Abstract
Hyperpolarized 83Kr surface quadrupolar relaxation (SQUARE) generates MRI contrast that was previously shown to correlate with surface-to-volume ratios in porous model surface systems. The underlying physics of SQUARE contrast is conceptually different from any other current MRI methodology as the method uses the nuclear electric properties of the spin I = 9/2 isotope 83Kr. To explore the usage of this non-radioactive isotope for pulmonary pathophysiology, MRI SQUARE contrast was acquired in excised rat lungs obtained from an elastase-induced model of emphysema. A significant 83Kr T1 relaxation time increase in the SQUARE contrast was found in the elastase-treated lungs compared with the baseline data from control lungs. The SQUARE contrast suggests a reduction in pulmonary surface-to-volume ratio in the emphysema model that was validated by histology. The finding supports usage of 83Kr SQUARE as a new biomarker for surface-to-volume ratio changes in emphysema.
Keywords: krypton-83, hyperpolarized noble gas MRI, pulmonary imaging, surface-sensitive contrast, animal model emphysema, nuclear electric quadrupolar relaxation
1. Introduction
Hyperpolarized krypton-83 (hp 83Kr) enables MRI contrast that is indicative of surface composition [1,2] and the surface-to-volume ratio (S/V) [3] in porous media. The T1-weighted MRI contrast is generated through surface quadrupolar relaxation (SQUARE) that causes S/V-dependent reduction in the hp 83Kr MR signal intensity as sketched in figure 1. Pulmonary SQUARE MRI contrast between major pulmonary airways and the alveolar regions has recently been demonstrated in excised rat lungs [4]. In this publication, the potential of hp 83Kr SQUARE contrast for lung pathophysiology is evaluated using ex vivo MRI of an established rat model of emphysema. The emphysema model was selected for this proof of concept study because of the well-known deterioration of the alveolar surface that is expected to cause increased SQUARE T1 times. Following the MRI acquisition, the alveolar surface deterioration can be quantified through histology.
Figure 1.
Illustration of the concept of hp 83Kr SQUARE contrast. The 83Kr atoms are depicted with the 10 possible spin states of this nuclear spin I = 9/2 isotope and with a non-spherical nucleus that possesses a nuclear electric quadrupolar moment. Hyperpolarized 83Kr atoms, drawn in blue, are depicted as occupying the lowest spin state (i.e. corresponding to the highest MRI signal intensity). However, longitudinal T1 relaxation will cause the spins to assume other states (atoms indicated in red), thus reducing the MRI signal intensity. Quadrupolar relaxation occurs when the electron cloud of 83Kr is forced out of the spherical shape. (a) In the bulk gas phase a non-spherical symmetry is caused by collisions (i) leading to the gas phase signal decay within minutes. (b) When surfaces are present, surface adsorption (ii) and possibly dissolution into deeper surface regions (iii) will lead to surface quadrupolar relaxation (SQUARE). Rapid exchange causes transfer of the SQUARE effect into the gas phase where the rapid signal decay is observed with typical T1 times approximately 1 s in rat lungs. See Material and methods section for further details.
Emphysema is a component of chronic obstructive pulmonary disease (COPD), the fourth leading cause of death worldwide [5]. The development of emphysema is mainly linked to cigarette smoking with a smaller proportion attributed to pollution, occupational exposure or intrinsic factors [6]. Over time, there is significant alveolar destruction with the resulting reduction in surface area for gas exchange with an accompanied loss of lung elasticity. Unfortunately, current routine investigations such as lung function tests often fail to diagnose the disease until the later stages [7] once a significant amount of damage has been done. There is therefore a need for new biomarkers to detect the disease in the early stages and to help separate COPD phenotypes [8].
Investigations of COPD and emphysema, in particular, have developed over the past decade with human studies using both computed tomography (CT) [9] and magnetic resonance imaging (MRI)-based techniques [10,11]. Hyperpolarized noble gas MRI [12–14] using 3He and 129Xe is able to provide measurements of ventilation and is able to delineate poorly ventilated and non-functioning lung regions [11,12]. Recently, visualization of delayed collateral ventilation into lung regions has been performed [15], providing information complementary to CT-based techniques. Furthermore, hp 3He and hp 129Xe provide measurements of the lung microstructure through the apparent diffusion coefficient (ADC) [16–24]. Using animal models of emphysema, the distinction between healthy and emphysematous tissue was possible through fractional ventilation generated hp 3He MRI contrast before an increase in alveolar diameter could be determined through histology [25,26]. Unlike fractional ventilation that is decreased in the disease model, ADC was found to be increased in long-term disease models developed over a six-month period [21]. A very promising new pulmonary MRI contrast can be obtained by probing the dissolved phase of hp 129Xe. A host of innovative new techniques, such as xenon polarization transfer contrast (XTC) [23,27–30], xenon alveolar capillary transfer (XACT) [31] and chemical shift saturation recovery spectroscopy (CSSR) [29,32,33] enable selective detection of gas phase xenon, tissue and plasma (TP) dissolved xenon and xenon interacting with red blood cells (RBC). For example, Dregely et al. [23] found a strong correlation between the XTC-based parameter (MXTC-F) and CT data. Using CSSR spectroscopy, Patz et al. [33] found a dramatic decrease in S/V in emphysema patients, while alveolar septal thickness and capillary transit time was not affected. In general, the findings suggest that a sensitive probe for S/V changes is the key to early emphysema diagnosis.
This work sets forth the verification of a new type of hyperpolarized noble gas modality for pulmonary studies, i.e. hp 83Kr SQUARE MRI contrast [4], which may provide a significant addition to existing methodology. Previous work exploring the underlying conceptual physics found SQUARE to be the dominant cause of 83Kr T1 relaxation observed in the gas phase in high S/V porous media such as lungs. See figure 1 and Material and methods section for an explanation of the SQUARE concept. The purpose of this work is to determine whether the SQUARE effect is sensitive enough to serve as a probe for disease-related lung physiological changes.
The potential significance of SQUARE MRI contrast is that it may enable a novel type of biomarker for pulmonary pathophysiology through a fundamentally different physical effect compared with those used in other pulmonary diagnostic techniques such as hp 3He ADC and hp 129Xe dissolved phase measurements. For example, the associated timescale of the ‘surface probing’ is 1–2 orders of magnitude longer than that of ADC measurements. The nature of the contrast generation may provide a methodology sensitive not only to S/V but also to the chemical composition of the surface. Furthermore, in model surfaces quadrupolar noble gas (i.e. 131Xe) relaxation was affected by microscopic surface fine structure [34,35]. Similarly, 83Kr SQUARE is likely to be sensitive to surface corrugation and may, therefore, provide different S/V values compared with dissolved xenon-based techniques [33].
Informed by the surface studies of previous models [1–3,36,37], the underlying hypothesis of this publication is that hp 83Kr SQUARE MRI contrast can serve as a biomarker for the alveolar S/V reduction caused by emphysema. Confirmation of the S/V hypothesis in a preclinical small animal model is a crucial milestone for the development of hp 83Kr MRI. The intratracheal elastase exposure of rat lungs to porcine pancreatic elastase (PPE) generates an established model of emphysema whereby initial inflammation is produced with the subsequent development of airspace enlargement and destruction [38–41]. The experimentally and regulatory less demanding ex vivo set-up [14,42] was used for the MRI measurements in this proof of concept work to set the basis for future in vivo preclinical and clinical studies.
2. Material and methods
2.1. SQUARE contrast
Figure 1 illustrates the mechanism of SQUARE with the nuclear spin I = 9/2 isotope 83Kr. Atomic nuclei are positively charged electric monopoles, however, the nucleus of any spin I > 1/2 isotopes is non-spherical leading to a non-uniform nuclear electric charge distribution. The resulting nuclear electric quadrupole moment can interact with the surrounding electrons if the noble gas atom is ‘distorted’—i.e. if the electronic cloud assumes a non-spherical symmetry. In the bulk gas phase, far away from surfaces (figure 1a), collisions events with other atoms cause rapidly fluctuating electron cloud distortions that results in 83Kr quadrupolar relaxation with T1 times of several minutes at ambient pressure. SQUARE (figure 1b) occurs when surface adsorption and possibly dissolution into deeper surface regions takes place. SQUARE can typically not be observed directly owing to strong line broadening and the limited number of atoms at the surface at any given time. However, the SQUARE effect is transferred into the gas phase through rapid exchange and, depending on the S/V ratio, can tremendously accelerate the decay of the hp gas phase signal. SQUARE contrast is, therefore, potentially sensitive to S/V, surface composition and surface temperature. SQUARE caused by high S/V alveolar region of rat lungs reduces the gas phase T1 time to approximately 1 s leading to the rapid hp 83Kr signal decay in the series of small flip angle spectra in figure 1b.
2.2. 83Kr Spin exchange optical pumping, compression and transfer
Hyperpolarized 83Kr was produced in batch mode by SEOP as described previously described in detail [43]. All MRI was performed using enriched 83Kr (99.925% 83Kr, CHEMGAS, Boulogne, France) to improve the available signal intensity. A 15% krypton 85% N2 (99.999% purity, Air Liquide, Coleshill, UK) mixture was used to reduce the consumption of expensive isotopically enriched 83Kr. SEOP build-up times of 12 min, corresponding to greater than 92% of the steady-state polarization were used to reduce the experimental duration. The hyperpolarized gas extraction unit described previously [4,44] was used to allow for below ambient pressure SEOP [4,43] performed at 55–65 kPa [43]. Overall, the method produced a 83Kr nuclear spin polarization of P = 16–17% after accounting for depolarization occurring in the gas extraction process [44]. An approximate volume of 12–16 ml of the hp gas mixture (1 : 6.7 Kr:N2) was obtained for lung imaging every 12 min. Since no viable method currently exist to separate hp 83Kr from the mixture, it is instructional to report the apparent spin polarization of approximately Papp = 2.5%. The apparent polarization is the nuclear spin polarization P times the fraction of krypton in the hp gas mixture [43]. A discussion of the nuclear spin polarization P for isotopes with nuclear spin I > 1/2 can be found in [45].
2.3. Hyperpolarized gas inhalation
The lungs were suspended in a 5% glucose solution (weight/volume) in the ventilation chamber as described in previous work [14,42]. The chamber was then placed in the centre of the superconducting magnet bore with the temperature kept constant at 295 K throughout the experiments. Active inflation of the lung was accomplished by pulling to a ventilation syringe volume (Vs) of 8 ml. Corresponding inhaled volumes (Vi) were measured separately using the water displacement technique on gas exhalation [14] and are shown in table 1. To limit gas trapping (particularly notable in the PPE-treated lungs) the ex vivo lungs were deflated over 30–60 s from Vs = 8 ml to maximum exhalation (Vs = 0 ml) as has been reported elsewhere [46,47] before hp 83Kr inhalation.
Table 1.
Demographic data from satellite subjects (histology only) and those used for hp 83Kr imaging (with subsequent histology). Summary of rat weights, whole lung mean alveolar area ± standard deviation of the mean, inhaled volumes (Vi) ± standard deviation corresponding to inflation (syringe) volume Vs = 8 ml with associated inhalation pressures ± standard deviation. No values for Vi were determined in the histology groups. Values omitted were not measured.
| lung usage | |||||
|---|---|---|---|---|---|
| identifier | rat weight (g) | whole lung MAA (104 μm2) | MRI: hp 83Kr MRI including histology, Histology: (satellite group) | inhaled volume in MRI, Vi (ml) | |
| control | CL.1 | 492 | — | MRI | 7.0 ± 0.3 |
| CL.2 | 555 | 2.5 ± 0.1 | MRI | 6.8 ± 0.1 | |
| CL.3 | 499 | 3.7 ± 0.6 | MRI | 6.8 ± 0.1 | |
| CL.4 | 400 | 1.9 ± 0.1 | histology | N/A | |
| CL.5 | 412 | 2.5 ± 0.4 | histology | N/A | |
| elastase (PPE) treated | EL.1 | 390 | 6.3 ± 1.1 | MRI | 5.9 ± 0.7 |
| EL.2 | 508 | 6.8 ± 1.1 | MRI | 6.1 ± 0.6 | |
| EL.3 | 416 | 5.1 ± 1.0 | MRI | 6.9 ± 0.3 | |
| EL.4 | 440 | 4.5 ± 0.6 | MRI | 7.3 ± 0.4 | |
| EL.5 | 513 | 3.6 ± 1.0 | MRI | 6.1 ± 0.2 | |
| EL.6 | 382 | 4.8 ± 1.4 | histology | N/A | |
| EL.7 | 436 | 10.8 ± 6.2 | histology | N/A |
2.4. Magnetic resonance imaging protocol
MRI experiments were performed using a vertical bore 9.4 T Bruker Avance III microimaging system (Bruker Corporation, Billerica, MA, USA) with a standard Bruker double saddle coil tuned to 83Kr resonance frequency of 15.40 MHz. The internal diameter (ID) of the coil was 30 mm.
Coronal images were acquired into 64 × 32 matrices using a variable flip angle (VFA) FLASH protocol (TE = 1.8 ms, TR = 12.6 ms) [48]. Rectangular RF pulses of constant duration of 0.3 ms and variable power levels were used in all experiments. The imaging protocol had a total acquisition time 0.405 s to minimize the effects of unwanted T1 decay during acquisition. To obtain T1-weighted images [4], each imaging sequence was started after a programmed time delay, τd, post inhalation. Typically, τd = 0.2 s, 0.7 s, 1.2 s, 1.7 s and 2.2 s were used, although some of the T1 maps were calculated from a series of four images with τd = 0.5 s, 1.0 s, 1.5 s and 2.0 s. The inhalation itself was accomplished manually by reducing the pressure in the artificial pleural cavity using the ventilation syringe [14,42]. Slight alternations in the timing of the manual inhalation procedure (approx.±0.2 s) were deemed acceptable. In all imaging experiments each individual image was acquired from a single inhalation cycle using one VFA FLASH acquisition and no signal averaging. The resulting FOV was 50.9 × 40.7 mm2.
2.5. Image reconstruction and T1 analysis
The raw 32 × 32 datasets were apodized using sine-bell squared function and zero filled to 64 points in each spatial domain before Fourier transformation. Final image resolution was 0.795 × 0.635 mm2 in the frequency encoding (longitudinal) and in the phase encoding (transverse) directions, respectively. These final 64 × 64 magnitude images were exported to IGOR Pro (v. 6.01, Wavemetrics, Lake Oswego, OR, USA) for T1 analysis.
The T1 datasets were created for each series of images by combining the images acquired at the individual time delays τd into a three-dimensional dataset where the first image in the set corresponded to the image acquired at the smallest time delay, τd as described in [4]. The T1 values outside of the 0 s < T1 ≤ 6 s range were rejected as physically not meaningful. The T1 values within the range of 0 s < T1 ≤ 6 s but located outside the lung contour region, or the region of interest (ROI), were also discarded. The ROI was determined from the first image in each T1 set. These final T1 maps were used to produce T1 histograms for each lung reported in this study.
The T1 data collected from the ROI in each T1 map were binned into 200 intervals with 0.03 s increment. The resulting histograms were analysed using build-in Multipeak 1.4 fitting procedure and automated peak picking. A bi-modal Gaussian distribution of T1 values in the lung was assumed because of the distinct alveolar and conducting airway compartments present in the lung. The results of the analysis returned the most probable (expected value, EV) relaxation time, 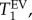 its probability and distribution measured as full width, half maximum, FWHM(T1) for each of the two Gaussian components.
its probability and distribution measured as full width, half maximum, FWHM(T1) for each of the two Gaussian components.
2.6. Model characterization: the elastase model and preparation for ex vivo MRI
Male Sprague-Dawley rats (260–300 g) were purchased from Harlan UK Ltd. (Bicester, UK). Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act 1986 were strictly observed. Experimental emphysema was induced by instilling one dose of 120 U kg−1 PPE (Merck Chemicals Ltd, Nottingham, UK) intratracheally at 1 ml kg−1 directly into the airways under general anaesthesia (inhaled isofluorane 4% with medical grade oxygen for 3–4 min) [40,49]. Control animals were similarly treated with 1 ml kg−1 sterile saline (Fresenius Kabi Ltd, Manor Park, UK) at the same time. At 28 days after intratracheal dosing animals were weighed and euthanized by overdose of sodium pentobarbital 200 mg kg−1 intra-peritoneal (Merial Animal Health, Harlow, UK). After confirmation of death, a catheter was inserted into the caudal vena cava to allow flushing of the pulmonary circulation with 20 ml heparin 100 IU ml−1 (Wockhardt UK Ltd, Wrexham, UK) in 0.9% saline solution (Baxter Healthcare Ltd, Thetford, UK) followed by 20 ml−1 Dublecco's phosphate buffer solution (D-PBS, Sigma-Aldrich Ltd, Gillingham, UK) to remove residual blood from the pulmonary circulation. The heart and lungs were subsequently removed en masse.
Lungs for ex vivo hp 83Kr imaging had a polytetrafluorethylene (PTFE) adapter tube inserted into the trachea 5–10 mm above the carina and sutured into place. The heart and lungs were then suspended in 5% glucose solution (weight/volume) (Baxter Healthcare Ltd, Thetford, UK) with the trachea pointing downwards as has previously reported [14,42]. The ex vivo lungs were repeatedly inflated with 8–10 ml of room air to check for gas leaks either from the suture around the trachea or the lungs themselves. The lungs were chilled to 278 K for transportation to the imaging facility during which time they were repeatedly inflated with 8–10 ml of air at 30–60 min intervals. Time from lung harvest to the start of imaging was no more than 8 h for each lung.
2.7. Model characterization: alveolar cross-section measurements
PPE-induced changes in air space were measured in both a satellite group of rats and on the ex vivo lungs used for hp 83Kr MRI. Lungs from both groups were similarly prepared by inflating to forced vital capacity (25 cm H2O) with 5% formalin–saline solution (Sigma-Aldrich Ltd, Gillingham, UK). The trachea was then tied off and the lungs and heart removed en bloc and placed in formalin. After at least 24 h in formalin, an experienced histologist processed the lungs. Sections were cut from the wax embedded samples using a microtome and stained with H&E.
Experimental emphysema was assessed by measuring average air space area using a method describe by Belloni et al. [50] and used previously [40]. Briefly, computer-assisted image analysis was performed using an Olympus BX40 microscope and Zeiss image-processing software (Imaging Associates, Bicester, UK). Using colour thresholding techniques, the total alveolar air space area and the number of air spaces was measured from five random fields per sample. From these figures the mean air space area was calculated. Any fields containing airways or vasculature were excluded. The person assessing the slides was blinded to the treatment. Details of control and PPE-treated lungs are shown in table 1 with average measurements of mean alveolar area (MAA) in the satellite group and in those lungs processed post ex vivo hp 83Kr MRI.
3. Results
3.1. Comparison hyperpolarized 83Kr magnetic resonance images and resultant T1 maps between control and porcine pancreatic elastase-treated groups
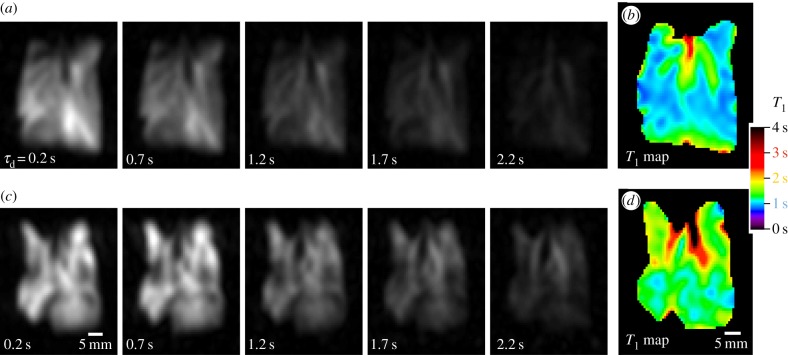
Examples of hp 83Kr MR images acquired using the series of increasing delay times, τd, and the resultant SQUARE T1 maps are displayed in figure 2. Compared with the control lungs, a significant heterogeneity of ventilation was found for in the PPE-treated lung. However, heterogeneous ventilation is not particular to emphysema as it can be caused by a variety of diseases and effects. For example, heterogeneous ventilation was also observed in two of the ex vivo control lungs shown in figure 3. Furthermore, ventilation MRI with a better image resolution can be obtained through hp 3He or hp 129Xe and the focus of this work was on T1 relaxation generated contrast. The hp 83Kr MR images in figure 2a demonstrate how increasing τd delay time between inhalation and image acquisition (i.e. from 0.2 s to 2.2 s) leads to a decrease in hp 83Kr signal intensity due to T1 relaxation. As sketched in figure 1a, the 83Kr gas phase relaxation outside the lung is in the order of minutes [51]. The fast 83Kr T1 relaxation found within the lung is therefore predominantly caused by interactions of the krypton atoms with the surrounding surface—i.e. by the SQUARE effect. Note ‘surface’ does not necessarily refer solely to the outermost surfactant layer and deeper levels, including cell membranes, may contribute as long as fast exchange transfers the depolarized 83Kr back into the alveolar gas phase where MRI signal detection takes place (only gas phase 83Kr signals are MR observable—see also figure 1). In any case however, the 83Kr relaxation time is expected to be sensitive to the S/V ratio and the purpose of this study is to investigate whether SQUARE can serve as an indicator for the emphysema model.
Figure 2.
Series of hp 83Kr MR images with resultant T1 maps in control and PPE-treated lungs. Each image was acquired using a new delivery of hp 83Kr. (a) VFA FLASH MRI with no slice selection in control lung CL.2, using a variable relaxation delay, τd, ranging from 0.2 to 2.2 s between hp gas inhalation and acquisition as indicated in the figure. Note that the major airways are less affected than the lung parenchyma by increasing τd values. (b) The resultant SQUARE T1 map for the control lung displays longer T1 values (green) for the major airways and shorter values for the alveolar region (blue). (c) VFA FLASH MRI as in (a) for the PPE-treated lung EL.1. The lung exhibits heterogeneity in ventilation that is likely caused by the PPE treatment but is not a clear indicator for emphysema. (d) The resultant SQUARE T1 map of the PPE-treated lung displays prolonged relaxation times (green) in the alveolar area compared with the control SQUARE T1 map above.
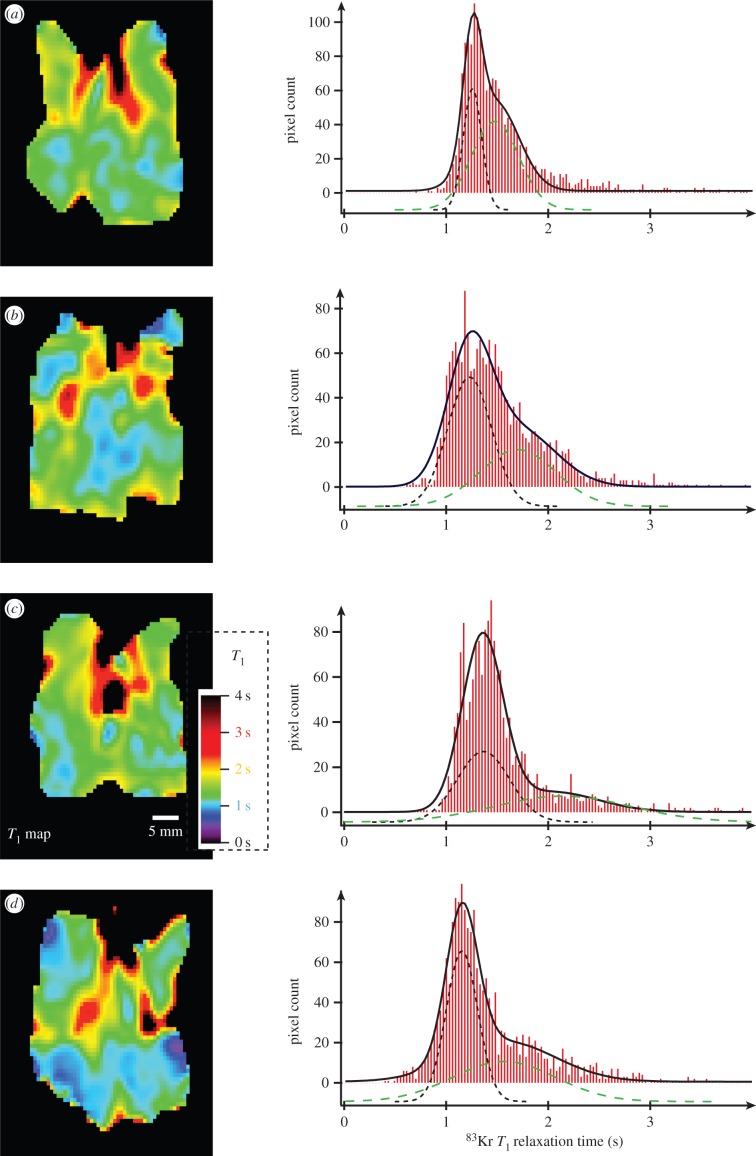
Figure 3.
83Kr MRI T1 maps (SQUARE contrast) of three control lungs and their corresponding histograms. The SQUARE T1 maps have been obtained as described in figure 2. Blue colours in the alveolar regions indicate short T1 values around 1 s. The histograms depict the frequencies (i.e. pixel count from the SQUARE T1 maps) of T1 values within 0.03 s intervals. Bimodal fitting leads to a narrow distribution of fast relaxing pixels centred around 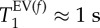 (black dotted line—displayed with negative pixel count offset for clarity) and a broader distribution centred around
(black dotted line—displayed with negative pixel count offset for clarity) and a broader distribution centred around  (green dashed line -negative pixel count axis offset). The solid black line displays the sum of the two Gaussian distributions. The characteristic T1 times are explained in insert in histogram (c) and the specific values for each lung and averaged data are listed in table 2 with lung CL.1 shown in (a), CL.2 in (b) and CL.3 in (c).
(green dashed line -negative pixel count axis offset). The solid black line displays the sum of the two Gaussian distributions. The characteristic T1 times are explained in insert in histogram (c) and the specific values for each lung and averaged data are listed in table 2 with lung CL.1 shown in (a), CL.2 in (b) and CL.3 in (c).
Although other factors, such as chemical composition affect SQUARE [3] the expected strong dependence on S/V ratios is the likely cause for regions with lower S/V, such as the major airways in the control lung, to experience a slower T1 relaxation and thus a slower depolarization rate. These areas remain ‘bright’ in the MR images while areas with higher S/V lead to ‘dark’ regions of faster depolarization. The T1 map calculated from the delay time τd-dependent signal decay leads to the actual SQUARE contrast images shown in figure 2b for the control lung and figure 2d for the elastase-treated lung. Prolonged relaxation times were found for the major airways compared with the alveolar region in the control lung in figure 2b (see also ref. [4]). PPE-treated lungs, serving as a model for emphysema, are imaged for the first time with hp 83Kr in this work. The hp 83Kr SQUARE T1 map of the lung shown in figure 2d exhibits increased T1 values for the alveolar region compared with control lungs. This is reflected in the SQUARE T1 map that displays an elevated level of green colour compared with figure 2b (far right).
Figure 3 depicts the T1 maps of all three control lungs. The data from the T1 maps were used for histogram analysis with frequencies of T1 values within 0.03 s intervals. The T1 relaxation time distribution of the histograms was analysed using bimodal Gaussian distribution function. The four characteristic T1 times from this fitting—i.e. the most probable (expected value, EV) relaxation time,  of each of the two Gaussian components and their distribution measured as full width, half maximum, FWHM(T1), are listed in table 2. The Gaussian distribution of the fast relaxing component centred around
of each of the two Gaussian components and their distribution measured as full width, half maximum, FWHM(T1), are listed in table 2. The Gaussian distribution of the fast relaxing component centred around 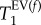 is indicated by a black dotted line in the histograms, whereas the slow relaxing group, centred around
is indicated by a black dotted line in the histograms, whereas the slow relaxing group, centred around  is displayed by the green dashed Gaussian curve. The sum of both components results in the black solid line that very closely resembles the histograms, suggesting that bimodal fitting is a good approach for extraction of parameters that are characteristic for the SQUARE behaviour of a lung.
is displayed by the green dashed Gaussian curve. The sum of both components results in the black solid line that very closely resembles the histograms, suggesting that bimodal fitting is a good approach for extraction of parameters that are characteristic for the SQUARE behaviour of a lung.
Table 2.
Characteristic T1 times from bimodal fitting of the histograms of all lungs used in this work.
 |
 |
 |
 |
||
|---|---|---|---|---|---|
| rat identifier | mean | mean | mean | mean | |
| control lung | CL.1 | 0.9958 | 0.19619 | 1.2353 | 0.53927 |
| CL.2 | 1.0130 | 0.091234 | 1.2189 | 0.30050 | |
| CL.3 | 1.0099 | 0.14987 | 1.3556 | 0.48057 | |
| average ± standard deviation | 1.00620 ± 0.009 | 0.14576 ± 0.053 | 1.2699 ± 0.075 | 0.44011 ± 0.124 | |
| combined histogram of control lungs (figure 5, blue histogram) | 1.0112 | 0.13073 | 1.2494 | 0.38797 | |
| elastase (PPE) treated lung | EL.1 | 1.2559 | 0.12770 | 1.4787 | 0.32787 |
| EL.2 | 1.2311 | 0.30498 | 1.7067 | 0.52674 | |
| EL.3 | 1.3697 | 0.28202 | 2.0474 | 0.63887 | |
| EL.4 | 1.1576 | 0.21975 | 1.5708 | 0.71203 | |
| average ± standard deviation | 1.25358 ± 0.088 | 0.23361 ± 0.079 | 1.7009 ± 0.249 | 0.55138 ± 0.167 | |
| combined histogram of elastase (PPE) treated lungs (figure 5, red histogram) | 1.2734 | 0.28201 | 1.7288 | 0.54928 | |
| EL.5 | 0.94994 | 0.1333 | 1.2257 | 0.4500 | |
Figure 4 shows the SQUARE T1 maps and histograms for PPE-treated lungs. SQUARE T1 maps and histograms display marked differences between PPE-treated and control lungs as can also be noted by the characteristic T1 times from the bimodal fitting listed in table 2. The combined histograms for all three control lungs are shown together with the combined histograms of the four elastase-treated lungs in figure 5.
Figure 4.
83Kr MRI T1 maps (SQUARE contrast) of four of five PPE-treated lungs and their corresponding histograms. Green colours have now become more prevalent in the alveolar regions indicating increased T1 values compared with those shown in figure 3. The histograms display the shift to larger T1 values with  (black dotted line),
(black dotted line), 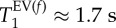 (green dashed line) with exact data listed in table 2. (a) Lung EL.1, (b) EL.2, (c) EL.3 and (d) EL.4. As in figure 3, the fast relaxing group has a narrower distribution than the slower relaxing group.
(green dashed line) with exact data listed in table 2. (a) Lung EL.1, (b) EL.2, (c) EL.3 and (d) EL.4. As in figure 3, the fast relaxing group has a narrower distribution than the slower relaxing group.
Figure 5.
Histogram of all added T1 frequencies. Data were obtained from the three control lungs (blue) in figures 3 and 4 elastase (PPE—red) treated animals (figure 4). The characteristic T1 data from bimodal fitting (as in figures 3 and 4) are listed in table 2 and are very similar to the averaged characteristic T1 values for control and elastase group. The sum of the bimodal fitting resulted to the blue solid line for the control group and the red solid line for the PPE group.
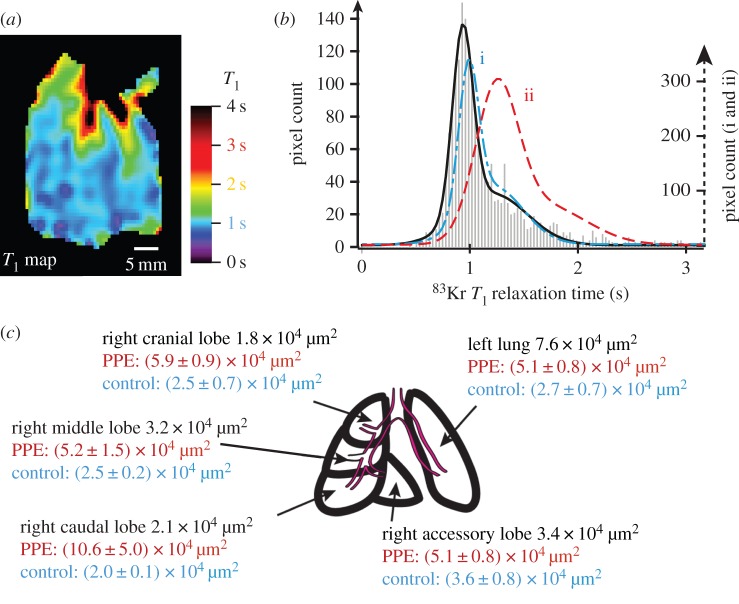
Figure 6 displays a fifth lung (EL.5) from the PPE group that exhibits SQUARE characteristics very similar to that of control lungs. However, table 1 shows that the averaged MAA is fairly low with MAA = 3.6 × 104 µm and thus is similar to that of the control group. The regional (lobar) MAA values are shown in figure 6c together with the averaged MAA values from the control and elastase groups. All lobes except for the left lung lobe of the lung display values in line with the control group. The left lung lobe exhibits increased MAA significantly above the average from the PPE group.
Figure 6.
83Kr MRI T1 maps (SQUARE contrast) of the PPE-treated lung EL.5 (a) and its corresponding histogram (b) with comparison of lobar mean alveolar area values (c). The grey bars indicate the experimental frequency of T1 values and the solid black line is the combined result from bimodal fitting (the two individual components not shown but characteristic times listed in table 2). For comparison the outline of the resulting histogram from figure 5 is shown for the control group (blue dash–dotted line—i) and the elastase group (red dashed line—ii). Both the appearance of the SQUARE contrast and the histogram demonstrate that this lung has similar 83Kr T1 values to those lungs in the control group. (c) The mean alveolar area from lobar histology of PPE-treated lung EL.5 after the MRI experiments (black numbers) suggest that the emphysema model did not develop in all but the left lobe when compared with the mean values for the control (blue text—average from four control lungs) and PPE-treated groups (red text—average from six PPE lungs).
4. Discussion
Two of the control lungs in figure 3 display clear ventilation heterogeneity as parts of the lungs remain dark in the hp 83Kr MR images. The cause of the heterogeneity is unknown but the lungs were transported over a 3-h period, subsequent to excision, between the location of animal holding facility at Imperial College and the hp MRI facility at Nottingham. Nevertheless, the analysis of the SQUARE T1 maps and histograms of all three control lungs led to comparable results with similar bimodal T1 distribution. Figure 5 depicts the combined T1 frequencies for the control group and for the elastase-treated animal lungs. The characteristic T1 values from bimodal fitting are listed in table 2 and show little variation within the two groups (i.e. control and PPE group). The pronounced increase in the  times for the PPE group in figure 5 and table 2 shows that the
times for the PPE group in figure 5 and table 2 shows that the  data can serve as an indicator for the development of the symptoms in the emphysema model. Presumably, the
data can serve as an indicator for the development of the symptoms in the emphysema model. Presumably, the  data are correlated to MAA. This correlation is expected from previous work with model surfaces but requires further corroboration in future studies. In any case however, the FWHM(T1) data are not associated with the MAA differences between the two groups. This view is further supported by the box diagrams in figure 7, which show no overlap in
data are correlated to MAA. This correlation is expected from previous work with model surfaces but requires further corroboration in future studies. In any case however, the FWHM(T1) data are not associated with the MAA differences between the two groups. This view is further supported by the box diagrams in figure 7, which show no overlap in 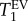 data between the two groups. However, the associated FWHM(T1) data almost completely overlap and are therefore unlikely to be useful as a biomarker for changes in MAA associated with the disease model.
data between the two groups. However, the associated FWHM(T1) data almost completely overlap and are therefore unlikely to be useful as a biomarker for changes in MAA associated with the disease model.
Figure 7.

Box diagram of the characteristic data listed in table 2. The MRI 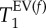 data between control and elastase (PPE) group do not overlap. The same observation is made for the
data between control and elastase (PPE) group do not overlap. The same observation is made for the  data. In contrast, the distribution of T1 values for the two modes of a histogram (characterized by FWHM(T1)) significantly overlaps between control and PPE group, indicating that no significant statistical difference to be expected from the T1 spread. However, the variation in
data. In contrast, the distribution of T1 values for the two modes of a histogram (characterized by FWHM(T1)) significantly overlaps between control and PPE group, indicating that no significant statistical difference to be expected from the T1 spread. However, the variation in 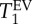 , in particular
, in particular 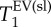 , between the individual animals is more pronounced in the PPE group than in the control animals as visible in the added data in figure 5.
, between the individual animals is more pronounced in the PPE group than in the control animals as visible in the added data in figure 5.
Figure 7 also provides the results of statistical significance testing using Student's t-test at a critical significance level of α = 0.05. The null hypothesis, i.e. assuming no statistically significant difference in  values between control and PPE group, was rejected for
values between control and PPE group, was rejected for 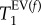 and
and 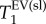 but not for
but not for 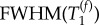 and
and  . This suggests that two parameters, namely
. This suggests that two parameters, namely 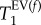 and
and 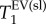 , are useful biomarkers for the elastase model of emphysema and may be sensitive to the increase in MAA.
, are useful biomarkers for the elastase model of emphysema and may be sensitive to the increase in MAA.
A correlation between MAA and SQUARE would be caused by the dependence on lung S/V ratio. A decreasing S/V is expected from increasing MAA in the emphysema model [21,26] and it is known from previous work with model surfaces [3] that T1 ∝ V/S. In this work, the T1 increase was not quantitatively correlated with the increase in MAA but a statistically significant increase in 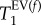 and
and  was observed for the emphysema model with increased MAA in histology compared with the control group. The bimodal appearance of the histogram could be caused by the presence of high S/V in the alveolar region and lower S/V within the airways. The alveoli may cause the fast relaxing mode described by
was observed for the emphysema model with increased MAA in histology compared with the control group. The bimodal appearance of the histogram could be caused by the presence of high S/V in the alveolar region and lower S/V within the airways. The alveoli may cause the fast relaxing mode described by  with a narrow
with a narrow 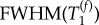 as the alveolar region will be fairly homogeneous. The airways, on the other hand, may contribute to the slow
as the alveolar region will be fairly homogeneous. The airways, on the other hand, may contribute to the slow  mode with a broad distribution and hence a larger
mode with a broad distribution and hence a larger  due to the higher variability in S/V within the various airway generations. Note the relative contribution (in pixel count) between the two modes may not be quantitative due to the different extent of depolarization during the initial 0.2 s (fixed) delay and image acquisition. If the slow mode is indeed (at least partially) caused by the airways, the increase in
due to the higher variability in S/V within the various airway generations. Note the relative contribution (in pixel count) between the two modes may not be quantitative due to the different extent of depolarization during the initial 0.2 s (fixed) delay and image acquisition. If the slow mode is indeed (at least partially) caused by the airways, the increase in  in the elastase-treated rat lungs would suggest that the S/V in the airways is affected by the disease model. Although this point was not further investigated, nitrogen chaser hp 83Kr MRI could be used for future exploration where the airways are largely purged with a small volume of N2 at the end of the inhalation [42].
in the elastase-treated rat lungs would suggest that the S/V in the airways is affected by the disease model. Although this point was not further investigated, nitrogen chaser hp 83Kr MRI could be used for future exploration where the airways are largely purged with a small volume of N2 at the end of the inhalation [42].
The fifth lung (EL.5) from the PPE-treated group was excluded from the statistical analysis because the MAA increase developed asymmetrically in the left lung lobe only, possibly owing to localized elastase deposition. From the data in figure 6c one would expect T1 values similar to those from control lungs in all lobes except for the left lung lobe that exhibited very high MAA values. The SQUARE T1 map and the corresponding histogram are indeed very similar to that of the control lungs, however, this also includes the T1 values found in the left lobe. One would expect that the unexposed part of the lung to shows control lung behaviour, as indeed observed, but one would also expect very long T1 times from the left lobe. However, the left lobe may have been damaged too excessively and some regions may, therefore, no longer be ventilated. These areas will not be able to contribute to the SQUARE T1 map. Although non-ventilated areas should appear as ‘dark’ regions in the hp 83Kr MR images, these zones may be masked by MR signals from unaffected regions that contribute to the non-slice selective images.
For this work, excised lungs have been used. Two of the control lungs exhibited ventilation defects that may have been caused by the excision procedure and long transport period between the laboratories. Although this situation is non-ideal, the experiments demonstrate that the SQUARE T1 contrast was little affected by any damage arising from lung excision, ex vivo transport, and ex vivo experiments. The MAA, obtained after MR image acquisition, were in the expected range for PPE and control group, except for EL.5 as discussed above.
This work demonstrated that hp 83Kr SQUARE can serve as a biomarker for the elastase model of emphysema, in all likelihood because SQUARE senses changes in S/V. If the absence of harmful X-ray radiation is a strong motivation to explore the various pulmonary MRI techniques, a further potential advantage arises with hp 83Kr: the SQUARE effect can be used as a pulmonary biomarker without the presence of pulsed magnetic field gradients (PFGs). Unlike ADC measurements where PFGs are required to generate the MRI contrast, SQUARE is solely caused by a relaxation rooted physical effect. Although PFGs are still needed for the MR image generation, hp 83Kr SQUARE may have a potential as a global lung surface biomarker for pulmonary screening without spatial resolution. Since the effect of relaxation can be measured remotely [37], SQUARE may be observed without an MRI scanner if the effect is remotely detected upon exhalation into a small bench-top NMR device.
The study presented here was focused on providing a first demonstration of hp 83Kr SQUARE usage as an MRI contrast and biomarker for pulmonary pathophysiology in an animal model. Remarkably, using completely different physical concepts from those described here, krypton gas usage has been previously reported for contrast generation in dual energy computed tomography (DECT) [52]. The high-volume inhalation of a mixture of 80% krypton and 20% O2 did not lead to adverse effects in COPD patients. Compared with CT, SQUARE MRI is expected to require lower krypton quantities for usable contrast generation. Finally, molecular oxygen does not dramatically accelerate 83Kr T1 relaxation and SQUARE measurements in rodent lungs [42] were unaffected by the presence of up to 40% O2.
5. Conclusion
Informed by the previous S/V dependence of SQUARE in model surfaces, the aim of this work was to demonstrate the ability of hp 83Kr SQUARE MRI to serve as a biomarker for the elastase model of emphysema. It was demonstrated that two characteristic  times, obtained from bimodal fitting of the histograms, enable statistically significant distinction between emphysema model and control lung. Beyond statistics, the difference between the control group and emphysema model can also be identified from visual inspection of the 83Kr SQUARE images shown in figures 3 and 4. The use of the simpler ex vivo model has allowed for rapid confirmation of the imaging technique despite some ventilation defects in the control group. Hp 83Kr SQUARE may serve as a potential biomarker for pulmonary disease-related S/V ratio changes. Future quantification of the effect and comparison with other hp noble gas modalities will provide further evaluation of this technique. Because neither the application of magnetic field gradients nor the detection within high magnetic fields is required for SQUARE measurements, this new biomarker should also be explored for potential pulmonary mass screening using small bench-top devices.
times, obtained from bimodal fitting of the histograms, enable statistically significant distinction between emphysema model and control lung. Beyond statistics, the difference between the control group and emphysema model can also be identified from visual inspection of the 83Kr SQUARE images shown in figures 3 and 4. The use of the simpler ex vivo model has allowed for rapid confirmation of the imaging technique despite some ventilation defects in the control group. Hp 83Kr SQUARE may serve as a potential biomarker for pulmonary disease-related S/V ratio changes. Future quantification of the effect and comparison with other hp noble gas modalities will provide further evaluation of this technique. Because neither the application of magnetic field gradients nor the detection within high magnetic fields is required for SQUARE measurements, this new biomarker should also be explored for potential pulmonary mass screening using small bench-top devices.
Acknowledgements
The authors would like to thank Samuel Patz, James Butler, Ian Hall and Peter Morris for stimulating discussions; Theodore Hughes-Riley for experimental components; Alan Dorkes and Ian Taylor for specialized equipment.
Competing Interests
We declare we have no competing interests.
Funding
This work was supported in part by the Medical Research Council under grant no. G0900785 and by the Royal Society through the Paul Instrument Fund.
References
- 1.Pavlovskaya GE, Cleveland ZI, Stupic KF, Meersmann T. 2005. Hyperpolarized krypton-83 as a new contrast agent for magnetic resonance imaging. Proc. Natl Acad. Sci. USA 102, 18 275–18 279. ( 10.1073/pnas.0509419102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cleveland ZI, Stupic KF, Pavlovskaya GE, Repine JE, Wooten JB, Meersmann T. 2007. Hyperpolarized Kr-83 and Xe-129 NMR relaxation measurements of hydrated surfaces: implications for materials science and pulmonary diagnostics. J. Am. Chem. Soc. 129, 1784–1792. ( 10.1021/ja065994t) [DOI] [PubMed] [Google Scholar]
- 3.Stupic KF, Cleveland ZI, Pavlovskaya GE, Meersmann T. 2006. Quadrupolar relaxation of hyperpolarized krypton-83 as a probe for surfaces. Solid State Nucl. Magn. Reson. 29, 79–84. ( 10.1016/j.ssnmr.2005.08.008) [DOI] [PubMed] [Google Scholar]
- 4.Six JS, et al. 2014. Pulmonary MRI contrast using surface quadrupolar relaxation (SQUARE) of hyperpolarized Kr-83. Magn. Reson. Imaging 32, 48–53. ( 10.1016/j.mri.2013.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. 2011. World Health Statistics 2011. [Google Scholar]
- 6.Mannino DM, Buist AS. 2007. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 370, 765–773. ( 10.1016/S0140-6736(07)61380-4) [DOI] [PubMed] [Google Scholar]
- 7.Vestbo J, et al. 2008. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). Eur. Respir. J. 31, 869–873. ( 10.1183/09031936.00111707) [DOI] [PubMed] [Google Scholar]
- 8.Han MK, et al. 2010. Chronic obstructive pulmonary disease phenotypes the future of COPD. Am. J. Resp. Crit. Care 182, 598–604. ( 10.1164/rccm.200912-1843CC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litmanovich D, Boiselle PM, Bankier AA. 2009. CT of pulmonary emphysema—current status, challenges, and future directions. Eur. Radiol. 19, 537–551. ( 10.1007/s00330-008-1186-4) [DOI] [PubMed] [Google Scholar]
- 10.Ley-Zaporozhan J, Ley S, Kauczor HU. 2008. Morphological and functional imaging in COPD with CT and MRI: present and future. Eur. Radiol. 18, 510–521. ( 10.1007/s00330-007-0772-1) [DOI] [PubMed] [Google Scholar]
- 11.Fain S, Schiebler ML, McCormack DG, Parraga G. 2010. Imaging of lung function using hyperpolarized helium-3 magnetic resonance imaging: review of current and emerging translational methods and applications. J. Magn. Reson. Imaging 32, 1398–1408. ( 10.1002/jmri.22375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu ZY, Araki T, Okajima Y, Albert M, Hatabu H. 2014. Pulmonary hyperpolarized noble gas MRI: recent advances and perspectives in clinical application. Eur. J. Radiol. 83, 1282–1291. ( 10.1016/j.ejrad.2014.04.014) [DOI] [PubMed] [Google Scholar]
- 13.Mugler JP, Altes TA. 2013. Hyperpolarized 129Xe MRI of the human lung. J. Magn. Reson. Imaging 37, 313–331. ( 10.1002/jmri.23844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lilburn DML, Hughes-Riley T, Six JS, Stupic KF, Shaw DE, Pavlovskaya GE, Meersmann T. 2013. Validating excised rodent lungs for functional hyperpolarized xenon-129 MRI. PLoS ONE 8, e73468 ( 10.1371/journal.pone.0073468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall H, et al. 2012. Direct visualisation of collateral ventilation in COPD with hyperpolarised gas MRI. Thorax 67, 613–617. ( 10.1136/thoraxjnl-2011-200864) [DOI] [PubMed] [Google Scholar]
- 16.Salerno M, de Lange EE, Altes TA, Truwit JD, Brookeman JR, Mugler JP. 2002. Emphysema: hyperpolarized helium 3 diffusion MR imaging of the lungs compared with spirometric indexes—initial experience. Radiology 222, 252–260. ( 10.1148/radiol.2221001834) [DOI] [PubMed] [Google Scholar]
- 17.Yablonskiy DA, Sukstanskii AL, Leawoods JC, Gierada DS, Bretthorst GL, Lefrak SS, Cooper JD, Conradi MS. 2002. Quantitative in vivo assessment of lung microstructure at the alveolar level with hyperpolarized He-3 diffusion MRI. Proc. Natl Acad. Sci. USA 99, 3111–3116. ( 10.1073/pnas.052594699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woods JC, et al. 2006. Hyperpolarized He-3 diffusion MRI and histology in pulmonary emphysema. Magn. Reson. Med. 56, 1293–1300. ( 10.1002/mrm.21076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sukstanskii AL, Yablonskiy DA. 2008. In vivo lung morphometry with hyperpolarized He-3 diffusion MRI: theoretical background. J. Magn. Reson. 190, 200–210. ( 10.1016/j.jmr.2007.10.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yablonskiy DA, Sukstanskii AL, Woods JC, Gierada DS, Quirk JD, Hogg JC, Cooper JD, Conradi MS. 2009. Quantification of lung microstructure with hyperpolarized He-3 diffusion MRI. J. Appl. Physiol. 107, 1258–1265. ( 10.1152/japplphysiol.00386.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephen MJ, et al. 2010. Quantitative assessment of lung ventilation and microstructure in an animal model of idiopathic pulmonary fibrosis using hyperpolarized gas MRI. Acad. Radiol. 17, 1433–1443. ( 10.1016/J.Acra.2010.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaushik SS, et al. 2011. Diffusion-weighted hyperpolarized 129Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn. Reson. Med. 65, 1154–1165. ( 10.1002/mrm.22697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dregely I, et al. 2011. Hyperpolarized xenon-129 gas-exchange imaging of lung microstructure: first case studies in subjects with obstructive lung disease. J. Magn. Reson. Imaging 33, 1052–1062. ( 10.1002/jmri.22533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirby M, et al. 2013. Pulmonary ventilation visualized using hyperpolarized helium-3 and xenon-129 magnetic resonance imaging: differences in COPD and relationship to emphysema. J. Appl. Physiol. 114, 707–715. ( 10.1152/japplphysiol.01206.2012) [DOI] [PubMed] [Google Scholar]
- 25.Spector ZZ, et al. 2005. Quantitative assessment of emphysema using hyperpolarized He-3 magnetic resonance imaging. Magn. Reson. Med. 53, 1341–1346. ( 10.1002/mrm.20514) [DOI] [PubMed] [Google Scholar]
- 26.Emami K, et al. 2008. Early changes of lung function and structure in an elastase model of emphysema—a hyperpolarized He-3 MRI study. J. Appl. Physiol. 104, 773–786. ( 10.1152/japplphysiol.00482.2007) [DOI] [PubMed] [Google Scholar]
- 27.Ruppert K, Mata JF, Brookeman JR, Hagspiel KD, Mugler JP. 2004. Exploring lung function with hyperpolarized Xe-129 nuclear magnetic resonance. Magn. Reson. Med. 51, 676–687. ( 10.1002/mrm.10736) [DOI] [PubMed] [Google Scholar]
- 28.Ruppert K, Mata JF, Wang HTJ, Tobias WA, Cates GD, Brookeman JR, Hagspiel KD, Mugler JP. 2007. XTC MRI: sensitivity improvement through parameter optimization. Magn. Reson. Med. 57, 1099–1109. ( 10.1002/mrm.21241) [DOI] [PubMed] [Google Scholar]
- 29.Patz S, et al. 2007. Hyperpolarized Xe-129 MRI: a viable functional lung imaging modality? Eur. J. Radiol. 64, 335–344. ( 10.1016/j.ejrad.2007.08.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hersman FW, et al. 2008. Large production system for hyperpolarized Xe-129 for human lung imaging studies. Acad. Radiol. 15, 683–692. ( 10.1016/j.acra.2007.09.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Driehuys B, Cofer GP, Pollaro J, Mackel JB, Hedlund LW, Johnson GA. 2006. Imaging alveolar-capillary gas transfer using hyperpolarized Xe-129 MRI. Proc. Natl Acad. Sci. USA 103, 18 278–18 283. ( 10.1073/pnas.0608458103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patz S, et al. 2008. Human pulmonary imaging and spectroscopy with hyperpolarized Xe-129 at 0.2T. Acad. Radiol. 15, 713–727. ( 10.1016/j.acra.2008.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patz S, Muradyan I, Hrovat MI, Dabaghyan M, Washko GR, Hatabu H, Butler JP. 2011. Diffusion of hyperpolarized 129Xe in the lung: a simplified model of 129Xe septal uptake and experimental results. New J. Phys. 13, 015009 ( 10.1088/1367-2630/13/1/015009) [DOI] [Google Scholar]
- 34.Pavlovskaya G, Blue AK, Gibbs SJ, Haake M, Cros F, Malier L, Meersmann T. 1999. Xenon-131 surface sensitive imaging of aerogels in liquid xenon near the critical point. J. Magn. Reson. 137, 258–264. ( 10.1006/jmre.1998.1688) [DOI] [PubMed] [Google Scholar]
- 35.Meersmann T, Deschamps M, Bodenhausen G. 2001. Probing aerogels by multiple quantum filtered Xe-131 NMR spectroscopy. J. Am. Chem. Soc. 123, 941–945. ( 10.1021/ja002747v) [DOI] [PubMed] [Google Scholar]
- 36.Cleveland ZI, Pavlovskaya GE, Stupic KF, Wooten JB, Repine JE, Meersmann T. 2008. Detection of tobacco smoke deposition by hyperpolarized krypton-83 MRI. Magn. Reson. Imaging 26, 270–278. ( 10.1016/j.mri.2007.06.009) [DOI] [PubMed] [Google Scholar]
- 37.Cleveland ZI, Pavlovskaya GE, Stupic KF, LeNoir CF, Meersmann T. 2006. Exploring hyperpolarized 83Kr by remotely detected NMR relaxometry. J. Chem. Phys. 124, 044312 ( 10.1063/1.2159493) [DOI] [PubMed] [Google Scholar]
- 38.Kuhn C, Tavassoli F. 1976. Scanning electron-microscopy of elastase-induced emphysema—comparison with emphysema in man. Lab. Invest. 34, 2–9. [PubMed] [Google Scholar]
- 39.Snider GL, Lucey EC, Stone PJ. 1986. Animal models of emphysema. Am. Rev. Respir. Dis. 133, 149–169. [DOI] [PubMed] [Google Scholar]
- 40.Birrell MA, Wong S, Hele DJ, McCluskie K, Hardaker E, Belvisi MG. 2005. Steroid-resistant inflammation in a rat model of chronic obstructive pulmonary disease is associated with a lack of nuclear factor-κB pathway activation. Am. J. Resp. Crit. Care 172, 74–84. ( 10.1164/rccm.200409-1257OC) [DOI] [PubMed] [Google Scholar]
- 41.Stevenson CS, Birrell MA. 2011. Moving towards a new generation of animal models for asthma and COPD with improved clinical relevance. Pharmacol. Therapeut. 130, 93–105. ( 10.1016/j.pharmthera.2010.10.008) [DOI] [PubMed] [Google Scholar]
- 42.Stupic KF, Elkins ND, Pavlovskaya GE, Repine JE, Meersmann T. 2011. Effects of pulmonary inhalation on hyperpolarized krypton-83 magnetic resonance T-1 relaxation. Phys. Med. Biol. 56, 3731–3748. ( 10.1088/0031-9155/56/13/001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Six JS, Hughes-Riley T, Stupic KF, Pavlovskaya GE, Meersmann T. 2012. Pathway to cryogen free production of hyperpolarized krypton-83 and xenon-129. PLos ONE 7, e2997 ( 10.1371/journal.pone.0049927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes-Riley T, Six JS, Lilburn DM, Stupic KF, Dorkes AC, Shaw DE, Pavlovskaya GE, Meersmann T. 2013. Cryogenics free production of hyperpolarized Xe and Kr for biomedical MRI applications. J. Magn. Reson. 237C, 23–33. ( 10.1016/j.jmr.2013.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stupic KF, Cleveland ZI, Pavlovskaya GE, Meersmann T. 2011. Hyperpolarized Xe-131 NMR spectroscopy. J. Magn. Reson. 208, 58–69. ( 10.1016/J.Jmr.2010.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frazer DG, Weber KC. 1976. Trapped air in ventilated excised rat lungs. J. Appl. Physiol. 40, 915–922. [DOI] [PubMed] [Google Scholar]
- 47.Hughes JMB, Rosenzweig DY. 1970. Factors affecting trapped gas volume in perfused dog lungs. J. Appl. Physiol. 29, 332–339. [DOI] [PubMed] [Google Scholar]
- 48.Zhao L, Mulkern R, Tseng CH, Williamson D, Patz S, Kraft R, Walsworth RL, Jolesz FA, Albert MS. 1996. Gradient-echo imaging considerations for hyperpolarized Xe-129 MR. J. Magn. Reson. B 113, 179–183. ( 10.1006/jmrb.1996.0173) [DOI] [PubMed] [Google Scholar]
- 49.Birrell MA, Wong S, Hardaker EL, Catley MC, McCluskie K, Collins M, Haj-Yahia S, Belvisi MG. 2006. IκB kinase-2-independent and -dependent inflammation in airway disease models: relevance of IKK-2 inhibition to the clinic. Mol. Pharmacol. 69, 1791–1800. ( 10.1124/mol.105.019521) [DOI] [PubMed] [Google Scholar]
- 50.Belloni PN, Garvin L, Mao CP, Bailey-Healy I, Leaffer D. 2000. Effects of all-trans-retinoic acid in promoting alveolar repair. Chest 117, 235S–241S. ( 10.1378/chest.117.5_suppl_1.235S) [DOI] [PubMed] [Google Scholar]
- 51.Cleveland ZI, Meersmann T. 2008. Density-independent contributions to longitudinal relaxation in Kr-83. Chemphyschem 9, 1375–1379. ( 10.1002/cphc.200800134) [DOI] [PubMed] [Google Scholar]
- 52.Hachulla AL, et al. 2012. Krypton ventilation imaging using dual-energy CT in chronic obstructive pulmonary disease patients: initial experience. Radiology 263, 253–259. ( 10.1148/Radiol.12111211) [DOI] [PubMed] [Google Scholar]