Abstract
Whereas a B cell–transcriptional profile has been recorded for operationally tolerant kidney graft patients, the role that B cells have in this tolerance has not been reported. In this study, we analyzed the role of B cells from operationally tolerant patients, healthy volunteers, and kidney transplant recipients with stable graft function on T cell suppression. Proliferation, apoptosis, and type I proinflammatory cytokine production by effector CD4+CD25− T cells were measured after anti-CD3/anti-CD28 stimulation with or without autologous B cells. We report that B cells inhibit CD4+CD25− effector T cell response in a dose-dependent manner. This effect required B cells to interact with T-cell targets and was achieved through a granzyme B (GzmB)–dependent pathway. Tolerant recipients harbored a higher number of B cells expressing GzmB and displaying a plasma cell phenotype. Finally, GzmB+ B-cell number was dependent on IL-21 production, and B cells from tolerant recipients but not from other patients positively regulated both the number of IL-21+ T cells and IL-21 production, suggesting a feedback loop in tolerant recipients that increases excessive B cell activation and allows regulation to take place. These data provide insights into the characterization of B cell–mediated immunoregulation in clinical tolerance and show a potential regulatory effect of B cells on effector T cells in blood from patients with operationally tolerant kidney grafts.
Keywords: B cells, regulation, apoptosis, granzyme B, kidney transplantation, tolerance
Tolerance in transplantation is defined as the maintenance of long-term, good, stable graft function in the absence of immunosuppression.1,2 Numerous studies have demonstrated that tolerance can easily be achieved in rodent models,3–5 including models for renal transplant.6 However, while it remains rare in human renal transplant it does exist, current estimates report roughly a hundred cases of operational tolerance, mainly patients not compliant with their immunosuppressive regimens.1,2,7 These patients, defined as “operationally tolerant,” are healthy, do not exhibit more infections or malignancies than healthy volunteers (HVs), and do not display clinical evidence of immune incompetence.2,8 Specific patterns have been associated with this operational tolerance; in particular, a B cell transcriptional signature that correlates with an increase in peripheral B cells has been reproducibly found.9–14 Several of these B cell markers are being currently tested and validated in multicenter studies around the world to predict patients who may benefit from immunosuppression withdrawal. In addition, some of these B cell markers are being examined for their involvement in the tolerance process; however, to date, none have been shown to have a role in tolerance induction or maintenance, and whether B cells are involved, or even have a potential role in tolerance, remains to be determined.
In transplantation, B cells are mainly known for their capacity to differentiate into plasma cells and produce antibodies that may be deleterious for the graft.15,16 However, B cells also have antibody-independent functions. They are able to produce cytokines and to present antigens, and thus to initiate or maintain an immune response.17,18 More recently, populations of regulatory B cells (Bregs) able to dampen the immune response have been highlighted as a “driving force” in autoimmune diseases, cancers, and transplantation.19–23 However, their nature, origin, phenotype, and mode of action in humans remain little known.
We previously reported that B cells from tolerant patients (TOLs) do not fully differentiate into plasma cells and that, during their differentiation, B cells from tolerant recipients produce higher levels of IL-10,24 suggesting that an imbalance between a lower number of deleterious plasma cells and a higher level of Bregs producing IL-10 may exist in these patients. In this article, we investigate the role of B cells in blood from this cohort of operationally TOLs, from patients with stable graft function under classic immunosuppression (STAs), and from HVs. We report that tolerant recipients exhibit a higher number of IL-21–dependent peripheral B cells that express granzyme B (GzmB), display a specific phenotype, and exhibit a contact- and GzmB-dependent, IL-10– and TGF-β–independent inhibition of effector T cell response. These results provide novel insights into the characterization of B cell–mediated immunoregulation in tolerance in the clinic.
Results
CpG-CD40 Prestimulated B Cells Inhibit CD4+CD25− T Cell Proliferation
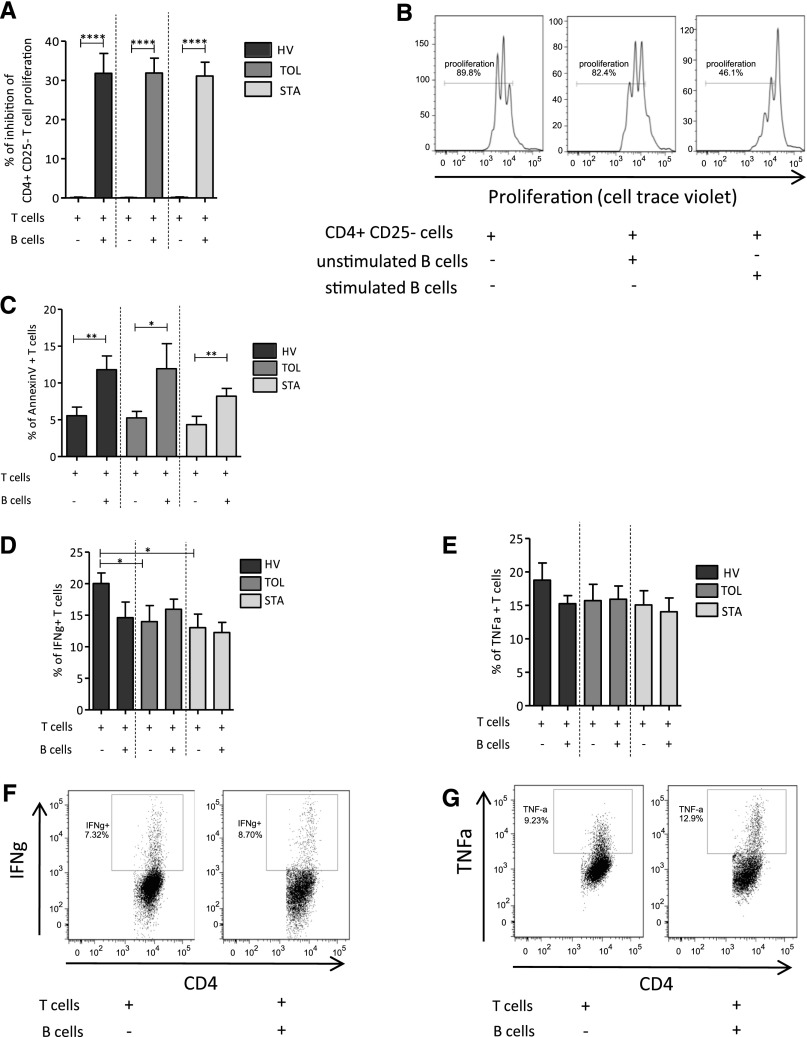
Human B cells have been shown to exhibit regulatory activity through the inhibition of various cell types, in particular through the inhibition of T cells. CD4+CD25− T cells were cocultured with CpG-CD40 stimulated or unstimulated autologous B cells, after polyclonal anti-CD3 and anti-CD28 activation, for 3 days and T cell proliferation was then analyzed, using CellTrace Violet staining. We found that prestimulated B cells from HVs, TOLs, and STAs significantly inhibit autologous CD4+CD25− T cell proliferation (Figure 1A), whereas unstimulated B cells have a lesser effect (Figure 1B). No difference was found in the number of total B cells and GzmB+ B cells or for B cell inhibition between men and women.
Figure 1.
CpG-CD40 prestimulated B cells inhibit CD4+CD25− T cell proliferation and induce T cell apoptosis without effect on type Th1 proinflammatory cytokine production. Effector T cell proliferation is followed by CellTrace Violet staining after 3 days activated with anti-CD3/anti-CD28 and cocultured with unstimulated B cells or B cells stimulated with CD40L and ODN 24 hours before the coculture. (A) Proportion of T cell proliferation inhibition when cocultured with or without prestimulated B cells in HVs (n=17), TOLs (n=12), and STAs (n=17) (mean±SEM; ****P<0.001). (B) Representative histograms of proliferation of T cells: alone, with unstimulated B cells, and with stimulated B cells from HVs. Apoptosis of effectors T cells is analyzed by Annexin V staining after 3 days of activation with anti-CD3/anti-CD28 and coculture with B cells prestimulated with CD40L and ODN 24 hours before the coculture. (C) Percentage of Annexin V T cells activated and cocultured with or without stimulated B cells in HVs (n=9), TOLs (n=10), and STAs (n=12) (mean±SEM; *P<0.05; **P<0.01). Secretion of IFN-γ and TNF-α is analyzed by intracellular staining in effector T cells after 3 days of coculture with B cells and anti-CD3/anti-CD28 activation. (D) Percentage of IFN-γ+ T cells activated and cocultured with or without B cells in HVs (n=17), TOLs (n=12), and STAs (n=14) (mean±SEM; *P<0.05). (E) Percentage of TNF-α+ T cells activated and cocultured with or without B cells in HVs (n=17), TOLs (n=12), and STAs (n=14) (mean±SEM). (F and G) Representative dot plots of the secretion of IFN-γ and TNF-α by T cells activated and cocultured with or without B cells from HVs.
CpG-CD40 Prestimulated B Cells Induce T Cell Apoptosis But Have No Effect on Proinflammatory Cytokine Production
Using Annexin V staining, apoptosis of CD4+CD25− T cells was measured at day 3 after anti-CD3/anti-CD28 activation and addition of prestimulated B cells to the culture. Prestimulated B cells and a 1:2 T cell/B cell ratio were used in all of the experiments. The addition of prestimulated B cells to the coculture induces a significant increase in CD4+CD25− T cell apoptosis in the three groups (Figure 1C). Interestingly, no difference was observed in apoptosis levels between cell trace+ and cell trace– T cells, confirming that the increase in apoptosis was not due to inhibition of T cell proliferation (data not shown). Type I helper T cell (Th1) proinflammatory cytokines (IFN-γ and TNF-α) were analyzed using intracellular staining after 3 days of coculture. IFN-γ T cell production was slightly lower when prestimulated B cells from HVs were added to the culture, but this was due to a slightly higher level of IFN-γ production by CD4+CD25− T cells from HVs only (Figure 1D). TNF-α production by T cells from the three groups of patients was unchanged when prestimulated B cells were added to the culture (Figure 1E). Representative pictures of IFN-γ and TNF-α production by T cells are displayed in Figure 1, F and G. Altogether, these data show that B cells from HVs, transplant TOLs, and STAs all inhibit T cell proliferation and induce T cell apoptosis but have no effect on Th1 proinflammatory cytokine production.
B Cell Inhibitory Effect on T Cells Is Dependent to GzmB and Is Contact Dependent
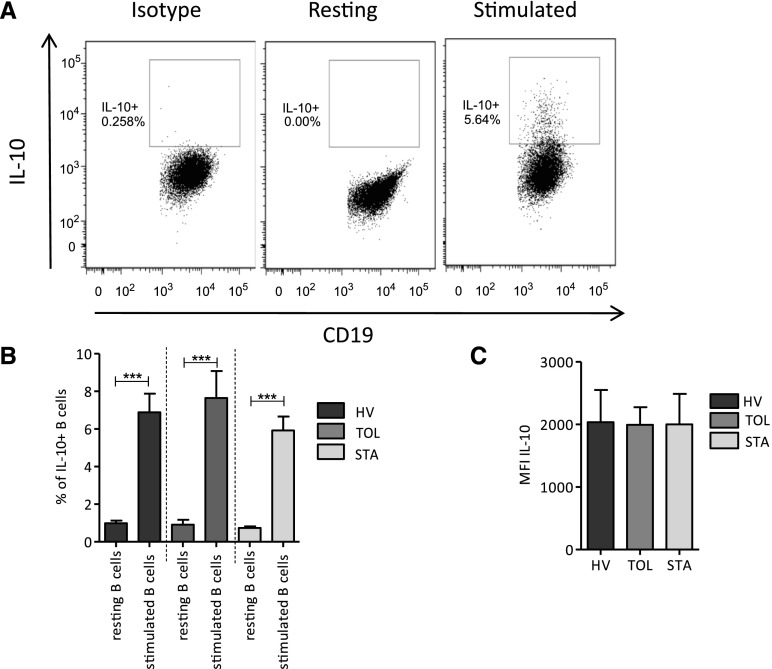
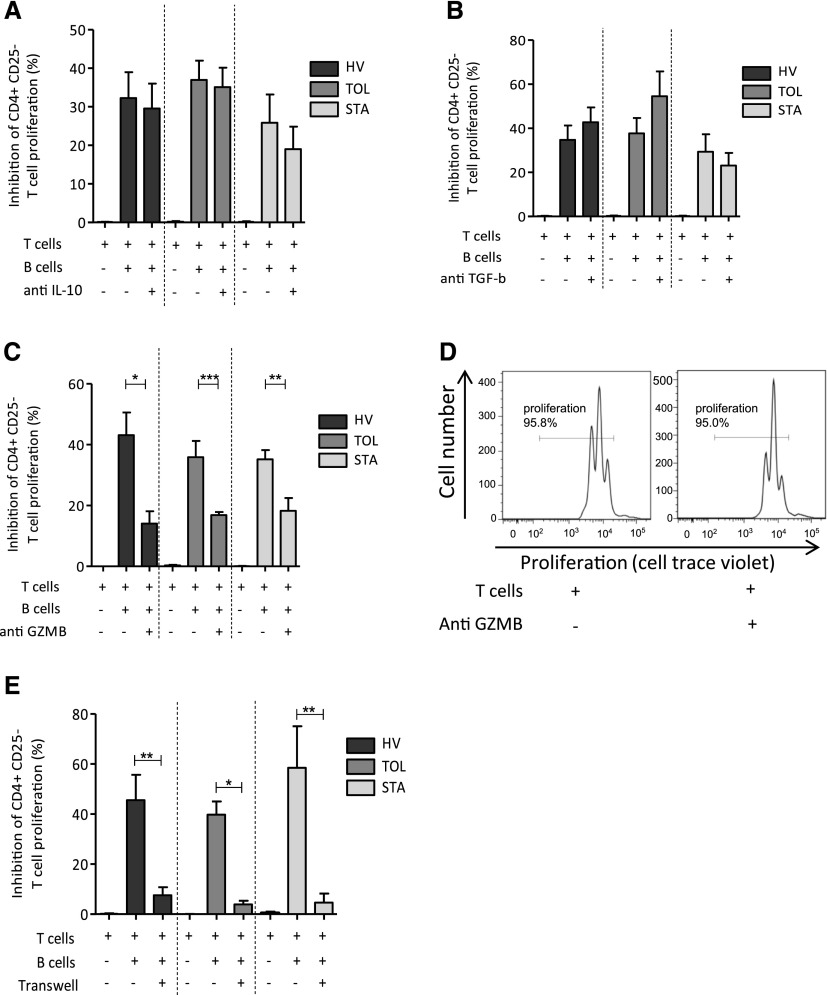
Having previously reported higher production of IL-10 by B cells from tolerant recipients during the differentiation process in vitro, as well as B cells having been shown to mainly display regulatory properties through IL-10, we decided to assess the role of IL-10 in our model. We looked at the frequency of IL-10–expressing B cells and the level of IL-10 expression by these B cells after 48 hours of CD40L and oligodeoxynucleotide (ODN) stimulation. As expected, although the resting B10 level was low, a significant and substantial increase in the frequency of B10 cells was found after activation (Figure 2A). No difference was observed in the frequency of B10 cells and in the relative amount of IL-10 expressed by B cells between the three groups of individuals (Figure 2, B and C). To assess the role of IL-10 in the coculture assay, we blocked its effect using anti–IL-10 antibody. We found that the blockade of IL-10 does not hinder the inhibitory effect of B cells on effector T cell proliferation (Figure 3A). Because other cytokines have been shown to play a role in the function of suppressive B cell populations, TGF-β and GzmB were similarly blocked by adding anti–TGF-β antibody and anti-GzmB peptide to the coculture at day 0. The blockade of TGF-β did not hinder the inhibitory effect of B cells on T cell proliferation (Figure 3B). However, for the three groups of patients, the addition of anti-GzmB peptide to the coculture significantly affects the suppressive effect of B cells on autologous CD4+CD25− T cell proliferation (Figure 3C), whereas GzmB inhibitor has no effect on T cell proliferation in the absence of B cells (Figure 3D).
Figure 2.
IL-10+ B cells and IL-10 secretion after 48-hour stimulation with CD40L/ODN. IL-10 expression was analyzed on B cells after 48-hour stimulation of PBMCs with CD40L and ODN. (A) Representative dot plot of IL-10 secretion in resting, stimulated B cells, and stimulated B cells staining with isotype control. (B) Percentage of IL-10+ B cells before and after activation in HVs (n=7), TOLs (n=9), and STAs (n=10) (mean±SEM; ***P<0.001). (C) Mean fluorescence intensity from IL-10 stimulated B cells in HVs (n=7), TOLs (n=9), and STAs (n=10).
Figure 3.
Regulation of effector T cell proliferation by B cells is contact and GzmB dependent. (A–C) T cell proliferation inhibition in HVs (n=12), TOLs (n=10), and STAs (n=7) when T cells are activated with anti-CD3/anti-CD8 and cocultured with B cells with or without anti–IL-10 blocking antibody (A), with or without anti–TGF-β blocking antibody in HVs (n=10), TOLs (n=8), and STAs (n=6) (B), or with or without GzmB inhibitor peptide in HVs (n=9), TOLs (n=9), and STAs (n=14). (D and E) Representative histograms of T cell proliferation with or without GzmB inhibitor peptide (D) and when T and B cells are cocultured in transwell (E) in HVs (n=6), TOLs (n=4), and STAs (n=3) (mean±SEM; *P<0.05; **P<0.01; ***P<0.001).
Because the inhibitory functions of B cells involving GzmB have been shown to act through a direct interaction of B cells with their target,25,26 transwell cocultures were performed to determine whether contact was required by B cells to inhibit T cell proliferation. As shown in Figure 3E, the inhibitory B cell effect disappeared when B and T cells were cultured in transwell, demonstrating that T cell–B cell interaction is necessary for the inhibitory B cell function. Altogether, these data show that B cells inhibit T cell proliferation via a GzmB pathway and depend on contact between the B and T cells.
Tolerant Recipients Have a Higher Number of B Cells, Which Act in a Dose-Dependent Manner and Express GzmB
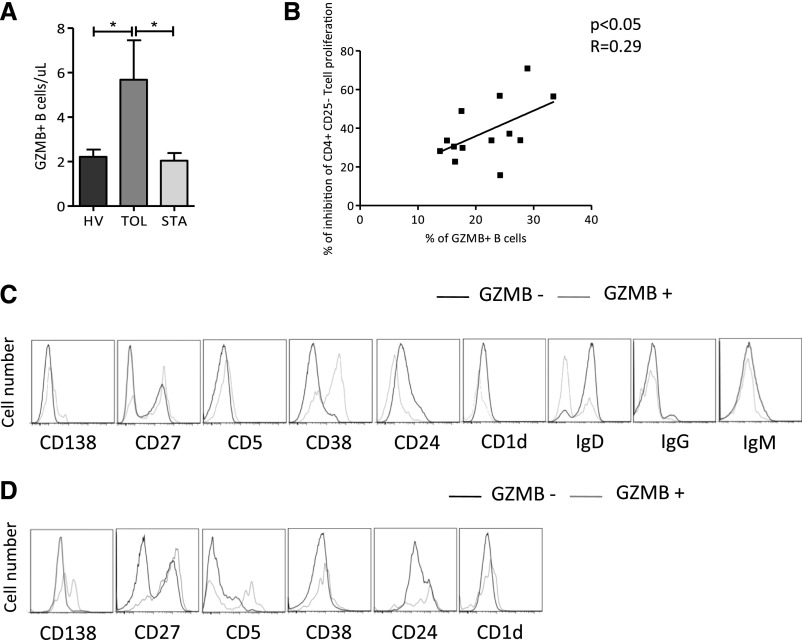
On a cell-by-cell basis, B cells from TOLs added to the coculture have the same ability as B cells from HVs and STAs to regulate autologous effector T cell proliferation in a contact- and GzmB-dependent manner (Figure 3, C and E). We previously reported on a higher number of total B cells in blood from TOLs.10,12,24 Here we found that the absolute value of GzmB-producing B cells was significantly higher in TOLs compared with HVs and STAs (P<0.05; Figure 4A) and the percentage of GzmB+ B cells is directly correlated with effector CD4+CD25− T cell proliferation inhibition (Figure 4B). Altogether, these data show that tolerant recipients have a higher number of peripheral B cells and GzmB+ B cells that are able to inhibit T cell response through a contact- and GzmB-dependent pathway and in a dose-dependent manner.
Figure 4.
Tolerant recipients have a higher number of B cells expressing GzmB and act in a dose-dependent manner. (A) Number of GZMB+ B cells per microliter of blood from HVs (n=6), TOLs (n=6), and STAs (n=6) (mean±SEM; *P<0.05). (B) Linear regression of percentage of GzmB+ B cells and percentage of inhibition of CD4+CD25− T cell proliferation (P<0.04). (C) Representative histograms for CD138, CD27, CD5, CD38, CD24, CD1d, IgD, IgG, and IgM expression within the GzmB+ (thin line) and GzmB− (thick line) in CD19+ B cells before stimulation. (D) Representative histograms for CD38 and CD138 within the GzmB+ (thin line) and GzmB− (thick line) in CD19+ B cells after stimulation.
Resting and Stimulated GzmB-Expressing B Cells Display a CD5+CD27+CD138+ Phenotype
The GzmB+ B cell phenotype was analyzed by flow cytometry before and after 3 days of coculture using CD19, CD20, CD138, CD38, CD27, CD24, CD5, CD1d, IgD, IgG, and IgM cell surface markers. Unstimulated GzmB+ B cells express a higher level of CD138, CD27, CD5, and CD38, and fewer IgD markers compared with unstimulated GzmB− B cells (Figure 4C). After stimulation, GzmB+ B cells have lower CD38 expression compared with GzmB− B cells (Figure 4D). Resting GzmB+ B cells represented around 2.7%±1.45% of total B cells from TOLs. Twenty-four hours of activation with CD40L and ADN following by three days of coculture with activated effector T cells greatly increases the expression of GzmB (20%±2.5% after activation) (Supplemental Figure 1, A and B). Altogether, these data show that tolerant recipients have a higher number of GzmB-expressing B cells with a CD138+CD27+CD5+CD38+IgD− phenotype.
B Cell Regulatory Transcriptional Profile Is Not Different in TOLs, HVs, and STAs
We investigated the expression of 60 markers selected either for their participation in the regulation of B cell functions (Supplemental Figure 2, A and B),26 or their implication in a tolerance-related B signature (Supplemental Figure 2, C and D)27 in purified and isolated B cells or PBMCs from samples from 32 individuals (10 TOLs, 10 HVs, and 12 STAs). Among these genes, only CD38 and CD1D exhibited a significant difference between STAs and HVs in B cells (Supplemental Figure 2A), whereas more than half were different in PBMCs (Supplemental Figure 2B). The second subset (Supplemental Figure 2, C and D) includes a panel of 35 markers previously linked to tolerance across blood transcriptomic studies and preferentially expressed in B cell subsets for 69% of them.27 Only two of these markers were different in B cells (EBF1 between TOLs and HVs; PLEKHG1 between STAs and HVs) (Supplemental Figure 2D), whereas all of them were different between TOLs and STAs in PBMCs (Supplemental Figure 2D). These data clearly show that B cells are not intrinsically different between the groups of patients and that, at least for these selected genes, the signature of B cells in blood from tolerant recipients is mainly the result of a higher number of B cells in PBMCs (Supplemental Figure 2).
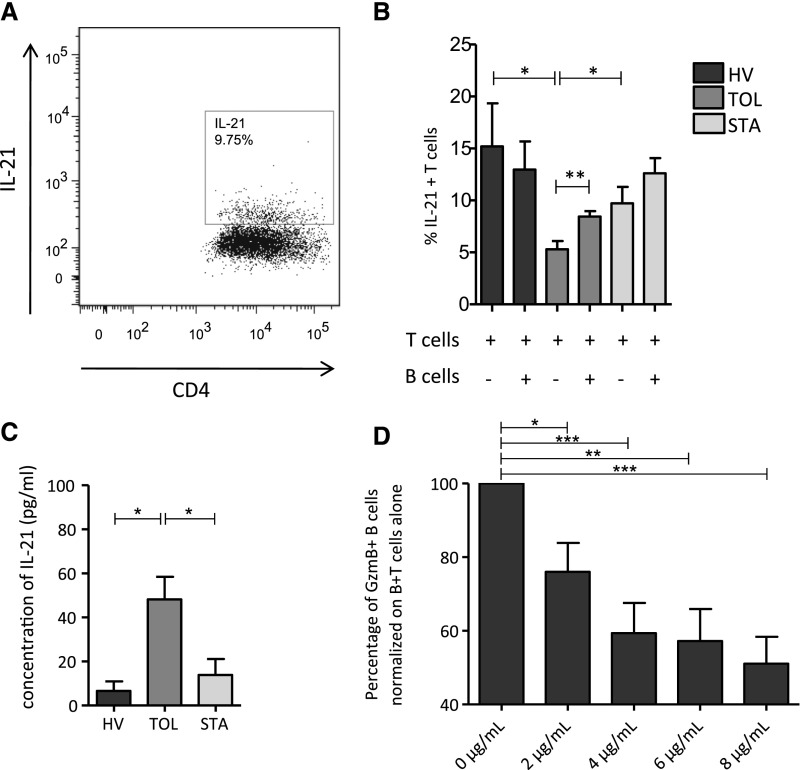
B Cells from Tolerant Recipients Regulate IL-21–Producing T Cell Levels; IL-21 Production by T Cells Regulates the Number of GZMB+ B cells
GzmB-expressing B cells have been shown to depend on T cell–produced IL-21, and IL-21 is also an important factor in B cell homeostasis. We found that in TOL stimulation with anti-CD3/anti-CD28 of CD4+CD25− T cells is associated with a lower number of IL-21–producing T cells compared with HVs and STAs (P<0.05). However, when B cells are added to the coculture, IL-21–producing T cells significantly increased in TOLs (P<0.01), whereas no difference, or even decreased IL-21 production, was observed in HVs and STAs (Figure 5, A and B). This correlates with a higher production of IL-21 by T cells stimulated by B cells from TOLs compared with those from HVs and STAs (P<0.05) (Figure 5C). Altogether, these data show that B cells from TOLs regulate the number of IL-21+ T cells and IL-21 production in vitro. Finally, to assess the role of IL-21 on GzmB-expressing B cells, we blocked its effect using anti–IL-21 antibody in the coculture assay. Increasing doses of anti–IL-21 (0, 2, 4, 6, and 8 μg/ml) significantly decreased the number of GzmB-expressing B cells (Figure 5D).
Figure 5.
B cells from tolerant recipients regulate IL-21–producing T cells levels and IL-21 production by T cells. (A) Dot plot of the secretion of IL-21 by activated CD4+CD25− T cells at day 3 of coculture with stimulated B cells. (B) Percentage of IL-21+ T cells at day 3 of activation and coculture with or without stimulated B cells in HVs (n=10), TOLs (n=12), and STAs (n=14) (*P<0.05; **P<0.01). (C) Concentration of IL-21 (pg/ml) in supernatants after 3 days of coculture, T cell coculture with B cells (mean±SEM; *P<0.05). (D) Percentage of GzmB+ B cells normalized on B cells plus T cells alone after increasing doses of IL-21 blocking molecule is added to coculture (0, 2, 4, 6, and 8 μg/ml) (*P<0.05; ***P<0.01).
Discussion
B cells may have a dual effect, acting as a driver and as a regulator of the immune system.15–18,28–32 We recently showed that tolerant recipients are characterized by a higher number of circulating B cells,10,12 a defect in terminal differentiation,24 and a B cell–transcriptional profile with overexpression of molecules associated with regulation.9,12 In animal models, that B cells have a role in tolerance is clearly suggested by the accumulation of B cells and formation of germinal centers in tolerant allografts and the ability of B cells to prolong graft survival.33 However, the role and nature of Bregs in the modulation of immune response in alloimmunity and in tolerance in humans remains unclear. Although tolerant kidney recipients clearly display a strong B-cell signature, with a higher number of B cells, their potential role in establishing and/or maintaining tolerance remains to be determined.
It is tempting to speculate that this increase in B cells with a specific inhibitory profile may be linked to the tolerance of these rare patients. Haynes et al. recently proposed an indirect pathway model in which a decrease in immunosuppression is associated with an increased number of indirect pathway regulatory T cells (Tregs) and of B cells, possibly Bregs, but the mechanisms have not been established.34 Silva et al. showed that peripheral B cells from tolerant recipients maintain the capacity to activate CD40-CD40L signaling, phosphorylate STAT3, and preserve B cell compartment diversity, suggesting a role for these B cells in tolerance.14 In this article, we explore the potential regulatory role of B cells in such patients. Because of the difficulty in identifying a unique Bregs population with a unique phenotype in humans, we analyzed the in vitro suppressive properties of B cells overall. We report a higher number of B cells with dose-dependent suppressive properties in blood from patients with a tolerant kidney graft. The inhibitory effect of B cells is dependent on GzmB and on the interaction of B cells with their T cell targets.
Much evidence suggests that activation is instrumental in Bregs activity.35 In this article, we show that prestimulated B cells are more efficient at suppressing effector T cell proliferation than nonstimulated B cells, a result in accordance with previous data showing that maturation and B cell activation were important parameters in this process.28,30–32,36 It may be a little counterintuitive to say that B cells need such activation to regulate the immune system because activated B cells are usually associated with stimulatory activity on other cell types. However, this has been extensively discussed and can be explained as the participation of B cells in a general feedback loop that both induces an immune process and prevents excessive inflammation or unwanted autoaggressive T cell response.37 Moreover, we clearly show that the inhibitory effect of B cells is dependent on GzmB, the expression of which occurs in resting B cells in vivo and is increased in stimulated B cells. Interestingly, as already described for the pro-B10 regulatory cell population, this leads to the conclusion that B cell–mediated regulation is probably inducible and that the effect of B cells is greatly influenced by the nature of the microenvironment.35,36,38–40
The expression of IL-10 has been shown to be a frequent characteristic of Bregs.41–43 Such cells, referred to as B10,32,44 are involved in the initiation, the onset, and the severity of various autoimmune diseases, and in transplantation.45–47 We show here that the suppressive properties of B cells are not dependent on IL-10. Anti–IL-10, used at doses able to efficiently block an IL-10 T cell–dependent response (data not shown), does not diminish the inhibitory effect of B cells on T cell proliferation. These data are in agreement with the work of Deng et al., who reported that anti-CD45RB treatment induces strong and antigen-specific tolerance, which is dependent on the presence of B lymphocytes and is independent from IL-10. In this model, IL-10 clearly counter-regulates tolerance induction, and even exerts a negative effect and causes histologic lesions of rejection.48 The same observations have been reported in other models.49 Interestingly, it has been reported that B cells from patients with chronic antibody-mediated rejection have a defect in suppressive properties,50 in contrast with B cells from STAs, which inhibit T cell proliferation and induce Treg generation through a TGF-β– and IDO–dependent pathway (personal communication, Nouël et al. Ann Rheum Dis abstract A8.32, 2014). In our situation, blocking TGF-β has no effect on the suppressive function of B cells, suggesting that TGF-β is not involved in B cell regulation either.
Interestingly, other regulation pathways have been shown to be involved in B cell regulatory activity. We found that GzmB blockade diminishes the B cell inhibitory effect and restores T cell proliferation. This is associated with a higher number of GzmB-producing B cells in blood from tolerant recipients and B cells that act in a contact- and a dose-dependent manner. GzmB is a 32-kD serine protease mainly known as a component of the cytotoxic granule of T cells and natural killer cells51,52 but is also produced by other cell types, such as B cells,53 Tregs,54 and plasmacytoid dendritic cells.55 GzmB mediates T cell apoptosis and T cell proliferation suppression, independently of the perforin component.55 In this work, the fact that a blockade of GzmB with anti-GzmB induces a diminishment of the B cell inhibitory effect indicates that GzmB is functional. Interestingly, we found that B cells not only inhibit T cell proliferation, but also induce their apoptosis. We did not find any involvement of the Fas-FasL pathway in this process (data not shown), but alternative pathways have been described as inducing apoptosis, and GzmB is one of them.56 These data suggest a pivotal role for GzmB in regulation by B cells in our patients through cell death induction and T cell proliferation inhibition, a mechanism already described for Tregs after polyclonal and antigen-specific activation.54 Interestingly, we found that B cells had no effect on TNF-α and IFN-γ production. Iwata et al. reported that IFN-γ and TNF-α production was dependant from IL-10.46 This is reinforced by the data from Lemoine et al., who reported that regulation of T cell proliferation was distinct from the differentiation of T cells into Th1 cells that depend on the production of IL-10.42 These observations are in accordance with ours and particularly reinforce our finding that IL-10 is not involved in B cell regulation in our present situation. To our knowledge, nothing has yet been reported on the effect of GzmB on IFN-γ and/or TNF-α production by B cells.
Several microenvironmental factors have been shown to be instrumental in B cell homeostasis. These factors include molecules such as IL-21.26,53 We first demonstrated that GzmB-expressing B cells are dependent on IL-21. Increasing doses of anti–IL-21 decrease the number of GzmB-expressing B cells in coculture. Moreover, when B cells are added to the coculture, IL-21–producing T cells increase significantly and there is greater production of IL-21 by T cells from TOLs. IL-21 is a key cytokine for GzmB gene transcription,26,57 which suggests that the increase in GzmB+ B cells in blood from tolerant recipients may be due to a direct effect of IL-21. These data are reinforced by the effective STAT3 phosphorylation in B cells from tolerant recipients,14 a key signal for the generation of GzmB activated B cells.57 Finally, the fact that tolerant recipients have a lower level of IL-21+ CD4+ T cells, and that B cells from these patients alone (and not HVs and STAs) increase IL-21 production by CD4+ T cells, strongly suggests a negative feedback loop in these patients, increasing excessive B cell activation and inflammation and allowing regulation to take place.
Bregs are able to control the immune response, but an excessive reaction from these cells may also promote tumor cell growth or chronic infection.26 We hypothesize that this fine-tuning of regulation by B cells and IL-21 production by T cells might be a key factor in tolerance maintenance.
We show that TOLs have more B cells in absolute value with regulatory properties, but on a cell-per-cell basis, their B cells have the same suppressive activity as B cells from STAs and HVs. These results are supported by transcriptional analysis that shows that the B cells are not intrinsically different between the groups of patients and that, at least for these selected genes, the B cell signature in blood from tolerant recipients’ results mostly from a higher number of B cells in PBMCs. It is not surprising that the greater suppressive effect in blood of tolerant recipients is due to a higher number of circulating GzmB+ cells, if we consider that clinical outcomes in numerous situations are mainly driven by the quantity of infiltrating and circulating cells, more than their quality.58 Interestingly, we find that GzmB+ B cells express a specific phenotype with upregulation of CD5, CD27, CD38, and CD138 markers. This is in accordance with our previous data reporting a higher level of memory CD27+ B cells and CD5+ B cells in blood from tolerant recipients.12 These data are also in accordance with the fact that CD5+ B cells constitutively express GzmB in an IL-21–dependent pathway59 and that CD5+ B cells express higher levels of IL-21–R than CD5− B cells.26 We also found that these cells express CD138 and CD38 and are IgD−, markers expressed by B cells secreting IL-10 and IL-35 and involved in suppression mechanisms,60 and also associated with plasma cell maturation.61 Interestingly, CD138 has also been shown to be associated with GzmB production, regulatory activity, and IL-21 dependence.57
In conclusion, we have demonstrated that TOLs have a higher absolute number of GzmB+ B cells with a plasma cell−like phenotype and with dose-dependent suppressive properties through the GzmB pathway. B cells decrease T cell proliferation and induce their apoptosis, two processes that may contribute to a tolerogeneic environment. GzmB-producing B cells are under the control of IL-21. The fact that there is more IL-21 in vitro in TOLs after B cell stimulation correlates with a greater number of IL-21–dependent circulating GzmBs and a higher inhibitory effect of B cells, which may act as pro-B10 that need to be activated to display an increased effect. In previous articles, we reported on a decreased number of circulating plasma cells and we showed that B cells from tolerant recipients do not fully differentiate into plasmablasts and are more sensitive to apoptosis.12,20 Here again, this is in accordance with the fact that in vitro unstimulated T cells from TOLs produce less IL-21, a molecule that directly acts on B cell differentiation.62,63 We hypothesize that these properties may contribute to a favorable tolerogeneic environment and favorable inversion of the B effector–plasma cell/Bregs balance in these patients and their lower antibody production. These data support a role for B cells in patients with operational tolerance. They also raise the question of whether it would be possible to trigger an ex vivo or in vivo increase to encourage any potential therapeutic effects, such as counteracting the alloimmune response or even promoting tolerance. These data also question the effectiveness of depleting B cells in order to control antibody-mediated rejection, rather than developing new strategies to maintain the fragile balance between the negative and beneficial effects of B cells in transplantation.
Concise Methods
Patients and HVs
Forty-one patients took part in the study and signed informed consent as follows: (1) TOLs with stable kidney graft function (creatinemia<150 µmol/L and proteinuria<1 g/24 h) in the absence of immunosuppression for at least 1 year (n=12) except for 2 who have creatinemia>150 µmol/L±20% but were stable over time, (2) STAs with stable kidney graft function (creatinemia<150 µmol/L and proteinuria<1 g/24 h) for at least 3 years under standard immunosuppression (calcineurin inhibitors and corticosteroids) (n=17), and (3) HVs without pathologies or infectious episodes in the previous 6 months (n=17) (Table 1).
Table 1.
Summary of clinical and demographic characteristic of patients and HVs
| Patient Group | Age (yr) | Sex (F/M) | Donor (LD versus NLV) | HLA Mismatches (n) | Time between Graft and Analysis (mo) | Creatinemia (μmol/L) | Proteinuria (g/24 h) | Time between Immunosuppression Withdrawal and Analysis (yr) |
|---|---|---|---|---|---|---|---|---|
| HV (n=17) | 8/17 | |||||||
| Median | 46.5 | |||||||
| SD | 10.8 | |||||||
| Minimum | 27.0 | |||||||
| Maximum | 61.0 | |||||||
| TOL (n=12) | 6/12 | 4/12 | ||||||
| Median | 58.0 | 3.0 | 220.6 | 90.0 | 0.1 | 10.5 | ||
| SD | 15.8 | 1.9 | 94.9 | 64.7 | 0.2 | 5.5 | ||
| Minimum | 31.4 | 0.0 | 71.4 | 66.0 | 0.0 | 1.0 | ||
| Maximum | 85.3 | 4.0 | 385.3 | 280.0 | 0.6 | 19.0 | ||
| STA (n=17) | 5/17 | 1/17 | ||||||
| Median | 57.6 | 4.0 | 120.4 | 124.0 | 0.1 | |||
| SD | 10.8 | 1.4 | 37.6 | 29.1 | 0.1 | |||
| Minimum | 40.4 | 1.0 | 66.4 | 74.0 | 0.0 | |||
| Maximum | 74,9 | 5.0 | 192.5 | 175.0 | 0.2 |
F, female; M, male; LD, living donor; NLV, nonliving donor.
Cell Culture
PBMCs were stimulated at 2×106 cells/ml for 48 hours in complete RPMI 1640 (Sigma-Aldrich, St. Louis, MO) containing L-glutamine, penicillin/streptomycin (Life Technologies, Carlsbad, CA), and 10% FCS (Lonza, Verviers, Belgium) in 96-well plates (Nunc, Langenselbold, Germany) at 37°C, 5% CO2. Stimulation was performed using CpG oligonucleotide (ODN 2006, 10 μg/ml; InvivoGen, San Diego, CA) and recombinant human soluble CD40L (R&D Systems, Minneapolis, MN). PMA (250 ng/ml), ionomycin (1 μg/ml), and brefeldin-A (10 μg/ml) (Sigma-Aldrich) were added for the last 5 hours of the B-cell culture. As a control, PBMCs were cultured in resting conditions for 48 hours.
Analyses of IL-10 Production by Stimulated B Cells
After 48 hours of culture, viability staining was performed using the aqua Live/Dead cell staining kit (Invitrogen/Life Technologies). B lymphocytes were stained with anti–CD19-PC7 (BD Biosciences, San Diego, CA), washed, fixed, and permeabilized using a permeabilization/fixation kit (BD Biosciences). Fcγ receptor inhibitor (eBiosciences, San Diego, CA) was used to avoid nonspecific staining. For detection of intracellular IL-10, staining was performed using anti–IL-10-phycoérythrine (PE) (clone JES3-9D7; BD Biosciences). Appropriate PE-conjugated isotype controls were used for gate setting for IL-10 expression. Unstimulated B cells were stained and used as a control for the gating strategy.
B Cell Functional Assays: B and T Cell Purification
PBMCs were obtained after Ficoll density centrifugation (Sigma-Aldrich) of fresh blood samples. B cells were purified by negative selection using a Human B Cell Isolation Kit II and an autoMACS PRO Separator, with purity >95% (Miltenyi Biotech, Gladbach, Germany) and stimulated for 24 hours with CD40L (1 μg/ml) and CpG-ODN (10 μg/ml) in a 96-well U-bottom plate at a concentration of 106 cells/ml. As a control, a proportion of PBMCs were kept at 4°C in complete medium for 24 hours. B cells were purified using the same technique, without stimulation. CD4+CD25− responding T cells were purified by negative selection using successively CD4+ T Cell Isolation Kit II and CD25+ Microbeads II (Miltenyi Biotech), according to the manufacturer’s instructions with a purity >95%.
Coculture Experiments
Coculture assays were performed for 72 hours by adding 1×105 autologous prestimulated B cells or unstimulated B cells to 0.5×105 CD4+CD25− responding T cells, stimulated with anti-CD3 and anti-CD28.2 dynabeads (at a 1:1 ratio of dynabeads/T cells) (Invitrogen, Oslo, Norway). After 72 hours of coculture, brefeldin-A was added at 10 μg/ml for 4 hours. Viability of T and B cells was checked by 4′,6-diamidino-2-phenylindole staining. The proliferation of CD4+CD25− responding T cells was measured after staining with CellTrace Violet (Invitrogen). T cell IFN-γ, IL-21, and TNF-α secretion was measured after permeabilization of responding T cells and staining with anti–CD4-PE (BD Biosciences), anti–IFN-γ-allophycocyanin (APC) and anti–TNF-α-fluorescéine isothiocyanate (FITC) (BD Biosciences). For measurement of T cell apoptosis, cells were stained with CD4-APC, CD19-PE-C7, and Annexin V-APC (BD Biosciences). To investigate whether B cells have a dose-dependent effect on T cells, the number of B cells was progressively increased in the coculture (50,000, 100,000, and 200,000), whereas the number of 50,000 T cells remained fixed.
Coculture Experiments Blockade
These coculture assays were performed under the same conditions using transwell polycarbonate inserts (0.4 μm; Corning, Inc.) or using anti–IL-10 (BD Biosciences), anti–TGF-β1 (Abcam, Inc., Cambridge, UK), or anti-GzmB, a peptide that irreversibly inhibits GzmB activity (Ac-IEPD-CHO; BioVision, San Francisco, CA). All antibodies were used at 10 μg/ml concentration.
Microarrays
Purified B cell samples from 32 individuals (10 TOLs, 10 HVs, and 12 STAs) were analyzed with whole-genome Agilent human microarray as previously described24 according to the manufacturer’s instructions (Agilent Technologies). Hybridization signals were normalized using a Lowess procedure.64 Probe conversion was performed using MADGene65 and those pertaining to the same gene were averaged. The expression of 60 markers selected either for their participation in the regulation of B cell functions26 and/or their implication in a tolerance-related B signature27 was then investigated. Significance of differential expression was evaluated using a one-tailed t test and results were compared with those obtained on PBMCs.27
GzmB+ B Cell Phenotyping
Anti–human mAbs included the following: CD19-V450 CD38-FITC, CD24-PE, CD5-APC, CD1d-PE, and GzmB-Alexa Fluor 700 from BD Biosciences. CD20-FITC and CD138 PE from Miltenyi Biotech were used for PBMC staining. PBMC suspensions were stained on ice using a predetermined optimal concentration of each antibody for 15–30 minutes. PBMCs were then fixed and permeabilized using a permeabilization/fixation kit (BD Biosciences). For detection of intracellular GzmB, staining was performed using anti–GzmB-A700.
IL-21 Measurement and Inhibition
Coculture assays were performed for 72 hours by adding 1×105 autologous prestimulated B cells or unstimulated B cells to 0.5×105 CD4+CD25− responding T cells stimulated with anti-CD3 and anti-CD28.2 dynabeads (at a 1:1 ratio of dynabeads/T cells) (Invitrogen). Cytokine production was measured in culture supernatants at 72 hours. IL-21 level was determined using BD CBA Human IL-21 FLEX SET as recommended by the manufacturer (BD Biosciences). GzmB production by B cells in B cell/T cell cocultures was measured at day 3 by blocking IL-21 with increasing doses (0, 2, 4, 6, and 8 μg/ml) of anti–IL-21 blocking molecule (recombinant IL-21R Fc chimera) (R&D Systems).
Statistical Analyses
Data are expressed as the means±SEM. Statistical analyses were performed using GraphPad Prism software, and P values were calculated by the Mann–Whitney test.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the patients and their families, whose trust, support, and cooperation were essential for the collection of the data used in this study. We also thank G. Blancho, Dr. D. Cantarovitch, J. Dantal, Dr. S. Ferrari-Lacraz, K. Hadaya, M. Hourmant, Dr. B. Hurault de Ligny, Dr. G. Lefrancois, C. Legendre, Dr. B. Le Mauff, Dr. H. Le Monies De Sagazan, Dr. A. Meurette, Dr. M. Rabant, J.F. Subra, Dr. J. Sayegh, Dr. A. Testa, Dr. F. Villemain, and Dr. J. Zuber for their help in this study.
This work was carried out with the support of CENTAURE and PROGREFFE foundation grants, a Roche Organ Transplantation Research Foundation and European Society for Organ Transplantation grant, and under the auspices of the IHU-Cesti project, which received French government financial support managed by the National Research Agency via the “Investment Into The Future” program (ANR-10-IBHU-005). The IHU-Cesti project is also supported by Nantes Metropole and the Pays de la Loire Region. This work was also supported by the Labex IGO project (ANR-11-LABX-0016-01) funded by the Investissements d’Avenir French Government program, managed by the French National Research Agency. The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013; grant agreement 305147 BIO-DrIM).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014040404/-/DCSupplemental.
References
- 1.Roussey-Kesler G, Giral M, Moreau A, Subra JF, Legendre C, Noël C, Pillebout E, Brouard S, Soulillou JP: Clinical operational tolerance after kidney transplantation. Am J Transplant 6: 736–746, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Brouard S, Pallier A, Renaudin K, Foucher Y, Danger R, Devys A, Cesbron A, Guillot-Guegen C, Ashton-Chess J, Le Roux S, Harb J, Roussey G, Subra JF, Villemain F, Legendre C, Bemelman FJ, Orlando G, Garnier A, Jambon H, Le Monies De Sagazan H, Braun L, Noël C, Pillebout E, Moal MC, Cantarell C, Hoitsma A, Ranbant M, Testa A, Soulillou JP, Giral M: The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am J Transplant 12: 3269–3307, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DSC, Hertl M, Goes NB, Wong W, Williams WW, Jr, Colvin RB, Sykes M, Sachs DH: HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 358: 353–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonard DA, Cetrulo CL, Jr, McGrouther DA, Sachs DH: Induction of tolerance of vascularized composite allografts. Transplantation 95: 403–409, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Poirier N, Blancho G, Vanhove B: Alternatives to calcineurin inhibition in renal transplantation: Belatacept, the first co-stimulation blocker. Immunotherapy 2: 625–636, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Jovanovic V, Lair D, Soulillou JP, Brouard S: Transfer of tolerance to heart and kidney allografts in the rat model. Transpl Int 21: 199–206, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Orlando G, Hematti P, Stratta RJ, Burke GW, 3rd, Di Cocco P, Pisani F, Soker S, Wood K: Clinical operational tolerance after renal transplantation: Current status and future challenges. Ann Surg 252: 915–928, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballet C, Roussey-Kesler G, Aubin JT, Brouard S, Giral M, Miqueu P, Louis S, van der Werf S, Soulillou JP: Humoral and cellular responses to influenza vaccination in human recipients naturally tolerant to a kidney allograft. Am J Transplant 6: 2796–2801, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Brouard S, Mansfield E, Braud C, Li L, Giral M, Hsieh SC, Baeten D, Zhang M, Ashton-Chess J, Braudeau C, Hsieh F, Dupont A, Pallier A, Moreau A, Louis S, Ruiz C, Salvatierra O, Soulillou JP, Sarwal M: Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci U S A 104: 15448–15453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis S, Braudeau C, Giral M, Dupont A, Moizant F, Robillard N, Moreau A, Soulillou JP, Brouard S: Contrasting CD25hiCD4+T cells/FOXP3 patterns in chronic rejection and operational drug-free tolerance. Transplantation 81: 398–407, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, Burlingham WJ, Marks WH, Sanz I, Lechler RI, Hernandez-Fuentes MP, Turka LA, Seyfert-Margolis VL, Immune Tolerance Network ST507 Study Group : Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 120: 1836–1847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallier A, Hillion S, Danger R, Giral M, Racapé M, Degauque N, Dugast E, Ashton-Chess J, Pettré S, Lozano JJ, Bataille R, Devys A, Cesbron-Gautier A, Braudeau C, Larrose C, Soulillou JP, Brouard S: Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int 78: 503–513, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, Chapman S, Craciun L, Sergeant R, Brouard S, Rovis F, Jimenez E, Ballow A, Giral M, Rebollo-Mesa I, Le Moine A, Braudeau C, Hilton R, Gerstmayer B, Bourcier K, Sharif A, Krajewska M, Lord GM, Roberts I, Goldman M, Wood KJ, Newell K, Seyfert-Margolis V, Warrens AN, Janssen U, Volk HD, Soulillou JP, Hernandez-Fuentes MP, Lechler RI: Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 120: 1848–1861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva HM, Takenaka MCS, Moraes-Vieira PMM, Monteiro SM, Hernandez MO, Chaara W, Six A, Agena F, Sesterheim P, Barbé-Tuana FM, Saitovitch D, Lemos F, Kalil J, Coelho V: Preserving the B cell compartment favors operational tolerance in human renal transplantation. Mol Med 18: 733–743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clatworthy MR: Targeting B cells and antibody in transplantation. Am J Transplant 11: 1359–1367, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stegall MD, Raghavaiah S, Gloor JM: The (re)emergence of B cells in organ transplantation. Curr Opin Organ Transplant 15: 451–455, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE: Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 1: 475–482, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Shlomchik MJ, Craft JE, Mamula MJ: From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol 1: 147–153, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Balkwill F, Montfort A, Capasso M: B regulatory cells in cancer. Trends Immunol 34: 169–173, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Chesneau M, Michel L, Degauque N, Brouard S: Regulatory B cells and tolerance in transplantation: From animal models to human. Front Immunol 4: 497, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fremd C, Schuetz F, Sohn C, Beckhove P, Domschke C: B cell-regulated immune responses in tumor models and cancer patients. OncoImmunology 2: e25443, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalampokis I, Yoshizaki A, Tedder TF: IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther 15[Suppl 1]: S1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang M, Rui K, Wang S, Lu L: Regulatory B cells in autoimmune diseases. Cell Mol Immunol 10: 122–132, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesneau M, Pallier A, Braza F, Lacombe G, Le Gallou S, Baron D, Giral M, Danger R, Guerif P, Aubert-Wastiaux H, Néel A, Michel L, Laplaud DA, Degauque N, Soulillou JP, Tarte K, Brouard S: Unique B cell differentiation profile in tolerant kidney transplant patients. Am J Transplant 14: 144–155, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Hagn M, Jahrsdörfer B: Why do human B cells secrete granzyme B? Insights into a novel B-cell differentiation pathway. OncoImmunology 1: 1368–1375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TFE, Beyer T, Reister F, Fabricius D, Lotfi R, Lunov O, Nienhaus GU, Simmet T, Kreienberg R, Möller P, Schrezenmeier H, Jahrsdörfer B: Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res 73: 2468–2479, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Lozano JJ, Pallier A, Martinez-Llordella M, Danger R, López M, Giral M, Londoño MC, Rimola A, Soulillou JP, Brouard S, Sánchez-Fueyo A: Comparison of transcriptional and blood cell-phenotypic markers between operationally tolerant liver and kidney recipients. Am J Transplant 11: 1916–1926, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Bouaziz JD, Yanaba K, Tedder TF: Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev 224: 201–214, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C: Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol 178: 7868–7878, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM: B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3: 944–950, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Mauri C, Gray D, Mushtaq N, Londei M: Prevention of arthritis by interleukin 10-producing B cells. J Exp Med 197: 489–501, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF: A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28: 639–650, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Le Texier L, Thebault P, Lavault A, Usal C, Merieau E, Quillard T, Charreau B, Soulillou JP, Cuturi MC, Brouard S, Chiffoleau E: Long-term allograft tolerance is characterized by the accumulation of B cells exhibiting an inhibited profile. Am J Transplant 11: 429–438, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Haynes LD, Jankowska-Gan E, Sheka A, Keller MR, Hernandez-Fuentes MP, Lechler RI, Seyfert-Margolis V, Turka LA, Newell KA, Burlingham WJ: Donor-specific indirect pathway analysis reveals a B-cell-independent signature which reflects outcomes in kidney transplant recipients. Am J Transplant 12: 640–648, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasare C, Medzhitov R: Control of B-cell responses by Toll-like receptors. Nature 438: 364–368, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderón Gómez E, Sweenie CH, Hao Y, Freitas AA, Steinhoff U, Anderton SM, Fillatreau S: TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol 180: 4763–4773, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Lampropoulou V, Calderon-Gomez E, Roch T, Neves P, Shen P, Stervbo U, Boudinot P, Anderton SM, Fillatreau S: Suppressive functions of activated B cells in autoimmune diseases reveal the dual roles of Toll-like receptors in immunity. Immunol Rev 233: 146–161, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Dang VD, Hilgenberg E, Ries S, Shen P, Fillatreau S: From the regulatory functions of B cells to the identification of cytokine-producing plasma cell subsets. Curr Opin Immunol 28: 77–83, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Fillatreau S: Cytokine-producing B cells as regulators of pathogenic and protective immune responses. Ann Rheum Dis 72[Suppl 2]: ii80–ii84, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O’Connor RA, Anderton SM, Bar-Or A, Fillatreau S, Gray D: B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209: 1001–1010, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, Ehrenstein MR, Mauri C: Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol 182: 3492–3502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemoine S, Morva A, Youinou P, Jamin C: Human T cells induce their own regulation through activation of B cells. J Autoimmun 36: 228–238, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Schmidl C, Hansmann L, Lassmann T, Balwierz PJ, Kawaji H, Itoh M, Kawai J, Nagao-Sato S, Suzuki H, Andreesen R, Hayashizaki Y, Forrest ARR, Carninci P, Hoffmann P, Edinger M, Rehli M, FANTOM consortium : The enhancer and promoter landscape of human regulatory and conventional T-cell subpopulations. Blood 123: e68–e78, 2014 [DOI] [PubMed] [Google Scholar]
- 44.DiLillo DJ, Matsushita T, Tedder TF: B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci 1183: 38–57, 2010. 20146707 [Google Scholar]
- 45.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C: CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 32: 129–140, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF: Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117: 530–541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nouël A, Simon Q, Jamin C, Pers JO, Hillion S: Regulatory B cells: An exciting target for future therapeutics in transplantation. Front Immunol 5: 11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng S, Moore DJ, Huang X, Lian MM, Mohiuddin M, Velededeoglu E, Lee MK, 4th, Sonawane S, Kim J, Wang J, Chen H, Corfe SA, Paige C, Shlomchik M, Caton A, Markmann JF: Cutting edge: Transplant tolerance induced by anti-CD45RB requires B lymphocytes. J Immunol 178: 6028–6032, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Singh A, Carson WF, 4th, Secor ER, Jr, Guernsey LA, Flavell RA, Clark RB, Thrall RS, Schramm CM: Regulatory role of B cells in a murine model of allergic airway disease. J Immunol 180: 7318–7326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nouël A, Ségalen I, Jamin C, Doucet L, Caillard S, Renaudineau Y, Pers JO, Le Meur Y, Hillion S: B cells display an abnormal distribution and an impaired suppressive function in patients with chronic antibody-mediated rejection. Kidney Int 85: 590–599, 2014 [DOI] [PubMed] [Google Scholar]
- 51.Ewen CL, Kane KP, Bleackley RC: A quarter century of granzymes. Cell Death Differ 19: 28–35, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoves S, Trapani JA, Voskoboinik I: The battlefield of perforin/granzyme cell death pathways. J Leukoc Biol 87: 237–243, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Jahrsdörfer B, Blackwell SE, Wooldridge JE, Huang J, Andreski MW, Jacobus LS, Taylor CM, Weiner GJ: B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood 108: 2712–2719, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ: Cutting edge: Contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol 174: 1783–1786, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Jahrsdörfer B, Vollmer A, Blackwell SE, Maier J, Sontheimer K, Beyer T, Mandel B, Lunov O, Tron K, Nienhaus GU, Simmet T, Debatin KM, Weiner GJ, Fabricius D: Granzyme B produced by human plasmacytoid dendritic cells suppresses T-cell expansion. Blood 115: 1156–1165, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tretter T, Venigalla RKC, Eckstein V, Saffrich R, Sertel S, Ho AD, Lorenz HM: Induction of CD4+ T-cell anergy and apoptosis by activated human B cells. Blood 112: 4555–4564, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Xu W, Narayanan P, Kang N, Clayton S, Ohne Y, Shi P, Herve MC, Balderas R, Picard C, Casanova JL, Gorvel JP, Oh S, Pascual V, Banchereau J: Human plasma cells express granzyme B. Eur J Immunol 44: 275–284, 2014 [DOI] [PubMed] [Google Scholar]
- 58.Cherukuri A, Rothstein DM, Clark B, Carter CR, Davison A, Hernandez-Fuentes M, Hewitt E, Salama AD, Baker RJ: Immunologic human renal allograft injury associates with an altered IL-10/TNF-α expression ratio in regulatory B cells. J Am Soc Nephrol 25: 1575– 1585, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hagn M, Ebel V, Sontheimer K, Schwesinger E, Lunov O, Beyer T, Fabricius D, Barth TFE, Viardot A, Stilgenbauer S, Hepp J, Scharffetter-Kochanek K, Simmet T, Jahrsdörfer B: CD5+ B cells from individuals with systemic lupus erythematosus express granzyme B. Eur J Immunol 40: 2060–2069, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grützkau A, Grün JR, Horn K, Kühl AA, Dörner T, Bar-Or A, Kaufmann SHE, Anderton SM, Fillatreau S: IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507: 366–370, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heine G, Drozdenko G, Grün JR, Chang H-D, Radbruch A, Worm M: Autocrine IL-10 promotes human B-cell differentiation into IgM- or IgG-secreting plasmablasts. Eur J Immunol 44: 1615–1621, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Berglund LJ, Avery DT, Ma CS, Moens L, Deenick EK, Bustamante J, Boisson-Dupuis S, Wong M, Adelstein S, Arkwright PD, Bacchetta R, Bezrodnik L, Dadi H, Roifman CM, Fulcher DA, Ziegler JB, Smart JM, Kobayashi M, Picard C, Durandy A, Cook MC, Casanova J-L, Uzel G, Tangye SG: IL-21 signalling via STAT3 primes human naive B cells to respond to IL-2 to enhance their differentiation into plasmablasts. Blood 122: 3940–3950, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding BB, Bi E, Chen H, Yu JJ, Ye BH: IL-21 and CD40L synergistically promote plasma cell differentiation through upregulation of Blimp-1 in human B cells. J Immunol 190: 1827–1836, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lamirault G, Meur NL, Roussel JC, Cunff MFL, Baron D, Bihouée A, Guisle I, Raharijaona M, Ramstein G, Teusan R, Chevalier C, Gueffet JP, Trochu JN, Léger JJ, Houlgatte R, Steenman M: Molecular risk stratification in advanced heart failure patients. J Cell Mol Med 14[6B]: 1443–1452, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baron D, Bihouée A, Teusan R, Dubois E, Savagner F, Steenman M, Houlgatte R, Ramstein G: MADGene: Retrieval and processing of gene identifier lists for the analysis of heterogeneous microarray datasets. Bioinformatics 27: 725–726, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.