [Google Scholar]
- 15.Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval of >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease- free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15(10):2661–2617. doi: 10.1245/s10434-008-9892-3. [DOI] [PubMed] [Google Scholar]
- 16.Stein DE, Mahmoud N, Anne PR, Rose DG, Isenberg GA, Goldstein SD. Longer time interval between completion of neoadjuvant chemoradiation and surgical resection does not improve downstaging of rectal carcinoma. Dis Colon Rectum. 2003;46(4):448–453. doi: 10.1007/s10350-004-6579-0. [DOI] [PubMed] [Google Scholar]
- 17.Panagiotopoulou IG, Parashar D, Mezher-Sikafi R, Parmar J, Wells AD, Menon M. Timing of surgery after neoadjuvant long-course chemoradiotherapy in the management of locally advanced rectal cancer. Clin Oncol. 2012;24(10) doi: 10.1016/j.clon.2012.09.002. e195. [DOI] [PubMed] [Google Scholar]
- 18.Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W. Pathologic complete response of primary tumor following Preoperative Chemoradiotherapy for Locally advanced rectal cancer, long-term outcomes and prognostic Significance of Pathologic Nodal Status (KROG 09-01) Ann Surg. 2010;252(6):998–1004. doi: 10.1097/SLA.0b013e3181f3f1b1. [DOI] [PubMed] [Google Scholar]


