Abstract
Hypoglycemia limits optimal glycemic control in type 1 diabetes mellitus (T1DM), making novel strategies to mitigate it desirable. We hypothesized that portal (Po) vein insulin delivery would lessen hypoglycemia. In the conscious dog, insulin was infused into the hepatic Po vein or a peripheral (Pe) vein at a rate four times of basal. In protocol 1, a full counterregulatory response was allowed, whereas in protocol 2, glucagon was fixed at basal, mimicking the diminished α-cell response to hypoglycemia seen in T1DM. In protocol 1, glucose fell faster with Pe insulin than with Po insulin, reaching 56 ± 3 vs. 70 ± 6 mg/dL (P = 0.04) at 60 min. The change in area under the curve (ΔAUC) for glucagon was similar between Pe and Po, but the peak occurred earlier in Pe. The ΔAUC for epinephrine was greater with Pe than with Po (67 ± 17 vs. 36 ± 14 ng/mL/180 min). In protocol 2, glucose also fell more rapidly than in protocol 1 and fell faster in Pe than in Po, reaching 41 ± 3 vs. 67 ± 2 mg/dL (P < 0.01) by 60 min. Without a rise in glucagon, the epinephrine responses were much larger (ΔAUC of 204 ± 22 for Pe vs. 96 ± 29 ng/mL/180 min for Po). In summary, Pe insulin delivery exacerbates hypoglycemia, particularly in the presence of a diminished glucagon response. Po vein insulin delivery, or strategies that mimic it (i.e., liver-preferential insulin analogs), should therefore lessen hypoglycemia.
Introduction
Hypoglycemia is a key barrier to optimal glycemic control in the management of type 1 diabetes mellitus (T1DM). Previous research has established the importance of aggressive control of hyperglycemia to mitigate microvascular (1–4) and possibly macrovascular (5–9) complications, but a principle limitation of this approach is increased hypoglycemia and its potential for devastating neurologic consequences (6,10–15). The homeostatic response to hypoglycemia is compromised in patients with T1DM for several reasons, including 1) the circulating insulin concentration does not fall in response to decreasing glucose concentrations, 2) the glucagon response is deficient (16–19), and 3) patients with antecedent hypoglycemia are predisposed to subsequent hypoglycemia because the sympathoadrenal response is diminished (18,20). Collectively, the presence of these abnormalities contributes to defective counterregulation and hypoglycemic unawareness.
Current therapy in T1DM is further limited by the necessity of injecting insulin into subcutaneous tissue, which delivers insulin into the peripheral (Pe) circulation, rather than the hepatic portal (Po) circulation. This approach results in a reversal of the normal insulin distribution, with higher insulin concentrations in the Pe circulation and lower insulin levels in the hepatic Po blood. A therapeutic balance must therefore be achieved, such that the excess of insulin in the Pe circulation and its effect on glucose uptake offsets the deficit of insulin at the liver and its effect on glucose production. Because Pe overinsulinization shifts the primary site of insulin action away from the liver and toward skeletal muscle, a conceivable result is a predisposition to hypoglycemia. Skeletal muscle has an inherently slower response time than the liver to fluxes in insulin, glucose, and counterregulatory factors (21–25). Further, skeletal muscle provides a larger “glucose sink” than the liver because it comprises a higher percentage of total body mass than the liver (26,27) and takes up glucose at all glycemic levels, as opposed to the liver, which takes up glucose poorly under hypoglycemic and only modestly under euglycemic conditions (28).
In the current study, we tested the hypothesis that Pe (as opposed to Po vein) insulin delivery leads to greater hypoglycemia, thereby placing a greater demand on the counterregulatory response system. We then tested the hypothesis that the propensity for increased hypoglycemia in response to Pe insulin treatment is exaggerated when the glucagon response to hypoglycemia is lost, as in individuals with T1DM, and can be minimized by Po insulin delivery.
Research Design and Methods
Animal Care and Surgical Procedures
Experiments were performed on conscious, adult male, 18-h overnight-fasted dogs weighing 19–25 kg, maintained on a daily diet of canned meat (400 g) and chow (550 g). Dogs were boarded in a surgical facility that met the standards of the Association for Assessment and Accreditation of Laboratory Animal Care guidelines, and the protocol was approved by the Vanderbilt University Medical Center Animal Care Committee.
Two weeks before the experiments, a laparotomy was performed for placement of Silastic infusion catheters in the jejunal and splenic veins, which drain into the hepatic Po circulation. Sampling catheters were placed in the femoral artery, Po vein, and hepatic vein and ultrasonic flow probes were placed around the hepatic artery and Po vein, as described previously (29,30). All dogs were healthy on the day of study, as evidenced by a leukocyte count <18,000/mm3, hematocrit >35%, good appetite, normal stooling, and healthy physical appearance.
Experimental Design
On the morning of the experiment, intravenous angiocatheters were placed in the cephalic and saphenous veins for infusion of [3-3H]glucose, human insulin, 20% dextrose, and somatostatin. The distal ends of the sampling catheters and flow probes were exteriorized from their subcutaneous pockets on the day of study through incisions made under local anesthesia (2% lidocaine). Dogs rested in a Pavlov harness throughout the experiment.
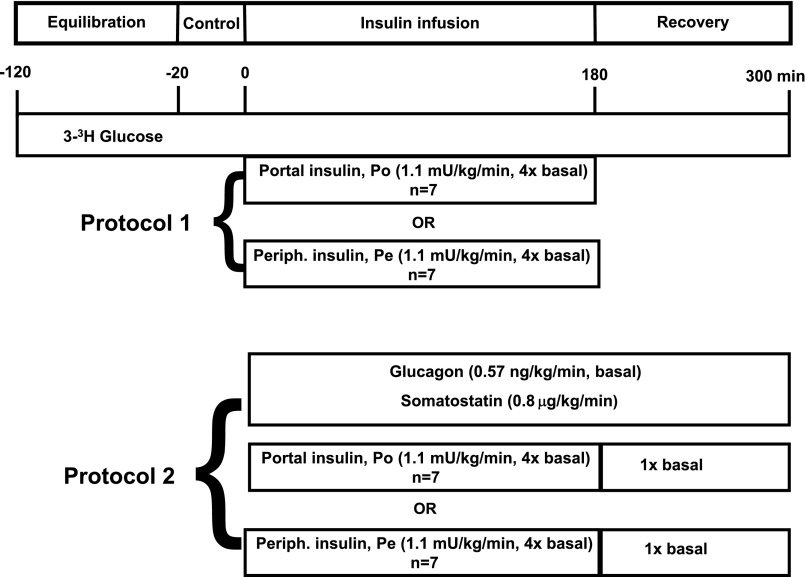
Using two protocols, each experiment consisted of a 100-min [3-3H]glucose equilibration period (−120 to −20 min), a 20-min basal period (−20 to 0 min), a 180-min insulin infusion period (0 to 180 min), and a 120-min recovery period (180 to 300 min), as shown in Fig. 1. At −120 min, a priming dose of [3-3H]glucose (38 μCi) was given, followed by a constant infusion at 0.32 μCi/min through the remainder of the experiment. During the infusion period, insulin was infused into the hepatic Po vein or a Pe vein at four times its basal secretion rate (1.1 mU/kg/min). The full counterregulatory hormone response to hypoglycemia was allowed to occur in one protocol (protocol 1 [Pr1]), whereas in the other protocol (Pr2), glucagon was fixed at a basal concentration from 0 to 300 min by infusing somatostatin (0.8 μg/kg/min) through a leg vein and glucagon at a basal rate (0.57 ng/kg/min) through the Po vein to mimic the diminished glucagon response seen in individuals with T1DM. Arterial plasma glucose samples were taken every 5 min during the infusion period, and glucose was infused as needed to maintain glucose at ∼40 mg/dL. During the recovery period in Pr1, insulin was withdrawn and no hormones were infused further. During the recovery period in Pr2, insulin was infused Po at a basal infusion rate (0.25 mU/kg/min), and the somatostatin and glucagon infusions were continued.
Figure 1.
Schematic representation of experimental protocols. Periph., peripheral.
Analytical Procedures
Plasma glucose, insulin, glucagon, catecholamines, cortisol, nonesterified fatty acids (NEFAs), blood lactate, alanine, glycerol, β-O-hydroxybutyrate (β-OHB), and hematocrit were measured as described elsewhere (31,32).
Calculations
The rates of glucose production (Ra) and utilization (Rd) were determined using a primed, constant infusion of [3-3H]glucose. Calculation of the data used a circulatory model described by Mari et al. (33) using canine parameters established by Dobbins et al. (34) and took into account exogenous glucose infusion when such occurred.
Net hepatic substrate balances and gluconeogenic and glycogenolytic fluxes were calculated as described elsewhere (35–37). A positive number for net hepatic gluconeogenic flux represents net gluconeogenic flux to glucose-6-phosphate, whereas a negative value represents net glycolytic flux. A positive value for net hepatic glycogenolytic flux represents net glycogen breakdown, whereas a negative number represents net glycogen synthesis. Nonhepatic glucose uptake was calculated as described elsewhere (38,39).
The changes in the counterregulatory hormone levels brought about by insulin infusion were assessed by calculating differences in area under the curve (ΔAUC) between the infusion period and the basal period using trapezoidal approximation. The changes in glucose turnover, glycogenolytic, gluconeogenic, and hepatic substrate balances were similarly assessed by calculating the ΔAUC between the period of interest and the basal period.
Statistics
Data were analyzed for differences between Po and Pe insulin delivery groups using SigmaStat software (SPSS, Chicago, IL). The statistical comparison between groups for time-course data used two-way, repeated-measures ANOVA. When significant differences in the responses between groups were found, post hoc analyses using the Holm-Šidák method were used to identify differences at specific time points between groups. The unpaired Student t test was used to compare ΔAUC data between groups. Data are presented as mean ± SEM.
Results
Insulin Concentrations
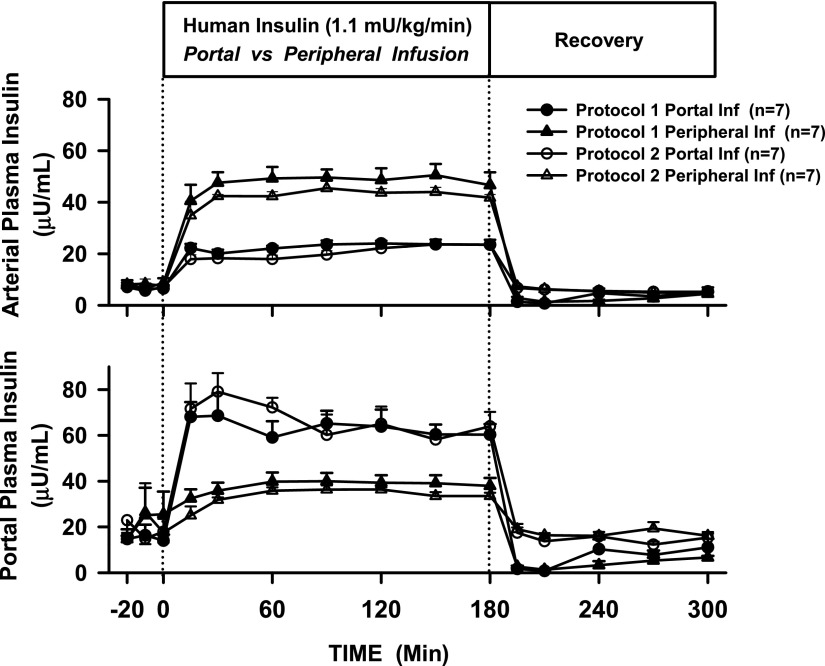
When insulin was infused Pe at four times the basal rate (n = 14), the insulin concentrations rose approximately sixfold in the artery and approximately twofold in the Po vein (Fig. 2) to 45 ± 3 and 36 ± 2 μU/mL, respectively. When insulin was infused Po at the same rate (n = 14), its level rose fourfold in both Pe and Po plasma to 22 ± 1 and 66 ± 4 μU/mL, respectively. In all groups, endogenous insulin secretion was inhibited, as indicated by C-peptide levels approaching zero (data not shown). During the recovery period in Pr1, plasma insulin initially dropped to nearly zero and then rose toward basal as endogenous insulin secretion resumed. During the recovery period in Pr2, insulin was infused intraportally at a basal rate in the presence of a somatostatin infusion, and as a result, the insulin levels rapidly returned to baseline values.
Figure 2.
Arterial (Pe) and hepatic (Po) vein plasma insulin concentrations in 18-h-fasted, conscious dogs during the basal (−20 to 0 min), experimental (0 to 180 min), and recovery (180 to 300 min) periods. Inf, infusion.
Pr1
Plasma Glucose Levels
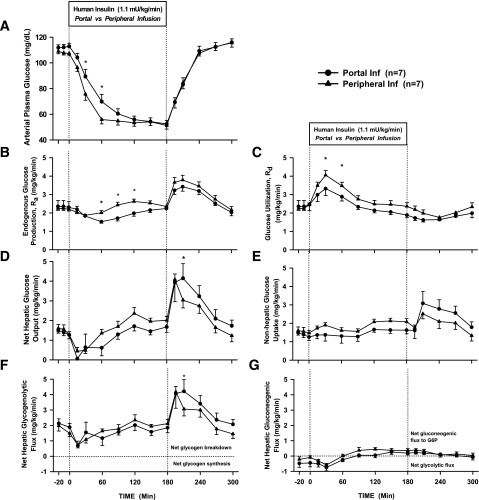
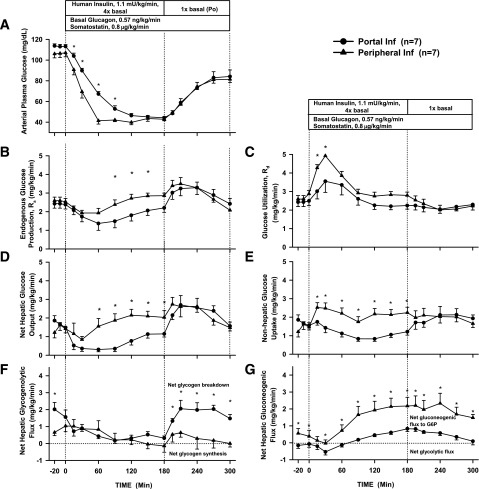
The arterial plasma glucose concentration fell more rapidly with Pe than with Po insulin infusion (Fig. 3A), reaching 56 ± 3 vs. 70 ± 6 mg/dL at 60 min, respectively (P = 0.04). Plasma glucose eventually reached a similar nadir (∼50 mg/dL) in both groups, but this occurred earlier in the Pe group (60 vs. 150 min). No dogs in Pr1 required a glucose infusion to prevent plasma glucose from falling to <40 mg/dL. Glucose quickly returned to basal levels in the recovery period, with little difference between the groups.
Figure 3.
Arterial plasma glucose concentrations (A), endogenous Ra (B), Rd (C), net hepatic glucose output (D), nonhepatic glucose uptake (E), net hepatic glycogenolytic flux (F), and net hepatic gluconeogenic flux (G) for Pr1 in 18-h-fasted, conscious dogs during the basal (−20 to 0 min), experimental (0 to 180 min), and recovery (180 to 300 min) periods (mean ± SEM). *P < 0.05 between groups. Inf, infusion; G6P, glucose-6-phosphate.
Glucose Turnover and Net Hepatic Glucose Balance
During the first hour of insulin infusion, the suppression of Ra was minimally greater with Po insulin than with Pe insulin (ΔAUC −14.8 ± 3.8 vs. −26.2 ± 13.8 mg/kg/60 min, Pe vs. Po, P = 0.41) (Fig. 3B). However, the rise in Rd was significantly greater with Pe insulin infusion (ΔAUC 76.4 ± 10.4 vs. 38.6 ± 9.2 mg/kg/60 min, Pe vs. Po, P = 0.01) (Fig. 3C), thus explaining the more rapid drop in plasma glucose in the Pe insulin group. In the last 30 min of the insulin infusion period, when glucose concentrations in both groups were equal (∼50 mg/dL) and essentially flat, Pe insulin infusion resulted in greater Rd (2.41 ± 0.20 vs. 1.91 ± 0.14 mg/kg/min, Pe vs. Po) and greater Ra (2.44 ± 0.13 vs. 2.19 ± 0.13 mg/kg/min, Pe vs. Po). Ra and Rd returned to basal rates as euglycemia was restored during the recovery period. The trends seen in glucose turnover were similar to those seen in the arteriovenous difference data (Fig. 3D and E). The initial suppression of glucose production was due to an insulin-induced fall in the net hepatic glycogenolytic flux and a small fall in the net hepatic gluconeogenic flux in both groups (Fig. 3F and G). Eventually, as the counterregulatory hormones rose, net hepatic glycogenolysis returned to the basal rate, but net hepatic gluconeogenic flux to glucose-6-phosphate increased and remained above baseline. During the recovery period, plasma glucose rapidly rose as a result of a rapid increase in glycogenolysis.
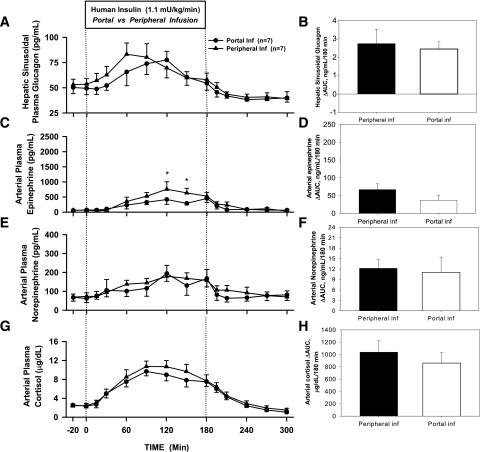
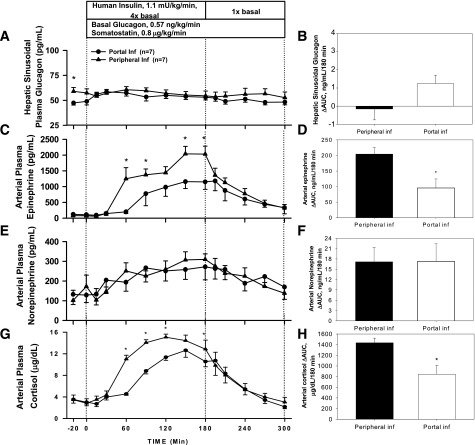
Counterregulatory Hormone Response
The ΔAUC for hepatic sinusoidal plasma glucagon was similar between Pe and Po insulin infusion (2.7 ± 0.8 vs. 2.4 ± 0.4 ng/mL/180 min, P = 0.81) (Fig. 4B), but glucagon peaked earlier with Pe insulin infusion (median [interquartile range] time to peak 60 [60–90] vs. 120 [90–120] min, P = 0.007) (Fig. 4A). The ΔAUC for arterial plasma epinephrine in the Pe insulin infusion group tended to be higher (67 ± 17 vs. 37 ± 14 ng/mL/180 min, Pe vs. Po, P = 0.32) (Fig. 4D) and tended to peak earlier (120 vs. 180 min, P = 0.22) (Fig. 4C). Norepinephrine rose steadily and similarly in both groups (Fig. 4E and F). The arterial plasma cortisol response to hypoglycemia was also similar in both groups (ΔAUC 1,038 ± 185 vs. 860 ± 171 µg/dL/180 min, P = 0.46) and peaked at the same time (90 min) (Fig. 4G and H). The counterregulatory hormone levels returned to baseline during the recovery period.
Figure 4.
Arterial plasma concentrations and ΔAUC for glucagon (A and B), epinephrine (C and D), norepinephrine (E and F), and cortisol (G and H) for Pr1 in 18-h-fasted dogs during the basal (−20 to 0 min), experimental (0 to 180 min), and recovery (180 to 300 min) periods (mean ± SEM). *P < 0.05 between groups. Inf, infusion.
Pr2
Plasma Glucose Levels
In Pr2, using the same insulin infusion rate as in Pr1 but with glucagon clamped, the fall in glucose was faster regardless of the route of insulin delivery. As in Pr1, however, glucose fell more rapidly with Pe than with Po insulin (Fig. 5A), reaching 41 ± 3 vs. 67 ± 2 mg/dL (P < 0.01) by 60 min. A nadir of ∼40 mg/dL was reached 60 min into the Pe insulin infusion compared with ∼45 mg/dL at 150 min with the Po insulin infusion. Five dogs in the Pe group versus one in the Po group required a low-rate glucose infusion (average 0.08 mg/kg/min vs. 0.02 mg/kg/min, respectively) to maintain glucose ≥40 mg/dL. Glucose rose more slowly in the recovery period in Pr2 than in Pr1 because the insulin infusion was returned to basal rather than being stopped.
Figure 5.
Arterial plasma glucose concentrations (A), endogenous Ra (B), Rd (C), net hepatic glucose output (D), nonhepatic glucose uptake (E), net hepatic glycogenolytic flux (F), and net hepatic gluconeogenic flux (G) for Pr2 in 18-h-fasted, conscious dogs during the basal (−20 to 0 min), experimental (0 to 180 min), and recovery (180 to 300 min) periods (mean ± SEM). *P < 0.05 between groups. Inf, infusion; G6P, glucose-6-phosphate.
Net Hepatic Glucose Balance and Glucose Turnover
In the first hour, there was minimal difference in suppression of Ra between the Pe and Po insulin infusion (ΔAUC −30.1 ± 13.6 vs. −36.4 ± 10.1 mg/kg/60 min, respectively) (Fig. 5B). Nevertheless, the rise in Rd was twice as great with Pe insulin as with Po insulin (ΔAUC 94.6 ± 15.1 vs. 47.3 ± 19.9 mg/kg/60 min, respectively, P = 0.06) (Fig. 5C), thus explaining the more rapid fall in plasma glucose. In the last 30 min of the insulin infusion period, when glucose concentrations for both groups were 40–45 mg/dL and essentially flat, Pe insulin infusion was associated with greater Rd (2.81 ± 0.17 vs. 2.21 ± 0.19 mg/kg/min, Pe vs. Po) and greater Ra (2.85 ± 0.14 vs. 2.22 ± 0.19 mg/kg/min, Pe vs. Po). Thus, as in Pr1, overall glucose turnover was greater with Pe infusion. In the recovery period, Ra and Rd returned to basal rates as euglycemia was restored. Similar trends were seen in the arteriovenous difference data (Fig. 5D and E).
Net hepatic glycogenolytic flux changed minimally during the first hour of Pe insulin infusion but decreased during Po insulin infusion (ΔAUC 4.3 ± 16.0 vs. −58.2 ± 16.2 mg/kg/60 min, respectively) (Fig. 5F). Net hepatic gluconeogenic flux decreased similarly in both groups in the first hour (ΔAUC −13.2 ± 7.4 vs. −10.5 ± 4.5 mg/kg/60 min, Pe vs. Po) (Fig. 5G). During the final 30 min of the infusion period, net hepatic glycogenolytic flux remained less than basal in both infusion groups (−0.09 ± 0.32 vs. 0.42 ± 0.15 mg/kg/min, Pe vs. Po). Net hepatic gluconeogenic flux, however, was increased in both groups, being three times higher with Pe insulin than with Po insulin (2.16 ± 0.50 vs. 0.72 ± 0.14 mg/kg/min, respectively). This was in contrast to Pr1, in which the net hepatic gluconeogenic flux was less in both groups (0.37 ± 0.12 vs. 0.23 ± 0.14 mg/kg/min, with Pe and Po insulin, respectively) (Fig. 3G). Although net hepatic glycogenolytic and gluconeogenic flux both returned to basal rates during the recovery period with Po insulin, net hepatic glycogenolysis remained suppressed and net hepatic gluconeogenic flux remained elevated with Pe delivery.
Counterregulatory Hormone Response
In Pr2, the plasma glucagon concentrations were basal and similar between Po and Pe during the insulin infusion (Fig. 6A and B). Without a rise in glucagon, the increase in epinephrine was much greater for both infusion groups, with a ΔAUC of 204 ± 22 for Pe vs. 96 ± 29 ng/mL/180 min for Po (P = 0.02, respectively) (Fig. 6C and D). Norepinephrine rose modestly and was not different between groups (Fig. 6E and F). With glucagon basal, cortisol rose to a greater extent in both groups, with the rise being greater in response to Pe insulin than to Po insulin (1,436 ± 86 vs. 843 ± 165 µg/dL/180 min, P < 0.01) (Fig. 6G and H). Counterregulatory hormone concentrations returned toward basal during the recovery period.
Figure 6.
Arterial plasma concentrations and ΔAUC for glucagon (A and B), epinephrine (C and D), norepinephrine (E and F), and cortisol (G and H) for Pr2 in 18-h-fasted dogs during the basal (−20 to 0 min), experimental (0 to 180 min), and recovery (180 to 300 min) periods (mean ± SEM). *P < 0.05 between groups. Inf, infusion.
Metabolite Response
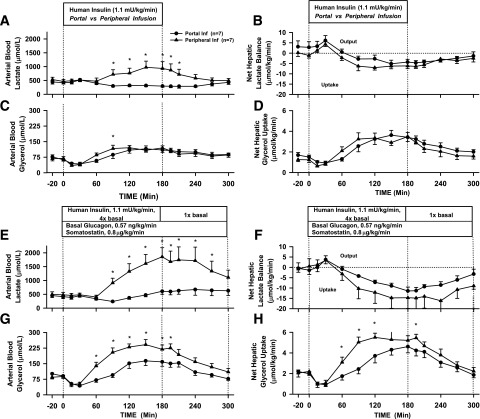
In Pr1, both infusion groups had a small rise in net hepatic lactate output at 30 min, followed by the liver switching to net lactate uptake (Fig. 7B) in parallel with the rise in plasma epinephrine. The rise in blood lactate levels that occurred in the face of elevated net hepatic lactate uptake indicates that muscle lactate production increased (Fig. 7A). The response was greater in the presence of Pe insulin than in Po insulin, concordant with the greater epinephrine response. A similar pattern was seen in Pr2 (Fig. 7E and F), but without a rise in glucagon, net hepatic lactate uptake increased to a greater extent in both groups with a larger difference between the Pe and Po groups compared with Pr1. Blood lactate levels rose nearly fourfold by the end of the infusion period with Pe insulin in Pr2 (Fig. 7E), compared with twofold in Pr1 (Fig. 7A). A rise in blood lactate levels, despite the large increase in net hepatic lactate uptake, indicates that muscle lactate production with Pe insulin was greater in Pr2 than in Pr1, consistent with the larger rise in epinephrine. Blood lactate levels with Po insulin infusion did not rise in Pr1 and rose minimally in Pr2.
Figure 7.
Arterial blood concentrations and net hepatic balances in Pr1 for lactate (A and B) and glycerol (C and D) and in Pr2 for lactate (E and F) and glycerol (G and H) in 18-h-fasted, conscious dogs during the basal (−20 to 0 min), experimental (0 to 180 min), and recovery (180 to 300 min) periods (mean ± SEM). *P < 0.05 between groups. Inf, infusion.
The arterial blood glycerol level and net hepatic glycerol uptake both initially fell in response to the rise in insulin in Pr1 but then rose during hypoglycemia in parallel with the rise in plasma epinephrine, and there was no difference between groups (Fig. 7C and D). Once again, because the rise in blood glycerol occurred in the face of increased net hepatic glycerol uptake, there must have been increased production of glycerol (lipolysis) by adipose tissue. A similar pattern was seen in Pr2, except there were significantly larger increases with Pe insulin than with Po insulin (Fig. 7G and H). NEFA kinetics in both protocols followed those of glycerol, with an initial suppression of NEFA levels and net hepatic uptake, followed by increases in both, concurrent with the rise in plasma epinephrine (Table 1). β-OHB production fell and then rose, as expected, given the changes in net hepatic NEFA uptake, with greater changes occurring in Pr2 than in Pr1. Blood alanine concentrations decreased modestly, while net hepatic alanine uptake increased slightly in response to hypoglycemia in both protocols, indicating that the fractional extraction of the amino acid by the liver increased. In the recovery periods, these parameters returned toward basal.
Table 1.
Blood alanine, free fatty acid, and β-OHB concentrations and balances for Pr1 and Pr2
| Control period (min) |
Experimental period (min) |
Recovery period (min) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −20 | 0 | 15 | 30 | 60 | 90 | 120 | 180 | 195 | 210 | 240 | 270 | 300 | ||
| Blood alanine level (μmol/L) | ||||||||||||||
| Pr1 | Pe insulin | 303.3 ± 29.7 | 290.3 ± 31.7 | 292.3 ± 27.5 | 303.1 ± 26.2 | 238.6 ± 26.8 | 217.4 ± 25.2 | 199.4 ± 30.5 | 186.6 ± 31.9 | 190.7 ± 41.5 | 180.9 ± 36.6 | 176.5 ± 33.9 | 185.3 ± 33.7 | 199.3 ± 30.9 |
| Po insulin | 263.2 ± 38.3 | 257.8 ± 31.7 | 256.3 ± 33.6 | 264.9 ± 35.7 | 220.5 ± 25.8 | 174.0 ± 21.7 | 150.8 ± 14.5 | 123.1 ± 14.3 | 114.0 ± 12.7 | 108.8 ± 8.5 | 112.5 ± 9.1 | 133.3 ± 12.0 | 167.1 ± 16.9 | |
| Pr 2 | Pe insulin | 282.2 ± 25.8 | 260.7 ± 23.9 | 270.5 ± 25.8 | 279.8 ± 27.4 | 214.0 ± 20.0 | 214.6 ± 20.8 | 238.4 ± 24.3 | 232.2 ± 25.3 | 259.3 ± 21.0 | 239.4 ± 18.4 | 242.5 ± 25.5 | 280.4 ± 25.8 | 286.4 ± 29.2 |
| Po insulin | 273.0 ± 19.4 | 255.6 ± 23.1 | 260.8 ± 22.3 | 272.5 ± 19.1 | 242.6 ± 21.3 | 190.4 ± 18.0 | 161.6 ± 15.5 | 153.3 ± 11.3 | 154.2 ± 10.8 | 142.8 ± 12.6 | 147.7 ± 19.1 | 175.9 ± 26.3 | 192.2 ± 31.3 | |
| Net hepatic alanine uptake (μmol/kg/min) | ||||||||||||||
| Pr 1 | Pe insulin | 2.5 ± 0.3 | 2.2 ± 0.4 | 1.8 ± 0.4 | 1.9 ± 0.4 | 2.7 ± 0.4 | 3.1 ± 0.5 | 3.0 ± 0.4 | 2.7 ± 0.2 | 3.1 ± 0.3 | 3.4 ± 0.5 | 3.0 ± 0.2 | 3.0 ± 0.3 | 2.9 ± 0.3 |
| Po insulin | 2.3 ± 0.5 | 2.4 ± 0.5 | 2.4 ± 0.5 | 2.6 ± 0.7 | 3.3 ± 1.2 | 2.8 ± 0.2 | 2.6 ± 0.4 | 2.6 ± 0.4 | 2.6 ± 0.4 | 2.6 ± 0.2 | 2.2 ± 0.2 | 2.6 ± 0.4 | 2.8 ± 0.4 | |
| Pr 2 | Pe insulin | 2.8 ± 0.3 | 2.4 ± 0.3 | 2.0 ± 0.3 | 2.1 ± 0.3 | 2.0 ± 0.3 | 2.7 ± 0.3 | 3.0 ± 0.2 | 3.0 ± 0.3 | 3.2 ± 0.2 | 3.2 ± 0.1 | 2.7 ± 0.5 | 4.1 ± 0.3 | 3.7 ± 0.3 |
| Po insulin | 2.7 ± 0.3 | 2.6 ± 0.4 | 2.2 ± 0.4 | 2.6 ± 0.4 | 2.9 ± 0.3 | 2.8 ± 0.4 | 2.6 ± 0.4 | 2.6 ± 0.2 | 3.3 ± 0.3 | 3.0 ± 0.3 | 3.1 ± 0.8 | 3.3 ± 0.3 | 3.4 ± 0.4 | |
| Blood NEFA level (μmol/L) | ||||||||||||||
| Pr1 | Pe insulin | 833.0 ± 109.4 | 858.1 ± 99.6 | 418.4 ± 53.6 | 280.0 ± 55.3 | 769.9 ± 127.3 | 1,014.5 ± 231.3 | 930.7 ± 204.5 | 785.0 ± 182.2 | 747.5 ± 146.6 | 905.3 ± 140.4 | 822.7 ± 126.2 | 846.9 ± 102.6 | 832.6 ± 117.8 |
| Po insulin | 771.0 ± 57.2 | 739.6 ± 55.2 | 532.9 ± 39.9 | 338.0 ± 39.9 | 527.2 ± 59.0 | 830.2 ± 166.4 | 971.2 ± 169.7 | 1,027.5 ± 136.1 | 980.1 ± 123.5 | 1,056.1 ± 87.5 | 1,002.7 ± 92.1 | 963.0 ± 97.3 | 893.4 ± 105.6 | |
| Pr2 | Pe insulin | 953.3 ± 71.4 | 988.5 ± 60.8 | 488.4 ± 126.4 | 349.1 ± 74.5 | 1,025.2 ± 203.5 | 1,151.6 ± 102.7 | 1,118.3 ± 101.8 | 1,068.5 ± 99.2 | 655.1 ± 101.0 | 832.0 ± 122.4 | 901.3 ± 90.8 | 804.2 ± 88.4 | 744.5 ± 94.3 |
| Po insulin | 984.6 ± 44.2 | 963.5 ± 37.1 | 695.0 ± 55.3 | 500.5 ± 76.0 | 540.3 ± 80.0 | 827.6 ± 105.2 | 1,265.9 ± 151.8 | 1,164.9 ± 70.2 | 986.1 ± 138.7 | 1,001.2 ± 76.0 | 1,223.3 ± 113.9 | 933.1 ± 126.7 | 813.8 ± 90.7 | |
| Net hepatic NEFA uptake (μmol/kg/min) | ||||||||||||||
| Pr1 | Pe insulin | 3.1 ± 1.1 | 2.1 ± 0.5 | 1.8 ± 0.8 | 0.7 ± 0.4 | 2.9 ± 0.9 | 3.1 ± 0.5 | 2.3 ± 0.8 | 2.6 ± 0.7 | 2.2 ± 0.4 | 3.8 ± 0.9 | 2.7 ± 0.7 | 2.6 ± 0.9 | 2.2 ± 0.4 |
| Po insulin | 2.3 ± 0.5 | 2.3 ± 0.8 | 1.8 ± 0.3 | 1.0 ± 0.3 | 1.8 ± 0.8 | 3.9 ± 1.3 | 2.9 ± 0.7 | 2.5 ± 1.1 | 3.4 ± 1.1 | 5.0 ± 1.9 | 2.5 ± 0.5 | 2.4 ± 0.8 | 3.3 ± 0.6 | |
| Pr2 | Pe insulin | 2.9 ± 0.7 | 2.0 ± 0.3 | 1.7 ± 0.5 | 0.5 ± 0.2 | 4.1 ± 1.3 | 3.1 ± 0.6 | 4.3 ± 0.5 | 4.4 ± 0.7 | 2.0 ± 0.7 | 2.4 ± 0.6 | 2.0 ± 1.1 | 3.6 ± 0.5 | 2.7 ± 0.6 |
| Po insulin | 2.5 ± 0.2 | 1.6 ± 0.7 | 2.8 ± 0.4 | 1.7 ± 0.5 | 2.1 ± 0.5 | 2.5 ± 0.4 | 4.4 ± 0.4 | 4.4 ± 0.6 | 3.0 ± 0.8 | 1.0 ± 0.4 | 3.5 ± 1.1 | 4.3 ± 1.2 | 3.0 ± 0.9 | |
| Blood β-OHB level (μmol/L) | ||||||||||||||
| Pr1 | Pe insulin | 28.8 ± 3.6 | 30.3 ± 4.7 | 23.3 ± 3.2 | 18.3 ± 1.8 | 25.8 ± 3.9 | 38.0 ± 9.8 | 31.4 ± 10.0 | 31.2 ± 8.4 | 30.1 ± 9.7 | 36.8 ± 13.1 | 33.3 ± 10.5 | 38.5 ± 9.1 | 43.3 ± 9.7 |
| Po insulin | 23.7 ± 4.0 | 23.8 ± 3.6 | 21.8 ± 3.2 | 16.3 ± 2.3 | 19.7 ± 3.2 | 34.2 ± 14.1 | 42.9 ± 16.3 | 37.4 ± 11.4 | 38.0 ± 12.5 | 49.8 ± 15.2 | 50.5 ± 10.6 | 38.0 ± 7.3 | 35.0 ± 6.3 | |
| Pr2 | Pe insulin | 56.6 ± 16.8 | 55.0 ± 15.2 | 30.3 ± 5.0 | 24.3 ± 3.2 | 70.8 ± 21.5 | 53.3 ± 18.7 | 35.7 ± 11.1 | 36.5 ± 7.2 | 25.9 ± 4.8 | 27.9 ± 5.8 | 28.9 ± 4.5 | 25.3 ± 2.7 | 27.1 ± 3.5 |
| Po insulin | 32.2 ± 5.3 | 31.3 ± 3.7 | 29.0 ± 4.5 | 20.8 ± 2.2 | 22.1 ± 1.4 | 42.6 ± 7.1 | 61.3 ± 7.3 | 55.0 ± 9.7 | 43.9 ± 8.3 | 43.0 ± 12.0 | 48.0 ± 14.0 | 39.4 ± 14.6 | 31.5 ± 10.9 | |
| Net hepatic β-OHB output (μmol/kg/min) | ||||||||||||||
| Pr1 | Pe insulin | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.3 | 0.6 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.3 | 1.0 ± 0.2 | 0.5 ± 0.7 |
| Po insulin | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.3 | 1.2 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.4 | 1.1 ± 0.3 | 1.2 ± 0.2 | 1.3 ± 0.3 | 1.0 ± 0.3 | |
| Pr2 | Pe insulin | 1.6 ± 0.6 | 1.6 ± 0.5 | 0.7 ± 0.2 | 0.6 ± 0.2 | 1.8 ± 0.8 | 1.5 ± 0.8 | 1.1 ± 0.4 | 1.0 ± 0.3 | 0.5 ± 0.2 | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.8 ± 0.3 | 0.6 ± 0.2 |
| Po insulin | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 | 1.3 ± 0.3 | 2.0 ± 0.3 | 1.6 ± 0.3 | 0.9 ± 0.3 | 1.0 ± 0.9 | 1.6 ± 0.7 | 1.3 ± 0.7 | 0.8 ± 0.5 | |
Data are shown as mean ± SEM.
Discussion
The current study sought to determine the extent to which the route of insulin delivery influences susceptibility to hypoglycemia. By infusing insulin at four times the basal rate into the hepatic Po or the Pe circulation, we demonstrated that the susceptibility to hypoglycemia was increased when insulin was delivered Pe as opposed to Po. When the glucagon response was prevented by clamping glucagon at a basal value (mimicking the diminished response seen in T1DM), the effect of Pe insulin delivery was even greater. This increased hypoglycemic propensity placed a larger demand on the adrenergic response to prevent plasma glucose from dropping to life-threatening concentrations. The effect of overinsulinizing the periphery (which increased Rd) was greater than the effect of underinsulinizing the liver (which increased Ra), thus explaining the greater hypoglycemia seen with Pe insulin infusion. A number of studies have examined differences between Pe and Po insulin delivery from the perspective of evaluating the efficacy of insulin delivery via intraperitoneal catheters (40–42). In general, slight improvements in glycemic control and glucose variability were seen. To our knowledge, however, ours is the first study specifically evaluating the effects of equivalent Pe versus hepatic Po insulin infusion on hypoglycemia.
In a previous study, we compared the effect on glucose kinetics of insulin infusion at a basal rate into the hepatic Po vein or a Pe vein (43). When basal insulin infusion was switched from the Po to the Pe route, there was a doubling in the arterial insulin concentration and a halving of the hepatic sinusoidal insulin concentration. This resulted in hepatic Ra rapidly rising by more than twofold while Rd rose slowly and to a lesser extent. As a consequence of this mismatch, arterial plasma glucose rose from 100 to 140 mg/dL. Thus, under basal insulin conditions, the effect of lowering insulin at the liver (which increased Ra) had more of an effect than increasing insulin at Pe tissues (which increased Rd). It follows that under basal conditions, the rate of insulin infusion into the Pe circulation would have to be greater than the rate of infusion into the Po vein to restrain Ra. In other words, control of the liver would require a higher Pe insulin level, with the consequence that one would move up the dose-response curve relating insulin to Rd. As that dose-response relationship steepens, errors in insulin dosing would have greater effect, resulting in an increase in hypoglycemic and hyperglycemic events. By comparison, when insulin is delivered into the hepatic Po vein, liver glucose production can be controlled with less insulin reaching the Pe tissues. This would result in a smaller increase in Rd (one would stay on the flatter part of the dose-response curve), effectively reducing glycemic variability and hypoglycemia while maintaining target glycemia.
A similar situation would occur under prandial conditions, with more insulin being required when it is given Pe than when it is given Po to bring about a normal increase in hepatic glucose uptake. As a result, the insulin level in Pe plasma would again be excessive, and in this case, glucose storage would shift away from the liver toward muscle. The current study delineates what would happen if one infused insulin at a rate four times basal via a Pe or Po vein route in the absence of a glucose load. As predicted, with Pe insulin delivery the effect on Rd dominated and hypoglycemia worsened. Clinically, when insulin is given subcutaneously, the dose would be reduced to prevent hypoglycemia, but to control hepatic glucose production, one would still be forced to overinsulinize the periphery, thus climbing the Rd/insulin dose–response curve and causing increased glycemic variability, including hyperglycemia and hypoglycemia. In contrast, insulin treatment targeted to the liver by Po vein insulin delivery or Pe delivery of a hepatopreferential insulin analog potently suppressed hepatic glucose production while concurrently stimulating whole-body glucose uptake minimally (39). Insulin delivery into a Pe vein was able to decrease hepatic glucose production equivalently but with the price of marked increase in muscle glucose uptake. Thus, insulin therapy directed at the liver would rely on a flatter portion of the Rd/insulin dose–response curve, and as a result, increases in insulin dosing to control hepatic glucose production and maintain euglycemia would be associated with less glycemic variability and hypoglycemia.
In Pr1, in which the counterregulatory hormone response remained intact, dogs receiving insulin Pe were able to establish a plasma glucose nadir of ∼50 mg/dL at 60 min. This was achieved by first rapidly increasing glucagon secretion, then later by increasing epinephrine secretion. By comparison, dogs receiving insulin Po established a plasma glucose nadir of ∼50 mg/dL at 150 min by reaching nearly the same peak hepatic sinusoidal plasma glucagon concentration, but at a later time point. Although their peak glucagon responses were similar, peak epinephrine levels were greater in the Pe group. In this way, the drop in plasma glucose brought about by the higher Pe insulin concentrations necessitated an earlier but equivalent rise in glucagon to “brake” the fall in glucose and a larger rise in epinephrine to “hold” the plasma glucose nadir when compared with Po delivery.
We next examined the effect of the route of insulin delivery in the absence of a glucagon response. Without this response, plasma glucose dropped more rapidly with both Pe and Po insulin infusion, underscoring the importance of glucagon as the first responder to hypoglycemia. In addition, in the absence of a rise in glucagon, the effect of Pe versus Po insulin infusion on hypoglycemia was significantly magnified. Whereas in Pr1 a glucagon rise enabled a recovery in net hepatic glycogenolytic flux back to basal levels, the lack of a glucagon rise in Pr2 resulted in a continued fall in glycogenolysis during the infusion period. As a result, a much greater adrenergic response was needed to bring about a similar rise in Ra to prevent severe hypoglycemia. This led to increased mobilization of gluconeogenic substrates from the periphery and a resultant rise in gluconeogenic flux, increasing Ra. This rise in gluconeogenic flux directly correlated with the extent of epinephrine rise across infusion groups and protocols. Despite the larger adrenergic response, low-dose intravenous glucose infusion was needed in five of seven Pe dogs to prevent plasma glucose from falling below 40 mg/dL, compared with only one of seven Po dogs requiring intravenous glucose. These data are consistent with previous studies that suggest a “partitioning” of the counterregulatory response to hypoglycemia (44). Whereas the early response depends on rising glucagon and resultant glycogenolysis, the later response relies on gluconeogenesis, because rising epinephrine leads to increased extrahepatic production of gluconeogenic substrates.
Several factors need to be considered in the practical application of these results to the treatment of T1DM. First, the Pe insulin resistance that is present in T1DM (45–50) may limit Rd in response to a given insulin infusion, thus minimizing the effect of its delivery on hypoglycemia. From a practical standpoint, however, insulin-resistant individuals would simply be given higher insulin doses to overcome the Pe insulin resistance. Thus, because their insulin doses would be titrated up, insulin delivered Pe would still likely result in greater propensity for glycemic variability and hypoglycemia.
A second consideration relates to the fact that patients with T1DM have an absent glucagon response to hypoglycemia and often have a blunted epinephrine response, especially when recent, antecedent hypoglycemia has occurred (18,20). The reliance on epinephrine was greatest with Pe insulin when the glucagon response was prevented (Pr2 Pe), with a 3-fold higher epinephrine response compared with when the glucagon response was intact (Pr1 Pe) and a 5.5-fold higher response compared with when the glucagon response was intact and insulin was delivered portally (Pr1 Po). This suggests that Pe insulin delivery would be even more problematic in T1DM patients who had antecedent hypoglycemia.
In summary, these studies support the hypothesis that Pe insulin delivery leads to greater hypoglycemia and increased glucose variability compared with equivalent insulin delivery into the hepatic Po circulation. The increased propensity for hypoglycemia associated with Pe insulin delivery stems from having higher insulin concentrations at muscle, leading to a larger increase in Rd than when insulin is delivered Po. This heightened susceptibility for hypoglycemia places a greater demand on the counterregulatory system to prevent severe hypoglycemia. When the glucagon response to hypoglycemia is defective, as is frequently the case in individuals with T1DM, there is an even greater likelihood of hypoglycemia in the presence of Pe insulin delivery. The fact that insulin has to be delivered Pe clearly plays a role in causing hypoglycemia and glycemic variability. These studies suggest that strategies to mimic endogenous insulin secretion into the Po circulation, such as intraperitoneal insulin delivery or use of hepatopreferential insulin analogs, should mitigate hypoglycemic risk and reduce fluctuations in glucose in patients with T1DM.
Article Information
Acknowledgments. The authors thank Patsy Raymer (Vanderbilt University) and the Vanderbilt Diabetes Research and Training Center Hormone Assay & Analytical Services Core for technical support. The authors also thank Phil Williams for technical assistance (Vanderbilt University Department of Surgery).
Funding. Hormone analysis was performed by the Hormone Assay & Analytical Services Core, which receives National Institutes of Health support from the Vanderbilt Diabetes Research and Training Center (DK020593) and the Vanderbilt Mouse Metabolic Phenotyping Center (DK059637). J.M.G. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Ruth L. Kirschstein National Research Service Award for Individual Postdoctoral Fellows (1F32DK100114-01A1) and by National Institute of Diabetes and Digestive and Kidney Diseases training grant T32DK007061. J.J.W. was supported by a National Institute of Diabetes and Digestive and Kidney Diseases Career Development Award (K01DK093799). Novo Nordisk provided funding for experiments. A.D.C. is the Jacquelyn A. Turner and Dr. Dorothy J. Turner Chair in Diabetes Research.
Duality of Interest. E.N., C.F., and C.L.B. are employed by Novo Nordisk. A.D.C. is consultant to Eli Lilly and Novo Nordisk and has received research funding from both companies. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M.G. wrote the manuscript and collected all data. G.K., M.F.S., D.W.N., B.F., M.S.S., J.R.H., E.J.A., E.P.D., N.R., J.J.W., D.S.E., E.N., C.F., and C.L.B. assisted in some aspects of the experiments or with the data analysis. A.D.C. was involved in all intellectual and financial aspects of the study. All authors provided input during the writing of the manuscript. A.D.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as an oral presentation at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
See accompanying article, p. 3353.
References
- 1.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Bogdanović R. Diabetic nephropathy in children and adolescents. Pediatr Nephrol 2008;23:507–525 [DOI] [PubMed] [Google Scholar]
- 3.Skyler JS. Diabetic complications. The importance of glucose control. Endocrinol Metab Clin North Am 1996;25:243–254 [DOI] [PubMed] [Google Scholar]
- 4.Lueder GT, Silverstein J; American Academy of Pediatrics Section on Ophthalmology and Section on Endocrinology . Screening for retinopathy in the pediatric patient with type 1 diabetes mellitus. Pediatrics 2005;116:270–273 [DOI] [PubMed] [Google Scholar]
- 5.Nathan DM, Lachin J, Cleary P, et al.; Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group . Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003;348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar A, Klein R, Klein BE, Moss SE. Association between glycosylated hemoglobin level and cardiovascular and all-cause mortality in type 1 diabetes. Am J Epidemiol 2007;166:393–402 [DOI] [PubMed] [Google Scholar]
- 8.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421–431 [DOI] [PubMed] [Google Scholar]
- 9.Olson JC, Erbey JR, Forrest KY, Williams K, Becker DJ, Orchard TJ. Glycemia (or, in women, estimated glucose disposal rate) predict lower extremity arterial disease events in type 1 diabetes. Metabolism 2002;51:248–254 [DOI] [PubMed] [Google Scholar]
- 10.Diabetes Control and Complications Trial Research Group . Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 11.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia 2002;45:937–948 [DOI] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Stettler C, Diem P. Risk of adverse effects of intensified treatment in insulin-dependent diabetes mellitus: a meta-analysis. Diabet Med 1997;14:919–928 [DOI] [PubMed] [Google Scholar]
- 13.Feltbower RG, Bodansky HJ, Patterson CC, et al. Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes: results from the Yorkshire Register of diabetes in children and young adults. Diabetes Care 2008;31:922–926 [DOI] [PubMed] [Google Scholar]
- 14.Frier BM. Morbidity of hypoglycemia in type 1 diabetes. Diabetes Res Clin Pract 2004;65(Suppl. 1):S47–S52 [DOI] [PubMed] [Google Scholar]
- 15.Beck RW, Hirsch IB, Laffel L, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 1973;182:171–173 [DOI] [PubMed] [Google Scholar]
- 17.Siafarikas A, Johnston RJ, Bulsara MK, O’Leary P, Jones TW, Davis EA. Early loss of the glucagon response to hypoglycemia in adolescents with type 1 diabetes. Diabetes Care 2012;35:1757–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest 1993;91:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross LA, Warren RE, Kelnar CJ, Frier BM. Pubertal stage and hypoglycaemia counterregulation in type 1 diabetes. Arch Dis Child 2005;90:190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsalikian E, Tamborlane W, Xing D, et al.; Diabetes Research in Children Network (DirecNet) Study Group . Blunted counterregulatory hormone responses to hypoglycemia in young children and adolescents with well-controlled type 1 diabetes. Diabetes Care 2009;32:1954–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong RB, Laughlin MH. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol 1983;344:189–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison DK, Birkenhake S, Knauf SK, Kessler M. Local oxygen supply and blood flow regulation in contracting muscle in dogs and rabbits. J Physiol 1990;422:227–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenway CV, Stark RD. Hepatic vascular bed. Physiol Rev 1971;51:23–65 [DOI] [PubMed] [Google Scholar]
- 24.Bergman RN. Orchestration of glucose homeostasis: from a small acorn to the California oak. Diabetes 2007;56:1489–1501 [DOI] [PubMed] [Google Scholar]
- 25.Sindelar DK, Balcom JH, Chu CA, Neal DW, Cherrington AD. A comparison of the effects of selective increases in peripheral or portal insulin on hepatic glucose production in the conscious dog. Diabetes 1996;45:1594–1604 [DOI] [PubMed] [Google Scholar]
- 26.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985) 2000;89:81–88 [DOI] [PubMed] [Google Scholar]
- 27.Seeley RR, Stephens TD, Tate P. Essentials of Anatomy and Physiology. Boston, WCB/McGraw-Hill, 1999 [Google Scholar]
- 28.Galassetti P, Chu CA, Neal DW, Reed GW, Wasserman DH, Cherrington AD. A negative arterial-portal venous glucose gradient increases net hepatic glucose uptake in euglycemic dogs. Am J Physiol 1999;277:E126–E134 [DOI] [PubMed] [Google Scholar]
- 29.Dobbins RL, Davis SN, Neal DW, Cobelli C, Jaspan J, Cherrington AD. Compartmental modeling of glucagon kinetics in the conscious dog. Metabolism 1995;44:452–459 [DOI] [PubMed] [Google Scholar]
- 30.Myers SR, McGuinness OP, Neal DW, Cherrington AD. Intraportal glucose delivery alters the relationship between net hepatic glucose uptake and the insulin concentration. J Clin Invest 1991;87:930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moghimzadeh E, Nobin A, Rosengren E. Fluorescence microscopical and chemical characterization of the adrenergic innervation in mammalian liver tissue. Cell Tissue Res 1983;230:605–613 [DOI] [PubMed] [Google Scholar]
- 32.Edgerton DS, Cardin S, Emshwiller M, et al. Small increases in insulin inhibit hepatic glucose production solely caused by an effect on glycogen metabolism. Diabetes 2001;50:1872–1882 [DOI] [PubMed] [Google Scholar]
- 33.Mari A, Stojanovska L, Proietto J, Thorburn AW. A circulatory model for calculating non-steady-state glucose fluxes. Validation and comparison with compartmental models. Comput Methods Programs Biomed 2003;71:269–281 [DOI] [PubMed] [Google Scholar]
- 34.Dobbins RL, Davis SN, Neal DW, Cobelli C, Cherrington AD. Pulsatility does not alter the response to a physiological increment in glucagon in the conscious dog. Am J Physiol 1994;266:E467–E478 [DOI] [PubMed] [Google Scholar]
- 35.Edgerton DS, Cardin S, Pan C, et al. Effects of insulin deficiency or excess on hepatic gluconeogenic flux during glycogenolytic inhibition in the conscious dog. Diabetes 2002;51:3151–3162 [DOI] [PubMed] [Google Scholar]
- 36.Winnick JJ, An Z, Moore MC, et al. A physiological increase in the hepatic glycogen level does not affect the response of net hepatic glucose uptake to insulin. Am J Physiol Endocrinol Metab 2009;297:E358–E366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winnick JJ, An Z, Kraft G, et al. Liver glycogen loading dampens glycogen synthesis seen in response to either hyperinsulinemia or intraportal glucose infusion. Diabetes 2013;62:96–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An Z, DiCostanzo CA, Moore MC, et al. Effects of the nitric oxide donor SIN-1 on net hepatic glucose uptake in the conscious dog. Am J Physiol Endocrinol Metab 2008;294:E300–E306 [DOI] [PubMed] [Google Scholar]
- 39.Edgerton DS, Moore MC, Winnick JJ, et al. Changes in glucose and fat metabolism in response to the administration of a hepato-preferential insulin analog. Diabetes 2014;63:3946–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shishko PI, Kovalev PA, Goncharov VG, Zajarny IU. Comparison of peripheral and portal (via the umbilical vein) routes of insulin infusion in IDDM patients. Diabetes 1992;41:1042–1049 [DOI] [PubMed] [Google Scholar]
- 41.Saudek CD, Duckworth WC, Giobbie-Hurder A, et al.; Department of Veterans Affairs Implantable Insulin Pump Study Group . Implantable insulin pump vs multiple-dose insulin for non-insulin-dependent diabetes mellitus: a randomized clinical trial. JAMA 1996;276:1322–1327 [PubMed] [Google Scholar]
- 42.Liebl A, Hoogma R, Renard E, et al.; European DiaPort Study Group . A reduction in severe hypoglycaemia in type 1 diabetes in a randomized crossover study of continuous intraperitoneal compared with subcutaneous insulin infusion. Diabetes Obes Metab 2009;11:1001–1008 [DOI] [PubMed] [Google Scholar]
- 43.Edgerton DS, Lautz M, Scott M, et al. Insulin’s direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest 2006;116:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frizzell RT, Hendrick GK, Biggers DW, et al. Role of gluconeogenesis in sustaining glucose production during hypoglycemia caused by continuous insulin infusion in conscious dogs. Diabetes 1988;37:749–759 [DOI] [PubMed] [Google Scholar]
- 45.Bergman BC, Howard D, Schauer IE, et al. Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. J Clin Endocrinol Metab 2012;97:1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donga E, van Dijk M, Hoogma RP, Corssmit EP, Romijn JA. Insulin resistance in multiple tissues in patients with type 1 diabetes mellitus on long-term continuous subcutaneous insulin infusion therapy. Diabetes Metab Res Rev 2013;29:33–38 [DOI] [PubMed] [Google Scholar]
- 47.Nadeau KJ, Regensteiner JG, Bauer TA, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab 2010;95:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schauer IE, Snell-Bergeon JK, Bergman BC, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes 2011;60:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yki-Järvinen H, Sahlin K, Ren JM, Koivisto VA. Localization of rate-limiting defect for glucose disposal in skeletal muscle of insulin-resistant type I diabetic patients. Diabetes 1990;39:157–167 [DOI] [PubMed] [Google Scholar]
- 50.Yki-Järvinen H, Taskinen MR, Kiviluoto T, et al. Site of insulin resistance in type 1 diabetes: insulin-mediated glucose disposal in vivo in relation to insulin binding and action in adipocytes in vitro. J Clin Endocrinol Metab 1984;59:1183–1192 [DOI] [PubMed] [Google Scholar]