Abstract
Background
Patients with homozygous familial hypercholesterolemia (HoFH) respond inadequately to existing drugs. We conducted a phase 3 study to assess the efficacy and safety of the microsomal triglyceride transfer protein inhibitor lomitapide in adults with HoFH.
Methods
Twenty-nine subjects enrolled into a single-arm, open-label study and maintained current lipid lowering therapy from six weeks before baseline through at least week 26. Lomitapide dose was escalated based on safety and tolerability from 5 mg to a maximum of 60 mg/day. The primary endpoint was mean percent change from baseline in LDL-C at week 26, after which patients remained on lomitapide through week 78 for safety assessment.
Findings
Twenty-three subjects completed weeks 26 and 78. The median dose of lomitapide was 40 mg/day. LDL-C was reduced by 50% from baseline at week 26 (4·3 ± 2·5 mmol/L vs. 8·7 ± 2·9 mmol/L, p<0.0001). Eight subjects achieved LDL-C <2·6 mmol/L at this time point. LDL-C was reduced by 44% at week 56 and 38% at week 78 (p<0.0001 for both). Gastrointestinal symptoms were the most common adverse event. Four patients had aminotransaminase > 5× ULN that resolved after dose reduction or temporary interruption of lomitapide. No subject permanently discontinued treatment due to liver abnormalities. Liver fat content assessed by nuclear magnetic resonance spectroscopy (NMRS; n=20) was 1·0 ± 1·3 % at baseline, 8·6 ± 8·1% at week 26 and remained stable up to week 78 (8·3± 5·3%).
Interpretation
These data demonstrate that lomitapide had a robust and durable efficacy in lowering LDL-C in patients with HoFH with an acceptable safety and tolerability profile.
INTRODUCTION
Homozygous familial hypercholesterolemia (HoFH) is a life-threatening disease most commonly caused by loss-of-function mutations in both alleles of the low-density lipoprotein (LDL) receptor gene. Mutations in other genes, including apolipoprotein (apo) B, proprotein convertase subtilisin/kexin type 9 (PCSK9), and the autosomal recessive hypercholesterolemia (ARH) LDL receptor adapter protein1, which alter the function of the LDL receptor or its ligand apoB, may also contribute to such a phenotype. As a consequence of impaired LDL receptor function, untreated total plasma cholesterol levels are typically greater than 13 mmol/L, resulting in premature and progressive atherosclerosis often leading to cardiovascular disease (CVD) before age 20 and death before age 301–3. Early initiation of aggressive treatment for these patients is therefore essential4.
Patients with HoFH respond inadequately to conventional drug therapies2,5–7, which generally lower LDL-cholesterol (LDL-C) through up-regulation of hepatic LDL receptors. Therefore, the current standard of care in HoFH includes LDL apheresis, which transiently reduces LDL-C by more than 50%8,9 and may delay the onset and progression of atherosclerosis7–9. However, even with the combined use of available drug therapies and apheresis, these patients still exhibit markedly elevated LDL-C levels and persistently high cardiovascular risk10. Liver transplantation has also been performed in patients with this disease11,12. In recent years alternative therapeutic approaches have been developed that target either apoB synthesis13 or the production of VLDL, the precursor of LDL14.
Lomitapide (Aegerion Pharmaceuticals Inc., Cambridge, MA) is an inhibitor of the microsomal triglyceride transport protein (MTP), a key protein in the assembly and secretion of apolipoprotein (apo) B-containing lipoproteins in the liver and intestine15. Lomitapide was shown to markedly reduce LDL-C levels in the Watanabe Heritable Hyperlipidemic rabbit, an animal model of HoFH16. We previously demonstrated that lomitapide administered orally for 16 weeks as monotherapy was effective in reducing LDL-C in 6 subjects with HoFH and that its efficacy was mediated by a reduction in LDL production14. To evaluate the long-term safety and efficacy of lomitapide when added to currently available lipid-lowering drug therapy with or without apheresis (standard of care), we performed a phase 3 multicenter, uncontrolled, open-label study in 29 HoFH patients over a 78 week treatment period. Safety assessments included an evaluation of the effects of chronic MTP inhibition on the liver.
METHODS
Subjects
Twenty-nine male and female adult subjects with HoFH, aged 18 years and older, were recruited from 11 centers in four countries (USA, Canada, South Africa and Italy). All subjects met diagnostic criteria for HoFH based either on clinical criteria (history of untreated total cholesterol >13 mmol/L and triglycerides <3.4 mmol/L and both parents with history of untreated total cholesterol >6.5 mmol/L) or on documented mutation(s) in both alleles of the LDL receptor or of other genes known to affect LDL receptor function. Exclusion criteria included: major surgery in the previous three months, congestive heart failure, history of liver disease or transaminases greater than two times the upper limit of normal (ULN), serum creatinine >221 µmol/L, recent malignancy, alcohol or drug abuse, known bowel disease or malabsorption, or chronic lung disease.
Study Protocol
Subjects were screened for eligibility 12 weeks prior to the first dose of lomitapide. Screening procedures included medical and medication history, review of current lipid-lowering therapies, physical examination, vital signs, 12-lead ECG, fasting lipid panel, safety laboratory assessments, and dietary counseling. All enrolled patients were required to enter a minimum 6-week run-in phase during which concomitant lipid-lowering therapies, including apheresis, the required low-fat diet were stabilized and the daily dietary supplementation of vitamin E and essential fatty acids initiated. At the end of the run-in phase, patients entered a 26-week efficacy phase, during which they received lomitapide in addition to their current lipid-lowering therapy. Lomitapide was initiated at a starting dose of 5 mg/day for the first two weeks and then escalated to 10, 20, 40, and 60 mg/day at 4-week intervals or until an individually determined maximum dose was achieved based on safety and tolerability. Patients remained at their maximum dose through the end of the 26-week efficacy phase. A fasting lipid and safety panel, including liver function tests, was obtained at baseline, prior to each dose escalation, and then every 4 weeks through week 26 (primary endpoint).
Following completion of the efficacy phase, patients entered a 52-week safety phase (Weeks 26–78) during which they continued to receive lomitapide and during which concomitant lipid-lowering therapies, including LDL apheresis, could be modified at the investigators discretion. Assessments during this phase were conducted every 5–10 weeks and at the end of treatment. Total treatment duration was 78 weeks. Eligible subjects completing the treatment phase were offered the option to enter a separate long-term study, in which subjects continued to receive lomitapide. Subjects who did not enter the long-term study discontinued lomitapide at week 78 and returned for a final follow up visit at week 84.
If subjects experienced confirmed ALT or AST elevations between five and 9·9 time ULN, or >100 U/L but <200 U/L above the baseline value, the dose of lomitapide was reduced to the previously tolerated dose level, with the possibility to re-escalate once transaminase elevations were resolved. Adverse events (AEs) were coded using MedDRA, Version 11.0. AEs were judged by the investigators as not related, unlikely, possibly, probably or definitely related to study drug and were reviewed regularly by an independent Data and Safety Monitoring Board (DSMB). The study was approved by each institution’s Institutional Review Board or Ethics Committee and all patients provided written, informed consent. This study was registered with ClinicalTrials.gov (NCT00730236).
Laboratory Analysis
Blood was drawn at baseline and at each visit following a 12 hour fast. Routine testing included a standard metabolic panel, a complete blood count, urinalysis and measurement of fat soluble vitamins and fatty acids. All testing was performed at a CDC-standardized lipid central laboratory (PPD, Highland Heights, KY, USA and Brussels, Belgium) or referred to a partnering laboratory for the measurement of vitamin K and essential fatty acids. In patients undergoing apheresis, samples for the fasting lipid profile were obtained shortly before the scheduled apheresis treatment and the timing of treatments (e.g. every 14 days) and study blood sampling was maintained throughout the study so that lipid assessments would be performed at the same point on the LDL-C rebound curve. Lipid and lipoprotein analyses were performed using serum. Total cholesterol, directly measured LDL-C and high density lipoprotein-cholesterol (HDL-C), and triglycerides were measured enzymatically. Non HDL-C and VLDL-C were calculated. Apolipoprotein (apo) A-I and apoB were measured by immunonephelometry.
Nuclear magnetic resonance spectroscopy (NMRS) for quantification of hepatic fat
Hepatic lipid content was assessed by NMRS imaging studies at baseline and at six-month intervals. All quantitative measurements were performed by a single radiologist who was blinded to the patient’s clinical status and liver function tests. NMRS was not performed in three patients who had contraindications to MRI. In these subjects a computed tomography (CT) scan or ultrasound was performed at the discretion of the local physician or if recommended by the DSMB.
Statistical analysis
The sample size calculation was based on an assumption of a 25% change from baseline in LDL-C at week 26 with a 30% standard deviation and 15% dropout rate. Using an alpha of 0·05 with 90% power, 20 subjects were needed. The statistical analyses were performed using SAS software (Version 9.1, SAS Institute; Cary, NC). Continuous variables were summarized by descriptive statistics (sample size, mean, standard deviation, median, minimum and maximum). Categorical variables were summarized by frequency (N) and percentages (%). Baseline values of lipid parameters were calculated using the average of two measurements taken 2 weeks a part (after 4 and 6 weeks of entering the run-in phase). The primary efficacy endpoint measure was the percent change from baseline in LDL-C concentration at the maximum tolerated dose after 26 weeks of treatment. Pre-specified secondary endpoints included percent changes in other lipid parameters, long-term safety and changes in hepatic fat content. All patients who received at least one dose of the study drug were considered in the assessment of the primary and secondary endpoints (intention-to-treat analysis) up to the end of the efficacy phase (week 26). Statistical significance of the percent changes from baseline to 26 weeks was assessed using a mixed linear model which assumes a missing-at-random mechanism. Analysis was also conducted in which missing data were imputed using the last observation carried forward method, as this was the statistical approach described in the original statistical analysis plan. Further secondary efficacy and safety analyses were performed during the safety phase (weeks 26–78); on-sample t-test were used to evaluate percent change from baseline at week 56 and 78. Statistical significance was defined as p value ≤0·05.
RESULTS
Study subjects
Of the 32 subjects with HoFH that were screened for eligibility, 31 entered the run-in period and 29 were enrolled in the study, with 23 (79%) completing both the efficacy phase (26 weeks) and the full study (78 weeks) (supplemental figure 1). Of the six subjects that discontinued the study, all occurred during the efficacy phase, the first 4 days after enrollment and the last at week 22; four were due to AEs (three were gastrointestinal [GI] events and one was headache); one was withdrawn for non-compliance with the protocol; and one withdrew consent for personal reasons.
The baseline characteristics of the subjects enrolled in the study are reported in the supplemental material and summarized in supplemental table 1. Briefly, all 29 subjects were either homozygotes or compound heterozygotes for mutations in the LDLR gene or genes affecting LRL receptor functionality. Twenty-seven subjects were being treated with statins, primarily rosuvastatin or atorvastatin, 22 with ezetimibe (all in combination with a statin), three with niacin, one with a fibrate and one with a bile acid sequestrant. Eighteen subjects were regularly undergoing apheresis with a frequency that ranged from weekly to every six weeks. Despite aggressive lipid lowering treatment, total cholesterol, LDL cholesterol and apoB were markedly elevated at baseline (table 1).
Table 1.
Lipid and lipoprotein levels at baseline and weeks 26, 56 and 78 (end of study) ^
| Baseline (n=29) |
26 weeks (n=23) |
%Change from baseline |
p value* |
Week 56 (n=23) |
%Change from baseline |
p value** |
Week 78 (n=23) |
%Change from baseline |
p value** |
|
|---|---|---|---|---|---|---|---|---|---|---|
| TC | 11·1±3·5 | 6·1±2·9 | −46 (−56, −35) | <0·0001 | 7·1± 3·7 | −39 (−51, −27) | <0·0001 | 7·3±3·9 | −35 (−48, −22) | <0·0001 |
| LDL-C | 8·7±2·9 | 4·3±2.5 | −50 (−62, −39) | <0·0001 | 5·1±3·2 | −44 (−57, −31) | <0·0001 | 5·4±3·4 | −38 (−52, −24) | 0·0001 |
| VLDL-C | 0·5±0·3 | 0·3±0.3 | −45 (−61, −29) | <0·0001 | 0·4±0·4 | −28 (−48, −10) | 0·0185 | 0·4±0·4 | −31(−54, −7) | 0·0389 |
| Non HDL-C | 10·0±3·4 | 5·1±2·8 | −50 (−61, −39) | <0·0001 | 5·9±3·6 | −44 (−57, −31) | <0·0001 | 6·2±3·8 | −39 (−53, −25) | <0·0001 |
| Triglycerides | 1·0(0·4–2·9) | 0·5 (0·1–1·7) | −45 (−61, −29) | <0·0001 | 0·7 (0·2–2·9) | −29 (−47, −11) | 0·0157 | 0·7 (0·2–4·1) | −31 (−54, −8) | 0·0368 |
| apoB | 2·6±0·8 | 1·3±0·7 | −49 (−60, −38) | <0·0001 | 1·5±0·8 | −45 (−57, −33) | <0·0001 | 1·5± 0·9 | −43 (−56, −29) | <0·0001 |
| Lp(a) | 0·8±0·6 | 0·6±0·4 | −15(−30, −0·9) | 0.0003 | 0·6±0·5 | −19·(−31, −8) | 0.0111 | 0·8±0·5 | −0·5(−17,6] | 0·5827 |
| HDL-C | 1·1±0·3 | 1·0±0·4 | −12 (−20, −4) | 0·0001 | 1·2±0·4 | 0·5 (−13, 15) | 0·954 | 1·1±0·3 | −5 (−13, 3) | 0·1396 |
| apoA-I | 1·2±0·3 | 1·0±0·2 | −14 (−17, −4) | 0·0003 | 1·1±0·3 | 0·77 (−11, 13) | 0·568 | 1·1±0·3 | −4 (−10, 3) | 0·1155 |
Data are reported in mmol/L or g/l (apo A-I, apoB and Lp(a)). Absolute values are mean ± SD or median (range), % Change from baseline values are mean (95% CI);
p-values from mixed model;
p-value from one-sample t-test.
Conversion to mg/dL for total cholesterol, VLDL-C, LDL-C, HDL-C, and non-HDL-C, divide by 0.02586.
Conversion to mmol/L for triglycerides, divide by 0.01129; Conversion to mg/dL for Apolipoproteins B and A, divide by 0.01
Compliance with study drug dosing, defined as >80% of capsules taken, was 93% during the efficacy phase and 95% during the safety phase. Of the six subjects who discontinued lomitapide treatment, two were receiving 5 mg, two were receiving 10 mg, one was receiving 20 mg and one was receiving 40 mg. Among the 23 subjects who completed the study, the maximal dose was 5 mg in one subject; 20 mg in five subjects; 40 mg in six subjects and 60 mg in 11 subjects at the end of the efficacy phase. The dose distribution remained similar at week 78.
Effects of lomitapide on plasma lipids and lipoproteins
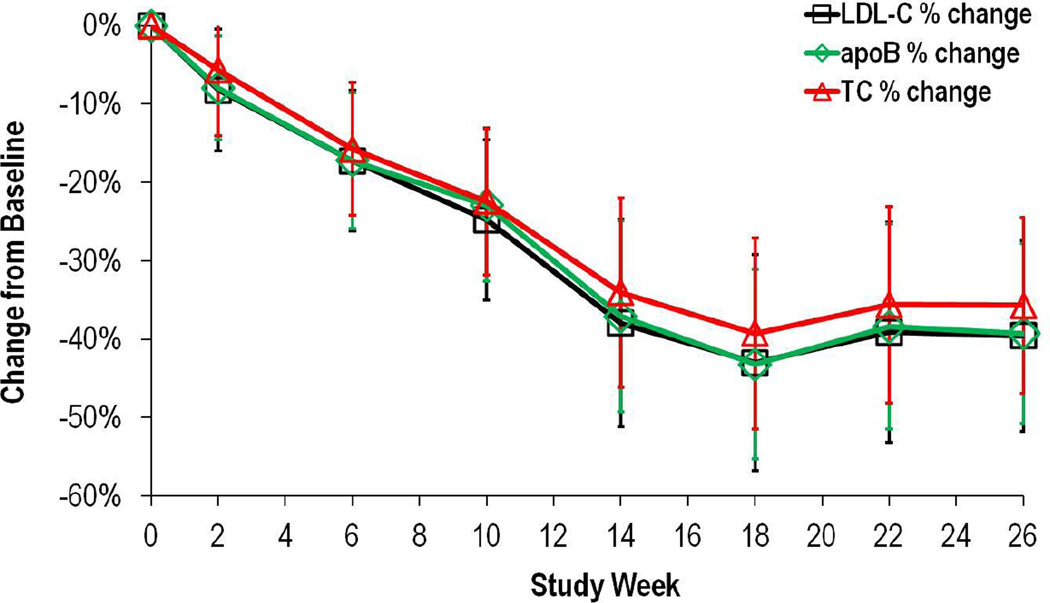
Mean LDL-C levels remained stable during the run-in phase, as shown by a mean percent change from screening in LDL-C of −1·2% (95% CI −15·66, 13·18) at week 0. Mean LDL-C decreased from 8·69 ± 2·95 mmol/L at baseline to 4·34 ± 2·48 mmol/L) at the end of the efficacy phase (week 26), a statistically significant percent change from baseline (−50%, 95% CI −62, −39, p<0·001) (table 1 and figure 1). Percent changes from baseline for key secondary end points (TC, apoB, and triglycerides) were consistent with those for LDL-C at week 26 (−46%, −49%, −45% respectively, table 1). Analysis performed using the last observation carried forward gave similar results.
Figure 1.
Mean percent changes in LDL-C, TC and apoB levels from baseline to week 26 (end of efficacy phase). Intention to treat analysis, n=29. Data are expressed as mean, 95%CI.
Overall, 19 subjects (83% of the subjects with data at week 26) experienced a decrease in LDL-C of > 25% with 52% having greater than a 50% reduction. Eight subjects achieved LDL-C levels <2·6 mmol/L (<100 mg/dL) at week 26, with one of these patients having levels <1·8 mmol/L (<70 mg/dL). Based on the LDL-C response, three subjects permanently discontinued LDL apheresis and three subjects permanently increased the time interval between apheresis treatments at some point during the safety phase (weeks 26–78). LDL-C efficacy at week 78 was −38% (95% CI −52, −24, p<0·0001) despite changes in concomitant lipid lowering therapy or any adjustment in lomitapide dose. Similar efficacy results were observed for total cholesterol, apoB and triglycerides (table 1).
Lp(a) levels were significantly reduced 15% and 19% from baseline at week 26 and 56 respectively (table 1), but were not significantly different at week 78.
A change of −12% in HDL cholesterol was observed (p<0·001) at week 26 that mirrored a −14% change in apoA-I (p<0·001). HDL-C and apoA-I returned to levels similar to those at baseline by week 78 (−5·0%, p=0·140 and −3·5%, p=0·116 for HDL cholesterol and apoA-I, respectively) (table 1).
Safety and Tolerability
A summary of adverse events reported during the efficacy and safety phase is presented in the Supplemental Material and in the supplemental table 4. The majority of subjects experienced at least one adverse event during both the efficacy (27 of 29, 93%) and safety (21 of 23, 91%) phases. The majority of the adverse events were assessed as mild to moderate in intensity. The most commonly reported types of events during treatment with lomitapide were GI in nature (93% and 74% during the efficacy and the safety phases, respectively). The three patients who discontinued the study due to GI disorders permanently stopped lomitapide by week 12 (see supplemental material for more details). There were no deaths during the study. Three (10%) of the 29 subjects experienced serious adverse events (SAEs) and included one subject with acute coronary syndrome/angina pectoris and lower respiratory tract infection, one subject with elective hysterectomy for menorrhagia and one subject with chest pain. All SAEs were assessed as unrelated or unlikely related to study treatment. No serious adverse events were reported between weeks 26 and 78.
Liver safety and liver fat
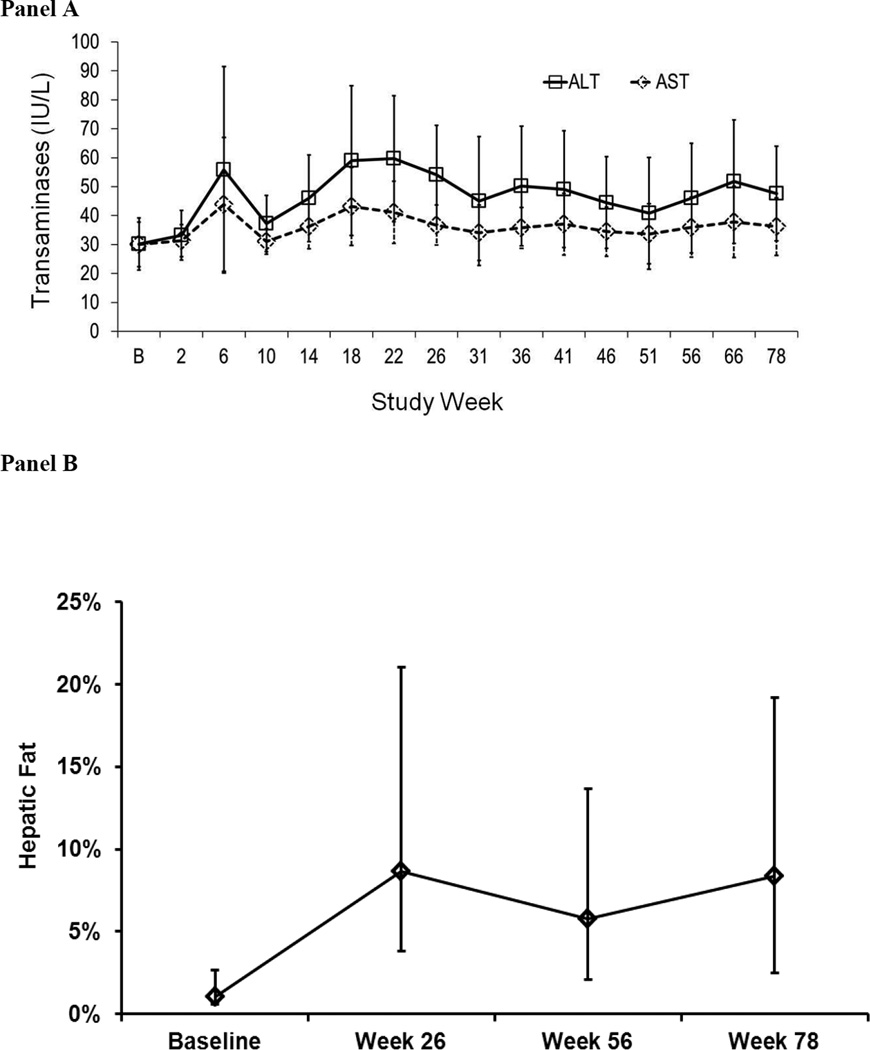
Mean ALT and AST levels over time are shown in Figure 2A. Ten subjects experienced elevations of ALT and/or AST >3× ULN once or more during the study. Four of these subjects exhibited ALT increases >5× ULN, accompanied in one such patient by a similar elevation in AST; these elevations occurred at lomitapide doses of 10 mg, 20 mg, 40 mg and 60 mg. No subject discontinued treatment permanently due to LFT elevations and all elevations were managed either by dose reduction or temporary interruption of lomitapide as per protocol. Of note, three of the four patients with LFT elevations >5× ULN reported consuming quantities of alcohol higher than those allowed per protocol. No subject experienced elevations in bilirubin or alkaline phosphatase levels.
Figure 2.
A. ALT and AST levels (mean, 95%CI) measured at baseline and at regular intervals during the study. Laboratory reference ranges were: ALT: Male (10–40 U/L), Female (10–33 U/L); AST: Male (10–43 U/L), Female (10–36 U/L). B. Percentage of Fat in the Liver (mean, 95%CI), as measured by MRI/NMRS at Baseline and 26, 56 and 78 weeks of lomitapide treatment (n=20).
Hepatic fat was measured non-invasively using NMRS. In the 20 subjects that had evaluable NMRS scans at baseline and week 26, 56 and 78, mean hepatic fat was 1·0% (range 0·0–5·0%) at baseline, 8·6% (range 0·0–33·6 %) at Week 26, 5·8% (range 0·0–16·5%) at Week 56 and 8.3% (range 0·0–19%) at Week 78 (Figure 2B). Change in percent hepatic fat was negatively associated with change in LDL-C. This association was statistically significant at week 26 and 56 (−0·50, p=0·0161 and −0·55, p=0·0083), but not so at week 78 (−0.2117, p=0.3618).
DISCUSSION
Patients with HoFH have an inadequate response to existing lipid-lowering drug therapies such as statins and ezetimibe7,17–19 and remain at very high risk for cardiovascular events and mortality. The results of the current open-label study demonstrate that treatment of HoFH patients with the MTP inhibitor lomitapide, administered concurrently with background lipid-lowering therapies including LDL apheresis, significantly reduced LDL-C levels by approximately 50%. More than one-third of the patients completing the efficacy phase had LDL-C levels <2·6 mmol/l, putting them close to the accepted therapeutic goals. This reduction is similar to that observed during lomitapide monotherapy in HoFH patients14, and indicates that lomitapide showed similar efficacy when added to existing concomitant treatment..
While cardiovascular outcome studies are not feasible given the rarity of HoFH, retrospective studies show that even a modest reduction in LDL- C, either by pharmacological intervention or LDL-apheresis, results in apparent improvement in morbidity and mortality6,8,9,20. Furthermore, observational studies clearly indicate that HoFH patients with some LDL receptor function (“receptor-defective”) have lower LDL-C levels and better prognosis than those with no LDL receptor function (“receptor-negative”)4. Thus, although we are unable to provide direct evidence, this magnitude of LDL-C reduction with lomitapide would be expected to reduce cardiovascular risk and improve survival.
LDL-C lowering was somewhat attenuated at the end of the study. This may be explained by the changes during the safety phase that were made in apheresis treatment or in concomitant lipid lowering therapy in some of the better responders, as well reductions in lomitapide dose in some of the subjects that experienced liver enzyme elevation or gastrointestinal tolerability issues.
We observed a statistically significant decrease in Lp(a) levels at week 26, that persisted up to week 56. The mechanism underlying this effect is not known, but a similar finding is observed also with other drugs affecting the secretion of apoB-containing lipoproteins by the liver17. The reason for loss of statistical significance in Lp(a) lowering at week 78 is not clear. Lp(a) levels are markedly affected by apheresis treatment21,22, thus it is possible that the changes in apheresis treatment that were allowed during the safety phase may have confounded the observed effect on Lp(a). Further studies are needed to test this hypothesis and clarify these findings.HDL-C and apoA-I levels were transiently decreased during the efficacy phase, a phenomenon observed in prior studies with lomitapide14,23. The mechanism(s) underlying these changes are not known and further studies will be necessary to explain this effect. Possible reasons may include the low fat diet or the inhibitory effects of lomitapide on dietary fat absorption; the reduced secretion of TG-rich lipoproteins, which carry apoA-I, from the gut and/or liver, as direct consequence of MTP inhibition, or a reduction in apoA-I production. Interestingly, the decrease in HDL-C levels was observed during the titration period, when the dose was gradually increased, and subsequently returned to levels approaching those at baseline once the dose was stabilized, suggesting that a compensatory mechanism may be in play. The clinical implications of this temporary reduction in HDL-C levels is unknown.This study was the first long-term study of any MTP inhibitor in humans and safety and tolerability were carefully assessed. Lomitapide, initiated at a low dose and escalated to an individualized maximum dose in the presence of a low-fat diet, was generally well tolerated. It is important to note that all three discontinuations due to GI events occurred during the titration phase. The incidence and the number of patients who experienced GI events improved during the safety phase suggesting that patients become more tolerant or learn to control their diet better, similarly to patients with abetalipoproteinemia15. Indeed, of the 23 subjects who completed the efficacy phase, all 23 remained on lomitapide for another 12 months and completed the entire protocol. As this was an open-label study in which investigators and patients were aware of the lomitapide dose and the lipid response, we cannot exclude the possibility that this influenced their reporting and assessment of adverse events.
Liver fat accumulation is intrinsically linked to the mechanism of action of MTP inhibitors, and has been the basis of concerns regarding the clinical use of this class of agents. The 18 month duration of this study afforded the first opportunity to assess the effect of chronic MTP inhibition on liver safety and liver fat. While ALT levels >3× ULN were seen in ten of 29 subjects, they were generally transient or resolved with dose reduction and were not associated with elevated bilirubin or alkaline phosphatase or evidence of impaired synthetic function.
As expected, mean hepatic fat increased from 1·0% to 8·6% at week 26, but no further increase was observed for the remainder of the study. As there is no a clear understanding of the clinical significance and long-term implications of the increase in hepatic fat as a result of lomitapide therapy, rigorous and standardized long-term monitoring will be necessary.
This study has several limitations that need to be considered when interpreting the results. This was an uncontrolled, open-label study. Since HoFH is a rare disease, our intent was to expose the maximum number of subjects to treatment for the duration of the study so that safety (especially surrounding the potential liver adverse events) could be assessed fully. Furthermore, based on the marked changes in LDL-C and apoB that were observed in the phase 2 study14 we expected to be able to easily discern the effect of lomitapide treatment from the potential effects of any variables that might confound the interpretation, such as regression to the mean. We acknowledge that the absence of a control group could bias the interpretation of the efficacy data, however we minimized this possibility with the introduction of a run-in period to stabilize low-fat diet and concomitant lipid lowering treatments and assess the effect, if any, of these factors, as well as establishing the baseline for lipid-related data as the average of two measurements taken two weeks apart at the end of the run in period. The inclusion of subjects receiving apheresis treatment may also potentially introduce a confounder for the assessment of LDL-C lowering. However, given the well-defined rules that were followed if apheresis treatment was present, we do not believe that the primary end-point results are confounded by the presence of such treatment. Finally, we believe that the subjects enrolled in this study are representative of the adult HoFH patients followed in the usual clinical setting and that the results obtained can be generalized and applied globally to different healthcare realities. In summary, lomitapide, added to a low fat diet and ongoing lipid-lowering treatment, was effective in substantially and stably reducing the levels of LDL-C and apoB in adult patients with HoFH and maintaining these effects over 1·5 years. While most subjects had at least one reported GI-related adverse effect and three of 29 subjects withdrew due to GI-related symptoms early in the study, the overall frequency of GI-related side effects diminished over time. The mean percent hepatic fat that was increased at six months remained stable thereafter. Overall, this study suggests that the benefit-risk of lomitapide in HoFH patients, who are at high risk of cardiovascular events and death at a young age, may be favorable.
Panel - Research in context
Systematic review
We searched PubMed for intervention studies in HoFH. This is a rare condition with untreated cholesterol levels greater than 13 mmol/L. Drug-based treatments were scarcely effective until the introduction of HMG-CoA reductase inhibitors (statins). Treatment at high doses of atorvastatin and rosuvastatin results in about 27% reduction in LDL-C19. Addition of ezetimibe to statin treatment may result in an additional 20% LDL-C reduction7. Apheresis treatment may acutely lower LDL-C level by 70–80% and result in a time-average reduction by 40–50% when performed regularly24. Recently a phase 3 randomized placebo control study assessing the efficacy of an anti-apoB ASO, mipomersen, showed a reduction LDL-C of approximately 25% in HoFH treated with maximal tolerated lipid-lowering drug therapy13.
Interpretation
Given the significantly elevated levels of LDL-C in untreated HoFH, and despite the treatment with multiple concomitant lipid-lowering therapies, these patients cannot currently reach LDL-C goals. This study expands the results obtained in a previously published phase 2 study. We report that lomitapide, when given in addition to currently available lipid-lowering therapy, results in an additional 50% reduction in LDL-C, potentially bringing these high risk patients closer to goal levels. In balance, the limitations due to the single-arm open label design and the safety considerations of potential dose-related transaminase elevations and liver-fat accumulation, are counterbalanced and we think outweighed by the significant LDL-C lowering effects of lomitapide in this severe disorder of unmet medical need. This study suggests that treatment with lomitapide may be a valuable drug in the management of hypercholesterolemia in HoFH patients.
Supplementary Material
Acknowledgements
The phase II study on which this study was based was funded by the Doris Duke Charitable Foundation. Diagnostic work-up for patients in South Africa is supported by of the Medical Research Council of South Africa (Cape Heart Group).
We especially thank all of the staff at the clinical sites as well as the study participants for their participation in this study.
Role of the funding source:
The study was supported by a grant from the FDA Office of Orphan Products Development (OOPD) awarded to Dr. Cuchel (FR-R-003098) and by Aegerion Pharmaceuticals, Inc. The University of Pennsylvania Clinical and Translational Research Center, in which study subjects at this site were seen, is supported by a grant for the NIH National Center for Research Resources (UL1-RR-024134) awarded to the University of Pennsylvania. None of the authors were paid to write this article by Aegerion Pharmaceuticals, Inc. or any other agency. Dr. Cuchel had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Dr. Cuchel received research grant, speaker honoraria and travel support for attending scientific meetings from Aegerion Pharmaceuticals. Dr. Blom received support for attending scientific meetings and speaking honoraria from Aegerion Pharmaceuticals. Dr. Sirtori owns equity in Aegerion Pharmaceuticals. Dr. Sasiela is a past employee and owns equity in Aegerion. L. Bloedon is an employee and owns equity in Aegerion Pharmaceuticals. Dr. Rader is an inventor on a patent related to lomitapide, serves as the chair of the Scientific Advisory Board for Aegerion, and owns equity in Aegerion. Dr. Sirtori and Dr. Rader were excluded from the day to day conduct of the study and were not involved in the care and management of study subjects.
Appendix
Phase 3 HoFH Lomitapide Study Investigators:
01 - University of Pennsylvania, Philadelphia, USA – M. Cuchel (lead PI), E.A. Maegher, D.M. Kolansky, B.S. Sachais
02 - Cedars-Sinai Heart Institute, Los Angeles, USA – P.K. Shah
11 - University of Cape Town, South Africa - D.J. Blom, A.D. Marais
12 - Netcare Private Hospital, Bloemfontein, South Africa – H. du T. Theron
13 - Clinical Research Unit, Pretoria, South Africa – A.M.E. du Plessis
22 - University of Montreal, Chicoutimi, Canada – D. Gaudet
23 - Robarts Research Institute, London, Canada – R.A. Hegele
31 - Università di Palermo, Italy- M Averna, A.B. Cefalù, D. Noto
32 - Ospedale Niguarda, Milano, Italy – C. Sirtori, A. Bondioli, M. Triolo, G. Mombelli
33 - Università di Ferrara, Italy – G.B.Vigna, E. Lodi, E. Menegatti, E. Tosini
35 - Università La Sapienza, Roma, Italy – C. Stefanutti, S. Di Giacomo
Footnotes
Authors’ contribution
MC, DJR, WJS, and LTB designed the study in collaboration with the investigators. MC, EAM, HTT, DJB, ADM, RAH, MA, PKS, DG, CS, GBV, AMED were site investigators who recruited subjects and collected data for this study. KJP was the statistician at the University of Pennsylvania that supervised the statistical analysis. MC, EAM, DJB, ADM, RAH, CS, GBV, LTB, DJR contributed to the interpretation of the data. MC and DJR were the primary writers of the manuscript; all authors critically reviewed and approved the manuscript.
Conflicts of interest
All other authors have no conflict of interest to declare.
REFERENCES
- 1.Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. J Clin Invest. 2003;111(12):1795–1803. doi: 10.1172/JCI18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein JL, Hobbs HH, Brown MS. Familial Hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill Information Services Company; 2001. pp. 2863–2913. [Google Scholar]
- 3.Macchiaiolo M, Gagliardi MG, Toscano A, Guccione P, Bartuli A. Homozygous familial hypercholesterolaemia. Lancet. 2012;379(9823):1330. doi: 10.1016/S0140-6736(11)61476-1. [DOI] [PubMed] [Google Scholar]
- 4.Kolansky DM, Cuchel M, Clark BJ, et al. Longitudinal evaluation and assessment of cardiovascular disease in patients with homozygous familial hypercholesterolemia. Am J Cardiol. 2008;102(11):1438–1443. doi: 10.1016/j.amjcard.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Marais AD, Firth JC, Blom DJ. Homozygous familial hypercholesterolemia and its management. Semin Vasc Med. 2004;4(1):43–50. doi: 10.1055/s-2004-822985. [DOI] [PubMed] [Google Scholar]
- 6.Raal FJ, Pilcher GJ, Panz VR, et al. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation. 2011;124(20):2202–2207. doi: 10.1161/CIRCULATIONAHA.111.042523. [DOI] [PubMed] [Google Scholar]
- 7.Gagne C, Gaudet D, Bruckert E. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation. 2002;105(21):2469–2475. doi: 10.1161/01.cir.0000018744.58460.62. [DOI] [PubMed] [Google Scholar]
- 8.Hudgins LC, Kleinman B, Scheuer A, White S, Gordon BR. Long-term safety and efficacy of low-density lipoprotein apheresis in childhood for homozygous familial hypercholesterolemia. Am J Cardiol. 2008;102(9):1199–1204. doi: 10.1016/j.amjcard.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 9.Thompson GR, Barbir M, Davies D, et al. Efficacy criteria and cholesterol targets for LDL apheresis. Atherosclerosis. 2009;208(2):317–321. doi: 10.1016/j.atherosclerosis.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Thompson GR, Catapano A, Saheb S, et al. Severe hypercholesterolaemia: therapeutic goals and eligibility criteria for LDL apheresis in Europe. Curr Opin Lipidol. 2010;21(6):492–498. doi: 10.1097/MOL.0b013e3283402f53. [DOI] [PubMed] [Google Scholar]
- 11.Starzl TE, Bilheimer DW, Bahnson HT, et al. Heart-liver transplantation in a patient with familial hypercholesterolaemia. Lancet. 1984;1(8391):1382–1383. doi: 10.1016/s0140-6736(84)91876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maiorana A, Nobili V, Calandra S, et al. Preemptive liver transplantation in a child with familial hypercholesterolemia. Pediatr Transplant. 2011;15(2):E25–E29. doi: 10.1111/j.1399-3046.2010.01383.x. [DOI] [PubMed] [Google Scholar]
- 13.Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9719):998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 14.Cuchel M, Bloedon LT, Szapary PO, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356(2):148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 15.Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta. 1997;1345(2):136–150. doi: 10.1016/s0005-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 16.Wetterau JR, Gregg RE, Harrity TW, et al. An MTP inhibitor that normalizes atherogenic lipoprotein levels in WHHL rabbits. Science. 1998;282(5389):751–754. doi: 10.1126/science.282.5389.751. [DOI] [PubMed] [Google Scholar]
- 17.Raal FJ, Pilcher GJ, Illingworth DR, et al. Expanded-dose simvastatin is effective in homozygous familial hypercholesterolaemia. Atherosclerosis. 1997;135(2):249–256. doi: 10.1016/s0021-9150(97)00168-8. [DOI] [PubMed] [Google Scholar]
- 18.Raal FJ, Pappu AS, Illingworth DR, et al. Inhibition of cholesterol synthesis by atorvastatin in homozygous familial hypercholesterolaemia. Atherosclerosis. 2000;150(2):421–428. doi: 10.1016/s0021-9150(99)00435-9. [DOI] [PubMed] [Google Scholar]
- 19.Marais AD, Raal FJ, Stein EA, et al. A dose-titration and comparative study of rosuvastatin and atorvastatin in patients with homozygous familial hypercholesterolaemia. Atherosclerosis. 2008;197(1):400–406. doi: 10.1016/j.atherosclerosis.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Sachais BS, Katz J, Ross J, Rader DJ. Long-term effects of LDL apheresis in patients with severe hypercholesterolemia. J Clin Apher. 2005;20(4):252–255. doi: 10.1002/jca.20036. [DOI] [PubMed] [Google Scholar]
- 21.Stefanutti C, D'Alessandri G, Russi G, et al. Treatment of symptomatic HyperLp(a)lipoproteinemia with LDL-apheresis: a multicentre study. Atheroscler Suppl. 2009;10(5):89–94. doi: 10.1016/S1567-5688(09)71819-7. [DOI] [PubMed] [Google Scholar]
- 22.Hovland A, Marcovina S, Hardersen R, Enebakk T, Mollnes TE, Lappegard KT. Three different LDL apheresis columns efficiently and equally reduce lipoprotein(a) concentrations in patients with familial hypercholesterolemia and small apolipoprotein(a) particles. Transfus Apher Sci. 46(1):73–76. doi: 10.1016/j.transci.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Samaha FF, McKenney J, Bloedon LT, Sasiela WJ, Rader DJ. Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia. Nat Clin Pract Cardiovasc Med. 2008;5(8):497–505. doi: 10.1038/ncpcardio1250. [DOI] [PubMed] [Google Scholar]
- 24.Hudgins LC, Gordon BR, Parker TS, Saal SD, Levine DM, Rubin AL. LDL Apheresis: an effective and safe treatment for refractory hypercholesterolemia. Cardiovasc Drug Rev. 2002;20(4):271–280. doi: 10.1111/j.1527-3466.2002.tb00097.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.