Abstract
Ulcerative dermatitis (UD) is a spontaneous idiopathic disease that often affects C57BL/6 mice or mice on a C57BL/6 background. UD is characterized by intense pruritus and lesion formation, most commonly on the head or dorsal thorax. Self-trauma likely contributes to wound severity and delayed wound healing. Histologically, changes are nonspecific, consisting of ulceration with neutrophilic and mastocytic infiltration and epithelial hyperplasia and hyperkeratosis. Diet appears to have a profound effect on the development and progression of UD lesions. We investigated the incidence and severity of UD in C57BL/6NCrl mice on a high-fat western-style diet (HFWD) compared with a standard rodent chow. In addition, we examined the protective effects of dietary supplementation with a multimineral-rich product derived from marine red algae on UD in these 2 diet groups. HFWD-fed mice had an increased incidence of UD. In addition, mice on a HFWD had significantly more severe clinical and histologic lesions. Dietary mineral supplementation in mice on a HFWD decreased the histologic severity of lesions and reduced the incidence of UD in female mice in both diets. In conclusion, a high-fat western-style diet may potentiate UD in C57BL/6NCrl mice. Insufficient mineral supply and mineral imbalance may contribute to disease development. Mineral supplementation may be beneficial in the treatment of UD.
Abbreviations: AIN76A, American Institute of Nutrition diet 76A; HFWD, high-fat western-style diet; KC, keratinocyte-derived cytokine; MS, mineralized red-algae supplement; RANTES, regulated on activation, normal T-cell expressed and secreted; UD, ulcerative dermatitis
Idiopathic, ulcerative dermatitis (UD) is a spontaneous, chronic disease generally affecting C57BL/6 mice and genetically engineered mice on a C57BL/6 background.2,22,46,47,56,57 Prevalence rates of 4.1% to 26% have been reported,2,12,13,21 with disease occurring predominately in female mice.12,21,45-47 UD is characterized by intense pruritus and lesion formation predominantly on the head or dorsal thorax. Self-trauma likely contributes to wound severity and delayed wound healing.2,6,21,22,27,42,46,47,56,57 Once UD develops, lesions are typically unresponsive or only partially responsive to treatment.21,22,42,56 The exact etiology of UD is unknown. Environmental factors, seasonal variation, microbial infection, follicular dystrophy, and immune complex vasculitis have all been proposed as predisposing factors for clinical disease development.2,16,21,22,26,46-48 Regardless, UD is an important clinical issue that often affects biomedical research by causing premature removal of mice from studies due to humane euthanasia for severe UD lesions.
Diet has a profound effect on disease incidence and progression. Specifically, a number of studies have shown that calorie restriction to approximately 60% of the normal intake reduces the incidence of UD12,37,52 in susceptible mice. One study reported that reducing dietary fat content fed to mice to 5% decreased the incidence of ulcerative lesions in the study population.28 In other studies, mice on an obesity-inducing diet (35% fat and high carbohydrate content) demonstrated increased UD lesions and impaired healing of induced wounds.41,55 In addition, deficient or excess dietary vitamin A or E content may predispose mice to UD development.16,22,24,48
In a study on the effects of a dietary multimineral supplement on dietary fat-influenced colonic carcinogenesis,8 there appeared to be a decreased incidence of UD in HFWD mice that received the multimineral supplement (study A in the current report). This mineralized red-algae supplement (MS) has been proposed as a supplement for osteoarthritis symptoms,17,18 bone support,3,7 and gastrointestinal health.5,6,8 The exact mechanism through which the MS exerts its effects is unknown; however, antioxidant or antiinflammatory properties have been proposed.8,23,33 To better evaluate this dermatitis-reducing effect, we followed the occurrence of UD in MS-supplemented and unsupplemented C57BL/6NCrl mice fed 2 diets—a standard rodent chow diet (American Institute of Nutrition 76A, AIN76A) and a high-fat, western-style diet (HFWD). We report 2 separate studies here. The first (study A) is the evaluation of the incidence of UD over a period of 15 mo in mice fed either the standard diet or HFWD.7,8 In a subset of HFWD-fed mice, we evaluated the UD modulatory effects of this MS. In the second study (study B, 19 mo), we compared the 2 diets and assessed the UD-modulatory effects of MS supplementation on UD under both dietary conditions.30,31,53 The purpose of these investigations was to determine whether the HFWD—like previous obesity-inducing diets (with higher fat and calorie content)—increased the incidence of UD and whether the MS decreased or impeded the development of UD in C57BL/6NCrl mice, irrespective of dietary fat content.
Materials and Methods
Experimental design.
Both studies A and B were collaborations between the University of Michigan's Department of Pathology, Department of Statistics, and Unit for Laboratory Animal Medicine. Study A was designed to investigate the effect of multimineral supplementation on the association between HFWD and colon carcinoma development and on bone structure and function.6-8 During the study, the research members and veterinary staff noted that mice on the HFWD were developing UD at a more rapid and severe rate when compared with mice in the other diet groups, and it appeared that the multimineral supplementation with a red algae- derived supplement (MS) had a modulatory effect on lesion development in the HFWD-fed group. Because study A showed strong evidence of mineral-associated effects on the development of UD and because this effect had been evaluated in HFWD only, a second study (study B) was planned to evaluate the modulatory effects of MS supplementation in both HFWD- and standard diet-fed animals. Additional evaluation parameters targeting UD were added.
Animals.
Study A involved 30 female and 30 male C57BL/6NCrl mice, and study B used 60 female and 60 male C57BL/6NCrl mice (age, 3 wk; Charles River Laboratories, Portage, MI). In both studies, mice were maintained under SPF conditions and were housed in an AAALAC-accredited facility at the Unit for Laboratory Animal Medicine, University of Michigan Medical School, in compliance with the US Public Health Service Policy on Humane Care and Use of Laboratory Animals34 and in accordance with the Guide for the Care and Use of Laboratory Animals.20 All procedures were approved by the University of Michigan IACUC, in accordance with federal policy. Mice were kept on a 12:12-h light:dark cycle, and temperature and relative humidity in the animal room were maintained at 22 °C ± 2 °C and 30% to 70%, respectively. Food and filtered (5 μm) tap water were provided free choice.
Sentinel mice and direct testing of colony animals were used to monitor the health status of our experimental animals. At the time of this study, all mice were free from pinworms, minute virus of mice, mouse parvovirus, epizootic diarrhea of infant mice virus, ectromelia virus, Sendai virus, pneumonia virus of mice, mouse poliovirus (Theiler meningoencephalitis virus), reovirus 3, mouse hepatitis virus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, murine adenovirus, and polyoma virus. Sentinel animals at this facility are not routinely screened for Helicobacter spp. or murine norovirus. In study B, all sentinel mice and a single colony mouse per box (20%), chosen at random, were examined for fur mites prior to scheduled euthanasia. A small amount of hair was plucked from each of 5 different locations (interscapular, sacral, abdomen, and both axillary regions). The hair was placed in mineral oil, and a cover slip was applied. Slides were examined by light microscopy for the presence of fur mites or eggs. All fur-mite examinations were negative. At the time of study A, routine testing for fur mites was not performed on colony or sentinel mice.
Diets.
AIN76A is a routinely used, certified, low-fat rodent diet. The HFWD is derived from AIN76A. However, it has several modifications designed to mimic the diet consumed by many people in Western society.26,44 The fat (corn oil) content is increased from 5 to 20 g%. The carbohydrate content is reduced, but the overall energy content (4.76 kcal/g in the HFWD compared with 3.90 kcal/gm in AIN76A) is increased. The HFWD has less fat and fewer overall calories than the previously used obesity-inducing diets.55 In addition to these modifications, the HFWD has reduced contents of calcium, vitamin D, folic acid and choline, and methionine is replaced with cysteine; all other vitamin, mineral and amino acids contents were essentially the same for both diets. The HFWD was formulated according to a previous study.30,31 All diets were formulated, manufactured, and provided by Research Diets (New Brunswick, NJ). The analyses of the AIN76A diet and HFWD have been published previously.30,31,38,39
The MS used in this study consists of the inorganic remains of the red marine alga, Lithothamnion calcareum.1 The MS is approximately 12% calcium and 1% magnesium by weight and contains measurable levels of 72 other trace minerals. The mineral product is sold as a food supplement under the name Aquamin (generally regarded as safe 000028; Marigot, Cork, Ireland) and has been used in previous human trials17,18 and rodent studies.3-5,7,8
In both studies mice, were grouped by sex, and 5 mice were housed per cage. In study A, mice were randomly divided into 3 diet groups, each consisting of 10 male and 10 female mice, which received AIN76A rodent chow, HFWD, or HFWD with MS. In study B, mice were randomly divided into 4 diet groups consisting of 15 male and 15 female mice per diet. The diet groups were the same as for study A, with the addition of a fourth diet group (AIN76A with MS). Mice in both studies were started on their respective diets at 3 wk of age. Mice in study A were euthanized at 15 mo, and mice in Study B were euthanized at 18 to 19 mo after the start of the study.
Premortem examination.
Mice in study A were evaluated for the incidence of UD over the course of the study, which was designed primarily as a colon polyp prevention study.8 In study B, mice were evaluated for incidence and severity of UD and for multiple clinical parameters associated with UD. Mice received a physical examination from a laboratory animal veterinarian on day 1 and every 2 wk until euthanasia or spontaneous death. In addition, mice underwent physical examination within 24 h of euthanasia. At each examination, mice were evaluated for alopecia, pruritus, excoriations, ulceration, and other dermatologic manifestations. When visible lesions were present, they were scored by using 4 criteria, as previously described.19 These criteria were each categorized on a scale of 0 to 3 in regard to the number of scratches in a 2-min period (0, no scratches; 1, less than 5; 2, 5 to 10; or 3, more than 10); character of the lesion (0, no lesion; 1, excoriations or single punctuate crust; 2, multiple or coalescing crust; and 3, erosion or ulceration), length of lesioned skin (0, 0 cm; 1, 1 cm; 2, 1 to 2 cm; and 3, greater than 2 cm), and body region(s) affected (0, none; 1, thoracic or abdominal region; 2, thoracic and abdominal region; and 3, any region involving the head). The average of the 4 scores combined, on a percentage scale, was used as an indication of disease severity.
Necropsy.
For both studies, mice were euthanized by carbon dioxide followed by bilateral pneumothorax. Skin samples of mice on study A were collected for histologic examination (see following section). Mice in study B were weighed within 24 h of euthanasia. Blood (>250 µL) was collected via cardiocentesis after euthanasia. Samples were allowed to clot for 10 min, and then serum was separated via centrifugation at 5200 × g for 8 min. Serum was immediately stored at –20 °C until further processing. The weight of the spleen was recorded. The spleen was collected for flow cytometric analysis and histologic examination. Mice were examined under a focal light for dermal lesions. Skin samples from all mice were collected for histologic examination. For these samples, sections were taken from lesional skin and adjacent skin in affected mice and from comparable skin areas (interscapular and dorsal neck) in unaffected mice.
Histologic evaluation.
Skin and spleen tissues were immersion-fixed in 10% neutral buffered formalin and processed for staining with hematoxylin and eosin. Skin slides were blinded and then reviewed by a board-certified veterinary pathologist for confirmation and characterization of UD. Slides were scored for the presence or absence of ulceration (that is, through the epidermis) and for inflammation with severity criteria as follows: minimal, focal or few foci with inflammation confined to the superficial dermis; moderate, multiple foci with inflammation extending to the deep dermis; severe, regionally extensive foci with inflammation occasionally extending to the subcutis; or marked, same as for the severe category, but with inflammation extending to the subcutis throughout a large portion of the lesion. In addition, slides were evaluated for epidermal thickness, hair follicle changes, and for the presence of mast cells. Spleen sections were evaluated (but not quantitated) with regard to cellular density and cellular composition.
Flow cytometric analysis—splenocytes.
Spleen cell populations underwent flow cytometry in study B. Approximately one third of the spleen was mechanically disrupted between frosted glass slides and passed over a 50-µm filter to yield a single-cell suspension. Processed splenocytes were counted to standardize the number of cells assayed and then stained for 4-color FACS analysis by using the following reagents from eBioscience (San Diego, CA): tube 1, antiCD45–APC-AF780 (pan-leukocyte marker), antiCD19–PE (B cells), antiCD3e–PerCP-Cy5.5 (T cells), and antiCD41–APC (megakaryocytes); tube 2, antiCD45–APC-AF780 (leukocytes), antiCD11b–PE (activated lymphocytes cells and granulocytes), antiGr1–PE-Cy7 (granulocytes and monocytes), and antiCD117 (c-kit)–APC (mast cells); tube 3, antiCD45–APC-AF780 and the isotype controls for tube 1 antibodies; tube 4, the isotype controls for tube 2 antibodies. All samples were fixed in a 2% buffered formalin solution and analyzed within 24 h of labeling. The samples were evaluated on an LSR II (BD Biosciences, San Jose, CA). For analysis of the splenocytes, 50,000 events were collected and analyzed by using the WinList 5.0 program (Verity Software House, Topsharn, ME). The percentage of each of the leukocyte populations was obtained by gating on the CD45+ population in a plot comparing CD45 with side scatter.
Serum cytokine analysis.
Serum cytokine analysis was performed for study B. A panel of murine cytokines including TNFα, IL6, IL1α, keratinocyte-derived cytokine (KC), macrophage chemotactic protein 1 (MCP1), and regulated upon activation, normal T-cell expressed and secreted (RANTES) protein was measured in serum samples by using a bead-based cytokine assay (BioPlex, BioRad Laboratories, Hercules, CA). The C-reactive protein in the same serum samples was assessed by using an ELISA kit (Life Diagnostics, West Chester, PA).
Data analysis.
Statistical analysis of UD incidence, effect of diet on incidence of dermatitis and sex differences were performed by using Meier–Kaplan survival curves, Cox regression, and the Fishers exact test (SSPS 19, IBM, Armonk, NY). Comparisons of lesion severity, spleen flow-cytometry data, and serum cytokine levels were analyzed by using the Student t test or ANOVA followed by paired group comparison (Student t test; GraphPad Software, La Jolla, CA, USA) or both. When comparing data from flow cytometry or cytokine assays, corrections for multiple comparisons correction were used. A P value of less than or equal to 0.05 was considered statistically significant.
Results
Presentation and incidence of UD.
We conducted 2 separate long-term studies: a 15-mo study (study A) using a total of 20 mice per diet group (n = 10 per sex) and a 19-mo study (study B) using 30 mice per diet group (n = 15 per sex). UD initially presented as punctate crusting lesions with pruritus indicated by increased scratching during scheduled clinical observations. Lesions frequently progressed to visible ulceration. In study B, where the time course of lesions was closely followed, the average age of UD onset in was 79 wk. UD increased in frequency with increasing time. Alopecia preceding lesion development was only seen in 3 mice (3.8% of the total mice that developed UD). This clinical picture followed the previous time course of lesions seen in our earlier study.19 The clinical progression mirrored that in numerous reports of UD in C57BL/6 in the association with pruritus and the increased incidence with age2,21,24,29,56 but was distinct from reports describing an UD in female C57BL/6J mice with extensive alopecia and variable pruritus preceding ulceration in young mice.16,28,48
During the course of study A, 20 of the 60 mice reached humane endpoints and required early euthanasia due to illness, including severity of UD, or died prematurely. Of these, 14 mice had UD; these mice were included in the analysis. The 6 mice that died or were euthanized prematurely due to other causes were excluded because the frequency of dermatitis could not be monitored in these animals for the full study period. In total, 18 mice of 54 mice (33.3%) in study A developed clinical UD. Of those, 3 of 18 that reached the end of study (16.6%) were from the AIN76A group, 10 of 17 (58.8%) were from the HFWD group, and 5 of 19 (26.3%) were from the HFWD with MS group (Table 1). Female mice were affected more often than were male (female, 11; male, 7). Among female mice, UD incidence was higher when mice received HFWD than either AIN76A (P = 0.0055) or HFWD with MS (P = 0.0001; Table 1).
Table 1.
Number of mice ([no. of male mice/no. of female mice]) that developed clinically evident UD by diet
| AIN76A | HFWD | Overall | ||
| Study A | ||||
| Clinical UDa | ||||
| Without mineral supplement | 3 (1/2)b | 10 (1/9) | 13 (2/11) | |
| With mineral supplement | ND | 5 (5/0)c | 5 (5/0)d | |
| Study B | ||||
| Clinical UDa | ||||
| Without mineral supplement | 4 (0/4)b | 13 (5/8) | 17 (5/12) | |
| With mineral supplement | 2 (2/0)e | 9 (3/6) | 11 (5/6) | |
| Resolved UD | ||||
| Without mineral supplement | 4 (1/3) | 1 (1/0) | 5 (2/3) | |
| With mineral supplement | 2 (2/0)e | 2 (2/0) | 4 (4/0) | |
| Clinical and resolved UDa | ||||
| Without mineral supplement | 8 (1/7) | 14 (6/8) | 22 (7/15) | |
| With mineral supplement | 4 (4/0)f | 11 (5/6) | 15 (9/6)d |
Significant (P < 0.05, 2-tailed Fisher exact test) difference between AIN76A and HFWD groups regardless of sex and supplementation status.
Significant (P < 0.05, 2-tailed Fisher exact test) difference between nonsupplemented AIN76A and HFWD groups regardless of sex.
Significant (P < 0.05, 2-tailed Fisher exact test) difference between supplemented and nonsupplemented female mice fed HFWD.
Significant (P < 0.05, 2-tailed Fisher exact test) difference between supplemented and nonsupplemented female mice.
Significant (P < 0.05, 2-tailed Fisher exact test) difference between supplemented AIN76A and HFWD groups regardless of sex.
Significant (P < 0.05, 2-tailed Fisher exact test) difference between supplemented and nonsupplemented female mice fed AIN76A.
During the course of study B, 29 of the 120 mice reached humane endpoints and required early euthanasia due to illness or died prematurely. Of these, 16 mice had UD; these mice were included in the analysis. The 13 mice that died or were euthanized prematurely due to other causes were excluded because the frequency of dermatitis could not be monitored in these animals for the full study period. In total, 37 (34.6%) of the 107 mice developed clinical UD (Table 1). Of the 37 mice that developed UD, 8 of 29 (27.6%) were from the AIN76A diet group, 4 of 27 (14.8%) were from the AIN76A diet group with MS, 14 of 23 (60.9%) were from the HFWD group, and 11 of 28 (39.3%) were from the HFWD group with MS. Of the 37 mice that developed UD, 9 (6 male and 3 female) had lesions that spontaneously resolved. Of the 9 mice whose disease resolved, 4 (44.4%) were from the AIN76A diet group, 2 (22.2%) were from the AIN76A diet group with MS, 1 (11.1%) was from the HFWD group, and 2 (22.2%) were from the HFWD group with MS. Statistically, mice fed HFWD had increased UD incidence as compared with mice on AIN76A (P < 0.02, Kaplan–Meier and Cox regression). Female mice had a trend toward higher incidence compared with that in male mice, but the difference was not statistically significant. More mice on either diet without MS developed UD more often than did mice provided MS (22% to 60% compared with 15% to 40%, respectively), although this difference was not statistically significant. However, when mice were compared according to sex, the difference in UD incidence between unsupplemented female mice (15% to 71%) compared with supplemented (6% to 29%) was statistically significant (P = 0.0292).
On the basis of the results of the 2 studies combined, we conclude that 1) the incidence of UD is higher in mice fed HFWD than in mice on the control diet and that 2) MS reduces the incidence of UD in female but not male mice, even though overall incidence is less in male mice.
Clinical severity assessment and histologic findings.
In study A, lesional skin was examined histologically in 16 mice in the AIN76A group (7 female, 9 male), 18 mice in the HFWD group (10 female, 8 male), and 18 mice in the MS-supplemented HFWD group (10 female, 8 male). Blinded histologic evaluation was performed for all skin sections in all 120 mice in Study B. Only mice with histologic UD had visible skin lesions at necropsy (Figure 1).
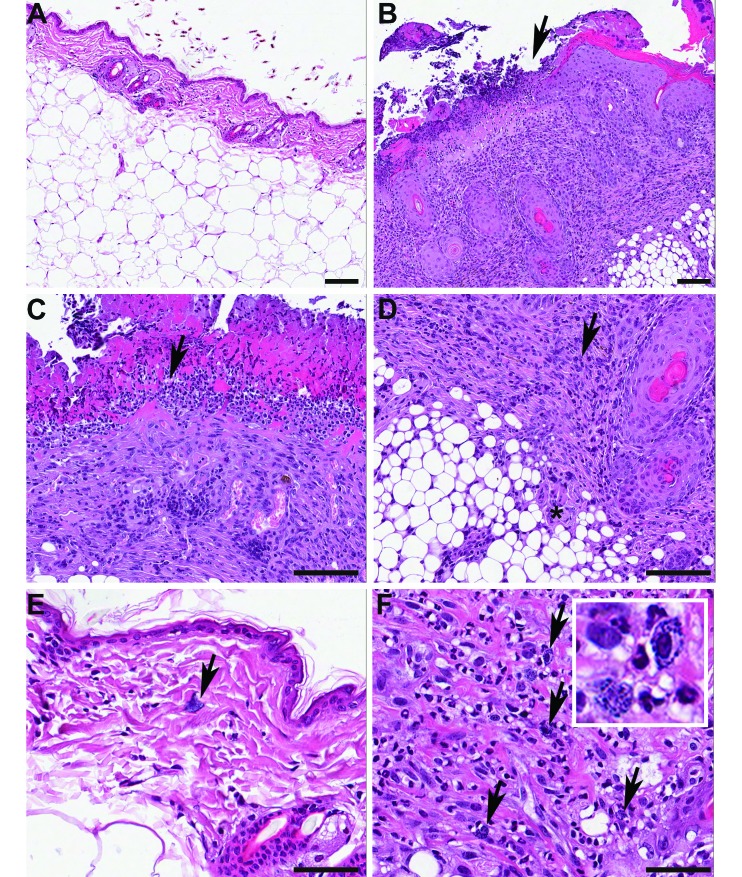
Figure 1.
Representative histologic appearance of UD. (A) Normal skin from an unaffected mouse. Note the thin epidermis and dermis above abundant subcutaneous fat. (B) A focus of ulcerative dermatitis in an affected animal, imaged at the same magnification as in A. The arrow denotes the junction between intact, markedly hyperplastic and hyperkeratotic epidermis (on the right) and a locally extensive area of ulceration (on the left). The ulcerated area is subtended by inflammation extending into the deep dermis and subcutis. (C) Higher magnification image of the area of ulceration, showing extensive serocellular crusting with numerous degranulated neutrophils (arrow) accompanying epidermal necrosis. (D) High magnification of the deeper portions of the ulcerated area, showing mixed mononuclear and granulocytic inflammation (arrow) in the dermis and extending into the subcutis (asterisk). (E) High magnification of normal skin, showing lack of inflammation in the dermis, with presence of occasional mast cells (arrow). (F) High magnification of the dermis in an animal with ulcerative dermatitis showing that the inflammatory infiltrate contains numerous mast cells (arrows, and inset). Hematoxylin and eosin stain; bar, 100 μm (A through D); 50 μm (E and F).
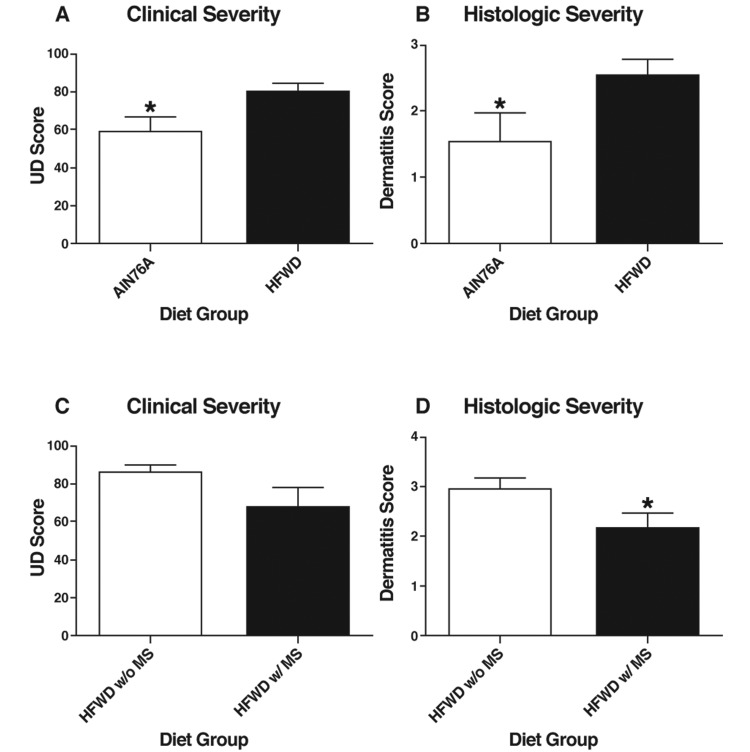
In study B, UD severity was assessed semiquantitatively by using both a clinical score and a histologic score. Clinical and histologic evaluation was performed in all mice. Mice fed AIN76A had less severe clinical and histologic UD than did mice on HFWD (Figure 2 A and B). The MS did not affect either the clinical or histologic severity in mice fed the control AIN76A diet (not shown). However, the severity of UD was reduced in the mice given HFWD with MS as compared with those fed unsupplemented HFWD. This reduction in clinical severity did not reach statistical significance, but MS supplementation produced a significant decrease in histologic severity, with regard to scores describing the size and depth of inflammatory foci (2.167 ± 0.305 compared with 2.950 ± 0.223, respectively, P = 0.0428; Figure 2 C and D).
Figure 2.
Study B. (A) Clinical and (B) histologic severity of UD lesions in mice on the AIN76A diet and HFWD, regardless of supplementation with mineralized red algae (MS). (C) Clinical and (D) histologic severity of UD lesions in the HFWD groups without and with MS. UD lesions were scored (scale, 0 to 100) according to scratching, character of lesion, length of lesions, and body regions affected. The histologic severity of dermatitis lesions was scored as 1, minimal; 2, moderate; 3, or 4, marked. Values represent mean ± SEM. *, P < 0.05 (Student t test).
Histologic alterations in affected mice in both studies consisted of localized to extensive ulceration with severe superficial neutrophilic and serocellular crusting. In addition, severe macrophagic, lymphocytic, and mastocytic inflammation extending deep into the dermis and subcutis (Figures 1 B through D) was present. Occasional mast cells were present in mice without dermatitis and in unaffected areas of skin from animals with dermatitis but were markedly increased in the affected areas (Figures 1 E and F). The epidermis adjacent to the affected areas showed marked hyperplasia and orthokeratotic hyperkeratosis. Severely affected mice had dermal fibrosis at the deep and lateral margins of the lesion. The affected areas also demonstrated follicular hyperkeratosis; however, this feature was not seen in the absence of ulcerative and inflammatory lesions. Follicular dystrophy, as described in young female mice of the C57BL/6J substrain that showed an alopecic and ulcerative dermatitis, was not apparent either adjacent to lesioned skin in affected mice or in unaffected skin from affected or unaffected mice.16,48 There were no abnormal histologic findings in skin from clinically normal animals.
Association between UD and biomarkers of systemic inflammation.
We compared the mice in study B that developed UD at any time during the study with mice those that did not develop UD, in regard to several parameters associated with systemic inflammation (Table 2). In brief, mice that developed UD weighed significantly less at euthanasia (both sexes: P = 0.0005; male: P = 0.001; female: not signficant), consistent with the high morbidity of this disease. Mice that developed UD had heavier spleens (both sexes: P = 0.05; male: P = 0.02; female: not significant). Histologically, spleen enlargement reflected an increase in extramedullary hematopoiesis, particularly of the myeloid (granulocyte–monocyte) lineage, reflective of an increased inflammatory demand (Figure 3).
Table 2.
General parameters and proinflammatory markers
| Male and female mice |
Male mice |
Female mice |
|||||
| With UD | Without UD | With UD | Without UD | With UD | Without UD | ||
| Body weight (g) | 37.3 ± 1.1a (36) | 43.8 ± 1.1 (73) | 39.8 ± 2.1a (16) | 47.3 ± 1.1 (38) | 35.3 ± 0.9 (20) | 39.9 ± 1.9 (35) | |
| Spleen weight (g) | 0.29 ± 0.03a (37) | 0.18 ± 0.03 (79) | 0.20 ± 0.04a (16) | 0.11 ± 0.02 (40) | 0.36 ± 0.04 (21) | 0.26 ± 0.07 (39) | |
| Kidney weight (g) | 0.48 ± 0.01a (37) | 0.44 ± 0.01 (78) | 0.52 ± 0.02 (16) | 0.49 ± 0.01 (40) | 0.46 ± 0.01a (21) | 0.38 ± 0.01 (38) | |
| Spleen flow cytometry (%)b | |||||||
| B cells | 37.4 ± 3.9a (26) | 55.7 ± 1.2 (71) | 48.6 ± 6.0a (12) | 61.2 ± 0.8 (38) | 27.8 ± 3.4a (14) | 49.1 ± 1.8 (33) | |
| T cells | 22.5 ± 1.3 (26) | 24.5 ± 0.6 (71) | 22.8 ± 0.5 (38) | 22.9 ± 1.6 (12) | 22.2 ± 2.0a (14) | 26.5 ± 1.1 (33) | |
| Granulocytes | 24.7 ± 3.7a (26) | 7.1 ± 0.4 (71) | 18.7 ± 5.7a (12) | 7.3 ± 0.6 (38) | 29.9 ± 4.7a (14) | 6.8 ± 0.6 (33) | |
| CRP (ng/mL) | 301 ± 94 (19) | 206 ± 23 (60) | 335 ± 70 (6) | 235 ± 39 (24) | 288 ± 128 (13) | 168 ± 15 (36) | |
| Plasma cytokine analysis | |||||||
| IL6 (pg/mL) | 26.5 ± 6.3a (34) | 2.5 ± 0.6 (70) | 19.1 ± 5.2a (15) | 2.2 ± 0.5 (37) | 32.3 ± 10.5a (19) | 2.8 ± 1.1 (33) | |
| IL1α (pg/mL) | 0.04 ± 0 (34) | 0.19 ± 0.05 (70) | 0.04 ± 0 (15) | 0.11 ± 0.03 (37) | 0.04 ± 0 (19) | 0.28 ± 0.11 (33) | |
| TNFα (pg/mL) | 2.5 ± 0.9a (35) | 1.0 ± 0.3 (70) | 1.6 ± 0.9 (15) | 0.8 ± 0.3 (37) | 3.1 ± 1.4 (19) | 1.4 ± 0.4 (33) | |
| RANTES (pg/mL) | 11.4 ± 1.4 (34) | 13.9 ± 0.7 (71) | 13.5 ± 2.6 (15) | 14.3 ± 1.0 (37) | 9.7 ± 1.4a (19) | 13.6 ± 0.9 (34) | |
| MCP1 (pg/mL) | 101 ± 21 (34) | 124 ± 10 (70) | 154 ± 44 (15) | 154 ± 14 (37) | 75 ± 21 (19) | 90 ± 10 (33) | |
| KC (pg/mL) | 42.9 ± 4.2a (34) | 27.2 ± 1.8 (70) | 37.8 ± 4.8 (15) | 29.1 ± 2.9 (37) | 46.8 ± 6.5a (19) | 25.0 ± 2.0 (33) | |
CRP, C-reactive protein.
Values are given as mean ± SEM (no. of animals analyzed).
P ≤ 0.05 (Student t test).
The percentage of each of the leukocyte populations was obtained by gating on the CD45+ population in a CD45 compared with side-scatter plot.
Figure 3.
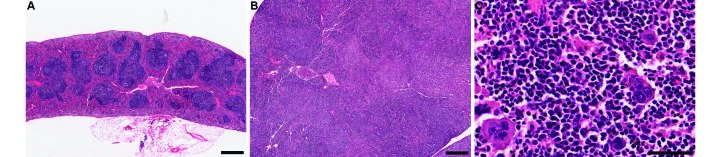
Representative histologic appearance of spleens from mice (A) without and (B) with UD. (A) Histologic appearance of a normal spleen in a mouse without dermatitis. (B and C) Histologic appearance of enlarged spleen in a mouse with ulcerative dermatitis. The architecture of the normal spleen is altered, in that (B) there are no well-defined lymphoid areas, and (C) the red pulp sinuses are markedly expanded by extramedullary hematopoietic elements. Hematoxylin and eosin stain; bar, 100 µm (A and B), 50 µm (C).
Splenic T lymphocyte, B lymphocyte, and granulocyte numbers were compared between mice that developed UD and those that did not. The percentage of B cells was lower (both sexes: P < 0.0001; male: P = 0.001; female: P < 0.0001) and the percentage of granulocytes was higher (both sexes: P < 0.0001; male: P = 0.001; female: P < 0.0001) in spleens of mice that developed UD as compared with mice that did not develop UD. Female mice that developed UD (but not male mice) showed a slight decrease (P < 0.04) in spleen T cell percentage (Table 2).
Analysis of circulating cytokines showed that mice that developed UD had significantly increased (P < 0.05) levels of the acute inflammatory cytokines IL6, TNFα, and KC. In contrast, mice that developed UD had slightly lower levels of the leukocyte chemoattractant RANTES (both sexes and male: not significant; female: P < 0.02), and there was no difference in serum MCP1, IL1α, or C-reactive protein (Table 2).
Discussion
C57BL/6 mice and genetically engineered mice on a C57BL/6 background are prone to developing (UD) as they age, and this propensity carries a significant risk for premature removal from studies due to morbidity. Although many publications have described various environmental and genetic influences in the development of UD, to date no specific pathogenesis or etiology has been described.2,21,46,47,56 Previous studies have shown that calorie-restriction diets significantly reduce the incidence and severity of UD,12,37,52 and further reducing dietary fat content below standard levels might be protective.28 Diets reduced in fat and calories protect against other forms of inflammatory skin disease as well: for example, our own previous studies demonstrated that caloric restriction mitigates the dermal irritation and inflammation resulting from topical retinoid use.53
In contrast to fat- and calorie-reduced diets, obesity-inducing diets increase the incidence of UD.29 The HFWD used in our study is similar to previous obesity-inducing diets in that the fat content is increased compared with that in normal mouse chow (AIN76A). However, the fat content is lower compared with that in obesity diets (20 g% as compared with 35 to 50 g% in obesity-inducing diets). In addition, the current HFWD has a modestly reduced carbohydrate content so that the overall calorie level (although increased relative to that in AIN76A) does not approach the levels in obesity diets. This HFWD does not induce diabetes.31 Nevertheless, HFWD similarly increased the incidence and severity of UD lesions, as previously reported with obesity-inducing diets.29 In study A, mice fed AIN76A had a UD incidence of 16.6% as compared with 58.8% for mice fed HFWD. In study B, the incidence of UD in mice fed the AIN76A diet was 27.6%, with 50% spontaneous lesion resolution, comparable to previous reports,2,12,13,21 whereas mice fed HFWD had an incidence of 60.9%, with only 12% spontaneous lesion resolution. Therefore our data confirm that increased fat and calories intake are risk factors for UD but suggest that increased UD does not require the severe diet manipulation seen in obesity-producing diets.
In the present study, we examined the effects of supplementation with calcium and multiple trace elements on UD development in both diets. The overall UD incidence and severity was decreased in response to the mineralized red-algae supplement (MS), but the reduction was only seen in female mice; male mice derived no protection from MS. These finding prompt 2 questions: first, how does the MS contribute to protection against UD development, and second, what accounts for these differences between female and male mice. The underlying mechanism of protection is not known. Calcium, the major cationic mineral in the supplement, plays a critical role in epithelial cell growth and differentiation in the skin as well as in other tissues.15,25 In addition, calcium may contribute to improved barrier function and reduce the effects of inflammatory stimuli on the skin. Although this finding needs to be verified experimentally, the same MS has been demonstrated to reduce inflammation in the mouse gastrointestinal tract.8 Although the MS doesn't appear to have a direct antioxidant effect,32 the individual trace elements found in the MS (for example, copper, zinc, selenium, manganese, and molybdenum) might play a role in the proposed antioxidant activity of the supplement.8,33,58 In addition, this MS had antiinflammatory effects in an in vitro model of inflammation33 and promoted colon epithelial cell differentiation.6,23,43 In the gastrointestinal tract, barrier function and inflammation are inversely correlated.14 Although calcium is critical to the function of the MS, other cationic trace elements in the MS also promote colon epithelial cell differentiation, and some of these work at nanomolar concentrations.9 Given these previous findings in the literature and the results in the current study, we hypothesize that early dietary supplementation of MS may prevent the development of UD in female C57BL/6NCrl mice by improving epidermal barrier function. Verification of this finding in additional substrains and evaluation of the reason for the apparent difference in protective ability between the sexes await additional experimentation.
The presence of UD influenced numerous parameters associated with systemic inflammation. Mice with UD had a lower body weight at euthanasia, likely a reflection of the high morbidity associated with this chronic disease. Splenomegaly, which is a consistent finding in UD and associated with extramedullary hematopoiesis, was present. Consistent with the systemic nature of the disease, mice with UD had a higher spleen granulocyte count and a lower B-cell count. These findings are consistent with the histologic features of UD, in which neutrophils and mast cells were the predominant inflammatory cells. Cytokine analysis showed that mice with UD had increased plasma levels of IL6, TNFα, and KC. These changes in cytokine content are consistent with both Th2 and Th17 differentiation; both Th2 and Th17 inflammatory responses occur in atopic dermatitis.35,36,40,49 Cytokine release from degranulating mast cells has been linked to intense itching.35 Whether any of these systemic manifestations of the disease are causative or simply a reflection of extensive tissue injury is unknown. Nevertheless, they provide potential targets for intervention.
Chronic UD in mice has been compared with a plethora of disease conditions in humans, including obsessive–compulsive disorder, trichotillomania, Tourette syndrome, and central centrifugal cicatricial alopecia.16,46,48 Many of these conditions are exacerbated or instigated by self-trauma. Prevention of self-trauma (for example, by nail-trimming) is an effective means of controlling progression in UD lesions.27 The instigating cause for the intense pruritus of UD in mice is still unclear. Follicular rupture secondary to follicular dystrophy similar to that in central cicatricial alopecia has been proposed as a mechanism,16,48 but the clinical presentation and histology of that condition (young mice with initial alopecia and follicular changes) is different from the presentation in our current study and many previous studies of UD.2,19,56,57
The clinical presentation and end-stage ulceration in UD is similar to that described in the atopic dermatitis of NC/Nga mice, in which atopic dermatitis develops after the application of mites or mite proteins but also occurs spontaneously in conventional housing conditions.35,51,54 Both UD and atopic dermatitis or eczema are characterized by intense pruritus that coincides with or precedes the development of clinically observable lesions. In addition, both conditions involve a robust inflammatory response notably involving mast cells. However, true atopic dermatitis or eczema is an allergic condition associated with a specific IgE response. Reportedly, C57BL/6 mice are low IgE responders in models of induced skin irritation.10 A subset of humans with the clinical appearance of atopic dermatitis or eczema but that lack a specific IgE response has been classified as having ‘intrinsic’ or ‘nonallergic’ dermatitis (also termed ‘atopiform dermatitis’). Intrinsic or nonallergic dermatitis or eczema lacks specific IgE triggering and may be the result of contact sensitization to an external antigen that acts as a hapten.50 Many of the cytokines increased in study B (that is, IL6 and KC) also are involved in the increased susceptibility of C57BL/6 mice to the irritant dermatitis induced by phthalic anhydride.10 Clinically, nonallergic or intrinsic dermatitis has a later age of onset and is more common in female subjects,11 both of which are features of UD in mice. Further evaluation of UD in C57BL/6NCrl mice in comparison to the clinical and molecular features of intrinsic (nonallergic) dermatitis may be helpful in defining pathogenesis.
In conclusion, the studies described here show that a typical western-style diet may potentiate the development of UD and that mineral supplementation may ameliorate the incidence and severity of this disease. Multiple factors in the western-style diet, including high-fat content and an inadequate mineral supply, may contribute to the underlying mechanism, and the elucidation of the potential protective effect of specific minerals in the face of an irritant challenge may be a useful future focus.
Acknowledgment
This study was supported in part by grant CA140760 from the NIH and by grant 11-0577 from the Agency for International Cancer Research, St Andrews, Fife, Scotland.
References
- 1.Adey WH, McKibbin DL. 1970. Studies on the maerl species Phymatolithon calcareum (Pallas) nov. comb. and Lithothamnium corallioides Crouan in the Ria de Vigo. Bot Mar 13:100–106. [Google Scholar]
- 2.Andrews AG, Dysko RC, Spilman SC, Kunkel RG, Brammer DW, Johnson KJ. 1994. Immune complex vasculitis with secondary ulcerative dermatitis in aged C57BL/6NNia mice. Vet Pathol 31:293–300. [DOI] [PubMed] [Google Scholar]
- 3.Aslam MN, Bergin I, Jepsen K, Kreider JM, Graf KH, Naik M, Goldstein SA, Varani J. 2013. Preservation of bone structure and function by Lithothamnion sp.-derived minerals. Biol Trace Elem Res 156:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslam MN, Bergin I, Naik M, Hampton A, Allen R, Kunkel SL, Rush H, Varani J. 2012. A multimineral natural product inhibits liver tumor formation in C57BL/6 mice. Biol Trace Elem Res 147:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aslam MN, Bergin I, Naik M, Paruchuri T, Hampton A, Rehman M, Dame MK, Rush H, Varani J. 2012. A multimineral natural product from red marine algae reduces colon polyp formation in C57BL/6 mice. Nutr Cancer 64:1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aslam MN, Bhagavathula N, Paruchuri T, Hu X, Chakrabarty S, Varani J. 2009. Growth-inhibitory effects of a mineralized extract from the red marine algae, Lithothamnion calcareum, on Ca2+-sensitive and Ca2+-resistant human colon carcinoma cells. Cancer Lett 283:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslam MN, Kreider JM, Paruchuri T, Bhagavathula N, DaSilva M, Zernicke RF, Goldstein SA, Varani J. 2010. A mineral-rich extract from the red marine algae Lithothamnion calcareum preserves bone structure and function in female mice on a western-style diet. Calcif Tissue Int 86:313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aslam MN, Paruchuri T, Bhagavathula N, Varani J. 2010. A mineral-rich red algae extract inhibits polyp formation and inflammation in the gastrointestinal tract of mice on a high-fat diet. Integr Cancer Ther 9:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attili D, Jenkins B, Aslam MN, Dame MK, Varani J. 2012. Growth control in colon epithelial cells: gadolinium enhances calcium-mediated growth regulation. Biol Trace Elem Res 150:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae CJ, Shim SB, Jee SW, Lee SH, Kim MR, Lee JW, Lee CK, Hwang DY. 2010. IL6, VEGF, KC, and RANTES are a major cause of a high-irritant dermatitis to phthalic anhydride in C57BL/6 inbred mice. Allergol Int 59:389–397. [DOI] [PubMed] [Google Scholar]
- 11.Bieber T. 2010. Atopic dermatitis. Ann Dermatol 22:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackwell BN, Bucci TJ, Hart RW, Turturro A. 1995. Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH31 open-formula diet. Toxicol Pathol 23:570–582. [DOI] [PubMed] [Google Scholar]
- 13.Brayton C. 2007. Spontaneous disease in commonly used mouse strains, p 648–649. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. The mouse in biomedical research, vol II: diseases. Boston (MA): Elsevier. [Google Scholar]
- 14.Clayburgh DR, Shen L, Turner JR. 2004. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest 84:282–291 [DOI] [PubMed] [Google Scholar]
- 15.Eckert RL. 1989. Structure, function, and differentiation of the keratinocyte. Physiol Rev 69:1316–1346. [DOI] [PubMed] [Google Scholar]
- 16.Everts HB, Silva KA, Montgomery S, Suo L, Menser M, Valet AS, King LE, Ong DE, Sundberg JP. 2012. Retinoid metabolism is altered in human and mouse cicatricial alopecia. J Invest Dermatol 133:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frestedt JL, Kuskowski MA, Zenk JL. 2009. A natural seaweed-derived mineral supplement (Aquamin F) for knee osteoarthritis: a randomised, placebo-controlled pilot study. Nutr J 8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frestedt JL, Walsh M, Kuskowski MA, Zenk JL. 2008. A natural mineral supplement provides relief from knee osteoarthritis symptoms: a randomized, controlled, pilot trial. Nutr J 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampton AL, Hish GA, Aslam MN, Rothman ED, Bergin IL, Patterson KA, Naik M, Paruchuri T, Varani J, Rush HG. 2012. Progression of ulcerative dermatitis lesions in C57BL/6Crl mice and the development of a scoring system for dermatitis lesions. J Am Assoc Lab Anim Sci 51:586–593. [PMC free article] [PubMed] [Google Scholar]
- 20.Institute of Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. p xii, 125. Washington (DC): National Academies Press. [Google Scholar]
- 21.Kastenmayer RJ, Fain MA, Perdue KA. 2006. A retrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci 45:8–12. [PubMed] [Google Scholar]
- 22.Lawson GW, Sato A, Fairbanks LA, Lawson PT. 2005. Vitamin E as a treatment for ulcerative dermatitis in C57BL/6 mice and strains with a C57BL/6 background. Contemp Top Lab Anim Sci 44:18–21. [PubMed] [Google Scholar]
- 23.Lee JC, Hou MF, Huang HW, Chang FR, Yeh CC, Tang JY, Chang HW. 2013. Marine algal natural products with antioxidative, antiinflammatory, and anticancer properties. Cancer Cell Int 13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mader JR, Mason MA, Bale LK, Gades NM, Conover CA. 2010. The association of early dietary supplementation with vitamin e with the incidence of ulcerative dermatitis in mice on a C57BL/6 background: diet and ulcerative dermatitis in mice. Scand J Lab Anim Sci 37:253–259 [PMC free article] [PubMed] [Google Scholar]
- 25.Menon GK, Elias PM, Lee SH, Feingold KR. 1992. Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res 270:503–512. [DOI] [PubMed] [Google Scholar]
- 26.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J, Fuchs CS. 2007. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. J Am Med Assoc 298:754–764. [DOI] [PubMed] [Google Scholar]
- 27.Mufford T, Richardson L. 2009. Nail trims versus the previous standard of care for treatment of mice with ulcerative dermatitis. J Am Assoc Lab Anim Sci 48:546. [Google Scholar]
- 28.Myers DD. 1997. C57BL/6J skin lesion problem eliminated. Bar Harbor (ME): The Jackson Laboratory. [Google Scholar]
- 29.Neuhaus B, Niessen CM, Mesaros A, Withers DJ, Krieg T, Partridge L. 2012. Experimental analysis of risk factors for ulcerative dermatitis in mice. Exp Dermatol 21:712–713. [DOI] [PubMed] [Google Scholar]
- 30.Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. 2008. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis 30:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. 2001. A western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis 22:1871–1875. [DOI] [PubMed] [Google Scholar]
- 32.O'Callaghan YC, Drummond E, O'Gorman DM, O'Brien NM. 2013. Antioxidant and proapoptotic effects of marine-derived, multimineral Aquamin supplemented with a pine-bark extract, Enzogenol, and a green-tea extract, Sunphenon. J Med Food 16:920–926. [DOI] [PubMed] [Google Scholar]
- 33.O'Gorman DM, O'Carroll C, Carmody RJ. 2012. Evidence that marine-derived, multimineral, Aquamin inhibits the NFκB signaling pathway in vitro. Phytother Res 26:630–632. [DOI] [PubMed] [Google Scholar]
- 34.Office of Laboratory Animal Welfare 2002. Public Health Service policy on humane care and use of laboratory animals amended. Bethesda (MD): Office of Laboratory Animal Welfare. [Google Scholar]
- 35.Ohmura T, Tsunenari I, Hayashi T, Satoh Y, Konomi A, Nanri H, Kawachi M, Morikawa M, Kadota T, Satoh H. 2004. Role of substance P in an NC/Nga mouse model of atopic dermatitis-like disease. Int Arch Allergy Immunol 133:389–397. [DOI] [PubMed] [Google Scholar]
- 36.Oyoshi MK, He R, Kumar L, Yoon J, Geha RS. 2009. Cellular and molecular mechanisms in atopic dermatitis. Adv Immunol 102:135–226. [DOI] [PubMed] [Google Scholar]
- 37.Perkins SN, Hursting SD, Phang JM, Haines DC. 1998. Calorie restriction reduces ulcerative dermatitis and infection-related mortality in p53-deficient and wildtype mice. J Invest Dermatol 111:292–296. [DOI] [PubMed] [Google Scholar]
- 38.Report of the American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies.1977. J Nutr 107:1340–1348. [DOI] [PubMed] [Google Scholar]
- 39.Research Diet. [Internet] 2014. AIN diets. [Cited 05 November 2014] Available at: http://www.researchdiets.com/opensource-diets/stock-diets/ain-diets.
- 40.Scharschmidt TC, Segre JA. 2008. Modeling atopic dermatitis with increasingly complex mouse models. J Invest Dermatol 128:1061–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seitz O, Schurmann C, Hermes N, Muller E, Pfeilschifter J, Frank S, Goren I. 2010. Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: a comparative study. Exp Diabetes Res 2010:476969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seta S. 2009. A simplified method for the treatment of mouse dermatitis. J Am Assoc Lab Anim Sci 48:608. [Google Scholar]
- 43.Singh NAM, Varani J, Chakrabarty S. 2013. Induction of calcium sensing receptor in human colon cancer cells by calcium, vitamin D and Aquamin: promotion of a more differentiated, less malignant and indolent phenotype. Mol Carcinog 54: 543–553. [DOI] [PubMed] [Google Scholar]
- 44.Slattery ML, Boucher KM, Caan BJ, Potter JD, Ma KN. 1998. Eating patterns and risk of colon cancer. Am J Epidemiol 148:4–16. [DOI] [PubMed] [Google Scholar]
- 45.Stowe HD, Wagner JL, Pick JR. 1971. A debilitating fatal murine dermatitis. Lab Anim Sci 21:892–897. [PubMed] [Google Scholar]
- 46.Sundberg J, Brown K, McMahon W. 1994Chronic ulcerative dermatitis in black mice, p 485–492. In: Sundberg JP. Handbook of mouse mutations with skin and hair abnormalities: animal models and biomedical tools. CRC series in dermatology. Boca Raton (FL): CRC Press. [Google Scholar]
- 47.Sundberg JP, Sundberg BA, King LE.Cutaneous changes in commonly used inbred mouse strains and mutant stocks, p 325–337. In: Mohr U. Pathobiology of the aging mouse. Washington (DC): ILSI Press. [Google Scholar]
- 48.Sundberg JP, Taylor D, Lorch G, Miller J, Silva KA, Sundberg BA, Roopenian D, Sperling L, Ong D, King LE, Everts H. 2010. Primary follicular dystrophy with scarring dermatitis in C57BL/6 mouse substrains resembles central centrifugal cicatricial alopecia in humans. Vet Pathol 48:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeda K, Gelfand EW. 2009. Mouse models of allergic diseases. Curr Opin Immunol 21:660–665. [DOI] [PubMed] [Google Scholar]
- 50.Tokura Y. 2010. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci 58:1–7. [DOI] [PubMed] [Google Scholar]
- 51.Tsudzuki M, Watanabe N, Wada A, Nakane Y, Hiroi J, Matsuda H. 1997. Genetic analyses for dermatitis and IgE hyperproduction in the NC/Nga mouse. Immunogenetics 47:88–90. [DOI] [PubMed] [Google Scholar]
- 52.Turturro A, Duffy P, Hass B, Kodell R, Hart R. 2002. Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J Gerontol A Biol Sci Med Sci 57:B379–B389. [DOI] [PubMed] [Google Scholar]
- 53.Varani J, Bhagavathula N, Aslam MN, Fay K, Warner RL, Hanosh A, Barron AG, Miller RA. 2007. Inhibition of retinoic acid-induced skin irritation in calorie-restricted mice. Arch Dermatol Res 300:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vestergaard C, Yoneyama H, Matsushima K. 2000. The NC/Nga mouse: a model for atopic dermatitis. Mol Med Today 6:209–210. [DOI] [PubMed] [Google Scholar]
- 55.Warden CH, Fisler JS. 2008. Comparisons of diets used in animal models of high-fat feeding. Cell Metab 7:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams-Fritze MJ, Carlson Scholz JA, Zeiss C, Deng Y, Wilson SR, Franklin R, Smith PC. 2011. Maropitant citrate for treatment of ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci 50:221–226. [PMC free article] [PubMed] [Google Scholar]
- 57.Williams LK, Csaki LS, Cantor RM, Reue K, Lawson GW. 2012. Ulcerative dermatitis in C57BL/6 mice exhibits an oxidative stress response consistent with normal wound healing. Comp Med 62:166–171. [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organization and Food and Agriculture Organization of the United Nations. 2004. Vitamin and mineral requirements in human nutrition, 2nd ed. Rome (Italy): World Health Organization. [Google Scholar]