Abstract
Purpose
To evaluate the feasibility of a novel planning concept that differentially redistributes RT dose away from functional liver regions as defined by 99mTc-sulphur colloid (SC) uptake on patient SPECT/ CT images.
Materials and methods
Ten HCC patients with different Child–Turcotte–Pugh scores (A5-B9) underwent SC SPECT/CT scans in treatment position prior to RT that were registered to planning CT scans. Proton pencil beam scanning (PBS) therapy plans were optimized to deliver 37.5–60.0 Gy (RBE) over 5–15 fractions using single field uniform dose technique robust to range and setup uncertainty. Photon volumetrically modulated arc therapy (VMAT) plans were optimized to the same prescribed dose and minimum target coverage. For both treatment modalities, differential hepatic avoidance RT (DHART) plans were generated to decrease dose to functional liver volumes (FLV) defined by a range of thresholds relative to maximum SC uptake (43–90%) in the tumor-subtracted liver. Radiation dose was redistributed away from regions of increased SC uptake in each FLV by linearly scaling mean dose objectives during PBS or VMAT optimization. DHART planning feasibility was assessed by a significantly negative Spearman’s rank correlation (RS) between dose difference and SC uptake. Patient, tumor, and treatment planning characteristics were tested for association to DHART planning feasibility using non-parametric Kruskal–Wallis ANOVA.
Results
Compared to conventional plans, DHART plans achieved a 3% FLV dose reduction for every 10% SC uptake increase. DHART planning was feasible in the majority of patients with 60% of patients having RS < −0.5 (p < 0.01, range −1.0 to 0.2) and was particularly effective in 30% of patients (RS < −0.9). Mean dose to FLV was reduced by up to 20% in these patients. Only fractionation regimen was associated with DHART planning feasibility: 15 fraction courses were more feasible than 5–6 fraction courses (RS < −0.93 vs. RS > −0.60, p < 0.02).
Conclusion
Differential avoidance of functional liver regions defined on sulphur colloid SPECT/CT is achievable with either photon VMAT or proton PBS therapy. Further investigation with phantom studies and in a larger cohort of patients may validate the utility of DHART planning for HCC radiotherapy.
Keywords: SPECT, Sulphur colloid, Functional avoidance, Dose painting, PBS, HCC
Liver function is critically important in the radiation therapy (RT) management of patients with hepatocellular carcinoma (HCC) [1]. In addition to risk of local intrahepatic progression, cirrhosis from chronic liver disease (viral hepatitis, alcohol injury, and steatosis) places HCC patients at higher risk for radiation induced liver disease (RILD) [2–4]. Risk of RILD reported in the literature is variable, ranging from 5% to 63% [5–7]. Reasons for this variability are likely multifactorial, including heterogeneity in patient characteristics, RILD definitions, and RT regimens. Other sources of variability may stem from the assumption that liver function is spatially homogenous, which is reflected by current anatomic liver dose objectives that presume uniform dose–responses from population-based normal tissue complication models [8,9]. These dose objectives ignore potential differences in radiosensitivity between cirrhotic, necrotic, and viable liver tissue. In patients with a high degree of liver function heterogeneity, it is unclear whether anatomic liver dose–volume objectives can effectively spare radiation to functional liver regions.
Incorporating functional liver imaging into RT planning may aid in addressing these issues. Several imaging modalities have been investigated for diagnostic imaging of liver function, including positron emission tomography (PET) with [18F]fluorodeoxygalactose [10,11], dynamic contrast-enhanced magnetic resonance imaging [12] with gadoxetic acid [13] or gadoxetate disodium [14,15]. Single photon emission tomography (SPECT) radiotracers of liver function include [99mTc]hepatobiliary iminodiacetic acid [16], [99mTc]galactosyl-human serum albumin [17], as well as [99mTc]sulphur and [99mTc]phytate colloid [18]. Sulphur colloid is taken up by the Kupffer cells of the reticuloen- dothelial system, which are intimately related to hepatocyte function. Sulphur colloid (SC) uptake has been shown to correlate to the blood serum marker indocyanine green, a well-established quantitative measure of liver function [18]. Furthermore, quantitative imaging parameters from colloid uptake such as the perfused hepatic mass were associated with explanted liver functional mass [19] and predicted clinical outcome [20]. While other imaging surrogates such as [99mTc] hepatobiliary iminodiacetic acid SPECT provide direct imaging of hepatocytes, they rely on dynamic SPECT/CT image acquisition which presents challenges for accurate and reproducible quantification. Both galactosyl-human serum albumin and colloid tracers rely on simpler pharmacokinetics to perform static image acquisition of albumin and Kupffer cells, respectively, which may prove advantageous for defining functional liver regions on quantitative images during radiotherapy planning or therapeutic response assessment.
Quantitative molecular imaging as a surrogate for liver function may provide objective measures by which to characterize spatial variation in liver function. Spatial heterogeneity in molecular images can guide the spatial modulation of radiation dose, a radiotherapy formalism known as ‘dose painting’ [21,22]. Intense investigation on the utility of dose painting has focused on non-uniform tumor dose escalation, primarily based on [18F]fluorodeoxyglucose PET as a surrogate for local failure risk [23,24], in a range of disease sites [25–28]. However, preliminary dose painting clinical trials in head-and-neck cancer [29,30] and non-small cell lung cancer [31] have not considered heterogeneity in normal tissue function. The concept of functional tissue avoidance is to spatially modify (i.e. paint) radiation doses such that normal tissue function is preserved while maintaining RT target dose coverage, which has been explored in avoidance planning of perfused lung [32,33]. Dose painting strategies in tumor versus strategies in normal tissue would ideally complement one another in a manner that substantially enhances the overall therapeutic ratio.
For primary HCC radiotherapy, dose painting based on functional liver heterogeneity has the potential to decrease complication rates of current treatment regimens and enable safer dose escalation strategies. The aim of this study was to develop a novel Differential Hepatic Avoidance Radiation Therapy (DHART) paradigm. As a proof of concept, we compared conventional RT and DHART plans in a cohort of HCC patients. The DHART planning paradigm using [99mTc] sulphur colloid SPECT/CT was implemented for both proton pencil beam scanning (PBS) therapy and photon volumetrically modulated arc therapy (VMAT). Preliminary results of feasibility are reported and implications for patients who may benefit from this treatment paradigm are discussed.
Materials and methods
Patient characteristics
After obtaining Institutional Review Board approval, ten patients (6 male, 4 female) with a median age of 65 years (range 41–83) were included in the study. All patients had a diagnosis of HCC and were ineligible for other liver-directed therapies. Each patient had a single HCC lesion, and gross tumor volumes (GTV) ranged from 1 cm3 to 434 cm3 (median 88 cm3). Six lesions were located in the periphery of the liver, with four located centrally. Six patients had received prior liver-directed therapy for HCC and presented with treatment failure, local recurrence, or new HCC lesions. Prior treatments included radiofrequency ablation (n = 3), transarterial chemoembolization (n = 5) or radioem-bolization (n = 1), and bland embolization (n = 1). The median number of prior liver directed therapies per patient was 4.5 (range 1–9). All patients had underlying cirrhosis with either well-compensated or mildly decompensated liver function, including Child–Turcotte–Pugh (CTP) A (n = 5) and CTP B (n = 5) respectively (range A5–B9). Cirrhosis was related to either hepatitis C (n = 6), alcohol intake (n = 3), non-alcoholic fatty liver disease (n = 2), hepatitis B (n = 1) or a combination of these factors. Six patients received stereotactic body RT (SBRT) in 5–6 fractions, while four received longer hypofractionated radiation courses of 15 fractions, with total doses ranging from 37.5 Gy to 60.0 Gy (RBE) in accordance with the NRG-GI001 cooperative trial protocol.
SPECT/CT image acquisition, reconstruction and registration
Patients underwent [99mTc] sulphur colloid (SC) SPECT/CT scans prior to definitive radiotherapy and were reproducibly immobilized in treatment position. SPECT/CT images were acquired on a Precedence™ (Philips Healthcare, Andover, MA) scanner comprising a dual head gamma camera and 16 slice CT scanner. Following the injection of 7 mCi (259 MBq) [99mTc] sulphur colloid, SPECT scans were acquired 15 min post-injection over a fixed time-averaged frame (64 views, 20 s/view, 180 degree arc). Emission images were corrected for scatter, collimation, and attenuation using a tidal breathing end-exhale position CT image. Reconstructions were performed with the Astonish™ (Philips Healthcare, Andover, MA) ordered subset expectation–maximization (OSEM) iterative algorithm over 2 iterations and 16 subsets that included a 10 mm Hanning filter and isotropic 4.64 mm voxels. Liver counts were normalized to spleen counts to form a relative liver-to-spleen uptake ratio, which facilitated inter-patient comparison of images.
Liver anatomy from the end-exhale attenuation correction CT acquired with each SPECT scan was registered to the reference liver anatomy from the end-exhale respiratory phase of a radiotherapy planning CT acquired the same day, either under free-breathing or active breathing control (ABC™, Elekta Inc., Stockholm, Sweden) breath-hold conditions. Rigid registration between the planning CT and SPECT/CT was performed in MIM 6.2™ (MIM Software Inc., Cleveland, OH) using built-in mutual information methods. The resulting spatial transformations estimated from CT-to-CT registration were applied to the respective SPECT images, and the rigidly translated/rotated matrices were resampled using a cubic spline filter onto a common planning grid in MIM. Deformable registration techniques were initially evaluated but did not provide sufficiently improved liver registration accuracy, particularly in the context of end-exhale CT scans and low spatial resolution SPECT, to warrant their implementation for this study.
Functional liver avoidance paradigm
In the absence of direct clinical evidence on the relationship between SC SPECT uptake and functional liver radiosensitivity, a simple modeling approach was adopted as a proof of concept. Under the assumptions that increased SC uptake is a surrogate for viable liver tissue at risk of radiation-induced complication and that SC avid areas are of higher preservation importance, the planning paradigm was designed to preferentially reduce dose to these regions. The regions were defined by applying multiple thresholds to the continuous SC SPECT uptake distribution to generate functional liver volumes (FLVxx%): 43%, 60%, 70%, 80% and 90% of the maximum liver-to-spleen uptake ratio (Fig. 1). The minimum threshold was chosen to match values reported in phantom and patient investigations that correlated quantitative [99mTc] colloid SPECT uptake with clinical liver function [34,35].
Fig. 1.
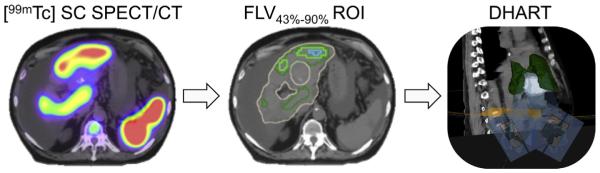
Workflow schematic for differential hepatic avoidance radiotherapy (DHART) planning. The [99mTc] sulphur colloid SPECT/CT uptake in the tumor-subtracted liver (khaki contour) was normalized to the spleen for all patients. Percentage of maximum uptake ratio thresholds were applied to generate discrete functional liver volumes (FLV43% – forest green contour, FLV70% – light green contour, FLV90% – light blue contour). These FLV served as avoidance regions in the DHART plans, yielding preferential irradiation through non-functional areas (fluence maps directed at PTV – white surface) and avoidance of concavely shaped functional areas (FLV43% – forest green surface). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The binned SC uptake distribution in the FLV was used to define dose–volume planning objectives for dose painting avoidance plans. The methodology of using discrete planning regions of interest (ROIs) to generate continuous voxel-based dose painting plans has been reported on in tumor dose escalation studies [36,37], which found that a finite number of regions are sufficient to accurately reproduce non-uniform dose distributions. This investigation adopted the same methodology but naturally translated the concept to functional normal tissue avoidance in order to increase the compatibility of DHART with commercially available planning systems. The number of FLVs needed for avoidance planning is directly related to the spatial resolution of the functional imaging modality, the gradients in uptake patterns, and the dose–response relationship. Due to lower spatial resolution SPECT imaging (5–10 mm), relatively shallow gradients in image intensity, and a presumed linear relationship between liver function and dose tolerance, five FLVs were utilized to generate functional avoidance plans.
The redistribution of dose away from high uptake FLVs was defined mathematically by the following equation:
| (1) |
The mean dose objective for a given FLV from threshold value xx% ❬DFLVxx%❭ was scaled from the mean dose objective to the GTV-subtracted anatomical liver volume ❬DALV❭ by the mean liver-to-spleen uptake ratio ❬UALV❭ divided by the mean FLV-tospleen uptake ratio ❬UFLVxx%❭. For example, if the mean SC uptake ratio in a given FLV was twice the SC uptake ratio in the anatomic liver, the resulting mean FLV dose objective would scale to half of the mean liver dose objective. This placed increasingly stringent dose objectives on regions of relatively increased SC uptake (Fig. 1).
Treatment planning
Patient target and normal structures were defined on the planning CT image using the MIM software by a board-certified radiation oncologist (SA). Critical structures included liver, bowel, duodenum, kidneys, oesophagus, stomach, and spinal cord. The image and structures sets were transferred to the RayStation 4.0™ treatment planning system (RaySearch Laboratories, Stockholm, Sweden). Proton PBS beams were designed for conventional plans without knowledge of functional liver SC SPECT/CT images following standard-of-care guidelines. Once conventional planning in each patient was completed, functional liver volume structures from SC SPECT/CT images were included in the DHART PBS plan design, which altered the beam angle configuration in some patient cases. Beam angles were altered to accept higher proton ranges for target coverage in order to avoid delivery through FLV. Photon VMAT plans utilized full arcs whereby preferential beam intensity from certain entrance angles was determined by the optimization objectives in the conventional and DHART plans.
Following the plan design, proton PBS and photon VMAT plans were optimized using identical objectives for target and anatomic organs-at-risk (OAR). Generally, patients with tumors less than 3 cm were planned with dose objectives for SBRT regimens (5–6 fractions), while patients with larger tumors were planned with objectives for mild hypofractionation regimens (15 fractions). OAR objectives included the following for SBRT regimens: mean dose to anatomic liver minus GTV (<7 Gy) and bilateral mean to kidneys (<10 Gy), as well as maximum doses (highest 0.5 cm3 volume) to small bowel (<30 Gy), oesophagus (<32 Gy), stomach (<30 Gy), duodenum (<30 Gy), colon (<22.3 Gy) and spinal cord +5 mm (<25 Gy). OAR objectives included the following for hypofractionated regimens: mean dose to anatomic liver minus GTV (<20 Gy) and bilateral mean to kidneys (<12 Gy), as well as maximum doses (highest 0.5 cm3 volume) to bowel (<45 Gy), oesophagus (<45 Gy), stomach (<36 Gy), duodenum (<45 Gy) and spinal cord +5 mm (<37.5 Gy). All of these conventional planning objectives were matched between each patient’s clinical plan and DHART plan. DHART plans included mean dose objectives to FLV following the formalism of Eq. (1). Proton PBS plans were optimized with an IBA Universal Nozzle™ (Ion Beam Applications, Belgium) to deliver single field uniform dose as a fraction of the total target dose prescription, which improved robustness to range uncertainty over intensity modulated proton therapy techniques. Proton longitudinal layer spacing and lateral spot spacing were scaled automatically as a function of the pristine Bragg peak width and spot size at each energy level. All proton plans were tested for robustness to 3% range uncertainty and 3 mm isotropic setup uncertainty, which sufficiently encompassed the recommended margins of dose calculation uncertainty for liver cancer proton therapy [38]. Photon VMAT plans were optimized with an Elekta Infinity linear accelerator (Elekta, Stockholm, Sweden) over two independent arcs at 4° angular gantry spacing and ±30° collimator settings to minimize inter-leaf leakage. Leaf motion was constrained to 0.3 cm per degree of each arc.
Plan evaluation and statistical analysis
Proton pencil beam and photon collapsed cone convolution dose distributions were calculated on isotropic 3 mm grids. All plans for each patient were scaled to match target coverage, either PTV D95% in hypofractionated cases or PTV D80% in SBRT cases. Dose–volume histograms were generated for relevant target, anatomical liver, and FLV structures in order to highlight differences due to functional avoidance.
A novel plan evaluation metric, the ‘SPECT-dose difference histogram’, which included both dosimetric and functional liver imaging information, was developed in MATLAB 8.2™ (MathWorks, Natick, MA). By simultaneously binning the SC SPECT liver-to-spleen ratios and the corresponding mean dose differences over spatially co-localized voxels, the SPECT-dose difference histogram captured whether dose was redistributed away from high functioning regions of the liver. DHART plans would ideally exhibit greater dose reduction (or more negative dose difference) relative to conventional plans as a function of increasing SC SPECT liver-to-spleen ratio while meeting the same remaining OAR objectives. The degree of dose redistribution in SPECT-dose difference histograms was quantified by the Spearman rank correlation coefficient RS and tested for statistical significance for each patient. Over the patient cohort, population-averaged SPECT-dose difference histograms and Spearman R were reported. Patient DHART plans with highly negative RS coefficients were considered more effective in avoiding functional liver than those with zero or positive RS coefficients.
Testing of statistical association between DHART plan effectiveness, as measured by negative rank correlation between SC SPECT uptake and dose difference, and patient characteristics was conducted. Patients were grouped by CTP class, prior treatment history, lesion location, lesion volume, and fractionation regimen. Differences in rank correlation coefficients between groups were tested using non-parametric Kruskal–Wallis ANOVA due to limited patient sample size for powering parametric tests.
Results
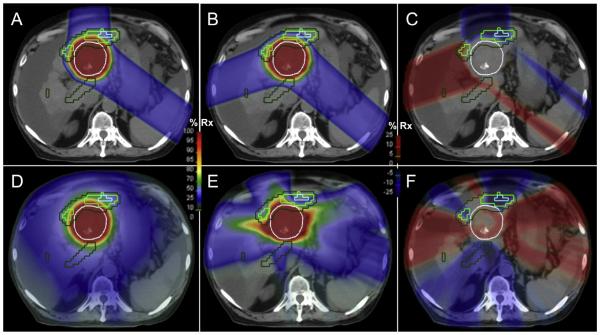
Conventional RT and DHART dose distributions are shown for an example patient in Fig. 2. The proton PBS conventional plan (Fig. 2A) utilized an anterior beam angle that minimizes range uncertainty in covering the target (PTV indicated by the white contour) and avoided treating through anatomical liver. However this beam compromised dose sparing to the functional liver volumes (FLV43–90% indicated by the colored contours). The proton PBS DHART plan (Fig. 2B) replaced this beam with a right lateral angle that has higher range uncertainty and passes through more anatomical liver, but avoided delivery through functional liver. Dose differences in proton plans (Fig. 2C) highlight the changes in beam angles, including small differences in the left posterior oblique beam to maintain robust target coverage under the functional avoidance paradigm. The photon VMAT conventional plan (Fig. 2D) conformed to the target shape but at the expense of high dose spilling isotropically into functional liver regions. The photon VMAT DHART plan (Fig. 2E) redistributed high dose to avoid functional liver and maintained target coverage from several arc segments, but did not conform as precisely to the target shape while allowing high dose to spill non-isotropically outside the target.
Fig. 2.
Conventional radiotherapy vs. DHART for an example HCC patient. Proton PBS dose distributions in an axial plane (A–C) that met either anatomic liver objectives (A) or functional liver objectives (B), with the resulting dose difference distribution (C). Photon VMAT dose distributions in the same axial plane (D–F) that met anatomic liver objectives (D) or functional liver objectives (E), with resulting dose difference distribution (F). Contours are shown for PTV (white), FLV43% (forest green), FLV70% (light green) and FLV90% (cyan). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Dose–volume histograms are shown for the same example patient in Fig. 3. While conventional and DHART proton PBS plans achieved statistically indistinguishable PTV dose distributions (indicated in Fig. 3A by black solid/dashed lines), there was significant reduction in dose to all FLV (indicated in Fig. 3A by colored lines), particularly to FLV90%. For instance, V20Gy in FLV90% was reduced from 24% to 3%, whereas V20Gy in anatomic liver-GTV did not change appreciably. The DHART photon VMAT plan (indicated in Fig. 3B by solid lines) also spared dose to all FLVs, including a reduction in FLV90% V20Gy from 45% to 2%. However, in order to maintain the same PTV coverage at D95%, the maximum PTV dose increased from 46 Gy to 51 Gy. Furthermore, the PTV dose heterogeneity, defined as the dose difference between D2% and D98% normalized by D50%, more than doubled from 6% to 13%. DHART was feasible for this patient case with both radiotherapy modalities, but in proton PBS it was distinguished by the beam angle selection while in photon VMAT it was distinguished by target heterogeneity.
Fig. 3.
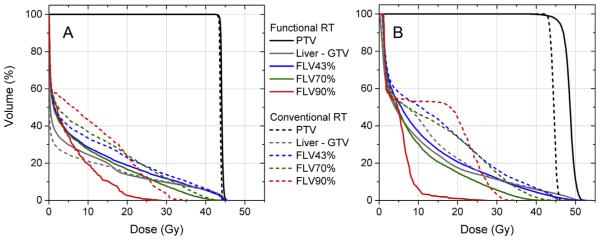
Dose–volume histograms comparing conventional radiotherapy and DHART for an example HCC patient. Conventional proton planning (A – dashed lines) yielded similar target coverage as functional liver avoidance planning (A – solid lines) but reduced dose to functional liver volumes (FLV), particularly FLV90% with the highest sulphur colloid SPECT liver-to-spleen uptake ratio. The dose to FLV was also reduced in the DHART photon plan (B – solid lines) compared to the conventional photon plan (B – dashes lines) but resulted in higher maximum PTV dose.
SPECT dose-difference histograms were calculated and displayed for the same example patient plans in Fig. 4. Each point represented a mean dose difference between DHART and conventional RT plans that was binned over a 2 percent interval in SC SPECT liver-to-spleen ratio within the GTV-subtracted liver structure. The data were also scaled by colour according to the absolute magnitude in conventional RT planned dose, where points in red represented regions of higher initial dose and points in blue represented regions of lower initial dose. The DHART proton PBS plan (Fig. 4A) was able to redistribute dose away from functional liver, where for example the dose to regions with at least 80% of the maximum liver-to-spleen uptake ratio was reduced by >20%. This came at the expense of increasing dose to regions with up to 20% of the maximum uptake ratio by >30%.
Fig. 4.

DHART plan dose difference vs. sulphur colloid (SC) SPECT uptake for an example HCC patient. Differences in dose to the GTV-subtracted liver in the DHART plan relative to conventional RT plan are shown using both proton PBS therapy (A) and photon VMAT (B), where each data point is scaled to the absolute dose color bar. Note that dose differences were negatively rank correlated to SC SPECT uptake (RS < −0.95, p < 0.001). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The DHART photon VMAT plan (Fig. 4B) was also able to reduce dose to functional liver. The slope of dose reduction was shallower than with proton PBS at low SC SPECT uptake values, while the maximum dose reduction reached 70%. Dose sparing appeared to be most effective in smaller regions of high uptake rather than in larger regions of moderate uptake. The highly negative rank correlation (RS = −0.99 for PBS, RS = −0.95 for VMAT) between dose difference and SC SPECT uptake indicated that DHART was technically feasible with both radiotherapy modalities in this patient.
Rank correlation of SPECT dose-difference histograms was tabulated for the patient cohort. A median RS of −0.55 (interquartile range 0.66) was achieved with proton PBS, while a median RS of −0.59 (interquartile range 1.24) was achieved with photon VMAT. Of the 10 patients, 6 patients had statistically significant negative rank correlations (RS < −0.5, p < 0.01) and 4 did not (RS < −0.3). Of the 6 patients with negative rank correlations, 3 had DHART plans with RS < −0.9.
When SPECT dose-difference histograms were averaged over all patients, the linear trend was a 3% reduction in planned dose using DHART per 10% increase in SC SPECT liver-to-spleen uptake ratio, shown visually for both proton PBS (Fig. 5A) and photon VMAT (Fig. 5B). Interestingly, this regression slope was not different between modalities (proton PBS slope = −0.25, photon VMAT slope = −0.27), which suggests that patient factors drove achievable dose reduction rather than radiotherapy technique. In cases where DHART was deemed feasible (6/10 patients), the median dose reduction to the largest functional liver volume (FLV43%) was 13% for proton PBS and 19% for photon VMAT.
Fig. 5.
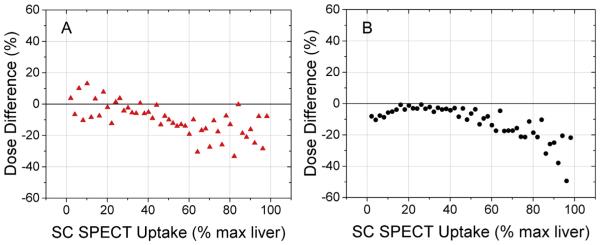
Population DHART plan dose difference vs. sulphur colloid (SC) SPECT uptake. Dose differences were binned for every 2% increase in SC SPECT uptake to yield population-averaged trends for proton PBS therapy (A) and photon VMAT (B). Note that both modalities have similar linear slopes of dose reduction (−0.25 vs. −0.27), yet inter-patient variability is larger for proton PBS in regions of high and low SC uptake that leads to a lower coefficient of determination compared to photon VMAT (R2 = 0.45 vs. 0.58).
Several patient characteristics were tested for statistical association to the rank correlation of SPECT dose-difference histograms as a surrogate for DHART feasibility. Patient groups included CTP class (A vs. B), prior treatment history (no vs. yes), lesion location (peripheral vs. central), GTV size (<median vs. >median), and radiotherapy regimen (hypofractionation vs. SBRT). Table 1 summarizes the results of the non-parametric Kruskal–Wallis ANOVA, which conservatively tested for differences in median and interquartile ranges between the patient groups. While larger and centrally located GTV showed statistical trends towards DHART feasibility, only patients undergoing hypofractionated RT could generate plans with significantly different rank correlation relative to patients undergoing SBRT (RS < −0.93 vs. RS > −0.60, p < 0.02).
Table 1.
Multivariate non-parametric Kruskal–Wallis (K–W) ANOVA for separating dose-reduction rank correlation (RS) by patient, tumor, and treatment characteristics. K–W p < 0.05 was considered statistically significant.
| Factor | Group | Proton PBS |
Photon VMAT |
||
|---|---|---|---|---|---|
| R S | K–W p | R S | K–W p | ||
| Child–Pugh Class | A | −0.41 | 1.00 | −0.93 | 0.52 |
| B | −0.61 | −0.96 | |||
| Prior Tx History | No | −0.28 | 0.39 | 0.29 | 0.29 |
| Yes | −0.61 | −0.74 | |||
| Lesion Location | Peripheral | −0.41 | 0.20 | −0.24 | 0.13 |
| Central | −0.80 | −0.95 | |||
| GTV Size | <median | −0.32 | 0.07 | 0.04 | 0.12 |
| >median | −0.90 | −0.95 | |||
| RT Fractionation | Hypofractionated | −0.93 | 0.01 | −0.99 | 0.02 |
| SBRT | −0.28 | −0.60 | |||
Discussion
Current RT planning for HCC patients only takes into account anatomic information when designing safe and effective treatment regimens. This study introduced a novel RT planning paradigm of differential hepatic avoidance RT (DHART) whereby regions of functional liver as defined by SC SPECT images were differentially spared through the use of dose painting techniques in proton PBS and photon VMAT RT. We demonstrated that DHART is technically feasible and its degree of usefulness is case-dependent. In the majority of the investigated patient cohort, dose could be reduced to areas of high SC SPECT uptake in proton PBS plans with different entrance angles or photon VMAT plans with increased target dose heterogeneity. This difference is fundamentally tied to the radiation modalities, as photon VMAT can achieve high dose conformality/avoidance through use of many different entrance angles across each arc, while proton PBS therapy can achieve integral dose sparing from delivery of few beams and favourable Bragg Peak depth-dose distributions. Proton PBS functional liver avoidance is bounded by incorporation of range/setup uncertainties and the broader scanning magnet-defined lateral penumbra, while photon VMAT functional avoidance is bounded by target dose homogeneity. DHART plans reduced a higher percentage of liver dose in hypofractionated treatment regimens than SBRT regimens. The cause of this observation is likely multifactorial, including stricter normal tissue constraints for SBRT that limit liver dose redistribution, variations in tumor location relative to critical structures, and dynamic range of spatially heterogeneous SC SPECT uptake. A larger cohort of patients would aid in the confirmation of these trends under sufficient statistical power.
Prior radiation avoidance feasibility studies have sought to avoid irradiation of discrete functional tissue volumes defined by perfused lung [32,33,39] or functional liver [40,41] estimated on SPECT imaging. Shirai and colleagues employed manual segmentation to define functional liver volumes on GSA SPECT and incorporated a functional dose–volume constraint (functional volume receiving 20 Gy or higher) in conformal radiotherapy plans, which resulted in less liver function deterioration as measured by Child– Turcotte–Pugh scores post therapy [40]. Their demonstration of technical feasibility and observed lower risk of RILD lends credibility to functional liver avoidance strategies. However, we have not yet reached consensus on whether discrete uniform avoidance or differential avoidance is preferable in HCC radiotherapy plan design. The uniform avoidance paradigm imposes sharp boundaries in dose–volume objectives from image thresholds that are especially challenging to define in heavily decompensated cirrhotic patients with global liver function deficits. On the other hand, the differential avoidance paradigm accounts for continuous functional image intensity gradients to redistribute radiation dose, making it less sensitive to image threshold techniques but potentially more sensitive to increased image voxel noise from scatter in large patients. Both avoidance approaches are susceptible to functional liver imaging artefacts of CT-based attenuation correction, particularly for SC SPECT uptake near the dome of the liver, that impact image quantitation and region delineation.
Within the framework of a proof of concept investigation, several simplifying assumptions were made when generating DHART plans: (1) higher SC uptake is equivalent to higher liver function, (2) sparing radiation dose to areas of higher SC uptake/ higher liver function is most important for reducing the risk of RILD, (3) deficits in liver function due to absorbed dose are governed by a linear relationship, and (4) relative SC uptake ratios measured by SPECT imaging are quantitatively accurate. If liver function is associated to the density of Kupffer cells that support viable hepatocytes, then the first assumption remains valid. On the other hand, if liver function is not simply correlated to cell density but to a host of other biological factors, then the assumption may potentially break down. Under the second and third assumptions, the radiation dose avoidance strategy was linearized to preferentially reduce dose to SC avid areas. While a reasonable first order approximation, the assumption does not address the complex radiation damage and recovery dynamics that affect global and regional liver function, which will play a larger role in refining liver radiation dose tolerance guidelines. The fourth assumption can be addressed by imaging of phantom test objects with known ground truth activity concentrations, which will inform on the quantitative accuracy and reproducibility of relative SC uptake ratios and SPECT imaging in the future.
Following demonstration of technical feasibility, the shape and magnitude of the relationship between radiation dose and SC SPECT image-based response must be characterized and ultimately correlated to endpoints of hepatic toxicity. The mathematical transformation of functional liver image intensity to radiosensitivity and RILD risk may not be linear but instead sigmoidal in shape. Longitudinal SC SPECT/CT imaging in response to known delivered regional radiation doses to the liver could elucidate this relationship by fitting data to radiobiological models of cell survival or normal tissue complication probability. Reports of correlation between molecular imaging response in tumors and radiation dose [42], as well as correlation of functional lung perfusion imaging to radiation dose [43,44], may inform on methods for functional liver dose–response modeling. Prediction models of hepatic function in response to radiation dose are currently under development to motivate physiologically adaptive radiation therapy [45] and could be adopted based on SC SPECT/CT imaging.
Dose–response modeling may also identify whether it is clinically relevant to reduce radiation dose below a critical magnitude to functional regions or to continuously redistribute dose as in this investigation. The answer depends on the shape of the dose–response curve and is likely impacted by the severity of pre-treatment liver cirrhosis. If a threshold exists both in dose and in SC SPECT/CT uptake at which RILD incidence rates begin to rise significantly, then functional liver planning objectives may avoid dosing any region of a certain volume to drop below this uptake threshold. However, limited spatial resolution of current SPECT imaging systems hampers the precise detection of a discrete dose–response threshold and so a continuous dose–response function may be more robust to imaging uncertainty. These are all topics of future investigation as part of a path towards in vivo radiosensitivity estimates of functional liver.
Continuous dose redistribution with DHART is a novel concept that presents an opportunity to increase the safety of current HCC treatment regimens. Dose painting for functional tissue avoidance in strategic combination with tumor dose intensification may improve the therapeutic ratio when managing HCC patients with definitive radiotherapy. Further investigation in a larger cohort of patients is warranted to test the clinical safety and efficacy of DHART.
Acknowledgements
This work was supported by a Research Scholar award from the Radiological Society of North America. The authors kindly acknowledge Michelle Wanner and the nuclear medicine technology staff for conducting the sulphur colloid SPECT/CT examinations, as well as Patricia Sponseller and Ning Cao for insightful discussion on planning parameters.
Footnotes
Conflict of interest
None to report.
References
- [1].El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- [2].Cheng JC, Wu JK, Huang CM, et al. Radiation-induced liver disease after radiotherapy for hepatocellular carcinoma: clinical manifestation and dosimetric description. Radiother Oncol. 2002;63:41–5. doi: 10.1016/s0167-8140(02)00061-0. [DOI] [PubMed] [Google Scholar]
- [3].Cheng JC, Wu JK, Huang CM, et al. Radiation-induced liver disease after threedimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol Biol Phys. 2002;54:156–62. doi: 10.1016/s0360-3016(02)02915-2. [DOI] [PubMed] [Google Scholar]
- [4].Liang SX, Zhu XD, Xu ZY, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys. 2006;65:426–34. doi: 10.1016/j.ijrobp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- [5].Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys. 2013;87:22–32. doi: 10.1016/j.ijrobp.2012.08.043. [DOI] [PubMed] [Google Scholar]
- [6].Culleton S, Jiang H, Haddad CR, et al. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014;111:412–7. doi: 10.1016/j.radonc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- [7].Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355–61. doi: 10.1016/j.ijrobp.2011.11.058. [DOI] [PubMed] [Google Scholar]
- [8].Russell AH, Clyde C, Wasserman TH, Turner SS, Rotman M. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27:117–23. doi: 10.1016/0360-3016(93)90428-x. [DOI] [PubMed] [Google Scholar]
- [9].Dawson LA, Normolle D, Balter JM, McGinn CJ, Ten Lawrence TS, Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–21. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- [10].Sorensen M, Frisch K, Bender D, Keiding S. The potential use of 2- [(1)(8)F]fluoro-2-deoxy-D-galactose as a PET/CT tracer for detection of hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2011;38:1723–31. doi: 10.1007/s00259-011-1831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sorensen M, Mikkelsen KS, Frisch K, Bass L, Bibby BM, Keiding S. Hepatic galactose metabolism quantified in humans using 2–18F-fluoro-2-deoxy-d-galactose PET/CT. J Nucl Med. 2011;52:1566–72. doi: 10.2967/jnumed.111.092924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cao Y, Wang H, Johnson TD, et al. Prediction of liver function by using magnetic resonance-based portal venous perfusion imaging. Int J Radiat Oncol Biol Phys. 2013;85:258–63. doi: 10.1016/j.ijrobp.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sirlin CB, Hussain HK, Jonas E, et al. Consensus report from the 6th International forum for liver MRI using gadoxetic acid. J Magn Reson Im. 2014;40:516–29. doi: 10.1002/jmri.24419. [DOI] [PubMed] [Google Scholar]
- [14].Ringe KI, Husarik DB, Sirlin CB, Merkle EM. Gadoxetate disodium-enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. AJR Am J Roentgenol. 2010;195:13–28. doi: 10.2214/AJR.10.4392. [DOI] [PubMed] [Google Scholar]
- [15].Cruite I, Schroeder M, Merkle EM, Sirlin CB. Gadoxetate disodium-enhanced MRI of the liver: part 2, protocol optimization and lesion appearance in the cirrhotic liver. AJR Am J Roentgenol. 2010;195:29–41. doi: 10.2214/AJR.10.4538. [DOI] [PubMed] [Google Scholar]
- [16].Wang H, Cao Y. Spatially resolved assessment of hepatic function using 99mTc-IDA SPECT. Med Phys. 2013;40:092501. doi: 10.1118/1.4816655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Beppu T, Hayashi H, Okabe H, et al. Liver functional volumetry for portal vein embolization using a newly developed 99mTc-galactosyl human serum albumin scintigraphy SPECT-computed tomography fusion system. J Gastroenterol. 2011;46:938–43. doi: 10.1007/s00535-011-0406-x. [DOI] [PubMed] [Google Scholar]
- [18].Zuckerman E, Slobodin G, Sabo E, Yeshurun D, Naschitz JE, Groshar D. Quantitative liver-spleen scan using single photon emission computerized tomography (SPECT) for assessment of hepatic function in cirrhotic patients. J Hepatol. 2003;39:326–32. doi: 10.1016/s0168-8278(03)00296-4. [DOI] [PubMed] [Google Scholar]
- [19].Hoefs JC, Wang F, Kanel G. Functional measurement of nonfibrotic hepatic mass in cirrhotic patients. Am J Gastroenterol. 1997;92:2054–8. [PubMed] [Google Scholar]
- [20].Everson GT, Shiffman ML, Hoefs JC, et al. Quantitative liver function tests improve the prediction of clinical outcomes in chronic hepatitis C: results from the Hepatitis C Antiviral Long-term Treatment Against Cirrhosis Trial. Hepatology. 2012;55:1019–29. doi: 10.1002/hep.24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ling CC, Humm J, Larson S, et al. Towards multidimensional radiotherapy (MD-CRT): Biological imaging and biological conformality. Int J Radiat Oncol. 2000;47:551–60. doi: 10.1016/s0360-3016(00)00467-3. [DOI] [PubMed] [Google Scholar]
- [22].Bentzen SM. Theragnostic imaging for radiation oncology: dose-painting by numbers. Lancet Oncol. 2005;6:112–7. doi: 10.1016/S1470-2045(05)01737-7. [DOI] [PubMed] [Google Scholar]
- [23].Aerts HJ, Bussink J, Oyen WJ, et al. Identification of residual metabolic-active areas within NSCLC tumours using a pre-radiotherapy FDG-PET-CT scan: a prospective validation. Lung Cancer. 2012;75:73–6. doi: 10.1016/j.lungcan.2011.06.003. [DOI] [PubMed] [Google Scholar]
- [24].Aerts HJ, van Baardwijk AA, Petit SF, et al. Identification of residual metabolicactive areas within individual NSCLC tumours using a pre-radiotherapy (18)Fluorodeoxyglucose-PET-CT scan. Radiother Oncol. 2009;91:386–92. doi: 10.1016/j.radonc.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Alber M, Paulsen F, Eschmann SM, Machulla HJ. On biologically conformal boost dose optimization. Phys Med Biol. 2003;48:N31–5. doi: 10.1088/0031-9155/48/2/404. [DOI] [PubMed] [Google Scholar]
- [26].Vanderstraeten B, De Gersem W, Duthoy W, De Neve W, Thierens H. Implementation of biologically conformal radiation therapy (BCRT) in an algorithmic segmentation-based inverse planning approach. Phys Med Biol. 2006;51:N277–86. doi: 10.1088/0031-9155/51/16/N02. [DOI] [PubMed] [Google Scholar]
- [27].Flynn RT, Bowen SR, Bentzen SM, Rockwell Mackie T, Jeraj R. Intensitymodulated x-ray (IMXT) versus proton (IMPT) therapy for theragnostic hypoxia-based dose painting. Phys Med Biol. 2008;53:4153–67. doi: 10.1088/0031-9155/53/15/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bowen SR, Flynn RT, Bentzen SM, Jeraj R. On the sensitivity of IMRT dose optimization to the mathematical form of a biological imaging-based prescription function. Phys Med Biol. 2009;54:1483–501. doi: 10.1088/0031-9155/54/6/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Duprez F, De Neve W, De Gersem W, Coghe M Madani I. Adaptive dose painting by numbers for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2010.03.028. [DOI] [PubMed] [Google Scholar]
- [30].Madani I, Duthoy W, Derie C, et al. Positron emission tomography-guided, focal-dose escalation using intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:126–35. doi: 10.1016/j.ijrobp.2006.12.070. [DOI] [PubMed] [Google Scholar]
- [31].van Elmpt W, De Ruysscher D, van der Salm A, et al. The PET-boost randomised phase II dose-escalation trial in non-small cell lung cancer. Radiother Oncol. 2012;104:67–71. doi: 10.1016/j.radonc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- [32].McGuire SM, Zhou S, Marks LB, Dewhirst M, Yin FF, Das SK. A methodology for using SPECT to reduce intensity-modulated radiation therapy (IMRT) dose to functioning lung. Int J Radiat Oncol Biol Phys. 2006;66:1543–52. doi: 10.1016/j.ijrobp.2006.07.1377. [DOI] [PubMed] [Google Scholar]
- [33].McGuire SM, Marks LB, Yin FF, Das SK. A methodology for selecting the beam arrangement to reduce the intensity-modulated radiation therapy (IMRT) dose to the SPECT-defined functioning lung. Phys Med Biol. 2010;55:403–16. doi: 10.1088/0031-9155/55/2/005. [DOI] [PubMed] [Google Scholar]
- [34].Groshar D, Slobodin G, Zuckerman E. Quantitation of liver and spleen uptake of (99m)Tc-phytate colloid using SPECT: detection of liver cirrhosis. J Nucl Med. 2002;43:312–7. [PubMed] [Google Scholar]
- [35].Iosilevsky G, Israel O, Frenkel A, et al. A practical SPECT technique for quantitation of drug delivery to human tumors and organ absorbed radiation dose. Semin Nucl Med. 1989;19:33–46. doi: 10.1016/s0001-2998(89)80034-0. [DOI] [PubMed] [Google Scholar]
- [36].Deveau MA, Bowen SR, Westerly DC, Jeraj R. Feasibility and sensitivity study of helical tomotherapy for dose painting plans. Acta Oncol. 2010;49:991–6. doi: 10.3109/0284186X.2010.500302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Korreman SS, Ulrich S, Bowen S, Deveau M, Bentzen SM, Jeraj R. Feasibility of dose painting using volumetric modulated arc optimization and delivery. Acta Oncol. 2010;49:964–71. doi: 10.3109/0284186X.2010.498440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schuemann J, Dowdell S, Grassberger C, Min CH, Paganetti H. Site-specific range uncertainties caused by dose calculation algorithms for proton therapy. Phys Med Biol. 2014;59:4007–31. doi: 10.1088/0031-9155/59/15/4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Das SK, Miften MM, Zhou S, et al. Feasibility of optimizing the dose distribution in lung tumors using fluorine-18-fluorodeoxyglucose positron emission tomography and single photon emission computed tomography guided dose prescriptions. Med Phys. 2004;31:1452–61. doi: 10.1118/1.1750991. [DOI] [PubMed] [Google Scholar]
- [40].Shirai S, Sato M, Noda Y, Kumayama Y, Shimizu N. Incorporating GSA-SPECT into CT-based dose–volume histograms for advanced hepatocellular carcinoma radiotherapy. World J Radiol. 2014;6:598–660. doi: 10.4329/wjr.v6.i8.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kudithipudi V, Gayou O, Thai NL, Kirichenko AV. Liver Stereotactic Radiation Therapy (SRT) with functional treatment planning for patients with Intermediate-Stage Hepatocellular Carcinoma (HCC) Int J Radiat Oncol. 2014;90:S372. [Google Scholar]
- [42].Bowen SR, Chappell RJ, Bentzen SM, Deveau MA, Forrest LJ, Jeraj R. Spatially resolved regression analysis of pre-treatment FDG, FLT and Cu-ATSM PET from post-treatment FDG PET: an exploratory study. Radiother Oncol. 2012 doi: 10.1016/j.radonc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose–volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76:S70–6. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Robbins ME, Brunso-Bechtold JK, Peiffer AM, Tsien CI, Bailey JE, Marks LB. Imaging radiation-induced normal tissue injury. Radiat Res. 2012;177:449–66. doi: 10.1667/rr2530.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang H, Feng M, Frey KA, Ten Haken RK, Lawrence TS, Cao Y. Predictive models for regional hepatic function based on 99mTc-IDA SPECT and local radiation dose for physiologic adaptive radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:1000–6. doi: 10.1016/j.ijrobp.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]