Abstract
When IVC are directly exhausted from a rodent housing room, the air quality of the room can become independent of the intracage air quality and may reduce the need for high room ventilation rates. This study assessed the effect of decreasing the ventilation rate in rodent rooms using direct-exhaust IVC systems. The study was conducted over 16 wk and compared conditions in 8 rodent rooms that had ventilation rates of 5 to 6 air changes per hour (ACH) with those in rooms at 10 to 12 ACH. At the low ventilation rate, rooms had higher CO2 concentrations, higher dew point temperature, and lower particulate levels and spent a greater percentage of time above the temperature set point than did rooms at the high rate. The levels of allergens and endotoxins in room air were the same regardless of the ventilation rate. Differences seen in parameters within cages at the 2 ventilation rates were operationally irrelevant. We detected no total volatile organic compounds in the room that were attributable to ammonia, regardless of the ventilation rate. Clearing the air of ethanol after a spill took longer at the low compared with high rate. However, ethanol clearance was faster at the low rate when the demand-control system was activated than at the high ventilation rate alone. Air quality in the room and in the cages were acceptable with room ventilation rates of 5 to 6 ACH in rodent rooms that use direct-exhaust IVC systems.
Abbreviations: ACH, air changes per hour; cfm, cubic feet per minute; DCV, demand controlled ventilation; TVOC, total volatile organic compounds
Many care programs for laboratory animals adopt the general recommendations of 10 to 15 air changes per hour (ACH) for animal rooms, as stated in the Guide for the Care and Use of Laboratory Animals,11 regardless of how the animals are housed or how the rooms and cages are ventilated. Reasons for these recommended ventilation rates include the need to supply adequate oxygen, remove thermal loads, dilute gaseous and particulate contaminants, adjust moisture content, maintain ideal temperature, and where needed, create air pressure differentials between adjoining spaces.
In animal rooms, the ventilation system must be able to maintain the temperature for rodents within 68 to 79 °F and minimize temperature fluctuations.11 Humidity should be maintained between 30% and 70%, because humidity above or below this range may increase preweaning mortality in mice.3 Ringtail in rats has been associated with low humidity,5,32 and high humidity can increase ammonia production within microisolator cages.4,6 Indoor air pollutants can be divided into the 3 basic categories: biologic, chemical, and particulates. In animal rooms, potential biologic pollutants include animal allergens and endotoxins as well as biologic agents that may be used by researchers. Chemical pollutants commonly present include CO2, ammonia, formaldehyde, anesthetic gases, cleaning and sanitizing solutions, and chemicals used by researchers. Particulates often enter a room through the supply air but can also be generated within the room by animal dander and bedding products and during husbandry procedures, such as changing cages and routine cleaning of the room.
IVC systems are increasingly becoming the standard for laboratory rodent housing, and many studies have examined the benefits of these systems. A particular benefit of IVC is improved air quality within the cage when compared with static microisolator cages, including lower ammonia, lower CO2, higher O2, lower humidity, and drier bedding.9,13,15-17,23,25 Another reported benefit of using IVC, especially when combined with handling animals in a cage-change station, is reduction of airborne allergens and particulates. Allergies to laboratory animals are recognized as an important occupational disease, with around one-third of persons working with laboratory animals reporting work-related allergic symptoms.2,7,10 Reducing exposure to animal allergens is considered the primary method for reducing the incidence of and relieving symptoms among affected personnel.8,28,33 Mouse allergens within rooms can be reduced as much as 50-fold by switching from open cages to filter -top cages, and additional reductions are possible by using IVC systems.18,22,28 Mouse allergens within the room are aerosolized and carried on particulates in the air.18,26 Levels of airborne endotoxin are high in animal housing areas and are positively correlated with airborne mouse urinary protein allergens.21,22 Respiratory symptoms can occur in personnel not sensitized to mouse allergens but exposed to high levels of airborne endotoxin, indicating that exposure to endotoxin is another important occupational health issue for animal care workers.20
Although several studies have examined air quality within cages at different intracage ventilation rates,24,25 few have examined air quality in rodent rooms or within cages at different room ventilation rates. One study using static cages found improved intracage parameters as the room ventilation rate was increased from 0 to 20 ACH.23 When a demand-controlled ventilation (DCV) system was used according to study parameters in a rodent facility with open-top cages, ventilation had to be increased 1.5% of the time over the base of 6 ACH to keep room air levels of total volatile organic compounds (TVOC) within the target range.29 These excessive levels typically occurred when cages were soiled, resulting in high ammonia levels.29 For 0.5% of the monitored time, ventilation had to be increased over the base of 6 ACH to keep the room air TVOC levels within the target range in a different facility with IVC that were exhausted into the room.29
One advantage of IVC is that the exhaust air from the ventilated cages can be delivered directly into the building exhaust system instead of being recirculated into the room. This feature effectively renders the quality of the room air independent of the animals housed and may reduce the need for high room ventilation rates.14 However, evidence-based criteria for ventilation rates in animal rooms using IVC systems are sparse. The purpose of the current study was to evaluate air quality in rodent rooms using direct-exhaust IVC between various room ventilation rates. Because the ventilation of the room is independent of the cage ventilation system, we hypothesized that room ventilation rates lower than those currently recommended in the Guide11 would not have any adverse effects on room or intracage air quality.
Materials and Methods
Facility.
This study was conducted in a 17,000 -ft2 facility with 18 rodent housing rooms, 22 procedure rooms, and cage wash and storage areas. The ventilation system for each housing room consisted of one supply system and 2 exhaust systems. One exhaust system was dedicated to the IVC racks via a thimble connection, which ensured that the building's exhaust did not override the rack exhaust blower. The other exhaust system was for general, room exhaust. Each system was controlled by an airflow control valve (Phoenix Controls, Acton, MA). Each room was equipped with an automated monitoring system (Aircuity, Newton, MA), which collects a 40-s sample of air from the general, room exhaust duct of each room in a continuous cycle such that each room was sampled approximately every 15 min. The air sample was collected from the general, room exhaust duct and should be representative of the room air since it was not from the IVC rack exhaust. For analysis, each air sample was sent to one of 2 centralized sensor suites via a network of air tubes. Each sensor suite comprised a set of sensors that measured total volatile organic compounds (TVOC; SEN-TVC-1 and -3, Aircuity; ±0.5 ppm of isobutylene), particulates 0.3 to 2.5 µm in diameter and reported as particulates per ft3 (SEN-PAR-1, Aircuity; ±25% of reading), CO2 (±45 ppm), and dew point temperature (SEN-C2D-3, Aircuity; ±0.9 °F). The TVOC sensor was calibrated to isobutylene and responds to ammonia with a response factor of 9.4 and to ethanol at 10.0. Thus, a sensor reading of 1 ppm indicates the presence of 1.0 ppm of isobutylene, 9.4 ppm of ammonia, 10.0 ppm of ethanol, or some other TVOC. The sensors were replaced every 6 mo with new, calibrated sensors. In the analysis, we compared the supply air parameters with the room air parameters; both air sources were evaluated by using the same set of sensors. In addition, each room was equipped with an exhaust duct probe that measured temperature every 1 min. The system had the capability to operate as a demand-controlled ventilation (DCV) system, automatically increasing air exchange rate in a room when a monitored parameter exceeded preset threshold values. For this study, the DCV portion of the system was used only during TVOC clearance testing.
Caging and husbandry.
All animals were housed in IVC (Tecniplast, Milan, Italy) that supplied 530 cm2 of floor space for mice and 904 cm2 for rats. Each cage consisted of the cage bottom, wire bar, water bottle, and a filter top (Reemay 2024, Dupont, Wilmington, DE). The HEPA-filtered ventilation blowers were set to supply (per minute) 11.6 L of air to each mouse cage and the exhaust was set at 8.1 L. For rats, the rate was 23.2 L for supply and 16.2 L for exhaust; these rates equated to an intracage air-exchange rate of 65 ACH. Ventilation in the rooms was at positive pressure relative to the hall with an offset of 50 to 100 cfm. For intracage measurements, each cage contained 5 adult female CD1 mice (Harlan, Indianapolis, IN), which were used for the health surveillance program. All animals in the facility were mice and rats on IACUC-approved research protocols and were used and maintained according to guidelines in the Guide.11 All animal handling within the room was conducted in a cage-change station (CS5 Evo, Tecniplast). Cages were changed routinely once every 14 d (mice) or 7 d (rats); open clean and dirty cages were temporarily kept on carts in the room as cages were changed.
Experimental design.
We selected 8 rooms for this study; 7 of these rooms housed mice, and the remaining room housed rats. The density of cages in the rooms ranged from 0.1 to 1.2 cages/ft2 in the mouse rooms and was 0.5 cages/ft2 in the rat room. The number of cages in each room was within 20% of the stated count throughout the course of the study. Each room was operated at a low ventilation rate of 5 to 6 ACH for two 4-wk periods and a high ventilation rate of 10 to 12 ACH for two 4-wk periods. The ACH rate was calculated from the supply airflow, which was set to supply 5.5 or 11.0 ACH for each room and period. To verify the correct airflow, a balometer airflow capture hood (Alnor Instrument, Skokie, IL) was used to measure the supply airflow in each room and period, and the results were within 10% of the setting. Rooms were randomized to the low or high ventilation rate at the start of the study and then switched after each 4-wk period. During each period, 4 rooms were at the low rate, and 4 rooms were at the high rate.
Allergen and endotoxin measurements.
Samples (n = 4 for each condition) were collected from 2 different rooms throughout the study. Air was sampled by use of an air sampling pump (model PCRX4, SKC, Eighty Four, PA) at (per minute) 2 L for 4 h for endotoxin and 4 L for 96 h for allergen testing. Samples for both allergen and endotoxin analysis were collected on 37-mm endotoxin cassettes with polycarbonate filers (pore size, 0.2 µm; EMSL Analytical, Cinnaminson, NJ). Both allergen and endotoxin levels were analyzed by EMSL Analytical. Endotoxins were tested by using a chromogenic (Kinetic-QCL) method, which is a qualitative, kinetic assay for the detection of gram-negative bacterial endotoxins. A quantitative ELISA method was used for Mus m1 allergen testing.
Intracage measurements.
Intracage measurements were obtained from 4 mouse cages, one each in 4 different rooms, throughout the study. Each cage contained 5 adult female CD1 mice (Harlan), which were provided free access to extruded diet (Harlan Teklad 2020) and autoclaved water through bottles. Ammonia concentration (n = 8 each condition) was determined by using tubes that measure 2 to 30 ppm ammonia (Accuro Pump, Dräger Safety, Lubeck, Germany). At 14 d after mice were placed in a clean cage with corncob bedding, the sampling tube was inserted into the cage through the water bottle port and placed at a level of approximately 1 cm above the bedding. Intracage CO2, temperature, and humidity were measured hourly by using a data logger (model CM-0018 CO2, Temperature, and % RH Data Logger, CO2 Meter, Ormond Beach, FL) that was placed between the filter top and wire bar inside the cage for the duration of each period.
TVOC clearance.
To determine whether ventilation rate altered the clearance of a spilled volatile organic compound, 10 mL of ethanol was poured onto a paper towel that was on the floor directly under the ceiling level room exhaust duct. This spill occurred 10 min before the next scheduled room sampling. In addition, the DCV system was tested to determine whether a high rate of air exchange cleared the air of TVOC after a significant level was detected.
Data analysis.
The air quality sensor and other data were compiled for each room and time period by using Excel (Microsoft, Redmond, WA) for data processing. Values are reported as mean ± SEM. A Student t test was used to compare all data (GraphPad Software, La Jolla, CA). Significance was defined as P value of less than 0.05, unless indicated otherwise. For comparing particulates with allergen levels, a linear regression calculator was used (GraphPad Software).
Results
Differences between room air and supply air.
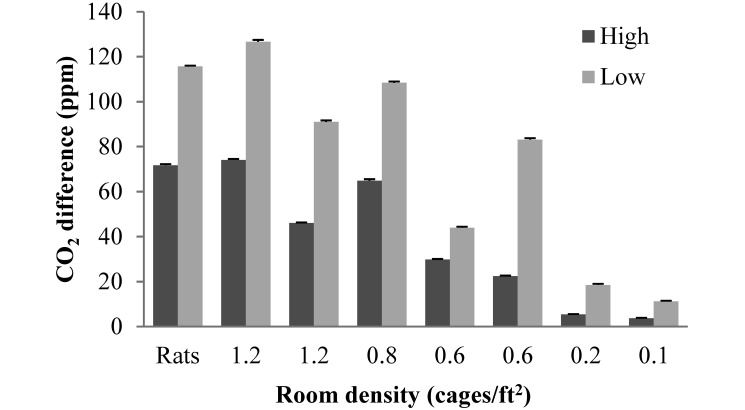
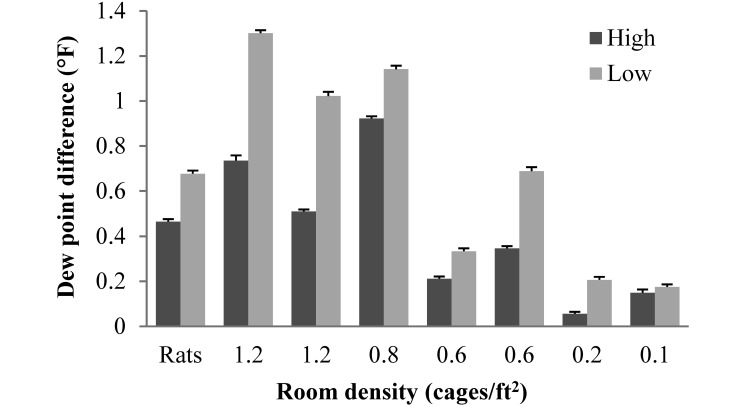
The highest levels of CO2 occurred when people were in the room, when peaks of around 100 ppm above the baseline room level were common. We also observed a diurnal fluctuation of CO2, with the nighttime level about 50 ppm higher than that during the day. Room air CO2 was significantly (P < 0.0001) higher in all rooms at the low ventilation rate (5 to 6 ACH) compared with the high ventilation rate (10 to 12 ACH; Figure 1). Room dew point temperature was significantly (P < 0.0001) higher in 7 of the 8 rooms at the low ventilation rate compared with the high ventilation rate (Figure 2). At both ventilation rates, both the CO2 level and dew point temperature tended to be higher in rooms with a greater density of animals. Particulates in the outside air averaged 950,284 ± 6756 particles/ft3 and after passing through an air inlet prefilter, the supply air averaged 365,285 ± 2801 particles/ft3. Particulates were significantly (P < 0.0001) higher in the rooms at the high ventilation rate (270,737 ± 1474 particles/ft3) than in the rooms at the low rate (241,509 ± 1282 particles/ft3).
Figure 1.
Difference in level of CO2 between the room and the supply air at the 2 ventilation rates (low, 5 to 6 ACH; high, 10 to 12 ACH) for each room, indicated as those housing rats (0.5 cages/ ft2) or as the number of mouse cages per ft2 of floor space. Data are presented as mean ± SEM. In all rooms, CO2 levels differed significantly (P < 0.0001) between the 2 ventilation rates.
Figure 2.
Difference in dew point temperature (°F) between the room and the supply air at the 2 ventilation rates (low, 5 to 6 ACH; high, 10 to 12 ACH) for each room, indicated as those housing rats (0.5 cages/ ft2) or as number of mouse cages per ft2 of floor space. Data are presented as mean ± SEM. Except for the room with 0.1 cages/ft2, dew point temperature differed significantly (P < 0.0001) between the 2 ventilation rates in all rooms.
Room temperature.
During the high-ventilation periods, 0.2% of the time the temperature was more than 2 °F below the set point, and 0.3% of the time it was above the set point by more than 2 °F. During the low-ventilation periods, 0.0% of the time the temperature was below the set point by more than 2 °F and 1.9% of the time above the set point by more than 2 °F. The maximal temperature deviation from the set point for any room and period was 5.0 °F above the set point and 3.0 °F below the set point.
Room allergen and endotoxin level.
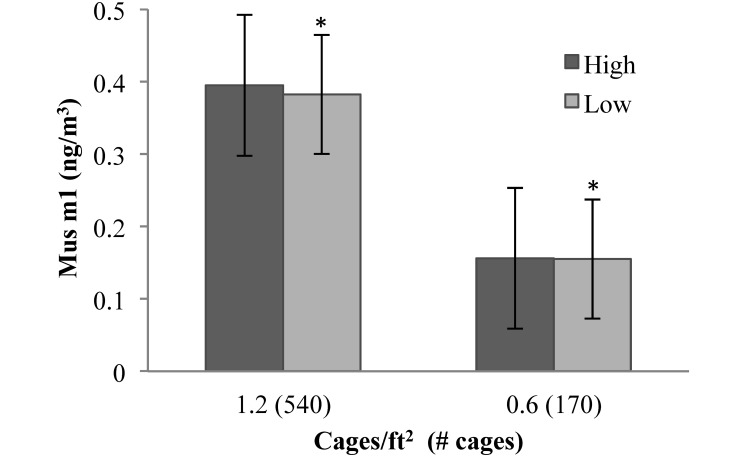
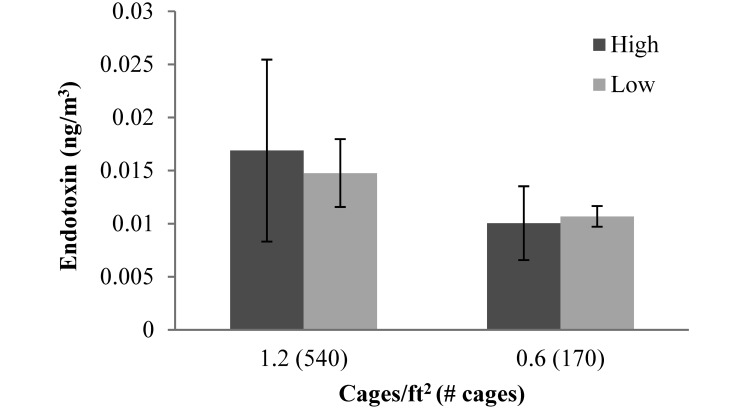
There were no differences in Mus m1 allergen (Figure 3) or endotoxin (Figure 4) at the different ventilation rates for each room. Both allergen and endotoxin tended to be higher in the room with a density of 1.2 cages/ft2 (540 cages) than in the room with 0.6 cages/ft2 (170 cages) at both ventilation rates. Correlations between room air allergen and particulates were not statistically significant (0.6 cages/ft2: r2 = 0.5423, P = 0.0591; 1.2 cages/ft2: r2 = 0.4091, P = 0.1219).
Figure 3.
Mus m1 Allergen level (ng/m3) in 2 rooms at the 2 ventilation rates (low, 5 to 6 ACH; high, 10 to 12 ACH). Data are presented as mean ± SEM. Allergen levels differed significantly (*, P < 0.037) between the 2 rooms at the low ventilation rate.
Figure 4.
Endotoxin level (ng/m3) in 2 rooms at the 2 ventilation rates (low, 5 to 6 ACH; high, 10 to 12 ACH). Data are presented as mean ± SEM. Endotoxin levels did not differ between rooms or ventilation rates.
Intracage parameters.
Temperature was 0.3 °F higher (P < 0.0001) within the cages at the low ventilation rate (Table 1). At the high ventilation rate, the intracage temperature was 2.8 °F higher than the room temperature; whereas at the low ventilation rate, the intracage temperature was 3.3 °F higher than the room temperature (P < 0.0001). Relative humidity within the cages was significantly (P < 0.0001) higher during high ventilation than during the low rate. No difference was seen in dew point temperature within the cages or the difference in dew point temperature between the room and within the cages at the different ventilation rates. CO2 within cages was significantly (P < 0.0001) lower in rooms with the low ventilation rate than in rooms at the high ventilation rate. No difference was seen in ammonia level within the cage as measured 14 d after mice were placed into a clean cage.
Table 1.
Intracage parameters (mean ± SEM). Temperature and dew point difference is between the room and cage.
| High ventilation rate | Low ventilation rate | |
| Temperature (°F) | 73.3 ± 0.02 | 73.6 ± 0.03a |
| Temperature difference (°F) | 2.8 ± 0.03 | 3.3 ± 0.03a |
| Relative humidity (%) | 48.1 ± 0.1 | 47.0 ± 0.1a |
| Dew point (°F) | 56.56 ± 0.07 | 56.47 ± 0.07 |
| Dew point difference (°F) | 10.43 ± 0.08 | 10.43 ± 0.08 |
| CO2 (ppm) | 1230.8 ± 3.5 | 1190.5 ± 3.4a |
| Ammonia (ppm) | 15.7 ± 3.9 | 14.8 ± 3.7 |
Significant (P < 0.0001) difference between the 2 ventilation rates.
TVOC levels.
The level of TVOC in the rooms was below the sensor's limit of detection for 99.7% of the time across all rooms and ventilation rates. We observed occasional spikes as high as 2.0 ppm of isobutylene in several rooms. These spikes typically lasted for 30 to 60 min and were associated with times when a researcher was using ethanol within the room.
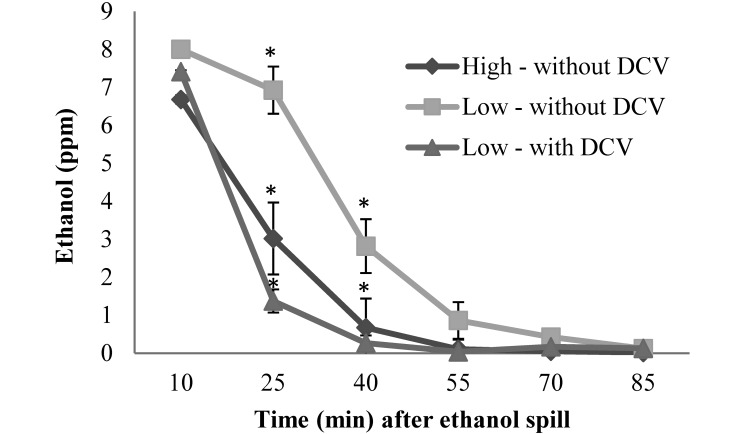
Ethanol clearance.
The level of ethanol in the room air peaked at 10 min after a spill. At the high ventilation rate, the ethanol level dropped to 45% of the peak value after 25 min, whereas at the low ventilation rate, the ethanol level dropped to 87% of the peak value after 25 min (P < 0.0001; Figure 5). At 40 min after an ethanol spill at the high ventilation rate, the ethanol level was 10% of the peak value, whereas the same decrease took 55 min at the low ventilation rate. With the DCV system activated, the sensors detected a high level of TVOC at 10 min after the spill, and the ventilation rate increased from 5 ACH to 15 ACH. Fifteen minutes later (25 min after the spill occurred), the room level of ethanol had dropped to 19% of the peak level.
Figure 5.
Clearance of a TVOC (ethanol) spill in animal rooms at different ventilation rates (low, 5 to 6 ACH; high, 10 to 12 ACH) and with and without the DCV system activated. Data shown as mean level ± SEM. Significant differences (*, P < 0.01) among means are indicated.
Discussion
This study shows that air quality is acceptable for both humans and animals at a room ventilation rate of 5 to 6 ACH in rodent rooms that are using direct-exhaust IVC systems. In particular, levels of Mus m1 allergen and endotoxin within the room did not differ at the 2 different room ventilation rates. No operationally relevant differences were detected in intracage environmental parameters. Although statistically significant increases in room CO2 and dew point temperature were seen during the low ventilation rates, the differences were within acceptable ranges and are likely operationally irrelevant. The TVOC sensor was unable to detect any prolonged elevation that could be attributed to increased levels of ammonia. However, the TVOC sensor occasionally detected spikes that corresponded to use of ethanol in the room. Clearing the air of ethanol after a spill took longer at the low ventilation rate than the high rate. However, with the DCV system activated and starting at a low ventilation rate, time to clearance was faster than at a high ventilation rate, indicating that demand-controlled ventilation is useful in situations in which hazardous chemicals are used.
CO2 has been used as a surrogate measure for the adequacy of ventilation. The 2010 ASHRAE standard for ventilation for acceptable indoor air quality states that when the CO2 level is less than 700 ppm above the level of outdoor air, the ventilation rate is adequate to dilute odors from human bioeffluents (body odors) most of the time.1 The safety limit set by OSHA for CO2 is an 8-h time-weighted average of 5000 ppm.19 Even though the cage exhaust was directly vented from the room and not recirculated within the room, we observed higher CO2 within the rooms compared with CO2 in the supply air. In our study cages were positively ventilated with 30% (per minute, 3.5 L for the mouse cages and 7.0 L for the rat cages) of the air pushed back into the room through the filter top. This effect was evident as a diurnal fluctuation of CO2, with higher levels in the room during the night, the period during which mice and rats are predominantly active. In the more heavily populated rooms, CO2 was 85 to 125 ppm above that of the outside air at the low ventilation rate and 45 to 75 ppm above the outdoor air level during high ventilation. Although these differences were highly significant statistically, the levels were still well below those considered hazardous, and thus we consider these differences to be operationally irrelevant.
To determine any effect of room ventilation rates on moisture in the air, we compared the amount of moisture in the exhaust and supply air. Because the temperature differed between the room air and the supply air, which was sampled before the reheat coils for each room, we report the amount of moisture as dew point temperature, which is a temperature-independent measure of the amount of moisture. Although the moisture was significantly higher in 7 of the 8 rooms at the low ventilation rate, the difference was only 0.25 °F, which is very small and operationally irrelevant.
Particulate levels in the outside air were almost 3 times higher than in the supply air, demonstrating the effectiveness of the prefilter through which incoming air passes. Interestingly, particulates in the room air were significantly lower than in the incoming supply air, most likely due to particulates settling out of the air while in the room. This explanation is supported by our observation of higher levels in the rooms during the high ventilation rate than during the low rates. Furthermore, we observed large daily fluctuations in supply air particulates, ranging from 10,000 to more than 1.6 million particles/ft3 (data not shown), presumably due to different outdoor environmental conditions. Particulate levels in the room air very closely followed this daily fluctuation, although about 35% lower. Taken together, these findings show that the biggest determinant of particulates in rodent room air was the level of particulates in the incoming air; very few, if any, particulates were generated in these rooms, presumably due to containment of animal-related particulates by the use of IVC and cage-change stations. This conclusion is consistent with the findings of another study.27
A key function for an animal facility's HVAC system is to maintain the temperature within prescribed limits with minimal fluctuations.11 Although we noted no difference in the amount of time the temperature was below the set point (± 2 °F) between the different ventilation rates, the amount of time the temperature exceeded the set point was greater during the low ventilation rate. Examination of the data (not shown) revealed that most of time during which the temperature was elevated occurred during several, unexpected warm periods in January and February, when the outdoor temperature exceeded 90 °F and the supply air cooler was unable to maintain its set point. Furthermore, most of the excursions occurred in 2 rooms that were located at the far end of the building's air supply. This pattern indicates that our facility's ventilation system may be somewhat limited, especially during unexpected warm periods. These results confirm the importance of monitoring temperature and responding as needed.
We noted no difference in the levels of allergen or endotoxin in room air at the different ventilation rates. Allergen and endotoxin were somewhat higher in the more densely populated rooms. This finding is consistent with those of other authors, who reported higher levels of allergens in rooms with a greater number of mice.18 However, unlike others, we were not able to show a clear correlation to allergens and particulates. Rather, our study suggested that the main determinant of the particulate level within a room was that in the supply air. Allergen levels within the room can be lowered by operating the IVC system with the cages negatively pressurized to the room.28 Even though our IVC was in a positive mode, room air allergen or endotoxin levels did not differ between the 2 different ventilation rates, although overall levels might have been lower if the cages had been in a negative mode.
Several studies have shown factors that affect intracage air quality with IVC include the type of IVC, ventilation rate within the cage, number and type of mice, days since last cage change, and bedding type.13,16,24,25,30,31 In our current study, somewhat surprisingly we saw lower levels of CO2 at the lower ventilation rates, even though the room levels of CO2 were higher at the lower ventilation rate. We also noted a higher temperature within the cages at the lower ventilation rate. These differences are most likely due to the sensor we used, which has a reported accuracy of ±30 ppm for CO2, ±0.5 °C for temperature, and ±3% for humidity. As measured by dew point temperature, the amount of moisture within the cage was same for both ventilation rates, but given that the temperature within the cage was slightly higher at the lower ventilation rate, the relative humidity was lower.
In the current study, the only periods during which the level of TVOC exceeded the limit of detection for the sensor was when a researcher used ethanol in the room, indicating that we never detected any level of ammonia within the rooms. Because we had no control over when researchers used ethanol within the rooms, we did not analyze TVOC levels at the different ventilation rates. Instead, we created a controlled spill of ethanol and examined the levels at different time points at the 2 different ventilation rates and with DCV activated. Others have performed similar studies of a TVOC spill in laboratories and found that at higher ventilation rates, the peak concentration is lower and clearance occurs faster, with the greatest improvement observed between 6 to 8 ACH and very little improvement above 12 ACH.12 In our study, we similarly observed a faster clearance of TVOC (ethanol) at the higher ventilation rate. When we used the DCV system, the TVOC sensor detected a high level 10 min after the spill occurred. As programmed, the DCV system increased the ventilation rate from a base of 5 to 6 ACH up to the maximum of 15 ACH. Our results indicate that this approach is very effective, because the air was actually cleared faster than occurred at a constant rate of 10 to 12 ACH. However, many hazardous chemicals that may be used or spilled in the facility may not be detected by the TVOC sensor, and therefore DCV systems may not increase ventilation to clear all contaminants equally well. In particular, sensors may not be able to detect some of the typical chemicals used in animal facilities, such as cleaning and sanitizing agents, anesthetic gases, and formaldehyde.
We chose the level of 5 to 6 ACH because this was the lowest rate at which all the air from racks could be directly exhausted and still maintain a positive pressure within the room. A typical room of 300 ft2 and containing 2 racks needed 100 cubic feet per minute (cfm) of exhaust from the racks, 100 cfm for the pressure offset, and a minimum of 50 cfm from the general room exhaust, for a total air supply of 250 cfm. With a 9-ft ceiling height, this equates to an ACH rate of 5.6. Setting ACH rates lower than the current standard of 10 to 15 ACH requires directly removing the air from the IVC rack exhaust to the building's exhaust system and not recirculating it into the room. In setting the supply and exhaust airflows, managers need to consider the overall room layout, design and functional ability of the HVAC system, IVC rack design, and required cage and room pressure differentials.
We estimate that at our facility in southern California, 1 cfm of air annually costs $3.50. Lowering the ventilation rate from 10 to 12 ACH to 5 to 6 ACH decreases the air usage by 225 to 315 cfm of air in a typical rodent room measuring 300 ft2. At US$3.50 per cfm, this decrease would save US$800 to US$1100 per room annually, resulting in significant energy and cost savings.
Acknowledgments
We thank the ACLAM Foundation for financially supporting this study. Additional thanks are extended to Scott Smith (Yardley-Zaretsky, Santa Ana, CA) for his assistance in adjusting the ventilation, Michael Phelan for statistical support, and UCI facility personnel.
References
- 1.American National Standard/American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc. 2010. ANSI/ASHRAE standard 62.1-2010, appendix C. Ventilation for acceptable indoor air quality. Atlanta (GA): American Society of Heating, Refrigeration and Air-Conditioning Engineers. [Google Scholar]
- 2.Bush RK, Stave GM. 2003. Laboratory animal allergy: an update. ILAR J 44:28–51. [DOI] [PubMed] [Google Scholar]
- 3.Clough G. 1982. Environmental effects on animals used in biomedical research. Biol Rev Camb Philos Soc 57:487–523. [DOI] [PubMed] [Google Scholar]
- 4.Corning BF, Lipman NS. 1991. A comparison of rodent caging systems based on microenvironmental parameters. Lab Anim Sci 41:498–503. [PubMed] [Google Scholar]
- 5.Flynn RJ. 1959. Studies on the etiology of ringtail of rats. Proc Anim Care Panel 9:155–160. [Google Scholar]
- 6.Gamble MR, Clough G. 1976. Ammonia build-up in animal boxes and its effect on rat tracheal epithelium. Lab Anim 10:93–104. [DOI] [PubMed] [Google Scholar]
- 7.Gordon S. 2001. Laboratory animal allergy: a British perspective on a global problem. ILAR J 42:37–46. [DOI] [PubMed] [Google Scholar]
- 8.Harrison DJ. 2001. Controlling exposure to laboratory animal allergens. ILAR J 42:17–36. [DOI] [PubMed] [Google Scholar]
- 9.Höglund AU, Renström A. 2001. Evaluation of individually ventilated cage systems for laboratory rodents: cage environment and animal health aspects. Lab Anim 35:51–57. [DOI] [PubMed] [Google Scholar]
- 10.Hunskaar S, Fosse RT. 1990. Allergy to laboratory mice and rats: a review of the pathophysiology, epidemiology, and clinical aspects. Lab Anim 24:358–379. [DOI] [PubMed] [Google Scholar]
- 11.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press [Google Scholar]
- 12.Klein RC, King C, Kosior A. 2009. Laboratory air quality and room ventilations rates. Chem Health Saf 16:36–42. [Google Scholar]
- 13.Lipman NS, Corning BF, Coiro MA., Sr 1992. The effects of intracage ventilation on microenvironmental conditions in filter-top cages. Lab Anim 26:206–210. [DOI] [PubMed] [Google Scholar]
- 14.Lipman NS. 1993. Strategies for architectural integration of ventilated caging systems. Contemp Top Lab Anim Sci 33:7–10. [PubMed] [Google Scholar]
- 15.Lipman NS. 1999. Isolator rodent caging systems (state of the art): a critical view. Contemp Top Lab Anim Sci 38:9–17. [PubMed] [Google Scholar]
- 16.Memarzadeh F, Harrison PC, Riskowski GL, Henze T. 2004. Comparison of environment and mice in static and mechanically ventilated isolator cages with different air velocities and ventilation designs. Contemp Top Lab Anim Sci 43:14–20. [PubMed] [Google Scholar]
- 17.Nagamine CM, Long CT, McKeon GP, Felt SA. 2012. Carbon dioxide and oxygen levels in disposable individually ventilated cages after removal from mechanical ventilation. J Am Assoc Lab Anim Sci 51:155–161. [PMC free article] [PubMed] [Google Scholar]
- 18.Ohman JL, Jr, Hagberg K, MacDonald MR, Jones RR, Jr, Paigen BJ, Kacergis JB. 1994. Distribution of airborne mouse allergen in a major mouse breeding facility. J Allergy Clin Immunol 94:810–817. [DOI] [PubMed] [Google Scholar]
- 19.Occupational Safety and Health Administration (OSHA). 2008. § 1910.1450 Occupational exposure to hazardous chemicals in laboratories.
- 20.Pacheco KA, McCammon C, Liu AH, Thorne PS, O'Neill ME, Martyny J, Newman LS, Hamman RF, Rose CS. 2003. Airborne endotoxin predicts symptoms in nonmouse-sensitized technicians and research scientists exposed to laboratory mice. Am J Respir Crit Care Med 167:983–990. [DOI] [PubMed] [Google Scholar]
- 21.Pacheco KA, McCammon C, Thorne PS, O'Neill ME, Liu AH, Martyny JW, Vandyke M, Newman LS, Rose CS. 2006. Characterization of endotoxin and mouse allergen exposures in mouse facilities and research laboratories. Ann Occup Hyg 50:563–572. [DOI] [PubMed] [Google Scholar]
- 22.Platts-Mills J, Custis N, Kenney A, Tsay A, Chapman M, Feldman S, Platts-Mills T. 2005 The effects of cage design on airborne allergens and endotoxin in animal rooms: high-volume measurements with an ion-charging device. Contemp Top Lab Anim Sci 44:12–16. [PubMed] [Google Scholar]
- 23.Reeb CK, Jones RB, Bearg DW, Bedigian H, Paigen B. 1997. Impact of room ventilation rates on mouse cage ventilation and microenvironment. Contemp Top Lab Anim Sci 36:74–79. [PubMed] [Google Scholar]
- 24.Reeb C, Jones R, Bearg D, Bedigan H, Myers D, Paigen B. 1998. Microenvironment in ventilated animal cages with differing ventilation rates, mice populations, and frequency of bedding changes. Contemp Top Lab Anim Sci 37:43–49. [PubMed] [Google Scholar]
- 25.Reeb-Whitaker CK, Paigen B, Beamer WG, Bronson RT, Churchill GA, Schweitzer IB, Myers DD. 2001. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim 35:58–73. [DOI] [PubMed] [Google Scholar]
- 26.Renström A, Björing G, Höglund AU. 2001. Evaluation of individually ventilated cage systems for laboratory rodents: occupational health aspects. Lab Anim 35:42–50. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum MD, VandeWoude S, Volckens J, Johnson T. 2010. Disparities in ammonia, temperature, humidity, and airborne particulate matter between the micro- and macroenvironments of mice in individually ventilated caging. J Am Assoc Lab Anim Sci 49:177–183. [PMC free article] [PubMed] [Google Scholar]
- 28.Schweitzer IB, Smith E, Harrison DJ, Myers DD, Eggleston PA, Stockwell JD, Paigen B, Smith AL. 2003. Reducing exposure to laboratory animal allergens. Comp Med 53:487–492. [PubMed] [Google Scholar]
- 29.Sharp GP. 2010. Demand-based control of lab air change rates. ASHRAE J 52:30–41. [Google Scholar]
- 30.Silverman J, Bays DW, Cooper SF, Baker SP. 2008. Ammonia and carbon dioxide concentrations in disposable and reusable ventilated mouse cages. J Am Assoc Lab Anim Sci 47:57–62. [PMC free article] [PubMed] [Google Scholar]
- 31.Smith E, Stockwell JD, Schweitzer I, Langley SH, Smith AL. 2004. Evaluation of cage microenvironment of mice housed on various types of bedding materials. Contemp Top Lab Anim Sci 43:12–17. [PubMed] [Google Scholar]
- 32.Taylor DK, Rogers MM, Hankenson FC. 2006. Lanolin as a treatment option for ringtail in transgenic rats. J Am Assoc Lab Anim Sci 45:83–87. [PubMed] [Google Scholar]
- 33.Thulin H, Bjorkdahl M, Karlsson AS, Renstrom A. 2002. Reduction of exposure to laboratory animal allergens in a research laboratory. Ann Occup Hyg 46:61–68. [DOI] [PubMed] [Google Scholar]