Abstract
This study compared the vector competence of four populations of Rhipicephalus sanguineus group ticks for the bacterium Ehrlichia canis, the agent of canine monocytic ehrlichiosis (CME). Ticks (larvae and nymphs) from the four populations—one from São Paulo state, southeastern Brazil (BSP), one from Rio Grande do Sul state, southern Brazil (BRS), one from Argentina (ARG), and one from Uruguay (URU)–were exposed to E. canis infection by feeding on dogs that were experimentally infected with E. canis. Engorged ticks (larvae and nymphs) were allowed to molt to nymphs and adults, respectively, which were tested by molecular analysis (E. canis-specific PCR assay) and used to infest naïve dogs. Through infestation of adult ticks on naïve dogs, after nymphal acquisition feeding on E. canis-infected dogs, only the BSP population was shown to be competent vectors of E. canis, i.e., only the dogs infested with BSP adult ticks developed clinical illness, seroconverted to E. canis, and yielded E. canis DNA by PCR. This result, demonstrated by two independent replications, is congruent with epidemiological data, since BSP ticks were derived from São Paulo state, Brazil, where CME is highly endemic. On the other hand, BRS, ARG, and URU ticks were derived from a geographical region (South America southern cone) where CME has never been properly documented. Molecular analysis of unfed adults at 30 days post molting support these transmission results, since none of the BRS, ARG, and URU ticks were PCR positive, whereas 1% of the BSP nymphs and 31.8% of the BSP adults contained E. canis DNA. We conclude that the absence or scarcity of cases of CME due to E. canis in the South America southern cone is a result of vector incompetence of the R. sanguineus group ticks that prevail on dogs in this part of South America.
Introduction
The Rhipicephalus sanguineus complex is a group of at least 12 morphologically closely related species, including Rhipicephalus sanguineus sensu stricto (s.s.) [1]. Until the end of the 20th century, the taxon R. sanguineus was thought to represent a single tick species with a nearly cosmopolitan distribution, mainly associated with domestic dogs [2,3]. During the last 10 years, a number of studies based on molecular [4,5,6,7], biological [4], and morphological [8] analyses revealed that at least two distinct species have been considered under the taxon R. sanguineus in Latin America. Moraes-Filho et al. [6] assigned these two species as ‘temperate’ and ‘tropical’, the former restricted to the southern cone of South America (Uruguay, Argentina, Chile, and the southernmost state of Brazil), and the later encompassing the rest of Latin America, from Mexico to Brazil. This distribution, based on genetic analysis, was corroborated by subsequent data [7]. Similarly to the current situation of Latin America, recent genetic studies showed that the taxon R. sanguineus s.s. was also applied to distinct genospecies on other continents [6,9,10,11], also corroborated by biological analysis [9]. In view of this unequivocally taxonomic problem, Nava et al. [1] recommended that, currently, it is not possible to assign the specific name R. sanguineus s.s. to any tick population of the world. Until this issue is not solved, the term “R. sanguineus group” should be employed instead of R. sanguineus s.s., in future studies [1]. We have adopted this recommendation in the present study.
The bacterium Ehrlichia canis is the etiological agent of canine monocytic ehrlichiosis (CME), a tick-borne disease of domestic dogs in many parts of the world [12], including South America [13]. Ticks of the R. sanguineus group are primary vectors of E. canis to dogs [14,15,16,17]. Because there is no transovarial transmission of E. canis in R. sanguineus group [14], dogs acquire the infection when infested by an infected nymph or adult tick that had acquired the infection in a previous developmental stage (transstadial transmission) or the same stage (intrastadial transmission) by feeding on an acutely or chronically infected dog [17,18].
According to a recent review [19] and subsequent studies [20,21,22,23], molecular detection of E. canis DNA has been achieved in blood samples from naturally infected dogs of all regions of Brazil where it has been attempted, except for the southernmost state, Rio Grande do Sul [24]. In addition, while canine seroprevalence values within 30–75% for E. canis have been reported in random samples of dogs from different regions of Brazil [22,25,26,27,28,29], studies in Rio Grande do Sul have reported seroprevalence values always below 5% [24,30,31,32], despite local abundance of R. sanguineus group ticks on dogs [33,34]. Because Rio Grande do Sul is the only part of Brazil where the ‘temperate species’ of the R. sanguineus group has been detected, in contrast to the widespread distribution of the ‘tropical species’ in the remaining regions of the country [6], we hypothesize that the low prevalence of E. canis in Rio Grande do Sul is related to possible vector incompetence of R. sanguineus group ticks from this region. Therefore, the present study evaluated a comparative analysis of the vector competence of different populations of R. sanguineus group, including one from São Paulo, southeastern Brazil, where CME is highly endemic [19], and one from Rio Grande do Sul. In addition, we also included one R. sanguineus group population from Uruguay and one from Argentina. These two populations were previously assigned to the ‘temperate species’ [6], and are also from areas where canine infection by E. canis has never been properly documented [35,36,37].
Materials and Methods
Ethics statement
This study has been approved by the Institutional Animal Care and Use Committee (IACUC) of the Faculty of Veterinary Medicine of the University of São Paulo (protocol 2100/2010). Rabbits were purchased from a commercial breeder (Criex, Mogi das Cruzes, São Paulo, Brazil) that produce these animals for research use only. Domestic dogs were obtained from the Department of Preventive Veterinary Medicine and Animal Health of the University of São Paulo under the coordination of one of the co-authors (MBL) of the present study. These dogs were bred for research use only.
Engorged females of the R. sanguineus group were collected from naturally infested dogs under owners’ consent in the following 4 geographical sites: São Paulo city (23°32’S, 46°38’W), state of São Paulo, southeastern Brazil (designated as BSP ticks); Cachoeira do Sul (30°02’S, 52°53’W), state of Rio Grande do Sul, southern Brazil (designated as BRS ticks); Rafaela (31°15’S, 61°29’W), Santa Fé Province, Argentina, designated as ARG ticks; and Montevideo (34°51’S, 56°10’W), Uruguay (designated as URU ticks). No specific permissions were required at the tick collection locations because R. sanguineus group ticks are unpleasant pests affecting domestic dogs in South America. These tick collections did not involve endangered or protected species. According to genetic data previously reported [6], BSP ticks correspond to the ‘tropical species’, whereas BRS, ARG, and URU ticks correspond to the ‘temperate species’. All engorged females were brought to the laboratory and allowed to lay eggs in an incubator set at 27°C, 85% RH and scotophase. Part of the hatched larvae were used for the experimental infestations on dogs described below, whereas another part of the larvae were fed on tick-naïve rabbits, as previously described [38], with the purpose of obtaining unfed nymphs to be used in the experimental infestations on dogs described below. During the study, larval, nymphal, or adult infestations consisted of ≈1,000, 300–500, or 100 ticks per host, respectively, while off-host developmental stages were held under the same conditions stated previously.
Dogs
For the present study, a total of 21 tick-naïve Beagle dogs were used. All dogs were provided by the animal facility of the Department of Preventive Veterinary Medicine and Animal Health of the University of São Paulo, where they were reared with no contact with ticks and under strict sanitary control. During the experiment, each dog was held individually within an enclosure (3.5 m x 2.5m). Two thirds of the enclosure had an open top in order to provide direct sunlight. Dogs were provided with water and a standard dry food diet (Pedigree Vital- Pro, Mars Brasil, Guararema, SP, Brazil) ad libitum, and environmental enrichment with dog toys. One week before experimental infestations, all dogs were shown to be negative for E. canis infection by both direct (E. canis-specific real-time PCR on blood sample) and indirect (serologic testing against E. canis antigens) diagnostic methods, using the protocols described below. Tick infestations on dogs were always performed inside cotton sleeves (10 to15 cm diameter) glued to the shaved back, as previously described [39]. During tick infestations, each dog received an Elizabethan collar in order to avoid removal of the cotton sleeve. Each infested dog had its cotton sleeve(s) opened daily for collection of ticks that had completed engorgement and had naturally detached.
During the experiment, medical routine checks were undertaken daily to monitor the overall health of the animals. No significant abnormality other than fever was observed. All E. canis-infected dogs recovered without clinical complications after doxycycline therapy (10 mg/Kg, 12/12 h P.O., for 28 days). Several weeks later, these animals were certified to be free of E. canis infection (tested by PCR and serology), and were sent for donation to accredited owners, together with the remaining dogs used in the present study.
Ehrlichia canis inoculum
For experimental infection of dogs with E. canis, we used frozen infected blood derived from a dog previously inoculated with the Jaboticabal strain of E. canis, as previously described [40]. Each canine inoculation consisted of defrosting 5 mL of infected blood in a water bath at 37°C, and subsequent intravenous inoculation of the dog. For the present study, we used a stock of E. canis (Jaboticabal strain) that has been maintained in the laboratory exclusively through canine passages, with no in vitro culture passage. This E. canis strain has been shown to be highly pathogenic for dogs that were experimentally infected via infected blood [40,41].
Molecular analyses
Ticks (unfed nymphs and adults) were processed individually for PCR as described previously using the guanidine isothiocynanate-phenol technique, as previously described [42], while canine blood samples (200 μL) were submitted to DNA extraction using the DNeasy Tissue Kit (QIAGEN, Chatsworth, CA, USA). Extracted DNA samples were tested for the presence of E. canis DNA by a real-time PCR assay using primers forward (5’-TTG CAA AAT GAT GTC TGA AGA TAT GAA ACA-3’) and reverse (5’-GCT GCT CCA CCA ATA AAT GTA TCY CCT A-3’), and the E. canis-specific taqman probe FAM-5’-AGC TAG TGC TGC TTG GGC AAC TTT GAG TGA A-3’BHQ-1, as previously described [43]. Primers and probe sequences correspond to portions of the dsb gene of E. canis. Because the dsb gene was shown to be highly polymorphic between Ehrlichia species, the above taqman PCR protocol was previously validated to be 100% specific for E. canis [43].
Serological testing
Individual canine serum samples were tested by the indirect immunofluorescence assay (IFA) using E. canis-infected DH82 cells as antigen, performed with the São Paulo strain of E. canis from Brazil [44]. Reactions were performed with fluorescein-conjugated anti-dog IgG (Sigma-Aldrich, St. Louis, MO). Serum was considered to contain antibodies reactive to E. canis if it displayed a reaction at the 1:80 dilution [45]. In each slide, a serum previously shown to be nonreactive (negative control) and a known reactive serum (positive control) were tested at the 1:80 dilution. Samples that reacted at the screening dilution (1:80) were then titrated using serial two-fold dilutions to determine endpoint titers.
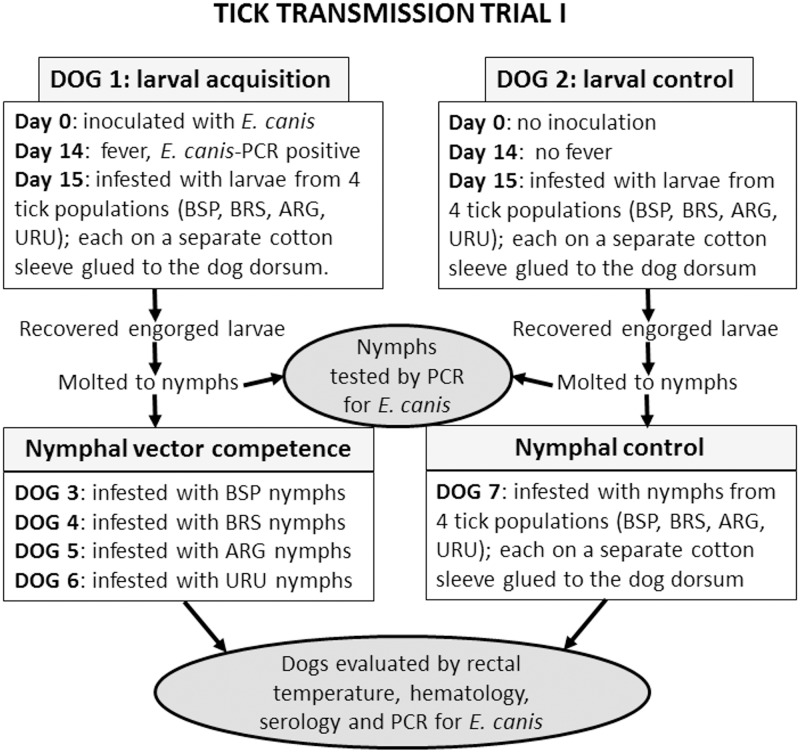
Tick transmission trial I: larval acquisition of E. canis followed by vector competence of nymphs
One dog (Dog 1), intravenously inoculated with E. canis at day 0, had its rectal temperature measured daily for 30 days. Fever (rectal temperature >39.5°) occurred from 14 to 22 days after inoculation (DAI). A blood sample collected at fever onset (14 DAI) revealed E. canis DNA by real-time PCR. On the same day, four independent cotton sleeves were glued to the shaved back of the dog. On the following day (15 DAI), each of the four cotton sleeves received unfed larvae of a tick population (BSP, BRS, ARG, or URU). Engorged larvae recovered from the four cotton sleeves were held in the incubator for molting to nymphs. All procedures described for this E. canis-inoculated dog were repeated in parallel with a non-inoculated dog (Dog 2), as an experimental control. Both dogs were tested by IFA at days 0, 14 and 28.
From each of the four tick populations (BSP, BRS, ARG, or URU), a sample of 100 unfed nymphs at ≈30 days after molting were tested by real-time PCR for E. canis DNA. Samples of unfed nymphs that molted from the engorged larvae that had fed on the E. canis-infected dog were used to infest tick-naïve dogs. In this case, four dogs were infested, each with nymphs from a tick population, i.e., Dog 3 with BSP nymphs, Dog 4 with BRS nymphs, Dog 5 with ARG nymphs, Dog 6 with URU nymphs. Another dog (Dog 7) was infested with nymphs that molted from the engorged larvae that had fed on the uninfected control dog (Dog 2). In this case, nymphs from each tick population fed inside one of four cotton sleeves glued to the shaved dorsum of this fifth dog. The number of engorged ticks recovered from each cotton sleeve were recovered and counted.
All dogs were monitored by daily rectal temperature for 63 consecutive days post infestation, and by real-time PCR, IFA and hematology using blood samples collected at 7-day intervals. For hematology, blood cells were counted in an automatic counter (Horiba ABX Brasil, São Paulo, Brazil) to determine total number of red blood cells, white blood cells and platelets, hemoglobin level, and globular volume. Procedures described for this trial are summarized in Fig 1.
Fig 1. Diagram illustrating experimental procedures of tick transmission trial I in the present study.
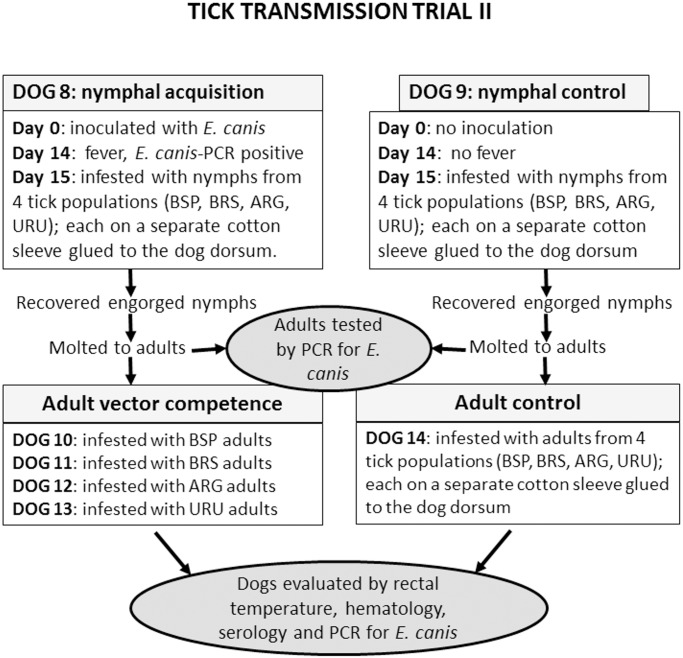
Tick transmission trial II: nymphal acquisition of E. canis followed by vector competence of adults
One dog (Dog 8), intravenously inoculated with E. canis at day 0, had its rectal temperature measured daily for 30 days. Fever (rectal temperature >39.5°) occurred from 14 to 24 DAI. A blood sample collected at fever onset (14 DAI) revealed E. canis DNA by real-time PCR. On the same day, four independent cotton sleeves were glued to the shaved back of the dog. On the following day (15 DAI), each of the four cotton sleeves received uninfected unfed nymphs of a tick population (BSP, BRS, ARG, or URU). Engorged nymphs recovered from the four cotton sleeves were held in the incubator for molting to adults. All procedures described for this E. canis-inoculated dog were repeated in parallel with a non-inoculated dog (Dog 9), as an experimental control. Both dogs were tested by IFA at days 0, 14 and 28.
From each of the four tick populations (BSP, BRS, ARG, or URU), a sample of 100 unfed adults at ≈30 days after molting were tested by real-time PCR for E. canis DNA. Samples of unfed adults that molted from the engorged nymphs that had fed on the E. canis-infected dog were used to infest tick-naïve dogs. In this case, four dogs were infested, each with adults from a tick population, i.e., Dog 10 with BSP adults, Dog 11 with BRS adults, Dog 12 with ARG adults, Dog 13 with URU adults. Another dog (Dog 14) was infested with adults that molted from the engorged nymphs that had fed on the uninfected control dog (Dog 9). In this case, adult ticks from each tick population fed inside one of four cotton sleeves glued to this fifth dog. The number of engorged ticks recovered from each cotton sleeve were recovered and counted.
Every dog infested with adult ticks was monitored during 63 consecutive days for rectal temperature, real-time PCR, IFA, and hematology, as described in tick transmission trial I. Procedures described for trial II are summarized in Fig 2.
Fig 2. Diagram illustrating experimental procedures of tick transmission trial II in the present study.
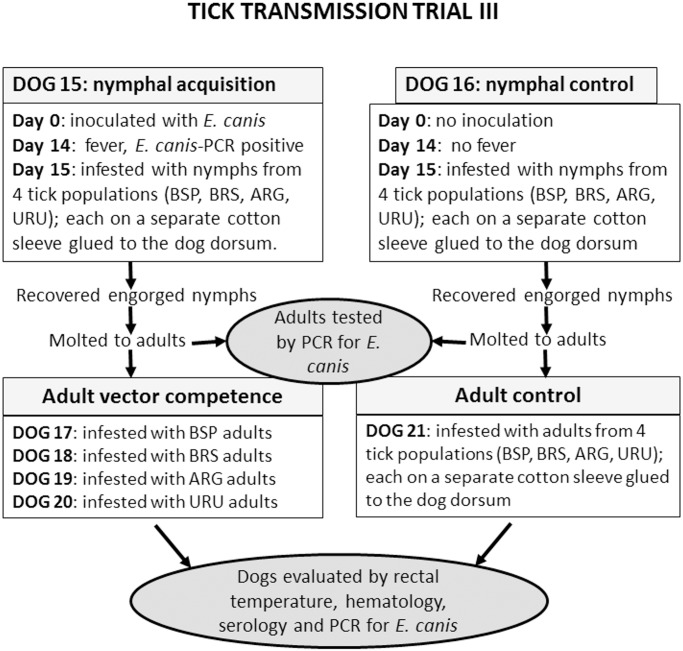
Tick transmission trial III: nymphal acquisition of E. canis followed by vector competence of adults
This trial was a replication of trial II. Therefore, all procedures performed in trial II were repeated in trial III, by using other tick-naïve dogs (Dogs 15 to 21) (Fig 3). The only difference was that unfed adults, derived from engorged nymphs of the four tick populations that had fed on the E. canis-infected blood (Dog 15), were tested by real-time PCR for E. canis DNA at 7 days post molting. In addition, another group of adults of BSP and BRS ticks were tested at 30 days post molting, and another group of BRS adults were tested at 90 days post molting.
Fig 3. Diagram illustrating experimental procedures of tick transmission trial III in the present study.
Results
Ehrlichia canis acquisition by dogs and ticks
All animals (Dogs 1, 8, 15) that were intravenously inoculated with E. canis-infected blood developed signs of CME, which started at the 14th DAI and lasted until at least the 22th DAI, when doxycycline therapy (10 mg/Kg, 12/12 h P.O., for 28 days) was initiated in order to prevent clinical complications. These dogs were infested by uninfected larvae (Dog 1) or nymphs (Dogs 8 and 15) at the 15 DAI. Because larval and nymphal feeding lasted from 3 to 6 days, all engorged ticks fed during the febrile period, before the beginning of doxycycline therapy. Blood samples from the three dogs were collected and tested positively for E. canis by real-time PCR at 14 and 21 DAI, which corresponded to the day before tick infestation and the day of detachment of the last engorged tick, respectively. Through IFA, the three dogs were serologically non-reactive (titer <80) to E. canis at DAI 0. Thereafter, their IFA titers were 640 or 1,280 at DAI 14, and 10,240 or 20,480 at DAI 28. In parallel, the control dogs of the three tick transmission trials (Dogs 2, 9, 16) were always afebrile, and negative by real-time PCR and IFA (titer <80) throughout the experimental period.
In trial I, a total of 650 to 890 engorged larvae of each of the four tick populations were recovered from Dog 1 (E. canis-infected) or Dog 2 (uninfected control dog). At least 80% of these larvae successfully molted to viable nymphs. Real-time PCR results were negative for E. canis in all tiks, except for 1% of nymphs tested from BSP that had fed as larvae on Dog 1 (Table 1).
Table 1. Real-time PCR results of unfed ticks (nymphs or adults) after molting from ticks (engorged larvae or nymphs, respectively) that had fed on Ehrlichia canis-infected dogs (Dogs 1, 8 or 15) or on uninfected control dogs (Dogs 2, 9 or 16).
| Tick transmission trial | Tick acquisition feeding | Tick population | Real-time PCR results for E. canis on molted ticks* | ||
|---|---|---|---|---|---|
| Tick stage | Days post molting | No. positive (%) | |||
| I | Fed as larvae on the E. canis-infected dog 1 | BSP | Nymph | 30 | 1 (1.0) |
| BRS | 30 | 0 (0) | |||
| ARG | 30 | 0 (0) | |||
| URU | 30 | 0 (0) | |||
| I | Fed as larvae on the uninfected dog 2 | BSP | Nymph | 30 | 0 (0) |
| BRS | 30 | 0 (0) | |||
| ARG | 30 | 0 (0) | |||
| URU | 30 | 0 (0) | |||
| II | Fed as nymphs on the E. canis-infected dog 8 | BSP | Adult | 30 | 7 (7) |
| BRS | 30 | 0 (0) | |||
| ARG | 30 | 0 (0) | |||
| URU | 30 | 0 (0) | |||
| II | Fed as nymphs on the uninfected dog 9 | BSP | Adult | 30 | 0 (0) |
| BRS | 30 | 0 (0) | |||
| ARG | 30 | 0 (0) | |||
| URU | 30 | 0 (0) | |||
| III | Fed as nymphs on the E. canis-infected dog 15 | BSP | Adult | 7 | 46 (46.0) |
| BRS | 7 | 16 (16.0) | |||
| ARG | 7 | 0 (0) | |||
| URU | 7 | 0 (0) | |||
| BSP | 30 | 27 (31.8) | |||
| BRS | 30 | 0 (0) | |||
| BRS | 90 | 0 (0) | |||
| III | Fed as nymphs on the uninfected dog 16 | BSP | Adult | 7 | 0 (0) |
| BRS | 7 | 0 (0) | |||
| ARG | 7 | 0 (0) | |||
| URU | 7 | 0 (0) | |||
*In all cases, 100 molted ticks were tested, except for BSP adults 30 days post molting in trial III, from which only 85 adult ticks were available for testing.
In trial II, a total of 245 to 280 engorged nymphs of each of the four tick populations were recovered from Dog 8 (E. canis-infected) or Dog 9 (uninfected control dog). At least 90% of these nymphs successfully molted to viable adults. Real-time PCR results were negative for E. canis in all ticks, except for 7% of the adults tested from BSP that had fed as nymphs on Dog 8 (Table 1).
In trial III, a total of 305 to 455 engorged nymphs of each of the four tick populations were recovered from Dog 15 (E. canis-infected) or Dog 16 (uninfected control dog). At least 90% of these nymphs successfully molted to viable adults. At 7 days post molting, real-time PCR results were negative for E. canis in all ticks, except for 46% of the adults tested from BSP and 16% of the adults tested from BRS that had fed as nymphs on Dog 15 (Table 1). At 30 days post molting, 31.8% of the BSP ticks were positive, and all BRS adults were negative. At 90 days post molting, BRS adult ticks remained negative.
Vector competence of nymphs (trial I)
Four dogs (Dogs 3, 4, 5, and 6) were infested by nymphs that had fed as larvae on the E. canis-infected Dog 1 (Fig 1). Each dog received nymphs from a single tick population, namely BSP, BRS, ARG, or URU. The number of engorged nymphs recovered per dog varied from 185 to 269 (Table 2). All dogs were clinically evaluated for 63 days after infestation. During this period, they remained afebrile (measure daily), negative by real-time PCR for E. canis (tested weekly), seronegative to E. canis (tested weekly), and had their hematological values within the normal range of health dogs (S1 Table). Dog 7 (control dog), which was infested with nymphs of the four tick populations that had fed as larvae on the uninfected control Dog 2, also remained afebrile, PCR negative, seronegative, and with hematological values within the range of healthy dogs.
Table 2. Results of the vector competence experiments of four populations of Rhipicephalus sanguineus sensu lato (BSP, BRS, ARG, URU) for Ehrlichia canis transmission to susceptible dogs.
| Dog No. | Tick infestation data | Canine data during 63 days after tick infestation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tick population | Tick stage | No. ticks infested per tick feeding cotton sleeve | No. ticks that engorged | PCR for E. canis | Seroconvertion to E. canis | Clinical data | |||||
| Fever | Low hemoglobin concentration | Low package cell volume | Low erythrocyte count | Low platelet counts | |||||||
| 3 | BSP | Nymph a | 300 | 269 | - | - | - | - | - | - | - |
| 4 | BRS | 185 | - | - | - | - | - | - | - | ||
| 5 | ARG | 219 | - | - | - | - | - | - | - | ||
| 6 | URU | 250 | - | - | - | - | - | - | - | ||
| 10 | BSP | Adult b | 100 | 68 d | + | + | - | + | + | + | + |
| 11 | BRS | 58 d | - | - | - | - | - | - | - | ||
| 12 | ARG | 72 d | - | - | - | - | - | - | - | ||
| 13 | URU | 59 d | - | - | - | - | - | - | - | ||
| 17 | BSP | Adult c | 100 | 71 d | + | + | - | + | + | + | + |
| 18 | BRS | 75 d | - | - | - | - | - | - | - | ||
| 19 | ARG | 61 d | - | - | - | - | - | - | - | ||
| 20 | URU | 74 d | - | - | - | - | - | - | - | ||
a Nymphs were exposed to E. canis infection by feeding as larvae on the E. canis-infected Dog 1 (trial I).
b Adult ticks were exposed to E. canis infection by feeding as nymphs on the E. canis-infected Dog 8 (trial II).
c Adult ticks were exposed to E. canis infection by feeding as nymphs on the E. canis-infected Dog 15 (trial III).
d Refer to naturally detached engorged females, plus male ticks that fed on the dogs until the natural detachment of the last engorged female.
-: negative or absent
+: positive or present
Vector competence of adults (trial II)
Four dogs (Dogs 10, 11, 12, and 13) were infested by adults that had fed as nymphs on the E. canis-infected Dog 8 (Fig 2). Each dog received adults from a single tick population, namely BSP, BRS, ARG, or URU. The number of engorged females recovered per dog varied from 21 to 34. When the last female completed engorgement (between 8 to 9 days after infestation), all male ticks were manually removed from the dogs. The number of male ticks recovered per dog varied from 31 to 48. All dogs were clinically evaluated for 63 days after infestation. During this period, all dogs remained afebrile, and all but one dog remained negative by real-time PCR for E. canis, seronegative to E. canis, and had their hematological values within the normal range of health dogs. The only exception was Dog 10 (infested with BSP adults), which became PCR-positive at 14 days after tick infestation, and remained PCR-positive at weekly intervals until 63 days after infestation, when trial II was ended (Table 2). During this period, Dog 10 showed marked alterations in its hematological values, namely hemoglobin concentration, package cell volume, erythrocyte and platelet counts below the reference range for healthy dogs (S1 Table). Dog 10 seroconverted to E. canis, as its IFA endpoint titers to E. canis were <80 at day 0, 160 at day 14, 2,560 at day 21, and 20,480 to 40,960 through days 28 to 63 after infestation. During this 63-day period, Dog 10 presented no fever, since its rectal temperature was always below 39.5°C.
Dog 14 (control dog), which was infested with adults of the four tick populations that had fed as nymphs on the uninfected control Dog 9, remained afebrile, PCR negative, seronegative, and with hematological values within the range of health dogs.
Vector competence of adults (trial III)
Four dogs (Dogs 17, 18, 19, and 20) were infested by adults that had fed as nymphs on the E. canis-infected Dog 15 (Fig 2). Each dog received adults from a single tick population, namely BSP, BRS, ARG, or URU. The number of engorged females recovered per dog varied from 31 to 40. When the last female completed engorgement (between 8 to 9 days after infestation), all male ticks were manually removed from the dogs. The number of male ticks recovered per dog varied from 31 to 35. All dogs were clinically evaluated for 63 days after infestation. During this period, all dogs remained afebrile, and all but one dog remained negative by real-time PCR for E. canis, seronegative to E. canis, and had their hematological values within the normal range of health dogs. The only exception was Dog 17 (infested with BSP adults), which became PCR-positive at 14 days after tick infestation, and remained PCR-positive at weekly intervals until 63 days after infestation, when trial III was ended (Table 2). During this period, Dog 17 showed marked alterations in its hematological values, namely hemoglobin concentration, package cell volume, erythrocyte and platelet counts below the reference range for healthy dogs (S1 Table). Dog 17 seroconverted to E. canis, as its IFA endpoint titers to E. canis were <80 at day 0, 320 at day 14, 10,240 at days 21 and 28, and 20,480 to 5,120 through days 28 to 63 after infestation. During this 63-day period, Dog 17 presented no fever.
Dog 21 (control dog), which was infested with adults of the four tick populations that had fed as nymphs on the uninfected control Dog 16, remained afebrile, PCR negative, seronegative, and with hematological values within the range of health dogs.
Discussion
We evaluated the vector competence of four geographic distinct populations of R. sanguineus group for the canine pathogen E. canis. In each of the three trials, acquisition feeding of ticks from the four populations were performed simultaneously on the same E. canis-infected dog. Therefore, we annulled the interference of host factors on this acquisition feeding process. The fact that we used an E. canis strain that has never been subjected to in vitro culture is also noteworthy, since it was previously reported that passages of E. canis in cell culture adversely affected its transmissibility by R. sanguineus group ticks [16].
Of the four groups of R. sanguineus tested for vector competence of E. canis (BSP, BRS, ARG and URU), only the BSP population was shown to be competent vector of E. canis, i.e., only the dogs infested with BSP adult ticks developed clinical illness, seroconverted to E. canis, and yielded E. canis DNA by PCR. This result, demonstrated by two independent replications (trials II and III), is congruent with epidemiological data, since BSP ticks were derived from the state of São Paulo, southeastern Brazil, where CME is highly endemic [25,46,47]. On the other hand, BRS ticks were derived from Rio Grande do Sul, the southernmost state of Brazil, where CME has never been properly documented. In the state of Rio Grande do Sul, canine serosurveys using E. canis antigens have reported 0 to 4.8% seropositive dogs [24,30,31,32]. When these reactive sera were titrated by IFA, they were generally characterized by low endpoint titers to E. canis [31,32]. A recent study in Rio Grande do Sul performed molecular analysis on 199 dogs for E. canis and Anaplasma platys. Whereas all dogs were negative for E. canis DNA, 14.07% contained A. platys DNA [24]. Because there is variable serologic cross-reactivity among E. canis and A. platys [48], it is possible that the low number of reactive dogs to E. canis antigens, previously reported in Rio Grande do Sul, could be a result of cross-reactivity to A. platys. The low E. canis-endpoint titers of these dogs [31,32] support this assumption.
In Uruguay, molecular detection of E. canis has never been reported. In one study, authors failed to detect ehrlichial DNA on a sample of 199 R. sanguineus group ticks collected from dogs [35]. In Argentina, two studies from different areas evaluated by molecular analyses 52 and 56 canine blood samples for Anaplasmataceae agents. Whereas all samples were negative for Ehrlichia agents, 13.5% and 37.5% of the dogs, respectively, were positive for A. platys [36,37]. Conversely, a recent study reported for the first time the molecular detection of E. canis DNA in the blood of 6 dogs from Argentina [49]. However, this report relied solely on 16S rRNA short sequences (318 nucleotides) and did not match 100% to any E. canis sequence available in GenBank. Because the 16S rDNA gene is highly conserved among Anaplasmataceae agents [50], this report requires confirmation.
In the trial II of present study, real-time PCR detected E. canis DNA in 7% of the unfed BSP adults 30 days post-molting, whereas all BRS, ARG, and URU ticks were negative by PCR. This result was corroborated by the competence vector evaluation, since only the dog infested by BSP adult ticks became infected by E. canis. In the trial III, similar results were obtained, since real-time PCR detected E. canis DNA in 31.8% of the unfed BSP adults and in none of the BRS, ARG, and URU adult ticks at 30 days post-molting. Again, only the dog infested by BSP adult ticks became infected by E. canis. On the other hand, when adult ticks were tested by real-time PCR at 7 days post-molting, 46.0 and 16.0% of BSP and BRS ticks, respectively, contained E. canis DNA. Since BRS ticks were shown to contain no E. canis DNA at 30 and 90 days post-molting, and because the dog infested with BRS ticks during trial III did not become infected by E. canis, we speculate that the 16% E. canis-positive ticks at 7 days post-molting was a result of residual E. canis DNA from the nymphal feeding in the tick body, which was not sufficient to cause an active infection in order to make the tick a competent vector of E. canis. This assumption is supported by a recent study that have successfully detected host DNA traces in at least 50% of post-molting ticks collected from the vegetation [51], which confirms that ticks are able to retain viable DNA for limited amount of time, acquired during blood feeding from a previous developmental stage. Finally, these results reinforce that molecular analyses alone does confirm vector competence of ticks for any investigated pathogen, i.e., experimental infestations on susceptible hosts must be done to confirm vector competence.
In trial I, where we tested the vector competence of R. sanguineus group nymphs for E. canis, none of the dogs became infected by E. canis, besides at least 1% of the BSP nymphs contained E. canis DNA at 30 days post-molting. While previous studies demonstrated that R. sanguineus group nymphs were able to transmit E. canis after a larval acquisition feeding, successful transmission was generally higher for adults than for nymphs [14,16]. The reasons for this variable vector competence of nymphs are unknown. However, it could be simply related to lower E. canis-infection rates among unfed nymphs, when compared to unfed adult ticks, as observed in the BSP ticks of the present study (Table 1), i.e., the lower the E. canis-infection rate, the lower the chance of an infected tick to complete engorgement on an infested dog. Finally, the explanation for lower ehrlichial infection rates in nymphs could be the much lower amount of infected blood that is ingested during larval acquisition feeding, in contrast to nymphal acquisition feedings.
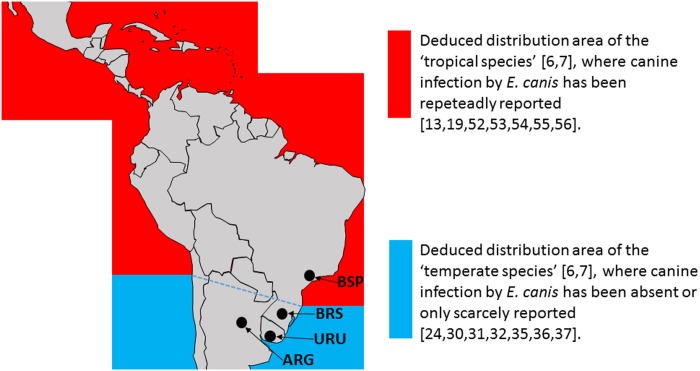
The results of the present study demonstrates that, whereas BSP ticks were competent vectors of E. canis, three other populations (BRS, ARG, URU) were not. According to a recent molecular study on R. sanguineus group in Latin America [6], BSP ticks correspond to the ‘tropical species’, while BRS, ARG, and URU ticks correspond to the ‘temperate species’ (Fig 4). The tropical species was reported to occur from Mexico to Brazil (except for the southernmost part of Brazil, which includes the state of Rio Grande do Sul), where canine infection by E. canis has been widely confirmed by molecular methods [13,19,52,53,54,55,56]. On the other hand, the ‘temperate species’ was reported in the Brazilian state of Rio Grande do Sul, Argentina, Uruguay, and Chile, from areas where E. canis has never been properly reported. Based on the results of the present study, we conclude that the absence or scarcity of cases of CME due to E. canis in the southern cone of South America is a result of vector incompetence of the R. sanguineus group ticks (‘temperate species’) that prevail on dogs in this part of South America. As stated by Nava et al. [1], further studies are required to elucidate the taxonomic position of the R. sanguineus group species complex in the world, which includes at least two species in South America, herein designated as ‘tropical species’ and ‘temperate species’.
Fig 4. Deduced distribution area of the ‘tropical species’ and the ‘temperate species’ of Rhipicephalus sanguineus group in Latin America. Ticks used in the present study were BSP (from São Paulo state, Brazil), BRS (from Rio Grande do Sul, Brazil), ARG (from Argentina), and URU (from Uruguay). The map is reprinted from http://www.usgs.gov/, and edited with Microsoft PowerPoint and Paint.
Supporting Information
(XLSX)
Acknowledgments
We are grateful to Santiago Nava, and José M. Venzal for providing engorged females of ticks from Argentina, and Uruguay, respectively, and to Rosangela Z. Machado for providing canine blood aliquots infected by E. canis Jaboticabal strain.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Fundação de Amparo a Pesquisa do Estado de São Paulo (Grant 2010/51002-5 to JM-F), Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grant 302832/2007-6 to MBL), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Grant 23038.005275/2011-79 to MBL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nava S, Beati L, Labruna MB, Szabó MP, Venzal JM, Guglielmone AA, et al. The taxonomic status of Rhipicephalus sanguineus (Latreille, 1806). Vet Parasitol. 2015; 208: 2–8. 10.1016/j.vetpar.2014.12.021 [DOI] [PubMed] [Google Scholar]
- 2. Pegram RG, Keirans JE, Clifford CM, Walker JB. Clarification of the Rhipicephalus sanguineus group (Acari, Ixodidoidea, Ixodidae). II. R. sanguineus (Latreille, 1806) and related species. Syst Parasitol. 1987; 10: 27–44. [Google Scholar]
- 3. Walker JB, Keirans JE, Horak IG. The genus Rhipicephalus (Acari: Ixodidae): a guide to the brown ticks of the world. Cambridge: CambridgeUniversity Press; 2000. [Google Scholar]
- 4. Szabó MP, Mangold AJ, Joao CF, Bechara GH, Guglielmone AA. Biological and DNA evidence of two dissimilar populations of the Rhipicephalus sanguineus tick group (Acari: Ixodidae) in South America. Vet Parasitol. 2005; 130: 131–140. [DOI] [PubMed] [Google Scholar]
- 5. Burlini L, Teixeira KR, Szabó MP, Famadas KM. Molecular dissimilarities of Rhipicephalus sanguineus (Acari: Ixodidae) in Brazil and its relation with samples throughout the world: is there a geographical pattern? Exp Appl Acarol. 2010; 50: 361–374. 10.1007/s10493-009-9321-8 [DOI] [PubMed] [Google Scholar]
- 6. Moraes-Filho J, Marcili A, Nieri-Bastos FA, Richtzenhain LJ, Labruna MB. Genetic analysis of ticks belonging to the Rhipicephalus sanguineus group in Latin America. Acta Trop. 2011; 117: 51–55. 10.1016/j.actatropica.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 7. Nava S, Mastropaolo M, Venzal JM, Mangold AJ, Guglielmone AA. Mitochondrial DNA analysis of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) in the Southern Cone of South America. Vet Parasitol. 2012; 190: 547–555. 10.1016/j.vetpar.2012.06.032 [DOI] [PubMed] [Google Scholar]
- 8. Oliveira PR, Bechara GH, Denardi SE, Saito KC, Szabó MP, Mathias MI, et al. Comparison of the external morphology of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) ticks from Brazil and Argentina. Vet Parasitol. 2015; 129: 139–147. [DOI] [PubMed] [Google Scholar]
- 9. Levin ML, Studer E, Killmaster L, Zemtsova G, Mumcuoglu KY. Crossbreeding between different geographical populations of the brown dog tick, Rhipicephalus sanguineus (Acari: Ixodidae). Exp Appl Acarol. 2012; 58: 51–68. 10.1007/s10493-012-9561-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dantas-Torres F, Latrofa MS, Annoscia G, Gianelli A, Parisi A, Otranto D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasit Vectors. 2013; 6: 213 10.1186/1756-3305-6-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu GH, Chen YZ, Song HQ, Lin RQ, Zhou DH, Zhu XQ. Complete mitochondrial genome sequence data provides evidence that dog tick Rhipicephalus sanguineus (Acari: Ixodidae) represents a species complex. Int J Biol Sci. 2013; 9: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harrus S, Waner T. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): an overview. Vet J. 2011; 187: 292–296. 10.1016/j.tvjl.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 13. Aguiar DM, Zhang X, Melo AL, Pacheco TA, Meneses AM, Zanutto MS, et al. Genetic diversity of Ehrlichia canis in Brazil. Vet Microbiol. 2013; 164: 315–321. 10.1016/j.vetmic.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 14. Groves MG, Dennis GL, Amyx HL, Huxsoll DL. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am J Vet Res. 1975; 36: 937–940. [PubMed] [Google Scholar]
- 15. Lewis GE, Ristic M, Smith RD, Lincoln T, Stephenson EH. The brown dog tick Rhipicephalus sanguineus and the dog as experimental hosts of Ehrlichia canis . Am J Vet Res. 1977; 38: 1953–1955. [PubMed] [Google Scholar]
- 16. Mathew JS, Ewing SA, Barker RW, Fox JC, Dawson JE, Kocan KM, et al. Attempted transmission of Ehrlichia canis by Rhipicephalus sanguineus after passage in cell culture. Am J Vet Res. 1996; 57: 1594–1598. [PubMed] [Google Scholar]
- 17. Bremer WG, Schaefer JJ, Wagner ER, Ewing SA, Rikihisa Y, Stich RW, et al. Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus . Vet Parasitol. 2005; 131: 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McClure JC, Crothers ML, Schaefer JJ, Stanley PD, Needham GR, Ewing SA, et al. Efficacy of a doxycycline treatment regimen initiated during three different phases of experimental ehrlichiosis. Antimicrob Agents Chemother. 2010; 54: 5012–5020. 10.1128/AAC.01622-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vieira RFC, Biondo AW, Guimarães AMS, Santos AP, Santos RP, Dutra LH, et al. Ehrlichiosis in Brazil. Rev Bras Parasitol Vet. 2011; 20: 1–12. [DOI] [PubMed] [Google Scholar]
- 20. Santos LGF, Melo ALT, Moraes-Filho J, Witter R, Labruna MB, Aguiar DM, et al. Molecular detection of Ehrlichia canis in dogs from the Pantanal of Mato Grosso State, Brazil. Rev Bras Parasitol Vet. 2013; 22: 114–118. [DOI] [PubMed] [Google Scholar]
- 21. Sousa KC, André MR, Herrera HM, de Andrade GB, Machado RZ, de Oliveira GP, et al. Molecular and serological detection of tick-borne pathogens in dogs from an area endemic for Leishmania infantum in Mato Grosso do Sul, Brazil. Rev Bras Parasitol Vet. 2013; 22: 525–531. 10.1590/S1984-29612013000400012 [DOI] [PubMed] [Google Scholar]
- 22. Tanikawa A, Labruna MB, Costa A, Aguiar DM, Justiniano SV, Mendes RS, et al. Ehrlichia canis in dogs in a semiarid region of Northeastern Brazil: serology, molecular detection and associated factors. Res Vet Sci. 2013; 94: 474–477. 10.1016/j.rvsc.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 23. Costa AP, Costa FB, Labruna MB, Silveira I, Moraes-Filho J, Soares JF, et al. A serological and molecular survey of Babesia vogeli, Ehrlichia canis and Rickettsia spp. among dogs in the state of Maranhão, northeastern Brazil. Rev Bras Parasitol Vet. 2015; 24: 28–35. 10.1590/S1984-29612015008 [DOI] [PubMed] [Google Scholar]
- 24. Lasta CS, dos Santos AP, Messick JB, Oliveira ST, Biondo AW, González FH, et al. Molecular detection of Ehrlichia canis and Anaplasma platys in dogs in Southern Brazil. Rev Bras Parasitol Vet. 2013; 22: 360–366. 10.1590/S1984-29612013000300007 [DOI] [PubMed] [Google Scholar]
- 25. Aguiar DM, Cavalcante GT, Pinter A, Gennari SM, Camargo LMA, Labruna MB. Prevalence of Ehrlichia canis (Rickettsiales: Anaplasmataceae) in dogs and Rhipicephalus sanguineus (Acari: Ixodidae) ticks from Brazil. J Med Entomol. 2007; 44: 126–132. [DOI] [PubMed] [Google Scholar]
- 26. Costa LM Jr, Rembeck K, Ribeiro MF, Beelitz P, Pfister K, Passos LM. Sero-prevalence and risk indicators for canine ehrlichiosis in three rural areas of Brazil. Vet J. 2007; 174: 673–676. [DOI] [PubMed] [Google Scholar]
- 27. Souza BM, Leal DC, Uzêda RS, De Alcântara AC, Ferreira F, Labruna MB, et al. Prevalence of ehrlichial infection among dogs and ticks in Northeastern Brazil. Rev Bras Parasitol Vet 2010; 19: 89–93. [DOI] [PubMed] [Google Scholar]
- 28. Spolidorio MG, Labruna MB, Machado RZ, Moraes-Filho J, Zago AM, Yoshinari NH, et al. Survey for tick-borne zoonoses in the state of Espirito Santo, southeastern Brazil. Am J Trop Med Hyg. 2010; 83: 201–206. 10.4269/ajtmh.2010.09-0595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melo AL, Martins TF, Horta MC, Moraes-Filho J, Pacheco RC, Labruna MB, et al. Seroprevalence and risk factors to Ehrlichia spp. and Rickettsia spp. in dogs from the Pantanal Region of Mato Grosso State, Brazil. Ticks Tick Borne Dis. 2011; 2: 213–218. 10.1016/j.ttbdis.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 30. Labarthe N, Campos MP, Barbarini O, Mckee W, Coimbra CA, Hoskins J. Serologic prevalence of Dirofilaria immitis, Ehrlichia canis, and Borrelia burgdorferi infections in Brazil. Vet Ther. 2003; 4: 67–75. [PubMed] [Google Scholar]
- 31. Saito TB, Cunha-Filho NA, Pacheco RC, Ferreira F, Pappen FG, Farias NAR, et al. Canine Infection by Rickettsiae and Ehrlichiae in Southern Brazil. Am J Trop Med Hyg. 2008; 79: 102–108. [PubMed] [Google Scholar]
- 32. Krawczak FS, Labruna MB, Sangioni LA, Vogel FSF, Soares JF, Lopes STDA. Serological survey on Ehrlichia sp. among dogs in the central region of Rio Grande do Sul. Rev Bras Parasitol Vet. 2012; 21: 415–417. [DOI] [PubMed] [Google Scholar]
- 33. Ribeiro VLS, Weber MA, Fetzer LO, Vargas CRB. Espécies e prevalência das infestações por carrapatos em cães de rua da cidade de Porto Alegre, RS, Brasil. Ciência Rural. 1997; 27: 285–289. [Google Scholar]
- 34. Evans DE, Martins JR, Guglielmone AA. A review of the ticks (Acari: Ixodidae) of Brazil, their hosts and geographic distribution 1. The state of Rio Grande do Sul, Southern Brazil. Mem Inst Oswaldo Cruz. 2000; 95: 453–470. [DOI] [PubMed] [Google Scholar]
- 35. Venzal JM, Agustín EP, Castro O, De Souza CG, Portillo A, Oteo JA. Study on seasonal activity in dogs and ehrlichial infection in Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) from southern Uruguay. Parasitol Latinoam. 2007; 62: 23–26. [Google Scholar]
- 36. Cicuttin GL, Vidal P, Nazarena De Salvo M, Beltrán FJ, Gury Dohmen FE. Molecular detection of Rickettsia massiliae and Anaplasma platys infecting Rhipicephalus sanguineus ticks and dogs, Bahía Blanca (Argentina). Rev Chilena Infectol. 2014; 31: 563–568. 10.4067/S0716-10182014000500008 [DOI] [PubMed] [Google Scholar]
- 37. Cicuttin GL, Brambati DF, Rodríguez Eugui JI, Lebrero CG, De Salvo MN, Anda P, et al. Molecular characterization of Rickettsia massiliae and Anaplasma platys infecting Rhipicephalus sanguineus ticks and domestic dogs, Buenos Aires (Argentina). Ticks Tick Borne Dis. 2014; 5: 484–488. 10.1016/j.ttbdis.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 38. Pinter A, Labruna MB, Faccini JL. The sex ratio of Amblyomma cajennense (Acari: Ixodidae) with notes on the male feeding period in the laboratory. Vet Parasitol. 2002; 105: 79–88. [DOI] [PubMed] [Google Scholar]
- 39. Piranda EM, Faccini JL, Pinter A, Pacheco RC, Cançado PH, Labruna MB. Experimental infection of Rhipicephalus sanguineus ticks with the bacterium Rickettsia rickettsii, using experimentally infected dogs. Vector Borne Zoonotic Dis. 2011; 11: 29–36. 10.1089/vbz.2009.0250 [DOI] [PubMed] [Google Scholar]
- 40. Aguiar DM, Saito TB, Hagiwara MK, Machado RZ, Labruna MB. Diagnostico sorologico de erliquiose canina com antigeno brasileiro de Ehrlichia canis. Ciencia Rural. 2007; 37: 796–802. [Google Scholar]
- 41. Castro MB, Machado RZ, de Aquino LP, Alessi AC, Costa MT. Experimental acute canine monocytic ehrlichiosis: clinicopathological and immunopathological findings. Vet Parasitol. 2004; 119: 73–86. [DOI] [PubMed] [Google Scholar]
- 42. Sangioni LA, Horta MC, Gennari SM, Soares RM, Galvão MAM, Labruna MB, et al. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg Infect Dis. 2005; 11: 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Doyle CK, Labruna MB, Breitschwerdt EB, Tang YW, Corstvet RE, Hegarty BC, et al. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time polymerase chain reaction of the dsb gene. J Mol Diagn. 2005; 7: 504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aguiar DM, Hagiwara MK, Labruna MB. In vitro isolation and molecular characterization of an Ehrlichia canis strain from São Paulo, Brazil. Braz J Microbiol. 2008; 39: 489–493. 10.1590/S1517-838220080003000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McBride JW, Corstvet RE, Breitschwerdt EB, Walker DH. Immunodiagnosis of Ehrlichia canis infection with recombinant proteins. J Clin Microbiol. 2001; 39: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diniz PPVP, Schwartz DS, de Morais HS, Breitschwerdt EB. Surveillance for zoonotic vector-borne infections using sick dogs from Southeastern Brazil. Vector-Borne Zoonotic Dis. 2007; 7: 689–697. [DOI] [PubMed] [Google Scholar]
- 47. Nakaghi ACH, Machado RZ, Costa MT, André MR, Baldani CD. Canine ehrlichiosis: clinical, hematological, serological and molecular aspects. Ciência Rural. 2008; 38: 766–770. [Google Scholar]
- 48. Mark Neer TM, Breitschwerdt EB, Greene RT, Lappin MR. Consensus statement on ehrlichial disease of small animals from the infectious disease study group of the ACVIM. American College of Veterinary Internal Medicine. J Vet Intern Med. 2002; 16: 309–315. [DOI] [PubMed] [Google Scholar]
- 49. Eiras DF, Craviotto MB, Vezzani D, Eyal O, Baneth G. First description of natural Ehrlichia canis and Anaplasma platys infections in dogs from Argentina. Comp Immunol Microbiol Infect Dis. 2013; 36: 169–173. 10.1016/j.cimid.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 50. Dumler JS, Barbet AF, Dasch GA, Palmer GH, Ray SC, Rurangirwa FR, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001; 51: 2145–2165. [DOI] [PubMed] [Google Scholar]
- 51. Scott MC, Harmon JR, Tsao JI, Jones CJ, Hickling GJ. Reverse line blot probe design and polymerase chain reaction optimization for blood meal analysis of ticks from the eastern United States. J Med Entomol. 2012; 49: 697–709. [DOI] [PubMed] [Google Scholar]
- 52. Unver A, Perez M, Orellana N, Huang H, Rikihisa Y. Molecular and antigenic comparison of Ehrlichia canis isolates from dogs, ticks, and a human in Venezuela. J Clin Microbiol. 2001; 39: 2788–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Romero LE, Meneses AI, Salazar L, Jiménez M, Aguiar DM, Labruna MB, et al. First isolation and molecular characterization of Ehrlichia canis in Costa Rica, Central America. Res Vet Sci. 2011; 91: 95–97. 10.1016/j.rvsc.2010.07.021 [DOI] [PubMed] [Google Scholar]
- 54. Vargas-Hernández G, André MR, Faria JL, Munhoz TD, Machado RZ, Tinucci-Costa M, et al. Molecular and serological detection of Ehrlichia canis and Babesia vogeli in dogs in Colombia. Vet Parasitol. 2012; 186: 254–260. 10.1016/j.vetpar.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 55. Santamaria A, Calzada JE, Saldaña A, Yabsley MJ, Gottdenker NL. Molecular diagnosis and species identification of Ehrlichia and Anaplasma infections in dogs from Panama, Central America. Vector Borne Zoonotic Dis. 2014; 14: 368–370. 10.1089/vbz.2013.1488 [DOI] [PubMed] [Google Scholar]
- 56. Pat-Nah H, Rodriguez-Vivas RI, Bolio-Gonzalez ME, Villegas-Perez SL, Reyes-Novelo E. Molecular Diagnosis of Ehrlichia canis in dogs and ticks Rhipicephalus sanguineus (Acari: Ixodidae) in Yucatan, Mexico. J Med Entomol. 2015; 52: 101–104. 10.1093/jme/tju010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.