Abstract
The respiratory epithelium functions as a central orchestrator to initiate and organize responses to inhaled stimuli. Proteases and antiproteases are secreted from the respiratory epithelium and are involved in respiratory homeostasis. Modifications to the protease/antiprotease balance can lead to the development of lung diseases such as emphysema or chronic obstructive pulmonary disease. Furthermore, altered protease/antiprotease balance, in favor for increased protease activity, is associated with increased susceptibility to respiratory viral infections such as influenza virus. However, nutritional antioxidants induce antiprotease expression/secretion and decrease protease expression/activity, to protect against viral infection. As such, this review will elucidate the impact of this balance in the context of respiratory viral infection and lung disease, to further highlight the role epithelial cell-derived proteases and antiproteases contribute to respiratory immune function. Furthermore, this review will offer the use of nutritional antioxidants as possible therapeutics to boost respiratory mucosal responses and/or protect against infection.
Keywords: protease/antiprotease, viral infection, nutritional antioxidants
nearly 40 different types of cells have been identified in the respiratory tract, including neutrophils, dendritic cells, macrophages, natural killer (NK) cells, B cells, and T cells (125). Because of this diverse population of cells, a central “orchestrator” is necessary for coordinating an appropriate immune response to inhaled stimuli. The respiratory epithelium is one of the first sites within the respiratory tract to be exposed to inhaled stimuli and functions as a focal orchestrator to organize and initiate appropriate responses in three distinct ways (Fig. 1). First, the respiratory epithelium forms a physical barrier through tightly regulated cell-cell interactions. These interactions promote basic biological functions, protect against invading pathogens, and establish mechanical strength. Second, epithelial cells express receptors and ligands that coordinate cellular responses and activate immune cells. These processes are important for detecting foreign stimuli, communicating among different cells, and guiding immune responses. Third, respiratory epithelial cells secrete soluble factors such as cytokines, chemokines, and host defense mediators such as proteases and antiproteases. These secreted, soluble factors are crucial for the overall host defense response. In the context of antimicrobial host defense responses, epithelial function can be modulated by intrinsic factors, such as age, and extrinsic factors, such as microbial infection, exposure to inhaled toxicants, and supplementation with nutritional antioxidants. As such, this review will examine the role of epithelial cell-derived proteases and antiproteases, in the context of lung disease and viral infection and offer a potential strategy to modulate this balance with nutritional antioxidants.
Fig. 1.

Respiratory epithelial cells function as an “orchestrator” to initiate, coordinate, and respond to inhaled stimuli. 1: Respiratory epithelial cells form a physical and mechanical barrier to protect against respiratory stimuli. 2: Respiratory epithelial cells express receptors and ligands that interact with neighboring epithelial cells, respiratory immune cells, and inhaled stimuli such as respiratory pathogens. 3: Respiratory epithelial cells secrete soluble factors such as cytokines, chemokines, and antimicrobial peptides to contribute to respiratory host defense.
Respiratory Viruses Infect Respiratory Epithelial Cells and Cause Exacerbations of Lung Disease
Respiratory epithelial cells are targets for respiratory viruses and differ in protease and/or antiprotease sensitivity.
Respiratory epithelial cells are the primary targets for respiratory viral infection based on extracellular ligands and residues that are found on these cells. More than 200 antigenically different viruses can infect respiratory epithelial cells (110). These viruses include coronaviruses, rhinoviruses, metapneumoviruses, enteroviruses, adenoviruses, respiratory syncytial viruses, measles virus, parainfluenza viruses, and influenza viruses. These respiratory viruses differ in genome composition, viral structure, specific target cell type, and sensitivity to components of the respiratory protease/antiprotease balance (Table 1).
Table 1.
Genome and structure, target cell type, and sensitivity to protease/antiprotease balance of respiratory viruses
| Respiratory Virus | Genome; Structure | Target Cell | Require Proteolytic Cleavage by Secreted Respiratory Proteases? | Sensitive to Secreted Respiratory Antiproteases? |
|---|---|---|---|---|
| Adenovirus | dsDNA; Nonenveloped | Epithelial cells using clathrin-dependent entry mechanisms (119) | No | No |
| Rhinoviruses | +ssRNA; Nonenveloped | Epithelial cells of the upper airway expressing ICAM-1 (69) | No | No |
| Enteroviruses | +ssRNA; Nonenveloped | Epithelial cells expressing ICAM-1 or DAF (129) | No | No |
| Coronaviruses | +ssRNA; Enveloped | Epithelial cells expressing ACE2 or DPP4 (107, 139) | Yes- cleave S protein (17, 19, 20, 64, 77, 157, 194) | Yes (96, 142) |
| Measles viruses | -ssRNA; Enveloped | Epithelial cells expressing nectin 4 (132, 133) | No | No |
| Metapneumoviruses | -ssRNA; Enveloped | Upper and lower airway epithelial cells expressing αVβ1 integrin (39) | Yes- cleave F protein (158) | No |
| Respiratory syncytial viruses | -ssRNA; Enveloped | Epithelial cells expressing sulfated glycosamino-glycans (70) | No | No |
| Parainfluenza viruses | -ssRNA; Enveloped | Epithelial cells expressing 2,3 or 2,6 linked sialic acid residues (55) | Yes- cleave F protein (3, 94) | Yes (52, 93, 94) |
| Influenza viruses | -ssRNA; Enveloped | Epithelial cells expressing 2,3 or 2,6 linked sialic acid residues (36) | Yes- cleave HA protein (10, 11, 16, 19, 22, 23, 56, 75, 148, 178, 194) | Yes (14, 91, 93, 95) |
Upon viral infection, respiratory epithelial cells replicate viral genomes and propagate infectious virions for productive viral infection. Additionally, respiratory epithelial cells mount intracellular immune responses to warn neighboring cells as well as orchestrate recruitment of immune cells to sites of infection (152). The respiratory epithelium also secretes soluble mediators such as type II transmembrane serine proteases (TTSPs) and antiproteases, such as secretory leukocyte protease inhibitor (SLPI), which are implicated in viral infection. Many respiratory viruses, such as coronaviruses, metapneumoviruses, parainfluenza viruses, and influenza viruses, are sensitive to TTSP-dependent cleavage (Table 1). However, whereas some respiratory viruses, such as coronaviruses, induce antiprotease expression, only parainfluenza and influenza are sensitive to SLPI activity (Table 1). Thus investigating the role of the protease/antiprotease balance in the context of a parainfluenza or influenza infection could elucidate mechanisms and strategies to prevent viral infections.
Understanding the role of the protease/antiprotease balance in the context of an influenza infection is of public health importance, since influenza infects 2–5 million people worldwide each year and can cause worldwide pandemics, as seen with the recent 2009 H1N1 strain (162). Moreover, in the US alone, over 20,000 people die because of influenza infection and related complications (29a). Influenza remains a significant public health burden due to yearly virus mutations and the ability of the virus to infect a wide range of hosts. Despite large-scale vaccination programs and the development of antiviral therapeutics, influenza-associated morbidity and mortality rates have not changed in recent years (99). This is of particular importance in years when the strains used in the influenza vaccine do not antigenically match the circulating influenza virus strains, as is the case in the current 2014–2015 influenza epidemic (54). Therefore, examining strategies such as modulating the protease/antiprotease balance may serve as alternative targets to prevent influenza infection.
HA must be proteolytically cleaved for productive IAV infection.
For influenza A virus (IAV) to infect cells, the viral membrane protein hemagglutinin (HA) must be proteolytically cleaved and activated by respiratory serine proteases. HA resides on the virion as a fusion-inactive precursor (HA0), and upon proteolytic cleavage a fusion-active trimer with disulfide-linked HA1 and HA2 subunits is generated (16, 18, 22, 23). After entry into the cell, the virion enters endosomal compartments, and upon acidification of the endosome viral and host membranes fuse together. The membrane fusion allows for viral entry into the cell, so viral replication can initiate (98).
Most HA0 will be cleaved at either a single arginine or lysine residue to generate active HA1 and HA2. For low-pathogenic IAV strains, respiratory trypsin-like serine proteases cleave at monobasic arginine or lysine residues (21, 102). However, highly pathogenic avian influenza viruses possess multibasic cleavage sites that can be cleaved by ubiquitous intracellular furin-like serine proteases (166, 182). This difference in HA cleavage motifs increases susceptibility for systemic, widespread infection to sites such as the central nervous system rather than localized, restricted infection to sites within the respiratory tract. Consequently, investigating strategies to block protease-induced HA activation and increase antiprotease activity may serve as a potential approach to protect against IAV infection.
Proteases and antiproteases of the Lung: a Delicate Enzymatic Balance
Epithelial cell-derived proteases.
Proteases are key components of respiratory host defense. In the healthy lung, proteases maintain tissue homeostasis and their activities are regulated by antiproteases. Elevated net protease activity is associated with lung destruction and the development of chronic lung diseases such as emphysema and chronic obstructive pulmonary disease (COPD) (1, 90). Lung destruction is induced by proteolytic degradation of extracellular matrix (ECM) components, including collagens, laminins, and elastin, with type I collagen serving as the major target (164). Cysteine proteases, matrix metalloproteinases (MMPs), and serine proteases are prevalent proteases found in the lung, with serine proteases serving as the most predominant class. Moreover, each family has unique target substrates, cellular sources, and active sites (Table 2).
Table 2.
Target substrates, sources, and activity of respiratory proteases
| Proteases in the Respiratory Tract | Target Substrates | Sources in the Lung | Activity |
|---|---|---|---|
| Cathepsin B, K, L, and S | Elastin, laminin, antiproteases, MHC II, respiratory viruses (20, 60, 144, 174) | Bronchial epithelial cells, macrophages, dendritic cells (26, 58) | Cysteine proteases with cysteine serving as a nucleophile for target motifs |
| MMP1, 2, 3, 7, 8, 9, 12, and 13 | Collagen, elastin, inflammatory cytokines such as IL-1β and IL-8, defensins, antiproteases (46, 84, 188) | Epithelial cells, alveolar macrophages, monocytes, neutrophils, mast cells, eosinophils (41, 51, 147) | Metal endopeptidases with conserved zinc binding site |
| Neutrophil-derived proteases (NE, cathepsin G, proteinase 3) | Elastin, collagen, fibronectin, laminin, proteoglycans, immunoglobulins, complement components, T cell receptors. antiproteases (47, 65, 116, 121, 167, 170, 180, 186) | Mature neutrophils (80) | Serine proteases with conserved catalytic triad of serine, aspartic acid, and histidine |
| TTSPs | Fibrinogen, urokinase, respiratory viruses (17, 23, 104, 158, 176) | Epithelial cells (25) | Serine proteases with conserved catalytic triad of serine, aspartic acid, and histidine |
Cathepsins.
Cysteine proteases, or cathepsins, are synthesized as proenzymes, require cleavage for activation, and contain conserved cysteine and histidine residues, with the cysteine serving as a nucleophile for target motifs. Cathepsin B, K, L, and S are highly abundant in the lung; cathepsin K, mainly produced in bronchial epithelial cells, is one of the most potent matrix-degrading proteases identified to date (26). Cathepsins cleave elastin, laminin, and collagen as well as antiproteases such as SLPI and are important for major histocompatibility complex II formation and antigen presentation (60, 144, 174). Cathepsins have been linked to respiratory virus infection since cathepsin L cleaves and activates the spike protein of severe acute respiratory syndrome coronavirus (SARS-CoV), and cathepsin B is induced during IAV infection in mice (20, 27).
MMPs.
MMPs are a family of metal endopeptidases that target ECM components. Secreted by immune cells and epithelial cells, MMPs possess a common prodomain and catalytic domain. Because MMPs are synthesized as inactive zymogens, the signal domain must be cleaved for MMPs activity. The catalytic domain contains a conserved zinc binding site that confers protease activity. MMP1, 2, 3, 7, 8, 9, 12, and 13 are the main MMPs found in the respiratory tract and are induced by growth factors, reactive oxygen species, and proinflammatory cytokines (42, 68, 100). MMP1, 8, and 13 cleave collagen, whereas MMP2 and 9 act as gelatinases and also degrade elastin and type IV collagen (46, 84).
Additionally, increased MMP activity is associated with inflammation. Baseline MMP levels such as MMP1, 2, 3, 8, and 9 are elevated in smokers and patients with COPD (40). Moreover, MMP9 cleaves proinflammatory cytokines such as interleukin-8 (IL-8), resulting in the release of a modified, more potent neutrophil chemotactic mediator (134). MMP12 degrades elastin but also inactivates key antiproteases in the lung such as SLPI (140). Although MMP activity has not been implicated in viral activation, MMPs are involved in virus-induced inflammation. In epithelial cell lines and mouse models, MMP2 and 9 are induced during IAV infection and contribute to the IAV-associated lung destruction (105, 185, 190). Tacon et al. (171) demonstrated that MMP levels are elevated in vitro and in vivo during human rhinovirus (HRV) infection, which was dependent on NF-κB, further illustrating the role of MMPs in virus-induced respiratory inflammation.
TTSPs.
Serine proteases make up the largest portion of respiratory proteases (66). Whereas neutrophil elastase (NE), proteinase 3, and cathepsins G are neutrophil-derived proteases, other serine proteases such as TTSPs are produced and secreted by the respiratory epithelium. TTSPs are a family of proteases that possess an NH2-terminal transmembrane domain, a stem region, and a COOH-terminal serine protease domain that contains the conserved catalytic triad composed of histidine, aspartic acid, and serine (25). Protease activity is attained through the nucleophilic serine, which attacks the substrate's carbonyl functional group. This activity generates an acyl intermediate, transfers a proton from the positively charged histidine to the substrate, and allows for the hydrolysis and cleavage of the substrate (5). TTSPs are synthesized as a single-chain, inactive proenzyme and require cleavage following a basic arginine or lysine (82). The catalytic domains are located on the terminal extracellular region to permit direct exposure to the extracellular environment.
TTSPs are comprised of four subfamilies: the human airway trypsin-like protease (HAT)/differentially expressed in squamous cell carcinoma (DESC) subfamily that include HAT, DESC1, and HAT-like 1–5; the hepsin/transmembrane protease serine (TMPRSS) subfamily contains hepsin, TMPRSS2-5 and 13, mosaic serine protease large-form, and enteropeptidase; the corin protease; and the matriptase subfamily that includes matriptase 1–3 and polyserase-1 (5). Although TTSPs are transmembrane proteases, release of the extracellular domains has been reported for several of the TTSPs such as matriptase, HAT, and TMPRSS2 (3, 189). Moreover, matriptase, HAT, and TMPRSS2 are widely expressed in epithelial tissues, including the respiratory tract.
Matriptase cleaves and activates protease-activated receptor 2 (PAR2), hepatocyte growth factor, and urokinase, indicating that matriptase plays a role not only in ECM degradation but also in epithelial cell differentiation and remodeling (104, 176). In the respiratory epithelium, matriptase is expressed on the basolateral side of the epithelial cells. Upon activation, matriptase migrates to the apical side of the epithelium, where it can be secreted into the lumen of the respiratory tract. Recent studies have revealed that intracellular matriptase localizes to the plasma membrane and endosomes, allowing for interaction and activation of the IAV virion (11, 73). Moreover, secreted matriptase cleaves HA protein on the IAV virion, including H1, but not H2 or H3, which activates the virion and allows for enhanced rates of replication (11, 73).
HAT cleaves fibrinogen, activates PAR2, and urokinase (11). In sputum samples from patients with chronic airway disorders such as bronchitis or asthma, secreted HAT levels are elevated (189). Moreover, Chokki et al. (34) demonstrated that HAT enhanced MUC5AC gene expression, resulting in increased mucus production. HAT is produced and secreted by ciliated epithelial cells and is not found in basal or goblet cells of the respiratory tract (175). HAT activates the SARS-CoV viral spike protein necessary for host cell entry (17). Moreover, HAT cleaves the monobasic site of the HA protein required for HA activation, enhancing IAV replication (21–23). Cleavage of HA by HAT has been shown to occur at the cell surface either during attachment and entry into host cells or during budding and shedding of virions from an infected cell (21).
TMPRSS2 possesses a wide range of functions specific to epithelial cell biology. Donaldson et al. (45) demonstrated that TMPRSS2 reduced epithelial sodium channel activity, indicating a role for TMPRSS2 in ion transport. TMPRSS2 can be expressed as a full-length 70-kDa protein or a variety of smaller, truncated forms, depending on tissue site and localization (3, 30). Additionally, TMPRSS2 is implicated in the activation of respiratory viruses. Cells expressing TMPRSS2 show enhanced replication of human metapneumovirus as well as increased activation and replication of SARS-CoV (19, 158). Bottcher et al. (22, 23) reported that cells stably expressing TMPRSS2 resulted in the monobasic cleavage of HA, which elevated IAV replication. In recent reports, TMPRSS2 has been shown to preferentially cleave H1N1, and to a lesser degree H3N2. Hatesuer et al. (75) revealed that viral titers in H1N1-infected TMPRSS2-deficient mice were significantly reduced compared with wild-type animals. Furthermore, this group reported that although H3N2 replicated at similar levels in both knockout and wild-type mice, the knockout mice had increased survival and body weight post-H3N2 infection (75).
Recent reports have further expanded on the HA specificity of TMPRSS2. One group demonstrated that TMPRSS2 knockout mice were protected from lethal H1N1 but not lethal H3N2 infection, whereas another group showed that TMPRSS2 knockout mice were protected against both H1N1 and H3N2 infection (148, 178). These opposing findings suggest that HA activation not only depends on HA structure but can vary among different viral strains bearing the same HA subtype. Despite these differences, there is substantial evidence implicating TTSPs in IAV pathogenesis.
Epithelial cell-derived antiproteases.
Antiproteases are a broad class of proteins that inhibit proteases and modulate immune responses in the lung. Respiratory antiproteases are comprised of four families: tissue inhibitors of metalloproteinases (TIMPs), serine protease inhibitors (serpins), trappin-2/elafin, and SLPI. Each antiprotease family has unique target substrates, cellular sources, and antiprotease function (Table 3). Although the primary function of antiproteases is to inhibit protease function, new and emerging studies indicate that antiproteases inhibit overt inflammatory cascades, protect against the development of chronic respiratory diseases, and block microbial infection. As such, antiproteases are critical components of respiratory host defense responses.
Table 3.
Target substrates, sources, and activity of respiratory antiproteases
| Antiproteases in the Respiratory Tract | Target Substrates | Sources in the Lung | Activity |
|---|---|---|---|
| TIMPs | MMPs (24) | Alveolar epithelial cells, alveolar macrophages, granulocytes (28, 97) | Insert reactive ridge NH2-terminal domain into MMP active site, inhibit apoptosis (103, 120) |
| Serpin | Serine and cysteine proteases, caspases, bacteria, viruses (9, 57, 138, 141, 154) | Epithelial cells, alveolar macrophages (35, 115, 159) | Insert RCL into the active site of the targeted protease, inhibit LPS-induced inflammation, block microbial infection (101, 130) |
| Trappin/Elafin | NE and proteinase-3, enveloped viruses (193) | Tracheal epithelial cells, club cells, type II cells, alveolar macrophages (123) | Contain WAP domain for antiprotease activity, inhibit viral infection (59, 150, 156, 187) |
| SLPI | NE, cathepsin G, chymase, bacteria, and viruses (62, 184) | Epithelial cells, neutrophils, macrophages (48, 121) | Contain WAP domain for antiprotease activity, antimicrobial effects due to NH2-terminal domain and high cationicity, block inflammatory cascades, inhibit viral infection (67, 91, 93, 150, 156, 172, 173, 187) |
MMPs, matrix metalloproteinases; NE, neutrophil elastase; RCL, reactive center loops; SLPI, secretory leukocyte protease inhibitor; TIMPs, tissue inhibitors of metalloproteinases; WAP, whey acidic protein domain.
TIMPs.
TIMP1-4 are produced by alveolar epithelial cells and alveolar macrophages (97). TIMPs inhibit MMP activity through the formation of an NH2-terminal reactive ridge domain that inserts into the active site of the target MMP (24, 103). The TIMP family share about 40% homology and have overlapping anti-MMP activity. TIMPs induce cell growth and proliferation. TIMP1 and 2 activate growth of keratinocytes, fibroblasts, and erythroid cells (15, 76).
Furthermore, TIMPs have been shown to be associated with chronic lung diseases such as asthma and COPD. Circulating granulocytes from patients with asthma have an impaired ability to release TIMP1, the main inhibitor of MMP9, after stimulation with phorbol 12-myristate 13-acetate (28). Additionally, although it has been shown that sputum samples from stable COPD patients contain elevated TIMP1 levels during a COPD exacerbation, others have reported that TIMP1 levels are decreased (40, 120). Moreover, Mercer et al. (120) demonstrated that during a COPD exacerbation there was a shift in the protease/antiprotease molar balance, with an increase in MMP9 and a decrease in TIMP1. In the context of viral infection, overexpressing TIMP-1 blocks respiratory syncytial virus syncytia formation (191). However, since MMP activity is directly implicated in viral activation and viral entry/fusion, there are limited studies that investigate the direct antiviral effects of TIMPs.
Serpins.
Serpins are the largest and most broadly distributed superfamily of protease inhibitors. The majority of serpins inactivate serine proteases, but a small portion of serpins also inhibits caspase and cysteine proteases (141, 154). Serpins exert their antiprotease activity by undergoing irreversible conformational changes that allow for the interaction and inactivation of serine proteases. To inhibit protease activity, serpins form long, flexible reactive center loops (RCL) that insert into the active site of the targeted protease. These RCL structures form above the serpin framework and allow for the NH2-terminal portion of the RCL to insert into the protease active site. At this time, the protease and serpin remain covalently linked, the protease structure is conformationally changed, and the protease activity is significantly reduced (101). This action leads to the permanent deactivation of both serpin and protease, often described as a “suicide” event.
SerpinA1 encodes the most abundant antiprotease in the lung, alpha-1 antitrypsin (α-1AT). Point mutations of α-1AT generates a misfolded version of this protein, resulting in an α-1AT deficiency (109). α-1AT-deficient individuals are at a high risk for developing emphysema and COPD (168, 169). Respiratory epithelial cells secrete α-1AT in both the apical and basolateral compartments of the cell (159). Additionally, serpins have been shown to block viral entry of enveloped viruses such as human immunodeficiency virus (HIV) and herpes simplex virus (9, 138). Smee et al. (161) showed that in vitro serpin antithrombin III treatment inhibited IAV infection nearly 100-fold more than ribavirin, a nucleoside inhibitor used to halt IAV replication. The authors found that this effect was dependent on viral HA with decreasing efficacy in order of H1N1>H3N2>H5N1 (161), suggesting that serpin activity may parallel the TTSP activity in the context of IAV infections, which is dependent on amino acid sequence, protein structure, and/or HA substrate accessibility. A recent study demonstrated that plasminogen activator inhibitor 1 (PAI-1), encoded by SerpinE1, inhibited HA cleavage and reduced infectivity of progeny virions (44). Specifically, these studies demonstrated that antiproteases, such as PAI-1, regulate the spread of IAV infection by blocking proteolytic maturation of newly formed virus particles and propagation of the infection. Thus protease/antiprotease balances may be important mucosal regulators of IAV spreading.
Trappin/elafin.
Trappin-2 is a cationic 9.9-kDa serine antiprotease secreted into the lung as a nonglycosylated preprotein. Upon proteolytic cleavage, trappin-2 is converted to elafin, a 6-kDa protein. Structurally, trappin-2 and elafin contain a cysteine-rich region, joined by four disulfide bonds, termed the whey acidic protein domain (WAP) (153). The structure of elafin is divided into two regions, the COOH-terminal region, which comprises the antiprotease active site, and NH2-terminal region, which allows for interactions with ECM proteins (149). Elafin specifically inhibits NE and proteinase-3 and is secreted from tracheal epithelial cells, club cells, type II cells, and alveolar macrophages (123, 187).
Trappin-2 and elafin function as alarm antiproteases, as both are produced in response to inflammatory stimuli (136). In respiratory epithelial cells, elevated elafin levels are protective against recombinant NE treatment and products derived from activated human neutrophils in vitro (160). In addition to functioning as an alarm antiprotease, elafin possesses antimicrobial effects. Ghosh et al. (59) revealed that recombinant elafin was protective during HIV infection only when elafin was preincubated with HIV, suggesting that elafin directly interacts with HIV virion and prevents viral infection. Interestingly, this group found that secreted elafin levels were elevated in the cervicovaginal lavages of HIV-negative women compared with HIV-positive women, albeit not statistically significant, suggesting that elafin secretions may be decreased after HIV infection. Similarly, after HRV infection, elafin is downregulated in subjects with COPD (113). These findings suggest that, despite elafin functioning as an alarm mediator, elafin secretions may be decreased after viral infection, thus enhancing susceptibility to viral infection.
SLPI.
SLPI is an additional alarm antiprotease that is highly expressed by epithelial cells, neutrophils, and macrophages. SLPI can be detected in respiratory secretions, saliva, seminal fluid, cervical mucus, tears, and cerebral spinal fluid (78, 151). Comprised of two closely related domains, the tertiary structure of SLPI resembles a boomerang (48). Similar to the trappin family, SLPI contains a cysteine-rich WAP COOH-terminal domain that is responsible for protease inhibition as well as the NH2-terminal domain that stabilizes the protease/antiprotease complex and exerts antimicrobial activity (192). The COOH-terminal active site, which confers antiprotease activity, is comprised of leucine and methionine residues (50, 181).
The main function of SLPI is to inhibit serine proteases such as NE (its main target), cathepsin G, elastase, and chymase (62, 184). SLPI further contributes to respiratory host defense by modulating inflammation and inducing wound healing. SLPI exerts cellular anti-inflammatory effects by preventing the degradation of regulatory components of the NF-κB pathway and by directly competing for NF-κB binding sites in the promoter regions of proinflammatory cytokines such as IL-8 and tumor necrosis factor-α (TNF-α) (67, 172, 173). SLPI is also implicated in wound healing as reported by Ashcroft et al. (8), who investigated the role of SLPI in a mouse model of dermal wounds. Using this model, the authors found that SLPI knockout mice had impaired wound healing due to increased NE activity.
SLPI possesses broad antimicrobial effects. The NH2-terminal domain of SLPI possesses extracellular antibacterial activity by disrupting Staphylococcus aureus and Escherichia coli membranes (79). Additionally, SLPI binds to Opa protein of Neisseria gonorrhoeae, which leads to the lysis of bacterial membranes (37). Fahey et al. (53) reported that increased extracellular SLPI secretions were correlated with elevated bactericidal activity and treatment with an anti-SLPI antibody significantly diminished bactericidal activity. SLPI has been shown to exert intracellular antibacterial effects by disrupting translation of E. coli during bacterial replication (124). Furthermore, SLPI exerts fungicidal and fungistatic activity toward Aspergillus fumigatus and Candida albicans that is also dependent on the NH2-terminal domain of SLPI (179).
In the context of viral infections, SLPI inhibits HIV, human papillomavirus (HPV), and respiratory viruses. McNeely et al. (117) first detailed the anti-HIV activity of SLPI in human saliva. The authors demonstrated that SLPI was highly expressed in the saliva and inhibited HIV infection in monocytes and T cells in dose-dependent fashion. Further studies revealed that SLPI exerts anti-HIV properties not through direct interaction with the virus, but rather through interacting with the target host cell (86). However, this group found that, despite decreasing viral load, SLPI did not alter the infectivity of progeny virions, suggesting that SLPI inhibits HIV at the early stages of viral infection (118).
SLPI is also associated with protection from human papillomavirus, with two recent studies demonstrating that high levels of SLPI, possibly induced by cigarette smoking, correlate with protection against HPV infection (81, 137). Additionally, SLPI is induced and protective during respiratory virus infection. Kido et al. (93) first demonstrated that SLPI decreased infectivity of IAV and Sendai viruses. Moreover, reports have revealed that intranasal administration with recombinant SLPI (rSLPI) decreased progeny virus nearly 3,000-fold compared with vehicle-treated mice infected with mouse-adapted IAV (95). Furthermore, the authors also reported that in mice administered rSLPI nearly all the progeny viruses released from infected cells were secreted as noninfectious HA0 precursor virions (95). Additionally, we have shown that recombinant human SLPI inhibits IAV replication in vitro (91).
Although SLPI contributes to respiratory host defense by decreasing inflammation and infection, SLPI can also induce proinflammatory responses. Mulligan et al. (126) demonstrated that posttranslational modifications of SLPI increased IL-8 secretion, neutrophil recruitment, and vascular permeability. Extracellular SLPI can be posttranslationally modified by respiratory proteases such as cathepsin L, MMP12, chymase, and NE, resulting in cleavage of SLPI (12, 116, 140, 174). Cleaved SLPI levels are elevated in COPD patients during respiratory infection, which renders SLPI nonfunctional (1, 113, 135). Moreover, we have shown that in nasal lavage fluid from smokers, SLPI is posttranslationally processed and cleaved (121). Reactive oxygen intermediates generated in the context of environmental exposures to oxidant pollutants, such as cigarette smoke, modify the active site and decrease the antiprotease properties of SLPI (29, 183). Whereas SLPI exerts powerful protective effects in the respiratory tract, posttranslational alterations and/or oxidative modifications can drastically shift the activity of SLPI toward potentially damaging effects and may reduce its antiviral properties.
The protease/antiprotease balance is a delicate interaction of enzymes and proteins that are involved in respiratory function. In the healthy lung, proteases are critical for cellular regeneration, cellular repair, and tissue homeostasis. Furthermore, antiproteases are important for protease inhibition and contribute to host defense. In the healthy lung, although there are many types of proteases and antiproteases expressed, a balance between proteases and antiproteases is established to ensure respiratory homeostasis. Balance will depend on the overall presence, timing of protease and antiprotease production/release, and activity. A shift in the balance toward increased protease expression and activity can lead to overt inflammation and the development of chronic lung disorders such as COPD and emphysema (1, 90). Moreover, respiratory serine proteases, such as the TTSP family, activate a variety of respiratory viruses for enhanced rates of viral infection. Additionally, antiproteases, such as SLPI, inhibit the function of serine proteases and block viral entry into the target cell. Hence the protease/antiprotease balance not only is a critical component for respiratory homeostasis but also is a powerful determinant of respiratory viral pathogenesis.
Nrf2 Decreases Oxidative Stress, Modifies Components of the Protease/antiprotease Balance, and Protects Against the Development of Lung Disease and Viral Infection
The respiratory epithelium is constantly being exposed to inhaled insults, which results in the generation of reactive oxygen species, free radicals, and peroxides (13). Oxidative stress has been associated with cancer, cardiovascular diseases, neurodegenerative diseases, and chronic inflammation (49, 89). Cells are equipped with various mechanisms, such as antioxidant responses, to block these damaging effects (71, 72). One mechanism involves nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a key transcription factor that regulates that transcription of many antioxidant and detoxifying enzymes (32, 63).
Under baseline conditions, Nrf2 is sequestered in the cytoplasm by kelch-like ECH-associated protein 1 (Keap1), and upon stimulation Nrf2 is released from Keap1 and Nrf2 translocates into the nucleus and binds to antioxidant response elements (ARE) (85). ARE binding sites are located on the promoter regions of phase II enzymes such as NADPH quinone oxidoreductase 1 (NQO1) and heme oxygenase-1 (HO-1) (33). The Nrf2-dependent phase II response has been implicated in the development of chronic lung diseases since antioxidant capacity and Nrf2 protein levels are decreased in the lungs of COPD patients (112). Iizuka et al. (83) reported that Nrf2-deficient mice exposed to cigarette smoke developed emphysema-like qualities and were unable to induce antiproteases such as SLPI. SLPI contains ARE regions on its promoter and we have previously demonstrated that Nrf2 agonists enhance SLPI expression in airway epithelial cells in an Nrf2-dependent manner (122), suggesting that oxidative stress responses modulate SLPI regulation.
In addition to its role in maintaining oxidant/antioxidant homeostasis, we and others have previously shown that Nrf2 is critical for respiratory epithelial responses to respiratory viral infection (31, 92). Nrf2 has been shown to regulate and decrease TMPRSS2 expression in prostate cancer cell lines, suggesting that in addition to inhibiting respiratory oxidative stress, Nrf2 alters components of the protease/antiprotease balance and protects against respiratory infection (155). Thus strategies activating Nrf2 may be a potential mechanism to not only reduce oxidative stress, but also protect against viral infection.
Antioxidants are an attractive strategy to modulate the protease/antiprotease balance and protect against chronic lung disease and viral infection.
Antioxidants have garnered much attention due to the inhibition of respiratory oxidative stress yet may also serve as therapies to modify the protease/antiprotease balance and inhibit viral infection (88). Antioxidants are a broad class of molecules that can be found in a variety of foods and have been shown to exert potent host defense effects. One potent class of antioxidants includes flavonoids, which are nonessential, polyphenolic phytonutrients that occur naturally in many plant-based foods and possess profound effects on human health (38). Over 8,000 flavonoid compounds have been identified, and they can be broken down into six groups: flavonols, flavones, isoflavones, flavanones, anthocyanidins, and flavan-3-ols (43). Flavan-3-ols (or commonly referred to as flavanols or catechins) are the most common flavonoid consumed in the American diet and can be found in red wine, tea, apple, grapes, and chocolate (7).
Flavan-3-ols found in green tea, such as epigallcatechin-3-gallate (EGCG), have been studied extensively in the context of a viral infection. EGCG has been shown to bind to CD4 T cell receptors and prevent interaction with gp120, a HIV envelope protein (87). Nance et al. (128) showed that EGCG was able to inhibit HIV infection of human T cells and macrophages at physiological doses. Because of these effects, use of EGCG as an anti-HIV therapeutic has entered preclinical investigation. Furthermore, EGCG possesses potent anti-influenza properties. EGCG agglutinates influenza viruses and inhibits viral replication, which was dependent on influenza subtype (127, 163). Ling et al. (108) corroborated these findings, showing that oral administration of EGCG in mice infected with influenza had nearly a 50% decrease in viral titers and a 50% increase in survival rates. We have shown that EGCG induced SLPI secretion, reduced TMPRSS2 secretion, and decreased influenza replication (91). Furthermore, we previously reported that EGCG blocked influenza entry into target epithelial cells (92). Together, these results suggest that antioxidant supplementation with flavan-3-ols may serve as an attractive therapy to protect against lung disease and infection.
SFN alters SLPI and TMPRSS2 levels, enhances lung host defense responses, and protects against infection.
Another potent antioxidant is sulforaphane (SFN), an isothiocyanate that induces respiratory host defense by decreasing oxidative stress and inflammation (111). SFN also exerts broad antimicrobial effects. SFN supplementation of alveolar macrophages from COPD patients increases clearance of Pseudomonas aeruginosa (74). Furthermore, SFN elevates bacterial detection and induces phagocytosis in alveolar macrophages from COPD patients, which is dependent on Nrf2 activation (74).
SFN is derived from the hydrolysis of glucosinolates found in cruciferous vegetables such as broccoli, brussels sprouts, and cabbage. Using in vitro models of respiratory epithelial cells, SFN supplementation enhances Nrf2 activity, induces cellular antioxidants such as HO-1 and NQO1, and inhibits proinflammatory cytokine release (106, 145, 165, 177). Furthermore, Riedl et al. (143) reported that in vivo SFN supplementation enhanced respiratory phase II enzyme expression, indicating that nutritional SFN supplementation induces respiratory antioxidant responses.
Beyond its antioxidant function, SFN may have important functions in modulating components of the protease/antiprotease balance. We have shown that SFN supplementation transcriptionally enhances SLPI expression, resulting in increased SLPI secretion in the nasal mucosa in an Nrf2-dependent fashion (122). SFN also inhibits MMP expression and inflammation (4, 114, 146). SFN decreases TTSPs, such as TMPRSS2, by downregulating androgen receptor (AR) signaling, a receptor involved regulation of TMPRSS2 expression (61). Recent reports from Schultz et al. (155) have confirmed and expanded these findings showing that Nrf2 negatively regulates AR transactivation of androgen response genes such as TMPRSS2. The authors found that nuclear factor (erythroid-derived 2)-like 1, a cytoplasmic transactivator of the AR, is sequestered in the nucleus when Nrf2 is induced. These studies reveal that SFN increases SLPI secretion and decreases TMPRSS2 expression in a variety of models, as summarized in Fig. 2.
Fig. 2.
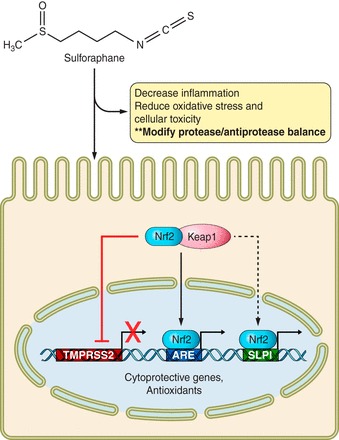
Sulforaphane (SFN) dampens inflammation, alleviates oxidative stress, and alters the protease/antiprotease balance. SFN enters the cell and induces Nrf2 release from its cytoplasmic repressor, Keap1. Nrf2 translocates into the nucleus and binds antioxidant response element (ARE) regions on various cytoprotective promoters. Secretory leukocyte protease inhibitor (SLPI) contains ARE sites on its promoter and SFN induces Nrf2-dependent activation of SLPI. Additionally, SFN decreases type II transmembrane serine proteases (TTSP) expression such as hepsin/transmembrane protease serine 2 (TMPRSS2).
Indeed, our studies indicate that SFN is protective against respiratory viruses such as IAV. We have shown that SFN decreases IAV replication by reducing IAV entry into respiratory epithelial cells (92). Furthermore, we and others have shown that SFN decreases TMPRSS2 expression, which we speculate is mediating the SFN-dependent protection against influenza (unpublished data) (61, 155). Furthermore, our group recently showed that sulforaphane-containing broccoli sprouts significantly decreased IL-6 and markers of viral replication in nasal lavage fluid from smokers in subjects inoculated with the live attenuated influenza virus vaccine (131). These results indicate that SFN may be a safe, low-cost intervention for decreasing influenza infection in susceptible populations such as smokers. On the basis of the data summarized above, we hypothesize that the anti-IAV effects of SFN may be mediated in a two-pronged approach: 1) by inducing SLPI into the nasal mucosa, which protects against influenza entry into respiratory epithelial cells, and 2) by decreasing TMPRSS2 secretion, which decreases HA activation and IAV replication (Fig. 3).
Fig. 3.

The protease/antiprotease balance regulates influenza A virus (IAV) infection and can be modulated by SFN supplementation. 1: For entry into target host cells, the hemagglutinin (HA) protein on the IAV virion must be proteolytically cleaved from HA0 (purple) into fusoactive HA1 and HA2 proteins (blue). 2: Respiratory serine proteases such as TMPRSS2 (TM), the human airway trypsin-like protease (HAT), and matriptase have been implicated in HA activation and IAV infection, which are likely to strain specific. Although antiproteases such as SLPI inhibit serine protease activity and protect against IAV infection, it remains unknown what HA-activating protease is inhibited by SLPI and how SLPI mediates these effects. 3: Lastly, SFN supplementation can be used to increase SLPI secretion, reduce TMPRSS2 secretion, and decrease viral entry and downstream IAV infection.
Summary
It is evident that respiratory epithelial cells contribute to respiratory health beyond barrier function and initiating immune responses. Respiratory epithelial cells secrete a variety of host defense mediators, including protease/antiprotease balance, that are involved in respiratory homeostasis and have been implicated in inflammation and viral infection. This balance can be modified by inhaled oxidants, such as cigarette smoke, and nutritional antioxidants, such as SFN. Thus employing the use of nutritional antioxidants to decrease specific proteases and increase antiproteases could protect against airway inflammation, lung disease, and infection. In addition, nutritional antioxidants could be a potential therapy during influenza pandemics, especially in the context when the influenza vaccine strains do not offer adequate protection match the circulating influenza virus strains, as seen in the current 2014–2015 influenza epidemic.
GRANTS
This research was funded by National Institutes of Health Grants R01HL095163 (I. Jaspers) and R01ES020897.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.M. and I.J. prepared figures; M.M. and I.J. drafted manuscript; M.M. and I.J. edited and revised manuscript; M.M. and I.J. approved final version of manuscript.
REFERENCES
- 1.Abboud RT, Vimalanathan S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int J Tuberc Lung Dis 12: 361–367, 2008. [PubMed] [Google Scholar]
- 2.Abe M, Tahara M, Sakai K, Yamaguchi H, Kanou K, Shirato K, Kawase M, Noda M, Kimura H, Matsuyama S, Fukuhara H, Mizuta K, Maenaka K, Ami Y, Esumi M, Kato A, Takeda M. TMPRSS2 is an activating protease for respiratory parainfluenza viruses. J Virol 87: 11930–11935, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afar DE, Vivanco I, Hubert RS, Kuo J, Chen E, Saffran DC, Raitano AB, Jakobovits A. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res 61: 1686–1692, 2001. [PubMed] [Google Scholar]
- 4.Annabi B, Rojas-Sutterlin S, Laroche M, Lachambre MP, Moumdjian R, Beliveau R. The diet-derived sulforaphane inhibits matrix metalloproteinase-9-activated human brain microvascular endothelial cell migration and tubulogenesis. Mol Nutr Food Res 52: 692–700, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Antalis TM, Buzza MS, Hodge KM, Hooper JD, Netzel-Arnett S. The cutting edge: membrane-anchored serine protease activities in the pericellular microenvironment. Biochem J 428: 325–346, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aron PM, Kennedy JA. Flavan-3-ols: nature, occurrence and biological activity. Mol Nutr Food Res 52: 79–104, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Ashcroft GS, Lei K, Jin W, Longenecker G, Kulkarni AB, Greenwell-Wild T, Hale-Donze H, McGrady G, Song XY, Wahl SM. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med 6: 1147–1153, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Asmal M, Whitney JB, Luedemann C, Carville A, Steen R, Letvin NL, Geiben-Lynn R. In vivo anti-HIV activity of the heparin-activated serine protease inhibitor antithrombin III encapsulated in lymph-targeting immunoliposomes. PLoS One 7: e48234, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baron J, Tarnow C, Mayoli-Nussle D, Schilling E, Meyer D, Hammami M, Schwalm F, Steinmetzer T, Guan Y, Garten W, Klenk HD, Bottcher-Friebertshauser E. Matriptase, HAT, and TMPRSS2 activate the hemagglutinin of H9N2 influenza A viruses. J Virol 87: 1811–1820, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaulieu A, Gravel E, Cloutier A, Marois I, Colombo E, Desilets A, Verreault C, Leduc R, Marsault E, Richter MV. Matriptase proteolytically activates influenza virus and promotes multicycle replication in the human airway epithelium. J Virol 87: 4237–4251, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belkowski SM, Masucci J, Mahan A, Kervinen J, Olson M, de Garavilla L, D'Andrea MR. Cleaved SLPI, a novel biomarker of chymase activity. Biol Chem 389: 1219–1224, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Benzie IF. Evolution of antioxidant defence mechanisms. Eur J Nutr 39: 53–61, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Beppu Y, Imamura Y, Tashiro M, Towatari T, Ariga H, Kido H. Human mucus protease inhibitor in airway fluids is a potential defensive compound against infection with influenza A and Sendai viruses. J Biochem 121: 309–316, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Bertaux B, Hornebeck W, Eisen AZ, Dubertret L. Growth stimulation of human keratinocytes by tissue inhibitor of metalloproteinases. J Invest Dermatol 97: 679–685, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Bertram S, Glowacka I, Blazejewska P, Soilleux E, Allen P, Danisch S, Steffen I, Choi SY, Park Y, Schneider H, Schughart K, Pohlmann S. TMPRSS2 and TMPRSS4 facilitate trypsin-independent spread of influenza virus in Caco-2 cells. J Virol 84: 10016–10025, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertram S, Glowacka I, Muller MA, Lavender H, Gnirss K, Nehlmeier I, Niemeyer D, He Y, Simmons G, Drosten C, Soilleux EJ, Jahn O, Steffen I, Pohlmann S. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J Virol 85: 13363–13372, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertram S, Glowacka I, Steffen I, Kuhl A, Pohlmann S. Novel insights into proteolytic cleavage of influenza virus hemagglutinin. Rev Med Virol 20: 298–310, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertram S, Heurich A, Lavender H, Gierer S, Danisch S, Perin P, Lucas JM, Nelson PS, Pohlmann S, Soilleux EJ. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One 7: e35876, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosch BJ, Bartelink W, Rottier PJ. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J Virol 82: 8887–8890, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottcher-Friebertshauser E, Freuer C, Sielaff F, Schmidt S, Eickmann M, Uhlendorff J, Steinmetzer T, Klenk HD, Garten W. Cleavage of influenza virus hemagglutinin by airway proteases TMPRSS2 and HAT differs in subcellular localization and susceptibility to protease inhibitors. J Virol 84: 5605–5614, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottcher E, Freuer C, Steinmetzer T, Klenk HD, Garten W. MDCK cells that express proteases TMPRSS2 and HAT provide a cell system to propagate influenza viruses in the absence of trypsin and to study cleavage of HA and its inhibition. Vaccine 27: 6324–6329, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Bottcher E, Matrosovich T, Beyerle M, Klenk HD, Garten W, Matrosovich M. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J Virol 80: 9896–9898, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 1803: 55–71, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem 284: 23177–23181, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buhling F, Waldburg N, Gerber A, Hackel C, Kruger S, Reinhold D, Bromme D, Weber E, Ansorge S, Welte T. Cathepsin K expression in human lung. Adv Exp Med Biol 477: 281–286, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Burster T, Giffon T, Dahl ME, Bjorck P, Bogyo M, Weber E, Mahmood K, Lewis DB, Mellins ED. Influenza A virus elevates active cathepsin B in primary murine DC. Int Immunol 19: 645–655, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Cataldo D, Munaut C, Noel A, Frankenne F, Bartsch P, Foidart JM, Louis R. Matrix metalloproteinases and TIMP-1 production by peripheral blood granulocytes from COPD patients and asthmatics. Allergy 56: 145–151, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Cavarra E, Lucattelli M, Gambelli F, Bartalesi B, Fineschi S, Szarka A, Giannerini F, Martorana PA, Lungarella G. Human SLPI inactivation after cigarette smoke exposure in a new in vivo model of pulmonary oxidative stress. Am J Physiol Lung Cell Mol Physiol 281: L412–L417, 2001. [DOI] [PubMed] [Google Scholar]
- 29a.Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 62: 1–43, 2013. [PubMed] [Google Scholar]
- 30.Chen YW, Lee MS, Lucht A, Chou FP, Huang W, Havighurst TC, Kim K, Wang JK, Antalis TM, Johnson MD, Lin CY. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol 176: 2986–2996, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho HY, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, Polack FP, Kleeberger SR. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am J Respir Crit Care Med 179: 138–150, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol Appl Pharmacol 244: 43–56, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal 8: 76–87, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Chokki M, Yamamura S, Eguchi H, Masegi T, Horiuchi H, Tanabe H, Kamimura T, Yasuoka S. Human airway trypsin-like protease increases mucin gene expression in airway epithelial cells. Am J Respir Cell Mol Biol 30: 470–478, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Cohen AB. Interrelationships between the human alveolar macrophage and alpha-1-antitrypsin. J Clin Invest 52: 2793–2799, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connor RJ, Kawaoka Y, Webster RG, Paulson JC. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology 205: 17–23, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Cooper MD, Roberts MH, Barauskas OL, Jarvis GA. Secretory leukocyte protease inhibitor binds to Neisseria gonorrhoeae outer membrane opacity protein and is bactericidal. Am J Reprod Immunol 68: 116–127, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corcoran MP, McKay DL, Blumberg JB. Flavonoid basics: chemistry, sources, mechanisms of action, and safety. J Nutr Gerontol Geriatr 31: 176–189, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Cseke G, Maginnis MS, Cox RG, Tollefson SJ, Podsiad AB, Wright DW, Dermody TS, Williams JV. Integrin alphavbeta1 promotes infection by human metapneumovirus. Proc Natl Acad Sci USA 106: 1566–1571, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Culpitt SV, Rogers DF, Traves SL, Barnes PJ, Donnelly LE. Sputum matrix metalloproteases: comparison between chronic obstructive pulmonary disease and asthma. Respir Med 99: 703–710, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Dahlen B, Shute J, Howarth P. Immunohistochemical localisation of the matrix metalloproteinases MMP-3 and MMP-9 within the airways in asthma. Thorax 54: 590–596, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davey A, McAuley DF, O'Kane CM. Matrix metalloproteinases in acute lung injury: mediators of injury and drivers of repair. Eur Respir J 38: 959–970, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 18: 1818–1892, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dittmann M, Hoffmann HH, Scull MA, Gilmore RH, Bell KL, Ciancanelli M, Wilson SJ, Crotta S, Yu Y, Flatley B, Xiao JW, Casanova JL, Wack A, Bieniasz PD, Rice CM. A serpin shapes the extracellular environment to prevent influenza a virus maturation. Cell 160: 631–643, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, Gabriel SE. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem 277: 8338–8345, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Donnelly LE, Rogers DF. Therapy for chronic obstructive pulmonary disease in the 21st century. Drugs 63: 1973–1998, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Doring G, Frank F, Boudier C, Herbert S, Fleischer B, Bellon G. Cleavage of lymphocyte surface antigens CD2, CD4, and CD8 by polymorphonuclear leukocyte elastase and cathepsin G in patients with cystic fibrosis. J Immunol 154: 4842–4850, 1995. [PubMed] [Google Scholar]
- 48.Doumas S, Kolokotronis A, Stefanopoulos P. Anti-inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect Immun 73: 1271–1274, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duthie GG, Duthie SJ, Kyle JA. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr Res Rev 13: 79–106, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Eisenberg SP, Hale KK, Heimdal P, Thompson RC. Location of the protease-inhibitory region of secretory leukocyte protease inhibitor. J Biol Chem 265: 7976–7981, 1990. [PubMed] [Google Scholar]
- 51.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax 61: 259–266, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elliott MB, Welliver RC Sr, Laughlin TS, Pryharski KS, LaPierre NA, Chen T, Souza V, Terio NB, Hancock GE. Matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in the respiratory tracts of human infants following paramyxovirus infection. J Med Virol 79: 447–456, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis 185: 1606–1613, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Flannery B, Clippard J, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, Petrie JG, McLean HQ, Belongia EA, Gaglani M, Berman L, Foust A, Sessions W, Thaker SN, Spencer S, Fry AM. Early estimates of seasonal influenza vaccine effectiveness — United States, January 2015. MMWR Morb Mortal Wkly Rep 64: 10–15, 2015. [PMC free article] [PubMed] [Google Scholar]
- 55.Fukushima K, Takahashi T, Ito S, Takaguchi M, Takano M, Kurebayashi Y, Oishi K, Minami A, Kato T, Park EY, Nishimura H, Takimoto T, Suzuki T. Terminal sialic acid linkages determine different cell infectivities of human parainfluenza virus type 1 and type 3. Virology: 464–465: 424–431, 2014. [DOI] [PubMed] [Google Scholar]
- 56.Galloway SE, Reed ML, Russell CJ, Steinhauer DA. Influenza HA subtypes demonstrate divergent phenotypes for cleavage activation and pH of fusion: implications for host range and adaptation. PLoS Pathog 9: e1003151, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gatto M, Iaccarino L, Ghirardello A, Bassi N, Pontisso P, Punzi L, Shoenfeld Y, Doria A. Serpins, immunity and autoimmunity: old molecules, new functions. Clin Rev Allergy Immunol 45: 267–280, 2013. [DOI] [PubMed] [Google Scholar]
- 58.Gerber A, Welte T, Ansorge S, Buhling F. Expression of cathepsins B and L in human lung epithelial cells is regulated by cytokines. Adv Exp Med Biol 477: 287–292, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. Trappin-2/elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology 129: 207–219, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibbons AM, McElvaney NG, Taggart CC, Cryan SA. Delivery of rSLPI in a liposomal carrier for inhalation provides protection against cathepsin L degradation. J Microencapsul 26: 513–522, 2009. [DOI] [PubMed] [Google Scholar]
- 61.Gibbs A, Schwartzman J, Deng V, Alumkal J. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc Natl Acad Sci USA 106: 16663–16668, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gipson TS, Bless NM, Shanley TP, Crouch LD, Bleavins MR, Younkin EM, Sarma V, Gibbs DF, Tefera W, McConnell PC, Mueller WT, Johnson KJ, Ward PA. Regulatory effects of endogenous protease inhibitors in acute lung inflammatory injury. J Immunol 162: 3653–3662, 1999. [PubMed] [Google Scholar]
- 63.Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol 647: 37–74, 2010. [DOI] [PubMed] [Google Scholar]
- 64.Glowacka I, Bertram S, Muller MA, Allen P, Soilleux E, Pfefferle S, Steffen I, Tsegaye TS, He Y, Gnirss K, Niemeyer D, Schneider H, Drosten C, Pohlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol 85: 4122–4134, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gompertz S, Stockley RA. Inflammation—role of the neutrophil and the eosinophil. Semin Respir Infect 15: 14–23, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Greene CM, McElvaney NG. Proteases and antiproteases in chronic neutrophilic lung disease - relevance to drug discovery. Br J Pharmacol 158: 1048–1058, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greene CM, McElvaney NG, O'Neill SJ, Taggart CC. Secretory leucoprotease inhibitor impairs Toll-like receptor 2- and 4-mediated responses in monocytic cells. Infect Immun 72: 3684–3687, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rec 87: 69–98, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greve JM, Davis G, Meyer AM, Forte CP, Yost SC, Marlor CW, Kamarck ME, McClelland A. The major human rhinovirus receptor is ICAM-1. Cell 56: 839–847, 1989. [DOI] [PubMed] [Google Scholar]
- 70.Hallak LK, Spillmann D, Collins PL, Peeples ME. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol 74: 10508–10513, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr 16: 33–50, 1996. [DOI] [PubMed] [Google Scholar]
- 72.Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic Res 25: 57–74, 1996. [DOI] [PubMed] [Google Scholar]
- 73.Hamilton BS, Gludish DW, Whittaker GR. Cleavage activation of the human-adapted influenza virus subtypes by matriptase reveals both subtype and strain specificities. J Virol 86: 10579–10586, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, Feller-Kopman D, Wise R, Biswal S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med 3: 78ra32, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hatesuer B, Bertram S, Mehnert N, Bahgat MM, Nelson PS, Pohlman S, Schughart K. Tmprss2 is essential for influenza H1N1 virus pathogenesis in mice. PLoS Pathog 9: e1003774, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett 298: 29–32, 1992. [DOI] [PubMed] [Google Scholar]
- 77.Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 88: 1293–1307, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hiemstra PS. Novel roles of protease inhibitors in infection and inflammation. Biochem Soc Trans 30: 116–120, 2002. [DOI] [PubMed] [Google Scholar]
- 79.Hiemstra PS, Maassen RJ, Stolk J, Heinzel-Wieland R, Steffens GJ, Dijkman JH. Antibacterial activity of antileukoprotease. Infect Immun 64: 4520–4524, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hiemstra PS, van Wetering S, Stolk J. Neutrophil serine proteinases and defensins in chronic obstructive pulmonary disease: effects on pulmonary epithelium. Eur Respir J 12: 1200–1208, 1998. [DOI] [PubMed] [Google Scholar]
- 81.Hoffmann M, Quabius ES, Tribius S, Hebebrand L, Gorogh T, Halec G, Kahn T, Hedderich J, Rocken C, Haag J, Waterboer T, Schmitt M, Giuliano AR, Kast WM. Human papillomavirus infection in head and neck cancer: the role of the secretory leukocyte protease inhibitor. Oncol Rep 29: 1962–1968, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hooper JD, Nicol DL, Dickinson JL, Eyre HJ, Scarman AL, Normyle JF, Stuttgen MA, Douglas ML, Loveland KA, Sutherland GR, Antalis TM. Testisin, a new human serine proteinase expressed by premeiotic testicular germ cells and lost in testicular germ cell tumors. Cancer Res 59: 3199–3205, 1999. [PubMed] [Google Scholar]
- 83.Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, Sakamoto T, Shimura M, Yoshida A, Yamamoto M, Sekizawa K. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells 10: 1113–1125, 2005. [DOI] [PubMed] [Google Scholar]
- 84.Imai K, Dalal SS, Chen ES, Downey R, Schulman LL, Ginsburg M, D'Armiento J. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am J Respir Crit Care Med 163: 786–791, 2001. [DOI] [PubMed] [Google Scholar]
- 85.Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med 36: 1208–1213, 2004. [DOI] [PubMed] [Google Scholar]
- 86.Jana NK, Gray LR, Shugars DC. Human immunodeficiency virus type 1 stimulates the expression and production of secretory leukocyte protease inhibitor (SLPI) in oral epithelial cells: a role for SLPI in innate mucosal immunity. J Virol 79: 6432–6440, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, Hori N, Watanabe T, Takahashi K, Nagawa H. Epigallocatechin gallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. J Allergy Clin Immunol 112: 951–957, 2003. [DOI] [PubMed] [Google Scholar]
- 88.Kelly FJ. Vitamins and respiratory disease: antioxidant micronutrients in pulmonary health and disease. Proc Nutr Soc 64: 510–526, 2005. [DOI] [PubMed] [Google Scholar]
- 89.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89–116, 2007. [DOI] [PubMed] [Google Scholar]
- 90.Kersul AL, Iglesias A, Rios A, Noguera A, Forteza A, Serra E, Agusti A, Cosio BG. Molecular mechanisms of inflammation during exacerbations of chronic obstructive pulmonary disease. Arch Bronconeumol 47: 176–183, 2011. [DOI] [PubMed] [Google Scholar]
- 91.Kesic MJ, Meyer M, Bauer R, Jaspers I. Exposure to ozone modulates human airway protease/antiprotease balance contributing to increased influenza A infection. PLoS One 7: e35108, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kesic MJ, Simmons SO, Bauer R, Jaspers I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic Biol Med 51: 444–453, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kido H, Beppu Y, Imamura Y, Chen Y, Murakami M, Oba K, Towatari T. The human mucus protease inhibitor and its mutants are novel defensive compounds against infection with influenza A and Sendai viruses. Biopolymers 51: 79–86, 1999. [DOI] [PubMed] [Google Scholar]
- 94.Kido H, Murakami M, Oba K, Chen Y, Towatari T. Cellular proteinases trigger the infectivity of the influenza A and Sendai viruses. Mol Cells 9: 235–244, 1999. [PubMed] [Google Scholar]
- 95.Kido H, Okumura Y, Yamada H, Le TQ, Yano M. Proteases essential for human influenza virus entry into cells and their inhibitors as potential therapeutic agents. Curr Pharm Des 13: 405–414, 2007. [DOI] [PubMed] [Google Scholar]
- 96.Kong SL, Chui P, Lim B, Salto-Tellez M. Elucidating the molecular physiopathology of acute respiratory distress syndrome in severe acute respiratory syndrome patients. Virus Res 145: 260–269, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kong YJ, Sun WX, Zhang YM, Shi YZ. [Relationships between the expressions of intercellular adhesion molecule-1 and tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9 in lung tissues of patients with chronic obstructive pulmonary disease]. Zhonghua Jie He He Hu Xi Za Zhi (Chinese J Tubercul Resp Dis) 31: 129–133, 2008. [PubMed] [Google Scholar]
- 98.Lakadamyali M, Rust MJ, Babcock HP, Zhuang X. Visualizing infection of individual influenza viruses. Proc Natl Acad Sci USA 100: 9280–9285, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med 363: 2036–2044, 2010. [DOI] [PubMed] [Google Scholar]
- 100.Lavigne MC, Thakker P, Gunn J, Wong A, Miyashiro JS, Wasserman AM, Wei SQ, Pelker JW, Kobayashi M, Eppihimer MJ. Human bronchial epithelial cells express and secrete MMP-12. Biochem Biophys Res Commun 324: 534–546, 2004. [DOI] [PubMed] [Google Scholar]
- 101.Law RH, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI, Whisstock JC. An overview of the serpin superfamily. Genome Biol 7: 216, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lazarowitz SG, Compans RW, Choppin PW. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology 46: 830–843, 1971. [DOI] [PubMed] [Google Scholar]
- 103.Lee MH, Rapti M, Murphy G. Delineating the molecular basis of the inactivity of tissue inhibitor of metalloproteinase-2 against tumor necrosis factor-alpha-converting enzyme. J Biol Chem 279: 45121–45129, 2004. [DOI] [PubMed] [Google Scholar]
- 104.Lee SL, Dickson RB, Lin CY. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem 275: 36720–36725, 2000. [DOI] [PubMed] [Google Scholar]
- 105.Lee YH, Lai CL, Hsieh SH, Shieh CC, Huang LM, Wu-Hsieh BA. Influenza A virus induction of oxidative stress and MMP-9 is associated with severe lung pathology in a mouse model. Virus Res 178: 411–422, 2013. [DOI] [PubMed] [Google Scholar]
- 106.Lee YJ, Lee SH. Sulforaphane induces antioxidative and antiproliferative responses by generating reactive oxygen species in human bronchial epithelial BEAS-2B cells. J Korean Med Sci 26: 1474–1482, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ling JX, Wei F, Li N, Li JL, Chen LJ, Liu YY, Luo F, Xiong HR, Hou W, Yang ZQ. Amelioration of influenza virus-induced reactive oxygen species formation by epigallocatechin gallate derived from green tea. Acta Pharmacol Sin 33: 1533–1541, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lomas DA. Molecular mousetraps, alpha1-antitrypsin deficiency and the serpinopathies. Clin Med 5: 249–257, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mackie PL. The classification of viruses infecting the respiratory tract. Paediatr Respir Rev 4: 84–90, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev 32: 687–726, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med 178: 592–604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Mallia P, Footitt J, Sotero R, Jepson A, Contoli M, Trujillo-Torralbo MB, Kebadze T, Aniscenko J, Oleszkiewicz G, Gray K, Message SD, Ito K, Barnes PJ, Adcock IM, Papi A, Stanciu LA, Elkin SL, Kon OM, Johnson M, Johnston SL. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in COPD. Am J Respir Crit Care Med 186: 1117–1124, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mao L, Wang HD, Wang XL, Qiao L, Yin HX. Sulforaphane attenuates matrix metalloproteinase-9 expression following spinal cord injury in mice. Ann Clin Lab Sci 40: 354–360, 2010. [PubMed] [Google Scholar]
- 115.Mason DY, Cramer EM, Masse JM, Crystal R, Bassot JM, Breton-Gorius J. Alpha 1-antitrypsin is present within the primary granules of human polymorphonuclear leukocytes. Am J Pathol 139: 623–628, 1991. [PMC free article] [PubMed] [Google Scholar]
- 116.Masuda K, Suga T, Takeuchi A, Kanesaki M, Imaizumi A, Suzuki Y. Specific cleavage of secretory leukoprotease inhibitor by neutrophil elastase and saliva. Biochem Pharmacol 48: 651–657, 1994. [DOI] [PubMed] [Google Scholar]
- 117.McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest 96: 456–464, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McNeely TB, Shugars DC, Rosendahl M, Tucker C, Eisenberg SP, Wahl SM. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood 90: 1141–1149, 1997. [PubMed] [Google Scholar]
- 119.Meier O, Greber UF. Adenovirus endocytosis. J Gene Med 5: 451–462, 2003. [DOI] [PubMed] [Google Scholar]
- 120.Mercer PF, Shute JK, Bhowmik A, Donaldson GC, Wedzicha JA, Warner JA. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res 6: 151, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meyer M, Bauer RN, Letang BD, Brighton LE, Thompson E, Simmen RC, Bonner J, Jaspers I. The regulation and activity of secretory leukoprotease inhibitor (SLPI) is altered in smokers. Am J Physiol Lung Cell Mol Physiol 306: L269–L276, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meyer M, Kesic MJ, Clarke J, Ho E, Simmen RC, Diaz-Sanchez D, Noah TL, Jaspers I. Sulforaphane induces SLPI secretion in the nasal mucosa. Respir Med 107: 472–475, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mihaila A, Tremblay GM. Human alveolar macrophages express elafin and secretory leukocyte protease inhibitor. Z Naturforsch C 56: 291–297, 2001. [DOI] [PubMed] [Google Scholar]
- 124.Miller KW, Evans RJ, Eisenberg SP, Thompson RC. Secretory leukocyte protease inhibitor binding to mRNA and DNA as a possible cause of toxicity to Escherichia coli. J Bacteriol 171: 2166–2172, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Muller L, Jaspers I. Epithelial cells, the “switchboard” of respiratory immune defense responses: effects of air pollutants. Swiss Med Wkly 142: w13653, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mulligan MS, Lentsch AB, Huber-Lang M, Guo RF, Sarma V, Wright CD, Ulich TR, Ward PA. Anti-inflammatory effects of mutant forms of secretory leukocyte protease inhibitor. Am J Pathol 156: 1033–1039, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakayama M, Suzuki K, Toda M, Okubo S, Hara Y, Shimamura T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res 21: 289–299, 1993. [DOI] [PubMed] [Google Scholar]
- 128.Nance CL, Siwak EB, Shearer WT. Preclinical development of the green tea catechin, epigallocatechin gallate, as an HIV-1 therapy. J Allergy Clin Immunol 123: 459–465, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Newcombe NG, Johansson ES, Au G, Lindberg AM, Barry RD, Shafren DR. Enterovirus capsid interactions with decay-accelerating factor mediate lytic cell infection. J Virol 78: 1431–1439, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nita I, Hollander C, Westin U, Janciauskiene SM. Prolastin, a pharmaceutical preparation of purified human alpha1-antitrypsin, blocks endotoxin-mediated cytokine release. Respir Res 6: 12, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Noah TL, Zhang H, Zhou H, Glista-Baker E, Muller L, Bauer RN, Meyer M, Murphy PC, Jones S, Letang B, Robinette C, Jaspers I. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: a randomized, double-blind study. PLoS One 9: e98671, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Noyce RS, Bondre DG, Ha MN, Lin LT, Sisson G, Tsao MS, Richardson CD. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog 7: e1002240, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Noyce RS, Richardson CD. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol 20: 429–439, 2012. [DOI] [PubMed] [Google Scholar]
- 134.Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol 22: 571–579, 2001. [DOI] [PubMed] [Google Scholar]
- 135.Parameswaran GI, Wrona CT, Murphy TF, Sethi S. Moraxella catarrhalis acquisition, airway inflammation and protease-antiprotease balance in chronic obstructive pulmonary disease. BMC Infect Dis 9: 178, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pfundt R, Wingens M, Bergers M, Zweers M, Frenken M, Schalkwijk J. TNF-alpha and serum induce SKALP/elafin gene expression in human keratinocytes by a p38 MAP kinase-dependent pathway. Arch Dermatol Res 292: 180–187, 2000. [DOI] [PubMed] [Google Scholar]
- 137.Quabius ES, Moller P, Haag J, Pfannenschmidt S, Hedderich J, Gorogh T, Rocken C, Hoffmann M. The role of the antileukoprotease SLPI in smoking-induced human papillomavirus-independent head and neck squamous cell carcinomas. Int J Cancer 134: 1323–1334, 2014. [DOI] [PubMed] [Google Scholar]
- 138.Quenelle DC, Hartman TL, Buckheit RW, Prichard MN, Lynn RG. Anti-HSV activity of serpin antithrombin III. Int Trends Immun 2: 87–92, 2014. [PMC free article] [PubMed] [Google Scholar]
- 139.Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495: 251–254, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ramadas RA, Wu L, LeVine AM. Surfactant protein A enhances production of secretory leukoprotease inhibitor and protects it from cleavage by matrix metalloproteinases. J Immunol 182: 1560–1567, 2009. [DOI] [PubMed] [Google Scholar]
- 141.Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell 69: 597–604, 1992. [DOI] [PubMed] [Google Scholar]
- 142.Ren Y, He QY, Fan J, Jones B, Zhou Y, Xie Y, Cheung CY, Wu A, Chiu JF, Peiris JS, Tam PK. The use of proteomics in the discovery of serum biomarkers from patients with severe acute respiratory syndrome. Proteomics 4: 3477–3484, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol 130: 244–251, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity 4: 357–366, 1996. [DOI] [PubMed] [Google Scholar]
- 145.Ritz SA, Wan J, Diaz-Sanchez D. Sulforaphane-stimulated phase II enzyme induction inhibits cytokine production by airway epithelial cells stimulated with diesel extract. Am J Physiol Lung Cell Mol Physiol 292: L33–L39, 2007. [DOI] [PubMed] [Google Scholar]
- 146.Rose P, Huang Q, Ong CN, Whiteman M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol 209: 105–113, 2005. [DOI] [PubMed] [Google Scholar]
- 147.Russell RE, Thorley A, Culpitt SV, Dodd S, Donnelly LE, Demattos C, Fitzgerald M, Barnes PJ. Alveolar macrophage-mediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteases. Am J Physiol Lung Cell Mol Physiol 283: L867–L873, 2002. [DOI] [PubMed] [Google Scholar]
- 148.Sakai K, Ami Y, Tahara M, Kubota T, Anraku M, Abe M, Nakajima N, Sekizuka T, Shirato K, Suzaki Y, Ainai A, Nakatsu Y, Kanou K, Nakamura K, Suzuki T, Komase K, Nobusawa E, Maenaka K, Kuroda M, Hasegawa H, Kawaoka Y, Tashiro M, Takeda M. The host protease TMPRSS2 plays a major role in in vivo replication of emerging H7N9 and seasonal influenza viruses. J Virol 88: 5608–5616, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sallenave JM. The role of secretory leukocyte proteinase inhibitor and elafin (elastase-specific inhibitor/skin-derived antileukoprotease) as alarm antiproteinases in inflammatory lung disease. Respir Res 1: 87–92, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sallenave JM. Secretory leukocyte protease inhibitor and elafin/trappin-2: versatile mucosal antimicrobials and regulators of immunity. Am J Respir Cell Mol Biol 42: 635–643, 2010. [DOI] [PubMed] [Google Scholar]
- 151.Sallenave JM, Si Tahar M, Cox G, Chignard M, Gauldie J. Secretory leukocyte proteinase inhibitor is a major leukocyte elastase inhibitor in human neutrophils. J Leukoc Biol 61: 695–702, 1997. [DOI] [PubMed] [Google Scholar]
- 152.Sanders CJ, Doherty PC, Thomas PG. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res 343: 13–21, 2011. [DOI] [PubMed] [Google Scholar]
- 153.Schalkwijk J, Wiedow O, Hirose S. The trappin gene family: proteins defined by an N-terminal transglutaminase substrate domain and a COOH-terminal four-disulphide core. Biochem J 340: 569–577, 1999. [PMC free article] [PubMed] [Google Scholar]
- 154.Schick C, Pemberton PA, Shi GP, Kamachi Y, Cataltepe S, Bartuski AJ, Gornstein ER, Bromme D, Chapman HA, Silverman GA. Cross-class inhibition of the cysteine proteinases cathepsins K, L, and S by the serpin squamous cell carcinoma antigen 1: a kinetic analysis. Biochemistry 37: 5258–5266, 1998. [DOI] [PubMed] [Google Scholar]
- 155.Schultz MA, Hagan SS, Datta A, Zhang Y, Freeman ML, Sikka SC, Abdel-Mageed AB, Mondal D. Nrf1 and Nrf2 transcription factors regulate androgen receptor transactivation in prostate cancer cells. PLoS One 9: e87204, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Scott A, Weldon S, Taggart CC. SLPI and elafin: multifunctional antiproteases of the WFDC family. Biochem Soc Trans 39: 1437–1440, 2011. [DOI] [PubMed] [Google Scholar]
- 157.Shirato K, Kawase M, Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol 87: 12552–12561, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shirogane Y, Takeda M, Iwasaki M, Ishiguro N, Takeuchi H, Nakatsu Y, Tahara M, Kikuta H, Yanagi Y. Efficient multiplication of human metapneumovirus in Vero cells expressing the transmembrane serine protease TMPRSS2. J Virol 82: 8942–8946, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Siegfried W, Rosenfeld M, Stier L, Stratford-Perricaudet L, Perricaudet M, Pavirani A, Lecocq JP, Crystal RG. Polarity of secretion of alpha 1-antitrypsin by human respiratory epithelial cells after adenoviral transfer of a human alpha 1-antitrypsin cDNA. Am J Respir Cell Mol Biol 12: 379–384, 1995. [DOI] [PubMed] [Google Scholar]
- 160.Simpson AJ, Wallace WA, Marsden ME, Govan JR, Porteous DJ, Haslett C, Sallenave JM. Adenoviral augmentation of elafin protects the lung against acute injury mediated by activated neutrophils and bacterial infection. J Immunol 167: 1778–1786, 2001. [DOI] [PubMed] [Google Scholar]
- 161.Smee DF, Hurst BL, Day CW, Geiben-Lynn R. Influenza virus H1N1 inhibition by serine protease inhibitor (serpin) antithrombin III. Int Trends Immun 2: 83–86, 2014. [PMC free article] [PubMed] [Google Scholar]
- 162.Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459: 1122–1125, 2009. [DOI] [PubMed] [Google Scholar]
- 163.Song JM, Lee KH, Seong BL. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res 68: 66–74, 2005. [DOI] [PubMed] [Google Scholar]
- 164.Starcher BC. Lung elastin and matrix. Chest 117: 229S–234S, 2000. [DOI] [PubMed] [Google Scholar]
- 165.Starrett W, Blake DJ. Sulforaphane inhibits de novo synthesis of IL-8 and MCP-1 in human epithelial cells generated by cigarette smoke extract. J Immunotoxicol 8: 150–158, 2011. [DOI] [PubMed] [Google Scholar]
- 166.Stieneke-Grober A, Vey M, Angliker H, Shaw E, Thomas G, Roberts C, Klenk HD, Garten W. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J 11: 2407–2414, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stockley RA. Neutrophils and protease/antiprotease imbalance. Am J Respir Crit Care Med 160: S49–S52, 1999. [DOI] [PubMed] [Google Scholar]
- 168.Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet 365: 2225–2236, 2005. [DOI] [PubMed] [Google Scholar]
- 169.Stoller JK, Aboussouan LS. A review of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med 185: 246–259, 2012. [DOI] [PubMed] [Google Scholar]
- 170.Sullivan AL, Dafforn T, Hiemstra PS, Stockley RA. Neutrophil elastase reduces secretion of secretory leukoproteinase inhibitor (SLPI) by lung epithelial cells: role of charge of the proteinase-inhibitor complex. Respir Res 9: 60, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tacon CE, Wiehler S, Holden NS, Newton R, Proud D, Leigh R. Human rhinovirus infection up-regulates MMP-9 production in airway epithelial cells via NF-κB. Am J Respir Cell Mol Biol 43: 201–209, 2010. [DOI] [PubMed] [Google Scholar]
- 172.Taggart CC, Cryan SA, Weldon S, Gibbons A, Greene CM, Kelly E, Low TB, O'Neill SJ, McElvaney NG. Secretory leucoprotease inhibitor binds to NF-kappaB binding sites in monocytes and inhibits p65 binding. J Exp Med 202: 1659–1668, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Taggart CC, Greene CM, McElvaney NG, O'Neill S. Secretory leucoprotease inhibitor prevents lipopolysaccharide-induced IkappaBalpha degradation without affecting phosphorylation or ubiquitination. J Biol Chem 277: 33648–33653, 2002. [DOI] [PubMed] [Google Scholar]
- 174.Taggart CC, Lowe GJ, Greene CM, Mulgrew AT, O'Neill SJ, Levine RL, McElvaney NG. Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor. J Biol Chem 276: 33345–33352, 2001. [DOI] [PubMed] [Google Scholar]
- 175.Takahashi M, Sano T, Yamaoka K, Kamimura T, Umemoto N, Nishitani H, Yasuoka S. Localization of human airway trypsin-like protease in the airway: an immunohistochemical study. Histochem Cell Biol 115: 181–187, 2001. [DOI] [PubMed] [Google Scholar]
- 176.Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem 275: 26333–26342, 2000. [DOI] [PubMed] [Google Scholar]
- 177.Tan XL, Shi M, Tang H, Han W, Spivack SD. Candidate dietary phytochemicals modulate expression of phase II enzymes GSTP1 and NQO1 in human lung cells. J Nutr 140: 1404–1410, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tarnow C, Engels G, Arendt A, Schwalm F, Sediri H, Preuss A, Nelson PS, Garten W, Klenk HD, Gabriel G, Bottcher-Friebertshauser E. TMPRSS2 is a host factor that is essential for pneumotropism and pathogenicity of H7N9 influenza A virus in mice. J Virol 88: 4744–4751, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tomee JF, Hiemstra PS, Heinzel-Wieland R, Kauffman HF. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J Infect Dis 176: 740–747, 1997. [DOI] [PubMed] [Google Scholar]
- 180.Tosi MF, Zakem H, Berger M. Neutrophil elastase cleaves C3bi on opsonized pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Invest 86: 300–308, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Van-Seuningen I, Davril M. Separation of the two domains of human mucus proteinase inhibitor: inhibitory activity is only located in the carboxy-terminal domain. Biochem Biophys Res Commun 179: 1587–1592, 1991. [DOI] [PubMed] [Google Scholar]
- 182.Vey M, Orlich M, Adler S, Klenk HD, Rott R, Garten W. Hemagglutinin activation of pathogenic avian influenza viruses of serotype H7 requires the protease recognition motif R-X-K/R-R. Virology 188: 408–413, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vogelmeier C, Biedermann T, Maier K, Mazur G, Behr J, Krombach F, Buhl R. Comparative loss of activity of recombinant secretory leukoprotease inhibitor and alpha 1-protease inhibitor caused by different forms of oxidative stress. Eur Respir J 10: 2114–2119, 1997. [DOI] [PubMed] [Google Scholar]
- 184.Vogelmeier C, Gillissen A, Buhl R. Use of secretory leukoprotease inhibitor to augment lung antineutrophil elastase activity. Chest 110: 261S–266S, 1996. [DOI] [PubMed] [Google Scholar]
- 185.Wang S, Quang Le T, Chida J, Cisse Y, Yano M, Kido H. Mechanisms of matrix metalloproteinase-9 upregulation and tissue destruction in various organs in influenza A virus infection. J Med Invest 57: 26–34, 2010. [DOI] [PubMed] [Google Scholar]
- 186.Weldon S, McNally P, McElvaney NG, Elborn JS, McAuley DF, Wartelle J, Belaaouaj A, Levine RL, Taggart CC. Decreased levels of secretory leucoprotease inhibitor in the Pseudomonas-infected cystic fibrosis lung are due to neutrophil elastase degradation. J Immunol 183: 8148–8156, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Williams SE, Brown TI, Roghanian A, Sallenave JM. SLPI and elafin: one glove, many fingers. Clin Sci (Lond) 110: 21–35, 2006. [DOI] [PubMed] [Google Scholar]
- 188.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286: 113–117, 1999. [DOI] [PubMed] [Google Scholar]
- 189.Yasuoka S, Ohnishi T, Kawano S, Tsuchihashi S, Ogawara M, Masuda K, Yamaoka K, Takahashi M, Sano T. Purification, characterization, and localization of a novel trypsin-like protease found in the human airway. Am J Respir Cell Mol Biol 16: 300–308, 1997. [DOI] [PubMed] [Google Scholar]
- 190.Yeo SJ, Kim SJ, Kim JH, Lee HJ, Kook YH. Influenza A virus infection modulates the expression of type IV collagenase in epithelial cells. Arch Virol 144: 1361–1370, 1999. [DOI] [PubMed] [Google Scholar]
- 191.Yeo SJ, Yun YJ, Lyu MA, Woo SY, Woo ER, Kim SJ, Lee HJ, Park HK, Kook YH. Respiratory syncytial virus infection induces matrix metalloproteinase-9 expression in epithelial cells. Arch Virol 147: 229–242, 2002. [DOI] [PubMed] [Google Scholar]
- 192.Ying QL, Kemme M, Simon SR. Functions of the N-terminal domain of secretory leukoprotease inhibitor. Biochemistry 33: 5445–5450, 1994. [DOI] [PubMed] [Google Scholar]
- 193.Zani ML, Nobar SM, Lacour SA, Lemoine S, Boudier C, Bieth JG, Moreau T. Kinetics of the inhibition of neutrophil proteinases by recombinant elafin and pre-elafin (trappin-2) expressed in Pichia pastoris. Eur J Biochem 271: 2370–2378, 2004. [DOI] [PubMed] [Google Scholar]
- 194.Zmora P, Blazejewska P, Moldenhauer AS, Welsch K, Nehlmeier I, Wu Q, Schneider H, Pohlmann S, Bertram S. DESC1 and MSPL activate influenza A viruses and emerging coronaviruses for host cell entry. J Virol 88: 12087–12097, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


