Abstract
Given the ubiquitous use of mice to study lung disease, it is curious that more investigators do not use repeated intubation to study mechanical and cellular changes in individual mice. One of the reasons for this limited use of intubation is that it is relatively difficult, despite there being several published studies that describe ways to achieve it. In this paper, we describe a complete procedure, including novel approaches that simplify this intubation, so that it can be routinely accomplished with relatively little training. The technique can also be set up with relatively little expense and expertise. This should make it possible for any laboratory to routinely carry out this intubation, thereby allowing longitudinal studies in individual mice and potentially increasing the statistical power by using each mouse as its own control.
Keywords: lung mechanics, broncoalveolar lavage, longitudinal studies, trachea
in 1999, our laboratory published a paper describing a method for intubation of the mouse lung (2). Such a technique has considerable utility in doing repeat pulmonary function or bronchoalveolar lavage (BAL) in individual mice in longitudinal studies (12). Since that original paper, there have been several other papers that have described different approaches to mouse intubation (5, 6, 8–11, 13). While all of these methods can be used successfully, they usually require considerable training and suffer from the fact that, as the intubation cannula approaches the tracheal opening, the visualization of that opening becomes obscured by the cannula itself. This makes the insertion of the cannula difficult at the most critical time. We recently developed simple technical improvements that not only eliminate this visualization problem, but also help to localize the cannula in the trachea and minimize air leakage. This new approach enables successful intubation with relatively little training or experience.
METHODS
The novel improvements of this method involve use of a simply constructed wedged intravenous (IV) catheter that incorporates a fiber-optic cable that serves as an introducer for the catheter. We will describe the system we use that works in most strains for 20- to 35-g mice. The method could easily be adaptable to larger or smaller mice, simply by changing the catheter and fiber-optic cable size.
Cannula.
For this size mouse, we use a 1-in.-long, 20-gauge IV catheter (BD Insylte, Sparks, MD or Jelco Optiva, Carlsbad, CA).
Fiber-optic cable.
We use a 2-ft-length of 0.5-mm optical fiber (Communication grade plastic fiber, #NT02-542, Edmund Optics, Barrington, NJ). The edge of the end that is inserted into the trachea is gently rounded by holding the fiber ∼2 cm from the end and then making small circles while dragging the tip for a few seconds on 1,000-grit emory paper (Fig. 1). The other end is inserted through a rubber stopper. This is most easily accomplished by first pushing a 18-gauge needle through the stopper, inserting the optical fiber through the needle bore, and then withdrawing the needle. This rubber stopper is connected to a 150-W halogen light source (e.g., NCL-150, Volpi, or any equivalent other or microscope light source). Since these commercial light sources are not designed for rubber stoppers, it is necessary to find a short length (2 in) of aluminum or brass tubing that fits over the light source's connecting stub (see Fig. 2). The size of this tubing will then dictate the size of the rubber stopper.
Fig. 1.

Rounded tip of 500-μm optical fiber.
Fig. 2.
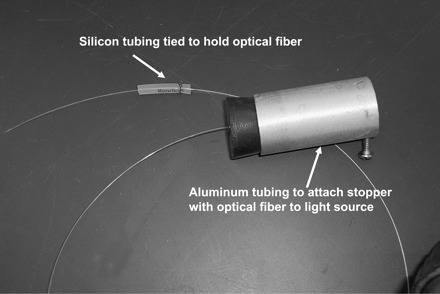
Five hundred-micrometer optical fiber with connection to light source and small piece of silicone tubing tied onto optical cable at other end to hold it in place in the Luer hub.
Cannula wedge.
One of the problems associated with intubation is that it is often very easy to insert the cannula too deep in the trachea, a problem that can result in only one lung being ventilated or to a unilateral drug delivery. To avoid this issue, we have used silicone rubber caulk to fabricate a wedge on the IV catheter at the appropriate location to set the catheter tip approximately midway between the larynx and carina. The catheter is inserted until the wedge meets increasing resistance to further insertion in the oropharynx. This also serves to provide an improved seal to lessen the possibility of air leakage. Another advantage is that, with repeated intubations, the placement of the catheter tip will be at the same point for all measurements.
To add this wedge to the IV cannula, we start by marking a point 15 mm from the catheter tip with a permanent marker. This marks the point where the cone begins and determines the depth at which the catheter tip will lie. Next we use a clear 1,000-μl pipette tip (no. 2079G, Molecular Bio Products, San Diego, CA), and cut the pipette tip with a razor blade just enough to allow entry of the catheter. To determine where to cut the other end of the pipette, it is laid adjacent to the IV catheter, such that the pipette tip is even with the pen mark on the catheter. The wider end of the pipette tip is then cut to a length that reaches the hub of the catheter. After cutting, the fit of this pipette mold is examined by sliding it over the catheter. To simplify removal of the mold, it is then cut with a razor in half along its full length and held closed with tape. To fabricate the cone on the catheter, the mold is first filled with silicone sealant (e.g., clear household silicone #01-30808, Loctite, Avon, OH), with care to avoid large air bubbles. The catheter, with the accompanying needle in place, is then advanced into the wide end of the mold until the Luer hub touches the end of the pipette tip. A small amount of silicone is expressed from both ends, which is easily wiped off. Although this silicone sealant normally cures in <24 h, we have found that, in this configuration with poor access for solvent vaporization, full curing will take from several days to a week. The mold is then removed by peeling the tape and carefully separating the halves of the mold. The completed intubation catheter with optical fiber light source ready for intubation is shown in Fig. 3. The completed catheters are disinfected by soaking overnight in 70% ethanol, which does not result in any deterioration of the catheter or wedge (3). These catheters are relatively simple to make, and with proper cleaning and disinfection, they can be reused many times over extended periods of time.
Fig. 3.
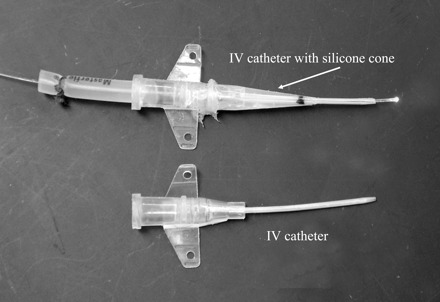
Twenty-gauge BD Insylte intravenous (IV) catheters, one with the fabricated cone and optical fiber in place ready for intubation.
PROCEDURE
Figure 4 shows the cannula and light source ready for intubation. The fiber-optic cable is inserted through the IV catheter until it extends 3–5 mm in front of the tip. To hold this position, it is useful to tie on a short (≈1.5 cm) piece of silicone rubber tubing (0.8 inner diameter × 4 mm outer diameter, Cole-Palmer, EW-96410-13) onto the fiber cable (Fig. 2). The tie should be tight enough to allow the cable to slide with some friction. This rubber tubing fits snuggly inside the Luer fitting of the IV catheter, thereby holding the fiber cable position with respect to the catheter.
Fig. 4.

Cannulation set up with angled support for mouse ready for intubation.
To test the intubation, we studied disease-free male BALB/c mice weighing 27.7 ± 0.40 g initially and 31.1 ± 0.27 g after 4 wk. They were anesthetized with ketamine (100 μg/g body wt) and xylazine (8 μg/g body wt) in saline via intraperitoneal injection. The protocol was approved by the Johns Hopkins Animal Care and Use Committee. The rubber stopper is connected to the light source, and the anesthetized mouse is placed on an adjustable-angled support suspended by its upper incisors, as previously described (2) and shown in Fig. 5. Using a forceps with tubing covering the tips, the tongue is gently pulled out of the mouth and held by the thumb and index finger. The middle finger is placed between the neck and plastic support. Traction on the tongue with the index finger and thumb is used to open the mouth, and to straighten the intubation path, the angle of the head is adjusted with the middle finger behind the neck, as shown in Fig. 5. This intubation procedure is also illustrated in a video taken through a microscope by Hamacher et al. (5). The fiber-optic cable serves as a light source to illuminate the tracheal opening, and when it is observed, it then serves as an introducer and is gently pushed through the vocal cords until the IV catheter begins to wedge in the oropharynx. The mouse oropharynx is quite narrow (1), and this allows the catheter to wedge snugly without damaging the trachea or vocal cords. The fiber cable is then withdrawn, and the IV catheter is then connected to the ventilator. If desired, it can also be secured with a suture to tissue around the mouth.
Fig. 5.
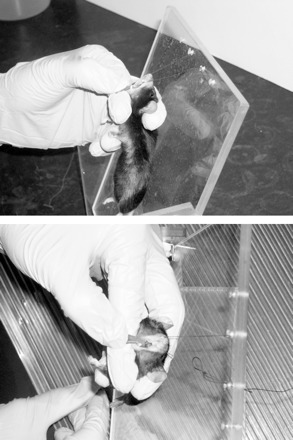
Two photos from different angles showing how to hold the mouse to straighten the intubation pathway for easy insertion of the cannula.
TESTING
As confirmation that the catheter is located in the trachea and not the esophagus or wedged into some soft tissue, an easy way to check this is with a small dental mirror. Chill the mirror in a −20°C freezer and keep cold until use. After placing the catheter, simply place the mirror at the Luer hub of the catheter. If the catheter is successfully placed, the exhaled breath will form a visible condensate on the mirror.
We did preliminary testing to check for leaks and to determine whether repeated intubation had any effects on subsequent measurements. We studied four BALB/c mice, anesthetized and intubated as described above. The mice were then connected to a constant-flow ventilator, with a rate of 2 Hz and tidal volume of 0.2 ml, and respiratory resistance was measured by the inspiratory occlusion method, as previously described (4). Figure 6 shows five weekly measurements in the four mice. Reproducibility is excellent, showing that at least at weekly intervals, there is no effect of the prior measurement. This is consistent with previously reported weekly assessments of mechanics and BAL cell profiles in individual BALB/c mice with a more difficult and potentially traumatic procedure (12).
Fig. 6.
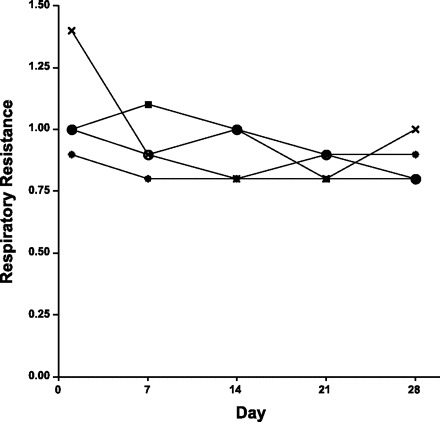
Reproducibility of weekly measurements of resistance in 4 BALB/c mice.
DISCUSSION
The procedure described here has several advantages. First the apparatus is simple and relatively inexpensive. The fabrication of the apparatus does not require any special tools or costly equipment. The use of a catheter introducer that also is the light source means that one never loses sight of the tracheal opening as the introducer approaches the tracheal opening. The use of a 0.5-mm introducer also serves to minimize trauma that might occur with an initial insertion of a larger cannula. We note here that a similar optical probe is available from a commercial vendor (Braintree Scientific, Braintree, MA). This device uses a battery-powered light source and optical fiber, but does not take advantage of the wedged catheter described in our system. Although we tested the procedure with repeat measurement of lung mechanics, such intubation could just as easily be used to instill chemicals or cells into the lung, as was recently described for repeated delivery of LPS (7). In addition, a prior report with a more primitive intubation procedure described the ability to do repeated BAL in individual mice (12), and this would be much more simply accomplished with the new intubation approach.
In practice, the method described here was demonstrated at a lung phenotyping course at the Jackson Laboratories in September 2008. This course had laboratory sessions in the afternoon, and, in one of these laboratories, intubation was attempted in 11-wk-old Balb/cJ mice. Of the 12 students who attempted intubation, all were able to do this successfully, despite the fact that none had ever attempted intubation before this course. In fact, in the one afternoon session, some of the students became sufficiently proficient to then teach some of the other students who had not yet tried it. This method thus has a considerable advantage, as it minimizes the number of mice needed for practice and should allow minimal damage in repeated studies.
In performing the intubation, there are several practical issues that should be mentioned. It is important to be as gentle as possible with the retraction of the tongue in the initial opening of the mouth. If unprotected forceps are used, it is easy to injure the tongue, and this can lead to death of the mouse. In first learning how to do the intubation, the most important thing is the use of the finger behind the neck to adjust the angle of the head to enable visualization of the tracheal opening. When done correctly, the vocal cords can easily be seen. It is this initial visualization step that usually requires the most time, since once the tracheal opening is seen, it is relatively simple to insert the fiber cable and IV catheter. Hamacher et al. described a unique intubation system with microscopic visualization (5). The online video of this intubation is also very instructive, although the means of positioning the head and neck is not entirely clear from this video and figure. While the system they describe seems to work very efficiently, it does require a dedicated microscope. Using the system and procedure we describe, the vocal cords and tracheal opening can be seen with the naked eye. With regard to insertion of the optical fiber, it is important to make sure the fiber has its edge smoothed. After cutting the cable to length, the edge is left relatively sharp, and it does not take much effort to pierce the tracheal wall.
Although the methods here were designed for intubation of adult mice, they could be adapted to younger mice or mice with different anatomical dimensions. Although the 0.5-mm optical fiber might not fit into a smaller gauge IV catheter, 0.25-mm optical fibers are available from the same source. We have also used a longer 1.25 mm in IV cannula (Jelco no. 4076, Smith's Medical) with success, although its Teflon construction makes it slightly more rigid than polyurethane. In addition, the longer length makes the resistance of this catheter ∼25% greater than the resistance of the 1-in. Optiva catheter (1 cmH2O·ml−1·s). This or any other catheter resistance of course needs to be subtracted from the total resistance measured with the mouse intubated. If different strains of mice have more narrow or wider pharynxes, it may also be necessary to either change the location where the silicone cone starts, or use a different pipette tip for a mold. We have not compared pharyngeal anatomical variations in different strains with regard to intubation.
In summary, the intubation procedure described here is inexpensive to fabricate and simple to use, and it should enable most investigators and laboratory technicians to quickly learn to successfully intubate mice with relatively little experience.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agrawal A, Singh SK, Singh VP, Murphy EC, Parikh I. Partitioning of nasal and pulmonary resistance changes during non-invasive plethysmography in mice. J Appl Physiol 105: 1975–1979, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown RH, Walters DM, Greenberg RS, Mitzner W. A method of endotracheal intubation and pulmonary functional assessment for repeated studies in mice. J Appl Physiol 87: 2362–2365, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Crnich CJ, Halfmann JA, Crone WC, Maki DG. The effects of prolonged ethanol exposure on the mechanical properties of polyurethane and silicone catheters used for intravascular access. Infect Control Hosp Epidemiol 26: 708–714, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Ewart SL, Levitt RC, Mitzner W. Respiratory system mechanics in mice measured by end-inflation occlusion. J Appl Physiol 79: 560–566, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Hamacher J, Arras M, Bootz F, Weiss M, Schramm R, Moehrlen U. Microscopic wire guide-based orotracheal mouse intubation: description, evaluation and comparison with transillumination. Lab Anim 42: 222–230, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Hastings RH, Summers-Torres D. Direct laryngoscopy in mice. Contemp Top Lab Anim Sci 38: 33–35, 1999. [PubMed] [Google Scholar]
- 7.MacDonald KD, McKenzie KR, Mitzner W, Zeitlin PL. Lung mechanics in heterozygous CF mice after repeated LPS dosing (Abstract). Am J Respir Crit Care Med 175: A930, 2007. [Google Scholar]
- 8.Rivera B, Miller S, Brown E, Price R. A novel method for endotracheal intubation of mice and rats used in imaging studies. Contemp Top Lab Anim Sci 44: 52–55, 2005. [PubMed] [Google Scholar]
- 9.Spoelstra EN, Ince C, Koeman A, Emons VM, Brouwer LA, van Luyn MJ, Westerink BH, Remie R. A novel and simple method for endotracheal intubation of mice. Lab Anim 41: 128–135, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Vergari A, Gunnella B, Rodola F, Frassanito L, Musumeci M, Palazzesi S, Casalinuovo IA. A new method of orotracheal intubation in mice. Eur Rev Med Pharmacol Sci 8: 103–106, 2004. [PubMed] [Google Scholar]
- 11.Vergari A, Polito A, Musumeci M, Palazzesi S, Marano G. Video-assisted orotracheal intubation in mice. Lab Anim 37: 204–206, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Walters DM, Wills-Karp M, Mitzner W. Assessment of cellular profile and lung function with repeated bronchoalveolar lavage in individual mice. Physiol Genomics 2: 29–36, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Zhao X, Wu N, Zhou J, Yang Y, Fang Y, Cheng W, Ma R, Tian Y, Huang L. A technique for retrograde intubation in mice. Lab Anim 35: 39–42, 2006. [DOI] [PubMed] [Google Scholar]


