Abstract
Information on common causes of death in people with CKD is limited. We hypothesized that, as eGFR declines, cardiovascular mortality and mortality from infection account for increasing proportions of deaths. We calculated eGFR using the CKD Epidemiology Collaboration equation for residents of Alberta, Canada who died between 2002 and 2009. We used multinomial logistic regression to estimate unadjusted and age- and sex-adjusted differences in the proportions of deaths from each cause according to the severity of CKD. Cause of death was classified as cardiovascular, infection, cancer, other, or not reported using International Classification of Diseases codes. Among 81,064 deaths, the most common cause was cancer (31.9%) followed by cardiovascular disease (30.2%). The most common cause of death for those with eGFR≥60 ml/min per 1.73 m2 and no proteinuria was cancer (38.1%); the most common cause of death for those with eGFR<60 ml/min per 1.73 m2 was cardiovascular disease. The unadjusted proportion of patients who died from cardiovascular disease increased as eGFR decreased (20.7%, 36.8%, 41.2%, and 43.7% of patients with eGFR≥60 [with proteinuria], 45–59.9, 30–44.9, and 15–29.9 ml/min per 1.73 m2, respectively). The proportions of deaths from heart failure and valvular disease specifically increased with declining eGFR along with the proportions of deaths from infectious and other causes, whereas the proportion of deaths from cancer decreased. In conclusion, we found an inverse association between eGFR and specific causes of death, including specific types of cardiovascular disease, infection, and other causes, in this cohort.
Keywords: CKD, epidemiology and outcomes, mortality, GFR, cardiovascular disease
Nondialysis-dependent CKD is common, affecting an estimated 8%–16% of the world’s population.1 Because of the prevalence of CKD and the independent relation between CKD and adverse outcomes—even for those with mild to moderate disease severity—the public health effect of CKD has been increasingly recognized. According to the 2010 Global Burden of Disease study, the number of deaths with CKD as the underlying cause increased by 82% from 1990 to 2010.1 CKD is associated with an increased risk of mortality caused by cardiovascular diseases (CVDs)2 and non-CVDs3–5; however, the proportions of death caused by specific causes and the differences in these proportions across stages of CKD have not been systematically described.
Characterizing cause of death serves several important functions, such as measuring disease burden, generating insight into mechanisms of disease, and indicating potential strategies for treatment and prevention. For example, the finding that sudden cardiac death is common among patients on hemodialysis led to work showing that other mechanisms other than underlying coronary artery disease were likely responsible for the high cardiovascular mortality in this population.6,7 Although studies of mortality for patients with CKD have focused primarily on risks of all-cause and cardiovascular mortality,8 several studies have also shown that CKD is associated with an increased risk of death from other causes, such as infection.3–5 To reduce the excess mortality associated with CKD, more detailed information on specific causes of death is needed to inform the design of future interventions.
Recognizing that patients with lower eGFR are known to be at higher risk of all-cause mortality, we used a large population–based dataset to examine the proportions of deaths attributable to various causes in people at varying levels of kidney function. We hypothesized that, at lower eGFR, cardiovascular mortality and mortality caused by infection would account for an increasing proportion of deaths compared with in those with eGFR>60 ml/min per 1.73 m2.
Results
Participant Flow and Characteristics
Over the study period, 3,897,684 residents of Alberta were identified. Of these potential participants, 3,816,620 were excluded; the primary reason for exclusion was no death (3,630,452 residents). Registration with Alberta Health (AH) was not available for 2332 (<1%) residents, and serum creatinine during the year before death was not available for 121,827 (3%) residents (Figure 1). A proteinuria measurement was missing for 12,160 (15%) participants.
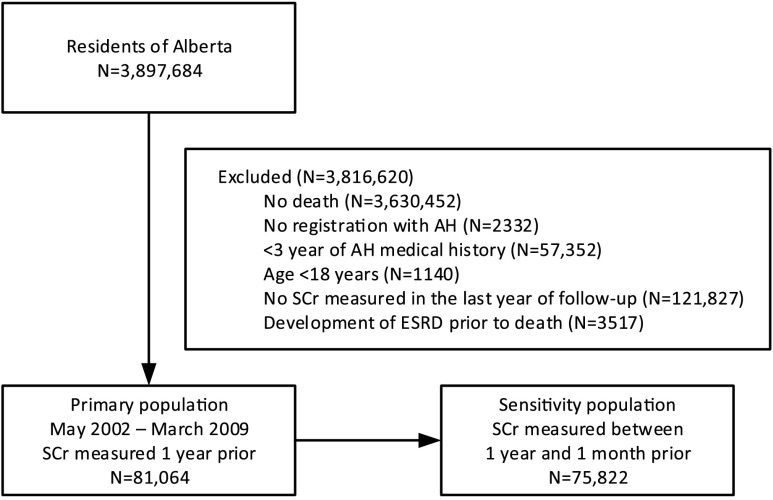
Figure 1.
Participant flow and characteristics for all residents of Alberta over the study period. The sensitivity analysis shown on the lower right, excluded all eGFR measurements in the month prior to death. SCr, serum creatinine.
Table 1 shows the baseline characteristics of the study participants according to the five categories of death. Among 81,064 people who died, the main cause of death was cancer (25,882 deaths; 31.9%) followed by cardiovascular deaths (24,494; 30.2%). Cause of death was missing for 4783 (5.9%) participants (Table 1). Participants who died from a cardiovascular or infectious cause were significantly older than those who died from cancer or an unknown or other cause. A greater proportion of Aboriginals died because of an infection than because of the other causes. Participants who died from cancer were younger and substantially less likely to have diabetes or hypertension. Within each of the main causes of death, the majority of participants had eGFR≥60 ml/min per 1.73 m2 and no proteinuria.
Table 1.
Demographic and clinical characteristics of participants who died during follow-up according to cause of death (n=81,064)
| Participant Characteristics | All Participants | CVD | Infection | Cancer | Othera | Missing | P Value |
|---|---|---|---|---|---|---|---|
| N | 81,064 (100) | 24,494 (30.2) | 3274 (4.0) | 25,882 (31.9) | 22,631 (27.9) | 4783 (5.9) | — |
| Age, yr | 83 (75–89) | 83 (73–90) | 73 (63–81) | 80 (68–88) | 78 (65–86) | <0.001 | |
| Men | 40,606 (50.1) | 11,866 (48.4) | 1544 (47.2) | 13,664 (52.8) | 11,056 (48.9) | 2476 (51.8) | <0.001 |
| Aboriginal | 1790 (2.2) | 368 (1.5) | 129 (3.9) | 420 (1.6) | 773 (3.4) | 100 (2.1) | <0.001 |
| Social assistance | 2923 (3.6) | 505 (2.1) | 169 (5.2) | 809 (3.1) | 1255 (5.5) | 185 (3.9) | <0.001 |
| Rural residence | 11,059 (13.6) | 3176 (13) | 429 (13.1) | 3702 (14.3) | 3070 (13.6) | 682 (14.3) | <0.001 |
| Comorbidities | |||||||
| Diabetes | 22,888 (28.2) | 7819 (31.9) | 1053 (32.2) | 5859 (22.6) | 6785 (30) | 1372 (28.7) | <0.001 |
| Hypertension | 55,871 (68.9) | 19,966 (81.5) | 2358 (72) | 15,240 (58.9) | 14,989 (66.2) | 3318 (69.4) | <0.001 |
| Charlson scoreb | 4 (2–6) | 4 (2–6) | 9 (8–10) | 3 (2–6) | 4 (2–8) | <0.001 | |
| eGFR, ml/min per 1.73 m2 | <0.001 | ||||||
| ≥60 without proteinuriac | 31,323 (38.6) | 7655 (31.3) | 904 (27.6) | 11,927 (46.1) | 8976 (39.7) | 1861 (38.9) | |
| ≥60 with proteinuria | 15,278 (18.9) | 3169 (12.9) | 770 (23.5) | 6146 (23.7) | 4211 (18.6) | 982 (20.5) | |
| 45–59 | 15,673 (19.3) | 5763 (23.5) | 674 (20.6) | 4141 (16) | 4176 (18.5) | 919 (19.2) | |
| 30–44 | 12,268 (15.1) | 5054 (20.6) | 588 (18) | 2606 (10.1) | 3338 (14.7) | 682 (14.3) | |
| 15–29 | 6522 (8.1) | 2853 (11.6) | 338 (10.3) | 1062 (4.1) | 1930 (8.5) | 339 (7.1) |
Other includes 5236 deaths caused by neurologic diseases and dementias, 4086 deaths caused by chronic lung disease, 3500 deaths caused by suicides and accidents, 3076 deaths caused by digestive diseases, 1583 deaths caused by diabetes, and an additional 5150 (6.4%) other deaths.
Charlson score includes AIDS/HIV, metastatic cancers, nonmetastatic cancers, CVA, chronic obstructive lung disease, dementia, diabetes, heart failure, mild liver disease, moderate/severe liver disease, myocardial infarction, paraplegia, peptic ulcer, peripheral vascular disease, and rheumatologic disease. The median and interquartile ranges are presented.
Proteinuria is defined as urine dipstick trace or greater, albumin-to-creatinine ratio≥3 mg/mmol, or protein-to-creatinine ratio≥15 mg/mmol.
Causes of Death According to eGFR Category
Figure 2 shows the unadjusted relative percentages of death by cause according to eGFR. Table 2 shows the percentages of deaths attributed to each cause as a function of eGFR. Within each category of eGFR, the cause of death was missing for <10% of participants.
Figure 2.
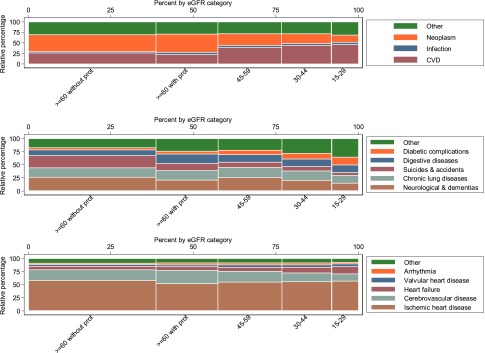
Unadjusted relative percentages for death by cause and eGFR. Top panel shows the relative percentages for death by eGFR category for each of the four main categories: CVD, neoplasm, infection, and other. The most common cause of death for those with eGFR>60 ml/min per 1.73 m2 and no proteinuria was cancer. The most common cause of death for those with eGFR<60ml/min per 1.73m2 was cardiovascular disease. Middle panel shows the relative percentages for death by eGFR category for the subclassification of other causes from top panel. Among participants without proteinuria and eGFR>60 ml/min per 1.73 m2, neurologic diseases (including dementia) were the most common cause of death. At lower eGFR, the proportions of death caused by unclassified and diabetic complications increased. Bottom panel shows the relative percentages for death by eGFR category for the subclassification of CVD deaths. For participants with eGFR>60 ml/min, death caused by ischemic heart disease (IHD) was the most common cause of cardiovascular death. The proportion of deaths caused by heart failure and valvular disease increased at lower eGFR. The height of each colored bar represents the percentage of participants for each cause of death within each category of eGFR. The width of each colored bar represents the percentage of participants for each eGFR category within each cause of death. The area of each colored bar represents the percentage of participants within each eGFR category and each cause of death. Prot, proteinuria.
Table 2.
Unadjusted and age- and sex-adjusted percentages (95% CIs) of deaths by cause and eGFR
| eGFR, ml/min per 1.73 m2 | CVD | Infection | Cancer | Other | Missing | Total |
|---|---|---|---|---|---|---|
| ≥60 without proteinuria | ||||||
| Unadjusted | 24.4 (24.0 to 24.9) | 2.9 (2.7 to 3.1) | 38.1 (37.5 to 38.6) | 28.7 (28.2 to 29.2) | 5.9 (5.7 to 6.2) | 31,323 |
| Age and sex adjusted | 26.0 (25.5 to 26.5) | 3.0 (2.8 to 3.2) | 36.2 (35.6 to 36.7) | 28.9 (28.4 to 29.4) | 5.9 (5.6 to 6.2) | |
| ≥60 with proteinuria | ||||||
| Unadjusted | 20.7 (20.1 to 21.4) | 5.0 (4.7 to 5.4) | 40.2 (39.5 to 41.0) | 27.6 (26.9 to 28.3) | 6.4 (6.0 to 6.8) | 15,278 |
| Age and sex adjusted | 21.7 (21.0 to 22.4) | 5.3 (4.9 to 5.6) | 38.5 (37.7 to 39.3) | 28.2 (27.4 to 28.9) | 6.4 (6.0 to 6.8) | |
| 45–59 | ||||||
| Unadjusted | 36.8 (36.0 to 37.5) | 4.3 (4.0 to 4.6) | 26.4 (25.7 to 27.1) | 26.6 (26.0 to 27.3) | 5.9 (5.5 to 6.2) | 15,673 |
| Age and sex adjusted | 33.3 (32.5 to 35.0) | 4.3 (3.9 to 4.6) | 28.7 (27.9 to 29.4) | 27.5 (26.8 to 28.3) | 6.3 (5.9 to 6.7) | |
| 30–44 | ||||||
| Unadjusted | 41.2 (40.3 to 42.1) | 4.8 (4.4 to 5.2) | 21.2 (20.5 to 22.0) | 27.2 (26.4 to 28.0) | 5.6 (5.2 to 6.0) | 12,268 |
| Age and sex adjusted | 37.1 (36.3 to 38.0) | 4.8 (4.4 to 5.2) | 23.7 (22.9 to 24.5) | 28.3 (27.5 to 29.2) | 6.1 (5.6 to 6.5) | |
| 15–29 | ||||||
| Unadjusted | 43.7 (42.5 to 44.9) | 5.2 (4.6 to 5.7) | 16.3 (15.4 to 17.2) | 29.6 (28.5 to 30.7) | 5.2 (4.7 to 5.7) | 6522 |
| Age and sex adjusted | 39.9 (38.7 to 41.1) | 5.2 (4.6 to 5.7) | 18.3 (17.3 to 19.3) | 30.9 (29.8 to 32.1) | 5.7 (5.1 to 6.3) | |
| Total | 24,494 | 3274 | 25,882 | 22,631 | 4783 | 81,064 |
Among participants with eGFR≥60 ml/min per 1.73 m2 and no proteinuria, death caused by cancer was the most common cause of death: 38.1% (95% confidence interval [95% CI], 37.5% to 38.6%). These results were similar after adjustment for age and sex (Table 2). For participants with eGFR≥60 ml/min per 1.73 m2 and proteinuria, cancer was the most common cause of death and responsible for 40.2% of deaths (95% CI, 39.5% to 41.0%). After age and sex adjustment, cancer and other causes were the most common causes of death in this category: 38.5% (95% CI, 37.7% to 39.9%) and 28.2% (95% CI, 27.4% to 28.9%), respectively. Among those with eGFR≥60 ml/min per 1.73 m2, the presence of proteinuria was associated with a modest decrease in the percentage of deaths caused by CVD (in both unadjusted and age- and sex-adjusted analyses).
Among participants with eGFR<60 ml/min per 1.73 m2, the primary cause of death was CVD; these results were similar after adjustment for age and sex. There was an inverse association between eGFR and the proportion of deaths from a cardiovascular cause (Table 2). The relationship between death caused by a cardiovascular cause and lower eGFR remained after adjustment for age and sex, although the magnitude was attenuated. At lower levels of eGFR, the proportion of deaths caused by cancer declined. A modest inverse relation was also observed for eGFR and the proportion of deaths caused by infectious and other causes (Table 2). Adjusted results were similar.
The proportions of deaths by cause and eGFR category were further subclassified by age: ≥70, 50–69.9, and 18–49.9 years old (data not shown). For those participants with an eGFR>60 ml/min per 1.73 m2 without proteinuria who died from CVD, the highest proportion of deaths was in the ≥70-year-old age group followed by the 50- to 69.9-year-old age group. The relationship between older age and deaths caused by CVD was similar at all levels of eGFR. Although the proportion of deaths caused by CVD was higher at lower eGFR among participants in the youngest age group, a similarly higher proportion of deaths caused by CVD at lower eGFR was observed in participants ages 50–69.9 and 18–49.9 years old. The highest proportion of deaths caused by cancer was observed in the 50- to 69.9-year-old age group; there was a similar pattern at all levels of eGFR. For deaths from other causes across all categories of eGFR, the highest proportion of deaths was in the 18- to 49.9-years-old age group.
Other Causes of Death According to eGFR Category
Other causes of death were further classified in Table 3. Among participants without proteinuria and eGFR≥60 ml/min per 1.73 m2, neurologic diseases (including dementia) were the most common cause of death: 26.1% of deaths (95% CI, 25.2% to 27.1%). After adjustment for age and sex, neurologic causes (including dementia) remained the most common cause of death (Table 3).
Table 3.
Unadjusted and age- and sex-adjusted percentages (95% CIs) of death by other causes and eGFR
| eGFR, ml/min per 1.73 m2 | Neurologic and Dementias | Chronic Lung Diseases | Suicides and Accidents | Digestive Diseases | Diabetic Complications | Other | Total |
|---|---|---|---|---|---|---|---|
| ≥60 without proteinuria | |||||||
| Unadjusted | 26.1 (25.2 to 27.1) | 17.9 (17.1 to 18.7) | 23.5 (22.6 to 24.3) | 11.0 (10.3 to 11.6) | 4.0 (3.6 to 4.4) | 17.5 (16.7 to 18.3) | 8976 |
| Age and sex adjusted | 28.9 (27.9 to 30.0) | 19.6 (18.6 to 20.5) | 16.3 (15.4 to 17.1) | 11.4 (10.7 to 12.1) | 4.5 (4.0 to 4.9) | 19.3 (18.4 to 20.2) | |
| ≥60 with proteinuria | |||||||
| Unadjusted | 20.8 (19.5 to 22.0) | 18.3 (17.1 to 19.4) | 13.6 (12.5 to 14.6) | 18.1 (16.9 to 19.2) | 5.1 (4.4 to 5.8) | 24.2 (23.0 to 25.5) | 4211 |
| Age and sex adjusted | 22.5 (21.1 to 23.9) | 18.5 (17.3 to 19.8) | 9.5 (8.6 to 10.3) | 18.2 (17.0 to 19.4) | 5.4 (4.7 to 6.1) | 26.0 (24.6 to 27.3) | |
| 45–59 | |||||||
| Unadjusted | 25.3 (24.0 to 26.6) | 19.4 (18.2 to 20.6) | 10.3 (9.4 to 11.2) | 14.2 (13.2 to 15.3) | 8.4 (7.6 to 9.2) | 22.3 (21.1 to 23.6) | 4176 |
| Age and sex adjusted | 20.8 (19.6 to 22.1) | 16.7 (15.5 to 17.8) | 12.7 (11.6 to 13.9) | 16.2 (15.0 to 17.4) | 9.0 (8.0 to 9.9) | 24.7 (13.3 to 26.1) | |
| 30–44 | |||||||
| Unadjusted | 20.2 (18.9 to 21.6) | 18.2 (16.9 to 19.5) | 8.4 (7.5 to 9.4) | 14.0 (12.8 to 15.1) | 10.9 (9.8 to 11.9) | 28.3 (26.8 to 29.8) | 3338 |
| Age and sex adjusted | 15.7 (14.6 to 16.9) | 15.2 (14.0 to 16.4) | 11.1 (9.9 to 12.3) | 15.8 (14.4 to 17.1) | 11.5 (10.3 to 12.6) | 30.8 (29.1 to 32.4) | |
| 15–29 | |||||||
| Unadjusted | 14.7 (13.1 to 16.3) | 14.9 (13.3 to 16.5) | 5.8 (4.8 to 6.8) | 14.0 (12.4 to 15.5) | 15.1 (13.5 to 16.7) | 35.5 (33.3 to 37.6) | 1930 |
| Age and sex adjusted | 11.3 (10.0 to 12.6) | 12.3 (10.9 to 13.6) | 7.3 (6.0 to 8.6) | 15.5 (13.8 to 17.2) | 15.7 (14.0 to 17.4) | 38.0 (35.7 to 40.2) | |
| Total | 5236 | 4086 | 3500 | 3076 | 1583 | 5150 | 22,631 |
For participants with eGFR≥60 ml/min per 1.73 m2 and proteinuria, undefined other causes and neurologic causes were the most common causes of death: 24.2% (95% CI, 23.0% to 25.5%) and 20.8% (95% CI, 19.5% to 22.0%). Among those with eGFR≥60 ml/min per 1.73 m2, the presence of proteinuria was associated with an increase in the proportions of deaths caused by digestive diseases, diabetic complications, and other causes (unclassified deaths); results were similar after adjustment for age and sex.
At lower eGFR, the proportions of deaths from neurologic causes, chronic lung diseases, and suicides and accidents decreased, whereas the proportions of death caused by unclassified causes and diabetic complications increased (Figure 2).
Subclassification of Cardiovascular Causes of Death by eGFR Category
Among participants who died of CVD, Table 4 shows further subclassification of these causes of deaths by eGFR category. For participants with eGFR>60 ml/min, death caused by ischemic heart disease (IHD) was the most common cause of cardiovascular death: 58.0% of cardiovascular deaths (95% CI, 56.9% to 59.1%) in both unadjusted and adjusted analyses.
Table 4.
Unadjusted and age- and sex-adjusted percentages (95% CIs) of death by cardiovascular causes and eGFR
| eGFR, ml/min per 1.73 m2 | IHD | CVA | Heart Failure | Valvular Heart Disease | Arrhythmia | Other | Total |
|---|---|---|---|---|---|---|---|
| ≥60 without proteinuria | |||||||
| Unadjusted | 58.0 (56.9 to 59.1) | 20.7 (19.8 to 21.6) | 6.5 (6.0 to 7.1) | 3.4 (3.0 to 3.8) | 2.7 (2.3 to 3.0) | 8.7 (8.1 to 9.4) | 7655 |
| Age and sex adjusted | 56.6 (55.5 to 57.7) | 21.6 (20.7 to 22.6) | 6.6 (6.0 to 7.1) | 3.3 (2.9 to 3.7) | 2.8 (2.4 to 3.1) | 9.1 (8.5 to 9.8) | |
| ≥60 with proteinuria | |||||||
| Unadjusted | 52.0 (50.3 to 53.8) | 25.2 (23.7 to 26.8) | 7.4 (6.5 to 8.3) | 3.6 (3.0 to 4.3) | 3.4 (2.8 to 4.1) | 8.3 (7.3 to 9.2) | 3169 |
| Age and sex adjusted | 50.1 (48.3 to 51.9) | 26.6 (25.0 to 28.1) | 7.5 (6.5 to 8.4) | 3.6 (3.0 to 4.3) | 3.6 (2.9 to 4.3) | 8.6 (7.6 to 9.6) | |
| 45–59 | |||||||
| Unadjusted | 55.1 (53.8 to 56.3) | 20.2 (19.2 to 21.3) | 8.0 (7.3 to 8.7) | 4.7 (4.1 to 5.2) | 3.5 (3.0 to 4.0) | 8.6 (7.8 to 9.3) | 5763 |
| Age and sex adjusted | 56.3 (55.0 to 57.6) | 19.4 (18.4 to 20.4) | 8.1 (7.4 to 8.8) | 4.6 (4.1 to 5.2) | 3.3 (2.8 to 3.7) | 8.4 (7.6 to 9.1) | |
| 30–44 | |||||||
| Unadjusted | 55.6 (54.2 to 56.9) | 16.8 (15.8 to 17.8) | 11.0 (10.1 to 11.8) | 4.8 (4.2 to 5.4) | 3.4 (2.9 to 3.9) | 8.5 (7.8 to 9.3) | 5054 |
| Age and sex adjusted | 57.3 (55.9 to 58.6) | 15.8 (14.8 to 16.8) | 11.0 (10.2 to 11.9) | 4.7 (4.1 to 5.3) | 3.0 (2.6 to 3.5) | 8.1 (7.4 to 8.9) | |
| 15–29 | |||||||
| Unadjusted | 56.8 (55.0 to 58.6) | 13.7 (12.4 to 14.9) | 13.9 (12.6 to 15.1) | 5.5 (4.7 to 6.3) | 2.8 (2.2 to 3.4) | 7.3 (6.4 to 8.3) | 2853 |
| Age and sex adjusted | 59.0 (57.1 to 60.8) | 12.6 (11.4 to 13.8) | 13.8 (12.5 to 15.1) | 5.3 (4.4 to 6.1) | 2.5 (1.9 to 3.0) | 6.9 (6.0 to 7.9) | |
| Total | 13,688 | 4790 | 2145 | 1044 | 766 | 2061 | 24,494 |
For those participants with eGFR≥60 ml/min per 1.73 m2 and proteinuria, IHD was the most common cause of death. However, among those with eGFR≥60 ml/min per 1.73 m2, the percentage of deaths caused by IHD was lower in the presence of proteinuria, whereas the proportions of deaths caused by cerebrovascular disease (CVA), heart failure, and arrhythmia were higher in the presence of proteinuria (in both unadjusted and age- and sex-adjusted analyses).
Deaths caused by IHD accounted for a similar proportion of deaths within each eGFR category (both adjusted and unadjusted analyses). Conversely, the percentage of deaths caused by heart failure increased at lower eGFR: 8.0% (95% CI, 7.3% to 8.7%), 11% (95% CI, 10.1% to 11.8%), and 13.9% (95% CI, 12.6% to 15.1%) for those with eGFR=45–59, 30–44, and 15–29 ml/min per 1.73 m2, respectively. The proportion of deaths caused by valvular disease increased modestly at lower eGFR. Conversely, the proportion of deaths caused by CVA decreased at lower eGFR. There was no clear relation between lower eGFR and the proportion of deaths caused by arrhythmia or other cardiac causes. These findings did not change appreciably with adjustment for age and sex.
Discussion
In this large population-based study, we found that the leading causes of death varied according to the presence and severity of CKD. Among Albertans with eGFR>60 ml/min per 1.73 m2 (with or without proteinuria) who died, the greatest proportion of deaths was from cancer. Among those with eGFR<60 ml/min per 1.73 m2 who died, the greatest proportion of deaths was from CVD. There were 19.3% and 13.9% more deaths caused by CVD in unadjusted and age- and sex-adjusted analyses, respectively, for those with eGFR=15–29 ml/min per 1.73 m2 compared with those with eGFR>60 ml/min per 1.73 m2 .The excess proportion of cardiovascular deaths seemed to be because of a larger proportion of deaths from heart failure and valvular heart disease at lower eGFR; there was no relation between lower eGFR and the proportion of deaths caused by IHD. We also found that lower eGFR was associated with lower proportion of deaths from cancer. Finally, although proteinuria is an important determinant of adverse outcomes9,10 (and the presence of proteinuria represents CKD), differences in the proportions of death attributed to different causes varied by eGFR but (among those with eGFR≥60 ml/min per 1.73 m2) not by proteinuria status.
Other data describing the risk of cause-specific death in the general population by eGFR category are limited. On the basis of data from a large population cohort from Taiwan, Gansevoort et al.11 identified an inverse relation between reduced eGFR and risk of death from CVD and a direct relation between deaths caused by cancer and declining eGFR (adjusted for age and sex). Our estimates of the proportions of deaths caused by CVD and cancer within each eGFR category were comparable with these findings with the exception of their finding of a higher proportion of cardiovascular deaths for individuals with eGFR=15–29 ml/min (39.9% of deaths caused by CVD in our study versus 59.8% in the Taiwanese cohort analyzed by Gansevoort et al.11). This difference is likely attributable to the inclusion of diabetic and CKD deaths as cardiovascular-related causes of deaths by Gansevoort et al.11
Our study extends the results of previous work in several ways. First, disaggregating cardiovascular causes of death is potentially useful from a clinical perspective, because these subcategories may require different interventions. Second, an analysis of the cause-specific deaths by eGFR serves to generate hypotheses on what pathophysiologic mechanisms may link worsening eGFR to specific causes of death. For instance, our finding that the majority of CVD deaths was caused by IHD is consistent with the association between CKD and IHD.12 However, contrary to our expectations, we found no clear association between lower eGFR and the proportion of deaths caused by IHD. Although IHD can contribute to other cardiac disorders, such as heart failure, our finding suggests that other processes more specific to CKD, such as arteriosclerosis or chronic volume overload, may contribute to increased cardiac workload at lower eGFR.13 Similarly, the excess proportion of deaths caused by valvular disease raises the hypothesis that valvular calcification may become increasingly important at lower eGFR.14 Third, CKD has been associated with an increased risk of a number of adverse health outcomes; characterizing the relative distribution of cause-specific deaths by eGFR category provides a comprehensive description of the cause-specific burden of disease in CKD. This information may be helpful for prioritizing resource allocation to future interventions.
Our finding that lower eGFR is associated with an increased mortality risk caused by infection is in keeping with other previous studies.4,5,15 On the basis of data from the Third National Health and Nutrition Examination Survey, Wang et al.15 found that, compared with those with eGFR≥60 ml/min, the hazards for infection-related mortality were 1.47 and 3.17 for those with eGFR=45–59 and <45 ml/min, respectively (adjusted for age, sex, and ethnicity). These findings may be because of the excess comorbidity and frailty associated with CKD,16 which presumably increase both the susceptibility to illness and the risk of death after illness develops.
Our study has several limitations. First, our findings are on the basis of data from a single Canadian province and may not be generalizable to other populations. Second, to define CKD, we used a single measurement of eGFR and proteinuria, a method that may have led to misclassification of exposure. Although we included three different measures of proteinuria, multiple measurements may lead to more accurate assessment of the risk of adverse events17; this limitation may explain why the risk of death from cardiovascular causes for those with eGFR>60 ml/min per 1.73 m2 did not seem to vary by the presence or absence of proteinuria. Third, data on cause of death and comorbidity from administrative databases have recognized shortcomings and therefore, could lead to misclassification.18 For instance, given that postoperative deterioration in renal function is an independent risk factor for death,19,20 we did a sensitivity analysis that excluded all eGFR measurements in the month before death; results were similar to those in the primary analysis. Fourth, there is no universally accepted scheme for classifying various causes of death into categories. The groupings that we used were on the basis of clinically relevant categories and informed by several validated algorithms,21 previously published data,22 and recommendations from experts.23,24 Fifth, our results must be interpreted carefully given that more severe CKD is much less common than milder forms: a disease that accounts for a lower proportion of deaths at higher eGFR may actually account for a substantially greater number of deaths than another disease that is responsible for a higher proportion of deaths among people with severe CKD.
In conclusion, the proportion of deaths from CVD or infection was higher at lower eGFR, with the former apparently driven by an increased proportion of deaths attributed to heart failure and valvular heart disease rather than IHD. These findings provide insight into the mechanism for the excess mortality seen among people with nondialysis-dependent CKD.
Concise Methods
Data Sources and Population
We used the Alberta Kidney Disease Network database, which incorporates data from AH (the provincial health ministry), the Northern and Southern Alberta Renal Programs, and the clinical laboratories in Alberta.25 We identified adults ages ≥18 years old who were Alberta residents, died between May of 2002 and March of 2009, and had an outpatient serum creatinine measured in the year before death. All people registered with AH were eligible for inclusion. All Alberta residents are eligible for insurance coverage by AH, and >99% of residents participate in this coverage. We excluded patients with kidney failure (defined as documented chronic dialysis or prior kidney transplant).
Cause of Death
Cause of death was classified into five broad categories using International Classification of Diseases 10-CA codes from AH Vital Statistics Branch: cardiovascular, infection, cancer, other, and not reported. The cause of death was considered to be the underlying cause rather than the immediate cause of death. In Alberta, the attending physician, medical examiner, or other certifier determines the cause of death. Specific codes are listed in Supplemental Table 1. Death caused by CVD was further subclassified into IHD, CVA, heart failure, valvular heart disease, and arrhythmia. Deaths caused by infection were categorized as abdominal, cardiac, kidney and genitourinary, neurologic, respiratory, and septicemia. Both malignant and nonmalignant cancers were included. Deaths caused by other causes were those for which a cause of death was recorded and other than cardiovascular, cancer, or infection related. Other causes were further classified into the following subcategories: neurologic and dementias, chronic lung diseases, suicides and accidents, digestive diseases, and diabetic complications. Where the proportions of deaths caused by a specific cause were very low, such as CKD, these causes were classified in a miscellaneous other category. Lastly, deaths with no reported cause of death were categorized as missing.
Covariates
Participants were divided into groups according to level of kidney function, which was estimated using the CKD Epidemiology Collaboration equation.26 The last outpatient eGFR 1 year before death was categorized as ≥60, 45–59.9, 30–44.9, or 15–29.9 ml/min per 1.73 m2. Those participants with eGFR≥60 ml/min per 1.73 m2 were further subdivided by the presence or absence of proteinuria. Proteinuria was defined as present if any of the following were present: trace urine dipstick or greater, albumin-to-creatinine ratio ≥3 mg/mmol, or protein-to-creatinine ratio ≥15 mg/mmol. The last value before death was used; if more than one value at that time point was available, measures were used in the following order of preference: first, albumin-to-creatinine ratio, second, protein-to-creatinine ratio, and third, dipstick. Demographic variables included age (categorized as 18–49.9, 50–69.9, and ≥70 years old), sex, Aboriginal (registered First Nations or recognized Inuit), social assistance, and rural/urban status. We used validated algorithms to define the Charlson comorbidities diabetes27 and hypertension28 at baseline using physician claims, hospitalization, and ambulatory care use data. The Charlson score was on the basis of the Deyo classification29 of the following comorbidities: CVA, peripheral vascular disease, congestive heart failure, cancer, chronic obstructive pulmonary disease, dementia, diabetes with and without complications, AIDS/HIV, metastatic solid tumor, myocardial infarction, mild liver disease, moderate/severe liver disease, paralysis, peptic ulcer disease, and rheumatic disease.
Statistical Analyses
We performed analyses with Stata/MP 11 (www.stata.com) and reported baseline descriptive statistics as counts and percentages or medians and interquartile ranges as appropriate. Chi-squared and Kruskal–Wallis tests were used to test for differences across groups. Using multinomial logistic regression models, we estimated risks of death for specific causes according to CKD stage and level of proteinuria. We reported unadjusted and age- and sex-adjusted proportions of death caused by the different causes. We also present the subcategories associated with cardiovascular and other causes of death. In sensitivity analyses, we eliminated eGFR values in the month before death and used the last value in the 11 earlier months. Repeating analyses after exclusion of all eGFR values obtained during the month before death yielded results that were very similar to the primary analyses (data not shown). The institutional review boards at the University of Alberta and the University of Calgary approved the study.
Disclosures
None.
Supplementary Material
Acknowledgments
This study is, in part, on the basis of data provided by Alberta Health and Alberta Health Services.
This work was supported by a team grant to the Interdisciplinary Chronic Disease Collaboration from the Alberta Heritage Foundation for Medical Research (AHFMR). M.J. is supported by a Kidney Research Scientist Core Education and National Training Program New Investigator Award (Kidney Foundation of Canada, Canadian Society of Nephrology, Canadian Institutes of Health Research). B.M. is supported by the Roy and Vi Baay Chair in Kidney Research. B.M. and M.T. are supported by an AHFMR Population Health Scholar Award, and M.T. is a Government of Canada Research Chair in the Optimal Care of People with Chronic Kidney Disease.
The interpretation and conclusions are those of the researchers and do not represent the views of the Government of Alberta.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Not All Deaths in CKD Are from a Broken Heart,” on pages 2307–2308.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014070714/-/DCSupplemental.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA: Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095–2128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couser WG, Remuzzi G, Mendis S, Tonelli M: The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80: 1258–1270, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PHM, Jenny NS, Stehman-Breen C, Gillen D, Bleyer AJ, Hirsch C, Siscovick D, Newman AB: Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 16: 3728–3735, 2005 [DOI] [PubMed] [Google Scholar]
- 4.James MT, Quan H, Tonelli M, Manns BJ, Faris P, Laupland KB, Hemmelgarn BR, Alberta Kidney Disease Network : CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis 54: 24–32, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Marks A, Macleod C, McAteer A, Murchie P, Fluck N, Smith WCS, Prescott GJ, Clark LE, Ali T, Black C: Chronic kidney disease, a useful trigger for proactive primary care? Mortality results from a large U.K. cohort. Fam Pract 30: 282–289, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Herzog CA, Strief JW, Collins AJ, Gilbertson DT: Cause-specific mortality of dialysis patients after coronary revascularization: Why don’t dialysis patients have better survival after coronary intervention? Nephrol Dial Transplant 23: 2629–2633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saravanan P, Davidson NC: Risk assessment for sudden cardiac death in dialysis patients. Circ Arrhythm Electrophysiol 3: 553–559, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M, Alberta Kidney Disease Network : Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJL, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR: Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol 28: 354–360, 2008 [DOI] [PubMed] [Google Scholar]
- 13.London GM, Marchais SJ, Guérin AP, Métivier F: Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens 14: 525–531, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Mizobuchi M, Towler D, Slatopolsky E: Vascular calcification: The killer of patients with chronic kidney disease. J Am Soc Nephrol 20: 1453–1464, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Wang HE, Gamboa C, Warnock DG, Muntner P: Chronic kidney disease and risk of death from infection. Am J Nephrol 34: 330–336, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker SR, Gill K, Macdonald K, Komenda P, Rigatto C, Sood MM, Bohm CJ, Storsley LJ, Tangri N: Association of frailty and physical function in patients with non-dialysis CKD: A systematic review. BMC Nephrol 14: 228, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bello A, Thompson S, Lloyd A, Hemmelgarn B, Klarenbach S, Manns B, Tonelli M, Alberta Kidney Disease Network : Multiple versus single and other estimates of baseline proteinuria status as predictors of adverse outcomes in the general population. Am J Kidney Dis 59: 364–371, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Smith Sehdev AE, Hutchins GM: Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med 161: 277–284, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Thakar CVT, Worley S, Arrigain S, Yared JP, Paganini EP: Influence of renal dysfunction on mortality after cardiac surgery: Modifying effect of preoperative renal function. Kidney Int 67: 1112–1119, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, Stegeman CA: Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol 16: 195–200, 2005 [DOI] [PubMed] [Google Scholar]
- 21.So L, Evans D, Quan H: ICD-10 coding algorithms for defining comorbidities of acute myocardial infarction. BMC Health Serv Res 6: 161, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang HE, Devereaux RS, Yealy DM, Safford MM, Howard G: National variation in United States sepsis mortality: A descriptive study. Int J Health Geogr 9: 9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, Edwards BK: SEER Cancer Statistics Review, 1975–2004, National Cancer Institute; Bethesda, MD, 2006. Available at: http://seer.cancer.gov/csr/1975_2004. Accessed August 27, 2014 [Google Scholar]
- 24.Canadian Institute for Health Information : International Statistical Classification of Diseases and Related Health Problems, Ottawa, ON, Canada, Canadian Institute for Health Information, 2009. Available at: http://www.cihi.ca/cihi-ext-portal/pdf/internet/icd_10_ca_vol1_2009_en. Accessed August 28, 2014 [Google Scholar]
- 25.Hemmelgarn BR, Clement F, Manns BJ, Klarenbach S, James MT, Ravani P, Pannu N, Ahmed SB, MacRae J, Scott-Douglas N, Jindal K, Quinn R, Culleton BF, Wiebe N, Krause R, Thorlacius L, Tonelli M: Overview of the Alberta Kidney Disease Network. BMC Nephrol 10: 30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hux JE, Ivis F, Flintoft V, Bica A: Diabetes in Ontario: Determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 25: 512–516, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Khan N, Hemmelgarn BR, Tu K, Chen G, Campbell N, Hill MD, Ghali WA, McAlister FA, Hypertension Outcome and Surveillance Team of the Canadian Hypertension Education Programs : Validation of a case definition to define hypertension using administrative data. Hypertension 54: 1423–1428, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.