Abstract
Left ventricular hypertrophy (LVH) is a common complication in patients with CKD and an independent risk factor for death. Changes in the levels of uremic solutes or Klotho have been reported to be related to CKD, whereas the relationships between these factors and CKD-associated LVH remain unclear. Here, we investigated the interaction between Klotho and indoxyl sulfate (IS), a typical uremic solute, in CKD-associated LVH. In a survey of 86 patients with CKD, a negative relationship was found between serum levels of IS and Klotho (r=−0.59, P<0.001). Furthermore, serum levels of IS and Klotho were independently associated with LVH (for IS: r=0.69, P<0.001; for Klotho: r=−0.49, P<0.001). In normal mice, intraperitoneal injection of IS for 8 weeks induced LVH accompanied by substantial downregulation of renal Klotho. Notably, IS-induced LVH was more severe in heterozygous Klotho-deficient (kl/+) mice. In vitro, treatment with Klotho strongly inhibited IS-induced cardiomyocyte hypertrophy by blocking oxidative stress and inhibiting p38 and extracellular signal–regulated protein kinase 1/2 signaling pathways. In a mouse model of CKD-associated LVH, the renal expression of Klotho was lower and the level of serum IS was higher than in healthy controls. Moreover, treatment of CKD mice with Klotho protein significantly restrained the development of LVH. Taken together, these results suggest that Klotho is an endogenous protector against IS-induced LVH, and the imbalance between Klotho and IS may contribute to the development of LVH in CKD.
Keywords: uremia, left ventricular hypertrophy, CKD
Cardiovascular disease (CVD) is the most common complication of CKD, and it is the leading cause of death among patients on dialysis.1–3 Left ventricular hypertrophy (LVH), a typical pathologic feature of uremic cardiomyopathy, is present in approximately 75% of patients with ESRD when they begin to undergo dialysis, and it was confirmed to be an independent risk factor for mortality of patients with CKD.4,5
Indoxyl sulfate (IS), which is known as an important uremic solute, is derived from dietary protein and excreted in urine. In patients with CKD, decreased GFR reduces IS excretion, producing a gradual increase in the serum IS concentration with the progression of CKD.6,7 Moreover, because IS is a protein-bound solute that cannot be effectively cleared by routine dialysis, a high concentration of IS in the circulation usually exists in patients both pre- and postdialysis and is considered to be associated with CVD.8–10 It has been shown that IS is involved in the development of atherosclerosis, because it can induce vascular endothelial cell dysfunction by enhancing oxidative stress.11,12 In addition, an in vitro study revealed that IS has a prohypertrophic effect on cultured cardiac myocytes through activation of the mitogen–activated protein kinase (MAPK) and NF-κB signaling pathways.13 AST-120, an oral charcoal adsorbent that can reduce oxidative stress by lowering serum IS level, was reported to prevent the progression of cardiac hypertrophy in CKD,8,14 indicating that IS might play an important role in the development of cardiac hypertrophy under uremic conditions.
Klotho is a putative antiaging gene predominantly expressed in renal tubular epithelial cells, and its secreted protein functions as a humoral factor.15 Previous studies showed that the antiaging function of Klotho is closely related to its remarkable antioxidative properties.16 Interesting, Klotho-deficient (kl/+) mice exhibit similar phenotypic features to patients who are uremic, including the rapid onset of CVD and early death.17,18 Therefore, more attention has been paid to the relationship between Klotho and cardiovascular complications of CKD.19 Our previous study showed that Klotho could inhibit IS–induced vascular endothelial cell dysfunction through inhibiting oxidative stress.20 However, Sun et al.21 recently reported that IS could downregulate the expression of renal Klotho by DNA hypermethylation. These studies suggest that there may exist a close relationship between IS and Klotho under pathophysiologic conditions. However, the interaction between IS and Klotho and their implications in cardiac hypertrophy in patients with CKD are unknown.
Here, we report a thorough analysis of the relationship between IS and Klotho and its association with LVH in patients with CKD as well as the in vivo and in vitro effects of IS and Klotho on cardiomyocyte hypertrophy and the underlying mechanisms. We show that Klotho has a potent capacity to counteract IS-induced LVH by inhibiting oxidative stress and its downstream signaling pathways.
Results
Serum Levels of IS and Klotho Are Negatively Related with Each Other, and Both of Them Have a Close Correlation with LVH in Patients with CKD
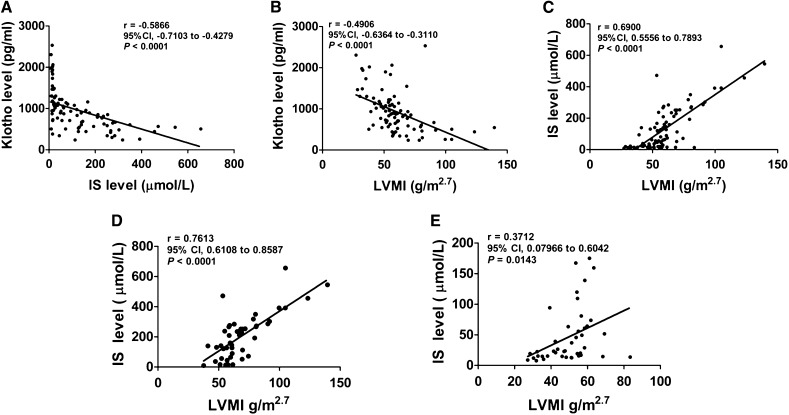
In total, 86 patients who were predialysis, including 38 women and 48 men with eGFR of 5–70 ml/min per 1.73 m2, were enrolled in this study. The median level of serum IS was 72.7 µmol/L (7.6–656 µmol/L). The serum level of Klotho decreased with the decline of eGFR, shown as 1293±454.7 pg/ml in patients with eGFR=30–59 ml/min per 1.73 m2, 851.5±389.6 pg/ml in patients with eGFR=15–29 ml/min per 1.73 m2, and 718.3±258.3 pg/ml in patients with eGFR<15 ml/min per 1.73 m2. Noticeably, an inverse correlation was found between the serum levels of Klotho and IS (r=−0.59, P<0.001) (Figure 1A). Referring to the setting range of left ventricular mass index (LVMI; normal range, <47 g/m2.7 in women and <50 g/m2.7 in men), LVH was detected in about 77% of the participants. Interestingly, we found that the increase of LVMI was negatively related to serum Klotho level (r=−0.49, P<0.001) and positively related to serum IS level (r=0.69, P<0.001) (Figure 1, B and C). Moreover, the regression line of IS/LVMI in patients with serum Klotho<883.5 pg/ml (median value) was much steeper than that in patients with serum Klotho greater than the median value (Figure 1, D and E), which was revealed by covariance analysis (F=12.188, P<0.001), indicating that the interactive relationship between IS elevation and left ventricular mass increase was more apparent in patients with low Klotho level. These data suggest that both IS and Klotho are possibly involved in the development of LVH in patients with CKD, and a close relationship may exist between IS and Klotho during this progression.
Figure 1.
Serum levels of Klotho and IS are associated with the development of LVH in patients with CKD. (A) The correlation between serum levels of Klotho and IS. (B) The correlation between serum Klotho level and LVMI. (C) The correlation between serum IS level and LVMI. (D and E) The correlation between serum IS level and LVMI in patients with serum Klotho level less than or more than median value (883.5 pg/ml). 95% CI, 95% confidence interval.
IS Injection Decreases the Expression of Renal Klotho and Induces More Severe LVH in kl/+ Mice
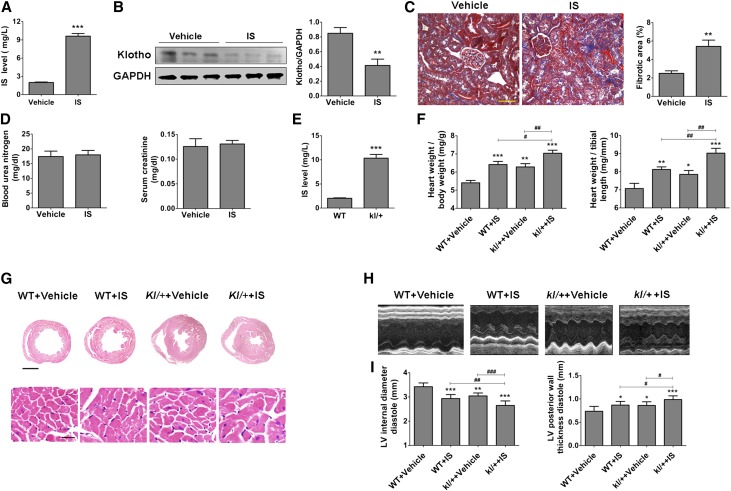
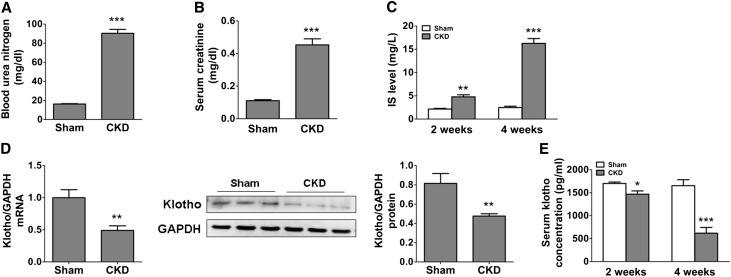
To further evaluate the relationships between IS, Klotho, and LVH, we treated C57BL/6J mice directly with IS (100 mg/kg per day) by intraperitoneal injection for 8 weeks and found that IS treatment resulted in an approximately 5.0-fold increase of IS in serum (Figure 2A). The increase in serum IS was accompanied by a significant downregulation of Klotho and a marked increase in collagen deposition in the kidneys (but without evident changes in BUN and serum creatinine) (Figure 2, B–D). However, a much higher level of serum IS was detected in heterozygous kl/+ mice relative to wild-type (WT) mice (Figure 2E), indicating that Klotho reduction may also cause IS increase.
Figure 2.
Intraperitoneal injection of IS induces severe LVH in kl/+ mice. (A) The serum IS level was significantly increased in mice intraperitoneally injected with IS (100 mg/kg per day) for 8 weeks. (B) The expression of Klotho in the kidney was significantly reduced after IS treatment, which was revealed by Western blotting (upper panel; 130-kD band). (C) An obvious increase of collagen deposition was observed in the kidneys in IS-injected mice, which was revealed by Masson staining. The fibrotic area (blue area) was quantified. Scale bar, 50 μm. (D) The BUN and serum creatinine were measured in IS-injected and vehicle-treated mice. Data are means±SEMs. **P<0.01, ***P<0.001 versus vehicle (n=8 mice). (E) The serum IS level was significantly elevated in kl/+ mice compared with WT mice. ***P<0.001 (n=8 mice). (F) The relative heart weight was markedly increased in IS-treated WT and kl/+ mice. (G) Representative gross pathology of midchamber sections of the heart (hematoxylin-eosin staining) and sections from the left ventricular midchamber free wall (hematoxylin-eosin staining) in WT and kl/+ mice treated with or without IS for 8 weeks. Scale bar, 200 μm in upper panel; 50 μm in lower panel. (H and I) The LVH was present in WT and kl/+ mice injected with IS, which was determined by echocardiography. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LV, left ventricular. *P<0.05; **P<0.01; ***P<0.001 versus WT+vehicle. #P<0.05; ##P<0.01; ###P<0.001 (n=5–8 mice).
Notably, an obvious increase in ventricular wall thickness and elevation of relative heart weight as well as decrease in left ventricular internal diameter diastole and increase in left ventricular posterior wall thickness diastole were observed in IS-injected WT and kl/+ mice. Moreover, relative to WT mice, kl/+ mice developed more severe LVH after injection with IS for 8 weeks (Figure 2, F–I). These results indicate that a high level of circulating IS is a causal factor for LVH, and the decrease of Klotho is probably implicated in IS-induced LVH.
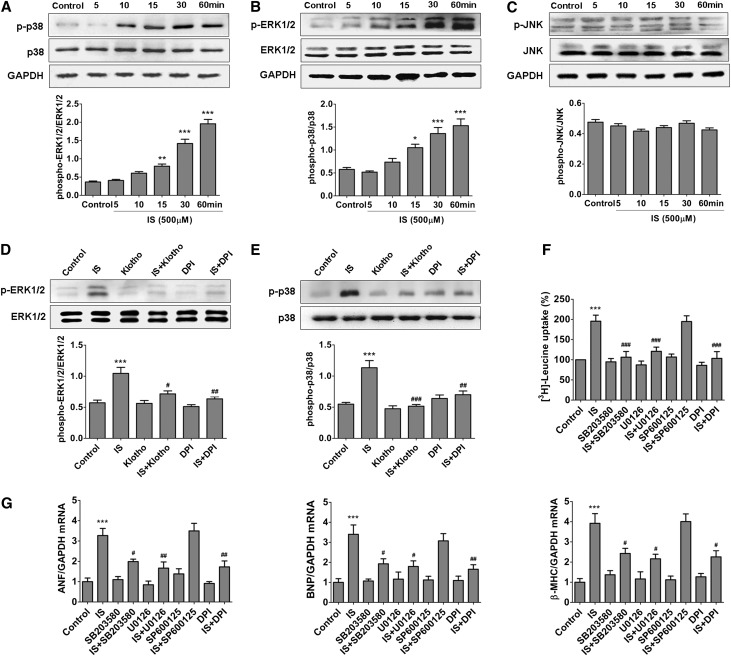
Klotho Has the Ability to Inhibit IS–Induced Cardiomyocyte Hypertrophy
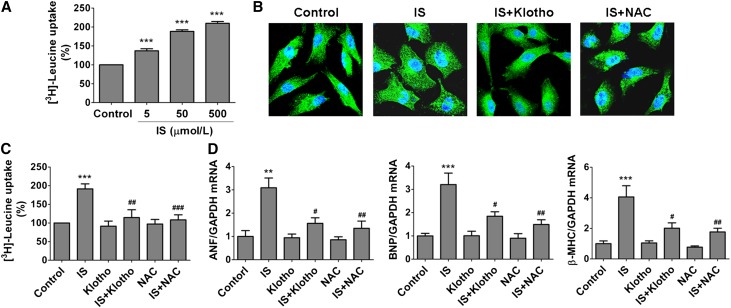
To reveal the direct effect of IS on cardiomyocyte hypertrophy and whether Klotho has a counteractive action against IS, we then treated the cultured neonatal rat cardiomyocytes (NRCMs) with IS in the absence or presence of Klotho. It was found that IS could dose dependently promote NRCMs uptake of 3H-leucine (Figure 3A), and the cells became obviously enlarged after being cultured with 500 μM IS for 48 hours accompanied by significant increases in the mRNA levels of atrial natriuretic factor (ANF), brain natriuretic peptide (BNP), and β-myosin heavy chain (β-MHC) (Figure 3, B–D), showing that IS has an inductive effect on cardiomyocyte hypertrophy. Notably, pretreatment with 400 pmol/L Klotho protein for 1 hour, similar to the effect of N-acety-L-cysteine, a reactive oxygen species (ROS) scavenger, significantly inhibited IS–induced cardiomyocyte hypertrophy, suggesting that Klotho has a distinct ability to suppress the action of IS on cardiomyocytes probably by inhibiting ROS signaling.
Figure 3.
Klotho suppresses IS–induced cardiomyocyte hypertrophy. (A) NRCMs were pretreated with different concentrations of IS for 48 hours, and then, the uptake of 3H-leucine was analyzed. (B) The cell size was determined by immunofluorescence staining using α-actinin antibody (green). (C) Pretreatment of NRCMs with 400 pmol/L Klotho or 5 mmol/L N-acety-L-cysteine (NAC) significantly inhibited the increase of 3H-leucine uptake induced by 500 μmol/L IS. (D) Pretreatment with 400 pmol/L Klotho or 5 mmol/L NAC significantly inhibited the increases of ANF, BNP, and β-MHC mRNA levels induced by 500 μmol/L IS, which was detected by real-time PCR. Experiments were repeated three times. Data are means±SEMs. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. **P<0.01; ***P<0.001 versus control. #P<0.05; ##P<0.01; ###P<0.001 versus IS treatment alone.
Repression of Oxidative Stress and Its Downstream Signaling Pathways Contributes to the Inhibitive Effect of Klotho on IS–Induced Cardiomyocyte Hypertrophy
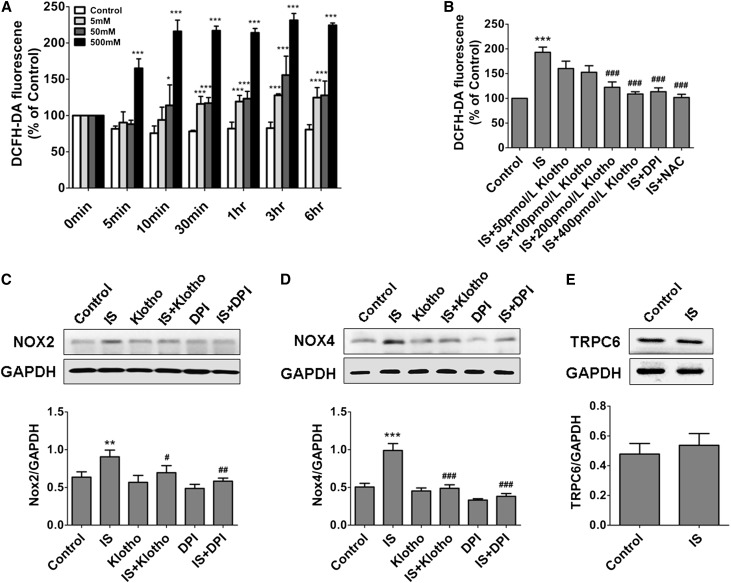
Because IS is characterized with oxidative property and oxidative stress plays an important role in myocardial hypertrophy,11,12,22 we next investigated the role of oxidative stress in IS–induced cardiomyocyte hypertrophy. As expected, IS could quickly promote ROS production in NRCMs, and incubation with 500 μmol/L IS for 10 minutes led to an about 2-fold increase in ROS level (Figure 4A). Notably, pretreatment with Klotho could dose dependently suppress IS–induced ROS production (Figure 4B). It was further found that the expressions of NADPH oxidase 2 (Nox2) and Nox4 were significantly upregulated in cardiomyocytes after 500 μmol/L IS treatment, whereas pretreatment with 400 pmol/L Klotho protein could inhibit IS-induced upregulation of Nox2 and Nox4, similar to the effect of diphenyleneiodonium chloride (DPI; an Nox inhibitor) (Figure 4, C and D). Because transient receptor potential canonical 6 (TRPC6) channels overactivation also contributes to cardiomyocyte hypertrophy and because Klotho can inhibit isoproterenol–induced TRPC6 upregulation,23 we then investigated the effect of IS on TRPC6 expression in NRCMs. The result showed that TRPC6 expression was not affected by IS (Figure 4E), hinting that TRPC6 channels were not involved in IS–induced cardiomyocyte hypertrophy.
Figure 4.
IS–induced ROS production in NRCMs is inhibited by Klotho. (A) The dose-dependent effects of IS on ROS production. (B) Pretreatment of NRCMs with 400 pmol/L Klotho, 5 mmol/L N-acety-L-cysteine (NAC), or 10 µmol/L DPI for 1 hour obviously inhibited IS–induced ROS production. (C and D) Pretreatment with 400 pmol/L Klotho and 10 µmol/L DPI significantly attenuated the IS-induced increase in the expressions of Nox2 and Nox4, which was revealed by Western blotting. (E) Treatment with 500 μmol/L IS did not affect the protein expression of TRPC6 channels in NRCMs, which was revealed by Western blotting. Experiments were repeated three times. Data are means±SEMs. DCFH-DA, 2′,7′-dichlorodihydrofluorescein diacetate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P<0.05; **P<0.01; ***P<0.001 versus control. #P<0.05; ##P<0.01; ###P<0.001 versus IS treatment alone.
It has been proven previously that activations of MAPK pathways, the downstream signals of ROS, play an important role in cardiomyocyte hypertrophy. We then explored the relationship between ROS overproduction and MAPK activation in NRCMs treated with IS. As shown in Figure 5, A–C, the phosphorylation of p38 and extracellular signal–regulated kinase 1/2 (ERK1/2) but not c-Jun N-terminal kinase (JNK) was markedly activated in cultured NRCMs after incubation with IS for 15–60 minutes. Interestingly, the activations of p38 and ERK1/2 could be significantly inhibited by pretreatment with 10 µmol/L DPI or 400 pmol/L Klotho protein (Figure 5, D and E). Moreover, like the effect of DPI, pretreatment with Klotho or the inhibitors of p38 and ERK1/2 (SB203580 and U0126) significantly inhibited IS-induced increases of 3H-leucine uptake and mRNA levels of ANF, BNP, and β-MHC (Figure 5, F and G). These results indicate that IS–induced cardiomyocyte hypertrophy is probably through activation of Nox/ROS/MAPK (p38 and ERK1/2) signaling pathways and that Klotho can counteract the action of IS.
Figure 5.
IS-induced activations of p38 and ERK1/2 signaling in NRCMs are inhibited by Klotho. After incubation with 500 μmol/L IS for 5, 10, 15, 30, and 60 minutes, the expression levels of (A) phosphorylated p38, (B) phosphorylated ERK1/2, and (C) phosphorylated JNK in NRCMs were detected by Western blotting. Pretreatment with 400 pmol/L Klotho and 10 µmol/L DPI significantly attenuated IS-induced activations of (D) p38 and (E) ERK1/2. Pretreatment with 10 µmol/L DPI, 20 µmol/L SB203580, or 10 µmol/L U0126 significantly attenuated (F) IS-induced increase of 3H-leucine uptake and (G) expressions of ANF, BNP, and β-MHC mRNA in NRCMs. Experiments were repeated three times. Data are means±SEMs. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P<0.05; **P<0.01; ***P<0.001 versus control. #P<0.05; ##P<0.01; ###P<0.001 versus IS treatment alone.
Intraperitoneal Injection of Klotho Protein Ameliorates LVH in CKD Mice
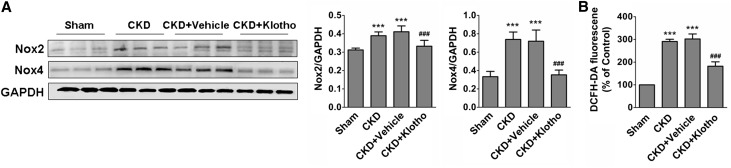
Finally, we assessed the therapeutic effect of exogenous Klotho protein on CKD-associated LVH in a mouse model of CKD. As shown in Figure 6, A–C, the levels of BUN and serum creatinine were dramatically increased in mice at 4 weeks after left nephrectomy. In contrast, Klotho expression in the remaining kidney tissue was remarkably decreased (Figure 6D). A gradual increase of IS accompanied by a reduction of serum Klotho was observed in the mice at 2 and 4 weeks after left nephrectomy (Figure 6, C and E). Compared with the sham group, the expressions of Nox2 and Nox4 as well as the ROS level were significantly increased in the myocardial tissue in CKD mice. Notably, treatment of CKD mice with exogenous Klotho protein (0.01 mg/kg per 48 hours) for 4 weeks led to significant inhibition of the expressions of Nox2 and Nox4 as well as a marked decrease in ROS production in the myocardial tissue (Figure 7).
Figure 6.
The serum level of IS was increased and the expression of renal Klotho was decreased in CKD mice. (A) BUN and (B) serum creatinine increased in CKD mice at 4 weeks after left nephrectomy. (C) The significant increase of serum IS was observed in CKD mice at 2 and 4 weeks after left nephrectomy. (D) The decreased expressions of Klotho mRNA and protein in the kidney were observed in CKD mice at 4 weeks after left nephrectomy. (E) A gradual reduction of serum Klotho level was observed in CKD mice at 2 and 4 weeks after left nephrectomy. Data are means±SEMs. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P<0.05; **P<0.01; ***P<0.001 versus sham group (n=6–8).
Figure 7.
Klotho inhibits the expression of Nox2 and Nox4 and ROS production in the myocardial tissues in CKD mice. (A) Klotho injection (100 mg/kg per day) for 4 weeks inhibited myocardial expressions of Nox2 and Nox4 in CKD mice. (B) The ROS production in myocardial tissues was reduced in CKD mice after intraperitoneal injection of Klotho for 4 weeks. Data are means±SEMs. DCFH-DA, 2′,7′-dichlorodihydrofluorescein diacetate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. ***P<0.001 versus sham group. ###P<0.001 versus CKD and CKD+vehicle (n=6).
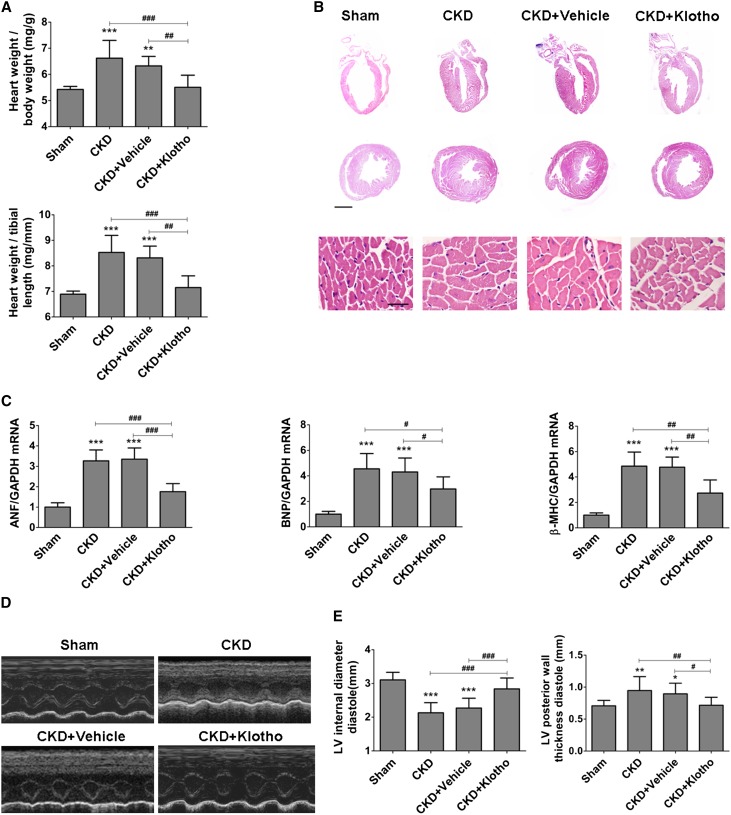
As expected, a majority of CKD mice developed LVH, whereas exogenous Klotho protein supplementation led to a significant alleviation of LVH as manifested by a reduction in the relative heart weight, a decrease in ventricular wall thickness, and a downregulation of mRNA expressions of cardiac BNP, ANF, and β-MHC as well as an increase in left ventricular internal diameter diastole and a decrease in left ventricular posterior wall thickness diastole (Figure 8). These results show that administration of exogenous Klotho protein is effective for the amelioration of CKD-associated LVH.
Figure 8.
Klotho treatment ameliorates LVH in CKD mice. (A) Klotho treatment reduced the ratio of heart weight to body weight and the ratio of heart weight to tibial length in CKD mice (n=12). (B) The representative gross pathology of sagittal and midchamber sections of the heart (hematoxylin-eosin staining) and sections from the left ventricular midchamber free wall (hematoxylin-eosin staining) in CKD mice at 4 weeks after the nephrectomy with or without Klotho treatment. Scale bar, 200 μm in upper panel; 50 μm in lower panel. (C) The mRNA levels of ANF, BNP, and β-MHC in myocardial tissues were decreased after Klotho treatment, which was detected by real-time PCR. (D and E) LVH in CKD mice was attenuated after Klotho treatment, which was assessed by echocardiography. Data are means±SEMs. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LV, left ventricular. *P<0.05; **P<0.01; ***P<0.001 versus sham. #P<0.05; ##P<0.01; ###P<0.001 (n=6).
Discussion
It is well known that there is a close relation between the heart and kidney under physiologic and pathologic conditions.2,3,24 This study discusses four points. (1) The development of LVH is positively related to the serum IS level and negatively related to the serum Klotho level in patients with CKD, and changes in IS and Klotho level are both ascribed to the renal dysfunction. (2) LVH can be induced by intraperitoneal injection with IS in mice accompanied by remarkable downregulation of renal Klotho expression, and IS-induced LVH is more severe in kl/+ mice. (3) Klotho, which is primarily produced in the kidneys and secreted into the serum, has a strong ability to counteract IS–induced cardiomyocyte hypertrophy by inhibiting oxidative stress and its downstream signal pathways p38 and ERK1/2. (4) Exogenous supplementation with Klotho protein is available for the treatment of LVH in CKD mice. Therefore, this study not only provides a new insight into the causation and treatment of CKD-associated LVH but also, expands our understanding of the relationship between the heart and kidney under uremic conditions.
It was previously believed that high BP and volume load were the key factors that triggered cardiac hypertrophy in patients who were uremic. However, LVH continues to progress in these patients when BP is controlled and the volume load is reduced.25 Therefore, some other causes arising from CKD are probably implicated in the development of LVH. As an important uremic solute, IS is gradually increased in the blood with the progression of CKD, and it cannot easily be removed from the blood through dialysis.26 In this study, we found that the maximum level of serum IS in patients with CKD was 656 µmol/L, which is consistent with the previous report that the highest level of serum IS in patients with CKD was above 500 µmol/L.27 Previously, Lekawanvijit et al.13 reported that IS has prohypertrophic and profibrotic effects on cultured cardiac myocytes and fibroblasts, suggesting that IS might be implicated in uremic cardiomyopathy. Here, we revealed that the development of LVH was positively related to serum IS level in patients with CKD. This finding is in accordance with the results of a recent clinical study that higher serum IS levels represent an increased risk of left ventricular diastolic dysfunction.28 More importantly, we found that LVH was induced in mice by administration of IS for 8 weeks, further confirming that high levels of IS in the blood may contribute to the development of LVH.
A typical characteristic of uremia is high oxidative stress, and many complications of uremia are related to oxidative stress injury. Nox isoforms are well known enzymes dedicated to ROS production. As reported, Nox2 and Nox4 are expressed in cardiomyocytes and myocardial fibroblasts, and their activation plays an important role in myocardial hypertrophy and/or cardiac remodeling.29,30 In general, Nox-derived ROS production activates redox–sensitive signaling pathways and stimulates the transcription of hypertrophy-related genes, eventually resulting in cardiac hypertrophy.31 In this study, a dose-dependent elevation of ROS production was detected in NRCMs after treatment with IS, and a remarkable increase in ROS level in the myocardial tissue was also observed in mice treated with IS (100 mg/kg per day) for 8 weeks (data not shown). In IS-treated NRCMs, the elevation of ROS production was accompanied by increased expression of Nox2 and Nox4. Comparatively, Nox4 was more sensitive to IS, which is consistent with previous findings that Nox4 is the major source of oxidative stress in the failing heart.27,30 Conversely, treatment with the Nox inhibitor DPI significantly constrained IS–induced ROS production and activations of p38 and ERK1/2, thereby inhibiting the hypertrophy of cardiomyocytes. Therefore, our findings not only support the view that IS has the property to promote cardiomyocyte hypertrophy13 but also, further unmask the underlying mechanism of the action of IS.
Klotho is a multifunctional factor and well known for its antiaging property. Klotho is expressed predominantly in renal tubular epithelial cells and contains two forms: membrane Klotho and secreted Klotho. Secreted Klotho is generated by membrane–anchored protease cleavage of the extracellular domain from membrane Klotho. Secreted Klotho is released into the blood circulation, acting like a hormone-like factor.32 Klotho is believed to play an important role in protecting renal function.33–35 Previous studies revealed that Klotho expression was significantly downregulated in patients with CKD and CKD animal models.36,37 In this study, we observed that the decrease of serum Klotho was closely related with the decline of eGFR in patients with CKD, which is in accordance with previous reports.38,39 However, a recent study performed by Seiler et al.40 showed that the plasma level of Klotho was not related to renal function in patients with CKD stages 2–4. We agree with the opinion that the discrepancy is probably caused by the study design and sampling strategies, including proportion of patients with different CKD stages, exclusion of specific intervention factors, and samples prepared from serum or plasma.41
It has been reported that uremic solutes, such as IS and p-cresyl sulfate, could distinctly inhibit Klotho gene expression in renal tubular epithelial cells by affecting cytosine-phosphate-guanine hypermethylation.21 We, therefore, presume that, during the progression of CKD, elevated IS may contribute to the downregulation of Klotho expression in renal tubular epithelial cells by increasing DNA hypermethylation, thereby leading to a marked decline of secreted Klotho in the blood. This viewpoint is supported by our finding that long-term IS administration resulted in a significant decrease of Klotho in mice. Interestingly, we also found that serum IS level was significantly increased in kl/+ mice relative to WT mice, indicating that Klotho reduction may conversely lead to the increase of IS.
Subsequently, we observed that long-term IS administration resulted in more severe LVH in kl/+ mice relative to WT mice. In line with animal studies, clinical data revealed that a steeper IS/LVMI relationship was found in patients with low Klotho (Figure 1, D and E). All of the findings show that Klotho reduction can aggravate IS-induced LVH. Of note, a slight but tangible LVH could be developed in kl/+ mice spontaneously, which is consistent with a previous report.18 In that study, Faul et al.18 concluded that the increased serum level of fibroblast growth factor 23 might contribute to the development of LVH in kl/+ mice. However, it cannot exclude the possibility that some other factors or complications arising from the deficiency of Klotho may also be implicated in the development of LVH.
Actually, in contrast to IS, Klotho protein is characterized by its antioxidative activity. As reported, Klotho-overexpressing mice exhibited both a longer lifespan and a substantial decrease in endogenous ROS production.16,17,42 In this study, our findings show that Klotho protein has a distinct capacity to inhibit IS–induced ROS production and the activations of p38 and ERK1/2 signaling pathways in cardiomyocytes, and treatment with exogenous Klotho protein can suppress the development of LVH in CKD mice. In addition, the IS-induced expressions of Nox2 and Nox4 could be significantly downregulated by Klotho treatment. A recent study showed that the upregulation of Klotho could suppress Nox2 expression in rat aortic smooth muscle cells by regulating cAMP/protein kinase A signaling,43 whereas cAMP/protein kinase A signaling was shown to be able to inhibit cardiomyocyte hypertrophy.44 These reports further support our finding that Klotho has the ability to inhibit cardiomyocyte hypertrophy, probably by suppressing Nox2/Nox4–derived ROS production and its downstream signaling.
In summary, this study reveals the interaction between uremic solute and Klotho and the implications in CKD-associated LVH, and it provides a deeper understanding of the relationship between the heart and the kidney under pathophysiologic conditions. Moreover, out study suggests that exogenous supplementation with Klotho may be a potential therapeutic approach for suppressing the progression of uremic cardiomyopathy.
Concise Methods
Selection of Patients
Patients ages 18–83 years old with primary chronic renal diseases who had not been receiving dialysis treatment were enrolled from the Department of Nephrology of Xinqiao Hospital (Chongqing, China). The exclusion criteria were congenital heart disease, BP>140/90 mmHg, heart failure, diabetes, pregnancy, HIV, polycystic kidney disease, renal cancer, and recent immunosuppressive therapy. Final data analysis was carried out in 86 patients who met the selected conditions. We collected the basic information and laboratory and echocardiographic parameters of the patients. The study protocol was approved by the Ethics Committee of Xinqiao Hospital, and the study was carried out in accordance with the Declaration of Helsinki.
LVMI Calculation in Patients
Echocardiograms were performed in recruited individuals on the first day of hospitalization with a single blind method by the same examiner. Two-dimensional M mode was carried out according to American Society of Echocardiography guidelines,45 and ventricular dimension and wall thickness were detected at end diastole and end systole using IE33–5S (Philips Medical System). LVMI was calculated by indexing the left ventricular mass value by the patient’s height; thus, LVH was defined as LVMI≥47 g/m2.7 in women and LVMI≥50 g/m2.7 in men.
Animals
Male C57BL/6J and Balb/c mice were purchased from Beijing HFK Biologic Technology (Beijing, China); kl/+ mice were provided by Jun Gu, and the mice were backcrossed to background mice for more than six generations to achieve congenic background.
Male C57BL/6J, heterozygous kl/+, or WT mice at 8 weeks of age were treated with a single daily intraperitoneal dose of 100 mg/kg IS for 8 weeks. In another experiment, a CKD mouse model was established as previously described.36 Briefly, male Balb/c mice at 12 weeks of age were first inflicted with 2/3 electrocoagulation of the right renal cortex and then received left total nephrectomy 2 weeks later. After left kidney nephrectomy, the mice were separated into four groups: sham (mice received a sham operation that included decapsulation of both kidneys), CKD, CKD+vehicle (0.01 mol/L PBS was administered through intraperitoneal injection), and CKD+Klotho (0.01 mg/kg Klotho protein dissolved in 0.01 mol/L PBS was administered through intraperitoneal injection). The recombinant mouse Klotho protein was purchased from R&D Systems (Minneapolis, MN). The intraperitoneal injections with Klotho protein were repeated every 48 hours for 4 weeks. After treatment, high-resolution echocardiography was performed under anesthesia, and left ventricular internal diameter diastole, left ventricular posterior wall thickness diastole, and short-axis M-mode views were recorded by the Vevo 770 Echocardiography Imaging System (VisualSonics, Toronto, ON, Canada). The mice were euthanized at appointed times, and the blood, heart, and kidney samples were obtained. The tibial length was recorded, and BUN and serum creatinine were measured. All animal studies were performed according to the guidelines established by the Institutional Animal Care and Use Committee of the Third Military Medical University.
ELISA Assay of Serum Klotho
The blood samples from patients and mice were collected and centrifuged for 10 minutes at 3000 rpm (4°C), and the sera were stored at −80°C until additional analysis. Serum Klotho was measured in duplicate using a human or mouse Klotho ELISA kit according to the manufacturer’s protocol (Cusabio, Cologne, Germany).
HPLC Assay of Serum IS
HPLC was performed to measure serum level of IS. The serum samples from the patients or mice were transferred to an autosampler, and chromatographic separation was performed on a hypersil ODS C18 column (150×4.6 mm, 5 μm; DIKMA). The mobile phase consisted of 15% acetonitrile in 85% Milli-Q water and 0.2% trifluoroacetic acid with a flow rate of 1 ml/min. The column temperature was set at 30°C. The detector wavelength was set at 295 nm for excitation and 390 nm for emission. The calibration curve was constructed within the range of 0.5–80.0 μg/ml.
Hematoxylin-Eosin and Masson’s Trichrome Staining
The hearts of mice were perfused by 4% paraformaldehyde and fixed in 10% paraformaldehyde for 2 days before being sectioned. After staining with hematoxylin-eosin, the sagittal, coronal, and microcosmic images of the heart tissue were observed under a light microscope. The kidney tissue paraffin sections were stained with a standard Masson’s trichrome method.
NRCMs
NRCMs were isolated and cultured as previously described.46 Briefly, the hearts of 1- to 2-day-old Sprague–Dawley neonatal rats were acquired and incubated with cold PBS. The left ventricles were collected, minced, and digested with 1.25% trypsin for mechanic disaggregation at room temperature followed with incubation with 0.08% collagenase II for 30 minutes at 37°C. The cardiac cells were filtered through a cell strainer (74-μm; BD Falcon), and the cell suspension was centrifuged at 1500 rpm for 10 minutes at 4°C. After being washed with PBS, the cells were resuspended in DMEM (Gibco, Grand Island, NY) with 15% FBS (Gibco), 0.16% glucose, 100 units/ml penicillin, and 100 µg/ml streptomycin (Gibco) at 37°C for 1 hour. Then, the cardiomyocytes were collected, washed, and plated in a plastic culture flask and grown in DMEM supplemented with 10% FBS and 0.1 mM bromodeoxyuridine (BrdU) for 48 hours before replacing with the normal medium (DMEM with 10% FBS) without BrdU.
Detection of ROS Production
The generation of intracellular ROS was detected using 2′,7′-dichlorodihydrofluorescein diacetate (Molecular Probes; Eugene, OR), an ROS–sensitive fluorescent dye. NRCMs were cultured in 96-well plates and then incubated with IS at different concentrations for different times. In parallel, NRCMs were preincubated with different concentrations of Klotho protein, 10 µmol/L DPI, or 5 mmol/L N-acety-L-cysteine (Sigma-Aldrich) for 1 hour and then incubated with 500 μmol/L IS for 6 hours. After IS treatment, the cells were loaded with the fluoroprobe 2′,7′-dichlorodihydrofluorescein diacetate (10 µmol/L) at 37°C for 40 minutes in 200 µl serum-free DMEM. The fluorescence level was observed under an inverted fluorescence microscope, and the fluorescence intensity was measured at 480-nm excitation and 525-nm emission with a microplate reader (Thermo Fisher Scientific, Pittsburgh, PA). In the animal study, ROS production was detected in the homogenate of heart tissue.
Measurement of 3H-Leucine
NRCMs were pretreated with 20 µmol/L p38 MAPK inhibitor (SB203580; Sigma-Aldrich), 10 µmol/L p44/42 MAPK (ERK1/2) inhibitor (U0126; Sigma-Aldrich), 15 µmol/L JNK MAPK inhibitor (SP600125; Sigma-Aldrich), or 400 pM Klotho for 1 hour and then incubated with 500 µmol/L IS for 48 hours before being exposed to 1 µCi 3H-leucine (PerkinElmer, Waltham, MA) for 18 hours. After treatment with 5% trichloroacetic acid for 30 minutes, the cells were scraped off and centrifuged. Then, a pellet of each sample was dissolved in 0.5 mol/L NaOH overnight at 4°C. The levels of 3H-leucine incorporation were measured using a scintillation fluid counter (Triathler LSC, Hidex, Finland).
Immunofluorescence Assay
The NRCMs were fixed in 4% paraformaldehyde and then permeabilized with 0.3% Triton X-100 at room temperature. After being blocked in goat serum (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at 37°C, the cells were incubated with mouse monoclonal actinin antibody (1:100; Santa Cruz Biotechnology) overnight at 4°C. After being washed with PBS, the cells were incubated for 1 hour with FITC–conjugated goat anti-mouse antibody (1:300; Santa CruzBiotechnology) in the dark. The nucleus was labeled with 4′,6-diamidino-2-phenylindole (1 mg/ml in PBS; Roche, Indianapolis, IN) for 5 minutes. Images were captured using a Leica TCS-SP5 laser-scanning confocal microscopy (Leica Microsystems, Inc., Bannockburn, IL).
Quantitative Real-Time PCR
Total RNA was extracted from the NRCMs and the heart and kidney tissues using TRIzol (Invitrogen, Carlsbad, CA). The RNA was reverse transcribed into cDNA, and quantitative real-time PCR was performed on an iCycler iQ (Bio-Rad, Hercules, CA) using a Real-Time PCR Assay Kit (TakaRa, Dalian, China). The primers used in the experiments are presented in Supplemental Table 1.
Western Blotting Analyses
The proteins isolated from NRCMs and heart and kidney tissue samples were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA). The membranes were incubated at 4°C overnight with the following primary antibodies: rabbit monoclonal phospho-p44/42 MAPK (Thr202/Tyr204) and p44/42 MAPK, rabbit monoclonal phospho-p38 MAPK (Thr180/Tyr182) and p38 MAPK, rabbit monoclonal phospho- stress-activated protein kinase /JNK (Thy183/T185) and stress-activated protein kinase /JNK (1:1000; Cell Signaling Technology, Beverly, MA), rabbit monoclonal Nox4 (1:500), rabbit polyclonal gp91phox, rabbit polyclonal Klotho (1:500; Abcam, Inc., Cambridge, UK), and glyceraldehyde-3-phosphate dehydrogenase (1:500; Santa Cruz Biotechnology, Dallas, TX). Then, the secondary antibodies were applied. The signals were developed with the ECL-Plus Western Blotting Detection System (GE Healthcare, Buckinghamshire, UK), and the densitometry analysis was performed with an image analysis system (Bio-Rad).
Statistical Analyses
The Spearman’s correlation coefficient was examined in the human study, and multivariable analysis was used to adjust for demographic factors, including age, sex, and body mass index. In animal studies, data are expressed as means±SEMs. Statistical analysis was performed using unpaired, two-tailed t test and one-way ANOVA with Tukey multiple comparison test. Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA) and SPSS 13.0 (SPSS Japan, Tokyo, Japan). A value of P<0.05 was considered significant (Table 1).
Table 1.
Nonstandard abbreviations and acronyms
| Abbreviations and Acronyms | Full Name |
|---|---|
| ANF | Atrial natriuretic factor |
| BNP | Brain natriuretic peptide |
| CVD | Cardiovascular disease |
| DPI | Diphenyleneiodonium chloride |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| IS | Indoxyl sulfate |
| LVH | Left ventricular hypertrophy |
| LVMI | Left ventricular mass index |
| MAPK | Mitogen-activated protein kinase |
| β-MHC | β-Myosin heavy chain |
| NAC | N-acety-L-cysteine |
| Nox | NADPH oxidase |
| NRCMs | Neonatal rat cardiomyocytes |
| ROS | Reactive oxygen species |
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by National Natural Science Foundation of China Research Grants 81270290, 81070168, and 30700316 and the project for overseas student from the Ministry of Human Resources and Social Security of the People’s Republic of China.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Loss of Klotho in CKD Breaks One’s Heart,” on pages 2305–2307.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014060543/-/DCSupplemental.
References
- 1.US Renal Data System : USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 2.Weiner DE: Public health consequences of chronic kidney disease. Clin Pharmacol Ther 86: 566–569, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Cerasola G, Nardi E, Palermo A, Mulè G, Cottone S: Epidemiology and pathophysiology of left ventricular abnormalities in chronic kidney disease: A review. J Nephrol 24: 1–10, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Silberberg JS, Barre PE, Prichard SS, Sniderman AD: Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int 36: 286–290, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Niwa T, Takeda N, Tatematsu A, Maeda K: Accumulation of indoxyl sulfate, an inhibitor of drug-binding, in uremic serum as demonstrated by internal-surface reversed-phase liquid chromatography. Clin Chem 34: 2264–2267, 1988 [PubMed] [Google Scholar]
- 7.Meert N, Schepers E, De Smet R, Argiles A, Cohen G, Deppisch R, Drüeke T, Massy Z, Spasovski G, Stegmayr B, Zidek W, Jankowski J, Vanholder R: Inconsistency of reported uremic toxin concentrations. Artif Organs 31: 600–611, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Fujii H, Nakai K, Fukagawa M: Role of oxidative stress and indoxyl sulfate in progression of cardiovascular disease in chronic kidney disease. Ther Apher Dial 15: 125–128, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group (EUTox) : Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Stanfel LA, Gulyassy PF, Jarrard EA: Determination of indoxyl sulfate in plasma of patients with renal failure by use of ion-pairing liquid chromatography. Clin Chem 32: 938–942, 1986 [PubMed] [Google Scholar]
- 11.Tumur Z, Niwa T: Indoxyl sulfate inhibits nitric oxide production and cell viability by inducing oxidative stress in vascular endothelial cells. Am J Nephrol 29: 551–557, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T: Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaB activation. Am J Nephrol 31: 435–441, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H: Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J 31: 1771–1779, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Lekawanvijit S, Kompa AR, Manabe M, Wang BH, Langham RG, Nishijima F, Kelly DJ, Krum H: Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS ONE 7: e41281, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negri AL: The klotho gene: A gene predominantly expressed in the kidney is a fundamental regulator of aging and calcium/phosphorus metabolism. J Nephrol 18: 654–658, 2005 [PubMed] [Google Scholar]
- 16.Couzin J: Physiology. Boosting gene extends mouse life span. Science 309: 1310–1311, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M: Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusano E: Mechanism by which chronic kidney disease causes cardiovascular disease and the measures to manage this phenomenon. Clin Exp Nephrol 15: 627–633, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Yang K, Nie L, Huang Y, Zhang J, Xiao T, Guan X, Zhao J: Amelioration of uremic toxin indoxyl sulfate-induced endothelial cell dysfunction by Klotho protein. Toxicol Lett 215: 77–83, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Sun CY, Chang SC, Wu MS: Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int 81: 640–650, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM: Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41: 2164–2171, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Xie J, Cha SK, An SW, Kuro-O M, Birnbaumer L, Huang CL: Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun 3: 1238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weidtmann B, Schunkert H: Kidney insufficiency and cardiovascular disease. Internist (Berl) 48: 770–778, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Siedlecki AM, Jin X, Muslin AJ: Uremic cardiac hypertrophy is reversed by rapamycin but not by lowering of blood pressure. Kidney Int 75: 800–808, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagugli RM, De Smet R, Buoncristiani U, Lameire N, Vanholder R: Behavior of non-protein-bound and protein-bound uremic solutes during daily hemodialysis. Am J Kidney Dis 40: 339–347, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Muteliefu G, Enomoto A, Jiang P, Takahashi M, Niwa T: Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Nephrol Dial Transplant 24: 2051–2058, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Sato B, Yoshikawa D, Ishii H, Suzuki S, Inoue Y, Takeshita K, Tanaka M, Kumagai S, Matsumoto M, Okumura S, Hayashi M, Matsubara T, Niwa T, Murohara T: Relation of plasma indoxyl sulfate levels and estimated glomerular filtration rate to left ventricular diastolic dysfunction. Am J Cardiol 111: 712–716, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Shanmugam P, Valente AJ, Prabhu SD, Venkatesan B, Yoshida T, Delafontaine P, Chandrasekar B: Angiotensin-II type 1 receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol 50: 928–938, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J: NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A 107: 15565–15570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anilkumar N, Sirker A, Shah AM: Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci (Landmark Ed) 14: 3168–3187, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y: Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242: 626–630, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Sun Z: Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 54: 810–817, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satoh M, Nagasu H, Morita Y, Yamaguchi TP, Kanwar YS, Kashihara N: Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol 303: F1641–F1651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M: Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286: 8655–8665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu J, Deng M, Zhao J, Huang L: Decreased expression of klotho gene in uremic atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun 391: 261–266, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y: Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280: 1015–1020, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Pavik I, Jaeger P, Ebner L, Wagner CA, Petzold K, Spichtig D, Poster D, Wüthrich RP, Russmann S, Serra AL: Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: A sequence suggested from a cross-sectional study. Nephrol Dial Transplant 28: 352–359, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto S, Ikebe M, Yuasa K, Yamanaka S, Sugiura T, Terada Y: Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 16: 722–729, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Seiler S, Wen M, Roth HJ, Fehrenz M, Flügge F, Herath E, Weihrauch A, Fliser D, Heine GH: Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int 83: 121–128, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Drüeke TB, Massy ZA: Circulating Klotho levels: Clinical relevance and relationship with tissue Klotho expression. Kidney Int 83: 13–15, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M: Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 280: 38029–38034, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Kuro-o M, Sun Z: Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell 11: 410–417, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ha CH, Kim JY, Zhao J, Wang W, Jhun BS, Wong C, Jin ZG: PKA phosphorylates histone deacetylase 5 and prevents its nuclear export, leading to the inhibition of gene transcription and cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A 107: 15467–15472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing Group. American Society of Echocardiography’s Guidelines and Standards Committee. European Association of Echocardiography : Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Louch WE, Sheehan KA, Wolska BM: Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol 51: 288–298, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.