Abstract
To dissect the role of vascular endothelial growth factor receptor-2 (VEGFR2) in Müller cells and its effect on neuroprotection in diabetic retinopathy (DR), we disrupted VEGFR2 in mouse Müller glia and determined its effect on Müller cell survival, neuronal integrity, and trophic factor production in diabetic retinas. Diabetes was induced with streptozotocin. Retinal function was measured with electroretinography. Müller cell and neuronal densities were assessed with morphometric and immunohistochemical analyses. Loss of VEGFR2 caused a gradual reduction in Müller glial density, which reached to a significant level 10 months after the onset of diabetes. This observation was accompanied by an age-dependent decrease of scotopic and photopic electroretinography amplitudes and accelerated loss of rod and cone photoreceptors, ganglion cell layer cells, and inner nuclear layer neurons and by a significant reduction of retinal glial cell line–derived neurotrophic factor and brain-derived neurotrophic factor. Our results suggest that VEGFR2-mediated Müller cell survival is required for the viability of retinal neurons in diabetes. The genetically altered mice established in this study can be used as a diabetic animal model of nontoxin-induced Müller cell ablation, which will be useful for exploring the cellular mechanisms of neuronal alteration in DR.
Introduction
Diabetic retinopathy (DR) is a leading cause of blindness in working-aged populations in developed countries and is traditionally regarded as a disorder of blood-retina barriers (BRBs). However, it is becoming increasingly clear that changes in neuronal function and viability occur independently from BRB abnormalities in patients with diabetes and in diabetic animals (1–5). Unfortunately, the molecular and cellular mechanisms in channeling signals for the alteration and survival of retinal neurons in DR are very much understudied. Müller glia, the major macroglia and retinal-supporting cells, span the whole retina from the inner limiting membrane to the outer limiting membrane. This geographic arrangement is ideal for Müller glia to serve as a cellular regulator for physiological and pathological responses in the retinal vasculature and neurons and allows Müller glia to play many essential roles in retinal metabolism, functions, maintenance, and protection by providing trophic factors, removing metabolic wastes, controlling extracellular space volumes and ion and water homeostasis, participating visual cycles, releasing neurotransmitters, regulating BRB function, and modulating innate immunity (for review, see [6]).
Vascular endothelial growth factor (VEGF or VEGF-A) is a pathogenic factor that plays a cardinal role in choroidal neovascularization in age-related macular degeneration and retinal neovascularization and in BRB breakdown in retinopathy of prematurity (ROP) and DR (for review see [7]). To dissect the role of Müller cell–derived VEGF in DR and ROP, we recently disrupted Müller cell–derived VEGF conditionally and demonstrated an essential role for Müller cells as a central cellular target to induce retinal inflammation, neovascularization, and vascular leakage and lesion in DR and ROP-like diseases (8,9).
To our surprise, VEGF disruption in retinal Müller glia did not cause any detectable alteration in neuronal function and densities, which was opposite to what was predicted in a previous study (10). Because we recognized that VEGF is a secreted protein and a partial reduction of retinal VEGF without blocking signaling mediated by the VEGF receptor (VEGFR) might not affect the integrity of retinal neurons, we decided to disrupt the major VEGF receptor, VEGFR2, in Müller glia conditionally and to investigate the effect of blocking VEGFR2-mediated signaling in Müller cells on retinal integrity in diabetes. This report summarizes our investigation into the effect of VEGFR2-mediated signaling in retinal Müller cells on neuronal integrity in diabetic conditional Vegfr2 knockout (KO) mice.
Research Design and Methods
Preparation of Conditional Vegfr2 KO Mice
All animal procedures complied with The Association for Research in Vision and Ophthalmology's “Statement for the Use of Animals in Ophthalmic and Visual Research” and were approved by local institutional animal care and use committees. Conditional Vegfr2 KO mice were generated by mating Müller cell–expressing Cre mice with floxed Vegfr2 mice (11,12). PCR analysis of a tail biopsy specimen was performed to identify the cre gene (with primer pair: 5′-AGG TGT AGA GAA GGC ACTTAG C-3′ and 5′-CTA ATC GCC ATC TTCCAG CAG G-3′) and the Vegfr2 gene (with primer pair: 5′-GGG TGC CAT AGCCAA TCA AAG ACG C-3′ and 5′-TAT CGG TGT TCC CCT GGG TGT GTG G-3′). Cre-mediated recombination was done by doxycycline feeding (at a concentration of 0.5 mg/mL in 5% sucrose for a week) or by intravitreal delivery (4 μg in 1 μL of 1× PBS), as described previously (11,13,14).
Diabetes was induced by streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO), as described previously (9). Briefly, freshly made STZ (55 mg/kg body weight in 10 mmol/L citrate buffer [pH 4.5]) was injected intraperitoneally to 2-month-old mice, daily for 5 consecutive days. Age-matched controls were injected with an equal volume of citrate buffer only. Mice with a blood glucose concentration exceeding 300 mg/dL were considered as diabetic. Mice with a blood glucose concentration exceeding 500 mg/dL were administered insulin therapy.
Measurement of Retinal Function With Electroretinography
Retinal function was measured with electroretinography (ERG), as described previously (8,9). Briefly, pupils were dilated with 0.5% tropicamide before the animals were kept in the dark overnight. Dim near-infrared light was used for ERG operators to perform experiments in the dark room. The dark-adapted mice were anesthetized and placed on a heating pad to keep body temperature. The corneal surface was anesthetized with proparacaine hydrochloride 1%. Retinal function was measured using a ColorDome Espion ERG recording system (Diagnosys, Lowell, MA). Scotopic ERG was recorded using a series of flashes with increasing light intensities (from 0.0004 to 2,000 cd ⋅ s/m2). After the brightest flash, mice were light-adapted in a 50 cd/m2 background dome for 10 min. Photopic ERG was recorded with a 2,000 cd ⋅ s/m2 flash.
Primary Müller Cell Cultures
Primary Müller cells were prepared as described previously and cultured with DMEM containing 10% tetracycline system approved FBS, 2 mmol/L glutamine, and 0.1% penicillin/streptomycin (8,15). To assess high glucose–induced alteration, primary Müller cells were cultured separately in high-glucose (HG), medium-glucose (25 mmol/L), and low-glucose (LG) medium (5 mmol/L, supplemented with mannitol to balance osmosis pressure) for 72 h. The media were changed daily. Passage 5 cells were analyzed. Cultures were incubated in serum-free medium overnight before the analysis.
Antibodies, Immunoblotting, and Immunohistochemistry
Mouse monoclonal anti–β-actin antibody (#A5441) was purchased from Sigma-Aldrich. Rabbit polyclonal anti-VEGF antibody (#SC-152), rabbit polyclonal antibody against glial cell line–derived neurotrophic factor (GDNF; #SC-328), rabbit polyclonal antibody against brain-derived neurotrophic factor (BDNF, #SC-546), and horseradish peroxidase–linked anti-rabbit, -mouse, or -goat IgG secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-phosphorylated AKT (pAKT) antibody (#9271), rabbit polyclonal antibody against AKT (#9272), and rabbit monoclonal anti-VEGFR2 antibody (#2479) were purchased from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal anti–S-opsin (#AB5407) and M-opsin (#AB5405) antibodies and monoclonal antibody against glutamine synthetase (GS, #MAB302) were from Millipore (Billerica, MA).
Immunoblotting was performed as described previously (8,9). Immunohistochemistry (IHC) with primary Müller cells was performed similarly as described previously (8). For IHC with paraffin-embedded samples, sections were deparaffinized with xylene. Antigen retrieval was performed by heating sections at 95°C for 20 min in citrate buffer (pH 6.3). Autofluorescence was quenched by incubating sections with 3% H2O2 in PBS for 20 min. Sections were blocked in 5% albumin in PBS for 1 h and were then incubated with primary antibodies overnight. The sections were washed with PBS three times and were incubated with appropriate secondary antibodies for 2 h. The sections were then washed three times, mounted, and imaged. For IHC with frozen retinal sections, the procedure was similar except that the deparaffinizing and antigen-retrieval steps were omitted. The TUNEL assay was performed with an in situ cell death detection kit (Roche, Indianapolis, IN), according to the manufacturer’s instruction.
Quantification of Neuronal and Cell Density
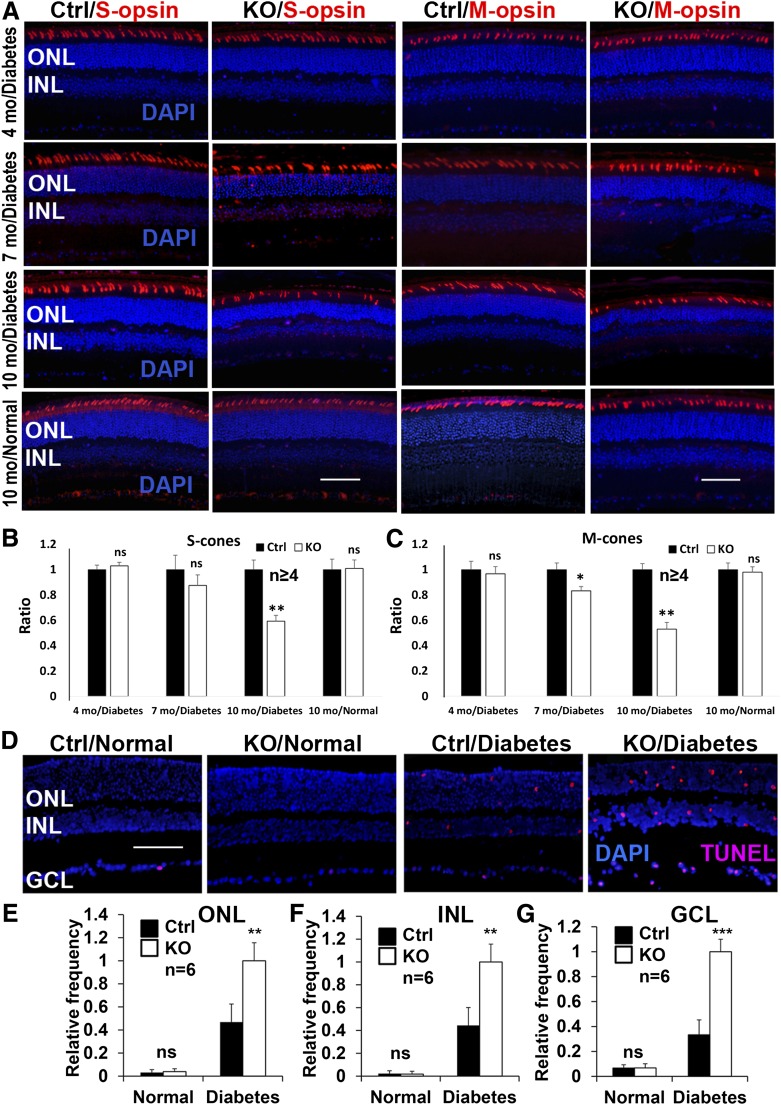
For retinal morphology, eyeballs were fixed at room temperature overnight in PerFix containing 4% paraformaldehyde, 20% isopropanol, 2% trichloroacetic acid, and 2% zinc chloride. The samples were kept in the 70% ethanol until paraffin embedding. Sections (5 μm thickness) were stained with hematoxylin and eosin (H&E) and examined under a light microscope. Outer nuclear layer (ONL) thickness was measured every 0.2 mm, as described previously (16). Inner nuclear layer (INL) thickness was determined by the average of eight distance points starting at 0.4 mm from the optic nerve head (ONH), as performed previously (9). For measurement of Müller cell, cone, ganglion cell layer (GCL), and TUNEL-positive cell density, the total number of positive cells in the retina was counted. The relative number of cells was determined by the ratio of total numbers versus the length of the retina. For IHC-stained Müller cells, those that demonstrated Müller glial-specific staining (line-like, white arrows in Fig. 1E and arrows in Fig. 2A) were considered positive.
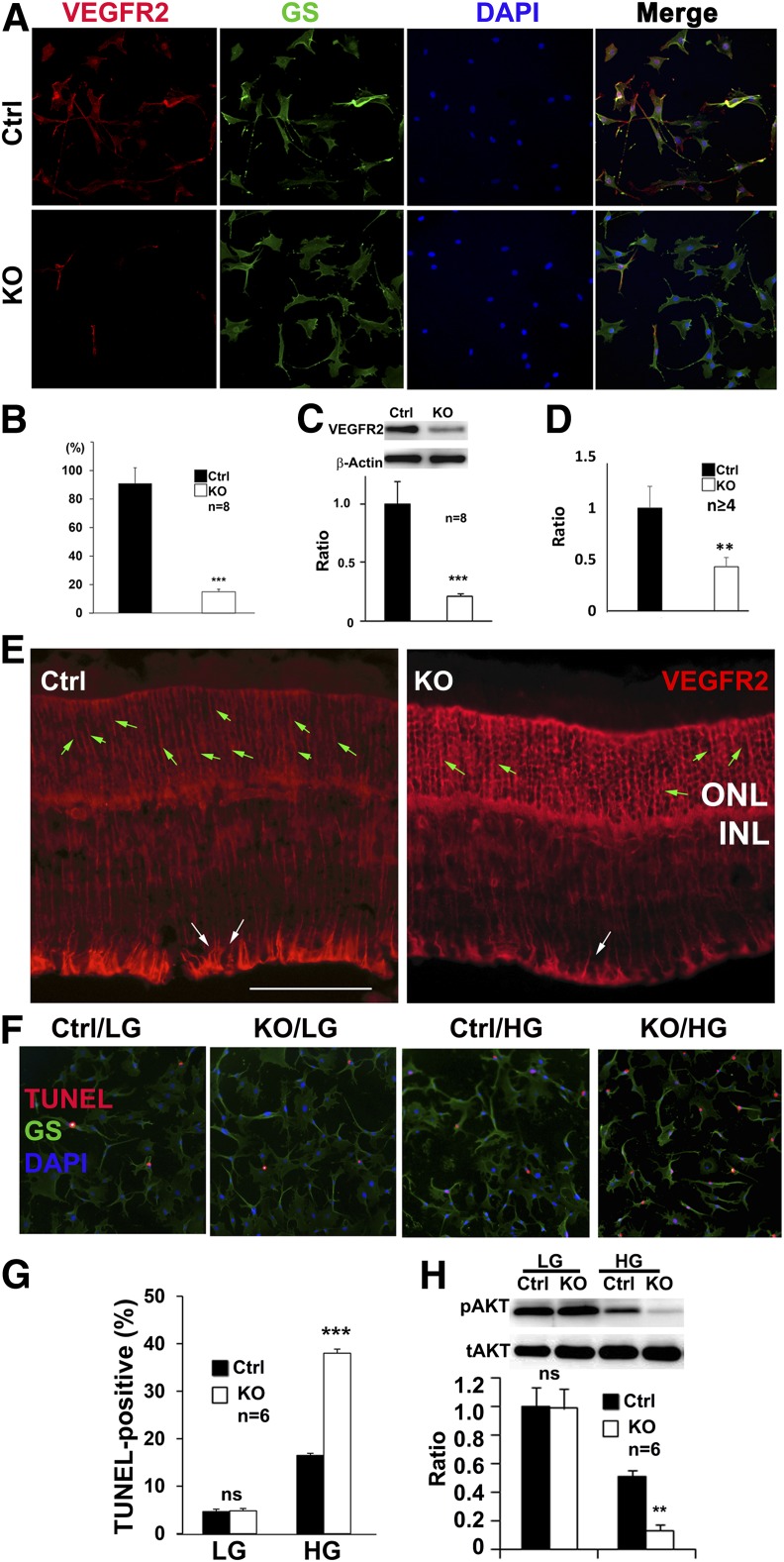
Figure 1.
Analysis of conditional Vegfr2 KO mice. Representative IHC images (A) and quantification (B) showing the loss of VEGFR2 staining in primary Müller cells of Vegfr2 KO (n = 8). Ctrl, control. C: Immunoblotting analysis showing reduced VEGFR2 expression in primary Vegfr2 KO Müller cells (n = 8). IHC images (E) and quantification (D) showing the reduction of VEGFR2-positive Müller cells in 1-month-old conditional Vegfr2 KO mice (n ≥ 4). Scale bar: 50 µm. Green arrows: VEGFR2-stained Müller glial cell body in ONL area. White arrows: area of VEGFR2-stained Müller cell body for quantification. The primary and secondary antibody controls showed no immunofluorescence (data not shown). Representative IHC images (F) and quantification (G) showing HG-elevated TUNEL-positive cells in primary Müller cells of Vegfr2 KO (n = 8). H: Immunoblotting analysis showing HG-accelerated reduction of pAKT in primary Müller cells with Vegfr2 KO (n = 6). tAKT, total AKT. Error bar: SD. ns, not significant. **P < 0.01; ***P < 0.001.
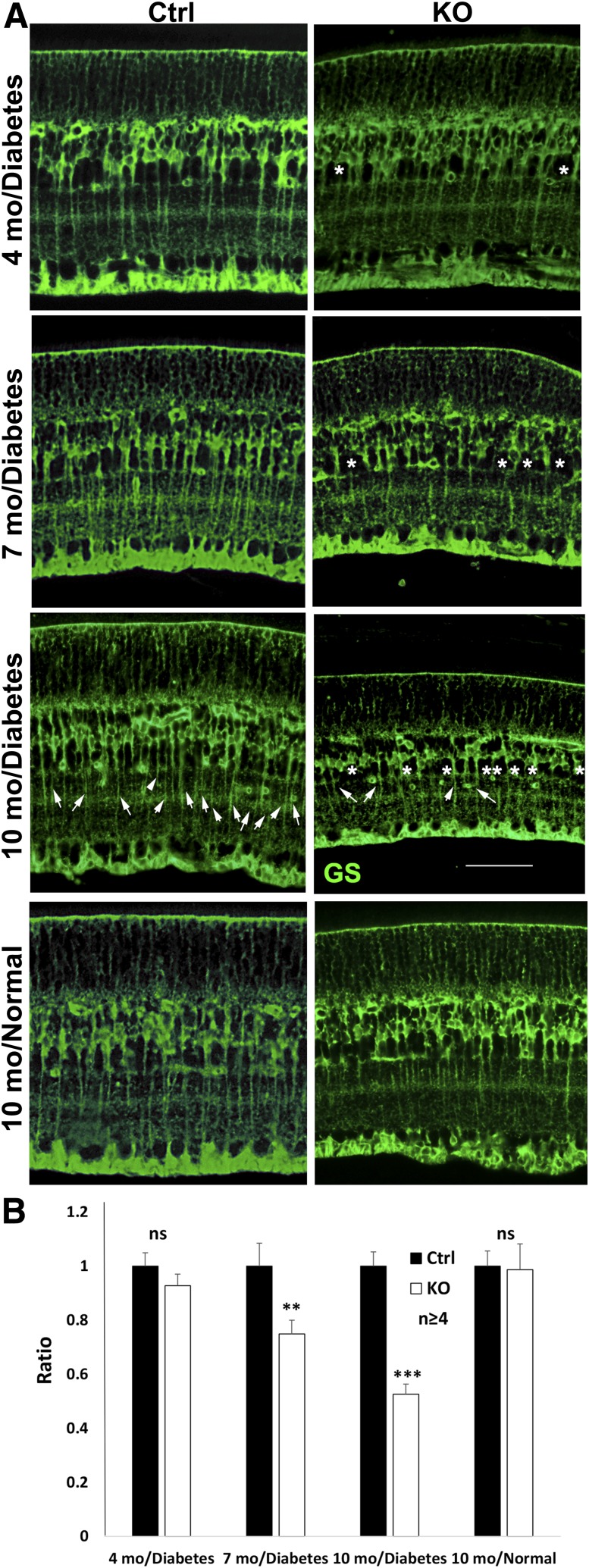
Figure 2.
Analysis of Müller cell density with IHC for GS in conditional Vegfr2 KO mice at 4, 7, and 10 months after the onset of diabetes. A: Representative images showing gradual loss of GS-positive cells and alteration of cell shapes (*) in Müller glia of diabetic conditional Vegfr2 KO mice. Arrows: Müller cell–specific GS staining for quantification. B: Statistical analysis with t test. Scale bar: 50 µm. n = 6 for 10 months in diabetes group, n ≥ 4 for other groups. Error bar: SD; Ctrl, control; mo, months; ns, not significant. **P < 0.01; ***P < 0.001.
Statistical Analysis
All data that required statistical analysis are expressed as mean ± SD. Pairwise statistical analysis was performed with the Student t test. A P value of <0.05 was considered statistically significant. For cell culture studies, at least three samples were used for each data point, and experiments were repeated at least once. For in vivo studies, at least six samples were used for each experimental point for critical data, and at least four samples were used for confirmatory data.
Results
Vegfr2 Disruption in Müller Cells
We previously generated a transgenic mouse expressing Cre recombinase in Müller cells, directed by the promoter of human vitelliform macular dystrophy-2. The Müller cell–specific expression pattern of this mouse line is probably a consequence of both vitelliform macular dystrophy promoter activity and transgene insertion control (11,17). These Cre mice were bred with mice carrying floxed Vegfr2 allele to generate conditional Vegfr2 KO mice (8). To determine the efficiency of Vegfr2 disruption in Müller glia, primary cell cultures were prepared from the conditional Vegfr2 KO mice and their littermate controls. The purity of the primary Müller cell cultures was examined by IHC using a Müller cell marker, GS. As shown in Fig. 1, almost all primary cells were GS-positive (10% were non–GS-positive cells). However, only 14% of the primary Müller cells derived from conditional Vegfr2 KO mice were VEGFR2-postive, indicating that 86% of the Müller cells had productive Cre-mediated recombination (Fig. 1A and B). This result is in agreement with an 87% reduction of VEGFR2 expression in primary Müller cells derived from the conditional Vegfr2 KO mice (cre+Vegfr2ff), compared with that of controls (cre+Vegfr2f+, Fig. 1C). Although slightly different, the data were in line with the loss of VEGFR2 staining in 63% of Müller cells in the conditional Vegfr2 KO mice (Fig. 1D and E, the calculation was based on the numbers indicated by the white arrows in panel E). Taken together, our data suggest that Vegfr2 was disrupted in most Müller cells in the conditional Vegfr2 KO mice. Interestingly, the loss VEGFR2 staining in Müller cells resulted in an increase in non-Müller cell–specific staining in ONL (Fig. 1D). Whether this observation is due to an IHC artifact or a reactive mechanism of photoreceptors in responding to VEGFR2 disruption in Müller glia is not clear.
Diabetes Induced the Loss of Retinal Müller Cells
To determine whether the disruption of VEGFR2 altered the survivability of Müller glia, we examined the density of Müller cells in conditional Vegfr2 KO mice. Under normal conditions, we did not observe any apparent alteration in Müller cell density in 1-year-old conditional Vegfr2 KO mice (Fig. 2). However, a gradual loss of Müller cell density in conditional Vegfr2 KO mice was observed ∼4 months after STZ injection. The Müller cell density was only 53% of that in the controls 10 months after the onset of diabetes (Fig. 2).
To dissect the mechanism(s) of VEGFR2 disruption-induced Müller cell loss in diabetes, we examined the degree of apoptosis and the expression level of the AKT survival signal in primary Müller cell cultures derived from conditional Vegfr2 KO mice. Although LG did not cause any apparent difference in the number of apoptotic cells (∼4% with no statistical significance between Vegfr2 KO cells and controls), HG elevated the number of TUNEL-positive cells to 38% in the primary Müller cells of Vegfr2 KO mice, which was more than twice as frequent as that in the controls (Fig. 1F and G).
We then examined the expression level of pAKT, an activated form of AKT survival signal, in primary Müller cells. As expected, we did not observe any apparent alteration in the expression of the pAKT survival signal in Vegfr2 KO cells grown in LG media compared with that of controls (Fig. 1H). Although HG media reduced the pAKT level to half of that in LG media in the control cells (Fig. 1H), HG significantly accelerated the reduction of the pAKT survival signal in the primary Müller cells with Vegfr2 KO, which was only 16% of that in LG media (Fig. 1H). Our data suggest that the loss of VEGFR2-mediated signaling resulted in a significant increase of apoptotic cells and an accelerated reduction of activated survival signals in primary Müller cells under diabetes-like conditions.
Retinal Morphology and Function in Conditional Vegfr2 KO Mice
To determine whether disruption of VEGFR2 in Müller cells affects retinal integrity, we examined retinal function with ERG and retinal morphology and neuronal density in retinal sections in conditional Vegfr2 KO mice. Scotopic and photopic ERG data indicated that there was no detectable difference between 1-year-old conditional Vegfr2 KO mice and their littermate controls under normal conditions (Fig. 3). Likewise, there was no detectable change in ONL thickness in the conditional KO mice compared with that in littermate controls under normal conditions (Fig. 4). The density of INL and GCL neurons was also unaltered under normal conditions (Fig. 4). These data indicate that disruption of VEGFR2 in Müller glia did not have an apparent effect on retinal neurons and their function under normal conditions.
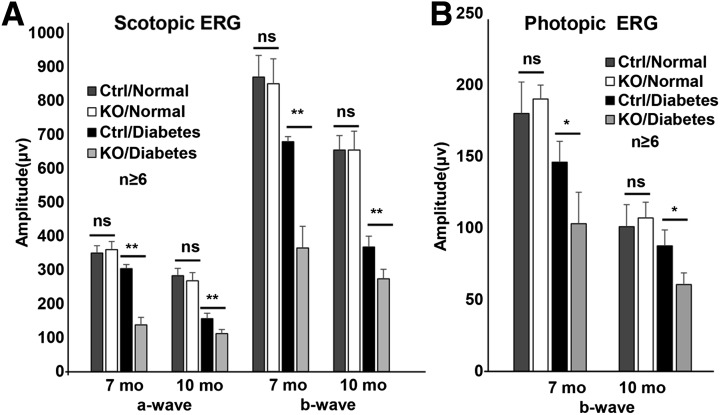
Figure 3.
Analysis of retinal function with scotopic (A) and photopic (B) ERG in conditional Vegfr2 KO mice 7 and 10 months after the onset of diabetes (n ≥ 6). Loss of VEGF caused an accelerated reduction of retinal function in diabetic conditional Vegfr2 KO mice. Error bar: SD. Ctrl, control; mo, month; ns, not significant. **P < 0.01; *P < 0.05.
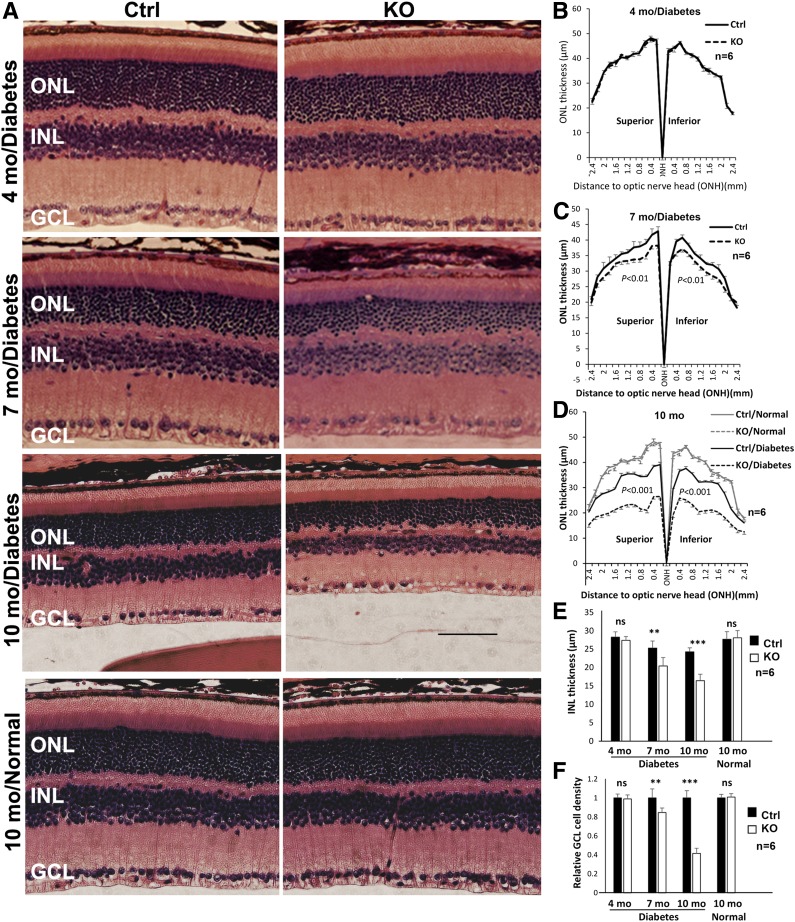
Figure 4.
Analysis of ONL and INL thickness and GCL nuclear density in H&E-stained retinal sections from diabetic conditional Vegfr2 KO mice. A: H&E-stained retinal sections. B–D: Morphometric analysis of ONL thickness (n = 6). E: Average INL thickness (n ≥ 4). F: Relative nuclear density in GCL (n ≥ 4). Loss of VEGFR2 in Müller cells significantly accelerated the loss of retinal neuron density in diabetic retinas. Error bar: SD. Ctrl, control; mo, month; ns, not significant. **P < 0.01; ***P < 0.001.
To evaluate whether VEGFR2-mediated signaling in Müller cells plays a role in neuronal integrity in DR, we induced diabetes in conditional Vegfr2 KO mice with STZ. Table 1 summarizes the average body weights and blood glucose levels in diabetic animals. As expected, there was a significant elevation of blood glucose and loss of body weight in diabetic animals. However, no detectable difference in the body weights and blood glucose levels was observed between conditional Vegfr2 KO mice and littermate controls (Table 1). Although there was no significant alteration of amplitudes of scotopic ERG a-wave and b-wave and photopic b-wave 4 months after STZ injection in diabetic conditional Vegfr2 KO mice (data not shown), they demonstrated an accelerated reduction in the amplitudes of scotopic ERG a-wave and b-wave and photopic b-wave at 7 and 10 months after STZ injection (at 2 months of age, Fig. 3). This result is coincided with a significant reduction in ONL thickness, S- and M-cone densities, and INL and GCL cell density in conditional Vegfr2 KO mice at 10 months after STZ injection (Figs. 4 and 5). Taken together, these data suggest that VEGFR2-mediated signaling in Müller cells was required for the maintenance of morphological and functional integrity of retinal neurons under diabetic conditions.
Table 1.
Average blood glucose levels and body weights in conditional Vegfr2 KO and control mice
| Animal group | Body weight (g) |
Blood glucose (mg/dL) |
||
|---|---|---|---|---|
| 4 months | 10 months | 4 months | 10 months | |
| Diabetic control | 22.67 ± 1.59* | 25.44 ± 1.34* | 399.22 ± 27.90* | 411.52 ± 23.15* |
| Diabetic KO | 22.30 ± 1.65* | 25.30 ± 1.18* | 407.52 ± 26.54* | 409.52 ± 24.43* |
| Nondiabetic control | 28.51 ± 1.42 | 32.81 ± 1.80 | 177.30 ± 12.90 | 173.56 ± 14.28 |
| Nondiabetic KO | 28.00 ± 1.74 | 32.22 ± 1.79 | 176.44 ± 12.78 | 174.15 ± 13.27 |
Time points represent months after STZ injection. Data are expressed as means ± SD (n = 20–30). No significance differences between KO and control animals were observed under any conditions.
*P < 0.001 in pairwise comparisons between diabetic and nondiabetic animals.
Figure 5.
IHC analysis of cone cell density and retinal apoptosis in diabetic conditional Vegfr2 KO mice. A–C: Representative IHC images (A) and statistical analysis (B and C) for diabetes-induced loss of S- and M-opsin–expressing cone cells in Vegfr2 KO mice (n = 6 for 10-month diabetes group; n ≥ 4 for other groups). Representative images (D) and analysis of diabetes-elevated TUNEL-positive cells in ONL (E), INL (F), and GCL (G) in conditional Vegfr2 KO mice 4 months after STZ injection (n = 6). Error bar: SD. Ctrl, control; mo, month; ns, not significant. *P < 0.05; **P < 0.01; ***P < 0.001.
Neuroprotective Mechanism(s) of VEGFR2-Mediated Signaling in Müller Glia in DR
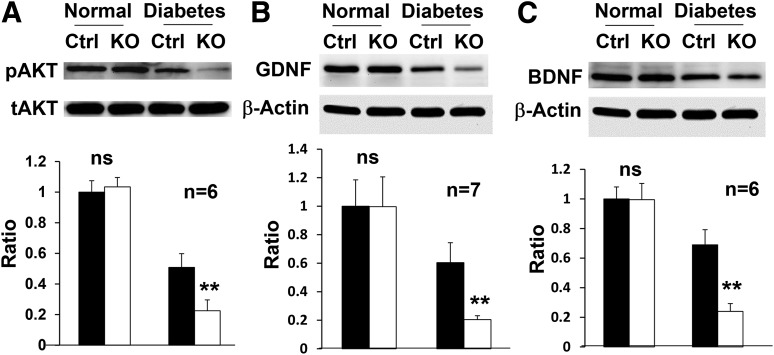
To determine the neuroprotective mechanism(s) of VEGFR2-mediated signaling in Müller glia in DR, we examined the degree of apoptosis in the retinas of conditional Vegfr2 KO mice. Under normal conditions, there was no significant alteration in the number of apoptotic cells in the retina between conditional Vegfr2 KO mice and controls (Fig. 5). However, loss of VEGFR2-mediated signaling in Müller cells significantly elevated diabetes-induced neuronal apoptosis 4 months after the STZ injection (Fig. 5). In addition, the level of activated cellular survival signal, pAKT, was only 44.3% of that of controls in the conditional Vegfr2 KO mice at 4 months after the onset of diabetes (Fig. 6A), which was less than 25% of that under normal conditions. These data suggest that the retinal neuronal loss in diabetic conditional Vegfr2 KO mice was likely a consequence of accelerated reduction of pAKT survival signals in retinal neurons, which triggered apoptosis and caused elevated retinal degeneration.
Figure 6.
Expression of survival and trophic factors in the retina of conditional Vegfr2 KO mice (n ≥ 6). Loss of VEGFR2 in Müller cells significantly exacerbated the reduction of pAKT (A) 4 months after STZ injection and GDNF (B) and BDNF (C) 10 months after the onset of diabetes in the retina of conditional Vegfr2 KO mice. tAKT, total AKT. Error bar: SD. Ctrl, control; ns, not significant. **P < 0.01.
To further delineate the neuroprotective mechanism(s) of VEGFR2-mediated signaling in DR, we compared the expression of trophic factors, particularly those that originate from Müller glia. Although there was no apparent alteration in the expression of ciliary neurotrophic factor, IGF-1, and erythropoietin (data not shown), the diabetic conditional Vegfr2 KO mice demonstrated an additional twofold loss of GDNF and BDNF (Fig. 6B and C), compared with controls, 4 months after the onset of diabetes. These results suggest that the depletion of Müller glial–derived trophic factors might play a role in causing neuronal degeneration in diabetic conditional Vegfr2 KO mice.
Discussion
VEGF is a major pathogenic factor for BRB diseases such as ROP and DR (7). However, VEGF signaling in neuronal integrity in DR has not been investigated seriously. Previous studies by others and us suggest that Müller glia are a potential cellular source of VEGF for retinal inflammation, neovascularization, vascular leakage, and vascular lesions in DR (8,9,18). Although we did not find any apparent role for Müller glia–derived VEGF in retinal integrity in our previous studies, the animal model was not designed to address whether VEGF signaling in Müller glia was required for their own survival and neuronal integrity in DR due to its inability to remove VEGF completely in the retina and to block VEGFR-mediated signaling in Müller cells in the animals. To address whether VEGF signaling is required for the survival of Müller glia and to determine the significance of VEGF signaling in the integrity of Müller glia and retinal neurons in DR, we disrupted VEGFR2, a major VEGF receptor, in the mouse Müller glia. Our data did not identify an apparent role for VEGFR2 under normal conditions; however, the loss of VEGFR2-mediated signaling in Müller cells caused a significant elevation of apoptotic Müller cells and a reduction of GS-positive staining cells in diabetes. A most likely scenario of this observation is that the loss of VEGFR2 induces a dramatic reduction of Müller cell density in diabetic conditions. This observation is supported by the previous observation that chronically elevated glucose contributes to Müller cell apoptosis (19). Although the VEGFR2-AKT pathway for Müller cell survival in DR has not been explored extensively, the receptor-mediated AKT survival pathway is commonly acknowledged in other stress-related settings.
Our findings support a significant role of the VEGFR2-AKT pathway in Müller cell survival. Our data also suggest that VEGFR2-mediated activation of AKT survival signals may be a major protective mechanism for retinal Müller glia under pathological conditions such as DR. Our results, together with the observation of retinal INL and ONL thinning in a rat ROP model of VEGF164 knockdown in Müller glia (20), support the notion that VEGF signaling in Müller cells may represent a stress-responsive neuroprotective mechanism, particularly for hypoxic conditions. This notion is in agreement with a previous study showing that VEGF signaling through VEGFR2 in Müller cells is essential to their own survival (10).
Müller glia span from the vitreal surface to subretinal space and cover all retinal layers, which is ideal for them to serve as major supporting cells by providing trophic factors in the retina (6). Because the loss of VEGFR2 signaling in Müller glia eventually leads to the loss of retinal neurons in diabetic conditional Vegfr2 KO mice, it is most likely that VEGFR2 signaling–mediated Müller glial self-protection may be a cellular mechanism to protect retinal neurons under pathological conditions such as in DR. Interestingly, among several trophic factors examined, we identified a significant reduction of BDNF and GDNF, two important neurotrophins, in diabetic conditional Vegfr2 KO mice, indicating that BDNF and GDNF cannot be compensated in our animal model and that neurotrophins may play a role in Müller glial self-protection or in supporting neuron survival in diabetic retinas. Müller glia produce BDNF and GDNF as trophic factors that are capable of protecting photoreceptors, ganglion neurons, and perhaps other neurons under stress conditions (21–26). Müller glia are considered as a source of BDNF and GDNF that may also have important functions for their own (27–30). Thus, we are not in a position to conclude whether the reduction of BDNF and GDNF plays a direct or indirect role (or both) in the loss of neuronal integrity in diabetic conditional Vegfr2 KO mice. However, at least two possibilities account for the reduction of BDNF and GDNF in our animal model. The reduction may be a direct consequence of Müller cell loss. Alternatively, VEGFR2-mediated signaling in Müller cells may stimulate the production of these trophic factors directly or indirectly. We are actively investigating these possibilities.
As a result of our experiment design, it is also possible that we might not identify all Müller glia–derived trophic factors required for neuroprotection in diabetic conditional Vegfr2 KO mice. Nevertheless, our data unequivocally suggest that VEGFR2-mediated signaling in Müller glia is a major survival mechanism for retinal neurons under pathological conditions such as in DR. One speculation might be perhaps that an initial role for VEGF upregulation in diabetic retinas is to protect retinal neurons. As a consequence, the uncontrollable VEGF upregulation in diabetic retinas leads to BRB breakdown and other pathological changes in DR.
In summary, we revealed a significant role for VEGFR2-mediated signaling in Müller glia under diabetic conditions, which, in turn, provides neuroprotection probably through trophic factor release directly or indirectly or perhaps other essential glial functions in DR-like disease. In addition, the conditional Vegfr2 KO mice established in this study can be used as a nontoxic model for diabetes-induced Müller cell ablation, which will be useful for exploring the cellular mechanisms of neuronal alteration in DR.
Article Information
Acknowledgments. The authors thank Dr. Gennadiy P. Moiseyev, of The University of Oklahoma, for critical reading of the manuscript, and the animal facilities at The University of Oklahoma Health Sciences Center and Dean McGee Eye Institute for mouse care.
Funding. The study received support from NIH grants GM104934, EY020900, and EY021725 (National Eye Institute Core), research grants from the Oklahoma Center for Adult Stem Cell Research and the Presbyterian Health Foundation, and an endowment from the Choctaw Nation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.F. designed and performed experiments, participated in data analysis, and wrote the draft manuscript. S.D. and M.Z. designed and performed experiments. D.M.S. guided IHC and microscopic experiments. C.W. participated in discussion. Z.Y. contributed to the revision. J.J.H. provided a new reagent and advice for the use of the reagent. Y.-Z.L. designed the study, participated in data analysis, and wrote the manuscript. Y.-Z.L. is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci 2004;45:3330–3336 [DOI] [PubMed] [Google Scholar]
- 2.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Nakamura M. New insights into the pathophysiology of diabetic retinopathy: potential cell-specific therapeutic targets. Diabetes Technol Ther 2000;2:601–608 [DOI] [PubMed] [Google Scholar]
- 3.Terasaki H, Hirose H, Miyake Y. S-cone pathway sensitivity in diabetes measured with threshold versus intensity curves on flashed backgrounds. Invest Ophthalmol Vis Sci 1996;37:680–684 [PubMed] [Google Scholar]
- 4.Aspinall PA. Rod-cone interaction: some indirect evidence. Acta Ophthalmol (Copenh) 1977;55:294–302 [DOI] [PubMed] [Google Scholar]
- 5.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 1998;102:783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichenbach A, Bringmann A. New functions of Müller cells. Glia 2013;61:651–678 [DOI] [PubMed] [Google Scholar]
- 7.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res 2008;27:331–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai Y, Ma JX, Guo J, et al. Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol 2009;219:446–454 [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 2010;59:2297–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saint-Geniez M, Maharaj AS, Walshe TE, et al. Endogenous VEGF is required for visual function: evidence for a survival role on Müller cells and photoreceptors. PLoS One 2008;3:e3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueki Y, Ash JD, Zhu M, Zheng L, Le YZ. Expression of Cre recombinase in retinal Müller cells. Vision Res 2009;49:615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haigh JJ, Morelli PI, Gerhardt H, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol 2003;262:225–241 [DOI] [PubMed] [Google Scholar]
- 13.Fu S, Zhu M, Wang C, Le YZ. Efficient induction of productive Cre-mediated recombination in retinal pigment epithelium. Mol Vis 2014;20:480–487 [PMC free article] [PubMed] [Google Scholar]
- 14.Fu S, Zhu M, Ash JD, Wang Y, Le YZ. Investigating the role of retinal Müller cells with approaches in genetics and cell biology. Adv Exp Med Biol 2014;801:401–405 [DOI] [PubMed] [Google Scholar]
- 15.Umapathy NS, Li W, Mysona BA, Smith SB, Ganapathy V. Expression and function of glutamine transporters SN1 (SNAT3) and SN2 (SNAT5) in retinal Müller cells. Invest Ophthalmol Vis Sci 2005;46:3980–3987 [DOI] [PubMed] [Google Scholar]
- 16.Zheng L, Anderson RE, Agbaga MP, Rucker EB 3rd, Le YZ. Loss of BCL-XL in rod photoreceptors: Increased susceptibility to bright light stress. Invest Ophthalmol Vis Sci 2006;47:5583–5589 [DOI] [PubMed] [Google Scholar]
- 17.Zhu M, Zheng L, Ueki Y, Ash JD, Le YZ. Unexpected transcriptional activity of the human VMD2 promoter in retinal development. Adv Exp Med Biol 2010;664:211–216 [DOI] [PubMed] [Google Scholar]
- 18.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A 1995;92:905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xi X, Gao L, Hatala DA, et al. Chronically elevated glucose-induced apoptosis is mediated by inactivation of Akt in cultured Müller cells. Biochem Biophys Res Commun 2005;326:548–553 [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Wang H, Culp D, et al. Targeting Müller cell-derived VEGF164 to reduce intravitreal neovascularization in the rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 2014;55:824–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A 1992;89:11249–11253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JE, Barde YA, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci 1986;6:3031–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmeer C, Straten G, Kügler S, Gravel C, Bähr M, Isenmann S. Dose-dependent rescue of axotomized rat retinal ganglion cells by adenovirus-mediated expression of glial cell-line derived neurotrophic factor in vivo. Eur J Neurosci 2002;15:637–643 [DOI] [PubMed] [Google Scholar]
- 24.Politi LE, Rotstein NP, Carri NG. Effect of GDNF on neuroblast proliferation and photoreceptor survival: additive protection with docosahexaenoic acid. Invest Ophthalmol Vis Sci 2001;42:3008–3015 [PubMed] [Google Scholar]
- 25.Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Müller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A 1998;95:3978–3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harada C, Harada T, Quah HM, et al. Potential role of glial cell line-derived neurotrophic factor receptors in Müller glial cells during light-induced retinal degeneration. Neuroscience 2003;122:229–235 [DOI] [PubMed] [Google Scholar]
- 27.Dai M, Xia XB, Xiong SQ. BDNF regulates GLAST and glutamine synthetase in mouse retinal Müller cells. J Cell Physiol 2012;227:596–603 [DOI] [PubMed] [Google Scholar]
- 28.Saito T, Abe T, Wakusawa R, et al. TrkB-T1 receptors on Muller cells play critical role in brain-derived neurotrophic factor-mediated photoreceptor protection against phototoxicity. Curr Eye Res 2009;34:580–588 [DOI] [PubMed] [Google Scholar]
- 29.Seki M, Tanaka T, Sakai Y, et al. Müller cells as a source of brain-derived neurotrophic factor in the retina: noradrenaline upregulates brain-derived neurotrophic factor levels in cultured rat Müller cells. Neurochem Res 2005;30:1163–1170 [DOI] [PubMed] [Google Scholar]
- 30.Politi L, Rotstein N, Carri N. Effects of docosahexaenoic acid on retinal development: cellular and molecular aspects. Lipids 2001;36:927–935 [DOI] [PubMed] [Google Scholar]