Abstract
We have designed a method for immobilizing the subjects of small-animal studies using a study group–specific 3D-printed immobilizer that significantly reduces interfraction rotational variation. A cone-beam CT scan acquired from a single specimen in a study group was used to create a 3D-printed immobilizer that can be used for all specimens in the same study group. 3D printing allows for the incorporation of study-specific features into the immobilizer design, including geometries suitable for use in MR and CT scanners, holders for fiducial markers, and anesthesia nose cones of various sizes. Using metrics of rotational setup variations, we compared the current setup in our small-animal irradiation system, a half-pipe bed, with the 3D-printed device. We also assessed translational displacement within the immobilizer. The printed design significantly reduced setup variation, with average reductions in rotational displacement of 76% ± 3% (1.57 to 0.37°) in pitch, 78% ± 3% (1.85 to 0.41°) in yaw, and 87% ± 3% (5.39 to 0.70°) in roll. Translational displacement within the printed immobilizer was less than 1.5 ± 0.3 mm. This method of immobilization allows for repeatable setup when using MR or CT scans for the purpose of radiotherapy, streamlines the workflow, and places little burden on the study subjects.
Abbreviation: ABS, acrylonitrile butadiene styrene
The technology surrounding small-animal irradiation systems is rapidly advancing, and targeted radiotherapy for small-animal species is quickly becoming a reality.1,7,8 Central to preclinical development and advances in radiotherapy research are irradiation systems that are completely scaled to small-animal proportions.4 A crucial component of small-animal irradiation systems is the reproducibility of subject positioning throughout treatment. Current image guidance capabilities in small-animal irradiation systems allow for translational repositioning of the subject before treatment, at the cost of reduced throughput. However, immobilization is needed to address concerns of rotational variation in setup, which cannot be overcome with table realignment alone.
Clinically, patient immobilization in human radiotherapy is achieved by using devices such as patient-specific aquaplasts and vaculocks. Although the use of analogous devices in small-animal radiotherapy is theoretically possible, it is inappropriate in practice. Few small-animal immobilization techniques have been described in the literature, and each has limitations regarding its application to small-animal radiotherapy. Two such techniques3,9 involve animal-specific molds, which are time-consuming to construct and may prolong the anesthesia time during the molding process. In addition, one group5 has presented a design that uses pegs to immobilize mice for positron emission tomography–CT systems, but the design is incompatible with the small coils used in small-animal MR imaging.
The use of 3D printing technology for medical purposes, including surgical planning, phantom development, and prosthetic applications, has been increasing. The 3D printing technology available for use in medicine has been reviewed recently6 and was the topic of a moderated scientific discussion at the 2014 meeting of the American Association of Physicists in Medicine.2 The 3D printing process can be summarized in 3 basic steps: image acquisition, image postprocessing, and 3D printing. The development of a 3D-printed immobilization device compatible with MR and CT systems would allow for reproducible setup in preclinical radiotherapy trials. For the purposes of radiotherapy, an ideal immobilization device would (1) provide reproducible rotational setup in fractionated treatment delivery, with or without the availability of image guidance; (2) allow for imaging in both CT and MR equipment; (3) function for multiple specimens in a study group; and (4) minimize the anesthesia time for study subjects.
Here we present a 3D-printed universal immobilization technique for mice that is compatible with MR and CT irradiation system geometries, addresses rotational variations in subject setup, places little additional burden on the subjects, and is easily adapted according to study requirements.
Materials and Methods
All animal studies were performed in the Small Animal Imaging Facility at The University of Texas MD Anderson Cancer Center (Houston, TX) under IACUC-approved protocols.
Creation of the immobilizer.
The immobilizer was created by using the following steps. First, a cone-beam CT scan of 1 mouse was acquired (X-RAD 225Cx Small Animal Image Guide Irradiation System, Precision X-Ray, North Branford, CT). To achieve the greatest immobilization possible, we extended the scan well past the attachments of all limbs to the axial skeleton. For imaging, the mouse was placed on a radiolucent material (Fine Turf Green Grass, Woodland Scenics, Linn Creek, MO). This product easily conforms to the natural shape of the animal without deformation due to gravity and has a nearly undetectable signal in CT images. We positioned a small amount of the product into the half-pipe bed currently used for small-animal irradiation at our institution (Figure 1); the mouse was then laid on top, and the material gently conformed to the mouse.
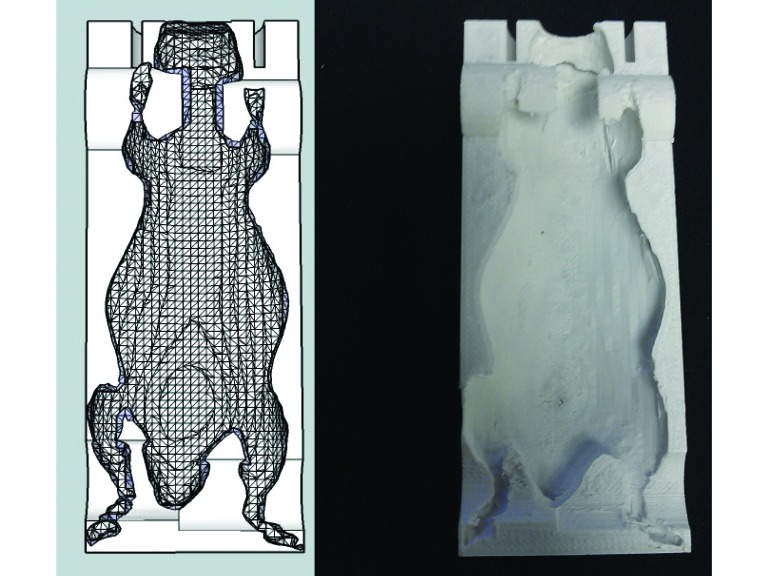
Figure 1.

A radiolucent product (green) was placed loosely in the half-pipe bed. One subject from the study group was then placed in the bed, and the product gently conformed to the mouse's shape. The study nose cone was used to allow for optimal incorporation into the immobilizer design.
Second, the DICOM images were transferred to a free Java-based image processing package (ImageJ version 1.47; http://rsb.info.nih.gov/ij/). A freely available plug-in (BoneJ version 1.3.11; http://bonej.org/isosurfaceversion) was used to create a resampled surface mesh design, which consisted of many small triangles. This design was exported as an STL (ASCII) file. Third, the STL file was imported into SketchUp (Trimble Navigation, Los Angeles, CA), in which the immobilizer design was created (Figures 2 and 3). The immobilizer contained a flat bottom, for use with the flat table-top of the irradiation system; we also created a more compact, rounded-bottom immobilizer for use with small MR coils. The general shape of the immobilizer and its functional components (for example, nose cone size, indentations for fiducials, other rigid positioning indicators) were easily incorporated into the design by using the SketchUp software.
Figure 2.
Top view of the immobilizer for use in CT and small-animal irradiation systems.
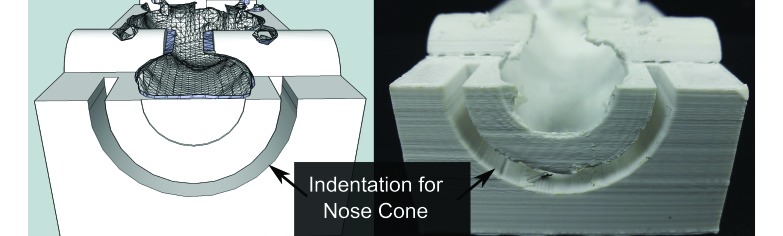
Figure 3.
Many study-specific alterations, such as an indentation for an anesthesia nose cone, can be made.
Fourth, the immobilizer design was exported as an STL file and sent to the 3D printer (Replicator 2X, MakerBot Industries, Brooklyn, NY). Two plastics were commercially available and compatible with the printer: polylactic acid and acrylonitrile butadiene styrene (ABS). We chose ABS because of its ruggedness and availability. The printer settings are shown in Figure 4. Finally after printing, needle-nose pliers, tweezers, and files were used as needed to remove rafts or supports added by the printer hardware and to smooth any rough edges on the immobilizer.
Figure 4.
The conditions under which the immobilizer was printed are specified. Note temperature parameters (extruder temperature, build-plate temperature) may need modification to each printing environment, although all tests were completed with settings within 5° of those cited.
Once a final design was determined, we printed 3 identical immobilizers to evaluate the replicability of the printing process. We also printed a couch clip, which secured the immobilizer to the preexisting table. The clip is necessary when using an MR-compatible immobilizer in the small-animal irradiation system. Because the table can be moved in 3 translational directions, the clip is not mandatory for positioning but it allows for quicker setup and minimizes gross movement of the immobilizer on the table.
Beam attenuation by the immobilizer.
To verify that the ABS material did not attenuate the beam to a degree that required dosimetric adjustments, 3 small, 3D-printed squares of 5-, 10-, and 20-mm thickness were placed in the beam. The small slabs were taped to the end of a 15 × 15-mm cone, and the beam was delivered under typical irradiation conditions (225 kV, 13 mA). A Farmer-type ionization chamber (IBA Dosimetry, Schwarzenbruck, Germany) was used to measure the exposure with and without material in the beam. The process was repeated 3 times for each of the 3 slab thicknesses. The average transmission was calculated over the 9 readings.
Quantification of rotational variation.
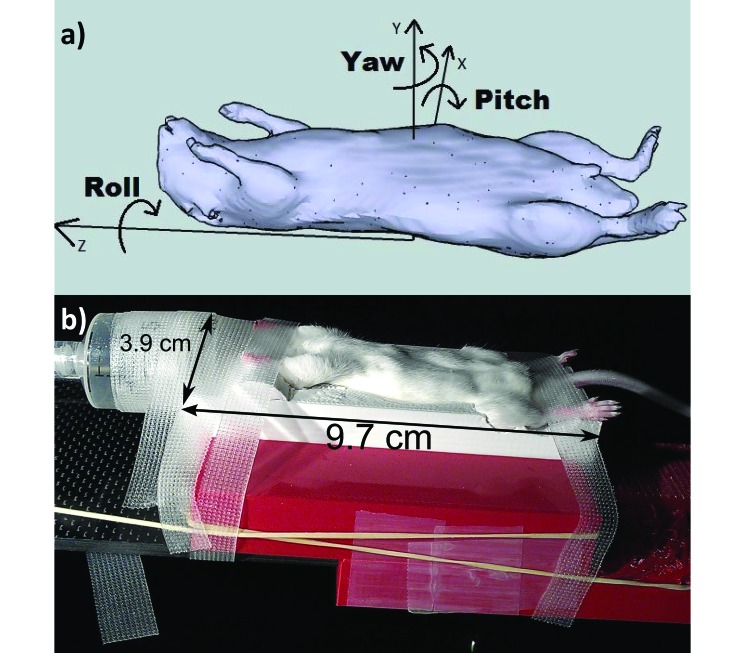
A retrospective review of a data set of 4 weekly cone-beam CT scans of 10 mice was used as a surrogate for interfraction reproducibility by using the current half-pipe setup. The first week's scan of each mouse was used as the reference scan, and the scan from each additional week was rigidly registered to this scan. This process yielded a set of 30 measurements of rotational variation in setup. Registration was performed by using the CT–CT cross-correlation algorithm implemented in Pinnacle Treatment Planning Software (Philips Medical System, Andover, MA). Rotation in the sagittal (pitch), coronal (yaw), and axial (roll) planes (Figure 5 A) was measured, and the mean, standard deviation, and range of displacement from the reference scan were calculated.
Figure 5.
(A) Depiction of axes of rotation in the sagittal (pitch), coronal (yaw), and axial (roll) planes. (B) A mouse positioned in the immobilizer.
To measure rotational variation in the immobilizer setup, 6 mice were each scanned 3 to 7 times. The mouse used for creation of the immobilizer was not used for quantification of rotational variation in setup, but all 6 mice scanned (as well as the one used to create the immobilizer) were similar in size. As in the half-pipe setup measurements, the first scan of each specimen was used as the reference from which rotational variation in subsequent scans was measured, thus providing a set of 21 data points.
A series of remeasurements and visual inspections of 10 random registrations was used to estimate uncertainty.
Translational displacement within the printed immobilizer.
To determine the reproducibility of translational setup of the mouse within the printed immobilizer, we compared a set of anatomic points with the location of a fiducial visible in the CT images. A fiducial was fixed to a printed indentation on the bottom of the immobilizer, and 4 image sets of 1 mouse were obtained. The lower jaw was chosen for superior–inferior displacement, the left coxofemoral articulation for left–right displacement, and the articulation of the seventh rib with the sternum for anterior–posterior displacement. These anatomic markers were chosen for 2 reasons. First, points of the axial skeleton are least influenced by the location of limbs, which are less often the subject of radiotherapy in mice. Second, compared with other sites, the locations of articulations and curved structures are most easily discernable on cone-beam CT images. The distance from these anatomic structures to the fixed fiducial was measured, and the standard deviation was calculated.
Feasibility in MR.
An immobilizer with dimensions compatible with the smallest MR coil was used to verify the feasibility of using the immobilizer in MR imaging. Images in the sagittal, coronal, and axial planes were acquired by using a 3-T MR system (Bruker Biosciences, Billerica, MA). The mouse was removed from the immobilizer in the MR scanner and replaced in the immobilizer in the cone-beam CT scanner. The MR and CT scans were then compared visually for similarity.
Results
Creation of immobilizer.
The printing process, once started, was self-regulated and took 2 to 4 h (Figure 2); alterations (Figure 3) required, at most, another 15 min. Immobilizers generated after repeating the printing process showed no macroscopic differences between each other.
Beam attenuation.
The percentage (mean ± 1SD) of attenuation though each of the 3 squares of ABS material was 98% ± 1%. Therefore, we concluded that no dosimetric adjustments were needed for the thickness of the ABS material in our application.
Quantification of rotational variation.
Table 1 shows the average, standard deviation, and range of rotational variation in each of the 3 dimensions in the half-pipe and 3D-printed immobilizer setups. The axes of rotation are illustrated in Figure 5 A. The roll plane presented the largest variation in positioning; given the geometry of the mouse and setup, this result was unsurprising. Using the immobilizer reduced roll variation by 87% ± 3% compared with the half-pipe setup, and overall rotational variation was reduced by an average of 80% ± 3% in all directions.
Table 1.
Rotational displacement in the half-pipe and 3D-printed immobilizer setups
| Half-pipe |
3D-printed immobilizer |
||||
| Mean ± 1 SD | Range | Mean ± 1 SD | Range | % Reduction | |
| Pitch, degrees | 1.57 ± 1.32 | −1.42 to 4.68 | 0.37 ± 0.35 | −0.89 to 1.25 | 76 ± 3 |
| Yaw, degrees | 1.85 ± 1.47 | −5.41 to 1.05 | 0.41 ± 0.34 | −0.64 to 1.33 | 74 ± 3 |
| Roll, degrees | 5.39 ± 4.64 | −12.93 to 18.86 | 0.70 ± 0.79 | −3.56 to 0.95 | 87 ± 3 |
All displacement values have an uncertainty of ±0.08.
Translational displacement within the printed immobilizer.
The translational reproducibility (defined as 1 SD of the distance to the fixed fiducial) within the printed immobilizer was 0.3 ± 0.3 mm in the left–right direction, 0.4 ± 0.3 mm in the anterior–posterior direction, and 1.5 ± 0.3 mm in the superior–inferior direction. Left–right and anterior–posterior translations were approximately equivalent to the thickness of 1 imaging slice.
Feasibility in MR.
MR and CT images were compared in the coronal view (Figure 6). On the basis of visual inspection, mouse positioning was similar in CT and MR using the printed immobilizer. In addition, the ABS material created no artifact and was not visible in the MR images.
Figure 6.
CT (left) and MR (right) images of a mouse in the 3D-printed immobilization device. No material artifacts are visible in the MR image.
Discussion
Preclinical radiotherapy trials require reproducibility in interfractional setup. Although image guidance and table controls overcome translational shifts in subject position, these features cannot address rotational variation in setup. In addition, image guidance prior to treatment reduces throughput and increases the amount of time subjects must spend under anesthesia.
This feasibility study outlines the process through which study-group–specific 3D-printed immobilizers can be created and evaluates their positive effect on small-animal reproducibility in radiotherapy. We have developed a study-specific, 3D-printed immobilization device for mice that allows quick and reproducible small-animal setup and reduces rotational variation by 80% on average. Translational displacement of the mouse within the immobilizer was, at most, 1.5 mm. This device is compatible with current CT and radiotherapy systems and can also be used in MR systems, allowing for excellent soft-tissue visualization.
The immobilizer may be used for applications including normal tissue irradiation, small-animal radiotherapy, and other preclinical investigations that depend on reproducibility in animal positioning. In addition, the design is easily customized to achieve study goals. Depending on the specifics of the study in which the immobilizer is implemented, additional uncertainties (for example, due to respiratory motion) are expected.
The 3D-printed immobilization device that we developed was used for mice of similar size and identical strain to that used to create the device. We speculate that a single immobilizer will accommodate most mice of the same strain, both nude and nonnude, that are similar in size (age) to the ‘model’ animal. Therefore, because only 1 additional scan of a study subject is required, the creation of a 3D-printed immobilizer places little extra burden is placed on the study cohort. End-to-end time to create the printed immobilizers ranged from 3.5 to 5.5 h, but, on average, only 1.5 h is required for scan acquisition, image processing, and postprinting alterations. Printing time, the remaining 2 to 4 h, is self-regulated. Finally, incorporation of the printed immobilizer may reduce the reliance on image guidance, improve the workflow, and streamline the process of subject setup.
References
- 1.Clarkson R, Lindsay PE, Ansell S, Wilson G, Jelveh S, Hill RP, Jaffray DA. 2011. Characterization of image quality and image-guidance performance of a preclinical microirradiator. Med Phys 38:845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehler E, Perks J, Rasmussen K, Bakic P. 2014. MO-A-9A-01. Innovation in medical physics practice: 3D printing applications. Med Phys 41:410–411. [Google Scholar]
- 3.Haney CR, Fan X, Parasca AD, Karczmar GS, Halpern HJ, Pelizzari CA. 2008. Immobilization using dental material casts facilitates accurate serial and multimodality small-animal imaging. Concepts Magn Reson Part B Magn Reson Eng 33B:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaffray D. 2008. TU-C-AUD C-05: glimpse of 2058 status of small-animal IGRT. Med Phys 35:2891. [Google Scholar]
- 5.Nelson GS, Perez J, Vilalta M, Ali R, Graves E. 2011. Facilitating multimodal preclinical imaging studies in mice by using an immobilization bed. Comp Med 61:499–504. [PMC free article] [PubMed] [Google Scholar]
- 6.Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, Giesel FL. 2010. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg 5:335–341. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Liu Z, Sultana S, Schreiber E, Zhou O, Chang S. 2007. A novel high resolution microradiotherapy system for small-animal irradiation for cancer research. Biofactors 30:265–270. [DOI] [PubMed] [Google Scholar]
- 8.Wong J, Armour E, Kazanzides P, Iordachita I, Tryggestad E, Deng H, Matinfar M, Kennedy C, Liu Z, Chan T, Gray O, Verhaegen F, McNutt T, Ford E, DeWeese TL. 2008. High-resolution, small-animal radiation research platform with X-ray tomographic guidance capabilities. Int J Radiat Oncol Biol Phys 71:1591-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanzonico P, Campa J, Polycarpe-Holman D, Forster G, Finn R, Larson S, Humm J, Ling C. 2006. Animal-specific positioning molds for registration of repeat imaging studies: comparative μPET imaging of 18F-labeled fluorodeoxyglucose and fluoromisonidazole in rodent tumors. Nucl Med Biol 33:65–70. [DOI] [PubMed] [Google Scholar]