Abstract
Tubulointerstitial fibrosis is common with ageing and strongly prognostic for ESRD but is poorly captured by eGFR or urine albumin to creatinine ratio (ACR). Higher urine levels of procollagen type III N-terminal propeptide (PIIINP) mark the severity of tubulointerstitial fibrosis in biopsy studies, but the association of urine PIIINP with CKD progression is unknown. Among community-living persons aged ≥65 years, we measured PIIINP in spot urine specimens from the 1996 to 1997 Cardiovascular Health Study visit among individuals with CKD progression (30% decline in eGFR over 9 years, n=192) or incident ESRD (n=54) during follow-up, and in 958 randomly selected participants. We evaluated associations of urine PIIINP with CKD progression and incident ESRD. Associations of urine PIIINP with cardiovascular disease, heart failure, and death were evaluated as secondary end points. At baseline, mean age (±SD) was 78±5 years, mean eGFR was 63±18 ml/min per 1.73 m2, and median urine PIIINP was 2.6 (interquartile range, 1.4–4.2) μg/L. In a case-control study (192 participants, 231 controls), each doubling of urine PIIINP associated with 22% higher odds of CKD progression (adjusted odds ratio, 1.22; 95% confidence interval, 1.00 to 1.49). Higher urine PIIINP level was also associated with incident ESRD, but results were not significant in fully adjusted models. In a prospective study among the 958 randomly selected participants, higher urine PIIINP was significantly associated with death, but not with incident cardiovascular disease or heart failure. These data suggest higher urine PIIINP levels associate with CKD progression independently of eGFR and ACR in older individuals.
Keywords: fibrosis, geriatric nephrology, progression of chronic renal failure, tubule, cells
CKD affects >13% of the United States population1 and is strongly associated with progression to ESRD, cardiovascular disease (CVD), and early death.2 Recognition of the public health implications of this problem has led to considerable efforts to understanding CKD risk factors and reduce its incidence and progression to prevent associated adverse health consequences.
Clinically, measurement of kidney function focuses almost exclusively on markers of glomerular function (serum creatinine and cystatin C concentrations) and glomerular capillary integrity (urine albumin to creatinine ratio [ACR] and proteinuria).3 In our clinical experience, however, we frequently observe extensive tubulointerstitial fibrosis in biopsy specimens, even among persons with relatively preserved eGFR and without albuminuria. This clinical finding was confirmed in a large series of healthy kidney donors who provided biopsy specimens at the time of kidney extraction for transplant. Tubulointerstitial fibrosis was common on biopsy and increased linearly with age; however, the degree of fibrosis was not associated with iothalamate measured GFR once age was taken into account.4 The degree of tubulointerstitial fibrosis on biopsy is strongly associated with progressive loss of GFR in nearly all etiologies of CKD that have been evaluated.5–7 Collectively, these data suggest that tubulointerstitial fibrosis is common in older age, prognostically important, and yet poorly captured by markers of kidney function available in contemporary clinical practice.
Renal fibrosis is characterized by deposition of various extracellular matrix components including type III collagen. Type III collagen is synthesized as a procollagen, and the amino-terminal propeptide (procollagen type III amino-terminal propeptide [PIIINP]) is largely cleaved during deposition of collagen in the extracellular matrix, and to a lesser extent during type III collagen breakdown. PIIINP is then released from the extracellular matrix into the urine or blood. Several prior studies evaluating kidney biopsy series have demonstrated that urine PIIINP concentrations are correlated with the severity of tubulointerstitial fibrosis on biopsy.8–10 In one study among kidney transplant recipients, PIIINP/creatinine (Cr) concentrations >100 ng/mmol were associated with declining eGFR; however, this study did not adjust for baseline eGFR or proteinuria.9 To our knowledge, no prior study has evaluated the association of urine PIIINP with loss of kidney function in older community-living individuals, an age group in which measurement of kidney function by creatinine is known to be insensitive to loss of GFR,11,12 proteinuria is infrequent, and renal fibrosis is particularly common.4
The purpose of this study was to determine the association of urine PIIINP concentrations with progression of CKD in community-living older adults. Our primary hypothesis was that higher urine PIIINP concentrations would identify individuals at greater risk for CKD progression and ESRD independent of eGFR and urine ACR. As a secondary objective, we also determined the associations of urine PIIINP with incident CVD, incident heart failure (HF), and all-cause mortality, which are common CKD-related outcomes in older persons.11,13
Results
Baseline Correlates of Urine PIIINP Measurements
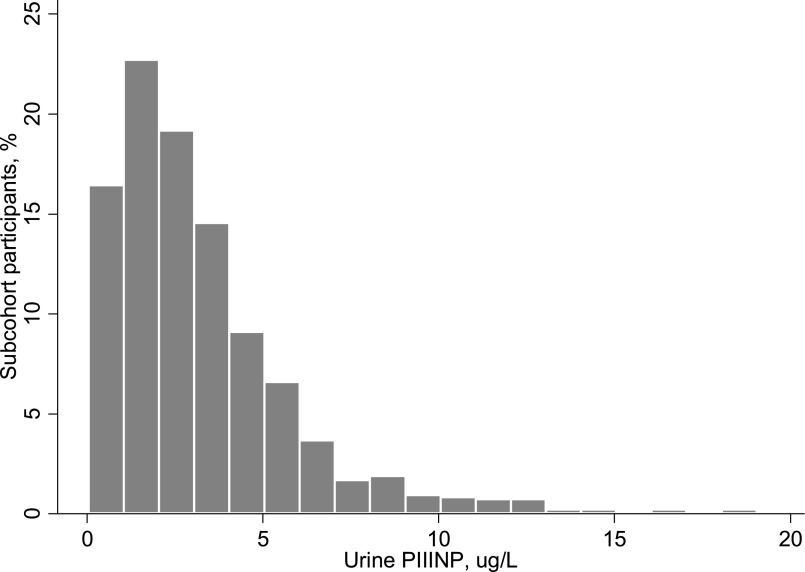
Among the randomly selected 958 subcohort, the mean age was 78±5 years, 60% were women, and 15% were black. The mean eGFR was 63±18 ml/min per 1.73 m2, and median urine ACR was 8 (interquartile range, 5–20) mg/g. The distribution of urine PIIINP was right skewed, with a median of 2.6 (interquartile range, 1.4–4.2) μg/L (Figure 1).
Figure 1.
Distribution of spot urine PIIINP concentrations in 958 randomly selected community-living older adult participants in the CHS.
Table 1 shows baseline characteristics by quartiles of urine PIIINP in the random subcohort. Compared with persons with lower urine PIIINP levels, those in the highest quartile were older, more frequently men and black, and more likely to have a history of stroke and diabetes. They also had higher plasma PIIINP concentrations and more advanced CKD on the basis of both lower eGFR and higher urine ACR. Among the subset of 289 individuals in the subcohort who had both baseline and follow-up eGFRs, the eGFR declined by approximately 5 ml/min per 1.73 m2 during 9 years follow-up in quartile 1 compared with approximately 10 ml/min per 1.73 m2 in quartile 4. Table 2 shows a correlation matrix of key measures. Urine PIIINP was moderately correlated with urine creatinine concentrations (r=0.66), likely because of the influence of urine tonicity on both markers. The correlation of urine PIIINP with urine PIIINP indexed to creatinine was 0.58. Correlations of urine PIIINP with plasma PIIINP, eGFR and urine ACR were present, but they were relatively modest (0.19, –0.17, and 0.25, respectively).
Table 1.
Baseline characteristics by quartiles of urine PIIINP in 958 randomly selected subcohort participants in the CHS
| Characteristic | Urine PIIINP Quartiles | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Range (μg/L) | ≤1.39 | >1.39–2.58 | >2.58–4.22 | >4.22 |
| n | 240 | 239 | 240 | 239 |
| Demographics | ||||
| Age±SD, y | 78±5 | 78±5 | 78±5 | 79±5 |
| Male, n (%) | 60 (25.0) | 81 (33.9) | 92 (38.3) | 145 (60.7) |
| Black, n (%) | 29 (12.1) | 27 (11.3) | 41 (17.1) | 49 (20.5) |
| Education (>high school), n (%) | 83 (34.6) | 98 (41.0) | 81 (33.8) | 90 (37.7) |
| Lifestyle factors | ||||
| Current smoking, n (%) | 18 (7.5) | 12 (5.0) | 23 (9.6) | 19 (7.9) |
| Alcohol (≥7 drinks/wk), n (%) | 28 (11.7) | 37 (15.5) | 34 (14.2) | 20 (8.5) |
| Prevalent CVD | ||||
| History of MI, n (%) | 29 (12.1) | 31 (13.0) | 23 (9.6) | 36 (15.1) |
| History of HF, n (%) | 23 (9.6) | 24 (10.0) | 13 (5.4) | 26 (10.9) |
| History of stroke, n (%) | 11 (4.6) | 15 (6.3) | 7 (2.9) | 32 (13.4) |
| Cardiovascular risk factors | ||||
| BMI±SD (kg/m2) | 26.1±4.4 | 26.6±4.8 | 27.6±5.0 | 27.2±4.6 |
| SBP±SD (mmHg) | 137.6±21.1 | 137.6±22.3 | 136.0±20.3 | 136.1±19.7 |
| DBP±SD (mmHg) | 69.2±10.0 | 69.8±12.4 | 69.3±10.8 | 70.4±11.4 |
| BP medication use, n (%) | 128 (53.3) | 141 (59.0) | 136 (56.7) | 126 (52.7) |
| Diabetes, n (%) | 20 (8.3) | 23 (9.6) | 39 (16.3) | 51 (21.5) |
| Total Chol.±SD (mg/dl) | 204.6±36.6 | 198.6±38.8 | 205.9±37.6 | 196.5±41.7 |
| Lipid medication use, n (%) | 32 (13.3) | 30 (12.6) | 27 (11.3) | 26 (10.9) |
| CRP (mg/L), median (IQR) | 2.3 (1.0–5.1) | 1.9 (0.9–3.9) | 2.7 (1.1–5.4) | 2.5 (1.3–6.4) |
| Plasma PIIINP±SD (μg/L) | 4.4±1.4 | 4.7±1.6 | 4.8±1.4 | 5.2±1.9 |
| Kidney function measures | ||||
| Baseline eGFR±SD (ml/min per 1.73 m2) | ||||
| In all participants (n=958) | 67.2±16.0 | 65.3±17.7 | 63.0±18.3 | 58.5±20.4 |
| In those with follow-up eGFR data (n=289) | 69.7±15.3 | 69.0±17.0 | 65.8±16.6 | 68.7±19.4 |
| Follow-up eGFR±SD (ml/min per 1.73 m2) (n=289) | 64.6±18.5 | 60.4±20.4 | 58.5±20.6 | 59.3±22.5 |
| Urine ACR, mg/g (IQR) | 6.4 (3.9–13.6) | 8.0 (4.7–16.6) | 7.5 (4.7–18.2) | 12.3 (7.0–52.6) |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; BP, blood pressure; Chol., cholesterol; CRP, C-reactive protein; IQR, interquartile range.
Table 2.
Correlation matrix of key variables relative to spot urine PIIINP concentrations in the 958 randomly selected subcohort participants in the CHS
| Urine PIIINP | eGFR | Urine ACR | Plasma PIIINP | |
|---|---|---|---|---|
| Urine PIIINP | 1.00 | |||
| eGFR | −0.17 | 1.00 | ||
| Urine ACR | 0.24 | −0.20 | 1.00 | |
| Plasma PIIINP | 0.19 | −0.22 | 0.08 | 1.00 |
Data show unadjusted Spearman correlations because all variables were right skewed with the exception of eGFR.
Urine PIIINP and Longitudinal Changes in Kidney Function
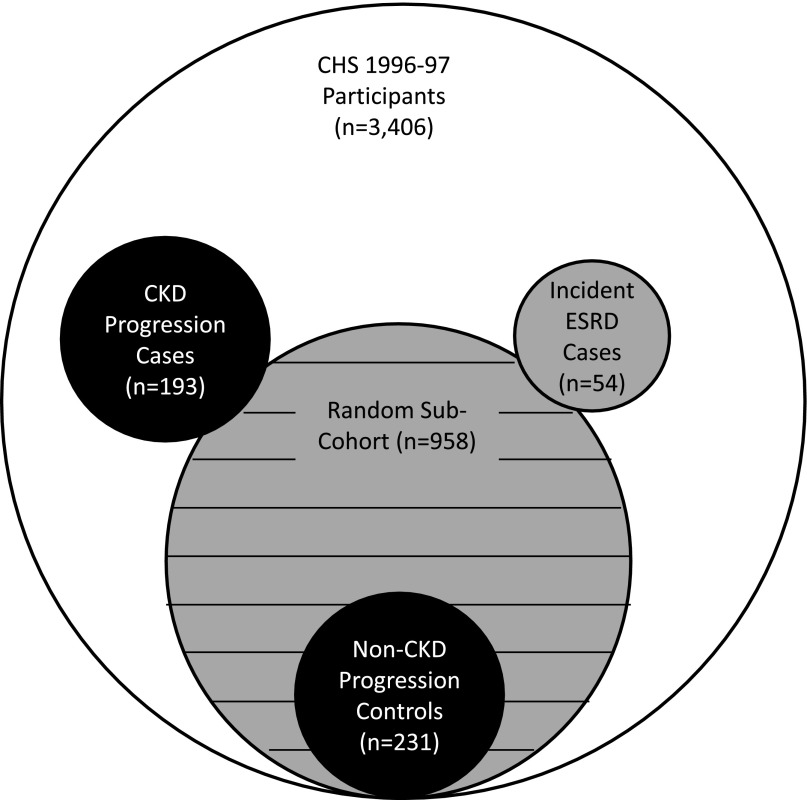
Among the 1001 Cardiovascular Health Study (CHS) participants who provided repeat blood specimens for eGFR measurement at the follow-up examination, the mean change in eGFR was –0.93±1.6 ml/min per 1.73 m2/y, and 192 (19%) had ≥30% decline in eGFR relative to baseline (CKD progression participants). Controls were those in the randomly selected subcohort who remained alive and provided repeat eGFR measurements at the follow-up examination, but had <30% decline in eGFR (n=231) (Figure 2). Compared with the lowest urine PIIINP quartile, those in the highest quartile had 2.6 times higher odds of CKD progression in models adjusted for age, sex, race, clinic site, and urine creatinine (Table 3). On a continuous scale, each doubling of urine PIIINP was associated with 35% higher odds of CKD progression. Additional adjustment for eGFR and ACR attenuated the association such that each doubling was associated with 23% higher odds of CKD progression, and the highest quartile was associated with 68% greater odds. Further adjustment for CVD and CKD risk factors did not meaningfully influence the association. When the final model was additionally adjusted for plasma PIIINP concentrations, the association did not change meaningfully (HR per doubling, 1.26; 95% confidence interval [95% CI], 1.03 to 1.56).
Figure 2.
Sampling for this study within the CHS. Black area represents participants included in the case-control study for the CKD progression outcome; gray area represents participants included in the case-cohort study for the incident ESRD outcome; and hashed gray area represents participants included in the prospective cohort study for the heart failure, CVD events, and all-cause mortality outcomes.
Table 3.
Association of urine PIIINP with CKD progression in community-living older adult participants in the CHS
| Urine PIIINP Quartiles | Urine PIIINP Continuous (Per Doubling) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Urine PIIINP range (μg/L) | ≤1.39 | 1.40–2.58 | 2.59–4.22 | >4.22 | |
| No. | 105 | 122 | 97 | 99 | |
| No. with CKD progression,a n (%) | 40 (38) | 56 (46) | 45 (46) | 51 (52) | |
| Demographic adjusted,b OR (95% CI) | 1.00 (reference) | 1.60 (0.92 to 2.80) | 1.92 (0.99 to 3.69) | 2.64 (1.28 to 5.46) | 1.35 (1.11 to 1.63) |
| Plus eGFR and urine albumin,c OR (95% CI) | 1.00 (reference) | 1.34 (0.76 to 2.36) | 1.40 (0.71 to 2.78) | 1.68 (0.78 to 3.64) | 1.23 (1.02 to 1.50) |
| Plus CVD risk factors,d OR (95% CI) | 1.00 (reference) | 1.33 (0.74 to 2.40) | 1.43 (0.70 to 2.93) | 1.69 (0.75 to 3.80) | 1.22 (1.00 to 1.49) |
OR, odds ratio.
CKD progression defined as ≥30% decline in eGFR at follow-up.
Adjusted for age, sex, race, education, clinic site, and urine creatinine.
Adjusted for demographic variable plus baseline eGFR and urine albumin.
Adjusted for demographic variables eGFR, urine albumin, plus smoking status, pack years, body mass index, diabetes, systolic blood pressure, blood pressure medication use, total cholesterol, lipid medication use, and C-reactive protein.
Results were similar when we evaluated the association of urine PIIINP concentrations with annualized change in eGFR within the randomly selected subcohort. In models adjusted for age, sex, race, clinic site, and urine creatinine concentration, each doubling in urine PIIINP was associated with a 0.20 (95% CI, 0.05 to 0.35) ml/min per 1.73 m2/y faster decline in eGFR. In the fully adjusted model, each doubling of urine PIIINP was associated with 0.12 (95% CI, –0.02 to 0.27) ml/min per 1.73 m2/y faster decline in eGFR.
In the secondary analysis, we evaluated 30% decline in eGFR by creatinine. Because participants were selected using 30% decline in eGFR by cystatin C, a priori, only some of the participants also had 30% decline in eGFR by creatinine, whereas others did not. Conversely, some met the definition of 30% decline by the creatinine-based outcome definition but were missing urine PIIINP measurements. Point estimates were similar to those in our primary analysis, but did not reach statistical significance (Supplemental Table 1).
There were 54 incident ESRD events during a median 9.5 years follow-up. When participants were categorized by quartiles of urine PIIINP, we observed no individuals in the lowest quartile developed ESRD, and 4, 13, and 37 individuals developed ESRD in quartiles 2–4, respectively (Table 4). With no ESRD events in quartile 1, it could not serve as the reference category. We therefore collapsed quartiles 1 and 2, which jointly served as the reference category for the ESRD outcome. In models adjusted for age, sex, race, clinic site, and urine creatinine concentration, individuals in the highest category of urine PIIINP were at 50-fold greater risk of ESRD than those in quartiles 1 or 2. In continuous analysis, each doubling was associated with >4-fold risk. When models were further adjusted for baseline eGFR and urine ACR, the associations were substantially attenuated; those in the highest quartile were at 3.2-fold higher risk, and each doubling of PIIINP conferred a 25% increased risk of ESRD, but both associations were no longer statistically significant. Further adjustment for CVD and CKD risk factors had a modest attenuating effect, and additional adjustment for plasma PIIINP had no further effect (HR per doubling, 1.16; 95% CI, 0.68 to 1.97).
Table 4.
Association of urine PIIINP concentrations with risk of ESRD in participants in the CHS
| Urine PIIINP Quartiles | Urine PIIINP Continuous (Per Doubling) | |||
|---|---|---|---|---|
| 1 and 2 | 3 | 4 | ||
| Range (μg/L) | ≤2.58 | 2.59–4.22 | >4.22 | |
| Events | 4 | 13 | 37 | |
| Person years at risk | 4457 | 2174 | 1975 | |
| Incidence rate (per 1000 person years) | 0.9 | 6.0 | 18.7 | |
| Demographic adjusted,a HR (95% CI) | 1.00 (reference) | 13.4 (4.12 to 43.36) | 50.9 (15.5 to 167.7) | 4.18 (3.05 to 5.73) |
| Plus eGFR and urine albumin,b HR (95% CI) | 1.00 (reference) | 2.42 (0.61 to 9.60) | 3.22 (0.81 to 12.91) | 1.25 (0.82 to 1.90) |
| Plus CVD risk factors,c HR (95% CI) | 1.00 (reference) | 2.36 (0.56 to 9.94) | 2.78 (0.57 to 13.59) | 1.16 (0.69 to 1.95) |
Adjusted for age, sex, race, education, urine creatinine, and clinic site.
Adjusted for demographic variable plus baseline eGFR and urine albumin.
Adjusted for demographic variables eGFR, urine albumin, plus smoking status, pack years, body mass index, diabetes, systolic blood pressure, blood pressure medication use, total cholesterol, lipid medication use, and C-reactive protein.
Urine PIIINP and Risk of CVD, HF, and Mortality
We observed no association of urine PIIINP with either incident CVD or HF in either the demographic or fully adjusted models. In contrast, we observed a statistically significant association with all-cause mortality (Table 5). There were 694 deaths during a median 9.9 (range, 0.3–15.5) years of follow-up. Compared with the lowest urine PIIINP quartile, those in the highest quartile were at 33% higher risk of death, and each SD (3.0 μg/L) higher urine PIIINP was associated with a 12% higher death risk in the fully adjusted model. Additional adjustment for plasma PIIINP had little effect on the final model (HR per SD, 1.11; 95% CI, 1.01 to 1.22).
Table 5.
Association of urine PIIINP with incident CVD, heart failure, and mortality in community-living older adult participants in the CHS
| Urine PIIINP Quartiles | Urine PIIINP Continuous Per SD (3.0 μg/L) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Urine PIIINP range (μg/L) | ≤1.39 | 1.40–2.58 | 2.59–4.22 | > 4.22 | |
| Incident CVDa | |||||
| No. of events | 75 | 64 | 82 | 68 | |
| No. at risk | 204 | 198 | 212 | 177 | |
| Incidence rate (per 1000 person years) | 39.1 | 33.8 | 44.3 | 48.0 | |
| Demographic adjusted,b HR (95% CI) | 1.00 (reference) | 0.87 (0.62 to 1.23) | 1.19 (0.83 to 1.71) | 1.18 (0.78 to 1.78) | 1.13 (0.97 to 1.31) |
| Fully adjusted,c HR (95% CI) | 1.00 (reference) | 0.77 (0.54 to 1.09) | 0.90 (0.61 to 1.33) | 0.84 (0.54 to 1.32) | 1.00 (0.85 to 1.18) |
| Incident HFd | |||||
| No. of events | 60 | 63 | 68 | 69 | |
| No. at risk | 217 | 215 | 227 | 213 | |
| Incidence rate (per 1000 person years) | 28.3 | 30.8 | 33.8 | 41.7 | |
| Demographic adjusted,b HR (95% CI) | 1.00 (reference) | 1.13 (0.78 to 1.62) | 1.28 (0.86 to 1.89) | 1.44 (0.94 to 2.21) | 1.12 (0.96 to 1.32) |
| Fully adjusted,c HR (95% CI) | 1.00 (reference) | 0.83 (0.57 to 1.21) | 0.79 (0.52 to 1.20) | 0.75 (0.47 to 1.19) | 0.90 (0.75 to 1.08) |
| Death | |||||
| No. of events | 163 | 166 | 165 | 200 | |
| No. at risk | 240 | 239 | 240 | 239 | |
| Incidence rate (per 1000 person years) | 65.9 | 68.3 | 70.4 | 100.2 | |
| Demographic adjusted,b HR (95% CI) | 1.00 (reference) | 1.11 (0.89 to 1.38) | 1.34 (1.05 to 1.72) | 1.87 (1.44 to 2.43) | 1.29 (1.19 to 1.40) |
| Fully adjusted,c HR (95% CI) | 1.00 (reference) | 0.93 (0.74 to 1.18) | 1.04 (0.80 to 1.34) | 1.30 (0.98 to 1.74) | 1.12 (1.03 to 1.23) |
Incident CVD defined as incident MI, stroke, or CVD death. Persons with prevalent CVD at baseline (n=167) were excluded.
Adjusted for age, sex, race, education, clinic site and urine creatinine.
Adjusted for age, sex, race, education, clinical site, urine creatinine, eGFR, urine albumin, smoking status, pack years, body mass index, diabetes, systolic blood pressure, blood pressure medication use, total cholesterol, lipid medication use, and C-reactive protein.
Persons with prevalent HF at baseline (n=86) were excluded.
Finally, we evaluated the associations of urine PIIINP with a composite outcome of death, 30% decline in eGFR, or dialysis. This analysis was limited to the 958 individuals within the random subcohort (Table 6). In the final model, each doubling of urine PIIINP was associated with 18% higher odds of the composite outcome (odds ratio, 1.18; 95% CI, 1.00 to 1.38).
Table 6.
Association of Urine PIIINP with the composite of death, 30% eGFR decline, or ESRD among the random subcohort participants in the CHS
| Urine PIIINP Quartiles | Urine PIIINP Continuous (Per Doubling) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Urine PIIINP range (μg/L) | ≤1.39 | 1.40–2.58 | 2.59–4.22 | >4.22 | |
| No. at risk | 174 | 183 | 178 | 198 | |
| No. with events,a n (%) | 109 | 117 | 126 | 151 | |
| Demographic adjusted,b OR (95% CI) | 1.00 (reference) | 1.12 (0.70 to 1.79) | 2.03 (1.19 to 3.46) | 2.78 (1.52 to 5.09) | 1.26 (1.05 to 1.52) |
| Plus eGFR and urine albumin,c OR (95% CI) | 1.00 (reference) | 0.91 (0.56 to 1.49) | 1.36 (0.77 to 2.40) | 1.40 (0.71 to 2.73) | 1.14 (0.98 to 1.33) |
| Plus CVD risk factors,d OR (95% CI) | 1.00 (reference) | 0.99 (0.60 to 1.63) | 1.62 (0.90 to 2.90) | 1.65 (0.83 to 3.30) | 1.18 (1.00 to 1.38) |
OR, odds ratio.
Within the random subcohort, 440 died, 72 had 30% decline in eGFR, and 14 had incident ESRD events. Because of overlaps, the total event number is 503.
Adjusted for age, sex, race, education, clinic site, and urine creatinine.
Adjusted for demographic variable plus baseline eGFR and urine albumin.
Adjusted for demographic variables eGFR, urine albumin, plus smoking status, pack years, body mass index, diabetes, systolic blood pressure, blood pressure medication use, total cholesterol, lipid medication use, and C-reactive protein.
Discussion
We found that the urine concentration of PIIINP—a marker of renal tubulointerstitial fibrosis—is associated with risk of CKD progression over 9 years in a large, community-living older adult population. This association was independent of baseline eGFR, urine ACR, and other CKD and CVD risk factors. Our preliminary findings suggest similar associations with incident ESRD; however, there were few events, and final models were not statistically significant. Higher urine PIIINP concentrations were also independently associated with all-cause mortality. No independent association was observed between urine PIIINP and incident CVD or HF events.
Currently, clinical assessment of CKD relies on eGFR and ACR; both evaluate glomerular health.3 However, kidney damage is not limited to the glomerulus. Tubular atrophy and tubulointerstitial fibrosis are common on biopsy in both CKD patients and in healthy older adults.4–7 Moreover, the severity of tubulointerstitial fibrosis on biopsy predicts progression to ESRD.5–7 Most participants in our study had eGFR≥60 ml/min per 1.73 m2 and low-grade or absent albuminuria at baseline. Although urine PIIINP concentrations were weakly correlated with these glomerular measures, urine PIIINP was independently associated with CKD progression during follow-up. If confirmed, these findings suggest that urine PIIINP may provide a noninvasive marker of renal tubulointerstitial fibrosis and a method to identify older individuals at particularly high risk for CKD progression beyond the information available by measurement of eGFR and urine ACR.
This study adds to a growing body of evidence that noninvasive assessment of kidney tubule health may provide complementary information above and beyond eGFR and ACR. We have shown that renal resistance to the hormonal actions of fibroblast growth factor-23 (a bone-derived hormone that induces renal phosphate excretion through its actions on renal tubule cells) is associated with higher risk of mortality and CVD events, outcomes that are strongly linked to CKD.14 Lower serum bicarbonate concentrations—a marker of metabolic acidosis and abnormal kidney tubule acid-base regulatory function—are independently associated with incident CKD development and more rapid CKD progression.15–17 Markers of kidney tubule injury and apoptosis (i.e., kidney injury molecule-1, IL-18, neutrophil gelatinase-associated lipocalin) have been associated with risk of CKD progression.18,19 Importantly, urine PIIINP differs from these markers because it identifies the degree of renal fibrosis, rather than kidney tubule cell injury per se. Collectively, these studies suggest that noninvasive measures may capture renal tubule dysfunction, injury, and fibrosis and may provide complementary information about the risk of CKD progression above and beyond eGFR and ACR. Future studies will require measurement of multiple markers of kidney tubule health concurrently to determine the degree to which they are correlated with one another and to identify which tubule health marker or set of markers provide the best method to predict CKD progression. In addition, participants in this study provided urine measurements in 1996–1997; therefore, these findings should be confirmed in patients receiving contemporary clinical practice and using freshly collected specimens. Whether measurement of urine PIIINP may improve clinical decision making and patient outcomes will be an important next step.
To our knowledge, this study is also the first to evaluate the association of urine PIIINP with longitudinal changes in kidney function while accounting for eGFR and ACR and the first to evaluate associated outcomes such as CVD, HF, and mortality. Nonetheless, prior studies support findings reported herein. Soylemezoglu and colleagues reported that patients with acute interstitial nephritis had urine PIIINP concentrations that were 4- to 5-fold higher than those with other forms of CKD, and about ten times higher than healthy controls.10 Similar findings were reported by Ghoul and colleagues, who studied 118 patients with CKD.8 Teppo and colleagues found that kidney transplant recipients with urine PIIINP/Cr>100 ng/mmol were more likely to lose GFR during follow-up than those with lower concentrations; however, they did not evaluate whether associations were independent of baseline eGFR, ACR, or other risk factors.9 All three of these studies found direct correlations between urine PIIINP concentrations and the severity of tubulointerstitial fibrosis on biopsy.
PIIINP is 44 kD in size and therefore smaller than albumin.20 It is possible that some PIIINP found in urine originates from glomerular filtration rather than tubule fibrosis, but this is unlikely to explain the associations reported here. The associations of urine PIIINP with CKD progression and all-cause mortality were independent of albuminuria, which served as a marker of nonspecific glomerular protein leak. Our results did not meaningfully change when we adjusted for plasma PIIINP concentrations. Prior studies in experimental animals comparing renal arterial and venous PIIINP concentrations failed to show renal extraction of PIIINP; most of its clearance was hepatic.21 Finally, Teppo and colleagues found that urine PIIINP concentrations were associated with tubulointerstitial fibrosis, whereas plasma PIIINP concentrations were not.9
The strengths of our study include its relatively large sample size; concurrent measurement of eGFR, ACR, and plasma PIIINP; availability of follow-up for change in kidney function and ESRD; and evaluation of a community-living older adult cohort with largely preserved eGFR and low-grade or absent albuminuria at baseline. The study also has important limitations. By necessity, for the CKD progression outcome, participants needed to live and return for a follow-up visit to provide repeat blood sampling to assess changes in kidney function. In this older cohort, many individuals died between visits, and others may have been too ill or debilitated to return, which may have introduced bias. We tried to mitigate this by evaluating ESRD in companion analyses because ESRD events could be captured irrespective of participation at the follow-up visit. Results were qualitatively similar, but the smaller number of incident ESRD participants provided limited statistical power. In addition, some older adults may have developed ESRD and chosen not to initiate dialysis and pursued palliative care; such individuals may have been captured in the associations of urine PIIINP with all-cause mortality. Serum creatinine was isotope dilution mass spectrometry (IDMS) standardized at the follow-up visit, but not at baseline; therefore, we chose to examine 30% decline in eGFR using cystatin C. We included a secondary analysis evaluating eGFR change defined by creatinine. The strength of association was qualitatively similar but did not reach statistical significance. This analysis was added post hoc. Because participants were selected for PIIINP measurement a priori on the basis of change in eGFR by cystatin C, the results may be influenced by the fact that only a subset of those with 30% decline by creatinine had available PIIINP measurements. Moreover, because creatinine measurements were IDMS standardized at one visit but not the other, laboratory drift in creatinine measurements between examinations may have led to misclassification. Nonetheless, point estimates appeared similar, albeit not statistically significant. Future studies are required to confirm these findings using creatinine-based eGFR measures. Some may consider adjustment for eGFR and ACR as overadjustment because declining eGFR will be intermediary between renal fibrosis and ESRD. However, our purpose was to determine whether urine PIIINP may have clinical utility in identifying risk of CKD progression and ESRD above and beyond kidney function measures routinely available in clinical practice.
In conclusion, in community-living older persons, urine PIIINP concentrations are associated with CKD progression independent of baseline eGFR, ACR, and CKD risk factors. Urine PIIINP is also independently associated with all-cause mortality. If confirmed, urine PIIINP measurement may provide a noninvasive method to assess the degree of renal fibrosis and to identify individuals at particularly high risk for subsequent CKD progression.
Concise Methods
Participants
The CHS is a community-based study of older adults designed to evaluate risk factors for CVD. The study design and protocols have been described previously.22,23 In brief, eligibility required age ≥65 years, expectation to remain in the area for 3 years after recruitment, no active cancer treatment, and the ability to provide informed consent. During 1989 and 1990, 5201 participants were recruited from four United States communities using Medicare eligibility lists. An additional 687 blacks were recruited in 1992–1993. In person examinations were performed annually through 1998–1999 and again in 2006–2007. Telephone interviews were conducted semiannually from 1989 to 1999 and biannually thereafter.
We measured urine PIIINP at the 1996–1997 study visit because it was the first visit at which spot urine specimens were obtained and stored. Figure 2 depicts the sampling design for this study. Among 3406 individuals who participated and provided blood at this visit, we excluded individuals with missing serum creatinine (n=1), cystatin C (n=0), or ACR (n=92). We then randomly selected a subcohort of 960 individuals, two of whom lacked sufficient urine to measure PIIINP, resulting in a subcohort of 958 individuals for analysis. Randomly selecting these individuals (i.e., not on the basis of the presence or absence of CKD progression, ESRD, CVD, HF, or death) allowed us to conduct both case-cohort and case-control analyses as subsequently described.
We defined CKD progression as ≥30% decline in eGFR on the basis of cystatin C from the 1996 to 1997 visit to the next CHS follow-up visit in which blood was obtained for eGFR measurement in 2005–2006.24 Among the 3406 individuals who participated and provided blood at the 1996–1997 visit, 1001 were alive and provided blood specimens again at the 2005–2006 visit. Among these, 192 had ≥30% decline in eGFR. These individuals were identified as participants and were all selected for urine PIIINP measurement. Of the 958 randomly selected subcohort participants from the total of 3406 with available specimens at the 1996–1997 visit, 289 were alive and provided blood for repeat eGFR measurement at the 2005–2006 visit. Among these, 59 had ≥30% decline in eGFR and were therefore already in the patient sample. The remaining 231 individuals with <30% decline in eGFR served as controls for the CKD progression analyses.
We also evaluated the association of urine PIIINP with incident ESRD using a case-cohort design. Among the 3406 individuals who participated and gave blood at the 1996–1997 study visit, we identified 54 subsequent ESRD participants. These individuals were selected as ESRD participants and had urine PIIINP measured from the 1996 to 1997 stored specimens. Among them, 14 originated within the subcohort.
Finally, we analyzed the association of urine PIIINP with risk of incident CVD, HF, and all-cause mortality. Each of these outcomes was common in this older adult cohort. Therefore, we conducted these analyses among the randomly selected subcohort using a standard prospective cohort design without additional case sampling.
In aggregate, the subcohort, CKD progression participants, and incident ESRD participants provided a study sample of 1122 individuals who had PIIINP measured.
Measurements
Urine PIIINP
Spot urine specimens were obtained at the time of the 1996–1997 study visit and stored at –70°C until 2013 when they were thawed and measured for PIIINP. We used a commercially available radioimmunoassay from Orion Diagnostica (Espoo, Finland). Measurements were made in duplicate in each sample, and results were averaged. In our laboratory, estimates of the interassay coefficient of variation (CV) ranged from 8.2% to 14.6%. We assessed short-term biologic variability of PIIINP/Cr by performing repeat measurements in seven healthy volunteers at 1, 3, and 7 days. Within-subject (CVI) and between-subject (CVG) CVs, and their ratio, the index of individuality (II), were calculated as previously described by Sakkinen and colleagues.25 The resulting value for PIIINP/Cr II was 0.25 (CVI/CVG=32.2/130.4), which compares well with values for plasma cholesterol (II=0.44) and C-reactive protein (II=0.53). The detectable range of the assay was between 0.05 and 50 μg/L. Among the 1122 samples, we found that 29 (2.6%) had urine PIIINP concentrations below the detectable limit. Values equal to the lower limit of detection (0.05 μg/L) were assigned to these samples. We also used the same radioimmunoassay to measure plasma PIIINP. Inter- and intra-assay coefficients of variation for both were <7.2%.26
Outcomes
GFR at both the 1996–1997 and 2005–2006 study visits was estimated using the equation that included serum cystatin C concentrations, age, sex, and race derived by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) study.27 Cystatin C measurements were made by a Siemens nephelometric assay at both study visits, as previously described.11 A change in eGFR≥30% from baseline defined CKD progression participants.24 Creatinine was not used for eGFR at either visit because the 2005–2006 creatinine measurements were IDMS standardized, whereas the 1996–1997 measurements were not.
To identify participants with incident ESRD, we merged CHS data with Centers for Medicare and Medicaid Services (CMS) claims data. We used the ESRD eligibility flag for fee-for-service Medicare, which begins on the first day of the forth month after dialysis initiation. The index date was the beginning of the eligibility period. Fee-for-service Medicare claims were available through the end of 2009. A prior linkage of CHS data with the United States Renal Data System (USRDS) identified CHS participants with ESRD through 2003.28 When comparing incident ESRDs identified by CMS versus USRDS linkage data through 2003, use of the CMS data had 70.1% sensitivity (95% CI, 59.4% to 79.5%) and 99.9% specificity (95% CI, 99.8% to 99.9%) for USRDS-defined ESRD participants. Using the CMS claims data we identified 54 incident ESRD participants from 1996 to 97 through 2009.
Methods of ascertainment and adjudication of CVD, HF, and mortality have been described previously.29–32 In brief, deaths are identified by review of obituaries, medical records, death certificates, the CMS’s health care utilization database for hospitalizations and from household contacts. On the basis of comparison with records from the National Death Index, ascertainment of mortality in CHS is estimated to be close to 100%.
All HF, CVD events, and CVD deaths are adjudicated by the CHS’s Events Committee. Participants with history of HF were excluded in analyses evaluating incident HF, whereas those with prevalent CVD (history of myocardial infarction [MI] or stroke) were excluded from incident CVD analyses. Incident HF required a physician’s diagnosis of HF, and adjudication by the Events Committee required symptoms, signs, chest radiographic findings, and treatment of HF.29,30 We evaluated a composite CVD outcome defined as the first occurring among incident fatal or nonfatal MI, incident fatal or nonfatal stroke, and CVD death. MI is ascertained from hospital records and on the basis of a clinical history of cardiac symptoms, elevated cardiac enzyme concentrations, and serial electrocardiographic changes.30 Cases of possible stroke are adjudicated by a committee of neurologists, neuroradiologists, and internists on the basis of interviews with patients, medical records, and brain imaging studies.31 CVD death was defined as death caused by atherosclerotic coronary heart disease or cerebrovascular disease.
Other Measurements
Information on covariates was obtained at the 1996–1997 study visit concurrent with urine PIIINP and included age, sex, race, clinic center, urine creatinine, and CVD risk factors, including systolic blood pressure, use of antihypertensive medications, diabetes (fasting glucose ≥126 mg/dl, nonfasting glucose ≥200 mg/dl, or use of antiglycemic medications or insulin), smoking status (current, former, or never), pack years of tobacco use, body mass index, total cholesterol, use of lipid lowering medications, and C-reactive protein concentrations.33
Statistical Analysis
We categorized participants into quartiles on the basis of urine PIIINP concentrations in the randomly selected subcohort and evaluated the distribution of potential confounders across urine PIIINP categories. Next, we evaluated the unadjusted correlations of urine PIIINP, eGFR, urine ACR, and plasma PIIINP levels using Spearman correlation coefficients.
We evaluated the association of urine PIIINP with CKD progression using logistic regression. Initial models were adjusted for age, sex, race, clinic site, and urine creatinine; the latter was used to adjust for differences in urine concentration at the time of collection without creating a ratio, to avoid potential influence of factors that affect the denominator (urine creatinine) rather than the numerator.34,35 A subsequent model additionally adjusted for baseline eGFR and urine ACR. A final model additionally included CVD and CKD risk factors (smoking status [current, former, never], pack years [continuous], body mass index [continuous], diabetes, systolic blood pressure [continuous], blood pressure medication use, total cholesterol [continuous], lipid medication use, and C-reactive protein concentrations [continuous]). Primary analysis evaluated eGFR defined by cystatin C, age, sex, and race, and participants were selected on the basis of this equation a priori. Some but not all of the participants also had a 30% decline when eGFR was calculated using serum creatinine and the CKD-EPI equation,36 and others who had 30% decline by the CKD-EPI equation did not have urine measurements of PIIINP. Creatinine measurements were IDMS standardized at one visit but not the other. Therefore, we evaluated CKD progression by creatinine only as a secondary outcome.
We evaluated the association of urine PIIINP with incident ESRD using modified Cox regression to account for the case-cohort approach.37,38 This analysis included all 958 persons within the random subcohort and all incident ESRD participants, regardless of whether they arose from the subcohort. Subcohort participants (ESRD noncases and subcohort cases before their failure) were weighted by the inverse of the sampling fraction. Cases that arose outside the subcohort were not weighted before their failure. All cases (irrespective of whether they arose in the subcohort or not) were assigned a weight of one at the time of failure. Robust variance estimators were computed. The series of models were adjusted identically to the CKD progression outcome.
Next, we evaluated associations of urine PIIINP with incident CVD, HF, and all-cause mortality. We used the 958 randomly selected subcohort sample only (no additional case sampling) and evaluated associations of urine PIIINP with each outcome using the standard Cox proportional hazards regression. For the evaluation of incident CVD events, we excluded 167 persons with prevalent CVD at the 1996–1997 visit, resulting in a sample size of 791 individuals for this outcome. Likewise, for the incident HF events outcome, we excluded 86 individuals with prevalent HF at the 1996–1997 visit, resulting in a sample size of 872 individuals for this outcome. Again, the series of adjustments were identical as for the CKD progression outcome decribed previously.
As a final step, we evaluated a composite outcome of death, 30% decline in eGFR, or ESRD. This analysis was limited to the 958 persons in the subcohort. Because 30% decline in eGFR was interval censored at the 2005–2906 examination, we evaluated associations using logistic regression for the composite outcome.
For each association, we evaluated the functional form using generalized additive models. For CKD progression and incident ESRD, there was an approximately linear association between log-transformed urine PIIINP and the outcome. For incident CVD, HF, and mortality, untransformed urine PIIINP provided a better linear fit. For the latter, we present risk estimates per SD increment of urine PIIINP.
We conducted sensitivity analyses for each outcome evaluating urine PIIINP indexed to urine creatinine, rather than adjusted for urine creatinine. In all cases, the results were similar (data not shown). All analyses were conducted using Stata version 12.1 (StataCorp., College Station, TX).
Disclosures
None.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute of Diabetes Digestive and Kidney Diseases (grant no. R01-DK098234) (to Drs. Ix and Shlipak); National Heart, Lung, and Blood Institute (grant no. R01-HL094555) (to Drs. Ix, Mukamal, Djousse, and Kizer); American Heart Association Established Investigator Award (award no. 14EIA18560026) (to Dr. Ix); and National Institute on Aging (grant nos. R01-AG027002) (Dr Sarnak). The CHS was supported by contracts HHSN268201200036C, HHSN268200800007C, N01-HC55222, N01-HC85079, N01-HC85080, N01-HC85081, N01-HC85082, N01-HC85083, N01-HC85086, and grant U01-HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke and from grant R01-AG023629 from the National Institute on Aging. This material is the result of work supported with resources of the VA San Diego Healthcare System.
Dr Biggs had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
A full list of principal CHS investigators and institutions can be found at https://chs-nhlbi.org/.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014070696/-/DCSupplemental.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT, CKD Prognosis Consortium : Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 369: 932–943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.KDIGO : 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, Textor SC, Stegall MD: The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 152: 561–567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Howie AJ, Ferreira MA, Adu D: Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transplant 16: 1163–1169, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Takebayashi S, Kiyoshi Y, Hisano S, Uesugi N, Sasatomi Y, Meng J, Sakata N: Benign nephrosclerosis: Incidence, morphology and prognosis. Clin Nephrol 55: 349–356, 2001 [PubMed] [Google Scholar]
- 8.Ghoul BE, Squalli T, Servais A, Elie C, Meas-Yedid V, Trivint C, Vanmassenhove J, Grünfeld JP, Olivo-Marin JC, Thervet E, Noël LH, Prié D, Fakhouri F: Urinary procollagen III aminoterminal propeptide (PIIINP): A fibrotest for the nephrologist. Clin J Am Soc Nephrol 5: 205–210, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teppo AM, Törnroth T, Honkanen E, Grönhagen-Riska C: Urinary amino-terminal propeptide of type III procollagen (PIIINP) as a marker of interstitial fibrosis in renal transplant recipients. Transplantation 75: 2113–2119, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Soylemezoglu O, Wild G, Dalley AJ, MacNeil S, Milford-Ward A, Brown CB, el Nahas AM: Urinary and serum type III collagen: Markers of renal fibrosis. Nephrol Dial Transplant 12: 1883–1889, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Kassirer JP: Clinical evaluation of kidney function--glomerular function. N Engl J Med 285: 385–389, 1971 [DOI] [PubMed] [Google Scholar]
- 13.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG, Cardiovascular Health Study : Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med 142: 497–505, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Dominguez JR, Kestenbaum B, Chonchol M, Block G, Laughlin GA, Lewis CE, Katz R, Barrett-Connor E, Cummings S, Orwoll ES, Ix JH, Osteoporotic Fractures in Men (MrOS) Study Research Group : Relationships between serum and urine phosphorus with all-cause and cardiovascular mortality: The Osteoporotic Fractures in Men (MrOS) Study. Am J Kidney Dis 61: 555–563, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driver TH, Shlipak MG, Katz R, Goldenstein L, Sarnak M, Hoofnagle A, Siscovick D, Kestenbaum B, De Boer IH, Ix JH: Low serum bicarbonate as a predictor of rapid kidney function decline and incident chronic kidney disease: The Multi-Ethnic Study of Atherosclerosis. (MESA). Am J Kidney Dis 64:534–541, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldenstein L, Driver TH, Fried LF, Rifkin DE, Patel KV, Yenchek RH, Harris TB, Kritchevsky SB, Newman AB, Sarnak MJ, Shlipak MG, Ix JH, Health ABC Study Investigators : Serum bicarbonate concentrations and kidney disease progression in community-living elders: The Health, Aging, and Body Composition (Health ABC) Study. Am J Kidney Dis 64: 542–549, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S: Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int 79: 356–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peralta CA, Katz R, Bonventre JV, Sabbisetti V, Siscovick D, Sarnak M, Shlipak MG: Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 60: 904–911, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhavsar NA, Köttgen A, Coresh J, Astor BC: Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 60: 233–240, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risteli J, Niemi S, Trivedi P, Mäentausta O, Mowat AP, Risteli L: Rapid equilibrium radioimmunoassay for the amino-terminal propeptide of human type III procollagen. Clin Chem 34: 715–718, 1988 [PubMed] [Google Scholar]
- 21.Bentsen KD, Henriksen JH, Bendtsen F, Hørslev-Petersen K, Lorenzen I: Splanchnic and renal extraction of circulating type III procollagen aminoterminal propeptide in patients with normal liver function and in patients with alcoholic cirrhosis. Hepatology 11: 957–963, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG: The Cardiovascular Health Study: Design and rationale. Ann Epidemiol 1: 263–276, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO: Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol 3: 358–366, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS, CKD Prognosis Consortium : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakkinen PA, Macy EM, Callas PW, Cornell ES, Hayes TE, Kuller LH, Tracy RP: Analytical and biologic variability in measures of hemostasis, fibrinolysis, and inflammation: Assessment and implications for epidemiology. Am J Epidemiol 149: 261–267, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Agarwal I, Glazer NL, Barasch E, Biggs ML, Djoussé L, Fitzpatrick AL, Gottdiener JS, Ix JH, Kizer JR, Rimm EB, Siscovick DS, Tracy RP, Zieman SJ, Mukamal KJ: Fibrosis-related biomarkers and risk of total and cause-specific mortality: The cardiovascular health study. Am J Epidemiol 179: 1331–1339, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, Seliger S, Siscovick D, Newman AB, Fried L: Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med 26: 379–385, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC: Predictors of congestive heart failure in the elderly: The Cardiovascular Health Study. J Am Coll Cardiol 35: 1628–1637, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S: Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 5: 278–285, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Price TR, Psaty B, O’Leary D, Burke G, Gardin J: Assessment of cerebrovascular disease in the Cardiovascular Health Study. Ann Epidemiol 3: 504–507, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J: Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol 5: 270–277, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, Polak JF, Tracy RP: C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: The cardiovascular health study. Circulation 112: 25–31, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Ix JH, de Boer IH, Wassel CL, Criqui MH, Shlipak MG, Whooley MA: Urinary creatinine excretion rate and mortality in persons with coronary artery disease: The Heart and Soul Study. Circulation 121: 1295–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ix JH, Wassel CL, Stevens LA, Beck GJ, Froissart M, Navis G, Rodby R, Torres VE, Zhang YL, Greene T, Levey AS: Equations to estimate creatinine excretion rate: The CKD epidemiology collaboration. Clin J Am Soc Nephrol 6: 184–191, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barlow WE, Ichikawa L, Rosner D, Izumi S: Analysis of case-cohort designs. J Clin Epidemiol 52: 1165–1172, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Therneau TM, Li H: Computing the Cox model for case cohort designs. Lifetime Data Anal 5: 99–112, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.