Abstract
The objective of this study was to determine whether the sodium-glucose transporter SGLT1 in the ventromedial hypothalamus (VMH) plays a role in glucose sensing and in regulating the counterregulatory response to hypoglycemia, and if so, whether knockdown of in the VMH can improve counterregulatory responses to hypoglycemia in diabetic rats or rats exposed to recurrent bouts of hypoglycemia (RH). Normal Sprague-Dawley rats as well as RH or streptozotocin (STZ)-diabetic rats received bilateral VMH microinjections of an adenoassociated viral vector containing either the SGLT1 short hairpin RNA (shRNA) or a scrambled RNA sequence. Subsequently, these rats underwent a hypoglycemic clamp to assess hormone responses. In a subgroup of rats, glucose kinetics was determined using tritiated glucose. The shRNA reduced VMH SGLT1 expression by 53% in nondiabetic rats, and this augmented glucagon and epinephrine responses and hepatic glucose production during hypoglycemia. Similarly, SGLT1 knockdown improved the glucagon and epinephrine responses in RH rats and restored the impaired epinephrine response to hypoglycemia in STZ-diabetic animals. These findings suggest that SGLT1 in the VMH plays a significant role in the detection and activation of counterregulatory responses to hypoglycemia. Inhibition of SGLT1 may offer a potential therapeutic target to diminish the risk of hypoglycemia in diabetes.
Introduction
The mechanisms the brain uses to detect and activate counterregulatory hormone responses to hypoglycemia have yet to be fully elucidated. In the brain, the dominant sensors appear to be located in the ventromedial hypothalamus (VMH) (1–3), and it has been proposed that some neurons in the VMH may use a sensing mechanism similar to that in pancreatic β-cells in that the uptake and oxidation of glucose determine their rate of firing (4). Glucose can enter neurons through a number of different transporters, including GLUTs and sodium-dependent glucose cotransporters (sodium-d-glucose cotransporters [SGLTs]). The latter operate by cotransporting glucose and sodium into the cell along a sodium gradient created by the actions of a Na+-K+ ATPase. Although the role of GLUTs in glucose sensing has been more widely studied, the role of SGLTs has received less attention.
The high-affinity SGLT1 is highly expressed in the intestinal mucosa of the small intestine, the proximal tubules of the kidneys, and the brain (5–8). The possibility that SGLTs may be involved in glucose sensing was first supported by studies showing that inhibition of SGLTs in the brain with intracerebroventricular delivery of phloridzin enhance food intake (9) and inhibit glucose-induced excitation of glucose-excited (GE) neurons in the VMH (4). Hence, in addition to the more widely theorized KATP-dependent mechanism of glucose sensing, it has been postulated that SGLTs may serve as a metabolism-independent mechanism that regulates the activity of brain glucose-sensing neurons because the entry of sodium can sufficiently depolarize the neuron without the need for the metabolism of glucose (10).
In the adult rat, it has been shown that SGLT1, 3a, and 3b are expressed in the hypothalamus and that these transporters may play a role in glucose sensing within hypothalamic GE neurons (11). In the current study, we focused on the 70–75 kDa high-affinity isoform SGLT1, the importance of which in hypoglycemic glucose counterregulation has yet to be explored. The objective of this study was to determine whether SGLT1 in the VMH plays a role in glucose sensing and specifically in regulating the counterregulatory response to insulin-induced hypoglycemia. To address this question, an adenoassociated virus (AAV) expressing a short hairpin RNA (shRNA) against SGLT1 was used to locally reduce SGLT1 expression in the VMH of nondiabetic and diabetic rats and to determine its impact on the response to a standardized hypoglycemic stimulus.
Research Design and Methods
Nine- to 11-week-old, male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 280–330 g were housed in the Yale Animal Resources Center in temperature- and humidity-controlled rooms on a 12-h light-dark cycle (lights on between 0700 and 1900 h). The animals had free access to water and a standard rodent chow diet (Prolab 3000; Agway) and were acclimatized to handling for 1 week before experimental manipulation. The animal care and experimental protocols were reviewed and approved by the Yale Institutional Animal Care and Use Committee.
shRNA Vectors
We validated the knockdown efficiency of a number of candidate shRNA plasmids in INS-1 cells inasmuch as the SGLT1 expressed in this cell line is similar to what is expressed in the VMH. We determined that the shRNA with sequence 5′-ACCTGAAAGTGCTTCCTCTTT-3′ had the greatest knockdown efficiency of SGLT1 (∼52%, P = 0.01) (data not shown). This shRNA was then commercially cloned into the rAAV2-U6-SGLT1-terminator-CAG-EGFP-WPRE-BGH-polyA vector (GeneDetect, Auckland, NZ). A control plasmid containing a scrambled RNA sequence, 5′-GGAATCTCATTCGATGCATAC-3′, was used as a negative control. The viruses were bilaterally microinjected into the VMH, and subsequently, vascular catheter and brain guide cannulas were implanted as described previously (12). The animals were then stratified into one of the following studies.
VMH SGLT1 Expression
Nondiabetic, hypoglycemia-naive control (n = 3), recurrently hypoglycemic (RH) (n = 3), and streptozotocin (STZ)-induced diabetic (n = 4) rats were sacrificed without undergoing a clamp procedure. The brains were rapidly removed and frozen. Frozen micropunches were then taken through the VMH, and SGLT1 mRNA was quantified in these samples as described below.
Acute Hypoglycemia Studies
This study assessed whether knockdown of SGLT1 in the VMH affects the counterregulatory response to an acute bout of hypoglycemia in nondiabetic, hypoglycemia-naive animals. Two weeks after AAV inoculation, both groups of animals, one receiving the scrambled RNA AAV and the other the SGLT1 shRNA AAV, were fasted overnight and on the following day, underwent hyperinsulinemic-hypoglycemic clamp (10 mU/kg/min) with or without a tritiated glucose infusion to monitor glucose kinetics. After collection of baseline blood samples, plasma glucose levels were lowered to ∼50 mg/dL and maintained there for ∼90 min. For the tracer study, a primed continuous infusion of [3H]glucose (0.105 μCi/min) was initiated 150 min before the clamp procedure, and then the tracer was infused at 0.120 μCi/min for the rest of the clamp. The change in the [3H]glucose infusion rate (GIR) was based on the changes in tritiated glucose activity observed in pilot experiments of identical design. Throughout the study, blood samples were collected at 30-min intervals to assess plasma glucagon and insulin concentrations and every 10 min for glucose tracer measurements (13). At the end of the study, the animals were sacrificed and the brains rapidly removed and frozen for analysis of SGLT1 mRNA expression.
RH Studies
Nondiabetic Sprague-Dawley rats that received either the scrambled or the SGLT1 shRNA AAV were used for this study. The RH regimen was initiated 10 days after surgery. This regimen involved giving an intraperitoneal injection of human regular insulin 10 units/kg (Eli Lilly, Indianapolis, IN) once daily for 3 consecutive days. Food was removed during this time, and plasma glucose levels were allowed to drop to ∼30–40 mg/dL for ∼2 h. Blood glucose was monitored from a tail nick every 30 min using the AlphaTRAK 2 glucometer (Abbott Laboratories, North Chicago, IL). After the third bout of hypoglycemia and once euglycemia was restored, the RH animals were fasted overnight. On the 4th day, the mice underwent a hyperinsulinemic-hypoglycemic clamp (10 mU/kg/min) during which plasma glucose was lowered to ∼50 mg/dL using a variable 20% dextrose infusion for 90 min. The brains were collected at the end of the study to validate the knockdown through quantification of SGLT1 mRNA levels in VMH micropunches.
Diabetes Study
Following inoculation with either the scrambled or the SGLT1 shRNA AAV, diabetes was induced using a single intraperitoneal injection of STZ 65 mg/kg in saline. Diabetic rats were not insulin treated, and plasma glucose concentrations in these animals averaged 300–400 mg/dL. Three weeks later, the animals underwent a hypoglycemic clamp study. For this purpose, a constant insulin infusion was given through a venous catheter at a rate of 50 mU/kg/min together with a variable 20% dextrose infusion. Plasma glucose levels in the diabetic animals before study onset (∼400 mg/dL) were first lowered to match the euglycemic levels observed in nondiabetic controls. Euglycemia was then maintained for 30 min, and subsequently, plasma glucose was lowered and maintained at ∼50 mg/dL for 90 min. At the end of the study, the brains were collected for quantification of SGLT1 mRNA expression to validate knockdown.
Plasma Glucose, Hormone, and Catecholamine Measurements
During the clamp studies, plasma glucose was measured at 10-min intervals using the glucose oxidase method (Analox Instruments, Lunenburg, MA). Blood samples for plasma hormones during the clamp were analyzed for insulin and glucagon using commercially available radioimmunoassay kits (Linco Research, St. Charles, MO). Plasma epinephrine and norepinephrine concentrations were assessed by high-performance liquid chromatography using electrochemical detection (Thermo Fisher Scientific, San Jose, CA). The tracer assay was performed as described previously (13).
SGLT1 mRNA Analysis
mRNA levels were evaluated with a brilliant SYBR green quantitative RT-PCR master mix kit (Bio-Rad). Total cellular RNA was isolated from frozen VMH micropunches using the RNeasy Plus Mini Kit (QIAGEN) according to the manufacturer’s instructions. The forward primer sequence for rat SGLT1 was 5′-GCCTACGGAACTGGAAGCTG-3′, and the reverse primer sequence was 5′-GACGGTGACGACGCTGATAG-3′. Quantitative RT-PCR reactions were made by combining 12.5 μL 2× SYBR RT-PCR Master Mix, 0.5 μL upstream primer (10 μmol/L), 0.5 μL downstream primer (10 μmol/L), 0.0625 μL StrataScript RT/RNase block enzyme mixture, 1.4375 μL RNase-free water, and 10 μL (20 ng/μL) RNA template. The quantitative RT-PCR was performed using a PCR Express thermal cycler (Hybaid Ltd., Ashford, Middlesex, U.K.) in which the mixture was heated to 65°C for 6.5 min for reverse transcription, heated to 25°C for 10 min and then to 50°C for 1 h and 85°C for 5 min, and cooled to 37°C for 30 min. A real-time PCR reaction was performed using the MYiQ2 Real-Time PCR Detection System (Bio-Rad) in which the mixture was heated to 95°C for 10 min and then cycled 45 times at 95°C for 15 s and 60°C for 1 min. To verify the specificity of the amplification reaction, melting curve analysis was performed. The size of the amplified product was confirmed by electrophoresis. The level of rat β-actin mRNA was determined in all of the samples, and the expression of SGLT1 was normalized to rat β-actin. The threshold cycle value was taken as the fractional cycle number at which the emitted fluorescence of the sample passes a fixed threshold above the baseline. The 2ΔΔCT method was used to calculate the relative differences between experimental and control groups as a fold change in gene expression (14). All reactions were run in a minimum of two independent assays.
Immunohistochemistry for SGLT1
Nondiabetic, hypoglycemia-naive rats (n = 4) were deeply anesthetized using sodium pentobarbital, the heart was exposed, and the animals perfused with PBS followed by 10% neutral-buffered formalin through the left ventricle. The brains were then removed and immersed in 30% sucrose in PBS for 18–36 h at 4°C. VMH sections (30 μm) were taken through the brain by means of a cryostat and stored at −20°C in cryoprotectant until histochemical processing (15). The immunohistochemistry protocol used in the current study was modified from previous studies (16). Briefly, after blocking, free-floating sections were incubated overnight with rabbit anti-SGLT1 (Cell Signaling) and rabbit anti-GFAP antibody (Abcam) or with anti-NeuN (Abcam) in fresh donkey serum. The following day, the sections were washed and incubated in biotinylated donkey anti-rabbit antibody for 1 h. The sections were washed again and incubated with a cocktail of Alexa Fluor 594–conjugated streptavidin and Alexa Fluor 488–conjugated antiserum. Tissues were mounted onto glass slides and imaged using an Olympus BX-50 fluorescence microscope. To assess the antibody specificity of the rabbit anti-SGLT1 used for these experiments, an SGLT1-specific blocking peptide was used (Cell Signaling).
Data Analysis
All data are expressed as mean ± SEM and were statistically analyzed using either Student t test or repeated-measures ANOVA followed by post hoc analysis using Prism 6.0 (GraphPad Software, San Diego, CA). Differences were considered significant at P < 0.05.
Results
SGLT1 Expression
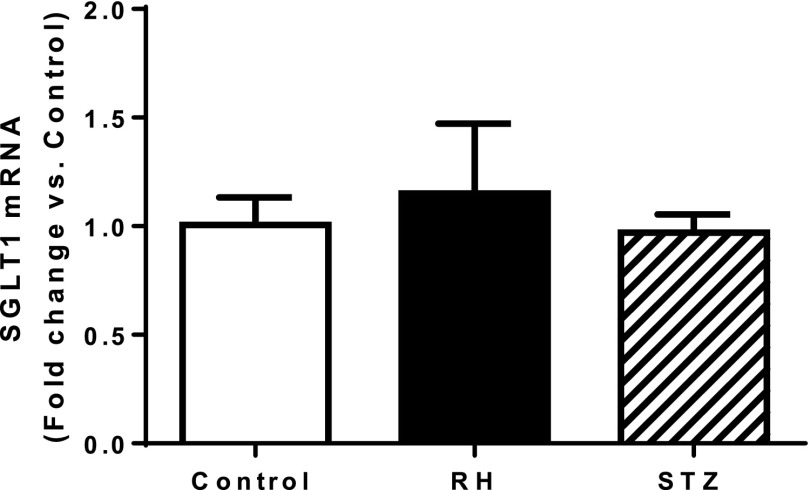
No significant differences in SGLT1 mRNA expression in the VMH were detected in control, RH, and STZ-diabetic animals (Fig. 1).
Figure 1.
Relative change in SGLT1 mRNA expression in the VMH of RH and STZ-diabetic rats compared with nondiabetic, hypoglycemia-naive control rats. Data are presented as mean ± SEM.
Acute Hypoglycemia Studies
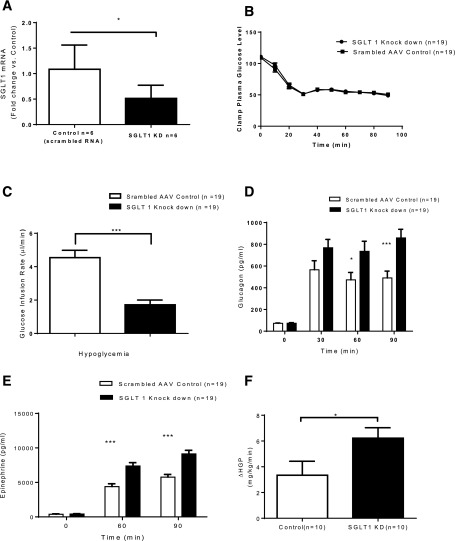
In nondiabetic, hypoglycemia-naive rats, the SGLT1 shRNA reduced VMH SGLT1 mRNA expression by 53% (P < 0.03) (Fig. 2A) compared with the scrambled RNA AAV controls. During the hypoglycemic clamp, plasma glucose and insulin levels were indistinguishable between the control (n = 19) and SGLT1 knockdown groups (n = 19) (Fig. 2B and Table 1). Despite this, the GIRs were significantly reduced in the SGLT1 knockdown group compared with the control group (P < 0.001) (Fig. 2C). The decrease in GIR was associated with an augmented glucagon and epinephrine response to hypoglycemia (∼60% and ∼67%, respectively) in the SGLT1 knockdown animals (Fig. 2D and E).
Figure 2.
Acute hypoglycemia study. A: VMH administration of SGLT1 shRNA reduced SGLT1 mRNA by ∼53%. B: Plasma glucose concentrations during the clamp in control and SGLT1 knockdown (KD) groups. C: Diminished GIRs in SGLT1 KD compared with control animals. Increased plasma glucagon (D) and epinephrine (E) responses to hypoglycemia in SGLT1 KD compared with control rats. F: Hepatic glucose production (HGP) during hypoglycemia was 88% greater in the SGLT1 KD group than in controls. Data are mean ± SEM. *P < 0.05 vs. SGLT1 KD; ***P < 0.001 vs. SGLT1 KD.
Table 1.
Plasma insulin concentrations in rats receiving VMH microinjections of scrambled RNA and SGLT1 shRNA during hypoglycemic clamp
| Experimental group | Scrambled RNA controls (μU/mL) (n = 19) | SGLT1 knockdown (μU/mL) (n = 19) |
|---|---|---|
| Baseline | 7 ± 1 | 6 ± 1 |
| Hypoglycemia (60 min) | 238 ± 13 | 232 ± 11 |
| Hypoglycemia (90 min) | 268 ± 20 | 274 ± 14 |
Data are presented as mean ± SEM.
In a subgroup of SGLT1 knockdown (n = 5) and control (n = 6) rats, tracer studies were performed to assess the mechanisms underlying the GIR differences observed between the two treatment groups. Inasmuch as specific activity was stable during the last 30 min of the clamp, steady-state equations were applied. During insulin-induced hypoglycemia, hepatic glucose production was increased by 88% in the SGLT1 knockdown group compared with controls (P < 0.05) (Fig. 2F). This effect was accompanied by increased responses of circulating glucagon and epinephrine.
Recurrent Hypoglycemia
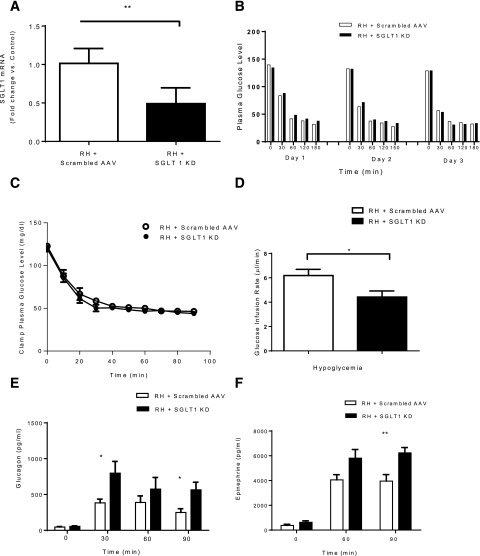
In RH rats, the SGLT1 shRNA decreased VMH SGLT1 mRNA by 51% (P < 0.005) (Fig. 3A). Blood glucose levels during the 3 days of recurrent insulin-induced hypoglycemia did not differ significantly between the control and SGLT1 knockdown groups (Fig. 3B). During the hyperinsulinemic clamp study, plasma glucose levels were indistinguishable between the control and SGLT1 knockdown groups both at baseline and during the period of hypoglycemia (Fig. 3C). GIRs during the hypoglycemic clamping period, however, were reduced by 30% (P < 0.03) compared with controls given the scrambled RNA AAV (Fig. 3D). The reduction in exogenous glucose requirements was also associated with increased glucagon and epinephrine secretion during the clamp (Fig. 3E and F).
Figure 3.
RH study. A: SGLT1 shRNA reduced VMH SGLT1 mRNA levels by ∼51% of rats exposed to RH. B: Blood glucose during the 3 days of insulin injection before clamp showed no significant difference between the control and SGLT1 knockdown (KD) groups. C: Plasma glucose concentrations during the clamp in the control and SGLT1 KD groups. D: GIRs during the clamp were diminished in RH + SGLT1 KD rats compared with RH + scrambled AAV. Plasma glucagon (E) and epinephrine (F) levels were increased in SGLT1 KD rats. Data are mean ± SEM. *P < 0.05 vs. RH + SGLT1 KD; **P < 0.01 vs. RH + SGLT1 KD.
Diabetes
In STZ-diabetic rats, VMH microinjection of SGLT1 shRNA reduced SGLT1 mRNA expression by 57% (P < 0.007) (Fig. 4A). Baseline glucose levels in the diabetic animals did not differ significantly in the SGLT1 knockdown (393 ± 50 mg/dL) and the scrambled AAV control (371 ± 36 mg/dL) groups. During the euglycemic and hypoglycemic phases of the clamp, plasma glucose and GIRs were comparable in both groups (Fig. 4B and C). However, during the hypoglycemic clamp, epinephrine responses were increased twofold in the SGLT1 knockdown group (Fig. 4D). No significant stimulatory effect on the impaired glucagon response in the diabetic rats was detected (Table 2).
Figure 4.
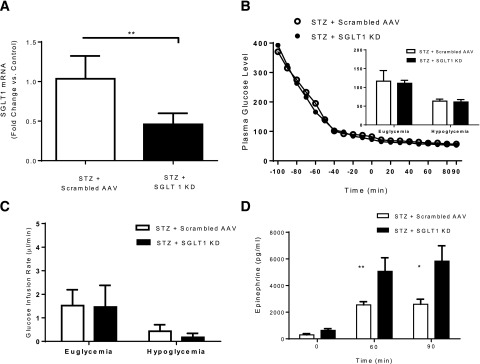
Diabetes study. A: SGLT1 shRNA AAV microinjection reduced VMH SGLT1 mRNA levels ∼57%. B: Plasma glucose concentrations during the clamp in control and SGLT1 knockdown (KD) groups. C: GIRs during the clamp were matched in STZ + SGLT1 KD rats compared with STZ + scrambled AAV rats. D: A significant increase in the epinephrine response to hypoglycemia in STZ-diabetic rats was found. *P < 0.05 vs. STZ + SGLT1 KD; **P < 0.01 vs. STZ + SGLT1 KD.
Table 2.
Plasma glucagon concentrations in STZ-diabetic animals (with scrambled AAV or SGLT1 shRNA AAV) at baseline and during hypoglycemic clamp
| Experimental group | STZ + scrambled RNA (pg/mL) (n = 5) | STZ + SGLT1 knockdown (pg/mL) (n = 6) |
|---|---|---|
| Baseline | 65 ± 6 | 71 ± 4 |
| Hypoglycemia (30 min) | 91 ± 30 | 105 ± 22 |
| Hypoglycemia (60min) | 108 ± 12 | 162 ± 41 |
| Hypoglycemia (90 min) | 150 ± 24 | 161 ± 32 |
Immunohistochemistry
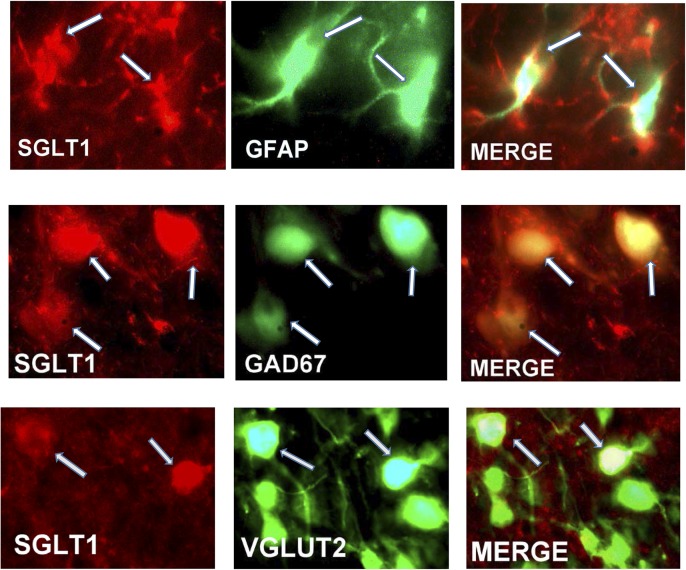
Dual immunohistochemical staining showed that SGLT1 was expressed in 98% of GABAergic, 2% of glutamatergic neurons, and 70% of astrocytes in the VMH (Fig. 5). The addition of an SGLT1-specific blocking peptide greatly diminished the immunoreactive-SGLT1 signal, suggesting that the antibody was specific for SGLT1 (Fig. 6).
Figure 5.
Top panels: Immunohistochemical staining for SGLT1 (red), the astrocytic marker GFAP (green), and the merged image showing colocalization of SGLT1 with GFAP (arrows). Middle and bottom panels: Immunohistochemical staining for SGLT1 (red), the neuronal markers GAD67 and VGLUT2 (green), and the merged image showing colocalization of SGLT1 with GAD67 and VGLUT2 (arrows).
Figure 6.
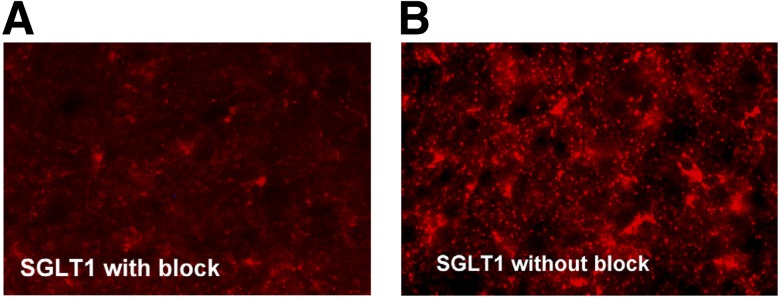
Immunohistochemical images showing that the addition of an SGLT1-specific blocking peptide can reduce the immunoreactive-SGLT1 signal in brain sections (A) compared with brain sections without the blocking peptide (B).
Discussion
It is well recognized that the brain plays an important role in hypoglycemia detection. The ability of glucose-sensing neurons within the VMH to fulfill this role is crucial for proper activation of the counterregulatory responses to hypoglycemia. Previous studies have suggested that the predominant glucose-sensing mechanism used by cells in the VMH to drive the response to glucose decrements is a KATP-dependent mechanism reliant on the oxidation of glucose. However, it has also been suggested that hypothalamic SGLTs might serve as an additional KATP-independent mechanism used to regulate glucose sensing (9). Nevertheless, the potential contribution of SGLTs in hypoglycemia sensing has received relatively little attention. Hence, the current study was undertaken to examine whether SGLT1, the predominant SGLT in the VMH, plays a role in monitoring circulating glucose and, in turn, the activation of counterregulatory responses. The current study provides evidence suggesting that inhibition of SGLT1 within the VMH augments the counterregulatory response to hypoglycemia, thus providing a potentially novel therapeutic target for improving sympathoadrenal responses to hypoglycemia in type 1 diabetes.
To assess whether SGLT1 contributes to the magnitude of the counterregulatory response to hypoglycemia, we selectively knocked down VMH SGLT1 in rats using an AAV expressing an shRNA against SGLT1 and subsequently subjected the animals to a hypoglycemic clamp study. Reducing SGLT1 expression by ∼50–60% and presumably the transport of glucose into VMH cells amplified both the glucagon and epinephrine responses to acute hypoglycemia in nondiabetic rats. This effect was accompanied by a reduction in the requirement for exogenous glucose as a result of a nearly twofold increase in hepatic glucose production. These data suggest that reducing the entry of glucose into cells expressing SGLT1 within the VMH enhances the hypoglycemic stimulus and, as a consequence, central nervous system activation of counterregulatory responses to hypoglycemia.
What was less clear from the current study was the cellular phenotype targeted by the knockdown procedure, that is, which specific cells were responsible for the observed effects. The immunohistochemical data demonstrate that SGLT1 is expressed in neurons (particularly GABA compared with glutamate neurons) as well as in a large percentage of astrocytes in the VMH. O’Malley et al. (11) previously reported that SGLT1 is expressed in hypothalamic GE neurons, which is in keeping with previous studies showing that phloridzin, a nonspecific inhibitor of SGLTs, can also reduce the activity of hypothalamic GE neurons (4). If these observations extend to the current VMH experiments, knocking down SGLT1 may enhance the glucoprivic stimulus in GE neurons, causing more pronounced GE neuron inhibition and, in turn, greater activation of counterregulatory responses. It is noteworthy in this regard that our previous studies have shown that a subclass of VMH GABAergic neurons appears to be glucose excitatory in nature (17). Thus, it is intriguing to speculate that SGLT1 inhibition might be suppressing VMH GABAergic neurons by limiting glucose entry into these neurons, which in turn enhances the glucoprivic stimulus induced by inhibition of the KATP-dependent pathway. In keeping with this possibility, we have previously reported that diminishing VMH GABA tone increases the counterregulatory response to hypoglycemia in nondiabetic rats (18) as well as in rodent models of type 1 diabetes and iatrogenic RH with impaired counterregulation (19,20).
The current immunohistochemical data also demonstrate that SGLT1 is expressed in the majority of astrocytes within the VMH. The functional role of these astrocytic transporters remains unclear. Astrocytes can temporarily supply neurons with fuel derived from stored glycogen, which could provide extra substrate during hypoglycemia (21–25). Furthermore, changes in astrocyte glucose uptake kinetics and/or metabolism might spare glucose for the neuron, thereby changing the balance between neuronal and astrocytic glucose oxidation. Indeed, this has been reported in RH rodents (26). Although it is difficult to be certain from the present quantitative PCR analysis (which was conducted in whole VMH punches) whether the reduction in SGLT1 expression might be localized to astrocytes or neurons, the data suggest that reducing glucose uptake through SGLT1s in the VMH can be beneficial for enhancing the counterregulatory responses to hypoglycemia during subsequent episodes of hypoglycemia.
Given that SGLT1 expression was not shown to be influenced by RH or diabetes, it is important to note that changes in SGLT1 expression are not likely to be driving the pathophysiology of either of these conditions. SGLT1 has a Km of ∼0.4 mmol/L for d-glucose, and its transport depends on the sodium gradient created by the actions of Na+-K+ ATPase. Brain glucose levels usually fluctuate between 0.75 and 2.5 mmol/L, and this would suggest that under normal circumstances or under conditions of hyperglycemia in diabetes, SGLT1 is likely saturated. However, the Km makes SGLT1 ideally suited to operate as an alternate gateway for glucose entry into brain cells during mild-moderate hypoglycemia (i.e., the conditions similar to those of the current study) where brain glucose levels can reach ∼0.5 mmol/L.
In summary, the current data demonstrate in three different rodent models (acute hypoglycemia, RH, and STZ-diabetes) that selective knockdown of SGLT1 in the VMH is capable of amplifying the counterregulatory responses to hypoglycemia. These findings indicate that SGLT1 within the VMH plays a significant role in hypoglycemia detection and that the ability to enhance the glucoprivic stimulus by reducing glucose entry through VMH SGLT1 transporters in neurons and astrocytes may offer a potential therapeutic target to diminish the risk of insulin-induced hypoglycemia in diabetes.
Article Information
Acknowledgments. The authors thank Aida Groszmann, Codruta Todeasa, Maria Batsu, and Ralph Jacob from the Yale Diabetes Research Center for invaluable assistance with the hormone assays in these studies.
Funding. This work was generously supported by research grants from the JDRF (4-2010-433) and the National Institutes of Health (P30-DK-045735 and R01-DK-020495). O.C. is the recipient of a Career Development Award from JDRF.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. X.F. researched the data and wrote the manuscript. O.C. and R.S. contributed to the protocol development and reviewed and edited the manuscript. Y.D., W.Z., and J.M. researched the data. R.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013, and the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
J.M. is currently affiliated with Regeneron Pharmaceuticals, Inc., Tarrytown, NY.
References
- 1.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 1997;99:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest 1994;93:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 1995;44:180–184 [DOI] [PubMed] [Google Scholar]
- 4.Yang XJ, Kow LM, Funabashi T, Mobbs CV. Hypothalamic glucose sensor: similarities to and differences from pancreatic beta-cell mechanisms. Diabetes 1999;48:1763–1772 [DOI] [PubMed] [Google Scholar]
- 5.Briski KP, Marshall ES. Induction of ependymal, glial, and neuronal transactivation by intraventricular administration of the SGLT1 Na+-D-glucose cotransporter inhibitor phlorizin. Neurochem Res 2001;26:783–792 [DOI] [PubMed] [Google Scholar]
- 6.Poppe R, Karbach U, Gambaryan S, et al. Expression of the Na+-D-glucose cotransporter SGLT1 in neurons. J Neurochem 1997;69:84–94 [DOI] [PubMed] [Google Scholar]
- 7.Thorsen K, Drengstig T, Ruoff P. Transepithelial glucose transport and Na+/K+ homeostasis in enterocytes: an integrative model. Am J Physiol Cell Physiol 2014;307:C320–C337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallner EI, Wada J, Tramonti G, Lin S, Kanwar YS. Status of glucose transporters in the mammalian kidney and renal development. Ren Fail 2001;23:301–310 [DOI] [PubMed] [Google Scholar]
- 9.Tsujii S, Bray GA. Effects of glucose, 2-deoxyglucose, phlorizin, and insulin on food intake of lean and fatty rats. Am J Physiol 1990;258:E476–E481 [DOI] [PubMed] [Google Scholar]
- 10.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 2003;52:1147–1154 [DOI] [PubMed] [Google Scholar]
- 11.O’Malley D, Reimann F, Simpson AK, Gribble FM. Sodium-coupled glucose cotransporters contribute to hypothalamic glucose sensing. Diabetes 2006;55:3381–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCrimmon RJ, Shaw M, Fan X, et al. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes 2008;57:444–450 [DOI] [PubMed] [Google Scholar]
- 13.Paranjape SA, Chan O, Zhu W, et al. Chronic reduction of insulin receptors in the ventromedial hypothalamus produces glucose intolerance and islet dysfunction in the absence of weight gain. Am J Physiol Endocrinol Metab 2011;301:E978–E983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Furuta T, Kaneko T. Neurokinin B-producing projection neurons in the lateral stripe of the striatum and cell clusters of the accumbens nucleus in the rat. J Comp Neurol 2004;480:143–161 [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Podolsky N, Sang Z, et al. The medial amygdalar nucleus: a novel glucose-sensing region that modulates the counterregulatory response to hypoglycemia. Diabetes 2010;59:2646–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu W, Czyzyk D, Paranjape SA, et al. Glucose prevents the fall in ventromedial hypothalamic GABA that is required for full activation of glucose counterregulatory responses during hypoglycemia. Am J Physiol Endocrinol Metab 2010;298:E971–E977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan O, Zhu W, Ding Y, McCrimmon RJ, Sherwin RS. Blockade of GABA(A) receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes 2006;55:1080–1087 [DOI] [PubMed] [Google Scholar]
- 19.Chan O, Cheng H, Herzog R, et al. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent hypoglycemia. Diabetes 2008;57:1363–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan O, Paranjape S, Czyzyk D, et al. Increased GABAergic output in the ventromedial hypothalamus contributes to impaired hypoglycemic counterregulation in diabetic rats. Diabetes 2011;60:1582–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science 1999;283:496–497 [DOI] [PubMed] [Google Scholar]
- 22.Pellerin L, Bouzier-Sore AK, Aubert A, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 2007;55:1251–1262 [DOI] [PubMed] [Google Scholar]
- 23.Pellerin L, Pellegri G, Bittar PG, et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci 1998;20:291–299 [DOI] [PubMed] [Google Scholar]
- 24.Choi IY, Seaquist ER, Gruetter R. Effect of hypoglycemia on brain glycogen metabolism in vivo. J Neurosci Res 2003;72:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruetter R. Glycogen: the forgotten cerebral energy store. J Neurosci Res 2003;74:179–183 [DOI] [PubMed] [Google Scholar]
- 26.Jiang L, Herzog RI, Mason GF, et al. Recurrent antecedent hypoglycemia alters neuronal oxidative metabolism in vivo. Diabetes 2009;58:1266–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]