Abstract
Objectives:
Burn injury is one of the most health-threatening problems in the world. Malva sylvestris (M. sylvestris) flowers have a high mucilage content and are used as a remedy for cut wound and dermal infected wounds in Iranian folklore Medicine. The purpose of this study was to investigate the effect of M. sylvestris cream on the second degree burn injury in rats.
Materials and Methods:
Five groups of 10 rats per group were burned with hot metal plate. Animals were administrated divided as control, normal saline, standard silver sulfadiazine 1% (SSD), 5% M. sylvestris, and 10% M. sylvestris into separate groups. Wound area, percentage of wound contraction, and histological and bacteriological assessments were evaluated.
Results:
Wound sizes were not significantly different among groups on 1st and 3rd days after burn injury, while they were significantly different among groups after 7th day post-burn injury. The average areas of wounds on the 15th day were 7.5±2.9, 6.7±2, 10.5±1.6, 4.7±2, and 4.5±2 cm2 for base cream, normal saline, SSD, 5% M. sylvestris, and 10% M. sylvestris, respectively. The results of histology exhibited well-formed horizontally-oriented collagen fibers in MS topical treatment groups. Microorganisms existed in the SSD group were most probably Staphilococcus epidermitis and for NS group were staphylococcus saprophiteccus.
Conclusion:
M. sylvestris cream improved histological changes of tissue components in the process of healing when compared with SSD cream. Therefore, it can be used as a topical treatment agent for burn wound.
Key Words: Malva sylvestris, Burns, Silver sulfadiazine, Rats, Wound healing
Introduction
Burn injury is one of the most health-threatening problems in the world (Forjuoh 2006 ▶). Over 6.6 million people world-wide suffer from burns and almost 265000 of them die annually (Penn et al., 2012 ▶; Mogosanu et al., 2013 ▶). About 1% of all deaths is related to burn injuries (Sadeghi-Bazargani and Mohammadi 2012 ▶). Prevention and management of wound infection are a major factor in wound care. There are many topical agents which are used for burn wound treatment (Khorasani et al., 2009 ▶; Hosseinimehr et al., 2010 ▶). The most important treatment for burn wound is silver sulfadiazine 1% cream (SSD) with antibacterial activity (Miller et al., 2012 ▶ ). SSD may cause side effects such as neutropenia, erythema multiforme, crystalluria, methemoglobinemia (Chung and Herbert 2001 ▶; Fong and Wood 2006 ▶; Beheshti et al., 2013 ▶), and delay wound healing. It is cautioned that SSD cream should not be used for long time on extended wounds (Atiyeh et al., 2007 ▶; Khorasani et al., 2009 ▶; Yaman et al., 2010 ▶). Wound healing process consists of inflammation, re-epithelialization, granulation, neovascularization, and wound contraction. Several natural products have been used for the management of burn wounds that could be considered as an alternative source of treatment of burn wounds. These products have been offered as more effective and cheaper treatment agents (Süntar et al., 2010 ▶; Nasiri et al., 2014 ▶; Bahramsoltani et al., 2014 ▶).
Malva sylvestris (Malvaceae), usually known as common mallow, is a native plant to Europe, North Africa, and Asia (Barros et al., 2010 ▶). In Iran, M. sylvestris is known as “Panirak” in folklore. M. sylvestris has high mucilage content and polysaccharides that are used for many purposes (Aliasl 2013 ▶; Samavati and Manoochehrizade 2013 ▶; Usami et al., 2013 ▶).This plant has antiulcerogenic activity which is probably related to its high mucilage content. (Samavati and Manoochehrizade 2013 ▶). M. sylvestris is consumed as a vegetable in Iran. The plant flowers are used as a remedy for cut wound, dermal infected wounds, eczema, and inflammatory disease such as gastritis, bronchitis (Pirbalouti et al., 2009 ▶) (Samavati and Manoochehrizade 2013 ▶), and rheumatism (Conforti et al., 2008 ▶) and is recommended for acne and skin care (Barros, Carvalho et al., 2010 ▶). Other properties of this plant were reported to be diuretic, laxative, spasmolytic, lenitive, choleretic, and antioxidant effects. M. sylvestris contains polyphenols, vitamin C, vitamin E, β-carotene (Barros, Carvalho et al., 2010 ▶), anthocyanidines, naphthoquinones, flavonoids or mucilaginous polysaccharides, tetrahydroxylated linear diterpene, monoterpenes, and phenol derivatives (Cutillo et al., 2006 ▶; Veshkorova et al., 2010 ▶; Razavi et al., 2011 ▶). Dellagreca et al. isolated eleven compounds from aqueous extracts of M. sylvestris such as 4-ydroxybenzoic acid, 4-methoxybenzoic acid, ferulic acid, methyl 2-hydroxydihydrocinnamate, and scopoletin as well as malvone A, 2-methyl-3-methoxy-5, and 6-dihydroxy-1,4-naphthoquinone (Pirbalouti et al. 2009 ▶, Dellagreca et al. 2009 ▶; Pirbalouti, Yousefi et al., 2009 ▶); DellaGreca et al., 2009 ▶). Gasparetto reported additional therapeutic properties related to flowers and aerial parts of M. sylvestris such as anti-inflammatory, anticancer, positive effectiveness on gingivitis, abscesses, tooth pain, urological disease, insect bites, and ulcerous wounds (Gasparetto et al., 2012 ▶).
The purpose of this study was to investigate the effect of M. sylvestris cream on the second degree burn wounds and compare its results with silver sulfadiazine in rats.
Material and Methods
Plant material
M . sylvestris flowers were procured from a herbal drug market (Sari, Iran) on September 2013. This herb was confirmed by a senior botanist, Prof. Mohammad Azadbakht, Department of Pharmacognosy, Faculty of pharmacy, Sari, Iran, and a voucher specimen (no. 1002) was deposited at the Department of Pharmacognosy, Mazandaran University of Medical Sciences. The flowers of M. sylvestris were dried at room temperature and powdered in a grinder. Aqueous ethanol (70%) was added to the powdered flowers and the mixture was kept at room temperature for 72 hours (Pirbalouti et al. 2009 ▶). After filtration, the solution was concentrated to dryness in reduced pressure under a rotary evaporator. Extract yield was 9.68% w/w. Hydro alcoholic extract of herbal flowers was dried to a powder with the use of a freeze dryer.
Formulation of M . sylvestris cream
The M. sylvestris extract was mixed with liquid paraffin (5 g), stearyl alcohol (5 g), cetyl alcohol (5 g), and span 60 (0.7 g) at 70 ˚C. It was prepared by adding tween 80 (1.8 g), propyl (0.015 g), methyl paraben (0.025 g), and glycerin (7g) in distillated 20.5 ml water and was heated to 70 ˚C. This formulation was prepared according to result of the previous studies and performed seven times formulation laboratory experience (Pirbalouti et al. 2009 ▶). Then, aqueous phase and oil phase were mixed and homogenized for 15 minutes. The cream was allowed to cool down at room temperature while being homogenized. The concentrations of M. sylvestris extract were 10% and 5% in topical creams. All formulations were stored at 4, 25, and 40 ˚C for two weeks and then the stability was evaluated. Consistency and uniformity of creams as well as inseparability of the aqueous and oil phase at different times were observed.
Animal study
The ethical and research committee of Mazandaran University of Medical Sciences approved the experimental protocol. All animals were obtained from animal house of the Mazandaran University of Medical Sciences. Male rats (n=50) weighing 160-200 grams, average age 10 weeks, were used and housed under standard condition at room temperature with a 12-h light/dark cycle, temperature approximately 22-23 ˚C for one week prior to the start of the experiment. Animals were allowed free access to laboratory food and water ad libitum. Each rat was weighted and anesthetized by intraperitoneally injection of 50 mg/kg sodium thiopental. Dorsum was shaved using an electric clipper and 70% alcohol was used to disinfect the dorsal area. Deep anesthetized rats were kept in a prone position. A deep second degree burn wound was induced by a hot metallic device (diameter: 5×2.5 cm2) warmed for 5 minutes within boiling water and put for 10 seconds on the dorsum of rat skin with an equal weight and pressure (Sayar et al., 2014 ▶; Akhoondinasab et al., 2014 ▶; Haghdoost et al., 2013 ▶). All animals were resuscitated with injection of 5 ml normal saline after burning. The burned animals were randomly divided into five groups of ten rats. Group 1 (NS) was control and rats were only washed with normal saline during dressing without any topical treatment. Group 2 was treated with base cream (BC) without any effective agent. Group 3 was treated with SSD 1% (Behvarzan Pharmaceutical Company, Iran). Animals in groups 4 and 5 were treated with 5% and 10% M. sylvestris cream. After topical application of creams, the wound was covered with the sterile plain gauze for 24 hours. The wound area was daily washed with normal saline in all groups, and then was dressed with cream for each group. In order to quantify the rate of wound healing, the wound area was evaluated using a ruler in 1, 3, 7, 10, 15, 20, 25 30, and 35 days after burn injury. The wound area was displayed as cm2. The area of wounds at each day was determined by a formula, which represented the area (cm2) by length and latitude rectangular. The rectangle area was calculated with length × width. Wound contraction was expressed as a reduction in percentage of original wound size. Percentage wound contraction on day X = [(area on day 0 – open area on day X) /area on day 0] × 100 (Tavares Pereira et al., 2012 ▶). Moreover, scales for the burn wound healing were evaluated according to histopathological components in all the groups (Haghdoost et al., 2013 ▶; Oliveira et al., 2013).
Histological study
Histological examinations of wound repair process were performed in 8 and 21 days after burn injury. After incision, samples were full thickness with 3 mm thickness. The samples were kept in formalin 10%. Tissue incisions were prepared in 5 micron thickness and were stained with hematoxylin-eosin and also specific collagen fibers staining. Masson trichrome stain was used for the examination of density of collagen fibers and blood vessel. Angiogenesis assessment (neovascularization), fibroblastic proliferation and presence of collagen fibers, re-epithelialization, complete healing, and infiltration of inflammatory cell were evaluated in tissue sections. Horizontal section of the microscopic high power field (HPF) was evaluated. Average value of the results of 10 HPF microscopic fields was calculated. The score were calculated as follow:
0 or (-) score was defined as the absence of vessels and cells such as macrophage in each HPF.
Angiogenesis of mild score was defined by the presence of 1 to 2 vessels, and for the cell study, it was defined as the presence of 1-2 cells such as macrophages which scored, 1 or (+).
Moderate score was 2 or (++), which meant the presence of 3-4 vessels for angiogenesis and 3-4 cells for cell study in each HPF.
Severe condition was 3 score or (+++) which was the presence of 5 or more cells or vessels in HPF.
Polymorphonuclear leukocytes (PNM) were used to assess pathological changes. These tissue sections were assessed by a pathologist blinded to the treatment assignment.
Histological criteria were defined according to a modified scoring system for surgical wound healing taken from previous studies (Velnar and Bailey, 2009 ▶; Tavares Pereira et al., 2012 ▶; Haghdoost et al., 2013 ▶; Akhoondinasab et al., 2014 ▶).
The sum of scores for wound healing was divided into three categories: extent of granulation tissue was scored based on seven parameters (the re-epithelization was monitored by evaluation of six components. The sum of scores for re-epithelization had a range from 0 to 18. Eighteen was the highest degree of re-epithelization on the 8th day and the new dermis was evaluated with five components. The sum of scores for granulation state was a range from -3 to 18. Eighteen was the highest degree of granulation tissue formation on the 8 days after burn injury. The sum of the scores for re-epithelization was a range from 0 to 18.
New dermis was evaluated with five components. The sum of the scores was between 0 and 15. The score for the best condition for new dermis was 15 in 21 days after burn injury. Each part of the categories or each component scored 0-3. The complete healing was evaluated on 21st day after burn injury. The sum of the scores for wound healing was devided into four groups as follow: 0 = no healing, (1-5) = low, (6-10) = moderate healing, and (11-15) = good healing). According to mathematical logic, the sum of the scales for each part of histopathological evaluation was devided into three groups: extent of granulation, new dermis, and re-epithelization. These criteria which were used as histological scores of wound healings are summarized in Table 1. This scoring system was used to determine the healing grade in each treatment group sample (Tavares Pereira et al., 2012 ▶; Edraki et al., 2014 ▶).
Table 1.
Scales for the burn wound healing according to histopathological components in M. sylvestris and control groups of second-degree burn
| Sum of components variables | Difinition of Scales |
|---|---|
| 1- Extent of granulation tissue (7 parameters) [(-3) -18] | (-3-0) = not healing (1-4) = low (5-12) = moderate healing (13-18) = good wound healing |
| 2- re-epithelization ( 6 parameters) [0-18] |
0 = not healing (1-6) = low (7-12) = moderate healing (13-18) = good wound healing |
| 3-new dermis (5 parameters) [0-15] |
0 = not healing (1-5) = low (6-10) = moderate healing (11-15) = good wound healing |
| 4-Sum of three components | [(-3) - 0] = not healing [1--18) = low (19-37) = moderate healing (38-57) = good wound healing |
Microbiological evaluation
Swabs were taken from the wounds during dressing change on 4th and 8th days. The collected swabs were immediately transferred to the laboratory for microbial tests. In the quantitative count study, 0.5 ml of normal saline was added to each sample tube. Each sample dilution was spread onto blood agar and MacConkey agar, and the plates were incubated at 37 °C for 24 hours and then the degree of contamination of injuries was evaluate. Diagnostic test for the colonies was applied to Novobiocin test.
Wound observation
Wound sites were assessed daily. Macroscopic visual evaluation was measured by direct observation of wounds during dressing each day. Tissue inflammation was evaluated by studying edema, secretion, redness, dark secretion or pus and wound bleeding, blistering, swelling, crust, during dressing at which follow-up visit, and wound area was recorded as being absent, redness, edema, or dirty and with light or dark secretion. The wound contraction was also evaluated .
Statistical analysis
Statistical analysis was carried out using the SPSS (Version 15) software. The data were tested for normality. One-way analysis of variance (ANOVA) was used for comparing quantity variables in the groups, followed by a post hoc multiple comparing test. Kruskal Wallis H test was used for qualitative variables between groups. The difference data were considered significant at p<0.05.
Results
The effect of burn injury on losing weight of rats is shown in Table 2. No significant differences were observed between M. sylvestris and control groups in the loss of weight after burn injury. Skin wound area was measured at 1, 3, 7, 10, 15, 20, 25, 30, and 35 days after the burn injury.
Table 2.
Mean weights (gram) of the rats after burn injury in different groups
| Group | 1 th day | 3 th day | 7 th day | 15 th day | 20 th day | 25 th day |
|---|---|---|---|---|---|---|
| BC | 184±16 | 182±15 | 190±14 | 203±15 | 215±19 | 224±20 |
| N/S | 183±15 | 180±16 | 184±14 | 206±10 | 212±13 | 216±16 |
| SSD | 185±12 | 182±11 | 185±12 | 195±12 | 207±15 | 216±13 |
| MS5% | 186±19 | 185±16 | 195±24 | 201±25 | 215±18 | 224±18 |
| MS1% | 184±17 | 183±17 | 187±19 | 201±16 | 214±11 | 229±9 |
| p value* | 0.991 | 0.956 | 0.678 | 0.766 | 0.801 | 0.739 |
BC: Base cream, N/S: Normal saline, SSD: Silver sulfadiazine, MS: M. sylvestris
p value of differences between groups.
The average area of wound on the 7th day was 11.4±3.7, 10.6±2.7, 13±1.6, 10±3, and 8.7±1.8 cm2 in base cream, NS, SSD, 5% M. sylvestris, and 10% M. sylvestris creams, respectively (p<0.05). The wound size had normal distribution which was shown using one-sample Kolmogorov-Smirnov test. Wound sizes were not significantly different between groups on 1st and 3rd days after burn injury. Wound area was significantly different between groups on 7th day of the post-burn injury and following multiple comparison Dunnett post hoc test showed that M. sylvestris 10% cream group exhibited lower wound size compared to SSD (p<0.05). There were not any significant differences between 5% M. sylvestris, 10% M. sylvestris and the base cream (Table 3).
Table 3.
Mean wound area (cm2) of the animals groups treated with various topical ointments in post burn injury.
| Group | 1 st day | 3 rd day | 7 th day | 10 th day | 15 th day | 20 th day | 25 th day | 30 th day |
|---|---|---|---|---|---|---|---|---|
| BC | 12.5±3 | 13.3±3 | 11.4±3.7 | 10.8±3 | 7.5±2.9 | 5±1.7 | 1.4±0.8 | 0.4±0.5 |
| N/S | 11.8±2.4 | 12.6±2.1 | 10.6±2.7 | 9±3.1 | 6.7±2 | 4.4±2 | 1±0.8 | 0.2±0.3 |
| SSD 1% | 13.3±2.5 | 13.3±2.5 | 13±1.6 | 12.6±2 | 10.5±1.6 | 5.5±1.5 | 3.1±1.3 | 1.8±0.7 |
| MS 5% | 12.9±3.2 | 13.5±3 | 10±3 | 9.7±2.6 | 4.7±2 | 0.8±0.7 | 0.41±0.44 | 0 |
| MS 10% | 11.6±2.4 | 12±1.9 | 8.7±1.8 | 8.4±2 | 4.5±2 | 0.4±0.5 | 0 | 0 |
| p value* | 0.575 | 0.675 | 0.014 | 0.011 | 0.001 | 0.001 | 0.001 | 0.001 |
BC: Base cream, N/S: Normal saline, SSD: Silver sulfadiazine, MS: Malva sylvestris.
In the base cream and SSD groups, skin irritation such as redness, edema, and slight secretion were observed in 5, 8, 12 days post-burn injury. The wound area was not different between 5% and 10% M. sylvestris 15 days after burn injury (p<0.32), meanwhile, it was different between SSD and base on day 8 which biopsy samples showed an increased macrophage infiltration and fibroblastic proliferation in animals that were treated with 10% M. sylvestrisor 5% M. sylvestris, which were better than SSD, NS, and base cream groups (Figure 1 and Table 4) (p<0.05).
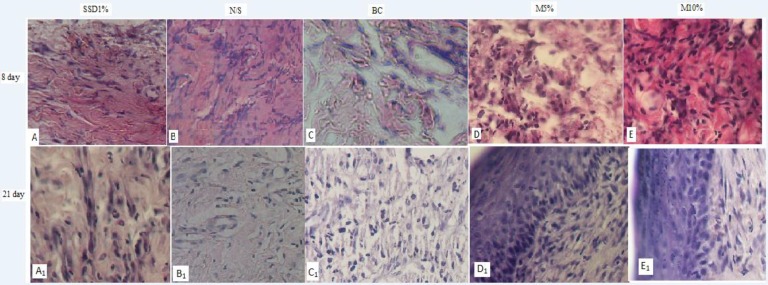
Figure 1.
Comparison of histopathology view among M. sylvestris (MS) treatment and control groups on 8th and 21st days after burn injury.( A, A1 = SSD1% group, B, B1= NS, C, C1 = BC, D, D1 = MS 5%, and E, E1= MS 10% group). [basic cream (BC), normal saline (NS), standard silver sulfadiazine treated (SSD 1%), M. sylvestris 5 % (MS5%), and M. sylvestris 10 % (MS 10%) creams], Macrophage infiltration, neovascularization activity and fibroblastic proliferation were better in M. sylvestris, compared with control groups on the 8th day. Moreover, degree of scar formation, collagenization organization, and new dermis formation in M. sylvestris were better in herbal treatment groups in comparison with control groups on 21st day
Table 4.
Extent of granulation tissue examined in different groups on 8th day after burn injury
| Components/Groups | BC | SSD 1% | N/S | MS 5% | MS 10% |
|---|---|---|---|---|---|
| Macrophage histocytic infiltration | 2 | 2 | 2 | 3 | 3 |
| Neovascularization | 1 | 2 | 2 | 1 | 3 |
| Fibroblastic proliferation | 2 | 2 | 2 | 3 | 2 |
| Matrix mucopolisacharid deposition | 2 | 2 | 2 | 2 | 2 |
| Degree of inflammation | 3 | 3 | 1 | 3 | 3 |
| Extent of bacterial colonization | -1 | -2 | -1 | -1 | -1 |
| Degree of granulation tissue formation | 1 | 2 | 1 | 1 | 2 |
| Sum | 10 | 11 | 9 | 12 | 14 |
BC: Base cream, N/S: Normal saline, SSD: Silver sulfadiazine, MS: M. sylvestris
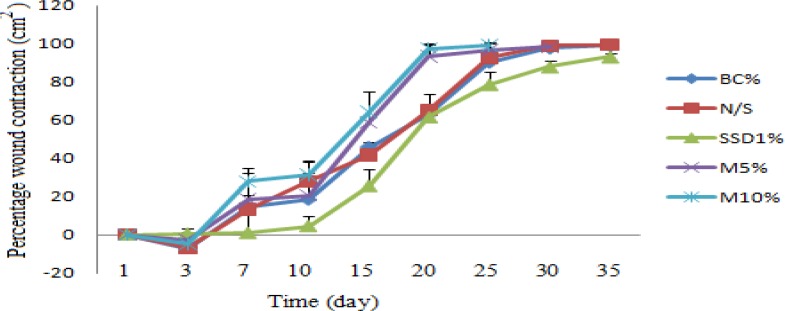
The percentage of wound contractions in different groups is shown in Table 5. It was significantly increased in 10% M. sylvestris group as compared to the SSD and control groups. Animals who received herbal treatment creams had a shorter healing time than rats in all control groups (Figure 2). Wound contraction started from day 4 in treatment group and day 5 in control groups. On day 7, animals treated with 10% M. sylvestris exhibited significant increase in the percentage of wound contraction as compared to other experimental groups. On day 20 of post-burn injury, 10% and 5% M. sylvestris creams exhibited more than 90% wound healing, whereas it was 63% , 65.1%, and 61.5% in rats treated with BC, NS, and SSD creams, respectively. On day 25, no scar was observed in animals treated with 10% and 5% M. sylvestris, while this improvement was observed for control groups on day 30. The time of wound healing in the herbal treatment group was about 10 days shorter than the SSD group. The herbal groups were treated 5-7 days faster than the BC group (Table 5 and Figure 3).
Table 5.
Comparison of the percentage of wound contraction among M. sylvestris (MS) and control groups
| Group |
3
rd
day
(%) |
7 th day (%) | 10 th day (%) | 15 th day (%) | 20 th day (%) | 25 th day (%) | 30 th day (%) | 35 th day (%) |
|---|---|---|---|---|---|---|---|---|
| BC | -7±6.5 | 14.6±16 | 18.4±9.8. | 45.9±9.6 | 63±9.3 | 90.2±3.5 | 97.6±33 | 98.8±1.8 |
| NS | -7±4.2 | 13.4±7.4 | 28±11 | 41.9±6.5 | 65.1±8.5 | 92.6±4.1 | 98.8±1.9 | 99.2±2(32D) |
| SSD 1% | .5±3 | .9±11.6 | 4.5±5.2 | 25.6±8.6 | 61.6±6.7 | 78.8±6.6 | 88±3.4 | 93.2±2 |
| MS 5% | -4±4.3 | 18.6±16.3.5 | 20.6±12 | 58.9±16.2 | 93.2±6.1 | 96.5±.3.4 | 98.3±2.9 | 100 |
| MS 10% | -4%±7.9 | 28±4.6 | 31.4±6.8 | 64.4±10.3 | 97.3±2.9 | 99±1.6 | 100 | 100 |
BC: Base cream, N/S: Normal saline, SSD: Silver sulfadiazine, MS: M. sylvestris.
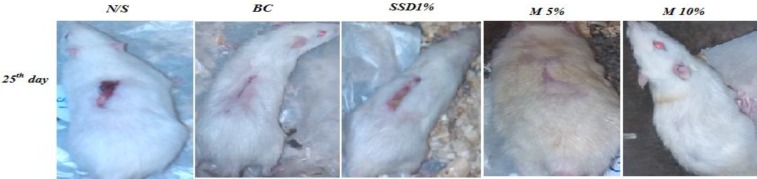
Figure 2.
Burn wound healing pattern in control groups [normal saline (NS), base cream (BC), and silver sulfadiazine 1% (SSD1%)] and herbal treated groups [M. sylvestris cream 5% and 10% (MS 5%,MS 10%)] in rats. The rate of healing in burn wounds created on rats were measured and photographed at regular intervals in both control groups and herbal treated rats during a 25-day period. Wound healing condition in (MS 10%) and MS 5% from ten rats were completed on 25 days
Figure 3.
Comparison of the percentage of wound contraction between M. sylvestris and control groups. M.sylvestris (MS) 5% and10% creams treated groupd showed faster time than control groups for wound contraction (BC: Base cream, N/S: Normal saline, SSD: Silver sulfadiazine, MS: M. sylvestris
The histopathology results of the condition of granulation tissue, matrix of organization, re-epithelialization, and new dermis generation are explained in Tables 4, 6, and 7. Histopathological results showed that the highest and the lowest extent of granulation tissue were observed in 10% M. sylvestris and NS groups, respectively (Table 4). Tissue re-epithelialization components were improved in herbal groups as compared to SSD and other control groups on 8 days after burn injury. Thickness of the granular cell layer and the maturation organization of squamous cells and orthokeratin in herbal treatment group were improved compared to control group. The sum of the re-epithelialization parameters score of herbal treatment was much more than SSD and N/S treatment group (Table 6 and Figure 1).
Table 6.
Comparison of the re-epithelialization components between Mavla sylvestris (MS) and control groups on 8th day post-burn injury
| Group/component |
Epidermal
thickness (0-3) |
Thickness of granular cell layer | Maturation organization of squamous cells | Extent of Keratin layer | Orthokeratin | Parakeratosis | Sum |
|---|---|---|---|---|---|---|---|
| BC | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| N/S | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| SSD 1% | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| MS 5% | 3 | 3 | 3 | 3 | 3 | 0 | 15 |
| MS 10% | 3 | 3 | 3 | 3 | 3 | 0 | 15 |
BC: Base cream, N/S: Normal saline, SSD: Silver sulfadiazine, MS: M. sylvestris.
Table 7.
Comparison of the new dermis formation between M. sylvestris (MS) and control groups on 21st day post-burn injury time
| Groups | Degree of scar formation | Collagenization organization | Extent of hair folliculs | Extent of lymphatic ducts | Degree of innervations | SUM |
|---|---|---|---|---|---|---|
| BC | 2 | 2 | 0 | 0 | 0 | 4 |
| N/S | 2 | 2 | 1 | 0 | 0 | 5 |
| SSD 1% | 2 | 2 | 1 | 0 | 0 | 5 |
| MS 5% | 3 | 2 | 1 | 1 | 1 | 8 |
| MS10% | 3 | 3 | 2 | 1 | 1 | 10 |
BC: Base cream, N/S: Normal saline, SSD: Silver sulfadiazine, MS: M. sylvestris.
Matrix of collagenization organization in 10% M. sylvestris group was better than all of the control groups at 21st day. Furthermore, the score of degree of scar formation was better in a herbal treatment group. These details are shown in Table 7. Histopathological results showed that complete wound healing and new dermis formation were observed in 10% and 5% M. sylvestris groups whereas SSD, NS, and base cream groups had moderate wound healing.
The extent of granulation tissue in herbal treatment groups was better than normal saline group. Moreover, parameters of the new dermis in M. sylvestris were better than SSD and other control groups. Three main histopathology components of wound healing in all groups are shown in Table 7.
Histopathological data showed that 10% and 5% M. sylvestris had better healing effects as compared with control groups (Table 8).
Table 8.
The sum of the score of three histopathological components for burn wound healing in M. sylvestris (MS) treatment and control groups
| Group |
Re-epithelialization
[0-15] |
Extent of granulation tissue[(-3)-18] | New dermis formation[0-15] |
Sum of score
[(-3)-58] |
|---|---|---|---|---|
| BC | 2 | 10 | 4 | 16 |
| NS | 1 | 9 | 5 | 15 |
| SSD 1% | 6 | 11 | 5 | 22 |
| MS 5% | 15 | 12 | 8 | 35 |
| MS 10% | 15 | 14 | 10 | 39 |
The period of re-epithelialization among the study groups were different.
The reepithelialization time for 10% M. sylvestris was 25 days after burns injury (99±1.6%). This time for SSD cream was 35 days (93.2±2%). A completed wound healing was observed in animals treated with 10% and 5% M. sylvestris on 30th and 35th days, respectively, while this time for SSD, NS, and BC creams were more than 35 days. We observed that wound care in the rats treated with herbal creams was five days shorter than the control groups.
Laboratory evaluation indicated no evidence of pathological bacteria in 4th and 8th days. On day 8, a little colorless secretion was observed on wounds in SSD and NS groups and bacteria were grown on blood agar culture media. Catalase positive gram positive cocci was observed in samples of these groups. The results of bacitrucin sensitive and coagulase tests for these samples were negative. This process was completed by novobiocin test which is used to differentiate coagulase-negative staphylococci. The SSD group sample was sensitive to the novobiocin test; on the contrary, NS group sample was not sensitive to this test. Finally, microorganism existed in the SSD group was most probably Staphilococcus epidermitis while for NS group, it was Staphylococcus saprophiteccus. MacConkey agar culture did not show any pathologic organism growth in these groups.
Discussion
The purpose of this study was to evaluate the effect M. sylvestris topical cream on burn wound healing in rats. The main result of this study showed a significant increase in burn wound contraction with topical 10% and 5% M. sylvestris cream during experimental trial, as compared with SSD, BC, and NS groups. The animals treated with the M. sylvestris showed a significant reduction in the wound area when compared with other groups. Wound healing in 10% and 5% M. sylvestris creams treatment groups was about 90% on the 20th day after burn injury, whereas it was 63%, 65.1%, and 61.5% in rats treated with BC, NS, and SSD creams, respectively. Pathological bacteria did not exist on burn wounds that treated with herbal creams. In previous studies, M. sylvestris was used topically for treatment of various diseases such as ulcers, dermatitis, swellings, abscesses, cough, bronchitis, inflammatory diseases, and burns (Chung and Herbert 2001 ▶; Camejo-Rodrigues et al., 2003 ▶; Razavi et al., 2011 ▶; Wang 2005 ▶; Pirbalouti et al., 2009 ▶). Many components of the M. sylvestris can be responsible for antimicrobial activity against pathogen microorganisms (Veshkorova et al., 2010 ▶; Razavi et al., 2011 ▶).
The chemical compositions of M. sylvestris were reported to be malvone A: 2-methyl-3-methoxy-5, 6-dihydroxy-1,4-naphthoquinone as flavonoids. Excretion of free radicals, antioxidant action, and anti-inflammatory properties of this plant showed to contribute to the treatment of wound (Pirbalouti and Koohpyeh 2011 ▶). Carotenoids, high vitamin C, carbohydrates and particularly sugars such as fructose and glucose and phenolics and high amount of ascorbic acid are present in the flowers of this plant (Barros, Carvalho et al., 2010 ▶). The results of bacteriology tests on the wound of M. sylvestris treated animals did not have showed any pathologic bacteria and doubtful secretion. Malvone A in M. sylvestris flower extract may be responsible for antibacterial activity (Pirbalouti and Koohpyeh 2011 ▶). Moreover, antioxidant, high vitamin C, and anti- inflammatory activities of M. sylvestris could be potent and effective properties of this plant for improving of wound healing and increasing wound contraction.
Our finding showed that 10% and 5% M. sylvestris could effectively prevent swelling, erythema, secretion, and other burn complications. The best results for wound contraction and short time of wound healing were obtained in animal treated by M. sylvestris cream. Delaying of the wound healing rate in control groups as compared to topical herbal treatment may be related to existence of bacteria in wounds or because of their histopathological lesions. Although many studies reported that SSD cream is widely used in burns units to reduce the risk of secondary infection and proposed as a standard treatment for burn wound, but we found microorganisms in SSD group. This might be the reason of many problems such as delay in wound repair (Fuller 2009 ▶; Hosseinimehr et al., 2010 ▶; Maghsoudi et al., 2011 ▶). Malvone A in M. sylvestris may be responsible for antimicrobial activity. This may be due to either the individual or additive effects of the phyto-constituents that accelerate the process of wound healing (Pirbalouti and Koohpyeh 2011 ▶). Razavi et al. have reported that the flower extract of M. sylvestris showed high antibacterial effects against some human pathogenic bacteria strains. They concluded that it can be considered as an antiseptic agent (Razavi et al., 2011 ▶). Antibacterial properties of M. sylvestris can help to prevent the wound infection. In recent experimental studies, application of the M. sylvestris cream on the wound was associated with significant wound healing (Razavi et al., 2011 ▶; Pirbalouti et al., 2009 ▶).
In histopathological assessment, the re-epithelialization was also found to be significantly better in animals treated with creams containing M. sylvestris. The results of histological evaluation showed that M. sylvestris significantly increased the rate of collagen turnover and wound reduction. Previous studies showed that M. sylvestris cream could increase well-organized bands of collagen and the number of fibroblasts and few inflammatory cells (Razavi et al., 2011 ▶; Pirbalouti et al., 2009 ▶). Collagen is the main protein in the extracellular matrix and provides strength and integrity to the dermis (Pirbalouti 2011 ▶; Razavi et al., 2011 ▶; Pirbalouti et al., 2009 ▶). The result of macrophage histiocytic infiltration and neovascularization showed well-formation in the 10% M. sylvestris group, but not the SSD and other control groups.
Our results also showed higher improvement of granulation tissue components such as collagen revenue in herbal treatment. Pirbalouti et al. reported that when using topical M. sylvestris in the treatment, collagen turnover significantly increased as compared to control groups. The findings of these two studies are similar (Pirbalouti and Koohpyeh 2011 ▶).
Collagen increase is the main component of healing process which improves and supports extracellular tissue and wound healing. It can be concluded that this biological activity of the plant may help wound contraction and rate of healing burn wounds.
Sayar et al. discussed that re-epithelialization occurred after 15 days with Hypericum perforatum (HP) treatment and 16.5 days with Calendula (as an herbal medicine) treatment (Sayar et al., 2014 ▶;), although re-epithelialization depends on thickness of the granular cell layer, epidermal thickness extent, maturation and organization of squamous cells, and migration of epithelial cells. Previous studies reported that re-epithelialization by some natural products in burn wound may be accelerated. Anti-inflammatory effect of some Malvacea and Boraginaceae species accelerated collagen fiber development and epithelium regeneration and improved epithelium thickness (Razavi et al., 2011 ▶; Mogosanu et al., 2013 ▶; Sayar et al., 2014 ▶;).
We found that re-epithelialization components in the M. sylvestris creams had a good epithelization on the 8th day after burn injury. These results showed no difference between 10% M. sylvestris and 5% M. sylvestris but the score in all control groups were lower as compared with herbal treatment groups. Moreover, we found that on 21st day, the rats treated with 10% and 5% M. sylvestris creams had a mature new dermis formation. The control group exhibited a wide area of ulcerations, a mild degree of scar formation, and other new dermis components. In our study, the new dermis formation was significantly higher on day 21 in the M. sylvestris group compared to the others. Macrophage histiocytic infiltration with congestions in the dermis, indicated that the healing in 10% and 5% M. sylvestris was better than other control groups. This variable was equal between 10% M. sylvestris and 5% M. sylvestris. Therefore, this plant can be considered as a wound healing agent. The wound repair process treated with M. sylvestris group was better than the standard SSD group and others which involves steps including the degree of inflammation, mucopolysacharid deposition, fibroblastic proliferation, macrophage infiltration, degree of granulation tissue, and neovascularization. M. sylvestris cream increased neovascularization, short period epithelialization and improved healing of the infection. Collagen plays an important role in the wound healing and it is an important component of connective tissue, which affords a structural framework for the renewal tissue. Collagen is produced by fibroblasts and facilitates the wound in gaining good quality during wound healing. The process of wound healing is very complex and occurs through coagulation, inflammation, debridement, and re-epithelialization phases. The role of proliferation, migration, and discrimination of squamous epithelial cells of epidermis of the healing process is important. In the last stage of the healing process, collagen deposition and remodeling occurs intradermis (Fuller 2009 ▶; Mekonnen et al., 2013 ▶; Mustafa et al., 2013). In the present study, parameters of the granulation tissue and re-epithelialization on 8th day post burn injury and parameters of new dermis on 21st day were better in M. sylvestris treatment as compared to other treatment groups. In most of the cases, 10% M. sylvestris cream showed a better healing effect than other groups. The biological activity of this plant may be attributed to its antioxidants such as polyphenols, vitamin C, vitamin E, b-carotene, and other important phytochemical (Barros et al., 2010 ▶). In folk medicine, the medicinal application of the common mallow is to treat specific disorders such as digestive, respiratory, genitourinary, muscular, and skeletal system, as well as skin disorders and injuries. Moreover, it possesses anti-inflammatory properties. It is also used as bronchodilator, expectorant, antitussive, and anti-diarrheal. It is highly recommended for acne and skin care, for centuries by European, North African, and Asian people (Sayar et al., 2014 ▶).
In our study, average of wound area, more than 90% percentage wound contraction, and wound healing time were 20 , 25, and 30-35 days for M. sylvestris, NS, BC, and SSD creams, respectively. This finding was similar to other reported studies (Veshkorova et al., 2010 ▶; Pirbalouti et al., 2009 ▶; Pirbalouti and Koohpyeh 2011 ▶). Nearly all histological data, including extent of granulation tissue (macrophage histocytic infiltration and neovascularization), re-epithelialization (epidermal thickness, thickness of granular cell layer, maturation organization of squamous cells, extent of keratin layer, and orthokeratin), and new dermis formation (degree of scar formation and collagenization organization) support the hypothesis that topical M. sylvestris cream is more efficient than standard common burn wound therapy in the treatment of second-degree burn wounds. The enhanced capacity for the histological promotion and acceleration of wound healing with the M. sylvestris could be explained by the anti-inflammatory, antibacterial, antioxidant properties, presence of mucilage, and high vitamin C content of this medicinal plant that is well documented in the previous studies (Barros et al., 2010 ▶; Pirbalouti and Koohpyeh 2011 ▶; Razavi al., 2011 ▶; Samavati and Manoochehrizade 2013 ▶; Pirbalouti et al., 2009 ▶).
The results of this study showed that M. sylvestris cream effectively improved various phases of the deep second burn wound and histology components of healing as compared to standard silver sulfadiazine and normal saline control groups. Therefore, this experimental study supports the recommendation of European, African, Asian, and Iranian traditional literatures about the use of this medicinal plant for wound healing. Topical administration of M. sylvestris cream resulted in faster healing burn wounds in vivo due to improvement in rates of wound contraction, facilitation of extent of granulation, reduction epithelialization time, new dermis formation, and prevention of infection burn wound parameters.
Acknowledgments
This study was supported by a grant from the Mazandara University of Medical Sciences, Sari, Iran. This research was the subject of a PhD thesis of Ebrahim Nasirias, PhD student of Mazandaran University of Medical Sciences.
Conflict of interest
The authors have declared no potential conflict of interest with respect to the authorship and/or publication of this study.
References
- Akhoondinasab MR, Khodarahm A, Akhoondinasab M, Saberi M, Iranpour M. Assessing effect of three herbal medicines in second and third degree burns in rats and comparison with silver sulfadiazine ointment. Burns. 2015;41:125–31. doi: 10.1016/j.burns.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Aliasl JFK. Traditional herbal remedies for burn wound healingin Canon of Avicenna. Jundishapur J Nat Pharm Prod. 2013;8:192–6. [Google Scholar]
- Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33:139–48. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Bahramsoltani R, Farzaei MH, Rahimi R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: an integrative review. Arch Dermatol Res. 2014;306:601–17. doi: 10.1007/s00403-014-1474-6. [DOI] [PubMed] [Google Scholar]
- Barros L, Carvalho AM, Ferreira IC. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: a comparative study of the nutraceutical potential and composition. Food Chem Toxicol. 2010;48:1466–72. doi: 10.1016/j.fct.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Beheshti A, Shafigh Y, Zangivand AA, Samiee-Rad F, Hassanzadeh G, Shafigh N. Comparison of topical sucralfate and silver sulfadiazine cream in second degree burns in rats. Adv Clin Exp Med. 2013;22:481–7. [PubMed] [Google Scholar]
- Camejo- Rodrigues J, Ascensao L, Bonet MA, Valles J. An ethnobotanical study of medicinal and aromatic plants in the natural park of Serra de Sao Maeda (Portugal) J Ethnopharmacol. 2003;89:199–209. doi: 10.1016/s0378-8741(03)00270-8. [DOI] [PubMed] [Google Scholar]
- Chung JY, Herbert ME. Myth: silver sulfadiazine is the best treatment for minor burns. West J Med. 2001;175:205–206. doi: 10.1136/ewjm.175.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti F, Sosa S, Marrelli M, Menichini F, Statti GA, Uzunov D, Tubaro A, Menichini F, Loggia RD. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J Ethnopharmacol. 2008;116:144–51. doi: 10.1016/j.jep.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Cutillo F, Abrosca B, Dellagreca M, Fiorentino A, Zarrelli A. Terpenoids and phenol derivatives from Malva silvestris. Phytochemistry. 2006;67:481–5. doi: 10.1016/j.phytochem.2005.11.023. [DOI] [PubMed] [Google Scholar]
- DellaGreca M, Cutillo FD, Abrosca B, Fiorentino A, Pacifico S, Zarrelli A. Antioxidant and radical scavenging properties of Malva sylvestris. Nat Prod Commun. 2009;4:893–6. [PubMed] [Google Scholar]
- Edraki M, Akbarzadeh A, Hosseinzadeh M, Tanideh N, Salehi A. Healing Effect of Sea Buckthorn, Olive Oil, and Their Mixture on Full-Thickness Burn Wounds. Adv Skin Wound Care. 2014;27:317–23. doi: 10.1097/01.ASW.0000451061.85540.f9. [DOI] [PubMed] [Google Scholar]
- Fong J, Wood F. Nanocrystalline silver dressings in wound management: a review. Int J Nanomedicine. 2006;1:441–9. doi: 10.2147/nano.2006.1.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forjuoh SN. Burns in low- and middle-income countries: a review of available literature on descriptive epidemiology, risk factors, treatment, and prevention. Burns. 2006;32:529–37. doi: 10.1016/j.burns.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Fuller FW. The Side Effects of Silver Sulfadiazine. J Burn Care Rehabil. 2009;30:464–70. doi: 10.1097/BCR.0b013e3181a28c9b. [DOI] [PubMed] [Google Scholar]
- Gasparetto JC, Martins CA, Hayashi SS, Otuky MF, Pontarolo R. Ethnobotanical and scientific aspects of Malva sylvestris L: a millennial herbal medicine. J Pharm Pharmacol. 2012;64:172–89. doi: 10.1111/j.2042-7158.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- Haghdoost F, Baradaran-Mahdavi MM, Zandifar A, Sanei MH, Zolfaghari B, Javanmard SH. Pistacia atlantica Resin Has a Dose-Dependent Effect on Angiogenesis and Skin Burn Wound Healing in Rat. Evid Based Complement Alternat Med. 2013;2013:1–8. doi: 10.1155/2013/893425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinimehr SJ, Khorasani G, Azadbakht M, Zamani P, Ghasemi M. Effect of aloe cream versus silver Sulfadiazine for healing burn wounds in rats. Acta Dermatovenerol Croat. Act Develop Cro. 2010;18:2–7. [PubMed] [Google Scholar]
- Khorasani GA, Hosseinimehr SJ, Azadbakht M, Zamani A, Mahadavi M. Aloe Versus Silver Sulfadiazine Creams for Second-Degree Burns:A Randomized Controlled Study. Surg Today. 2009;39:587–591. doi: 10.1007/s00595-008-3944-y. [DOI] [PubMed] [Google Scholar]
- Maghsoudi H, Monshizadeh S, Mesgari M. A comparative study of the burn wound healing properties of saline-soaked dressing and silver sulfadiazine in rats. Indian J Surg. 2011;73:24–7. doi: 10.1007/s12262-010-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekonnen A, Sidamo T, Asres K, Engidawork E. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalancho petitiana A. Rich(Crassulaceae) leaves in mice. J Ethnopharmacol . 2013;145:638–46. doi: 10.1016/j.jep.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Miller AC, Rashid RM, Falzon L, Elamin EM, Zehtabchi S. Silver sulfadiazine for the treatment of partial-thickness burns and venous stasis ulcers. J Am Acad Dermatol. 2012;66:159–65. doi: 10.1016/j.jaad.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Mogosanu GD, Popescu FC, Busuioc CJ, Lascar I, Mogoanta L. Comparative study of microvascular density in experimental third-degree skin burns treated with topical preparations containing herbal extracts. Rom J Morphol Embryol. 2013;54:107–113. [PubMed] [Google Scholar]
- Kulac M, Aktas C, Tulubas F, Uygur R, Kanter M, Erboga M, Ceber M, Topcu B, Ozen OA. The effect of topical treatment with curcumin on burn wound healing in rats. J Mol Hist. 2013;44:83–90. doi: 10.1007/s10735-012-9452-9. [DOI] [PubMed] [Google Scholar]
- Nasiri E, Hosseinimehr SJ, Azadbakht M, Madani SA. A review of natural products for burn healing based on the Iranian traditional medicine. J Mazandaran Univ Med Sci. 2014;23:263–280. [Google Scholar]
- De Oliveira AP, Franco Ede S, Rodrigues Barreto R, Cordeiro DP, de Melo RG, de Aquino CM, E Silva AA, de Medeiros PL, da Silva TG, Góes AJ, Maia MB. 2013. Effect of Semisolid Formulation of Persea Americana Mill (Avocado) Oil on Wound Healing in Rats. Evid Based Complement Alternat Med. 472382:1–8. doi: 10.1155/2013/472382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- Pirbalouti AG, Koohpyeh A. Wound Healing Activity of Extracts of Malva sylvestris and Stachys lavandulifolia. Int J Biol. 2011;3:174–179. [Google Scholar]
- Pirbalouti AG, Yousefi M, Nazari H, Karimi I, Koohpayeh A. Evaluation of Burn Healing Properties of Arnebia euchroma and Malva sylvestris. E J Bio. 2009;5:62–66. [Google Scholar]
- Razavi SM, Zarrini GR, Molavi G, Ghasemi G. Bioactivity of Malva Sylvestris L a Medicinal Plant from Iran. Iran J Basic Med Sci. 2011;14:574–579. [PMC free article] [PubMed] [Google Scholar]
- Sadeghi-Bazargani H, Mohammadi R. Epidemiology of burns in Iran during the last decade (2000-2010): review of literature and methodological considerations. Burns. 2012;38:319–29. doi: 10.1016/j.burns.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Samavati V, Manoochehrizade A. Polysaccharide extraction from Malva sylvestris and its anti-oxidant activity. Int J Biol Macromol. 2013;60:427–36. doi: 10.1016/j.ijbiomac.2013.04.050. [DOI] [PubMed] [Google Scholar]
- Sayar H, Gergerlioglu N, Seringec N, Ozturk P, Bulbuloglu EG. Comparison of efficacy of topical phenytoin with hypericin in second-degree burn wound healing: An experimental study in rats. Med Sci Monit Basic Res. 2014;20:36–46. doi: 10.12659/MSMBR.890337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süntar IP, Akkol EK, Yilmazer D, Baykal T, Kirmizibekmez H, Alper M, et al. Investigations on the in vivo wound healing potential of Hypericum perforatum L. J Ethnopharmacol. 2010;127:468–77. doi: 10.1016/j.jep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Tavares Pereira DDS, Lima-Ribeiro MHM, Pontes-Filho NT, Carneiro-Le˜ao ADA, Santos Correia MTD. Development of AnimalModel for Studying Deep Second-Degree-Thermal Burns. J Biomed Biotechnol. 2012;2012:1–7. doi: 10.1155/2012/460841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami A, Kashima Y, Marumoto S, Miyazawa M. Characterization of aroma-active compounds in dry flower of Malva sylvestris L by GC-MS-O analysis and OAV calculations. J Oleo Sci. 2013;62:563–70. doi: 10.5650/jos.62.563. [DOI] [PubMed] [Google Scholar]
- Velnar T, Bailey T, Smarkoli V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J Int Med Res. 2009;37:1528–42. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- Veshkurova O, Golubenko Z, Pshenichnov E, Arzanova I, Uzbekov V, Sultanova E, Salikhov S, Williams HJ, Reibenspies JH, Puckhaber LS, Stipanovic RD. Malvone A, a phytoalexin found in Malva sylvestris (Family Malvaceae) Phytochemistry. 2010;67:2376–2379. doi: 10.1016/j.phytochem.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Wang ZY. Impact of anthocyanin from Malva sylvestris on plasma lipids and free radical. J For Res. 2005;16:228–232. [Google Scholar]
- Yaman I, Durmus AS, Ceribasi SMY. Effects of nigella sativa and silver sulfadiazine on burn wound healing in rats. Vet Med. 2010;55:619–24. [Google Scholar]