Abstract
Gavage is a widely performed technique for daily dosing in laboratory rodents. Although effective, gavage comprises a sequence of potentially stressful procedures for laboratory animals that may introduce bias into experimental results, especially when the drugs to be tested interfere with stress-dependent parameters. We aimed to test vehicles suitable for drug delivery by voluntary ingestion in rats. Specifically, Male Wistar rats (age, 2 to 3 mo) were used to test nut paste (NUT), peanut butter (PB), and sugar paste (SUG) as vehicles for long-term voluntary oral administration of losartan, an angiotensin II receptor blocker. Vehicles were administered for 28 d without drug to assess effects on the glucose level and serum lipid profile. Losartan was mixed with vehicles and either offered to the rats or administered by gavage (14 d) for subsequent quantification of losartan plasma levels by HPLC. After a 2-d acclimation period, all rats voluntarily ate the vehicles, either alone or mixed with losartan. NUT administration reduced blood glucose levels. The SUG group had higher concentrations of losartan than did the gavage group, without changes in lipid and glucose profiles. Our results showed that NUT, PB, and SUG all are viable for daily single-dose voluntary ingestion of losartan and that SUG was the best alternative overall. Drug bioavailability was not reduced after voluntary ingestion, suggesting that this method is highly effective for chronic oral administration of losartan to laboratory rodents.
Oral administration, whether by gavage or voluntary ingestion, is a common method for providing a single daily dose of drug to animal models. Gavage is widely performed for precise oral dosing in rodents, particularly in efficacy, toxicity, and drug discovery studies. This technique, applicable in unanesthetized animals, is a rapid and efficient mean of accurately delivering fixed doses of the drug to be tested.30 However, gavage comprises a sequence of procedures that are potentially stressful for laboratory animals. The removal of an animal from its cage, its manual restraint, and the insertion of a flexible or rigid dosing cannula into the esophagus with direction to the stomach all cause high levels of stress, even in trained animals.2,5 In addition, the use of gavage is associated with welfare issues when it is done by inexperienced people or in long-term studies.13 Indeed, gastroesophageal aspiration and pulmonary injury represent recurrent complications of gavage dosing of rodents that can be triggered by technical errors.8 Moreover, the use of gavage for various drugs and vehicles can elicit a significant stress response in a vehicle- and dose-volume–dependent fashion.5
When testing antihypertensive drugs, a noninvasive and stress-free method for drug delivery is crucial, because any source of external stress on rodents can significantly increase heart rate and blood pressure3-5,18 and therefore potentially confound the experimental results.
Several alternatives to gavage, which require less human involvement, have been tested in recent years. The administration of a drug mixed with an attractive vehicle by voluntary ingestion seems to be an effective, noninvasive method.12,15 In previous attempts to refine the gavage procedure, nut paste has been successfully used for analgesic drug delivery1,12,15,17 and is a promising option for estrogen administration.14 Other alternatives involve the use of pill dosing method,32 flavored gelatin (‘jello’) preparations,10 sugar cookie dough,7 honey,19 and syringe-feeding.2
In our study, we used nut paste (NUT), peanut butter (PB), and sugar paste (SUG) as drug vehicles due to their palatability, consistency, and previously reported results. We aimed to investigate the suitability of these vehicles for voluntary oral administration and, as an alternative method to gavage, for the chronic administration of losartan to laboratory rats. We hypothesized that low amounts of these vehicles, even when administered to rats over a long period of time, would not induce relevant changes in blood glucose or lipid profiles and would efficiently deliver the tested drug, as assessed by losartan serum concentrations. Accordingly, we further hypothesized that the serum concentrations of the drug delivered by voluntary ingestion would be at least as high as that obtained by using gavage.
Materials and Methods
Animals.
These experiments were performed in 41 male Wistar rats (Rattus norvegicus L.; age, 2 to 3 mo; body weight [mean ± 1 SD], 283 ± 5 g) obtained from the NOVA Medical School (Lisbon Portugal) animal facility. This inhouse colony was established by using rats acquired from Charles River Laboratories (Portage, Michigan). Rats were housed individually in polycarbonate cages with wire lids (Tecniplast, Buguggiate, Varese, Italy), under 12:12-h light:dark cycles (08:00 am to 08:00 pm), at a room temperature 22 ± 2.0 °C and relative humidity 60% ± 10%. Rats were maintained on standard laboratory diet (RM1, SDS Diets, Essex, United Kingdom) and reverse-osmosis–purified water, given free choice. Corncob bedding (Probiológica, Lisbon, Portugal) was used and changed once a week. Rats were SPF according to FELASA recommendations.21
Applicable institutional and governmental regulations concerning ethical use of animals were followed, according to the NIH Principles of Laboratory Animal Care (NIH publication no. 85-23, revised 1985),22 the European Guidelines for the Protection of Animals Used for Scientific Purposes (European Union Directive 2010/63/EU),29 and Portuguese Decree-Law number 113/2013.9 Experimental procedures were previously approved (protocol no. 21/2013/CEFCM) by the Institutional Ethics Committee of the NOVA Medical School for animal care and use in research.
Experimental protocol.
We tested 3 vehicles for long-term (14 d) oral administration of losartan, an antihypertensive drug (angiotensin II type 1 receptor antagonist). In light of their consistency, palatability, and use in previous reports,1,7,12,14-16 we selected nut paste (NUT; Nutella , Ferrero Ibérica, Llobregat, Spain), peanut butter (PB; Skippy , Unilever, London, United Kingdom), and sugar paste (SUG; SweetArt, Entertraining, Lisbon, Portugal) for the current study. NUT ingredients include sugar, vegetable oil, hazelnuts (13%), skim milk powder (8.7%), fat-reduced cocoa powder (7.4%), soy lecithin (emulsifier), and vanillin (flavoring). The caloric content of NUT is 21.75 kJ/g; protein content is 7.1%; fat content 30.3%; and sugar content is 54.7%. PB is composed of roasted peanuts, sugar, hydrogenated vegetable oils, and salt. The caloric content is 26.10 kJ/g; protein content is 22.2%; fat content is 49.4%; and carbohydrate content is 23.8%. The ingredients of SUG are sugar, cornstarch, glucose, vegetable fat, vegetable gums, stabilizer E420, preservatives E202, and dyes E102 and E131. The caloric content of SUG is 16.50 kJ/g; protein content is 3.5%; fat content is 6.6%; and carbohydrate content is 86.6%.
After weaning, rats were randomized into 6 groups (n = 6 rats each): NUT only (0.5 g) for 28 d; PB only (0.5 g) for 28 d; SUG only (0.5 g) for 28 d; NUT with 10 mg/kg losartan daily for 14 d; PB with 10 mg/kg losartan daily for 14 d; and SUG with 10 mg/kg losartan daily 14 d. The control group of 5 rats received 10 mg/kg losartan by oral gavage daily for 14 d. All groups that received NUT, PB, or SUG were acclimated for 2 d, during which they were fed 0.5 g of the respective vehicle once daily to minimize neophobia1 and prevent incomplete ingestion incidents. Drug or vehicle deliveries were considered incomplete when any vehicle was found in either the culture dish used to present the vehicle, on the side of the cage, or in bedding beyond 1 h after presentation.
In the first set of experiments, the vehicles were administered without drug for 28 d to determine whether rats would voluntarily ingest them throughout this prolonged time period and to test the vehicles’ effects on the blood glucose level and serum lipid profile. In the second set of experiments, the vehicles were weighed, mixed with the respective amount of drug (losartan 10 mg/kg, Cinfa, Pamplona, Navarra, Spain), and offered to the animals in 92 × 16 mm polystyrene culture dishes (Sarstedt, Numbrecht, Germany). The culture dishes were placed vertically and were attached to the inner cage wall by using adhesive tape (Figure 1). The vehicles, alone or mixed with the drug, were given to the animals at approximately the same time each day (11:00 to 12:00 am). The administration schedule, the ingestion profile (1, complete; 2, incomplete), and the time between vehicle or vehicle–drug presentation and consumption (1, within 5 min; 2, between 5 min and 1 h; 3 more than 1 h) were recorded. The control rats underwent a 7-d handling acclimation period. Each day, the same person handled each rat for 2 min and accustomed it to the gavage position, in a procedure room located outside the animal holding room. Handling and gavage training were performed on the same schedule (11:00 am to 12:00 am) as for voluntary administration. Gavage was performed by using a stainless steel curved gavage-feeding needle with a round tip (16-gauge; tip diameter, 3 mm; length, 75 mm; Fine Science Tools, North Vancouver, British Columbia, Canada).
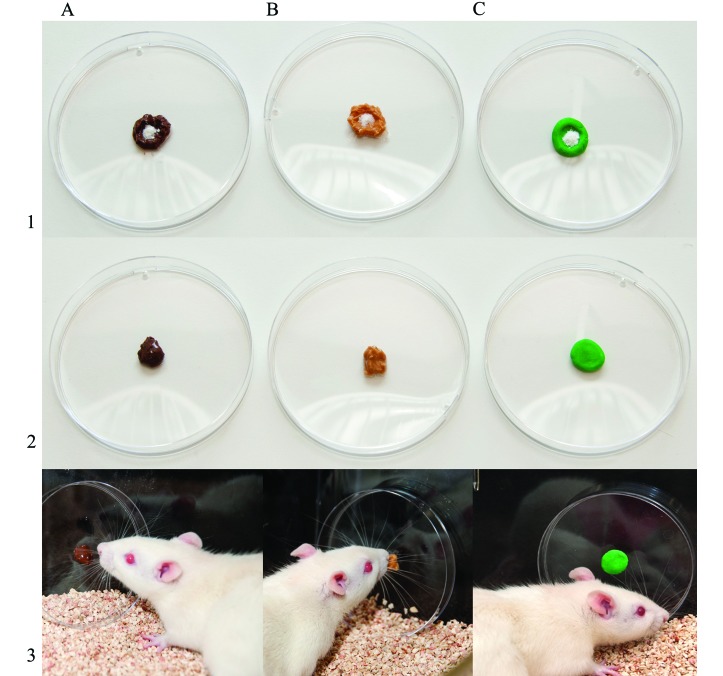
Figure 1.
Three vehicles for voluntary ingestion of losartan: (A) nut paste; (B) peanut butter, and (C) sugar paste. To prepare the drug for voluntary ingestion by rats, (1) weighed losartan powder was placed on top of the vehicle. (2) The vehicle–drug mixture was shaped into a ball ready for delivery. (3) Rats ingested the vehicle–drug mixture from a polystyrene culture dish that was placed vertically in the cage.
Rats were weighed at baseline and then once weekly throughout the entire study. The amounts of losartan were adjusted weekly to ensure a daily dose of 10 mg/kg. Losartan was prepared daily, immediately before administration by gavage or in vehicle. For gavage, losartan was dissolved in reverse-osmosis–purified water (2 mL). When losartan was administered in one of the test vehicles, the required amount of drug for each rat was mixed with 0.5 g of the respective vehicle. During the vehicle-only experiments, water intake was measured weekly, and the blood glucose level and serum lipid profile were assessed at baseline and then at 2 to 3 h after administration, on day 14, and on day 28.
At the end of the experiments, rats were anesthetized at 2 to 3 h after drug delivery by intraperitoneal injection with medetomidine (0.5 mg/kg; Domitor, Pfizer Animal Health, New York, NY) and ketamine (75 mg/kg body weight; Imalgene 1000, Merial, Lyon, France), and cardiocentesis was performed to collect blood for further drug quantification. The rats were then euthanized by an intracardiac overdose of sodium pentobarbital followed by cervical dislocation.
Blood sampling.
Tail-vein sampling.
For the vehicle-only experiments, blood samples were collected from the tail vein of conscious rats at baseline, day 14, and day 28. Rats were placed in a conventional plastic restrainer (model 554-BSRR, Plas-Labs, Lansing, MI) at a room temperature 24 to 27 °C. A scalpel blade was used to make an incision in the lateral tail vein. Approximately 100 μL of blood was collected from each rat by using a capillary tube (Hirschmann Laborgerate, Eberstadt, Germany), transferred to microfuge tubes, and kept on ice. This procedure also was used to collect 2 drops of blood for glucose quantification. After collection, bleeding was stopped by compression for a few seconds, and the area was cleaned with povidone–iodine. Serum samples were centrifuged in a microfuge at 2000 × g (4 °C) for 10 min, immediately decanted, and stored at −80 °C until analysis. According to prior recommendations, the restrainer was washed and dried after each use to prevent pheromonally induced stress.23
Cardiocentesis.
For the lorsartan-dosing experiments, blood collection was performed without thoracotomy by using a 20-gauge needle and a 10-mL syringe. For plasma sampling, approximately 3 mL of blood was collected into an EDTA-containing vacuum phlebotomy tube, kept on ice, and immediately centrifuged at 2000 × g (4 °C) for 10 min. Plasma samples were decanted prior to storage at −80 °C until analysis.
Measurement of blood glucose level and serum lipid profile.
Blood glucose level (in mg/dL) was determined by using a digital glucometer (Precision Xceed, Abbott Diabetes Care, Oxon, United Kingdom). The serum levels of triglycerides, total cholesterol, HDL-cholesterol, and LDL-cholesterol were determined spectrophotometrically (Rx Daytona Analyser, Randox Laboratories, Crumlin, United Kingdom) by using enzymatic colorimetric assay kits (Randox Laboratories).
HPLC analysis.
Losartan plasma concentrations were determined by HPLC according to a minor modification of a previously described method.34 Briefly, losartan was dissolved in methanol yielding a stock solution of 1 mg/mL. Calibration samples were prepared by adding known amounts of the diluted stock solution to rat plasma and covered a range of 1 to 10 μg/mL. Subsequently, calibration samples (200 μL) were extracted with 3 mL of ethyl acetate. After centrifugation (2000 × g, 4 °C, 10 min), the organic phase was evaporated to dryness at 60 °C in a vacuum concentrator (Speed-Vac, Labconco, Kansas City, MO). The resulting residue was reconstituted in 150 μL of mobile phase, and 100 μL was injected into the HPLC column.
HPLC was accomplished by using a solvent-delivery pump (model LC 9-A, Schimadzu, Kyoto, Japan), autosampler (model 7725i, Schimadzu), UV–VIS spectrophotometric detector (model SPD-6 AV, Schimadzu), column (250 × 4 mm; particle size, 5 μm; LiChrospher 100 RP-18; Merck, NJ) protected by a guard-column (4 × 4 mm; particle size, 5 μm; LiChrospher 100 RP-18e; Merck, Kenilworth, NJ), and a column oven (model CTO-10AS VP, Schimadzu). Data acquisition and processing were performed by using Shimadzu Class VP 7.X software. The mobile phase consisted of solution A (methanol: acetonitrile: sodium dihydrogen phosphate monohydrate buffer; 10 mM, pH 3, 50:10:40 v/v/v) and solution B (methanol). All HPLC solvents were purchased from VWR International (Carnaxide, Portugal). Prior analysis, both solutions were degassed for 15 min by sonication. The elution program consisted of an isocratic flow of solution A for 9.5 min, followed by a linear gradient of A:B (20:80 v/v, 2.5 min), an isocratic step of A:B (20:80, 4 min), and finally a return to the initial conditions in 2 min. The analytical run was performed with at a mobile phase flow rate of 0.8 mL/min, 25 °C, and detection wavelength of 230 nm. Analytical standards of losartan for HPLC analysis were purchased from Sigma– Aldrich (Sintra, Portugal).
Statistics.
All data are represented as mean ± SEM. One-way ANOVA with the Bonferroni multiple-comparison test or Tukey multiple-comparison test was used, whenever appropriate, to evaluate the vehicles’ effects on glycemia and the lipid profile. A 2-way repeated-measures ANOVA with a posthoc comparison test was used to compare the effects of the vehicle effect on mean body weight and water intake throughout the 28-d experiment. Comparison of losartan plasma concentrations was performed by using one-way ANOVA with the Dunnett multiple-comparison posthoc test, with the gavage value serving as the control. Statistical analysis was performed by using Prism (version 5.01, GraphPad Software, San Diego, CA). The threshold for statistical significance was set at a P value of less than 0.05.
Results
Vehicles and vehicles-drug mix ingestion.
During the 2-d acclimation period, losartan-free NUT, PB, and SUG were made available in the cages (Figure 1). By day 2 of acclimation, all rats took less than 1 h (and most less than 5 min) to voluntarily ingest all of the vehicle presented. After acclimation, no incomplete drug ingestion incidents were observed, and ingestion of the vehicle-drug mixture was immediate (less than 5 min) for all rats.
Physiologic data, blood glucose levels, and lipid profile.
None of the vehicles significantly affected mean body weight or water intake throughout the 28 d of experiments. Vehicle ingestion did not induce any deviation in the weight variation observed in animals of this age in this population. The average weight gain was 90 g among the 18 animals in these experiments, and the daily water intake was 14.8 mL on average during the 28 d.
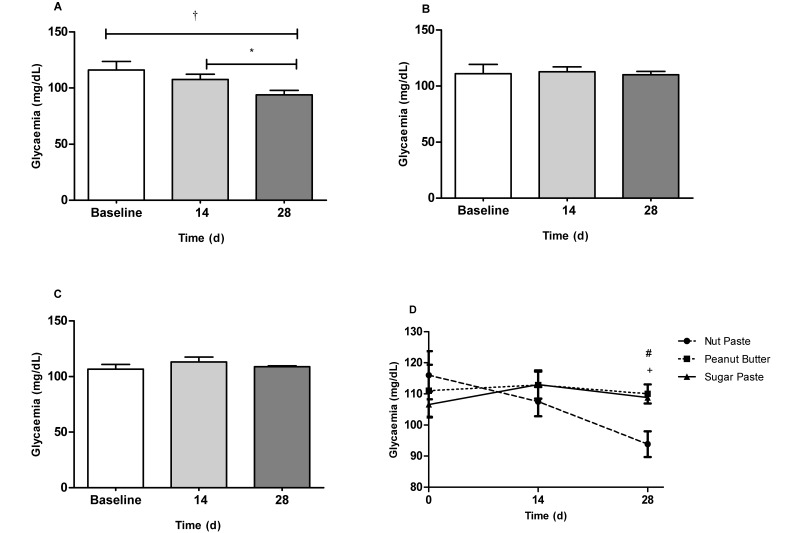
In contrast to the PB and SUG groups, the NUT rats had a significant (P < 0.05) decrease in the mean blood glucose level during the 28-d experiment (Figure 2 A). Specifically, at the 28-d time point, the mean glycemia of NUT group was lower than that of the PB (P < 0.05) and SUG (P < 0.01) groups (Figure 2 D). The glycemic profile remained unchanged in the PB and SUG groups (Figure 2 B and C).
Figure 2.
Blood glucose values (mg/dL) in the serum of Wistar rats fed 0.5 g of (A) nut paste, (B) peanut butter, or (C) sugar paste. (D) Comparison of the effects of the 3 vehicles on glycemia at the 14- and 28-d time points. Data are expressed as mean ± SEM; n = 6 for all groups. *, P < 0.05; †, P < 0.01 according to one-way ANOVA with the Bonferroni multiple-comparison test. #, P < 0.05 (nut paste compared with sugar paste); +, P < 0.01 (nut paste compared with peanut butter) according to one-way ANOVA with the Tukey multiple-comparison test.
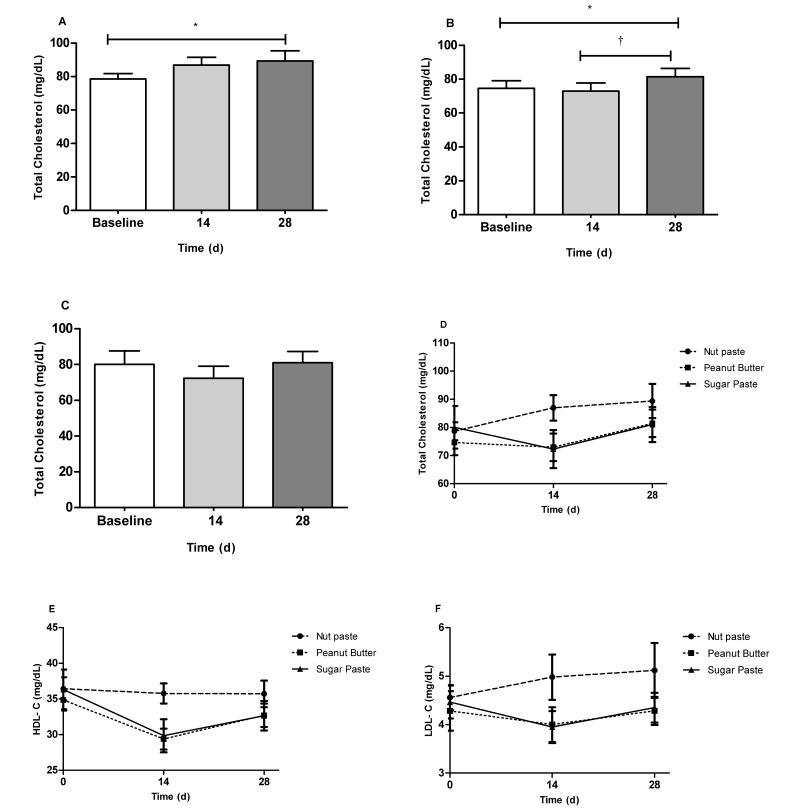
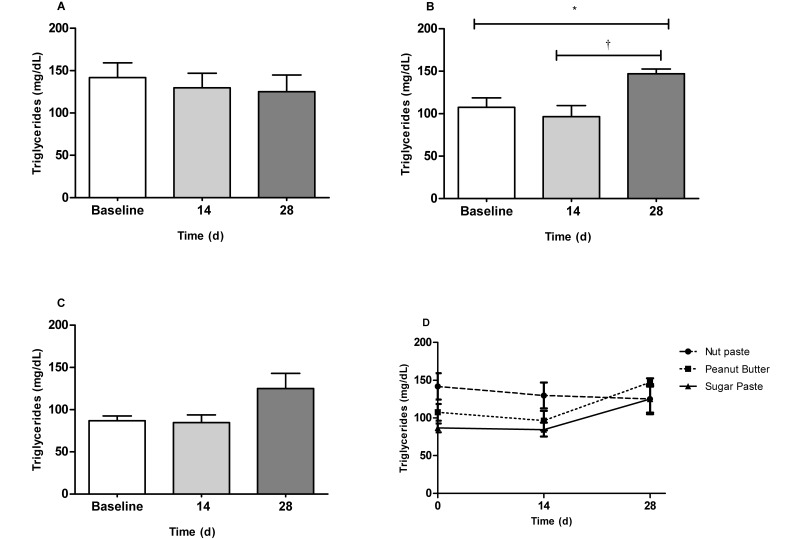
Regarding the lipid profile, serum levels of total cholesterol gradually increased in both the NUT (P < 0.05; baseline compared with 28 d) and PB (P < 0.05; baseline compared with 28 d) groups (Figure 3 A and B) but remained constant in the SUG group (Figure 3 C). However, the mean total cholesterol level did not differ between the 3 groups at either the 14-d or 28-d time point (Figure 3 D). Serum triglyceride concentrations did not differ throughout the 28-d study in either the NUT (Figure 4 A) or SUG (Figure 4 C) groups but increased significantly (P < 0.05; baseline compared with 28 d; P < 0.01 14 d compared with 28 d) in the PB group (Figure 4 B). Serum triglyceride values did not differ among the 3 groups at either the 14-d or 28-d time point (Figure 4 D). Compared with baseline values, HDL-cholesterol concentrations were decreased at day 14 for both the SUG (P < 0.01) and PB (P < 0.001) group. These values progressively increased and, at the end of the experiment, were similar to those observed at baseline; in addition, concentrations in the NUT group remained constant throughout the study. HDL-cholesterol values did not differ among vehicles at the 3 time points (Figure 3 E). Finally, no significant changes in LDL-cholesterol values were observed for either the NUT, PB or SUG groups throughout the experimental period (Figure 3 F).
Figure 3.
Total cholesterol concentrations (mg/dL) in the serum of Wistar rats fed 0.5 g of (A) nut paste, (B) peanut butter, or (C) sugar paste. *, P < 0.05; †, P < 0.01 according to one-way ANOVA with the Bonferroni multiple-comparison test. Comparison of the effects of the 3 vehicles on (D) total cholesterol, (E) HDL-cholesterol, and (F) LDL-cholesterol levels at 14- and 28-d time points by one-way ANOVA with the Tukey multiple-comparison test. Data are expressed as mean ± SEM (n = 6 for all groups).
Figure 4.
Mean triglyceride concentrations (mg/dL) in the serum of Wistar rats fed 0.5 g of (A) nut paste, (B) peanut butter, or (C) sugar paste.*, P < 0.05; †, P < 0.01 according to one-way ANOVA with the Bonferroni multiple-comparison test. Comparison of the effects of the 3 vehicles on triglyceride levels at the 14- and 28-d time points. Data are expressed as mean ± SEM (n = 6 for all groups).
Losartan plasma concentrations.
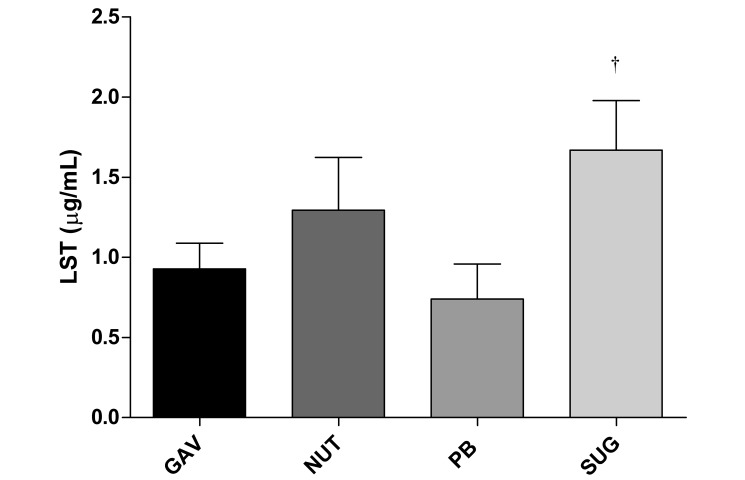
After 14 d of losartan daily administration by gavage the mean plasma concentration was 0.9 ± 0.09 μg/mL. A slight but nonsignificant decrease of the plasma concentration of losartan was observed in the PB group (0.7 ± 0.09 μg/mL). However, when losartan was given along with NUT, its plasma concentration tended to be higher than that in the gavage group (1.3 ± 0.15 μg/mL), although this difference did not reach statistical significance. When SUG vehicle was used, a significantly higher losartan plasma concentration was achieved (1.7 ± 0.14 μg/mL; P < 0.01 compared with gavage). These results are presented in Figure 5.
Figure 5.
Plasma concentrations of losartan (μg/mL) in rats from blood collected by cardiocentesis after 14 d of daily ingestion by gavage (GAV; n = 5), nut paste (NUT; n = 6), peanut butter (PB; n = 6), or sugar paste (SUG; n = 6). Data are expressed as mean ± SEM. †, P < 0.01 compared with gavage according to one-way ANOVA with the Dunnett multiple-comparison test.
Discussion
We tested NUT, PB, and SUG as vehicles for chronic losartan administration to rats to avoid the potential stress induced by orogastric administration (gavage). Voluntary ingestion proved to be an effective method for a controlled daily dose administration, according to a define timetable, that was independent of handling and restraint procedures. Moreover, as does gavage, this approach mimics the single-dose administration method widely used in human clinical practice.
We used individually housed, adult male Wistar rats to avoid postulated effects of estrogen on blood lipids.11 Although some scientists recommend that rats should ideally be housed in groups to decrease the stress levels,25 we single-housed all of our rats to ensure correct dosage and to confirm the complete ingestion of drug-containing vehicle or vehicle only. In terms of palatability, NUT, PB, and SUG were attractive to the rats, given that they ingested the products completely and almost immediately. Furthermore, the consistency of these vehicles facilitates the weighing and the mixing of powder, thereby ensuring the accuracy of the drug dose. The amount of vehicle given to the animals (0.5 g) was sufficient to hold the weighed amount of drug but was not so large to cause noteworthy metabolic changes. Offering the mixture in a culture dish placed vertically inside the cage proved to be an effective means to ensure complete ingestion. As previously reported, placing NUT in a container on the floor of the cage is not recommended, given that neophobic rats tend to bury the novel item and thus mix the vehicle with the bedding material.1
Although some authors have recommended a period of as long as 5 d for acclimating to mice the vehicles,14 in our study, 2 d was sufficient to ensure that no neophobic behavior occurred. To minimize the stress levels associated with handling and restrained, a 1-wk period of acclimation was defined in the gavage group.
Oral gavage has recently been refined from using metal gavage needle to a sterile flexible feeding tube which reduce the risk of trauma, perforation and cross contamination.20 However, the stress related to orogastric administration is not overcome by this approach.
Losartan is the first orally available angiotensin receptor antagonist without agonist properties.26 After oral administration, losartan is rapidly absorbed, reaching maximal concentrations 1 to 2 h postadministration.26 This drug is freely soluble in water, and the reported bioavailability of losartan with a 50 mg tablet is 32.6%.26 Dose administration with meals slows the rate of absorption and reduces the area under the plasma concentration–time curve of losartan and its metabolite by approximately 10%.27 Food can have a significant effect on drug absorption through several mechanisms, including delay in gastric emptying, increases in splanchnic blood flow, and changes in gastrointestinal secretions. These effects can alter tablet disintegration, drug dissolution, and drug transit through the gastrointestinal tract. Given these effects, we decided to offer the vehicle–drug mixtures during the first few hours of the light phase to ensure that drug ingestion took place during a period with decreased food intake typically. The amount of vehicle used did not interfere with losartan bioavailability. In addition, only maximal concentrations were measured in the present study. Losartan has an extensive first-pass effect, with an oral bioavailability of 32%, indicating that transcellular intestinal absorption (P-glycoprotein) and biotransformation (CYP3A4) affect its bioavailability and lead to drug–nutrient interactions.26
The site for blood sampling can strongly influence several routinely tested parameters, including glucose, total cholesterol, triglycerides, and HDL-cholesterol, and highlights the need to standardize sampling sites, especially when repeated blood sampling is required.6 To avoid this experimental bias, we collected blood from the tail vein only on 3 different days for measurement of the blood glucose level and serum lipid profile. Accordingly, no differences were observed among groups of rats when basal levels (that is, prior to gavage or vehicle administration) were compared. Moreover, previous studies indicate no significant age-related changes in glycemia24 or lipid profiles31 are present during the time period of our study. Therefore, any changes observed in these parameters are due to the vehicles’ composition and not are age-related.
Analysis of the results suggested that NUT, PB and SUG are possible alternatives to gavage for chronic administration of losartan, given that their ingestion was not associated with weight gain or increased water ingestion and that all were consumed completely by the rats. However, after taking into account the glycemic and lipid profiles, SUG proved to be the most suitable vehicle for the administration of this antihypertensive drug for 2 main reasons. First, the mean plasma concentration of losartan was significantly higher than that achieved after gavage. Second, SUG did not induce any significant change in either glycemia or the lipid profile during the 28-d study. In contrast, the use of PB seems to be the least appropriate due to the associated changes in the rats’ lipid profile and the 22% reduction in the plasma concentration of losartan when compared with that achieved after gavage. Even though NUT decreased glucose and increased total cholesterol, the losartan plasma concentration in this group was 29% higher than that after gavage. These results suggest that SUG facilitates losartan absorption, leading to increased bioavailability.
In our opinion, the higher losartan concentrations attained in the SUG group, compared with gavage, might be explained by 3 different mechanisms: delayed absorption, increased absorption, or decreased biotransformation. In fact, the high content of sugar might increase the rate or extent of drug absorption given that sucrose fatty acid esters improve the intestinal absorption of poorly absorbable drugs via transcellular and paracellular pathways.33 In addition, a high carbohydrate content may decrease CYP450 activity, particularly when high doses of drug are administered.28
Substances that have an unpleasant odor or flavor could discourage voluntary ingestion and thus compromise the success of this method. Therefore, we suggest that pilot studies are warranted to ensure that all rats successfully ingest the drug–vehicle mixture.
The results presented in this report support that the use of voluntary oral administration is a viable alternative for chronic administration of a fixed dose of losartan. In fact, our results suggest that SUG is better suited than is gavage for oral daily administration of losartan. Furthermore, these data provide evidence that SUG is an optimal vehicle for losartan administration in rats and overcomes several shortcomings associated with gavage. SUG did not reduce the bioavailability of losartan, suggesting that this approach is an effective oral dosing method for the chronic administration of this drug to rats. This refinement might be useful in the safety evaluation of drugs, particularly for those that show stress-associated toxic effects.
Acknowledgments
We thank Isabel Ribeiro da Silva for her technical assistance. The present work was supported by Fundação para a Ciência e Tecnologia (FCT) grant (PTDC/SAU-TOX/ 112264/2009) and CEDOC (Portugal). Lucília N Diogo holds a FCT grant (SFRH / BD / 48335 / 2008).
The authors have no conflicts of interest to declare.
References
- 1.Abelson KS, Jacobsen KR, Sundbom R, Kalliokoski O, Hau J. 2012. Voluntary ingestion of nut paste for administration of buprenorphine in rats and mice. Lab Anim 46:349–351. [DOI] [PubMed] [Google Scholar]
- 2.Atcha Z, Rourke C, Neo AH, Goh CW, Lim JS, Aw CC, Browne ER, Pemberton DJ. 2010. Alternative method of oral dosing for rats. J Am Assoc Lab Anim Sci 49:335–343. [PMC free article] [PubMed] [Google Scholar]
- 3.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 4.Bonnichsen M, Dragsted M, Hansen AK. 2005. The welfare impact of gavaging laboratory rats. Anim Welf 14:223–227. [Google Scholar]
- 5.Brown AP, Dinger N, Levine BS. 2000. Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci 39:17–21. [PubMed] [Google Scholar]
- 6.Chan YK, Davis PF, Poppitt SD, Sun X, Greenhill NS, Krishnamurthi R, Przepiorski A, McGill AT, Krissansen GW. 2012. Influence of tail versus cardiac sampling on blood glucose and lipid profiles in mice. Lab Anim 46:142–147. [DOI] [PubMed] [Google Scholar]
- 7.Corbett A, McGowin A, Sieber S, Flannery T, Sibbitt B. 2012. A method for reliable voluntary oral administration of a fixed dosage (mg/kg) of chronic daily medication to rats. Lab Anim 46:318–324. [DOI] [PubMed] [Google Scholar]
- 8.Damsch S, Eichenbaum G, Tonelli A, Lammens L, Van den Bulck K, Feyen B, Vandenberghe J, Megens A, Knight E, Kelley M. 2010. Gavage-related reflux in rats: identification, pathogenesis, and toxicological implications. [(review)] Toxicol Pathol 39:348–360. [DOI] [PubMed] [Google Scholar]
- 9.Decree no. 113/2013 implementing EU Directive No. 2010/63 on animal protection for scientific purposes. [Internet] 2013. Diário da República, I Série, no. 151, 7 August 2013. [Cited 18 August 2015] Available at: http://faolex.fao.org/docs/pdf/por126282.pdf [Article in Portuguese]
- 10.Flecknell PA, Roughan JV, Stewart R. 1999. Use of oral buprenorphine (‘buprenorphine jello’) for postoperative analgesia in rats—a clinical trial. Lab Anim 33:169–174. [DOI] [PubMed] [Google Scholar]
- 11.Ganong WF. 2002. Handbook of medical physiology. EGC, Jakarta. [Google Scholar]
- 12.Goldkuhl R, Hau J, Abelson KS. 2010. Effects of voluntarily ingested buprenorphine on plasma corticosterone levels, body weight, water intake, and behaviour in permanently catheterised rats. In Vivo 24:131–135. [PubMed] [Google Scholar]
- 13.Huang-Brown KM, Guhad FA. 2002. Chocolate, an effective means of oral drug delivery in rats. Lab Anim (NY) 31:34–36. [DOI] [PubMed] [Google Scholar]
- 14.Isaksson IM, Theodorsson A, Theodorsson E, Strom JO. 2011. Methods for 17β-oestradiol administration to rats. Scand J Clin Lab Invest 71:583–592. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen KR, Kalliokoski O, Hau J, Abelson KSP. 2011. Voluntary ingestion of buprenorphine in mice. Anim Welf 20:591–596. [DOI] [PubMed] [Google Scholar]
- 16.Kalliokoski O, Abelson KS, Koch J, Boschian A, Thormose SF, Fauerby N, Rasmussen RS, Johansen FF, Hau J. 2010. The effect of voluntarily ingested buprenorphine on rats subjected to surgically induced global cerebral ischaemia. In Vivo 24:641–646. [PubMed] [Google Scholar]
- 17.Kalliokoski O, Jacobsen KR, Hau J, Abelson KS. 2011. Serum concentrations of buprenorphine after oral and parenteral administration in male mice. Vet J 187:251–254. [DOI] [PubMed] [Google Scholar]
- 18.Kramer K, Voss HP, Grimbergen JA, Mills PA, Huetteman D, Zwiers L, Brockway B. 2000. Telemetric monitoring of blood pressure in freely moving mice: a preliminary study. Lab Anim 34:272–280. [DOI] [PubMed] [Google Scholar]
- 19.Küster T, Zumkehr B, Hermann C, Theurillat R, Thormann W, Gottstein B, Hemphill A. 2012. Voluntary ingestion of antiparasitic drugs emulsified in honey represents an alternative to gavage in mice. J Am Assoc Lab Anim Sci 51:219–223. [PMC free article] [PubMed] [Google Scholar]
- 20.Morton DB, Jennings M, Buckwell A, Ewbank R, Godfrey C, Holgate B, Inglis I, James R, Page C, Sharman I, Verschoyle R, Westall L, Wilson AB, Joint Working Group on Refinement 2001. Refining procedures for the administration of substances. Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab Anim 35:1–41. [DOI] [PubMed] [Google Scholar]
- 21.Nicklas W, Baneux P, Boot R, Decelle T, Deeny AA, Fumanelli M, Illgen-Wilcke B, FELASA Working Group on Health Monitoring of Rodent And Rabbit Colonies 2002. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim 36:20–42. [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health Guide for the care and use of laboratory animals 1985. NIH publication 85–23. U.S. Department of Health, Education and Welfare.
- 23.Parasuraman S, Raveendran R, Kesavan R. 2010. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother 1:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro RT, Afonso RA, Guarino MP, Macedo MP. 2008. Loss of postprandial insulin sensitization during aging. J Gerontol A Biol Sci Med Sci 63:560–565. [DOI] [PubMed] [Google Scholar]
- 25.Sharp JL, Zammit TG, Azar TA, Lawson DM. 2002. Stress-like responses to common procedures in male rats housed alone or with other rats. Contemp Top Lab Anim Sci 41:8–14. [PubMed] [Google Scholar]
- 26.Sica DA, Gehr TW, Ghosh S. 2005. Clinical pharmacokinetics of losartan. Clin Pharmacokinet 44:797–814. [DOI] [PubMed] [Google Scholar]
- 27.Simpson KL, McClellan KJ. 2000. Losartan: a review of its use, with special focus on elderly patients. Drugs Aging 16:227–250. [DOI] [PubMed] [Google Scholar]
- 28.Sonawane BR, Coates PM, Yaffe SJ, Koldovsky O. 1983. Influence of dietary carbohydrates (alpha-saccharides) on hepatic drug metabolism in male rats. Drug and Nutr Interact 2:7–16. [PubMed] [Google Scholar]
- 29.The European Parliament and the Council of the European Union 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Official Journal of the European Union: L 276:33–79. [Google Scholar]
- 30.Turner PV, Vaughn E, Sunohara-Neilson J, Ovari J, Leri F. 2012. Oral gavage in rats: animal welfare evaluation. J Am Assoc Lab Anim Sci 51:25–30. [PMC free article] [PubMed] [Google Scholar]
- 31.Uchida K, Nomura Y, Kadowaki M, Takase H, Takano K, Takeuchi N. 1978. Age-related changes in cholesterol and bile acid metabolism in rats. J Lipid Res 19:544–552. [PubMed] [Google Scholar]
- 32.Walker MK, Boberg JR, Walsh MT, Wolf V, Trujillo A, Duke MS, Palme R, Felton LA. 2012. A less stressful alternative to oral gavage for pharmacological and toxicological studies in mice. Toxicol Appl Pharmacol 260:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto A, Katsumi H, Kusamori K, Sakane T. 2014. Improvement of intestinal absorption of poorly absorbable drugs by various sugar esters. Yakugaku Zasshi 134:47–53. [Article in Japanese] [DOI] [PubMed] [Google Scholar]
- 34.Yeung PK, Jamieson A, Smith GJ, Fice D, Pollak PT. 2000. Determination of plasma concentrations of losartan in patients by HPLC using solid phase extraction and UV detection. Int J Pharm 204:17–22. [DOI] [PubMed] [Google Scholar]