Abstract
Using the Evans Blue procedure, we previously found strain-related differences in plasma volumes in 5 inbred rat strains. Because albumin binds strongly with Evans blue, this protein is important in the Evans blue method of plasma volume determination. Therefore, we speculated that interstrain differences in plasma albumin concentration (PAC) could distort calculated plasma volumes. To address this concern, we used ELISA techniques to measure PAC in these inbred rat strains. In study A, the blood volume was measured by using Evans blue dye, and albumin was measured at the start of hemorrhage. In study B, blood volume was not measured, and albumin was measured twice, near the start and end of hemorrhage (approximately 14 min apart). Neither study revealed any interstrain differences in PAC, which decreased after hemorrhage in all 5 strains. No correlation was found between PAC and plasma volume, survival time, blood lactate, or blood base excess. Percentage changes in PAC during hemorrhage were greater in salt-sensitive compared with Lewis rats. Moreover, these percentage changes were associated with survival time in Fawn hooded hypertensive rats. Our data show that the plasma volumes we measured previously were not misrepresented due to variations in PAC.
Abbreviation: STAH, survival time after hemorrhage
We have been conducting studies with inbred rat strains to identify quantitative trait loci or genes that regulate early survival ability after hemorrhage.23,24 For such studies, comparable hemorrhage volumes among these inbred rat strains were of crucial importance. Comparable hemorrhage volumes are, in turn, highly dependent on accurate plasma volume measures and associated blood-volume calculations. Using Evans blue dye, a quantitative procedure for blood volume that has been used for decades,7,10 we previously measured plasma volumes and found them to differ among inbred rat strains.24 This dye binds almost exclusively to plasma albumin when the molar ratio of dye to albumin is 8:1 or less.32
Albumin is the most abundant plasma protein, constituting about 60% of measured serum protein, and is synthesized almost exclusively by the liver.31 Albumin has multiple physiologic functions,13,30,31one of which is to provide 80% of the colloid osmotic pressure of the blood,31 which is important in maintaining fluid balance between capillaries and the interstitium.25,28 Plasma albumin concentrations are a potential predictor of survival in patients with various pathologic conditions19 and after trauma.40 In addition, albumin is pivotal for the Evans blue dye method for blood volume determination, which is dependent on the high binding affinity of Evans blue dye to serum albumin.15,32
In evaluating our earlier blood volume studies, we realized that the potential existed for strain-dependent differences in plasma albumin concentrations to influence our blood volume determinations. If albumin concentrations were significantly lower in one strain than another, then less dye might be bound to albumin and the dye might be eliminated from the blood more rapidly. Such a phenomenon could then lead to overestimation of the blood volume (more rapid clearance providing lower plasma concentrations would be interpreted as a larger volume for dilution). Knowing that the multiple Evans blue binding sites on each albumin molecule15 would help compensate for putative differences in albumin concentrations, we hypothesized that albumin concentrations would not be a confounding factor in our blood-volume determinations. Nevertheless, this hypothesis needed to be proven. Moreover, we wondered whether—as in human trauma studies40—there was any association between plasma albumin and survival after, or various indices of, severe hemorrhage. To address these concerns, we conducted 2 studies to compare plasma albumin concentrations and survival time after severe hemorrhage among the inbred rat strains in which we had previously measured plasma volume.
Materials and Methods
All rats were maintained at an AAALAC-accredited facility. This study was approved by the IACUC of the US Army Institute of Surgical Research (Fort Sam Houston, Houston, TX). Animals received humane care in accordance with the Guide for the Care and Use of Laboratory Animals.20 We used 5 inbred rat strains: Brown Norway/Mcwi (BN; study A: Charles River Laboratories, Wilmington, MA; study B: Medical College of Wisconsin, Milwaukee, WI); Fawn hooded hypertensive (FHH; Physiogenix, Milwaukee, WI); Dahl salt-sensitive (SS; Charles River Laboratories), dark agouti (DA) and Lewis (LEW) (Harlan, Indianapolis, IN) were used for the study. These 5 strains were found to be the most divergent in survival time after hemorrhage (STAH) in a previous study in which blood volume had been measured.24 Moreover, congenic and consomic strains, which could expedite analyses of quantitative trait loci in future studies, were available many of these strains. We used for 2 suppliers for BN rats because Charles River stopped supplying this particular strain. All rats were male, were shipped at approximately 10 wk of age, and were held for an 18- to 24-d acclimation period before experimentation. Rats were maintained individually in filter-top microisolation plastic cages containing rodent bedding (catalog no. T7084, Harlan Teklad Rodent Bedding, Harlan, Madison, WI) and kept at 19 to 23 °C, with lights on from 0600 to 1800. Food (catalog no. 2018, Harlan) and tap water in bottles were constantly available. Vendor surveillance records indicated the rats were free of known bacterial, viral, parasitic pathogens, including cilia-associated respiratory bacillus, Toolan H1 virus, Kilham rat virus, pneumonia virus of mice, lymphocytic choriomeningitis virus, rat parvovirus, rat cytomegalovirus, Sendai virus, rat coronavirus/sialodacryoadenitis, rat minute virus, reovirus, and Theiler murine encephalomyelitis virus, Bordetella bronchiseptica, Corynebacterium kutscheri, Salmonella spp., Streptococcus moniliformis, Mycoplasma pulmonis, Pasturella spp., Helicobacter spp., and common ecto- and endoparasites.
All surgical procedures were conducted under aseptic conditions as described previously.23 Rats were anesthetized with 2% to 5% isoflurane (Forane, Baxter Healthcare, Deerfield, IL) in 100% oxygen. The left common carotid artery was isolated and then catheterized by using PE50 tubing with a PE10 tip (Clay Adams, Parsipany, NJ). Each catheter was pretreated with tridodecylmethylammonium chloride–heparin (Polysciences, Warrington, PA) before placement. The catheter was passed dorsally beneath the skin, exteriorized in the intrascapular region, filled with 100 μL of sterile glycerol, and sealed with a sterile stainless-steel wire plug (1 cm × 0.635 mm). Rats were injected subcutaneously with buprenorphine (2.5 μg/100 g body weight) as an analgesic and with 10 mL/400 g body weight of sterile 0.9% saline to provide hydration during recovery. Rats were maintained under a warm lamp for approximately 4 h and routinely monitored to ensure recovery from anesthesia and surgery, after which they were returned to the animal room in the vivarium.
In study A (BN, DA, FHH: n = 9; LEW, n = 8; and SS, n = 10), blood volumes were measured on the day of surgery by using the Evans blue dye technique as described previously.24 In study B (DA, n = 8; BN, FHH, Lew, and SS, n = 9), the average strain-specific blood volumes determined in study A were used to calculate the volume of blood to be removed. Estimated numbers of rats per strain (n = 10) needed for each study were computed by using a power analysis that took into account the desired power (probability of rejecting a false null hypothesis), the variability (standard deviation) of the most meaningful measure being considered (STAH), the least difference of interest between 2 true means, the critical probability level, and the number of treatment groups in the study. However, experimental losses prevented the achievement of n = 10 in most cases. Rats were randomly assigned to day of surgery, order of surgery on each day, and order of hemorrhage on each day. Approximately 24 h after surgical catheterization, all rats underwent a conscious controlled hemorrhage, wherein 47% of the total blood was removed during 26 min. Rats were observed for a maximum of 4 h after commencement of hemorrhage. At the end of 4 h, surviving rats were euthanized with an intravascular injection of sodium pentobarbital (150 mg/kg). To avoid potential confounding effects of any diurnal variations on measures, both surgeries and hemorrhages were always conducted between 0700 and 1200.
In study A, blood (the ‘initial’ sample) was collected for albumin analysis during a 4-min period toward the beginning (min 4 through 7, approximately 3 mL) of the 26-min hemorrhage. In study B, blood was obtained at 2 time points, separated by 14 min: at the start of minute 2 to the start of minute 5 (3 min, approximately 2 mL; the initial sample); and at the start of minute 18 to the start of minute 24 (6 min, approximately 2.3 mL; the ‘final’ sample). For albumin, blood samples were collected in heparinized syringes and centrifuged at 1900 × g for 5 min. The top (plasma) layer was rapidly frozen in liquid nitrogen and then stored at –80 °C. For study A, arterial blood gases, electrolytes, and metabolites were measured (Cobas b221 Blood Gas System, Roche Diagnostics, Indianapolis, IN). For study B, a handheld system (iSTAT System with CG8 cartridge; Abbott Laboratories, Abbott Park, IL) was used. For these measures, blood was collected in heparinized syringes during the first 1 min (studies A and B) and last 2 min (study B) of hemorrhage. Two different instruments were used for measuring arterial blood measures because the Roche blood gas system was unavailable during study B. Because we did not directly compare measures between studies, the necessary use of 2 different instruments has not confounded our data interpretation. Arterial blood gases and metabolites were measured to determine the presence of any strain-dependent associations between such measures and survival ability after controlled, conscious hemorrhage.
Albumin was quantified by using a rat albumin ELISA kit (Bethyl Laboratories, Montgomery, TX). The manufacturer's protocol was slightly modified to allow correct validation of the assay for appropriate accuracy and precision. Briefly, the manufacturer's procedure included: 1) coat wells of a 96-well plate with coating antibody, then wash plate; 2) incubate the plate with blocking solution and wash; 3) add samples or standards, incubate at room temperature for 1 h, and wash; 4) add diluted horseradish-peroxidase–conjugated detection antibody, incubate for 1 h, and wash; 5) add 3,3,5,5-tetramethylbenzidine substrate solution, protect from light, and incubate for 15 min at room temperature; and add stop solution and measure absorbance at 450 nm on a plate reader.
Intraassay variation resulted due to differences in absorbance at the extremes of the 96-well plate; the albumin standards measured in the first 3 columns (triplicates) of the plate had lower absorbance when compared with the same standards measured in the last 3 columns of the same plate. The differences in absorbance were possibly due to different incubation times of samples and standards (step 3 of manufacturer's procedure) at well 1 compared with well 96. On average, it took approximately 20 min to add all ingredients to the 96-well plate. Therefore the incubation time at step 3 was up to approximately 33% longer in well 1 compared with well 96, resulting in higher coefficients of variation (CV) for samples loaded at the ends of the plates. Thus, to minimize this variation, the time to achieve equilibrium for antigen-antibody binding at step 3 was measured. At step 3, samples were incubated for various time points: 1, 1.5, 2.5, 3.5, 4, 6, 12, 18, 24, 36, and 48 h. At 24 h of incubation and longer, a plateau in absorbance was evident, indicating that an equilibrium condition of albumin binding to the coated antibody had occurred. In addition, at this incubation time there were lower CV between samples loaded in the extreme wells. Consequently, the incubation time at step 3 was increased to 24 h; all other steps followed the manufacturer's protocol.
After optimization, the ELISA procedure was validated for inter- and intraassay variation and accuracy. The CV for absorbance was 7.9% among assays for the internal control. For most standards, duplicates samples yielded CV of 4.5% to 8.3% for all assays conducted. However the lowest 2 standard concentrations had CV of 13% and 10.5%. All samples had absorbance values toward the higher end of the standard curve. Accuracy—measured by adding 4 known and different amounts of standards to the unknown internal control sample—averaged 105.5% over 3 independent determinations, and average slopes of expected compared with measured (b = 1.00) did not differ from 1 (P > 0.05).
After completion of validation procedures, plasma concentrations of albumin in rats were measured in both studies. Data were analyzed by using SAS version 9.2 (SAS Institute, Cary, NC). One-way ANOVA (PROC ANOVA) was used to test for strain differences in albumin concentrations of study A and in change (initial – final) and percentage change in albumin concentration in study B. Percentage change was calculated by dividing the change in albumin concentration by the initial value and multiplying the quotient by 100%. Repeated-measures ANOVA (PROC MIXED) were used in study B to compare the albumin concentrations of the initial and final samples. The initial albumin concentration of study A was compared with that of study B by using 2-way ANOVA. Tests for differences among individual means included the a posteriori Student–Newman–Keuls test (1-way ANOVA) and the PDIFF procedure of SAS with adjustment for multiple comparisons by using the false-discovery rate (2-way and repeated-measures ANOVA).5 Pearson correlation analyses were conducted by using PROC CORR. Because correlation coefficients and their associated probability levels can be deceptive, all data were plotted (using PROC GPLOT), and the scatter of data points around the best-fit line and outliers were noted. High correlation coefficients due to an outlier were eliminated. Only those correlations associated with coefficients of determinations (r2) of 0.5 or greater were considered potentially biologically relevant; that is, 50% or more of the variation in one variable is due to variation in the second variable. Data were analyzed for homogeneity of variance (Levene test) and for normality of distribution (PROC Univariate Normal with associated Kolmogorov–Smirnov test). Data were transformed where necessary to meet assumptions of ANOVA. A probability level of 0.05 or less was considered significant.
Results
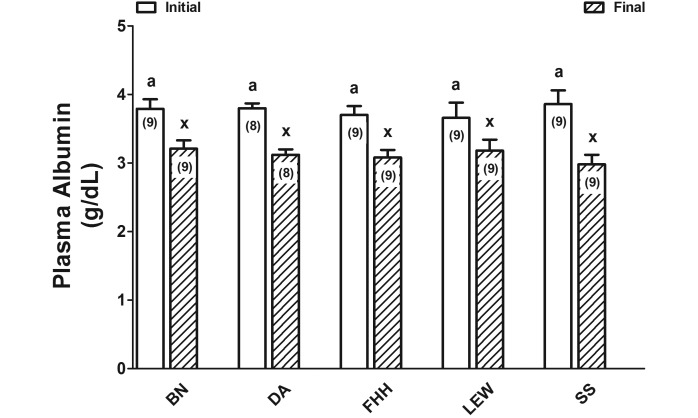
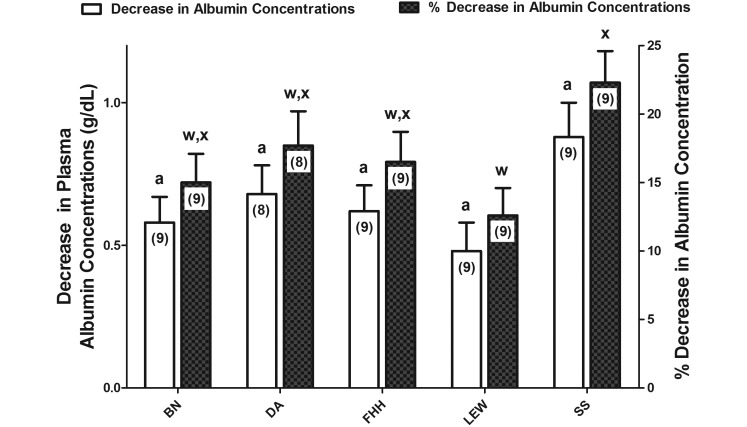
For all 5 inbred rat strains, the initial plasma albumin concentration (g/dL) did not differ between study A and study B (P = 0.31) or among strains (P > 0.05; Table 1; Figure 1) for both the initial (studies A and B) and final sample (study B, P = 0.98; Figure 1). Final plasma albumin concentrations were lower (P < 0.0001; Figure 1) than the initial concentrations in all 5 strains. Changes in albumin concentrations—which were highly variable among the rats in each strain—did not differ among inbred rat strains despite the almost 2-fold numerical difference between SS and LEW rats (P = 0.09; Figure 2). However, the percentage changes in albumin concentrations were greater in SS compared with LEW rats (P = 0.047; Figure 2).
Table 1.
Differences in plasma albumin and Evans Blue dye concentrations in inbred rats strains for which blood volumes were determined by using the Evans Blue dye procedure (study A)
| Inbred rat strain | Albumin concentration |
Evans blue dye concentration (µM) | Ratio [Evans Blue] : [Albumin] | |
| g/dL | µM | |||
| Brown Norway (n = 9) | 3.86 ± 0.18a | 593.2 ± 28.4a | 107.3 ± 1.9b | 0.18 ± 0.01c |
| Dark agouti (n = 9) | 3.59 ± 0.15a | 551.9 ± 23.7a | 128.5 ± 2.3a | 0.24 ± 0.01 a,b |
| Fawn hooded hypertensive (n = 9) | 3.84 ± 0.18a | 591.7 ± 27.1a | 121.8 ± 4.9a | 0.21 ± 0.01 b,c |
| Lewis (n = 8) | 3.31 ± 0.10a | 509.7 ± 16.0a | 123.8 ± 3.8a | 0.24 ± 0.01a |
| Salt-sensitive (n = 10) | 3.48 ± 0.08a | 535.8 ± 11.9a | 117.7 ± 4.9 a,b | 0.22 ± 0.01 a,b |
Albumin concentrations are presented in 2 different units: g/dL is the normal unit designation as well as allows for comparison with Figure 1; uM allows for comparison with the concentration of Evans Blue dye. Values with different superscripted letters differ significantly (P < 0.05).
Figure 1.
Plasma concentrations of albumin (mean ± SEM) in rats from study B wherein blood volumes were not measured. The 5 inbred rat strains were Brown Norway (BN), dark agouti (DA), Fawn hooded hypertensive (FHH), Dahl salt-sensitive (SS), and Lewis (LEW). Two-way ANOVA with repeated measures indicated no interstrain differences (P = 0.98), a significant (P < 0.0001) difference based on sample (initial compared with final), and an absence of interaction between strain and sample (P = 0.095). Different superscripted letters indicate values that differ significantly (P < 0.001). Numbers within bars represent the number of rats for each measure.
Figure 2.
Decreases and percentage decreases in albumin concentrations during severe (47% blood volume) hemorrhage in 5 inbred rat strains. The 5 inbred rat strains were Brown Norway (BN), dark agouti (DA), Fawn hooded hypertensive (FHH), Dahl salt-sensitive (SS), and Lewis (LEW). Decreases represent the difference between the initial and final concentrations. Percentage decreases were calculated by dividing these decreases by the initial value and this quotient multiplied by 100%. One-way ANOVA revealed an absence of interstrain differences (P = 0.09; open bars with superscripted a). Percentage decreases were lower for LEW compared with SS rats (P = 0.04); filled bars with different superscripted letters indicate significantly (P < 0.05) different values.
In study A, wherein blood volume was determined in each rat on the day of surgery, Evans blue concentrations differed (P = 0.0052) among inbred rat strains and were approximately 5-fold less than the albumin concentration (Table 1). In addition, the ratio of the concentrations of Evans blue to albumin differed among inbred rat strains (P = 0.0006; Table 1). Finally, in neither study were there relevant correlations—either within a given strain or across all strains—between plasma albumin concentrations and other phenotypes of interest (for example, survival time, plasma volume, mean arterial pressure, base excess, blood carbonate, blood lactate, and so forth; data not shown) that both met the criteria detailed in the methods section and that were reproduced in both studies. However, FHH rats showed a positive association between the percentage decrease in plasma albumin concentration and STAH (r = 0.69; P = 0.04). Given that the r2 value (0.48) associated with this measure was very close to our defined threshold value (r2 = 0.50), we accepted the association as potentially relevant.
Discussion
In the current work, we measured plasma albumin concentrations in the 5 inbred rat strains for which we had previously shown strain-dependent differences in plasma and blood volumes.24 Inbred strains that differed in blood and plasma volumes24 did not differ in plasma albumin concentration, nor were than any correlations between plasma volume and albumin concentration. To ascribe differences in plasma volume to differences in albumin concentrations, one must remember that each albumin molecule has 8 to 14 binding sites for Evans blue.15,32 Therefore, plasma albumin concentrations would have to decrease markedly before significantly less Evans blue would be bound and presumably more rapidly cleared from the blood,9 thereby leading to the false conclusion of a larger blood volume. The concentrations of Evans blue dye achieved through our injections and the concentration of albumin present strongly suggest that dye would be bound equivalently in all inbred rat strains. In the current study, we did not measure equilibrium dissociation constants (Kd) for the binding of Evans Blue to albumin. The most recent study to measure Kd for multiple species (human, rabbit, bovine, canine)15 determined an average Kd of 8.33 × 10−7 M for those species. Given the concentrations of albumin and Evans blue present in the current study and assuming 13 binding sites for Evans blue per albumin molecule and a fraction of albumin-bound dye of greater than 0.99,2,15 then a 100-fold increase in the Kd (decrease in affinity) would have to occur before one would measure decreases in the concentration of bound Evans blue as calculated by the method of Scatchard.27 Theoretically, this situation would lead to associated increases in free Evans blue dye, which could be eliminated from the blood more rapidly.39 Such data and considerations strongly suggest that previously measured blood volume differences were not due to variable albumin concentrations or affinity. Therefore, our previously-stated hypothesis and the validity of our original blood volume measures24 have been confirmed.
Additional findings of the current studies include: 1) plasma albumin concentrations did not correlate with STAH in the 5 inbred rat strains we evaluated; 2) final albumin concentrations during late hemorrhage were lower than initial concentrations in all 5 strains; and 3) Evans blue dye injected 24 h prior did not appear to affect ELISA-based quantification of plasma albumin. Lack of correlation with STAH suggests that plasma albumin concentration (initial or final) may not be a useful predictor of survival ability for these inbred rat strains undergoing conscious controlled hemorrhage. Numerous studies have shown an association between increased plasma albumin concentrations and improved health outcomes.30 In addition, some earlier studies have reported increased albumin concentrations to be a predictor of more positive outcomes after trauma40 and ischemic stroke.12 Furthermore, albumin has been successfully used in resuscitation fluid to improve survival in specific cohorts of patients.6 However, in patients with brain injury, albumin may increase mortality,34 and its use in hypovolemic patients remains controversial.14 Because of the many supportive functions of blood albumin,14,31 we had hypothesized that plasma albumin concentration would be correlated with survival time, but at the time points measured, our data do not support this hypothesis. It is noteworthy, however, that the percentage changes in albumin concentrations between the initial and final samples were correlated with STAH in FHH rats. The potential relevance of this association is discussed later.
Although the current study is the first to demonstrate reductions in plasma albumin concentrations in these 5 inbred rat strains, albumin concentration previously has been observed to rapidly decrease after trauma and hemorrhage in dogs16 and after hemorrhage in rats.1 What is new and worthy of consideration are the relative changes of albumin during the hemorrhage and how these changes may reflect biologic processes that act—in a strain-dependent manner—to maintain homeostasis during and after the hemorrhage challenge.
Decreased albumin concentrations undoubtedly reflect the hemodilution that occurs, through transcapillary refill, during and immediately after severe hemorrhage.4,18 This dilution involves passage of protein-free interstitial fluid into the intravascular space.4,11 However, there is also evidence for a redistribution of blood from the microvasculature to the macrovasculature immediately after hemorrhage,26 although this redistribution would not decrease the albumin concentration. Plasma concentrations of albumin reflect the net effects of its synthesis, catabolism, leakage from the intravascular space (transcapillary escape), and replacement by means of the lymphatic system.28,33 The passage of solutes—such as albumin—out of the vascular space involves primarily 2 transport routes: 1) through the endothelial cells (transcellular) and 2) between the endothelial cells through interendothelial junctions (paracellular).25 During normal nonpathologic functioning albumin is transported exclusively transcellularly through caveolae, in an energy-dependent process, from the luminal side of the endothelium to the interstitial side.25,28 Some of this transport is in conjunction with albumin-binding proteins, including albondin.29 Albumin subsequently is returned to the vascular system after passage through the interstitium through the lymphatics.3 Some pathologic conditions (sepsis and hemorrhage plus resuscitation) have been associated with both increased caveolae-associated albumin transport8,37 and paracellular transport;17,41 that is, increased albumin capillary permeability and transfer of albumin from the blood into tissue. The effects of hemorrhage itself on capillary albumin permeability are undoubtedly complex and are both time-and tissue-dependent. Indeed, different studies have found both increases35 and decreases38 in albumin capillary permeability during or soon after hemorrhage. Regardless of which of the described mechanisms are associated with changes in plasma albumin concentrations in our study, our data suggest that these mechanisms differed between LEW and SS rats. Moreover, in FHH, these mechanisms appear to have influenced STAH. Therefore, our results encourage future genomic investigations into factors controlling plasma refill and capillary albumin permeability as intermediaries of survival after severe hemorrhage.
Finally, only the Evans blue dye procedure was used to measure plasma volume. As noted earlier, this method is an independent, well-established and validated technique that has been used for decades to measure plasma volume.7,10 However, this procedure has some potential confounding factors, including interference due to lipemia and hemolysis7 and overestimation of blood volume when increased capillary permeability leads to decreased plasma albumin concentrations.21 All of these factors can lead to potential inaccuracies in blood volume estimation. Although other techniques are available (for example, 125I-albumin, hydroxylethyl starch22), the repeatability, relative ease of use, absence of deleterious effects on the animal compared with use of 125I-albumin,36and wide acceptance of the Evans blue procedure7 argued for its use in our studies. The current results indicating the absence of differences in albumin concentrations among the inbred rat strains studies further support this argument.
In conclusion, our data indicate an absence of interstrain differences in plasma albumin concentration among the 5 inbred rat strains tested and thereby confirm the validity of our previously published blood-volume measures, which made use of the albumin-dependent Evans blue procedure. Furthermore, the absence of correlation between albumin levels and STAH indicate that albumin concentrations may not be useful predictors of survival time after controlled, conscious hemorrhage in these inbred rats. However, our data do suggest—albeit indirectly—the presence of strain-dependent differences in hemorrhage-induced changes in cellular mechanisms associated with capillary albumin permeability and concomitant plasma refill that may positively influence survival after hemorrhage in FHH rats.
Acknowledgments
We thank Mariam Calderon and Dr Thomas Oh (US Army Institute of Surgical Research) for their assistance with rat experiments and LTC Kenneth Jacobsen (Attending Veterinarian) for information concerning rat housing and pathogen status of our inbred rat strains. We also thank Dr Bijan Kheirabadi for his critical comments on the paper. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense. This research was conducted on funds from the US Army Medical Research and Materiel Command.
References
- 1.Alfaro V, Pesquero J, Palacios L. 1999. Acid–base disturbance during hemorrhage in rats: significant role of strong inorganic ions. J Appl Physiol 86:1617–1625. [DOI] [PubMed] [Google Scholar]
- 2.Allen TH, Orahovats PD. 1950. Combination of toluidine dye isomers with plasma albumin. Am J Physiol 161:473–482. [DOI] [PubMed] [Google Scholar]
- 3.Aukland K, Reed RK. 1993. Interstitial–lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev 73:1–78. [DOI] [PubMed] [Google Scholar]
- 4.Barton RN, Passingham BJ. 1982. Early responses to hemorrhage in the conscious rat: effects of corticosterone. Am J Physiol 243:R416–R423. [DOI] [PubMed] [Google Scholar]
- 5.Benjamini JD. 1995. Controlling the false-discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc, B 57:289–300. [Google Scholar]
- 6.Caironi P, Gattinoni L. 2009. The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus 7:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien S, Gregerson MI. 1962. Determination of body fluid volumes, p 1–105. In: Nastuk WL. Physical techniques in biological research IV—special methods. New York (NY): Academic Press. [Google Scholar]
- 8.Childs EW, Udobi KF, Hunter FA, Dhevan V. 2005. Evidence of transcellular albumin transport after hemorrhagic shock. Shock 23:565–570. [PubMed] [Google Scholar]
- 9.Crooke AC, Morris CJ. 1942. The determination of plasma volume by the Evans blue method. J Physiol 101:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson AB, Evans HM, Whipple GH. 1920. Blood volume studies III. Behavior of large series of dyes introduced into the circulating blood. Am J Physiol 51:232–256. [Google Scholar]
- 11.Drucker WR, Chadwick CD, Gann DS. 1981. Transcapillary refill in hemorrhage and shock. Arch Surg 116:1344–1353. [DOI] [PubMed] [Google Scholar]
- 12.Dziedzic T, Slowik A, Szczudlik A. 2004. Serum albumin level as a predictor of ischemic stroke outcome. Stroke 35:e156–e158. [DOI] [PubMed] [Google Scholar]
- 13.Emerson TE., Jr 1989. Unique features of albumin: a brief review. Crit Care Med 17:690–694. [DOI] [PubMed] [Google Scholar]
- 14.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. 2012. Human serum albumin: from bench to bedside. Mol Aspects Med 33:209–290. [DOI] [PubMed] [Google Scholar]
- 15.Freedman FB, Johnson JA. 1969. Equilibrium and kinetic properties of the Evans blue–albumin system. Am J Physiol 216:675–681. [DOI] [PubMed] [Google Scholar]
- 16.Getzen LC, Pollak EW, Wolfman EF., Jr 1977. Serum protein concentration during hemorrhagic shock. Surg Gynecol Obstet 144:42–44. [PubMed] [Google Scholar]
- 17.Gong P, Angelini DJ, Yang S, Xia G, Cross AS, Mann D, Bannerman DD, Vogel SN, Goldblum SE. 2008. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem 283:13437–13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haddy FJ, Scott JB, Molnar JI. 1965. Mechanism of volume replacement and vascular constriction following hemorrhage. Am J Physiol 208:169–181. [DOI] [PubMed] [Google Scholar]
- 19.Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. 2008. Albumin levels predict survival in patients with systolic heart failure. Am Heart J 155:883–889. [DOI] [PubMed] [Google Scholar]
- 20.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 21.Jones JG, Wardrop CA. 2000. Measurement of blood volume in surgical and intensive care practice. Br J Anaesth 84:226–235. [DOI] [PubMed] [Google Scholar]
- 22.Kalra PR, Anagnostopoulos C, Bolger AP, Coats AJ, Anker SD. 2002. The regulation and measurement of plasma volume in heart failure. J Am Coll Cardiol 39:1901–1908. [DOI] [PubMed] [Google Scholar]
- 23.Klemcke HG, Baer DG, Pankratz VS, Cox A, Cortez DS, Garrett MR, Joe B, Ryan KL. 2008. Is survival time after hemorrhage a heritable, quantitative trait? An initial assessment. Shock 29:748–753. [DOI] [PubMed] [Google Scholar]
- 24.Klemcke HG, Joe B, Calderon ML, Rose R, Oh T, Aden J, Ryan KL. 2011. Genetic influences on survival time after severe hemorrhage in inbred rat strains. Physiol Genomics 43:758–765. [DOI] [PubMed] [Google Scholar]
- 25.Komarova Y, Malik AB. 2010. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 72:463–493. [DOI] [PubMed] [Google Scholar]
- 26.LaForte AJ, Lee LP, Rich GF, Skalak TC, Lee JS. 1992. Fluid restitution and shift of blood volume in anesthetized rabbits subject to cyclic hemorrhage. Am J Physiol 262:H190–H199. [DOI] [PubMed] [Google Scholar]
- 27.Limbird LE. 1986. Cell surface receptors: a short course on theory and methods. Boston (MA): Martinus Nijhoff Publishing. [Google Scholar]
- 28.Mehta D, Malik AB. 2006. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86:279–367. [DOI] [PubMed] [Google Scholar]
- 29.Merlot AM, Kalinowski DS, Richardson DR. 2014. Unraveling the mysteries of serum albumin—more than just a serum protein. Front Physiol 5:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson JP, Wolmarans MR, Park GR. 2000. The role of albumin in critical illness. Br J Anaesth 85:599–610. [DOI] [PubMed] [Google Scholar]
- 31.Peters JT. 1960. Serum albumin, p 133–181. In: Putnam FW. The plasma proteins, vol 1 New York (NY): Academic Press. [Google Scholar]
- 32.Rawson RA. 1943. The binding of T1824 and structurally related diazo dyes by the plasma proteins. Am J Physiol 138:708–717. [Google Scholar]
- 33.Ruot B, Papet I, Bechereau F, Denis P, Buffiere C, Gimonet J, Glomot F, Elyousfi M, Breuille D, Obled C. 2003. Increased albumin plasma efflux contributes to hypoalbuminemia only during early phase of sepsis in rats. Am J Physiol Regul Integr Comp Physiol 284:R707–R713. [DOI] [PubMed] [Google Scholar]
- 34.SAFE Study Investigators. Australian and New Zealand Intensive Care Society Clinical Trials Group. Australian Red Cross Blood Service. George Institute for International Health. Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, Bishop N, Kai Lo S, Vallance S. 2007. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med 357:874–884. [DOI] [PubMed] [Google Scholar]
- 35.Schumacher J, Binkowski K, Dendorfer A, Klotz KF. 2003. Organ-specific extravasation of albumin-bound Evans blue during nonresuscitated hemorrhagic shock in rats. Shock 20:565–568. [DOI] [PubMed] [Google Scholar]
- 36.Thomsen JK, Fogh-Andersen N, Bulow K, Devantier A. 1991. Blood and plasma volumes determined by carbon monoxide gas, 99mTc-labelled erythrocytes, 125I–albumin, and the T1824 technique. Scand J Clin Lab Invest 51:185–190. [DOI] [PubMed] [Google Scholar]
- 37.Tiruppathi C, Shimizu J, Miyawaki-Shimizu K, Vogel SM, Bair AM, Minshall RD, Predescu D, Malik AB. 2007. Role of NFκB-dependent caveolin 1 expression in the mechanism of increased endothelial permeability induced by lipopolysaccharide. J Biol Chem 283:4210–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tucker VL, Bravo E, Weber CJ, Wisner DH. 1995. Blood-to-tissue albumin transport in rats subjected to acute hemorrhage and resuscitation. Shock 3:189–195. [DOI] [PubMed] [Google Scholar]
- 39.Wilde WS, Hill JH, Wilson E, Schielke GP. 1971. Exchange of free and albumin-bound Evans blue in interstitium of hamster kidney. Am J Physiol 220:1991–1999. [DOI] [PubMed] [Google Scholar]
- 40.Yukl RL, Bar-Or D, Harris L, Shapiro H, Winkler JV. 2003. Low albumin level in the emergency department: a potential independent predictor of delayed mortality in blunt trauma. J Emerg Med 25:1–6. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Sun K, Liu YY, Zhang YP, Hu BH, Chang X, Yan L, Pan CS, Li Q, Fan JY, He K, Mao XW, Tu L, Wang CS, Han JY. 2013. Ginsenoside Rb1 ameliorates lipopolysaccharide-induced albumin leakage from rat mesenteric venules by intervening in both trans- and paracellular pathways. Am J Physiol Gastrointest Liver Physiol 306:G289–G300. [DOI] [PubMed] [Google Scholar]