Abstract
Objective
Gallstone disease (GSD) is related to multiple cardiovascular risk factors; the present study was to prospectively examine the association between GSD and ischemic heart disease (IHD).
Approach and Results
We examined the association of GSD with IHD among 199,292 men and 288,081 women aged 30–79 years in the China Kadoorie Biobank study. Participants with cancer, heart disease, and stroke at baseline were excluded. Cox proportional hazards regression model was used to estimate the association of GSD with IHD. The prevalence of self-reported GSD was 3.7% in men and 7.3% in women at baseline. During 3,431,124 person-years of follow-up between 2004 and 2013 (median, 7.2 years), we documented 10,245 incident IHD cases in men and 14,714 in women. As compared with men without GSD at baseline, the multivariate-adjusted hazard ratio for IHD was 1.11 (95% confidence interval [CI], 1.02–1.22) for men with GSD; the respective hazard ratio was 1.27 (95% CI, 1.20–1.34) in women and 1.23 (95% CI, 1.17–1.28) in the whole cohort. The sex difference in IHD risk associated with GSD was statistically significant (P=0.009 for interaction with sex). In addition, we found the association between GSD and IHD was stronger in non-hypertensive than hypertensive women (P<0.001 for interaction).
Conclusions
In this large prospective study, the presence of GSD was associated with an increased risk of incident IHD, independent of other risk factors of cardiovascular disease. Our findings suggest novel prevention strategy to mitigate heart disease through improvement of gastrointestinal health.
Keywords: longitudinal cohort study, ischemic heart diseases, risk factor, Chinese
Introduction
Gallstone disease (GSD), a condition with crystalline deposits in the gallbladder, is one of the most common and costly gastrointestinal disorders resulting in hospital admission in developed countries.1 To a relatively lower degree, GSD is also a common health problem in Asian populations such as Chinese;2 and the prevalence has been increasing along with growing adoption of western lifestyle and epidemic of obesity.3
Patients with GSD have higher prevalence of cardiovascular risk factors such as obesity, type 2 diabetes, dyslipidemia, hyperinsulinemia, and sedentary lifestyle.1 In addition, a recent study linked gut microbiota dysbiosis with the presence of cholesterol gallstone.4 Emerging evidence has implicated gut microbiota as a novel factor for cardiovascular disease (CVD).5, 6 Several previous studies have related presence of GSD with an increased CVD risk including outcomes of coronary heart disease, stroke, and cardiovascular disease mortality. 7-13 However, these studies were largely limited by cross-sectional design or small sample size. Prospective investigations on the relation between GSD and CVD risk in large cohorts are sparse.
Therefore, we aimed to prospectively examine the association between a history of GSD and the risk of incident ischemic heart disease (IHD) in a large cohort of 0.5 million of adult Chinese – the China Kadoorie Biobank (CKB) study.14, 15 In addition, we particularly assessed potential interactions between GSD and conventional CVD risk factors.
Materials and Methods
Materials and Methods are available in the online-only supplement.
Results
At baseline, 5.8% of 487,373 participants reported the presence of GSD (men 3.7%; women 7.3%). Age- and site-adjusted baseline characteristics according to the presence of GSD are presented in Table 1. As compared with participants without GSD, those with GSD were older, more likely to be urban residents, less likely to smoke tobacco and drink alcohol, less physically active, had higher BMI, WC, and prevalence of diabetes, chronic hepatitis/cirrhosis, and peptic ulcer (Table 1). Women with GSD had an earlier age at the first diagnosis and longer duration than men with GSD.
Table 1. Baseline characteristics of 487,373 participants according to the presence of gallstone disease.
| Baseline characteristics | Men |
Women |
||||
|---|---|---|---|---|---|---|
| With GSD | Without GSD | P Value | With GSD | Without GSD | P Value | |
| No. of participants | 7,436 | 191,856 | -- | 20,909 | 267,172 | -- |
| Age (year) | 53.9 | 51.8 | <0.001 | 53.3 | 50.3 | <0.001 |
| Urban area (%) | 55.8 | 42.1 | <0.001 | 45.4 | 43.3 | <0.001 |
| Currently married (%) | 94.5 | 92.9 | <0.001 | 89.9 | 89.4 | 0.016 |
| Middle school and above (%) | 62.8 | 57.6 | <0.001 | 46.7 | 43.0 | <0.001 |
| Current regular smoker (%) | 57.7 | 62.3 | <0.001 | 2.3 | 2.3 | 0.789 |
| Current regular drinker (%) | 28.3 | 34.2 | <0.001 | 1.8 | 2.1 | 0.001 |
| Physical activity (MET-hr/day) | 21.1 | 22.7 | <0.001 | 19.8 | 20.9 | <0.001 |
| Average weekly consumption (day)* | ||||||
| Red meat | 4.0 | 3.9 | 0.089 | 3.4 | 3.5 | 0.177 |
| Fresh vegetables | 6.9 | 6.8 | 0.048 | 6.85 | 6.83 | 0.016 |
| Fresh fruits | 2.4 | 2.1 | <0.001 | 2.8 | 2.6 | <0.001 |
| BMI (kg/m2) | 23.9 | 23.4 | <0.001 | 24.2 | 23.7 | <0.001 |
| WC (cm) | 83.5 | 81.8 | <0.001 | 80.0 | 78.7 | <0.001 |
| Prevalence of (%) | ||||||
| Hypertension | 34.5 | 36.0 | 0.007 | 31.3 | 32.3 | 0.001 |
| Diabetes | 6.5 | 5.0 | <0.001 | 6.6 | 5.5 | <0.001 |
| Chronic hepatitis/cirrhosis | 4.4 | 1.6 | <0.001 | 1.4 | 0.8 | <0.001 |
| Peptic ulcer | 8.5 | 5.1 | <0.001 | 5.3 | 2.6 | <0.001 |
| Postmenopausal (%) | -- | -- | -- | 51.7 | 50.7 | <0.001 |
| Family history of heart attack (%) | 6.8 | 6.7 | 0.745 | 6.7 | 5.8 | <0.001 |
| Characteristics of GSD† | ||||||
| Age at the first diagnosis (year) | 45.3 | -- | -- | 44.3 | -- | -- |
| Duration since the first diagnosis (year) | 8.2 | 9.2 | ||||
The results are presented as adjusted means or percentages. All variables are adjusted for age and survey sites, as appropriate. MET denotes metabolic equivalent task; BMI, body mass index; WC, waist circumference; GSD, gallstone disease.
The average weekly consumptions of red meat, fresh vegetables, and fruits were calculated by assigning participants the midpoint of their consumption category.
There were statistically significant differences in both the age at the first diagnosis and the duration since the first diagnosis between men and women (P<0.001).
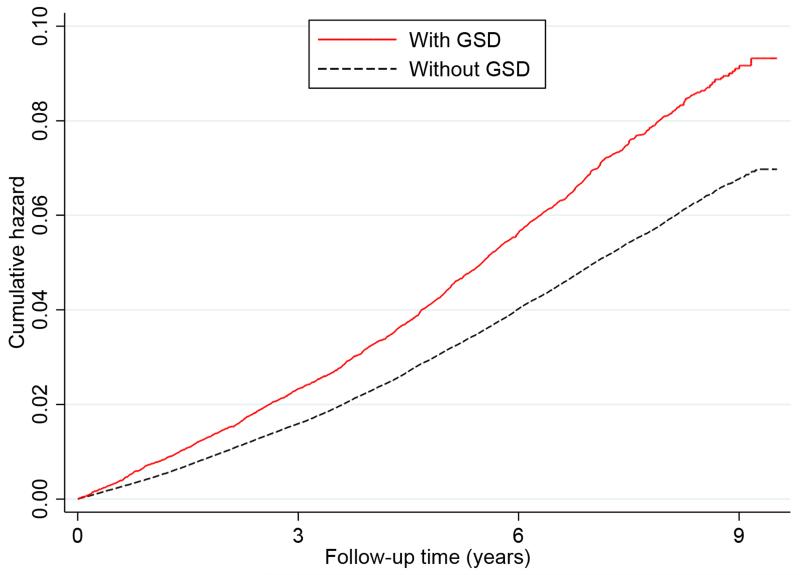
During a median follow-up of 7.2 years (interquartile range: 1.91 years; total person-years, 3,431,124), we documented 10,245 incident IHD cases in men (1,034 [10.1%] fatal, 9,211 [89.9%] nonfatal) and 14,714 in women (767 [5.2%] fatal, 13,947 [94.8%] nonfatal). Incidence rates according to the presence of GSD were 7.1 and 9.8 deaths per 1,000 person years for participants without and with GSD, respectively. Figure 1 presents the Nelson-Aalen curves of the cumulative hazard of IHD according to the presence of GSD. The cumulative incidence of IHD was statistically significantly higher in participants with GSD than in those without GSD (log-rank test: P<0.001). In age-adjusted analyses, the presence of GSD was significantly associated with an increased risk of incident IHD. Further adjustment for other potential confounders, especially BMI, hypertension, diabetes, and several lifestyle risk factors did not substantially attenuate these associations (Table 2). As compared with men without GSD at baseline, the multivariate-adjusted HR for IHD was 1.11 (95% CI, 1.02–1.22) for men with GSD; the respective HR in women was 1.27 (95% CI, 1.20–1.34), and 1.23 (95% CI, 1.17–1.28) in the whole cohort. We found that the association between GSD and IHD was significantly stronger in women than in men (P=0.009 for interaction with sex). These associations were not materially changed in sensitivity analyses with additional adjustment for the presence of digestive system diseases, replacement of BMI by WC, or exclusion of diabetic patients (Table 2).
Figure 1. Nelson-Aalen cumulative hazard for incident ischemic heart disease according to the presence of gallstone disease (GSD).
Log-rank test, P<0.001
Table 2. Hazard ratios (95% CIs) for association between gallstone disease and incident ischemic heart disease.
| Total |
Men |
Women |
||||
|---|---|---|---|---|---|---|
| Without GSD | With GSD | Without GSD | With GSD | Without GSD | With GSD | |
| Case/person-years | 23,017/3,233,478 | 1,942/197,646 | 9,772/1,337,533 | 473/50,998 | 13,245/1,895,946 | 1,469/146,648 |
| Model 1 | 1.00 | 1.26 (1.21–1.32) | 1.00 | 1.15 (1.05–1.26) | 1.00 | 1.29 (1.23–1.37) |
| Model 2 | 1.00 | 1.24 (1.18–1.30) | 1.00 | 1.12 (1.02–1.23) | 1.00 | 1.28 (1.22–1.36) |
| Model 3 | 1.00 | 1.23 (1.17–1.28) | 1.00 | 1.11 (1.02–1.22) | 1.00 | 1.27 (1.20–1.34) |
| Sensitivity analyses | ||||||
| Model 4 | 1.00 | 1.22 (1.17–1.28) | 1.00 | 1.11 (1.01–1.22) | 1.00 | 1.26 (1.20–1.34) |
| Model 5 | 1.00 | 1.22 (1.16–1.28) | 1.00 | 1.11 (1.01–1.21) | 1.00 | 1.26 (1.20–1.34) |
| Model 6 | 1.00 | 1.23 (1.17–1.29) | 1.00 | 1.12 (1.01–1.24) | 1.00 | 1.27 (1.20–1.35) |
CIs denotes confidence intervals; GSD, gallstone disease. Model 1 were adjusted for age. Model 2 additionally included sex (for whole cohort only), level of education, marital status, alcohol consumption, smoking status, physical activity, intake frequencies of red meat, fresh fruits, and vegetables, prevalent hypertension, prevalent diabetes, family history of heart attack, menopausal status (for women only). Model 3 additionally included body mass index (BMI). On the basis of model 3, model 4 additionally included the histories of digestive system diseases including chronic hepatitis/cirrhosis and peptic ulcer; model 5 replaced BMI with WC; model 6 excluded diabetic patients from the analyses.
We further performed stratified analyses according to the duration of GSD from the first diagnosis to the baseline. Similar associations were observed among participants with different durations of GSD in the whole cohort. Notably, the association of GSD with incident IHD appeared to be stronger in women than men among those who reported their duration of GSD since the first diagnosis more than ten years (Table 3).
Table 3. Hazard ratios (95% CIs) for association between gallstone disease and incident ischemic heart disease according to the duration (years) since the first diagnosis.
| Duration | Without GSD |
With GSD |
||
|---|---|---|---|---|
| Case/person-years | HR | Case/person-years | HR (95% CI) | |
| Total | 23,017/3,233,478 | |||
| 0– | 1.00 | 523/54,333 | 1.22 (1.12–1.34) | |
| 3– | 1.00 | 696/73,867 | 1.21 (1.13–1.31) | |
| 10– | 1.00 | 723/69,235 | 1.25 (1.16–1.34) | |
| Men | 9,772/1,337,533 | |||
| 0– | 1.00 | 142/15,293 | 1.16 (0.98–1.37) | |
| 3– | 1.00 | 184/19,358 | 1.20 (1.04–1.39) | |
| 10– | 1.00 | 147/16,325 | 0.99 (0.84–1.16) | |
| Women | 13,245/1,895,946 | |||
| 0– | 1.00 | 381/39,041 | 1.24 (1.12–1.38) | |
| 3– | 1.00 | 512/54,510 | 1.22 (1.12–1.33) | |
| 10– | 1.00 | 576/52,910 | 1.35 (1.24–1.46) | |
CIs denotes confidence intervals; GSD, gallstone disease. The multivariate models were adjusted for the following baseline factors: age, sex (for whole cohort only), level of education, marital status, alcohol consumption, smoking status, level of physical activity, intake frequencies of red meat, fresh fruits, and vegetables, prevalent hypertension, prevalent diabetes, family history of heart attack, menopausal status (for women only), and body-mass index.
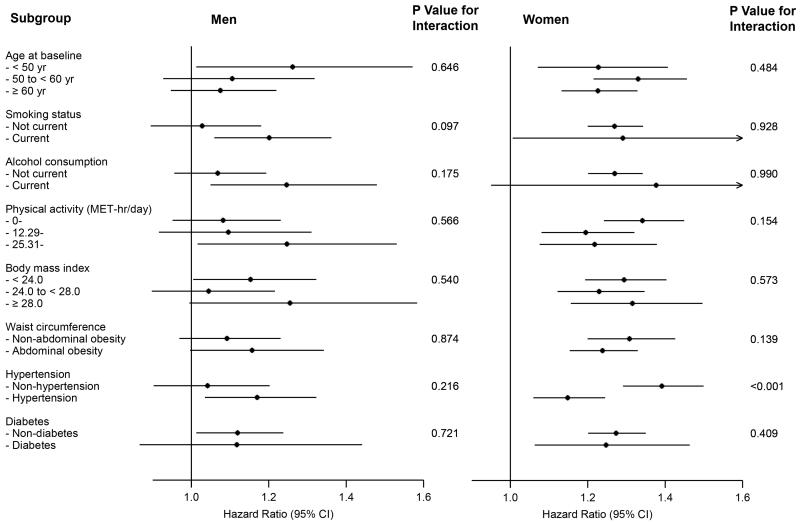
We also analysed the associations between GSD and incident IHD according to other potential baseline risk factors; the positive associations were generally similar across subgroups stratified according to age, smoking status, alcohol consumption, level of physical activity, BMI, abdominal obesity measured by WC, and the presence of diabetes (all P values for interaction > 0.05) (Figure 2, Table I in the online-only Data Supplement). Statistically significant difference across strata was observed for the presence of hypertension in women (P<0.001 for interaction) but not in men (P=0.216 for interaction), with a stronger association in non-hypertensive than hypertensive women.
Figure 2. Subgroup analyses of association between gallstone disease and ischemic heart disease according to potential baseline risk factors.
Adjustments were made for: age, education, marital status, alcohol consumption, smoking status, physical activity, intakes of red meat, fresh fruits, and vegetables, hypertension, diabetes, family history of heart attack, menopausal status (for women only), and body-mass index.
Discussion
In this large prospective study with more than 3.4 million person-years of follow-up, we found that presence of GSD was associated with a significantly increased risk of incident IHD; and such association was independent of traditional cardiovascular risk factors. The association was stronger in women than in men; and was stronger in non-hypertensive than hypertensive women.
Our findings are consistent with the results from four previous smaller prospective studies. In those studies, a history of GSD was associated with an increased risk of coronary heart disease (odds ratio [OR]=1.75; 95% CI: 1.13–2.69) among men in the Framingham Heart Study,9 cardiovascular disease mortality (HR=1.4; 95% CI: 1.2–1.7) in a general US population from the third US National Health and Nutrition Examination Survey,10 cardiovascular disease including myocardial infarction and stroke (HR=1.24, 95% CI: 1.02–1.50) in the German arm of the European Prospective Investigation into Cancer and Nutrition (EPIC),11 and coronary heart disease (HR=1.42; 95% CI, 1.28–1.58) in a Taiwan population identified from the Taiwan National Health Insurance Research Database (NHIRD).12 In addition, in a retrospective cohort study based on Taiwan NHIRD, persons with GSD were also found to have an increased risk of developing ischemic (HR=1.28; 95% CI, 1.25–1.31) and hemorrhagic stroke (HR=1.33; 95% CI, 1.25–1.41).13
Several potential mechanisms may account for the association between GSD and cardiovascular risk. Presence of GSD has been related to a variety of cardiovascular risk factors such as obesity,16,17 hyperinsulinemia,18, 19 insulin resistance,20 diabetes mellitus,19, 21 and metabolic syndrome.22 However, in our study, the association of GSD with IHD risk remained significant after adjustment for obesity, hypertension, diabetes, and common lifestyle risk factors, suggesting other mechanisms might be also involved.
In a recent study, it was found that GSD was related to gut microbiota dysbiosis;4 probably through distorted secretion of bile acids that play a key role in regulating abundance or metabolism of gut microbiota.23 Gut microbiota has recently emerged as a novel risk factor for CVD; and several studies have associated gut microbiota related metabolites such as trimethylamine-N-oxide (TMAO) and L-carnitine with risk of CVD and mortality.6, 24 In addition, abundance of gut microbiota has been related to elevated levels of CVD risk factors including inflammation and dyslipidemia through metabolic endotoxemia.25, 26 We assume the association between GSD and IHD might be at least partly through affecting gut microbiota metabolism; our findings would motivate further investigation on this hypothesis.
In our study, the risk for incident IHD associated with GSD differed by sex and was stronger in women than men. Estrogen has been suggested to be an important risk factor for the formation of cholesterol gallstones, leading to observations that cholesterol gallstones are more common in women than in men; and such sex difference begins from puberty and continues through the childbearing years.27 Sustained effect of estrogen on gallstone formation may also account for a potentially more important etiologic role of GSD in the development of IHD in women than in men. This result was different from findings from previous studies, which reported either no sex difference 10-12 or stronger association in men.9 The discrepant observations may be partly explained by population diversity. In addition, the previous studies are largely in small size and might be underpowered to detect such sex difference. How sex may affect the association between GSD and IHD warrants further investigations.
In addition, we observed statistically significant interaction between GSD and hypertension on IHD risk; and stronger association was observed in nonhypertensive than hypertensive women. GSD and hypertension may partially share common pathogenic mechanisms that contribute to the increased risk of IHD. It is possible that hypertensive patients were already at a high risk of IHD, and GSD status added only modestly deleterious effect on the relative scale. However, it is notable that the absolute risk associated with hypertension among women with GSD was much greater than those without hypertension. In addition, antihypertensive therapy or lifestyle modifications among hypertensive patients may play an antagonistic role on the pathways involved in the development of IHD among women with both conditions. Further study is needed to clarify the possible biologic mechanisms underlying differential effects of GSD on IHD by hypertension. No interaction was observed between GSD and hypertension on IHD risk in men, probably partly due to less important etiologic role of GSD in men than in women.
Our study had several strengths. To our knowledge, this was thus far the largest prospective study assessing time-dependent association between GSD and incident IHD. We carefully controlled for a wide range of established and potential confounders associated with CVD. We excluded participants with a history of CVD at baseline to minimize the influence of possible reverse causation. However, our results need to be interpreted in the context of a few limitations. First, one of the main limitations was that the study relied on self-reporting of the presence of GSD, potentially leading to misclassification of exposures. However, previous smaller prospective studies using diagnosed GSD by gallbladder ultrasonography 10 or based on medical records showed similar findings.12 In addition, in a prospective study design, measurement errors with regard to GSD may be non-differential and the association is more likely to be biased toward the null. Second, we did not collect specific information on the subtypes of gallstone. However, since most gallstones in the Chinese population have been of the cholesterol type since the early 1990s,28 we expected that lack of such information might only have minor impact on the results. Our data were also not able to identify the severity of GSD such as asymptomatic, symptomatic, or having performed cholecystectomy. This could limit our in-depth analysis to explore this association of interest to a further extent. Third, while we have carefully adjusted for a broad range of known or potential risk factors, residual confounding by other unmeasured or unknown factors such as dyslipidemia and hyperinsulinemia was still possible. For example, if dyslipidemia confounds the observed association between GSD and IHD but was not controlled for, it would have potentially led to an overestimation of observed association and biased our results away from the null value.
Conclusions
In summary, in thus far the largest prospective cohort of the Chinese population, we observed that the presence of GSD was associated with an increased risk of incident IHD. Our findings lend further support to the potential role of GSD in affecting cardiovascular risk; and suggest novel prevention strategy to mitigate heart disease through improvement of gastrointestinal health. Additional studies are warranted to confirm the association and to elucidate the potential biological mechanism.
Supplementary Material
Significance.
Gallstone disease (GSD) is one of the most common and costly gastrointestinal disorders resulting in hospital admission. GSD is related to multiple cardiovascular risk factors; however, prospective investigations on the relation between GSD and cardiovascular risk in large cohorts are sparse. In this study, the presence of GSD was independently associated with an increased risk of incident ischemic heart disease (IHD). Our findings suggest novel prevention strategy to mitigate heart disease through improvement of gastrointestinal health.
Acknowledgements
The chief acknowledgment is to the participants, the project staff, and the China National Center for Disease Control and Prevention (CDC) and its regional offices for access to death and disease registries. The Chinese National Health Insurance scheme provides electronic linkage to all hospital treatment.
China Kadoorie Biobank collaborative group
a) International Steering Committee: Liming Li (PI), Junshi Chen, Rory Collins, Richard Peto, Zhengming Chen (PI).
b) Study Coordinating Centres:
International Co-ordinating Centre, Oxford: Zhengming Chen, Garry Lancaster, Xiaoming Yang, Alex Williams, Margaret Smith, Ling Yang, Yumei Chang, Iona Millwood, Yiping Chen, Sarah Lewington. National Co-ordinating Centre, Beijing: Yu Guo, Jun Lv, Zheng Bian, Canqing Yu, Pei Pei, Huiyan Zhou, Yunlong Tan, Can Hou, Lei Guo, Bingyang Han, Shuzhen Qu, Ge Chen. Regional Co-ordinating Centres, 10 areas in China: Qingdao CDC: Zengchang Pang, Shutao Pang, Shaojie Wang, Yongmei Liu, Ranran Du, Yajing Zang, Liang Cheng. Licang CDC: Silu Lv, Junzheng Wang, Wei Hou. Heilongjiang Provincial CDC: Jiyuan Yin, Shumei Liu, Zhigang Pang, Xue Zhou, Huijun Wang. Nangang CDC: Liqiu Yang, Bo Yu, Yanjie Li, Jing Qi, Huaiyi Mu, Qin’ai Xu, Meiling Dou. Hainan Provincial CDC: Jianwei Du, Shanqing Wang, Ximin Hu, Hongmei Wang, Jinyan Chen, Yan Fu, Zhenwang Fu, Xiaohuan Wang, Hua Dong. Meilan CDC: Min Weng, Xiangyang Zheng, Yijun Li, Huimei Li. Jiangsu Provincial CDC: Ming Wu, Jinyi Zhou, Ran Tao, Jie Yang. Suzhou CDC: Jie Shen, Yihe Hu, Yan Lu, Yan Gao, Liangcai Ma, Aiyu Tang, Shuo Zhang, Jianrong Jin. Guangxi Provincial CDC: Zhenzhu Tang, Naying Chen, Ying Huang. Liuzhou CDC: Mingqiang Li, Jinhuai Meng, Rong Pan, Qilian Jiang, Jingxin Qin, Weiyuan Zhang, Yun Liu, Liuping Wei, Liyuan Zhou, Ningyu Chen, Jun Yang, Hairong Guan. Sichuan Provincial CDC: Xianping Wu, Ningmei Zhang, Xiaofang Chen, Xuefeng Tang. Pengzhou CDC: Guojin Luo, Jianguo Li, Xiaofang Chen, Jian Wang, Jiaqiu Liu, Qiang Sun. Gansu Provincial CDC: Pengfei Ge, Xiaolan Ren, Caixia Dong. Maiji CDC: Hui Zhang, Enke Mao, Xiaoping Wang, Tao Wang. Henan Provincial CDC: Guohua Liu, Baoyu Zhu, Gang Zhou, Shixian Feng, Liang Chang, Lei Fan. Huixian CDC: Yulian Gao, Tianyou He, Li Jiang, Huarong Sun, Pan He, Chen Hu, Qiannan Lv, Xukui Zhang. Zhejiang Provincial CDC: Min Yu, Ruying Hu, Le Fang, Hao Wang. Tongxiang CDC: Yijian Qian, Chunmei Wang, Kaixu Xie, Lingli Chen, Yaxing Pan, Dongxia Pan. Hunan Provincial CDC: Yuelong Huang, Biyun Chen, Donghui Jin, Huilin Liu, Zhongxi Fu, Qiaohua Xu. Liuyang CDC: Xin Xu, Youping Xiong, Weifang Jia, Xianzhi Li, Libo Zhang, Zhe Qiu.
Sources of Funding
This work was supported by grants (81390544, 81390541) from the National Natural Science Foundation of China; by a grant (2011BAI09B01) from the National Key Technologies Research and Development Program in the 12th Five-year Plan, Chinese Ministry of Science and Technology; by a grant (088158/Z/09/Z) from the Wellcome Trust in the UK; by a grant from the Kadoorie Charitable Foundation in Hong Kong. Dr Qi is supported by NIH grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States – Israel Binational Science Foundation Grant 2011036. Dr Qi was a recipient of the American Heart Association Scientist Development Award (0730094N). The funders had no role in the study design, data collection, data analysis and interpretation, writing of the report, or the decision to submit the article for publication.
Abbreviations
- CKB
China Kadoorie Biobank
- CVD
cardiovascular disease
- GSD
gallstone disease
- IHD
ischemic heart disease
- MET
metabolic equivalent task
- WC
waist circumference
Footnotes
Disclosures
None.
References
- 1.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230–239. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 2.Center for Health Statistics and Information, MOH . [An analysis report of National Health Services Survey in China, 2008] Peking Union Medical College Press; Beijing: 2009. [Google Scholar]
- 3.Wang S, Marquez P, Langenbrunner J. Toward a healthy and harmonious life in china: Stemming the rising tide of non-communicable diseases. Human development unit; East Asia and Pacific region. World Bank; Washington, DC: 2011. [Google Scholar]
- 4.Wu T, Zhang Z, Liu B, Hou D, Liang Y, Zhang J, Shi P. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics. 2013;14:669. doi: 10.1186/1471-2164-14-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlsson FH, Fak F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Backhed F, Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang WHW, Wang ZE, Levison BS, Koeth RA, Britt EB, Fu XM, Wu YP, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. New Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang ZY, Sheng X, Xu CY, Li WW, Chang XX, Sun LY, Yang XB, Yu LF. Gallbladder gallstone disease is associated with newly diagnosed coronary artery atherosclerotic disease: a cross-sectional study. PloS One. 2013;8:e75400. doi: 10.1371/journal.pone.0075400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendez-Sanchez N, Bahena-Aponte J, Chavez-Tapia NC, Motola-Kuba D, Sanchez-Lara K, Ponciano-Radriguez G, Ramos MH, Uribe M. Strong association between gallstones and cardiovascular disease. Am J Gastroenterol. 2005;100:827–830. doi: 10.1111/j.1572-0241.2005.41214.x. [DOI] [PubMed] [Google Scholar]
- 9.Bortnichak EA, Freeman DH, Jr., Ostfeld AM, Castelli WP, Kannel WB, Feinleib M, McNamara PM. The association between cholesterol cholelithiasis and coronary heart disease in framingham, massachusetts. Am J Epidemiol. 1985;121:19–30. doi: 10.1093/oxfordjournals.aje.a113978. [DOI] [PubMed] [Google Scholar]
- 10.Ruhl CE, Everhart JE. Gallstone disease is associated with increased mortality in the united states. Gastroenterology. 2011;140:508–516. doi: 10.1053/j.gastro.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirth J, Giuseppe R, Wientzek A, Katzke VA, Kloss M, Kaaks R, Boeing H, Weikert C. Presence of gallstones and the risk of cardiovascular diseases: The epic-germany cohort study. Eur J Prev Cardiol. 2015;22:326–334. doi: 10.1177/2047487313512218. [DOI] [PubMed] [Google Scholar]
- 12.Olaiya MT, Chiou HY, Jeng JS, Lien LM, Hsieh FI. Significantly increased risk of cardiovascular disease among patients with gallstone disease: a population-based cohort study. PloS One. 2013;8:e76448. doi: 10.1371/journal.pone.0076448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei CY, Chung TC, Chen CH, Lin CC, Sung FC, Chung WT, Kung WM, Hsu CY, Yeh YH. Gallstone disease and the risk of stroke: A nationwide population-based study. J Stroke Cerebrovasc Dis. 2014;23:1813–1820. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Lee L, Chen J, Collins R, Wu F, Guo Y, Linksted P, Peto R. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC) Int J Epidemiol. 2005;34:1243–1249. doi: 10.1093/ije/dyi174. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Chen J, Collins R, Guo Y, Peto R, Wu F, Li L, China Kadoorie Biobank collaborative Group China Kadoorie Biobank of 0.5 million people: Survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volzke H, Baumeister SE, Alte D, Hoffmann W, Schwahn C, Simon P, John U, Lerch MM. Independent risk factors for gallstone formation in a region with high cholelithiasis prevalence. Digestion. 2005;71:97–105. doi: 10.1159/000084525. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Prospective study of abdominal adiposity and gallstone disease in us men. Am J Clin Nutr. 2004;80:38–44. doi: 10.1093/ajcn/80.1.38. [DOI] [PubMed] [Google Scholar]
- 18.Misciagna G, Guerra V, Di Leo A, Correale M, Trevisan M. Insulin and gall stones: a population case control study in southern italy. Gut. 2000;47:144–147. doi: 10.1136/gut.47.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruhl CE, Everhart JE. Association of diabetes, serum insulin, and C-peptide with gallbladder disease. Hepatology. 2000;31:299–303. doi: 10.1002/hep.510310206. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y, Sung E, Ryu S, Park YW, Jang YM, Park M. Insulin resistance is associated with gallstones even in non-obese, non-diabetic korean men. J Korean Med Sci. 2008;23:644–650. doi: 10.3346/jkms.2008.23.4.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haffner SM, Diehl AK, Mitchell BD, Stern MP, Hazuda HP. Increased prevalence of clinical gallbladder disease in subjects with non-insulin-dependent diabetes mellitus. Am J Epidemiol. 1990;132:327–335. doi: 10.1093/oxfordjournals.aje.a115662. [DOI] [PubMed] [Google Scholar]
- 22.Chen LY, Qiao QH, Zhang SC, Chen YH, Chao GQ, Fang LZ. Metabolic syndrome and gallstone disease. World J Gastroenterol. 2012;18:4215–4220. doi: 10.3748/wjg.v18.i31.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WHW, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caesar R, Fak F, Backhed F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J Intern Med. 2010;268:320–328. doi: 10.1111/j.1365-2796.2010.02270.x. [DOI] [PubMed] [Google Scholar]
- 26.Brown JM, Hazen SL. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med. 2015;66:343–359. doi: 10.1146/annurev-med-060513-093205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang HH, Liu M, Clegg DJ, Portincasa P, Wang DQ. New insights into the molecular mechanisms underlying effects of estrogen on cholesterol gallstone formation. Biochim Biophys Acta. 2009;1791:1037–1047. doi: 10.1016/j.bbalip.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu X, Zhang S, Huang Z. [The trend of the gallstone disease in China over the past decade] Zhonghua Wai Ke Za Zhi [Chinese Journal of Surgery] 1995;33:652–658. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.