Abstract
Caffeine is one of the most widely consumed behavioral substances. We have previously shown that caffeine- and theophylline-induced inhibition of renal reabsorption causes diuresis and natriuresis, an effect that requires functional adenosine A1 receptors. In this study, we tested the hypothesis that blocking the Gi protein-coupled adenosine A1 receptor via the nonselective adenosine receptor antagonist caffeine changes Na+/H+ exchanger isoform 3 (NHE3) localization and phosphorylation, resulting in diuresis and natriuresis. We generated tubulus-specific NHE3 knockout mice (Pax8-Cre), where NHE3 abundance in the S1, S2, and S3 segments of the proximal tubule was completely absent or severely reduced (>85%) in the thick ascending limb. Consumption of fluid and food, as well as glomerular filtration rate, were comparable in control or tubulus-specific NHE3 knockout mice under basal conditions, while urinary pH was significantly more alkaline without evidence for metabolic acidosis. Caffeine self-administration increased total fluid and food intake comparably between genotypes, without significant differences in consumption of caffeinated solution. Acute caffeine application via oral gavage elicited a diuresis and natriuresis that was comparable between control and tubulus-specific NHE3 knockout mice. The diuretic and natriuretic response was independent of changes in total NHE3 expression, phosphorylation of serine-552 and serine-605, or apical plasma membrane NHE3 localization. Although caffeine had no clear effect on localization of the basolateral Na+/bicarbonate cotransporter NBCe1, pretreatment with DIDS inhibited caffeine-induced diuresis and natriuresis. In summary, NHE3 is not required for caffeine-induced diuresis and natriuresis.
Keywords: caffeine, fluid homeostasis, natriuresis, NBCe1, Npt2a
one of the most widely consumed behavioral active substances is caffeine. Caffeine belongs to the group of methylxanthines (1,3,7-trimethylxanthine). After ingestion, the most widely recognized effects of caffeine include increased arousal, alertness, vigilance, and locomotor activity, as well as sleep disturbance or diuresis (16). When moderate amounts of caffeine are consumed in the form of coffee (∼50–400 mg/cup) or energy drinks (<300 mg/can), plasma caffeine concentrations are in the range of 10–50 μmol/liter (3, 37, 54). Some of the effects of caffeine are attributed to antagonism of adenosine A1 and/or A2A receptors that caffeine blocks nonselectively (16). In contrast, although caffeine has been proposed to inhibit phosphodiesterases (PDE) and therefore cAMP/cGMP breakdown, the concentrations required to inhibit PDEs (∼1,000 μmol/liter) are significantly higher compared with the concentrations required for the antagonistic effect on adenosine receptors (14).
In contrast to the well described physiological effects of caffeine, the molecular origin of caffeine-induced diuresis and natriuresis is still unknown. We previously demonstrated that the diuretic and natriuretic effects of caffeine require functional adenosine A1 receptors (52). Similarly, caffeine self-administration induced a significant increase in total fluid intake in wild-type mice, but not in adenosine A1 receptor knockout mice, which is consistent with the absence of a diuretic effect of caffeine in this model (51). Natriuresis resulting from systemic administration of selective A1 adenosine receptors antagonists is most likely caused by an inhibitory effect on proximal tubular reabsorption (26–28, 41, 68). Adenosine A1 receptors are Gi protein-coupled receptors that reduce cAMP levels when activated (15). Contrastingly, caffeine acts as a competitive antagonist on adenosine A1 receptors to increase cAMP (9, 17). In the proximal tubule, cAMP inhibits Na+/H+ exchanger isoform 3 (NHE3) (24, 43, 66, 67), and 24-h caffeine treatment in rats reduces NHE3 protein expression (31), suggesting that caffeine's diuretic and natriuretic effects may be due to NHE3 inhibition. This mechanism is supported by studies in renal proximal tubular cell lines and opossum kidney cells, where adenosine was shown to inhibit NHE3 via adenosine A1 receptors (12). Other transporters regulated by adenosine A1 receptor activation include the Na+/phosphate (7, 8) and Na+/HCO3− cotransporters (59). Interestingly, adenosine A1 receptor blockade via theophylline (1,3-dimethylxanthine) inhibited basolateral HCO3− conductance in isolated perfused rabbit proximal convoluted tubules, an effect mediated by elevated intracellular cAMP levels (59).
In this study, we tested the hypothesis that renal tubular NHE3 mediates caffeine-induced diuresis and natriuresis. We generated tubulus-specific NHE3 knockout mice and examined the effects of caffeine on the renal excretion of fluid and electrolytes in these mice compared with control mice. Our results demonstrate that tubular NHE3 is not required for the diuretic and natriuretic effect of caffeine. Furthermore, caffeine does not affect the abundance or localization of NHE3, the Na+/phosphate cotransporter Npt2a, or the Na+/bicarbonate transporter NBCe1. However, caffeine-induced diuresis and natriuresis is inhibited by DIDS, implying that Na+/HCO3− cotransporter(s) are involved in the renal effects of caffeine.
MATERIALS AND METHODS
Animals.
All animal experimentation was conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD) and was approved by the local Institutional Animal Care and Use Committee, VA San Diego Healthcare System, San Diego, CA. Mice were housed under a 12:12 h light:dark cycle in standard rodent cages with free access to standard rodent chow (Harlan Teklad 7001, Madison, WI) and tap water. Floxed NHE3 (NHE3loxlox) female mice (33) were crossed with hemizygous male Pax8-Cre mice (5), that express the Pax8-Cre transgene specifically in the tubulus system (5). From the filial (F) 1 progeny, male mice heterozygous for the floxed NHE3 allele (NHE3lox/wild-type) and Pax8-Cre were bred to NHE3loxlox mice to generate final breeder pairs in the F2 progeny. To generate male experimental tubulus-specific NHE3 knockout (NHE3loxloxCre) mice, female NHE3loxlox mice were bred to male NHE3loxloxCre mice. Mice were genotyped by PCR from genomic DNA isolated from ear punch with the following primers: NHE3loxlox, 5′-AGC CAA GGA TAA TTC TGA AGA C-3′ and 5′-TGC CTA CTG TTC CTT GGT GAA G-3′. Cycling parameters for PCR were as follows: segment 1 94°C, 5 min (one cycle) and segment 2 94°C, 30 s; 60°C, 1 min; 68°C, 3 min (40 cycles). The wild-type allele gave rise to a PCR product of 200 bp; the presence of a 160 bp fragment indicated the Neo cassette-disrupted NHE3 gene. Pax8-Cre, 5′-TCT CCA CTC CAA CAT GTC TGC-3′, 5′-CCC TCC TAG TTG ATT CAG CCC-3′, and 5′-AGC TGG CCC AAA TGT TGC TGG-3′. Cycling parameters for PCR were as follows: segment 1 94°C, 5 min (one cycle) and segment 2 94°C, 30 s; 58°C, 30 s; 72°C, 30 sec (35 cycles). The wild-type allele gave rise to a PCR product of 389 bp, and the Pax8-Cre allele generates a 673-bp fragment.
Two-bottle choice test.
Male control (NHE3loxlox) or NHE3loxloxCre mice were housed in their home cages with free access to food. Mice were given free access to either tap water (short bottle tip) or caffeinated solution (long bottle tip; 0.3 g/liter caffeine in tap water) on alternate days for 6 days (51). This conditioning is used to train the mice to the visual and gustatory presentation of caffeinated solution or water. After this conditioning procedure, mice were given free access to both bottles for a period of 2 wk. To avoid position preference, the positions of the bottles were switched daily. Consumption of water or caffeinated solution was determined daily as well as body weight and food intake. The mean values were determined for each mouse over the 2-wk period.
Metabolic cage experiments in conscious mice: renal caffeine effects.
Male control and NHE3loxloxCre mice were randomized to acute application of vehicle (sterile water, 3% of body wt) or caffeine (45 mg/kg body wt; Sigma-Aldrich, St. Louis, MO) given by oral gavage (52). The mice were placed in metabolic cages (Tecniplast, Hohenpeissenberg, Germany) without access to food or water for a 3-h period during which quantitative urine collections were performed. The amount of urine produced was determined gravimetrically and flow rate calculated. The urine was analyzed as described below.
Effect of DIDS on renal caffeine effects.
Male control mice were randomized to acute application of vehicle (0.85% NaCl, 2 μl/g body wt ip) or DIDS [NBC inhibitor (35, 36), 40 mg/kg, 2 μl/g body wt ip; Acros Organics, Geel, Belgium] 30 min before application of a caffeine via oral gavage (45 mg/kg, 3% of body wt). DIDS was dissolved in sterile 0.85% NaCl solution by heating at 60°C for 15 min. Mice were housed in metabolic cages without access to food or water and, a 3-h quantitative urine collection was performed. The amount of urine produced was determined gravimetrically and flow rate calculated. Urine was analyzed as described below.
Effect of caffeine on abundance, phosphorylation, and trafficking of NHE3 and NBCe1.
Male control mice were randomized to acute application of vehicle (0.85% NaCl, 1% of body wt) or caffeine via oral gavage (45 mg/kg body wt) and anesthetized 1 h after application. The right kidney was immediately removed for Western blotting (see below), and the left kidney perfused via the left cardiac ventricle at a pressure of ∼120 mmHg with 1X phosphate buffered saline (pH 7.4, Mediatech, Manassas, VA) followed by 4% paraformaldehyde in phosphate buffered saline (Affymetrix, Santa Clara, CA) and processed for kidney sectioning (see below) (13).
Measurement of GFR in conscious mice.
Glomerular filtration rate (GFR) measurements were performed by determining the plasma kinetics of the GFR marker FITC-Sinistrin (Fresenius-Kabi, Linz, Austria) following a bolus intravenous injection as previously described (48). Briefly, FITC-Sinistrin (2% in 0.85% NaCl, which was also used to establish a standard curve) was injected into the retro-orbital plexus (2 μl/g body wt) during brief isoflurane anesthesia. At 3, 5, 7, 10, 15, 35, 56, and 75 min after injection, blood was collected from the tail vein into a Na+-heparinized 10-μl microcap (Hirschmann Laborgeräte, Eberstadt, Germany). After centrifugation, plasma was diluted 1:10 in 0.5 mol/liter HEPES (pH 7.4) and fluorescence determined with a Nanodrop ND-3300 fluorospectrometer (Thermo Scientific, Wilmington, DE) by pipetting 2 μl of samples onto the pedestal. GFR was calculated by a two-compartment model of two-phase exponential decay (GraphPad Prism, San Diego, CA).
Urine analysis and blood analysis.
Sodium and potassium concentrations were analyzed by flame photometry (Cole-Parmer, Vernon Hills, IL). Chloride (Pointe Scientific, Canton, MI), calcium, glucose, and magnesium (Thermo Fisher Scientific, Middletown, VA) were determined photometrically by commercial assays. Urine osmolality was measured by vapor pressure (Vapro, Wescor, Salt Lake City, UT). Urinary pH was determined with a pH electrode (9810BN, Thermo Fisher Scientific). Blood chemistry was determined by an OPTI CCA blood gas analyzer with a B60 type cassette (OPTIMedical, Roswell, GA).
Immunohistochemistry.
All procedures have been previously described in detail (42). Primary antibodies used were against NHE3 (25), Npt2a (19), CLC-K (AB5392, EMD Millipore, Billerica, MA), and NBCe1 (39). Labeling was visualized by use of a peroxidase-conjugated secondary antibody (P448, Dako, Glostrup, Denmark) and visualized with 0.05% 3,3′-diaminobenzidine tetrachloride (Kemen Tek, Copenhagen, Denmark). Microscopy was performed with a Leica DMRE light microscope equipped with a digital camera (Leica Microsystems, Wetzlar, Germany). All microscope and camera settings were identical when obtaining images from different genotypes and experimental conditions.
Immunofluorescent labeling of kidney sections, confocal laser scanning microscopy, and image quantification.
Tissue preparation, sectioning, and labeling were performed as previously described (53). Goat anti-rabbit Alexa 488 conjugated secondary antibodies (Invitrogen, Carlsbad, CA) were used for visualization of labeling. Sections were mounted with glycerol-based mounting medium containing antifade reagent (Dako, Carpinteria, CA). A Leica TCS SL confocal microscope with an HCX PL APO 63× oil objective lens (numerical aperture: 1.40) was used for imaging of labeled sections (Leica Microsystems). The procedure for semiquantitative imaging and semiquantitative protein abundance, in addition to the assessment of apical-to-basolateral pixel intensity has been described in detail previously (4, 53).
Immunoblot analysis.
Renal tissue was homogenized in buffer containing protease inhibitor cocktail (250 mmol/liter sucrose, 10 mmol/liter triethanolamine, Sigma-Aldrich and Roche Applied Science, respectively, Indianapolis, IN) and Halt phosphatase inhibitor cocktail (Thermo Scientific). The homogenate was centrifuged at 4,000 g for 15 min. Pellets were resuspended and used for Western blotting. Equal lane loading (total NHE3: 4 μg, pS552 NHE3: 4 μg, pS605 NHE3: 10 μg) was achieved by a Bio-Rad DC Protein assay (Bio-Rad Laboratories, Richmond, CA) (50). Samples were resolved on 4 to 12% NuPAGE gels in MOPS buffer (Invitrogen). Gel proteins were transferred to nitrocellulose membranes and immunoblotted with antibodies against NHE3 (dilution 1:1,000; Millipore), pS552 NHE3 (1:1,000; Novus Biologicals, Littleton, CO) and pS605 NHE3 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA). Chemiluminescent detection was performed with enhanced chemiluminescence (Amersham, Piscataway, NJ). Densitometric analysis was performed with ImageJ Software v1.46 (National Institutes of Health, Bethesda, MD). β-Actin was used as a loading control (dilution 1:5,000; Sigma-Aldrich).
Statistical analysis.
Data are expressed as means ± SE. Unpaired and paired Student's t-test or Mann-Whitney U-test were performed as appropriate to analyze for statistical differences between groups. For comparison of pixel distribution using semiquantitative immunofluorescent labeling, two-way ANOVA was used to test whether the curves (association) were significantly different between the experimental conditions. Significance was considered at P < 0.05.
RESULTS
Renal caffeine effects occur without changes in NHE3 localization, phosphorylation, or abundance.
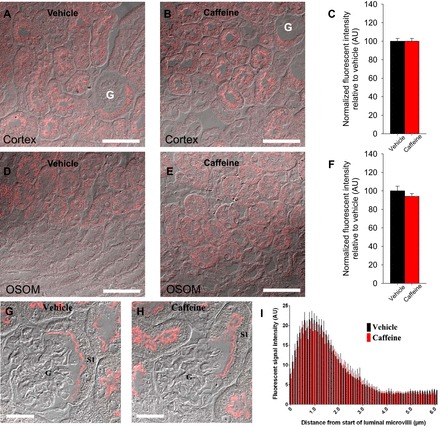
Our initial hypothesis centered on a role for NHE3 in mediating caffeine-induced diuresis and natriuresis. We thus examined NHE3 abundance and localization in mice kidneys following acute caffeine administration. NHE3 staining was observed in the S1, S2, and S3 segment of the proximal tubule 1 h after vehicle application (Fig. 1). Semiquantitative analysis indicated that in the S1 and S3 segments, the majority of NHE3 resides in the apical brush border, distributed approximately over the first 1–2 μm of the microvilli. Caffeine treatment did not change the labeling intensity or the distribution of NHE3 (Fig. 1). Concordantly, caffeine treatment did not significantly affect the total abundance of NHE3 or the phosphorylation of NHE3 at inhibitory sites S552 and S605 (29, 72) in renal membranes (Fig. 2).
Fig. 1.
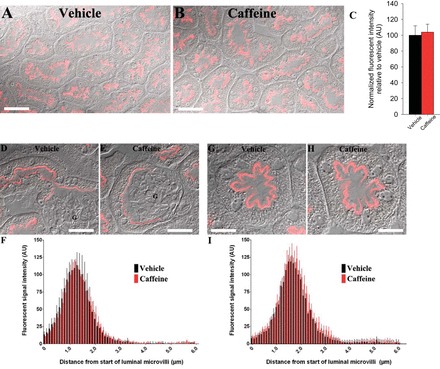
Effect of caffeine on Na+/H+ exchanger isoform 3 (NHE3) localization and abundance. Representative confocal microscopy images of renal cortical NHE3 expression in kidney sections from vehicle-treated (A) or caffeine-treated (B) mice. Total NHE3 fluorescent intensity was not significantly different between the conditions (C). D–H: representative differential interference contrast overlayed quantitative laser scanning microscopy images of NHE3 labeling in S1 (D–F) or S3 (G–I) segments of the kidney following vehicle or caffeine administration. F and I: mean fluorescent signal intensity of NHE3 labeling measured from the apical brush border was not significantly different between vehicle and caffeine-treated mice. Data is an average from a minimum of 6 cells/mouse. G, glomerulus; n = 6/condition. Scale bar = 50 μm (A and B), 25 μm (D and E), and 15 μm (G and H).
Fig. 2.
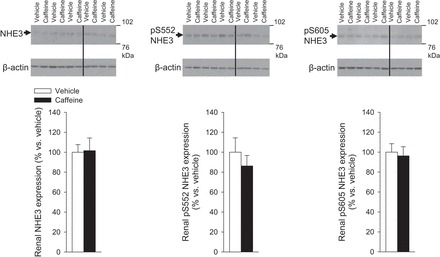
Caffeine-induced diuretic and natriuretic response is independent of NHE3 abundance and phosphorylation at serine 552 (pS552) or serine 605 (pS605) in renal membranes. Kidneys of control (NHE3loxlox) mice were harvested 1 h after application of vehicle (water, 1% of body wt) or caffeine (45 mg/kg body wt by oral gavage). Data are expressed relative to the mean of the vehicle-treated control group, which was set as 100%. Black lines indicate where the original blots were spliced. n = 5/group.
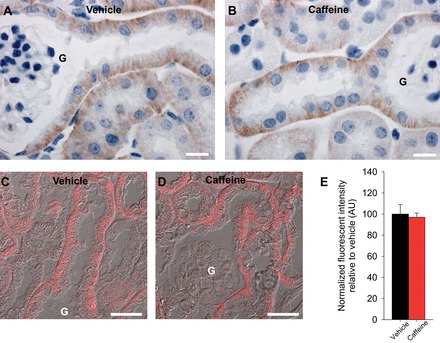
Confirmation of NHE3 knockout in kidney tubules.
As, for example, an effect of caffeine on NHE3 could be via direct inhibition of the transporter rather than abundance and/or localization changes, we aimed to develop a tubulus-specific NHE3 knockout mouse. Qualitative immunohistochemistry in control mice identified NHE3 in the S1-3 segments of the proximal tubule as well as the thick ascending limb (Fig. 3A). This labeling was greatly diminished in NHE3loxloxCre mice. Semiquantitative immunofluorescence labeling and confocal microscopy confirmed the absence (>98%) of cortical NHE3 staining in NHE3loxloxCre mice (Fig. 3, B and D). Analysis of labeling of NHE3 in the thick ascending limb in the region of the inner stripe of outer medulla (Fig. 3, C and D) demonstrated severely reduced NHE3 (∼85% reduction) abundance in NHE3loxloxCre mice compared with control mice. Urinary pH in spontaneously collected urine was significantly more alkaline from NHE3loxloxCre mice compared with control mice (7.7 ± 0.1 vs. 7.0 ± 0.1, P < 0.05); however, there were no differences in blood pH (control: 7.33 ± 0.02; NHE3loxloxCre: 7.34 ± 0.02, not significant [NS]) or blood bicarbonate (control: 26.5 ± 1.2; NHE3loxloxCre: 25.2 ± 0.9 mmol/liter, NS) between genotypes. Urine osmolality was comparable between genotypes (control: 2,292 ± 74; NHE3loxloxCre: 2,219 ± 95 mmol/kg, NS).
Fig. 3.
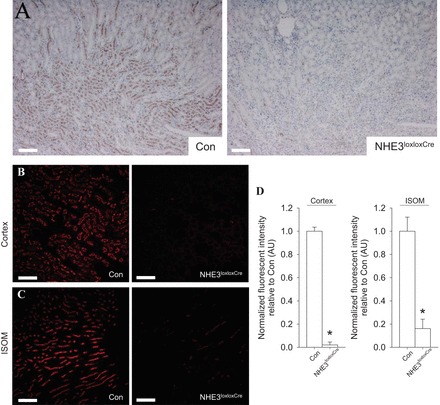
Knockout of NHE3 in renal tubules. A: representative images of diaminobenzidine-stained kidneys shows NHE3 in the cortex and medulla of NHE3loxlox mice (Con). In contrast, the NHE3 staining is almost completely absent in tubulus-specific NHE3 knockout mice (NHE3loxloxCre). B: representative confocal microscopy images of renal cortical NHE3 expression in kidney sections from Con and NHE3loxloxCre mice. C: representative confocal microscopy images of NHE3 in inner stripe of outer medulla (ISOM) sections from Con and NHE3loxloxCre mice. NHE3 in Con mice is consistent with thick ascending limb localization, with labeling significantly reduced in NHE3loxloxCre mice. D: quantification of NHE3 labeling intensity (mean fluorescent signal) shows that total NHE3 labeling intensity in the cortex is almost completely absent and severely reduced (<85%) in the ISOM of NHE3loxloxCre compared with Con mice. n = 5/genotype; *P < 0.05 vs. Con. Scale bar = 200 μm (A) and 100 μm (B and C).
Caffeine self-administration increases total fluid and food intake in both genotypes.
Baseline consumption of fluid (control: 6.2 ± 0.3; NHE3loxloxCre: 6.2 ± 0.3 ml/day, NS) and food (control: 4.6 ± 0.2; NHE3loxloxCre: 4.2 ± 0.2 g/day, NS) was not significantly different between genotypes. To study the diuretic effects of caffeine, mice were subjected to caffeine self-administration via a two-bottle choice test and given a choice between caffeinated solution (0.3 g/l) and water (Fig. 4). Total fluid and food intake increased comparably in control and NHE3loxloxCre mice by ∼30% and ∼13%, respectively. The mean consumption of water and caffeinated solution was not significantly different between control and NHE3loxloxCre mice, and no significant change in body weight was observed, indicating that mice were in fluid balance. To exclude that a lower GFR, a finding previously reported in conventional NHE3 knockout mice (6, 34), was masking the renal caffeine effects, we measured GFR in conscious mice. Control and NHE3loxloxCre mice had a comparable baseline GFR (524 ± 34 vs. 586 ± 36 μl/min, NS), supporting the idea that NHE3loxloxCre mice have intact fluid homeostasis.
Fig. 4.
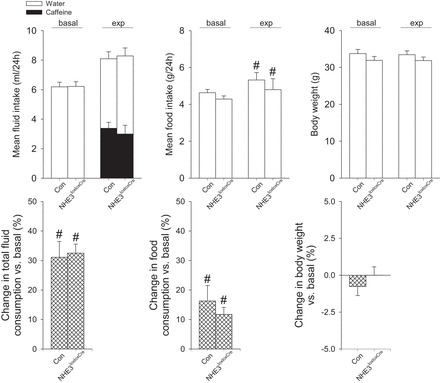
Mean intake of water and caffeinated solution in NHE3loxlox (Con) and NHE3loxloxCre mice under basal conditions with access to water alone (basal) and over a 2-wk experimental period (exp) with choice of water or caffeinated solution (0.3 g/liter). Total fluid and food intake increased in both genotypes during the experimental period; however, no difference in the consumption of caffeinated solution, fluid, or food were observed, and the changes in total fluid consumption, food intake, and body weight were comparable between genotypes. n = 12/genotype; #P < 0.05 vs. basal same genotype.
Acute caffeine effects in conscious animals.
As caffeine self-administration did not identify differences in diuresis, we hypothesized that we might unmask the contribution of NHE3 by acute oral application of caffeine. Application of vehicle did not show significant differences in any of the measured absolute renal excretions between control and NHE3loxloxCre mice except for a significantly higher urinary pH in NHE3loxloxCre mice (Fig. 5), which may be a consequence of impaired proximal tubule bicarbonate reabsorption (33, 56). In control mice, caffeine significantly increased absolute urinary excretion of fluid, Na+, Cl−, K+, glucose, Ca2+, and Mg2+, represented by an increased total osmolar excretion. NHE3loxloxCre mice showed a comparable renal response to caffeine; however, urinary K+ excretion was significantly higher, while urinary Ca2+ excretion was significantly lower compared with caffeine-treated control mice. Caffeine treatment did not affect urinary pH in either genotype.
Fig. 5.
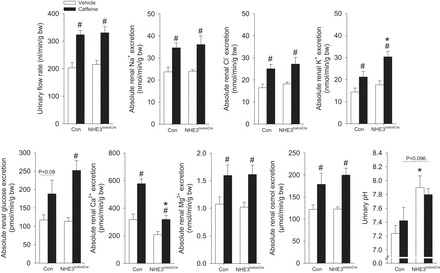
Urinary flow rate and urinary excretions of Na+, K+, Cl−, glucose, Ca2+, Mg2+, total osmoles, and urinary pH in metabolic cage experiments of vehicle-treated (sterile water, 3% of body wt by oral gavage) or caffeine-treated (45 mg/kg body wt) NHE3loxlox (Con) and NHE3loxloxCre mice. n = 12/genotype; *P < 0.05 vs. Con;+ #P < 0.05 vs. vehicle same genotype.
Renal caffeine effects occur without changes in Npt2a abundance or localization.
Another possible mechanism explaining how caffeine could cause diuresis and natriuresis relates to the observation that adenosine stimulates phosphate transport in opossum kidney epithelial cells (10). In this study (Fig. 6), and as described previously (13), Npt2a was detected throughout the proximal tubule, with greater abundance in the early (S1) segment. Vehicle application identified that the majority of Npt2a resides in the apical brush border membrane, distributed approximately over the first 3 μm of the microvilli. Caffeine treatment did not change the labeling intensity or the distribution of Npt2a (Fig. 6).
Fig. 6.
Effect of caffeine on Npt2a localization and abundance. Representative confocal microscopy images of renal Npt2a abundance in kidney sections from vehicle- or caffeine-treated control (NHE3loxlox) mice in cortex (A and B) or outer stripe outer medulla (OSOM) (D and E). Total Npt2a fluorescent intensity was not significantly different between the conditions (C and F). G and H: representative differential interference contrast overlayed quantitative laser scanning microscopy images of Npt2a labeling in S1 segments of the kidney following vehicle or caffeine administration. I: mean fluorescent signal intensity of Npt2a labeling measured from the apical brush border was not significantly different between vehicle- and caffeine-treated mice. Data is an average of a minimum of 14 cells/mouse. G, glomerulus; n = 6/condition. Scale bar = 50 μm (A, B, D, and E) and 25 μm (G and H).
DIDS pretreatment inhibits caffeine-induced diuresis and natriuresis.
Our previous micropuncture studies suggested a role for NBCe1 in mediating the acute diuretic effect in response to selective adenosine A1 receptor blockade (40). To further examine a potential role of NBCe1 for mediating the caffeine effects, we randomized control mice to treatment with DIDS [nonselective NBCe1 inhibitor (23, 35)] or vehicle 30 min prior to application of caffeine. In contrast to vehicle plus caffeine administration, DIDS plus caffeine showed a significantly lower urinary flow rate, alongside lower urinary Na+ and Cl− excretion (Fig. 7). In contrast, absolute urinary K+, glucose, Ca2+, Mg2+, and total osmolar excretion were not different between caffeine-treated control mice with pretreatment of DIDS or vehicle.
Fig. 7.
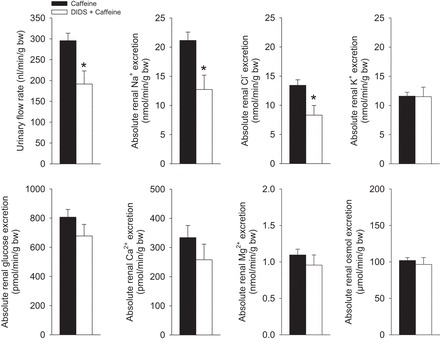
Pretreament with DIDS impairs caffeine-induced diuresis and natriuresis. For comparison of vehicle effects see Fig. 4. Control (NHE3loxlox) mice respond to application of DIDS (40 mg/kg ip) + caffeine (45 mg/kg, 3% of body wt via oral gavage) with a significantly lower urinary flow rate and urinary Na+ and Cl− excretion compared with vehicle (0.85% NaCl solution, 2 μl/g body wt) + caffeine. n = 12/condition. *P < 0.05 vs. vehicle + caffeine.
DIDS effects are independent of NBCe1 abundance or trafficking.
We examined if the reduced diuresis and natriuresis observed with DIDS was caused by caffeine affecting NBCe1 abundance or trafficking. NBCe1 staining was observed in the basolateral membrane of the S1 and early S2 segments of the proximal tubule 1 h after vehicle application. Caffeine treatment did not change the labeling intensity or the distribution of NBCe1 (Fig. 8).
Fig. 8.
In the mouse kidney NBCe1 is localized to the basolateral membrane of S1 and early S2 segments of the proximal tubule. Representative images of diaminobenzidine-stained kidneys shows basolateral NBCe1 abundance in the S1 and early S2 segment of the proximal tubule (A), and caffeine application did not clearly change this staining pattern (B). C and D: representative differential interference contrast overlayed quantitative laser scanning microscopy images of NBCe1 in S1 proximal tubule cells. Mean fluorescent signal intensity of NBCe1 labeling was not significantly different between vehicle- and caffeine-treated mice. G, glomerulus; n = 6/condition. Scale bar = 10 μm (A and B) and 25 μm (C and D).
Renal caffeine effects occur without changes in CLC abundance or localization.
As DIDS has also been shown to affect CLCs (46), we examined the effects of caffeine on CLC-K2 abundance and trafficking. However, immunohistochemistry (technical issues limited analysis by confocal microscopy) did not show any clear differences between vehicle or caffeine administered mice (Fig. 9).
Fig. 9.
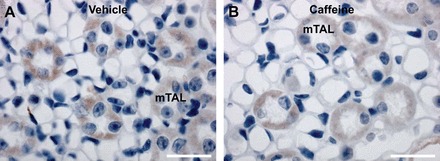
In the mouse kidney ClC-K2 is localized to the medullary thick ascending limb. Representative images of diaminobenzidine-stained kidneys show ClC-K2 in the thick ascending limb (A), and caffeine application did not clearly alter this staining pattern (B). mTAL, medullary thick ascending limb; n = 6/condition. Scale bar = 25 μm.
DISCUSSION
Caffeine is a widely consumed substance, but induces renal effects including diuresis and natriuresis. The mechanisms behind these effects were previously unknown. We have reported that adenosine A1 receptors mediate the diuretic and natriuretic effects of the methylxanthines caffeine and theophylline (52); however, the involved transport protein(s) have so far not been identified. In the current study we generated renal tubulus-specific NHE3 knockout mice and demonstrated that NHE3 is not required for the diuretic and natriuretic effects of caffeine. Additional experiments excluded the Na+/phosphate cotransporter Npt2a being responsible for the caffeine-induced effects. In contrast, DIDS (unspecific Na+/bicarbonate cotransporter NBCe1 blocker) was found to block caffeine-induced diuresis and natriuresis, although this effect was independent of significant changes in NBCe1 abundance or distribution.
Our initial studies of the renal effects of caffeine centered on the role of NHE3. Previous studies have demonstrated that NHE3 is regulated via adenosine A1 receptors (11, 12), and adenosine A1 receptor knockout mice lack caffeine-induced diuresis and natriuresis (52), suggesting that NHE3 may be responsible for the caffeine effects. One mechanism for alteration of NHE3 function is via redistribution. For example, dopamine acutely decreases apical membrane NHE3 abundance in the mouse proximal tubule within 1 h (2), or a 20 min elevation in blood pressure causes redistribution of NHE3 from the brush border membrane to the base of the microvilli (71). Thus we hypothesized that caffeine-induced redistribution of NHE3 from the brush border membrane, resulting in diuresis and natriuresis. In contrast to our hypothesis, we found that caffeine did not significantly affect abundance or localization of NHE3 in the S1-3 segments of the proximal tubule. Additionally, changes in phosphorylation of NHE3 at S552 and S605, which were shown to accompany diuresis and natriuresis following activation of glucagon-like peptide 1 receptors (50) or activation of the cAMP/PKA signaling pathways by uroguanylin (32), were not observed following caffeine treatment.
To completely rule out a role of NHE3 for mediating caffeine effects, a tubulus-specific NHE3 knockout mouse was generated by interbreeding NHE3loxlox mice with Pax8-Cre mice. In contrast to the proximal tubule S1-2 segment-specific NHE3 knockout mouse (33) generated by interbreeding with a Sglt2-Cre mouse, the current model had a complete absence of NHE3 in the S1-3 segments of the proximal tubule. In addition, no NHE3 expression was detected in the cortical thick ascending limb, and a severely reduced NHE3 expression was detected in the medullary portions of the thick ascending limb. Baseline physiological parameters were unremarkable in NHE3loxloxCre mice except for a more alkaline urinary pH. The more alkaline urinary pH was also evident in our metabolic cage experiments after vehicle application. In contrast to conventional whole-body NHE3 knockout mice, a lower GFR (not present in NHE3loxloxCre mice; see below) and therefore a reduced filtered bicarbonate load is not a compensating mechanism. The more alkaline urinary pH in NHE3loxloxCre mice might relate to the 50–60% inhibited bicarbonate reabsorption seen in conventional NHE3 knockout mice, which show only a mild metabolic acidosis (56, 65). Of note, the metabolic acidosis is not a consistent finding in conventional NHE3 knockout mice (44). Our NHE3loxloxCre mice show comparable blood pH and bicarbonate concentrations as well as food intake relative to control mice, while excreting a more alkaline urine, suggesting a homeostatic mechanism can be activated in the absence of tubular NHE3 to keep blood pH constant. The precise mechanism(s) and the organ(s) involved remain to be determined. Taken together, our novel NHE3loxloxCre mouse model challenges the commonly accepted role of NHE3 for fluid and pH homeostasis. The current results warrant further studies to determine the overall role of NHE3 in the kidney for salt homeostasis and blood pressure regulation. Possible compensatory changes in the expression and/or localization of other transport proteins and/or channels are also warranted.
In metabolic cage experiments, caffeine self-administration resulted in an identical diuretic response between tubulus-specific NHE3 knockout mice and control mice. However, acute caffeine administration resulted in a higher kaliuresis and lower Ca2+ excretion in NHE3loxloxCre mice relative to control mice. The reasons for these altered responses remain to be determined. We speculate that they could relate to changes in salt transport systems downstream of the proximal tubule and further examination of these various pathways would be informative. As the data provided substantial evidence that NHE3 was not directly responsible for mediating the renal effects of caffeine, we examined alternative mechanisms. Na+-dependent phosphate transport in human kidney cells is stimulated by adenosine A1 receptor agonists (60). Furthermore, KW3902 and 8-Cyclopentyl-1,3-dipropylxanthine (both selective adenosine A1 receptor antagonists) inhibit Na+-dependent phosphate uptake in cultured rat proximal tubule cells via stimulation of cAMP (7, 8). We hypothesized that redistribution of Npt2a from the proximal tubule brush border membrane (13, 22) would occur following caffeine administration. In vehicle-treated control mice, Npt2a was detected in the proximal tubule with a similar distribution as described previously (13), with the majority of Npt2a residing in the first 3 μm of the microvilli. Unlike the clear effect of PTH on Npt2a (within a similar time frame) (13), caffeine administration had no significant effect on Npt2a distribution or abundance.
Our previous micropuncture experiments employing KW3902 indicated that Na+/bicarbonate transporters may be responsible for KW3902-induced diuresis and natriuresis (40). NBCe1 accounts for the reabsorption of ∼80% of filtered bicarbonate (30) and, like NHE3 and Npt2a, is regulated via the protein kinase A/cAMP pathway (20, 21). Therefore, NBCe1 was an alternative molecular player in the caffeine-induced effects. We detected NBCe1 in the basolateral membrane of cells in the S1 and early S2 segment of the mouse proximal tubule, an expression pattern comparable to that described in the rat (39, 55). Furthermore, treatment of mice with DIDS (an unspecific NBCe1 blocker) clearly inhibited caffeine-induced diuresis and natriuresis. However, we detected no clear effect of caffeine on either NBCe1 abundance or distribution, suggesting that the caffeine-induced diuresis and natriuresis might be an inhibitory effect on NBCe1 transport activity rather than NBCe1 localization. Of note, mutated NBCe1 (G486R) had normal membrane abundance while showing impaired (∼50% reduced) transport activity and, as a consequence, caused renal proximal tubular acidosis (58). Why would DIDS decrease caffeine-induced diuresis and natriuresis? In theory, if DIDS targeted the same transport protein(s) as caffeine, one would expect to see an identical or even increased renal response to caffeine. However, DIDS clearly inhibited caffeine-induced diuresis and natriuresis without affecting kaliuresis. At this stage we can only postulate reasons for these responses: 1) caffeine has effects on transport protein(s) other than NBCe1 which, as a prerequisite, require NBCe1 to be functional for maximal caffeine-induced diuresis and natriuresis; 2) ATP and/or UTP acting via P2 receptors inhibit renal salt and water reabsorption (49); as DIDS can act as a P2 receptor antagonist (47, 69), DIDS may increase salt and water reabsorption in distal nephron segments thereby antagonizing the renal caffeine effects, 3) caffeine's effect via a membrane bound receptor is inhibited by DIDS; and 4) in vivo application of DIDS alters, for example, vasopressin secretion which counteracts the caffeine effects.
Another point of discussion from our studies is related to GFR effects following NHE3 gene deletion. Conventional NHE3 knockout mice and NHE3 knockout mice with transgenic expression of NHE3 in the small intestine show a 20–30% lower whole kidney GFR compared with control mice (6, 70). In addition, single nephron GFR is decreased in conventional NHE3 knockout mice, an effect attributable to activation of tubuloglomerular feedback (TGF) (34). Supporting a previous study that provided indirect evidence that kidney function, determined via plasma creatinine measurements, was unaltered in mice with knockout of NHE3 in the S1-2 segment of the proximal tubule (Sglt2-Cre) (33), we found a comparable GFR between conscious control and NHE3loxloxCre mice. Of note, blocking NHE3 by S3226 (a potent inhibitor) during in vivo microperfusion did not significantly alter the TGF response measured by the fall in early proximal tubular stop flow pressure when the loop of Henle perfusion rate was increased (63). Taken together, this possibly indicates that expression of NHE3 outside the kidney tubule and/or other factors contribute to the GFR differences observed in other NHE3 knockout models. Similarly, considering the overall mild phenotype of different kidney-specific NHE3 knockout mouse models in respect to acid base and fluid homeostasis, the role of NHE3 in proximal tubule may need to be reconsidered, including the role of NHE3 for altering the set point for Na+-fluid volume homeostasis.
Our study is not without limitations. To our knowledge, the only available tool to block Na+/bicarbonate transporters is DIDS, which also inhibits other transporters and channels, including Cl−/bicarbonate transporters (45) or Cl− channels (ClC-K1 and/or 2) (1). However, blockade of Cl− channels would be predicted to result in nephrogenic diabetes insipidus (38), and the addition of caffeine should theoretically exacerbate its diuretic and natriuretic action. In addition, our studies did not show an effect of caffeine on ClC-K2 redistribution. The development of novel selective antagonists or floxed NBCe1 mice [conventional NBCe1 knockout mice die before weaning (18)] will help to test for a role of caffeine and selective adenosine A1 receptor antagonism for the diuretic and natriuretic effect. Methylxanthines are also nonselective adenosine receptor antagonists and these studies were not designed to confirm that they are proximal diuretics, a finding previously concluded from lithium clearance studies (57, 62). Similarly, it was recommended that caffeine-containing products be avoided in all studies of lithium clearance (61), and in the light of these findings these rules have been followed in hundreds of human studies. Finally, since adenosine receptors are expressed along the entire nephron (64), we cannot exclude that other transport proteins downstream of the proximal tubule are involved in the caffeine-induced diuresis and natriuresis.
GRANTS
This work was supported by a Bastyr University Faculty Research Seed Grant (to J.A. Dominguez Rieg), O'Brien Center for Acute Kidney Injury Research Grant P30DK079337, Diabetes Endocrinology Research Center P30DK063491 (to T. Rieg), American Heart Association 15BGIA22410018 (to T. Rieg), Satellite Healthcare, a not-for-profit renal care provider (to T. Rieg), UCSD Academic Senate (to T. Rieg), Boehringer Ingelheim (to M. Busslinger), Department of Veterans Affairs Merit Review Award (to M. Soleimani), and Center on Genetics of Transport and Epithelial Biology at University of Cincinnati. Further funding (to R.A. Fenton) is provided by the Lundbeck Foundation, the Danish Medical Research Council, and the Novo Nordisk Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.A.F. and T.R. conception and design of research; R.A.F., S.d.l.M.C., J.A.D.R., and T.R. performed experiments; R.A.F., S.B.P., S.d.l.M.C., J.A.D.R., and T.R. analyzed data; R.A.F., S.B.P., J.A.D.R., and T.R. interpreted results of experiments; R.A.F. and T.R. prepared figures; R.A.F. and T.R. drafted manuscript; R.A.F., S.B.P., S.d.l.M.C., M.S., M.B., J.A.D.R., and T.R. edited and revised manuscript; R.A.F., S.B.P., S.d.l.M.C., M.S., M.B., J.A.D.R., and T.R. approved final version of manuscript.
REFERENCES
- 1.Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ, CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: ion channels. Br J Pharmacol 170: 1607–1651, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacic D, Kaissling B, McLeroy P, Zou L, Baum M, Moe OW. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int 64: 2133–2141, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benowitz NL. Clinical pharmacology of caffeine. Annu Rev Med 41: 277–288, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Bohn AB, Norregaard R, Stodkilde L, Wang Y, Bertelsen LB, Fenton RA, Matchkov VV, Bouzinova EV, Horsman MR, Frokiaer J, Stodkilde-Jorgensen H. Treatment with the vascular disrupting agent combretastatin is associated with impaired AQP2 trafficking and increased urine output. Am J Physiol Regul Integr Comp Physiol 303: R186–R198, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard M, Souabni A, Busslinger M. Tissue-specific expression of cre recombinase from the Pax8 locus. Genesis 38: 105–109, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Brooks HL, Sorensen AM, Terris J, Schultheis PJ, Lorenz JN, Shull GE, Knepper MA. Profiling of renal tubule Na+ transporter abundances in NHE3 and NCC null mice using targeted proteomics. J Physiol 530: 359–366, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai H, Batuman V, Puschett DB, Puschett JB. Effect of KW-3902, a novel adenosine A1 receptor antagonist, on sodium-dependent phosphate and glucose transport by the rat renal proximal tubular cell. Life Sci 55: 839–845, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Cai H, Puschett DB, Guan S, Batuman V, Puschett JB. Phosphate transport inhibition by KW-3902, an adenosine A1 receptor antagonist, is mediated by cyclic adenosine monophosphate. Am J Kidney Dis 26: 825–830, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Chavez-Valdez R, Wills-Karp M, Ahlawat R, Cristofalo EA, Nathan A, Gauda EB. Caffeine modulates TNF-alpha production by cord blood monocytes: the role of adenosine receptors. Pediatr Res 65: 203–208, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Coulson R, Johnson RA, Olsson RA, Cooper DR, Scheinman SJ. Adenosine stimulates phosphate and glucose transport in opossum kidney epithelial cells. Am J Physiol Renal Fluid Electrolyte Physiol 260: F921–F928, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Di Sole F, Casavola V, Mastroberardino L, Verrey F, Moe OW, Burckhardt G, Murer H, Helmle-Kolb C. Adenosine inhibits the transfected Na+-H+ exchanger NHE3 in Xenopus laevis renal epithelial cells (A6/C1). J Physiol 515 (Pt 3): 829–842, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Sole F, Cerull R, Petzke S, Casavola V, Burckhardt G, Helmle-Kolb C. Bimodal acute effects of A1 adenosine receptor activation on Na+/H+ exchanger 3 in opossum kidney cells. J Am Soc Nephrol 14: 1720–1730, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Fenton RA, Murray F, Dominguez Rieg JA, Tang T, Levi M, Rieg T. Renal phosphate wasting in the absence of adenylyl cyclase 6. J Am Soc Nephrol 25: 2822–2834, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredholm BB. Theophylline actions on adenosine receptors. Eur J Respir Dis 109: 29–36, 1980. [PubMed] [Google Scholar]
- 15.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev 63: 1–34, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51: 83–133, 1999. [PubMed] [Google Scholar]
- 17.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol 61: 443–448, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3− cotransporter. J Biol Chem 282: 9042–9052, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Giral H, Lanzano L, Caldas Y, Blaine J, Verlander JW, Lei T, Gratton E, Levi M. Role of PDZK1 protein in apical membrane expression of renal sodium-coupled phosphate transporters. J Biol Chem 286: 15032–15042, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross E, Fedotoff O, Pushkin A, Abuladze N, Newman D, Kurtz I. Phosphorylation-induced modulation of pNBC1 function: distinct roles for the amino- and carboxy-termini. J Physiol 549: 673–682, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross E, Hawkins K, Abuladze N, Pushkin A, Cotton CU, Hopfer U, Kurtz I. The stoichiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. J Physiol 531: 597–603, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo J, Song L, Liu M, Segawa H, Miyamoto K, Bringhurst FR, Kronenberg HM, Juppner H. Activation of a non-cAMP/PKA signaling pathway downstream of the PTH/PTHrP receptor is essential for a sustained hypophosphatemic response to PTH infusion in male mice. Endocrinology 154: 1680–1689, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heyer M, Muller-Berger S, Romero MF, Boron WF, Fromter E. Stoichiometry of the rat kidney Na+-HCO3− cotransporter expressed in Xenopus laevis oocytes. Pflügers Arch 438: 322–329, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Kahn AM, Dolson GM, Hise MK, Bennett SC, Weinman EJ. Parathyroid hormone and dibutyryl cAMP inhibit Na+/H+ exchange in renal brush border vesicles. Am J Physiol Renal Fluid Electrolyte Physiol 248: F212–F218, 1985. [DOI] [PubMed] [Google Scholar]
- 25.Kim GH, Ecelbarger C, Knepper MA, Packer RK. Regulation of thick ascending limb ion transporter abundance in response to altered acid/base intake. J Am Soc Nephrol 10: 935–942, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Knight RJ, Bowmer CJ, Yates MS. The diuretic action of 8-cyclopentyl-1,3-dipropylxanthine, a selective A1 adenosine receptor antagonist. Br J Pharmacol 109: 271–277, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kost CK Jr, Herzer WA, Rominski BR, Mi Z, Jackson EK. Diuretic response to adenosine A(1) receptor blockade in normotensive and spontaneously hypertensive rats: role of pertussis toxin-sensitive G-proteins. J Pharmacol Exp Ther 292: 752–760, 2000. [PubMed] [Google Scholar]
- 28.Kulick A, Panico C, Gill P, Welch WJ. Low salt intake increases adenosine type 1 receptor expression and function in the rat proximal tubule. Am J Physiol Renal Physiol 295: F37–F41, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurashima K, Yu FH, Cabado AG, Szabo EZ, Grinstein S, Orlowski J. Identification of sites required for down-regulation of Na+/H+ exchanger NHE3 activity by cAMP-dependent protein kinase. phosphorylation-dependent and -independent mechanisms. J Biol Chem 272: 28672–28679, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz I, Zhu Q. Structure, function, and regulation of the SLC4 NBCe1 transporter and its role in causing proximal renal tubular acidosis. Curr Opin Nephrol Hypertens 22: 572–583, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Ha JH, Kim S, Oh Y, Kim SW. Caffeine decreases the expression of Na+/K+-ATPase and the type 3 Na+/H+ exchanger in rat kidney. Clin Exp Pharmacol Physiol 29: 559–563, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Lessa LM, Carraro-Lacroix LR, Crajoinas RO, Bezerra CN, Dariolli R, Girardi AC, Fonteles MC, Malnic G. Mechanisms underlying the inhibitory effects of uroguanylin on NHE3 transport activity in renal proximal tubule. Am J Physiol Renal Physiol 303: F1399–F1408, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Li HC, Du Z, Barone S, Rubera I, McDonough AA, Tauc M, Zahedi K, Wang T, Soleimani M. Proximal tubule specific knockout of the Na(+)/H(+) exchanger NHE3: effects on bicarbonate absorption and ammonium excretion. J Mol Med 91: 951–963, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol Renal Physiol 277: F447–F453, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Lu J, Boron WF. Reversible and irreversible interactions of DIDS with the human electrogenic Na/HCO3 cotransporter NBCe1-A: role of lysines in the KKMIK motif of TM5. Am J Physiol Cell Physiol 292: C1787–C1798, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Lyons JC, Ross BD, Song CW. Enhancement of hyperthermia effect in vivo by amiloride and DIDS. Int J Radiat Oncol Biol Phys 25: 95–103, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Mandel HG. Update on caffeine consumption, disposition and action. Food Chem Toxicol 40: 1231–1234, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Matsumura Y, Uchida S, Kondo Y, Miyazaki H, Ko SB, Hayama A, Morimoto T, Liu W, Arisawa M, Sasaki S, Marumo F. Overt nephrogenic diabetes insipidus in mice lacking the CLC-K1 chloride channel. Nat Genet 21: 95–98, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Maunsbach AB, Vorum H, Kwon TH, Nielsen S, Simonsen B, Choi I, Schmitt BM, Boron WF, Aalkjaer C. Immunoelectron microscopic localization of the electrogenic Na/HCO(3) cotransporter in rat and ambystoma kidney. J Am Soc Nephrol 11: 2179–2189, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Miracle CM, Rieg T, Blantz RC, Vallon V, Thomson SC. Combined effects of carbonic anhydrase inhibitor and adenosine A1 receptor antagonist on hemodynamic and tubular function in the kidney. Kidney Blood Press Res 30: 388–399, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizumoto H, Karasawa A. Renal tubular site of action of KW-3902, a novel adenosine A1-receptor antagonist, in anesthetized rats. Jpn J Pharmacol 61: 251–253, 1993. [DOI] [PubMed] [Google Scholar]
- 42.Moeller HB, Knepper MA, Fenton RA. Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int 75: 295–303, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murtazina R, Kovbasnjuk O, Chen TE, Zachos NC, Chen Y, Kocinsky HS, Hogema BM, Seidler U, de Jonge HR, Donowitz M. NHERF2 is necessary for basal activity, second messenger inhibition, and LPA stimulation of NHE3 in mouse distal ileum. Am J Physiol Cell Physiol 301: C126–C136, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan W, Borovac J, Spicer Z, Hoenderop JG, Bindels RJ, Shull GE, Doschak MR, Cordat E, Alexander RT. The epithelial sodium/proton exchanger, NHE3, is necessary for renal and intestinal calcium (re)absorption. Am J Physiol Renal Physiol 302: F943–F956, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93: 803–959, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picollo A, Liantonio A, Didonna MP, Elia L, Camerino DC, Pusch M. Molecular determinants of differential pore blocking of kidney CLC-K chloride channels. EMBO Rep 5: 584–589, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998. [PubMed] [Google Scholar]
- 48.Rieg T. A High-throughput method for measurement of glomerular filtration rate in conscious mice. J Vis Exp 10: e50330, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Rieg T, Gerasimova M, Murray F, Masuda T, Tang T, Rose M, Drucker DJ, Vallon V. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol 303: F963–F971, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rieg T, Schnermann J, Vallon V. Adenosine A1 receptors determine effects of caffeine on total fluid intake but not caffeine appetite. Eur J Pharmacol 555: 174–177, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Rieg T, Steigele H, Schnermann J, Richter K, Osswald H, Vallon V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J Pharmacol Exp Ther 313: 403–409, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Rieg T, Tang T, Uchida S, Hammond HK, Fenton RA, Vallon V. Adenylyl cyclase 6 enhances NKCC2 expression and mediates vasopressin-induced phosphorylation of NKCC2 and NCC. Am J Pathol 182: 96–106, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riesenhuber A, Boehm M, Posch M, Aufricht C. Diuretic potential of energy drinks. Amino Acids 31: 81–83, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Immunolocalization of the electrogenic Na+-HCO-3 cotransporter in mammalian and amphibian kidney. Am J Physiol Renal Physiol 276: F27–F38, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Shirley DG, Walter SJ, Skinner J, Noormohamed FH. The natriuretic effect of lithium in man: is the proximal tubule involved? Scand J Of Clin Lab Invest 55: 635–642, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki M, Vaisbich MH, Yamada H, Horita S, Li Y, Sekine T, Moriyama N, Igarashi T, Endo Y, Cardoso TP, de Sa LC, Koch VH, Seki G, Fujita T. Functional analysis of a novel missense NBC1 mutation and of other mutations causing proximal renal tubular acidosis. Pflügers Arch 455: 583–593, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Takeda M, Yoshitomi K, Imai M. Regulation of Na(+)-3HCO3- cotransport in rabbit proximal convoluted tubule via adenosine A1 receptor. Am J Physiol Renal Fluid Electrolyte Physiol 265: F511–F519, 1993. [DOI] [PubMed] [Google Scholar]
- 60.Tang Y, Zhou L. Characterization of adenosine A1 receptors in human proximal tubule epithelial (HK-2) cells. Receptors Channels 9: 67–75, 2003. [PubMed] [Google Scholar]
- 61.Thomsen K. Lithium clearance: a new method for determining proximal and distal tubular reabsorption of sodium and water. Nephron 37: 217–223, 1984. [DOI] [PubMed] [Google Scholar]
- 62.Thomsen K, Schou M. Renal lithium excretion in man. Am J Physiol 215: 823–827, 1968. [DOI] [PubMed] [Google Scholar]
- 63.Vallon V, Schwark JR, Richter K, Hropot M. Role of Na(+)/H(+) exchanger NHE3 in nephron function: micropuncture studies with S3226, an inhibitor of NHE3. Am J Physiol Renal Physiol 278: F375–F379, 2000. [DOI] [PubMed] [Google Scholar]
- 64.Vitzthum H, Weiss B, Bachleitner W, Kramer BK, Kurtz A. Gene expression of adenosine receptors along the nephron. Kidney Int 65: 1180–1190, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Wang T, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Giebisch G, Aronson PS. Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol Renal Physiol 277: F298–F302, 1999. [DOI] [PubMed] [Google Scholar]
- 66.Weinman EJ, Shenolikar S, Kahn AM. cAMP-associated inhibition of Na+-H+ exchanger in rabbit kidney brush-border membranes. Am J Physiol Renal Fluid Electrolyte Physiol 252: F19–F25, 1987. [DOI] [PubMed] [Google Scholar]
- 67.Weinman EJ, Steplock D, Shenolikar S. NHERF-1 uniquely transduces the cAMP signals that inhibit sodium-hydrogen exchange in mouse renal apical membranes. FEBS Lett 536: 141–144, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Wilcox CS, Welch WJ, Schreiner GF, Belardinelli L. Natriuretic and diuretic actions of a highly selective adenosine A1 receptor antagonist. J Am Soc Nephrol 10: 714–720, 1999. [DOI] [PubMed] [Google Scholar]
- 69.Wildman SS, Marks J, Turner CM, Yew-Booth L, Peppiatt-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol 19: 731–742, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woo AL, Noonan WT, Schultheis PJ, Neumann JC, Manning PA, Lorenz JN, Shull GE. Renal function in NHE3-deficient mice with transgenic rescue of small intestinal absorptive defect. Am J Physiol Renal Physiol 284: F1190–F1198, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Yip KP, Tse CM, McDonough AA, Marsh DJ. Redistribution of Na+/H+ exchanger isoform NHE3 in proximal tubules induced by acute and chronic hypertension. Am J Physiol Renal Physiol 275: F565–F575, 1998. [DOI] [PubMed] [Google Scholar]
- 72.Zhao H, Wiederkehr MR, Fan L, Collazo RL, Crowder LA, Moe OW. Acute inhibition of Na/H exchanger NHE-3 by cAMP. Role of protein kinase a and NHE-3 phosphoserines 552 and 605. J Biol Chem 274: 3978–3987, 1999. [DOI] [PubMed] [Google Scholar]


