Abstract
Self-injurious behavior (SIB) occurs within laboratory-housed NHP at low frequency but can have a devastating effect on animal research and wellbeing. One barrier to the study and clinical management of these cases is the cost of equipment and personnel time to quantify the behavior according to the current standard of observation and to score remotely obtained video recordings. In studies of human SIB, in which direct observation is difficult or prohibited, researchers have demonstrated that quantifying the tissue damage resulting from SIB can be a useful proxy to represent the underlying behavior. We hypothesized that the nature of wounds resulting from SIB in NHP could be used in a similar manner to measure the abnormal behavior. Using a cohort of rhesus macaques with high-incidence SIB, we examined severity, distribution, and number of wounds and compared them with observed incidences of SIB during a 12-wk experiment. We found that the number, severity, and distribution of physical wounds were associated with the incidences of biting behavior observed during the 2 wk prior to measurement. We also found that an increased number of wounds was associated with increased severity. Animals with wounds of moderate severity were more likely to also have severe wounds than were macaques with wounds that were lower than moderate in severity. This work is the first representative study in NHP to find that behavioral SIB correlates with physical wounding and that increases in the frequency and number of the body regions affected correlates with the severity of wounding.
Abbreviation: SIB, self-injurious behavior
Self-injurious behavior (SIB) is a potentially devastating problem that occurs at low but predictable frequencies among captive NHP. Individual housing and social isolation of young rhesus macaques have been associated with early development of SIB, with some studies identifying self-directed biting as early as 32 d of age.2,12,13,15 In addition, male rhesus macaques are at increased risk for developing SIB.5,11 However, many cases are not diagnosed until animals produce noticeable wounds.14 On the basis of behavioral observation and examination of clinical records, the estimated prevalence of SIB in research NHP ranges from 5% to 25%,1,11,14 but whether this variance is real or due to differences in detection is unclear. In addition, there is a disconnect between the way the behavior typically is measured (for example, biting incidents observed by remotely obtained videorecordings) and how clinical decisions regarding the fate of these animals are made (that is, severity of wounding).
Several barriers to the assessment of SIB have impaired the treatment and study of the behavior. SIB is an infrequent behavior that does not always result in physical wounds, may be challenging to observe directly, and, in some cases, can be elicited or altered by the presence of an observer.14 Most studies therefore have used video analysis to assess SIB,2,9,19 a method that is not always practical in a clinical setting. Technical difficulties associated with videorecording include the purchase and maintenance of equipment in a working vivarium and the ability to have good visualization within a cage. Moreover, the personnel costs of reviewing extensive videorecordings may be prohibitive. Previous studies2,9,19 have used representative sampling to observe SIB but have yet to fully evaluate the effect of time of day or optimal length of sample observation.
Clinical monitoring of physical wounds is an alternative way to quantify SIB. In humans with SIB, wound assessment has been used to evaluate the behavior in situations where direct observation was infeasible, with the presence and severity of wounds serving as outcome measures of the interventions.7,8 This practice of wound characterization relies on the finding that patients with greater frequency of SIB and multiple types of wounding are also at an increased risk for an increased severity of SIB.10,21 To our knowledge, this method has not yet been applied to NHP in the literature and likely presents challenges. The infrequent nature of wounding proves to be problematic for assessing treatment efficacy in individual cases, because discerning whether observed improvement is due to an intervention or to the seemingly random ebb and flow of wounding frequency and severity can be difficult. Identifying new cases requires knowledge of common wounding patterns and a willingness to recognize injuries as SIB. The severity of individual wounds and frequency of wounding each has been used to monitor ongoing cases of SIB and to determine ‘endpoints,’ but little is known about the relationship of wound severity and frequency to the behavior itself. Another consideration is that the frequency and severity of SIB likely indicate the degree of impairment to the psychologic wellbeing of the animal.
We hypothesize that the severity and distribution of wounds on rhesus macaques reflect and correlate to observed incidences of biting behavior. In addition, we hypothesize that the severity and distribution of wounds are related to one another and can be combined and used as the basis of a scoring system that is quick, repeatable, and reflective of the underlying frequency of biting in many cases. This type of scoring system might be used by veterinarians to effectively track the progress of SIB cases where video observation is not possible.
Materials and Methods
Animals.
This study used a cohort of 16 male rhesus macaques (average age, 7 y) that had a high incidence of clinically significant wounds reported to our veterinary staff. These animals had previously been used for an unrelated longitudinal study, which required they be housed singly, after being purchased from an outside source without record of rearing history other than being individually housed as young animals (median age, 504 d). The animals were individually housed for 5 y prior to this study at our facility but under the direction of another investigator. Throughout the course of this study, animals were singly housed as part of a separate ongoing experiment, the treatment conditions of which were analyzed in conjunction with each of the analyses included in this study and determined not to significantly contribute to any of them. Animals were maintained in an AAALAC-accredited facility, and all experiments described were evaluated and approved by the Johns Hopkins University School of Medicine Animal Care and Use Committee. Animals were maintained on a 14:10-h light:dark cycle, offered free access to water, fed a standard laboratory NHP diet daily (Harlan, Indianapolis, IN), and once-daily food enrichment (for example, fruit, vegetables) offered by our staff with whom the animals were accustomed to working. In addition, the macaques in this study received the standard forms of enrichment at our facility, including music and toys in the cage, and some of the macaques had ‘visual blocks’ attached to the fronts of their cages. A visual block is an opaque barrier that covers a portion (no more than a third) of the front of the cage, allowing a macaque to disrupt visual contact from conspecifics directly across from it. Macaques also had direct visual access with conspecifics.
Behavioral monitoring.
Macaques were habituated to video monitoring equipment and personnel for 2 wk before recording of behavior began. During the 2 wk immediately preceding an associated wound assessment, 1 h of continuous video was scored from each of 5 separate days, totaling 5 h scored in each 2-wk block. From Monday through Friday, video recording equipment was set up before 0830, left to record with no staff in the room, and then removed at 1200 to allow for room sanitation. The hour selected for scoring was always from 0900 to 1000 and included an event during which someone entered the room and offered each macaque a food treat but excluded other room activities, such as feeding and cage cleaning. The decision to score the videorecording from the same time period each day reflects our desire to minimize effects due to known fluctuations in SIB incidence throughout the day.14 Within the 12-wk study, these 2-wk blocks corresponded to weeks 3 to 4, 7 to 8, and 11 to 12 and therefore were compared only with the 3 observations for wound scoring, which were recorded during weeks 4, 8, and 12.
For the purposes of the behavioral evaluation, self-biting was defined as: opening the mouth, placing teeth on any part of the body, and pulling the body part away from the mouth, or placing any part of the body in the mouth and closing the mouth around the body part. Self-biting did not include placing fingers in the mouth during grooming or eating or lip smacking on the body during grooming. Each incidence of teeth closure, pulling movement, or head bob toward a body part in the mouth was scored as a separate instance by a single reviewer blinded to the wound scores associated with that videorecording. Whether a particular incident of biting led to a wound could not be determined, but many more biting incidents than wounds were detected.
Wound observation.
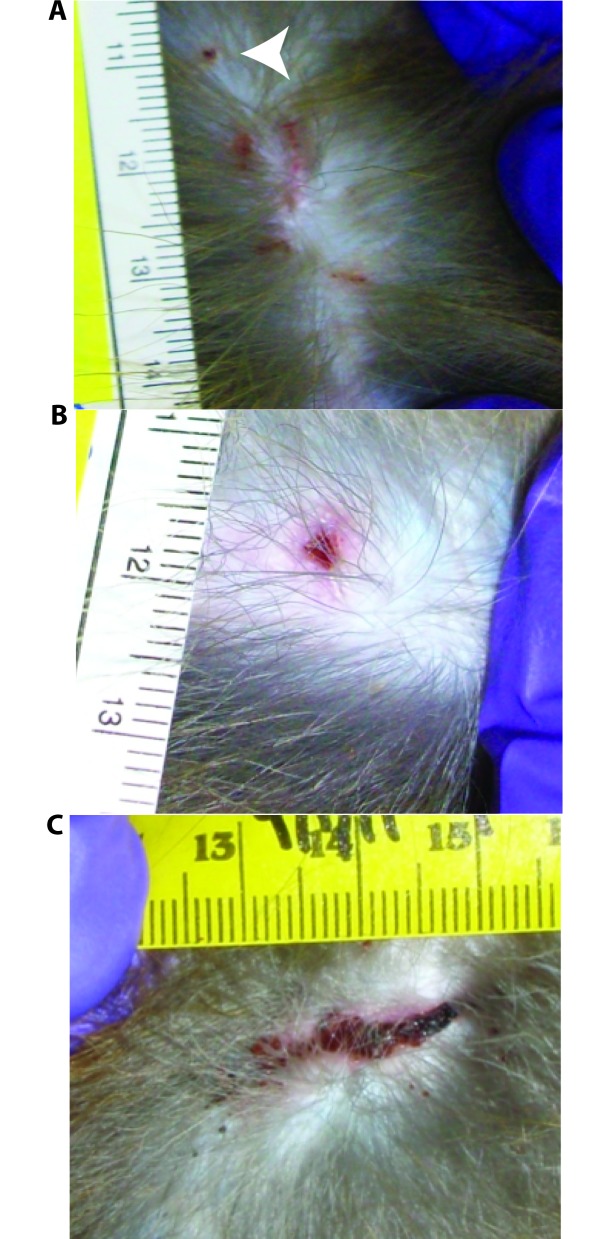
Macaques were sedated (10 mg/kg ketamine IM) biweekly over 12-wk period and examined for the presence of wounds. Performing physical examinations and wound severity assessments on a biweekly basis allows for adequate healing of normal wounds.4,16 In addition, we confirmed that preexisting wounds were not a significant factor in determining wound score (see Results). The body was allocated into 4 quadrants for the purpose of physical examination: right thorax, left thorax, right lower abdomen, and left lower abdomen, each with the associated thoracic or pelvic limbs. Individual wounds were counted and recorded for each quadrant. Regardless of whether they were old or new, wounds were assigned a severity score according to the following criteria: 0, no wounds; 1, ‘pinprick’ wounds not penetrating the dermis (consistent with hair pulling or mild biting); 2, scratches not penetrating the dermis (consistent with excoriation or mild biting); 3, wounds deeper than the dermis in which no diameter was greater than 1 cm (consistent with a wound resulting from canine puncture); and 4, wounds deeper than the dermis and greater than 1 cm in any dimension (consistent with a severe, tearing bite; Figure 1). At each examination, the distribution of wounds was defined as the number of quadrants in which wounds were present, and the maximal wound severity was defined as the highest severity of any single wound for the entire body. The total number of wounds was determined by adding together the numbers of wounds in all quadrants. A total of 122 wound examinations were performed on the 16 macaques over the 12-wk study period. Two macaques were removed from this study when they became incompatible to their concurrent primary study and had received only 5 wound examinations each, whereas all other animals had 8 examinations over the course of the study. A veterinarian not involved with the study made all clinical decisions about the treatment of wounds. None of the wounds observed during the course of this study required primary closure (suturing) or antibiotics.
Figure 1.
Images of wound-score classifications for representative wounds, including (A) superficial pinprick wounds (white arrow; score, 1), surface scratches (score, 2), (B) deep puncture (score, 3), and (C) deep laceration (score, 4). Measurements are in centimeters; panel A shows 4 scratches with scores of 2 and 1 pinprick wound with score of 1; the overall score for the area is 2.
Data analysis.
All graphical and statistical analyses were performed by using Prism 6.0 (GraphPad Software, San Diego, CA) or Stata (StataCorp, College Station, TX). A P value less than 0.05 was considered significant. Repeated-measures ANOVA was used to analyze whether the preceding wound severity was a significant factor in determining the subsequent wound severity. All other analyses used a mixed-model linear regression, with subject set as a fixed-effect independent variable to account for repeated measures and unequal sample sizes. The Holm–Bonferroni method was used to correct for multiple comparisons.
Results
To confirm that biweekly wound assessment allowed sufficient time for a severe wound to heal, we examined the wound scores recorded 2 wk after the most severe wounds (score, 4) were found. In these 19 recorded cases, the same quadrants that had scored a 4 just 2 wk prior scored 0 in 3 cases, 1 in 3 cases, 2 in 6 cases, 3 in 2 cases, and 4 in 5 cases. Because a severity of 1 indicates a pin-prick and 4 a deep laceration, the 6 cases for which a 0 or a 1 was recorded after 4 should be considered to demonstrate complete healing of the original wound. Furthermore, we analyzed whether the maximal wound severity recorded at a given time was affected by the maximal severity found during the 2 wk prior. Using a mixed-model ANOVA with repeated measures, we found that although the animal itself was a significant determinant of the maximal wound severity (that is, animals tended to have similar wound severities from week to week, P = 0.0139), neither the previous maximal severity nor the interaction between subject and previous severity was a significant factor (P = 0.7546 and P = 0.6730, respectively). In other words, the wound score on a given macaque during a given week was determined by the identity of the animal and not by the previous score. Furthermore, the incidence of observed SIB behavior was associated with the number of wounds (P = 0.025; z score, 2.25) and their distribution (P = 0.024; z score, 2.25). The maximal wound severity was associated with the incidence of observed SIB (P = 0.005; z score, 2.83) and highly associated with the distribution of wounds (P < 0.0005; z score, 16.01).
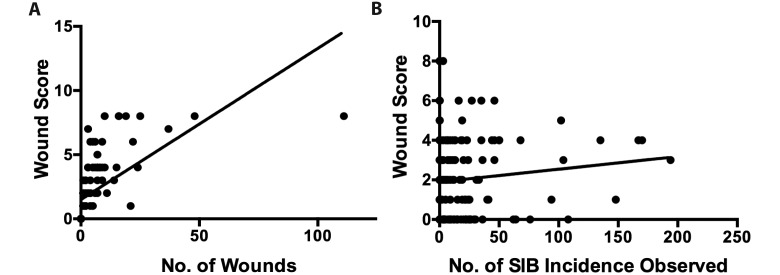
Because maximal wound severity and wound distribution were associated with each other, we devised a wound-scoring system to represent each macaque during each independent exam. Each quadrant was represented by its most severe wound, and the 4 numbers were summed to give an overall wound score for that exam. Thus, the maximal score for a quadrant was 4, and the maximal score that a macaque could receive during any exam was 16. We found that this wound score was associated with both the number of wounds (P < 0.0005; z score, 8.38; Figure 2 A) and the incidence of observed SIB (P = 0.008; z score, 2.63; Figure 2 B).
Figure 2.
(A) Wound score correlates with the total number of wounds at each time point (P < 0.0005; z score, 8.38). (B) Wound score correlates with the incidence of observed SIB (P = 0.008; z score, 2.63). The graphical representations in panels A and B do not display the variable of time, the third dimension of the statistical analysis performed.
Clinical observations indicated that macaques with more severe SIB tended to have wounds that were distributed more broadly over their bodies. We tested this observation by examining the relationship between the distribution of wounds and maximal wound severity. The number of affected quadrants was associated with the maximal wound severity (P < 0.0005; z score, 5.01; Figure 3). Our data demonstrated that this wound score was repeatable, with effective matching among individual macaques on repeated measures of > 99% confidence (matching P < 0.001) and can be measured reliably by independent trained observers (interrater reliability, >95%).
Figure 3.
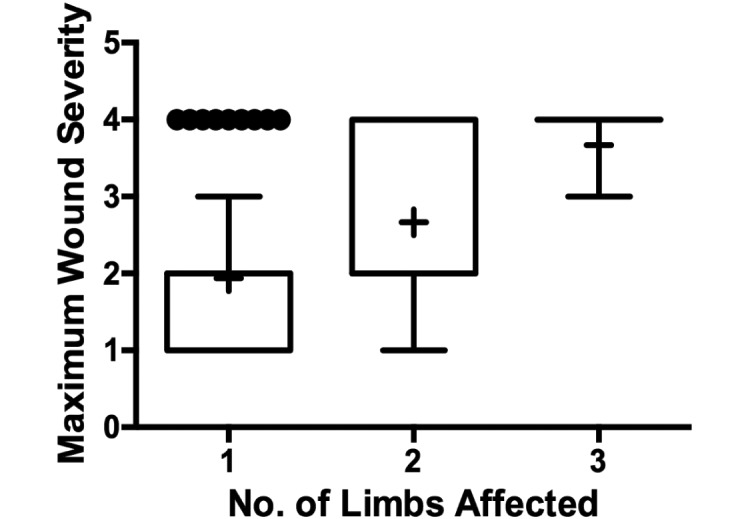
Macaques in which the number of quadrants with wounds increased show also showed an increase in the most severe wound overall (P = 0.0001). This graphical representation does not display the variable of time, the third dimension of the statistical analysis performed.
Discussion
Given the relationships of the distribution and severity of wounds to biting frequency, the wound scoring system we developed adequately reflects SIB in rhesus macaques as revealed through traditional behavioral observation but requires less input cost and no additional specialized training. The number, severity, and distribution of wounds all reflect the observed frequency of biting behavior in our cohort. In addition, the maximal wound severity in a macaque correlates with the number and distribution of wounds, even though the definition of severity does not rely on either of these other characteristics. Although individual clinical cases have provided ancillary evidence of an association between increased frequency and distribution of wounds with increased severity of SIB, our study is the first thorough examination of this relationship. Consistent with the understanding of human SIB, it seems logical that an increased frequency of the disease would be associated with an escalating severity of wounds and with more wounds with higher severity scores. Understanding this relationship places greater importance on the early identification of macaques with mild wounds or nonwounding biting behavior for targeted clinical interventions to prevent or delay the progression of SIB severity.
Our emphasis on the most severe wound rather than total number of wounds corrects for the confounding variable of the delayed wound healing, a phenomenon common to infections and depressives states,6,20 frequently associated with SIB.17 Performing physical examinations and wound severity assessments on a biweekly basis allows for sufficient healing of wounds under typical conditions.4,16 Other methods used in human medicine to observe physical wounds include measuring wound size or area as an indicator of SIB severity.22 The measurement of wounds brings technical challenges in NHP and, although methods using photographs and computer-assisted analysis exist,18,22 they remained limited by their attendant equipment and software requirements. An additional challenge to using wound size or area in NHP is a lack of understanding regarding how the behavior of SIB correlates with these measures. One potential critique of assessing severity involves how to account for preexisting wounds or injuries present on sequential examinations. As defined, our method does not distinguish between wounds made, for example, 5 min compared with 1 mo prior to examination. Old wounds pose a challenge regarding assessing the nature of wounding. If the macaque allows wounds to heal during the interval between examinations, then the severity of that wound decreases at subsequent examinations. However, the severity score reflects the situation in which these animals might inflict new wounds that are more severe than the original wound or alter its ability to heal. In our experience, macaques with SIB have a tendency to aggravate old wounds and to wound themselves again in similar locations.
Our wound score has the benefit of being directly tied to the clinical outcome most typically used by veterinarians to determine humane endpoints in NHP SIB cases. We anticipate that our scoring system might be used to monitor known SIB macaques and in future studies investigating underlying mechanisms, triggers, or treatments. The wound score system might be used to describe baseline wounding patterns prior to therapeutic intervention, with repeated wound scores being assigned at regular intervals during and after therapeutic intervention. This use of the wound score system supports the quantifiable assessment of SIB treatment efficacy. Furthermore, this method can be used in conjunction with behavioral observation or as an alternative method in cases where behavioral observations are not possible.
The observation of behavior remains important, however, because it is the only way to monitor macaques whose self-biting behaviors don't cause wounds, to detect temporal and activity patterns to the behavior that may identify triggers or causes, and perhaps to allow for discrimination between wounds secondary to SIB and those caused in other ways. In addition, behavioral observation and wound scores in NHP are similar to the use of plasma compared with hair sampling for the assessment of cortisol levels, in that one provides an instantaneous ‘snapshot’ of the syndrome, whereas the other is more representative of the process over prolonged time.3 One method is not inherently better than the other, but they answer different questions. In addition, not all wounds are self-injurious, so care must be taken when applying the wound score technique to animals without a known history of SIB. The severity scores of 1 and 2, in particular, indicate superficial wounds that could be mimicked by skin conditions such as papillary dermatitis or mild cage-induced lesions.
For similar reasons, our system needs to be used with discretion in the case of socially housed NHP, which can inflict wounds upon each other. Although the recent emphasis on increased social housing hopefully would lead to pair-housing of all NHP, exemptions for single housing due to research concerns likely will be used in the interim. When screening a colony of NHP for any condition, the most sensitive test that is inexpensive and efficient is typically selected. We feel that our approach will be valid not only for those NHP that are granted an exception for single housing but also as an initial screen for NHP that have SIB and could then be assessed further by using behavioral analysis tools. Future work might examine the difference between self-inflicted wounds and those inflicted by a conspecific. In addition, it should be considered that not all animals displaying SIB cause wounding, and in such cases, behavioral observation is the only feasible method of measuring the behavior. In the current study, we used a cohort of male rhesus macaques because of their high incidence of SIB; more studies are needed to determine whether our scoring system appropriately represents SIB in female rhesus macaques and other species of NHP.
Veterinarians and their staff might implement wound scoring as a part of routine physical exams and in cases for which routine behavior observation is not possible. Although for the purposes of this study we performed wound examinations biweekly in an attempt to fully characterize the macaques, we do not believe these examinations need to be performed so frequently and instead recommend a longer time interexam interval. This system has been used at our institution in conjunction with behavior observation since the original study and at an increased time interval of 4 to 6 wk for known SIB macaques. In at least one instance, a macaque not identified through behavioral observation was identified as having SIB during this routine screening. Ideally, this wound score system would be used in conjunction with behavioral assessment. In facilities without dedicated behaviorists or with rotating veterinarians, our wound score system would enable consistent and reliable tracking of SIB cases over time, facilitating decisions regarding the efficacy of treatment and humane endpoints. In addition, this system might be a valuable tool in the study of SIB itself, because this scoring allows for an adjunct measure of the behavior and of the severity of the disorder.
Acknowledgments
We acknowledge the following funding sources: NIH ORIP T32 OD011089, NIH ORIP K01 OD018244, NIH ORIP P40 OD013117, P40 RR019995, and Johns Hopkins University School of Medicine Research Animal Resources. In addition, we thank the following people for their assistance in the experiments described: Melanie Albano, Nicole Azene, Tori Baxter, Caroline Garrett, Tracey Graham, Kristy Koenig, and Theresa Meade.
References
- 1.Bayne KDS, Suomi S. 1995. Nonhuman primate wounding prevalence: a retrospective analysis. Lab Anim (NY) 24:40–44. [Google Scholar]
- 2.Bellanca RU, Crockett CM. 2002. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. Am J Primatol 58:57–69. [DOI] [PubMed] [Google Scholar]
- 3.Dettmer AM, Novak MF, Novak MA, Meyer JS, Suomi SJ. 2009. Hair cortisol predicts object permanence performance in infant rhesus macaques (Macaca mulatta). Dev Psychobiol 51:706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giacometti L. 1967. The healing of skin wounds in primates. I. The kinetics of cell proliferation. J Invest Dermatol 48:133–137. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb DH, Capitanio JP, McCowan B. 2013. Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): animal's history, current environment, and personality. Am J Primatol 75:995–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gouin JP, Kiecolt-Glaser JK. 2011. The impact of psychological stress on wound healing: methods and mechanisms. Immunol Allergy Clin North Am 31:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grace NC, Thompson R, Fisher WW. 1996. The treatment of covert self-injury through contingencies on response products. J Appl Behav Anal 29:239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwata BA, Pace GM, Kissel RC, Nau PA, Farber JM. 1990. The Self-Injury Trauma (SIT) scale: a method for quantifying surface tissue damage caused by self-injurious behavior. J Appl Behav Anal 23:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempf DJ, Baker KC, Gilbert MH, Blanchard JL, Dean RL, Deaver DR, Bohm RP., Jr 2012. Effects of extended-release injectable naltrexone on self-injurious behavior in rhesus macaques (Macaca mulatta). Comp Med 62:209–217. [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr PL, Muehlenkamp JJ, Turner JM. 2010. Nonsuicidal self-injury: a review of current research for family medicine and primary-care physicians. J Am Board Fam Med 23:240–259. [DOI] [PubMed] [Google Scholar]
- 11.Lutz C, Well A, Novak M. 2003. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. Am J Primatol 60:1–15. [DOI] [PubMed] [Google Scholar]
- 12.Lutz CK, Davis EB, Ruggiero AM, Suomi SJ. 2007. Early predictors of self-biting in socially housed rhesus macaques (Macaca mulatta). Am J Primatol 69:584–590. [DOI] [PubMed] [Google Scholar]
- 13.Mason WA, Sponholz RR. 1963. Behavior of rhesus monkeys raised in isolation. J Psychiatr Res 1:299–306. [DOI] [PubMed] [Google Scholar]
- 14.Novak MA. 2003. Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. Am J Primatol 59:3–19. [DOI] [PubMed] [Google Scholar]
- 15.Rommeck I, Gottlieb DH, Strand SC, McCowan B. 2009. The effects of 4 nursery-rearing strategies on infant behavioral development in rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 48:395–401. [PMC free article] [PubMed] [Google Scholar]
- 16.Roth GS, Kowatch MA, Hengemihle J, Ingram DK, Spangler EL, Johnson LK, Lane MA. 1997. Effect of age and caloric restriction on cutaneous wound closure in rats and monkeys. J Gerontol A Biol Sci Med Sci 52A:B98–B102. [DOI] [PubMed] [Google Scholar]
- 17.Sansone RA, Sansone LA. 2013. Preventing wounds from healing: clinical prevalence and relationship to borderline personality. Innov Clin Neurosci 10:23–27. [PMC free article] [PubMed] [Google Scholar]
- 18.Thawer HA, Houghton PE, Woodbury MG, Keast D, Campbell K. 2002. A comparison of computer-assisted and manual wound size measurement. Ostomy Wound Manage 48:46–53. [PubMed] [Google Scholar]
- 19.Tiefenbacher S, Novak MA, Jorgensen MJ, Meyer JS. 2000. Physiological correlates of self-injurious behavior in captive, socially reared rhesus monkeys. Psychoneuroendocrinology 25:799–817. [DOI] [PubMed] [Google Scholar]
- 20.Walburn J, Vedhara K, Hankins M, Rixon L, Weinman J. 2009. Psychological stress and wound healing in humans: a systematic review and meta-analysis. J Psychosom Res 67:253–271. [DOI] [PubMed] [Google Scholar]
- 21.Whitlock J, Muehlenkamp J, Eckenrode J. 2008. Variation in nonsuicidal self-injury: identification and features of latent classes in a college population of emerging adults. J Clin Child Adoles Psychol 37:725–735. [DOI] [PubMed] [Google Scholar]
- 22.Wilson DM, Iwata BA, Bloom SE. 2012. Computer-assisted measurement of wound size associated with self-injurious behavior. J Appl Behav Anal 45:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]