Abstract
We showed previously that prior exposure to a modified ultrasound regimen prevents kidney ischemia-reperfusion injury (IRI) likely via the splenic cholinergic anti-inflammatory pathway (CAP) and α7 nicotinic acetylcholine receptors (α7nAChR). However, it is unclear how ultrasound stimulates the splenic CAP. Further investigating the role of the spleen in ischemic injury, we found that prior splenectomy (–7d) or chemical sympathectomy of the spleen with 6-hydroxydopamine (6OHDA; –14d) exacerbated injury after subthreshold (24-minute ischemia) IRI. 6-OHDA–induced splenic denervation also prevented ultrasound-induced protection of kidneys from moderate (26-minute ischemia) IRI. Ultrasound-induced protection required hematopoietic but not parenchymal α7nAChRs, as shown by experiments in bone marrow chimeras generated with wild-type and α7nAChR–/– mice. Ultrasound protection was associated with reduced expression of circulating and kidney-derived cytokines. However, splenocytes isolated from mice 24 hours after ultrasound treatment released more IL-6 ex vivo in response to LPS than splenocytes from sham mice. Adoptive transfer of splenocytes from ultrasound-treated (but not sham) mice to naïve mice was sufficient to protect kidneys of recipient mice from IRI. Ultrasound treatment 24 hours before cecal ligation puncture–induced sepsis was effective in reducing plasma creatinine in this model of AKI. Thus, splenocytes of ultrasound-treated mice are capable of modulating IRI in vivo, supporting our ongoing hypothesis that a modified ultrasound regimen has therapeutic potential for AKI and other inflammatory conditions.
Keywords: acute renal failure, cholinergic anti-inflammatory pathway, neuroimmune, spleen, inflammation
AKI is a major health burden without major pharmacologic advances in its prevention or treatment.1 To improve outcomes in AKI, a better understanding of the pathophysiology of AKI is necessary to develop novel therapies in well designed clinical trials.2 Much work has been done on the intrinsic cellular and molecular mechanisms of AKI,3 but extrarenal factors are key modulators of AKI. Kidney ischemia-reperfusion injury (IRI), a form of AKI, results in a systemic inflammatory response4 that affects multiple organ systems.5 Multiorgan dysfunction in response to AKI likely accounts for the high mortality associated with this disorder.5,6 Inflammatory molecules are found in both the arterial supply to and venous outflow from the kidney as soon as 1 minute after AKI.7 These data suggest that the kidney is a source of inflammatory molecules after AKI, but they also suggest that the release of inflammatory mediators from other unidentified peripheral tissues immediately after AKI could modulate kidney injury. The contribution of renal and nonrenal sources of inflammatory mediators in the development of AKI remains unclear.
Previous studies have suggested an important role of the spleen in AKI. Splenectomy before kidney IRI exacerbated lung injury through proinflammatory mechanisms that may involve removal of protective splenic IL-10.8 Splenectomy also reduced the protective efficacy of the anti-inflammatory agent, chloroquine, in AKI.9 These results suggest that the spleen has an important role in modulating inflammation after AKI. Not all studies, however, have shown a protective role of the spleen in ischemic organ injury. Splenectomy reduces ischemic damage in both the liver10 and brain.11 These studies demonstrate the need to better understand the function of the spleen in kidney IRI.
Recently, we reported that a simple ultrasound-based protocol reduced tissue inflammation and prevented kidney IRI in mice.12 This effect was dependent on an intact spleen and functional α7 nicotinic acetylcholine receptors (α7nAChRs), which is consistent with the hypothesis that ultrasound stimulates the splenic cholinergic anti-inflammatory pathway. As described by Tracey,13 the cholinergic anti-inflammatory pathway is initiated via activation of the adrenergic splenic nerve and culminates with the activation of α7nAChRs. However, it is still unclear how ultrasound initiates this pathway and protects kidneys from AKI. In this study we further explored the importance of the spleen and the splenic cholinergic anti-inflammatory pathway in modulating kidney IRI. We now report that the protective effect of ultrasound depends on sympathetic innervation of the spleen and hematopoietic α7nAChRs and that ultrasound-induced modulation of splenocyte function is sufficient to confer protection from IRI.
Results
Splenectomy or Splenic Denervation Exacerbates Injury in a Subthreshold Ischemia-Reperfusion Model
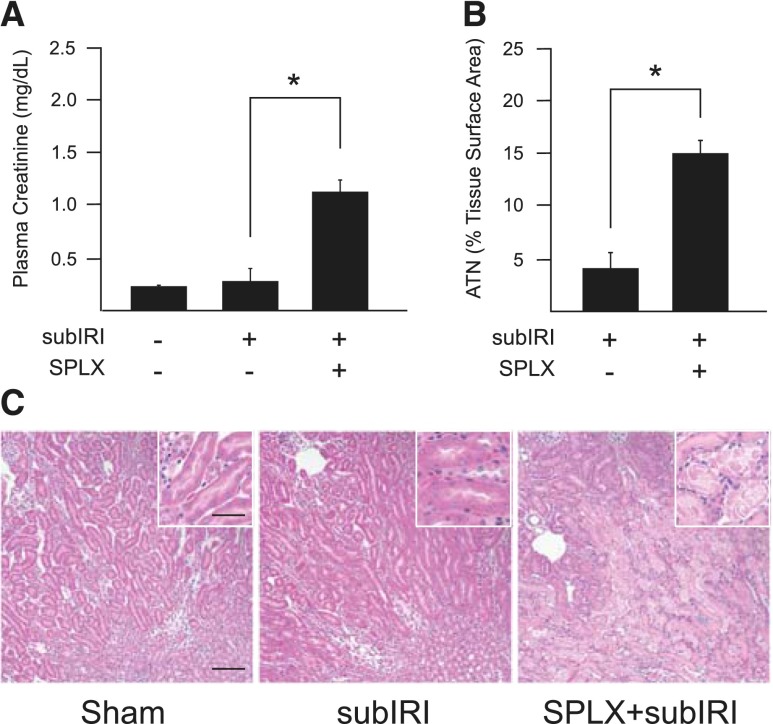
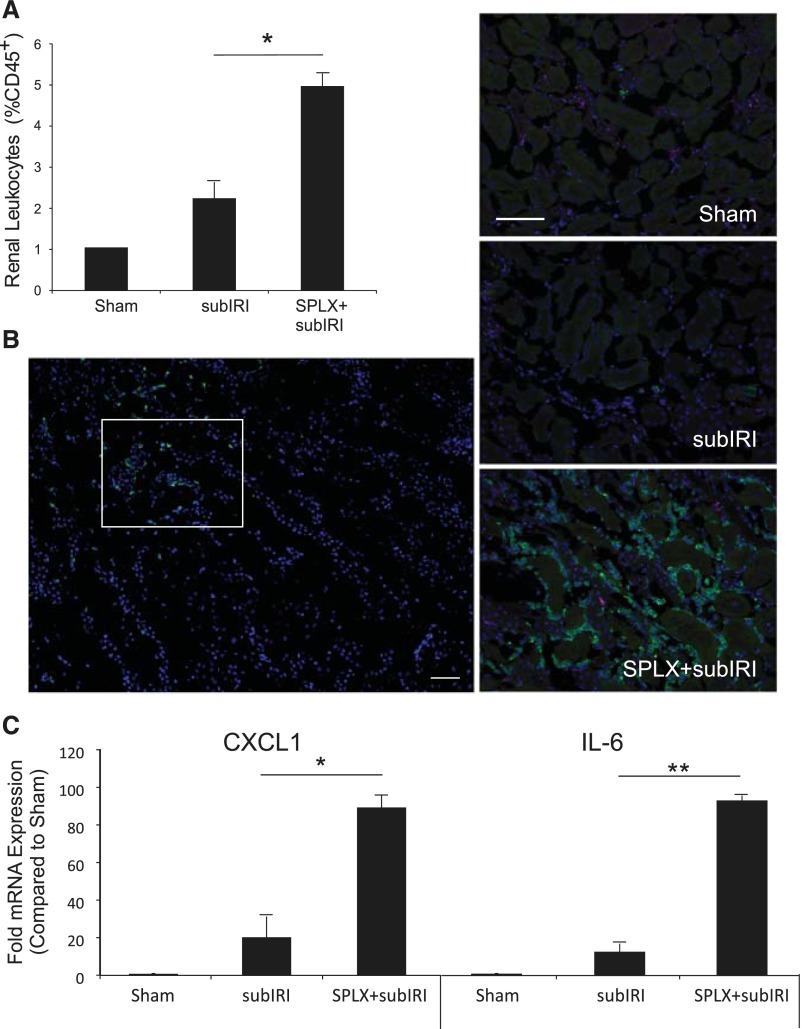
Our data suggest that the spleen is an important target in mediating the protective effect of ultrasound.12 Others have demonstrated that splenectomy before kidney IRI increased lung injury but not kidney injury.8 It is possible that an immune modulatory function of the spleen in protecting the kidney from IRI may be missed if the injury is too severe. Therefore, in the studies on splenectomy we used a reduced ischemic time of 24 minutes (subthreshold ischemia-reperfusion injury [subIRI]) to determine if splenectomy exacerbates AKI. Subthreshold IRI was not sufficient to alter plasma creatinine or cause acute tubular necrosis in mice with sham splenectomy; however, mice that were splenectomized 1 week before subIRI had significantly higher plasma creatinine (1.2 mg/dl, P<0.001), extensive acute tubular necrosis (Figure 1), and increased renal inflammation (Figure 2). The percentage of CD45+ leukocytes and 7/4+ neutrophils and the level of expression of chemokines/cytokines CXCL1 and IL-6 was greater in mice splenectomized 1 week before subIRI (Figure 2). Interestingly, similar increases in plasma creatinine were observed in Rag1–/– mice. Plasma creatinine in Rag1–/– mice exposed to sham splenectomy and subIRI was 1.1±0.08mg/dl, a value significantly lower than those splenectomized a week before (1.7±0.07mg/dl, P<0.001, n=7–8). Combined, these data suggest that prior splenectomy exacerbates IRI, and this effect is independent of splenic T and B lymphocytes.
Figure 1.
SPLX exacerbates kidney IRI. Mice underwent SPLX or sham surgery 7 days before a subIRI (24 minutes of ischemia, 24 hours of reperfusion), which was not sufficient alone to cause AKI. Prior SPLX exacerbated subthreshold injury and resulted in elevated plasma creatinine (A) and robust ATN (B) (scored from hematoxylin and eosin samples) (C). ATN, acute tubular necrosis; SPLX, splenectomy; subIRI, subthreshold ischemia-reperfusion injury. n=6–13. *P≤0.001. Scale bar is 100 μm in the main panel and 50 μm in the inset.
Figure 2.
Prior SPLX increases renal inflammation after subIRI. (A) Leukocyte infiltration (CD45+ cells as a percent of total kidney cells) measured in kidneys by flow cytometry 24 hours after subIRI was higher in mice with prior SPLX. (B) Neutrophil infiltration was highest in the outer medulla (upper left quadrant of large panel) and sparse in the inner medulla (lower right region) and other areas of kidney after subIRI. White box indicates area sampled for photographs in smaller panels at right. Neutrophils (green, FITC-7/4 immunofluorescence) were densely sequestered in the outer medulla of kidneys from mice with prior SPLX. Blue, DAPI-labeled nuclei. Scale bar is 50 μm. (C) Renal mRNA expression of CXCL1 and IL-6 was significantly greater in mice with SPLX plus subIRI compared with subIRI alone. Sham, sham ischemia-reperfusion injury surgery; SPLX, splenectomy; SPLX+subIRI, splenectomy plus subthreshold ischemia-reperfusion injury; subIRI, sham splenectomy plus subthreshold ischemia-reperfusion injury. n=4–11. *P<0.001; **P=0.003.
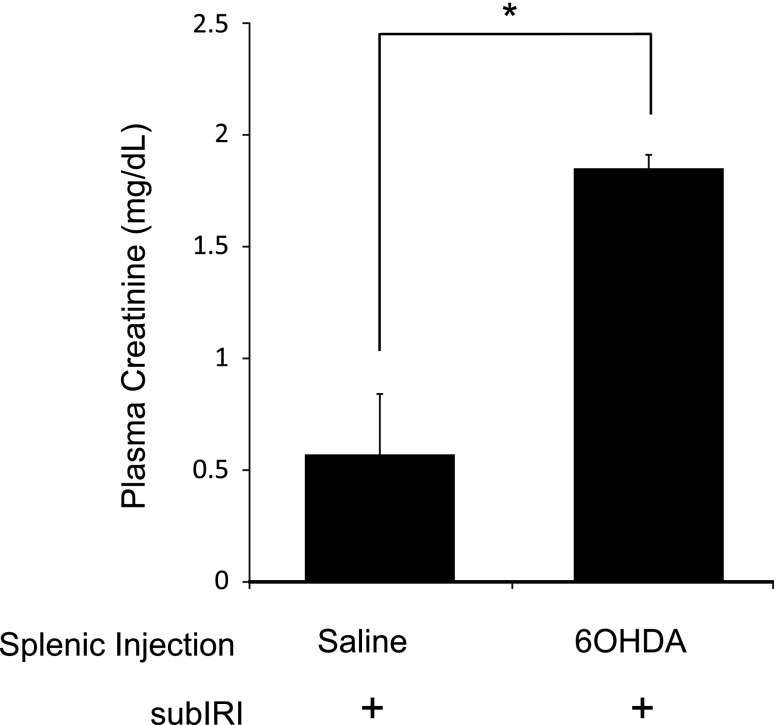
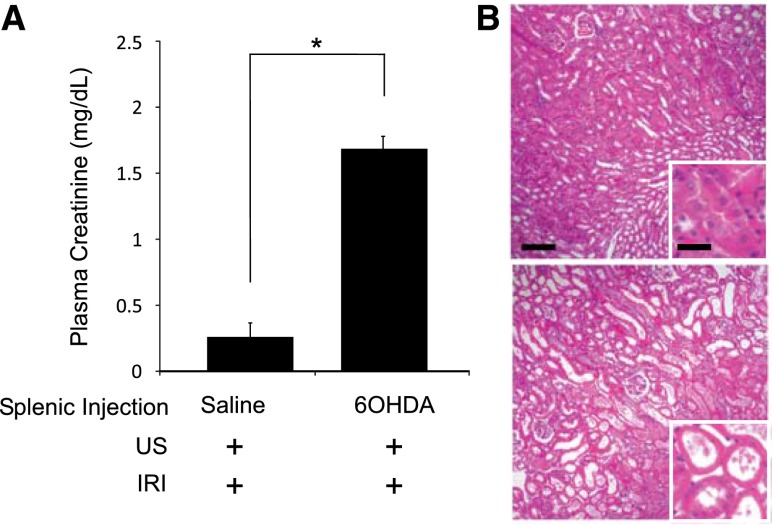
Activation of the cholinergic anti-inflammatory pathway requires catecholaminergic input to the spleen.14 To explore the role of this input in splenic modulation of AKI, the spleen was chemically denervated by locally injecting 6-hydroxydopamine (6-OHDA), a neurotoxin that destroys catecholaminergic neurons.15 Similar to splenectomy, splenic 6-OHDA, as little as 15 µg injected 14 days before subIRI, exacerbated kidney injury as shown by increased plasma creatinine (P<0.001) and acute tubular necrosis compared with mice with splenic vehicle (saline) injections before subIRI (Figure 3).
Figure 3.
Splenic sympathectomy exacerbates subIRI. Mice received splenic injections of saline (60 μl) or 6-OHDA (120 μg in 60 μl saline) 14 days before subIRI. Mice with splenic 6-OHDA injections had elevated plasma creatinine. n=3–12. *P<0.001.
Protective Effect of Ultrasound is Dependent on the Splenic Nerve and Hematopoietic α7nAChRs
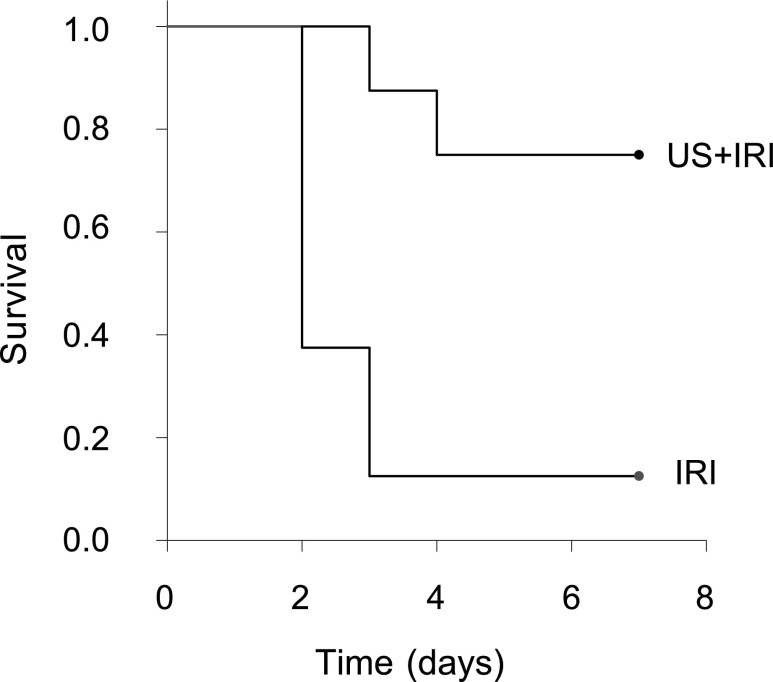
We have shown that prior exposure to a modified ultrasound regimen (up to 48 hours before ischemia) prevents AKI and the subsequent development of renal fibrosis in mice exposed to renal IRI.12 Here, the same ultrasound regimen 24 hours before our standard bilateral kidney IRI (26 minutes of ischemia) reduced kidney injury as assessed by plasma creatinine (P=0.002; data not shown) and significantly (P=0.004) improved mouse survival (Figure 4).
Figure 4.
Prior ultrasound (US) exposure improves survival after kidney IRI. Mice were exposed to a modified ultrasound regimen 24 hours before bilateral IRI (26 minutes of ischemia, 24 hours of reperfusion). Mice were maintained for a week, and survival was determined using log-rank Kaplan–Meier survival analysis. n=8. P=0.004 (IRI versus US plus IRI).
Our previous data suggest that ultrasound prevents IRI by stimulating the cholinergic anti-inflammatory pathway,12 which can be initiated by activation of the splenic nerve.13 To determine whether the splenic nerve is needed for the protective effect of ultrasound treatment, the spleen was chemically sympathectomized by direct injection with 6-OHDA 14 days before IRI. Prior splenic 6-OHDA treatment abolished the protective effect of ultrasound after IRI compared with vehicle-treated mice (Figure 5).
Figure 5.
Protective effect of prior ultrasound exposure is abolished by splenic sympathectomy. Mice received splenic injections of saline or 6-OHDA (as in Figure 3) 14 days before the ultrasound (US) and IRI (26 minutes of ischemia) treatments. Splenic injections of 6-OHDA abolished the protective effects of US as determined by (A) plasma creatinine and (B) histologic assessment (hematoxylin and eosin) of acute tubular necrosis. Scale bar is 100 μm in the main panel and 50 μm in the inset. n=5. *P<0.001.
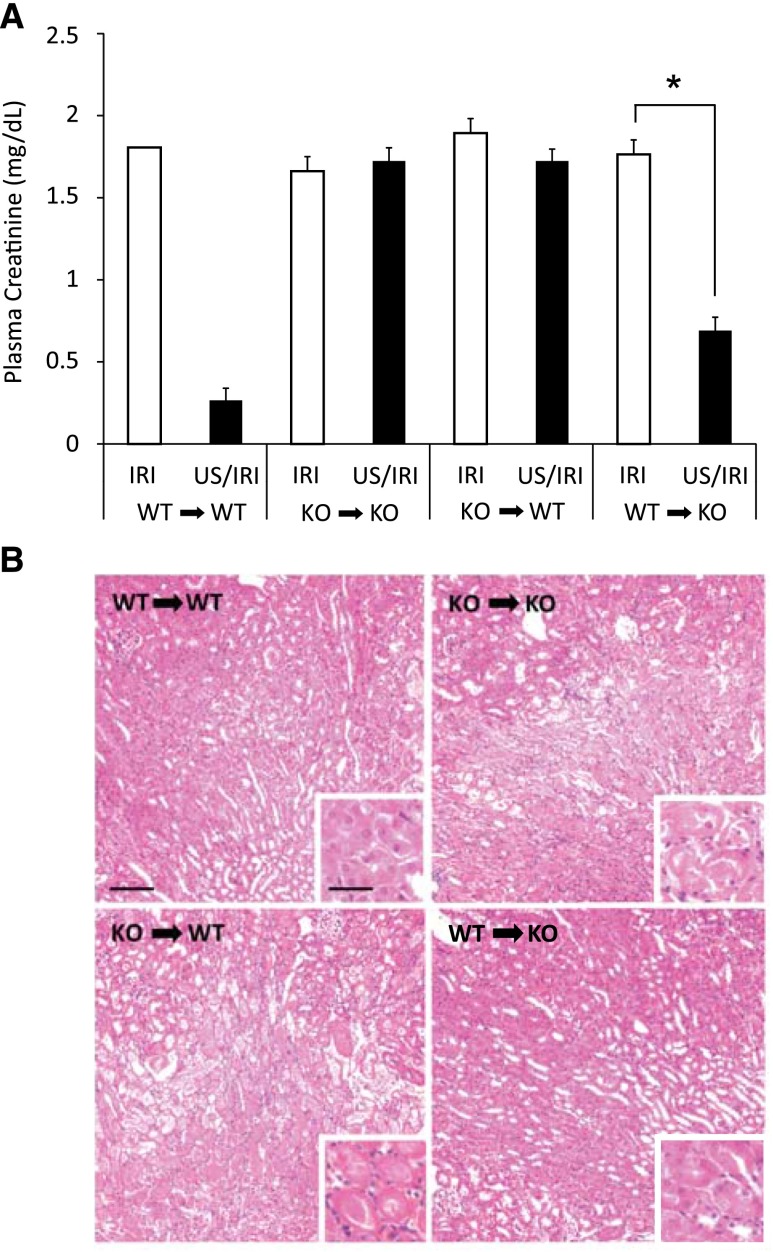
Although the splenic nerve is believed to stimulate the cholinergic anti-inflammatory pathway, binding of acetylcholine to the α7nAChR is considered the key step in the pathway.13 We showed that α7nAChR agonists prevent kidney IRI, and the protective effect of ultrasound is dependent on α7nAChRs.12 Nicotine reduces inflammation and protects the kidney from IRI in an α7nAChR-dependant manner, but the contribution of hematopoietic versus parenchymal α7nAChRs was not examined.16 To determine whether hematopoietic or parenchymal α7nAChRs mediate the protective effect of ultrasound, we generated bone marrow chimeras with wild-type and α7nAChR–/– mice. Ultrasound pretreatment was protective only in mice with bone marrow cells from wild-type mice regardless of the parenchymal genotype, therefore pointing to the essential role of hematopoietic α7nAChRs in ultrasound protection from IRI (Figure 6).
Figure 6.
Hematopoietic α7nAChRs are required for the protective effects of prior ultrasound (US) exposure. α7nAChR–/– or WT mice were lethally irradiated, reconstituted with bone marrow from either WT or α7nAChR–/– (KO) mice, and 8–10 weeks later were exposed to ultrasound and IRI (26 minutes of ischemia). Only mice with WT bone marrow reconstitution (WT→WT or WT→KO), regardless of the parenchymal phenotype, benefitted from the ultrasound treatment, as determined by (A) plasma creatinine and (B) hematoxylin and eosin analysis of morphology. Scale bar is 100 μm in the main panel and 50 μm in the inset. n=4−7. *P<0.001.
Ultrasound Reduces Circulating and Kidney Cytokines after IRI
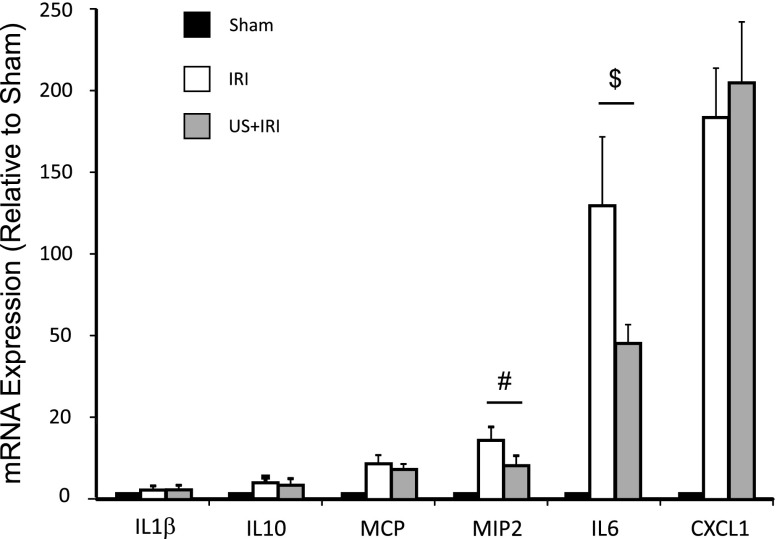
Inflammation is a key component of IRI, and stimulation of the cholinergic anti-inflammatory pathway has been shown to reduce systemic cytokine production16 Therefore, the protective effect of ultrasound may involve modulation of systemic inflammatory pathways. IRI-induced increases in circulating IL-6, IL-10, LIF, IL-15, MCP-1, MIP-2, and TNFα concentrations (Table 1) and kidney IL-6 and MIP2 mRNA expression (Figure 7) were reduced by ultrasound treatment 24 hours before IRI.
Table 1.
IRI-induced increases in plasma cytokine concentrations are reduced by prior ultrasound treatment
| Treatment | Plasma Cytokine Concentrations (pg/ml) | ||||||
|---|---|---|---|---|---|---|---|
| IL-6 | IL-10 | LIF | IL-15 | MCP-1 | MIP-2 | TNF-α | |
| Sham | 61.9 | 6.7 | 2.3 | 14.9 | 23 | 4.4 | 8.2 |
| IRI | 398.5±63.2 | 58±8.6 | 8.2±1.2 | 17.8±1.8 | 128.4±14.8 | 26.9±4.3 | 15.2±1.5 |
| Ultrasound plus IRI | 121.4±59.9 | 3.9±8.1 | 2.8±1.5 | 9.6±1.5 | 43.7±14.1 | 7.3±4.1 | 5.3±1.4 |
| P value | 0.015 | 0.003 | 0.014 | 0.012 | 0.004 | 0.014 | 0.002 |
Values are mean±SEM. P values are for comparisons between IRI and ultrasound plus IRI. n=5–6.
Figure 7.
Ultrasound (US) blunts the IRI-induced increases in renal mRNA expression of IL-6 and MIP2. Mice were exposed to US 24 hours before IRI (26 minutes of ischemia). Then 24 hours after IRI, kidney samples were assayed for mRNA expression of key chemokines/cytokines by real-time PCR. Only IL-6 and MIP2 (CXCL2) expression (levels relative to sham) differed between the IRI and US plus IRI groups. n=13–14. $P=0.003; #P=0.02.
Ultrasound Modulates Splenocyte Function: ex vivo and in vivo Adoptive Transfer Studies
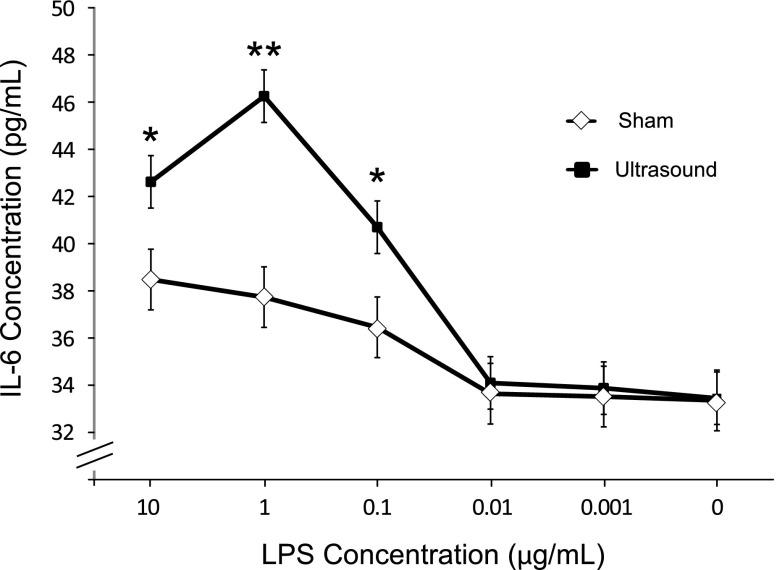
To determine whether splenocytes from ultrasound-treated mice respond differently to inflammatory stimuli, splenocytes were isolated from ultrasound- or sham-treated animals 24 hours after treatment. Cells were then incubated ex vivo with increasing concentrations of LPS, and IL-6 production was measured 16 hours later. IL-6 is known to have both pro- and anti-inflammatory effects.17 In contrast with the decreases in circulating IL-6 and kidney mRNA levels in ultrasound-treated animals previously described, IL-6 production ex vivo in response to LPS concentrations of 0.1–10 µg/ml was greater in splenocytes from ultrasound-treated mice compared with cells from sham mice (Figure 8). These results demonstrate functionally that splenocytes behave differently when exposed to ultrasound, therefore supporting either a direct or indirect effect of ultrasound treatment on splenocytes. The result is different from what we observed in vivo likely because of the simple artificial culture conditions relative to the complex microenvironment of the spleen and the difference in stimulus (IRI versus LPS).
Figure 8.
Splenocytes from ultrasound-treated mice have heightened IL-6 production when stimulated with LPS. Splenocytes were isolated 24 hours after ultrasound or sham treatment. Splenocytes were cultured (1×105 cells/well) and stimulated with LPS for 18 hours. The supernatants were then assayed for IL-6 by ELISA. n=3. *P=0.02; **P<0.001.
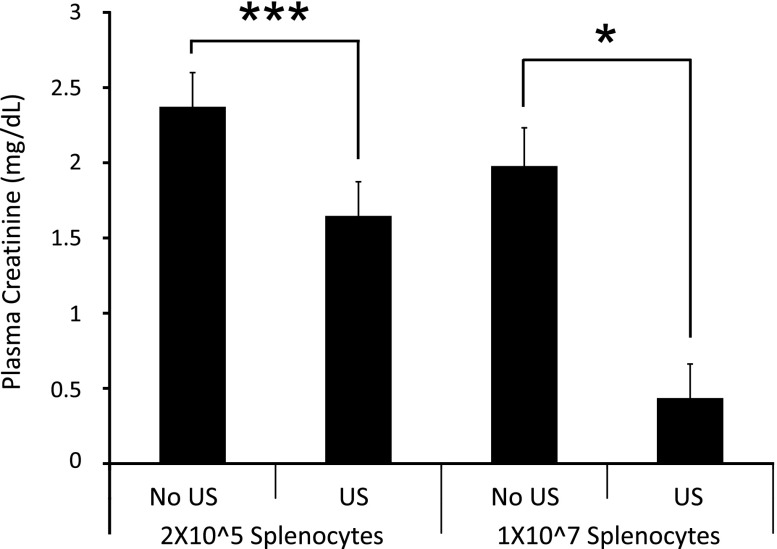
To determine if ultrasound modulates splenocyte response to inflammatory stimuli in vivo, splenocytes were isolated from mice 24 hours after ultrasound (or sham) treatment and were adoptively transferred (2×105 or 1×107 cells, intravenously) to naïve recipient mice 24 hours before IRI. Ultrasound did not appear to change the relative composition of populations of splenocytes, as determined by flow cytometry; the proportions of Ly6C+, CD3+, CD4+, and CD11b+ cells were similar in spleens from control and ultrasound-treated mice. However, adoptive transfer of splenocytes from ultrasound-treated mice produced a dose-dependent decrease in plasma creatinine (Figure 9) in recipient mice after IRI compared with transfer of splenocytes from sham-treated animals.
Figure 9.
Adoptive transfer of splenocytes from ultrasound (US)-treated mice confers protection from IRI. Splenocyte single-cell suspensions were isolated from mice 24 hours after US or sham (no US) treatment and were injected (2×105 or 1×107 cells/mouse, intravenously) into naïve mice 24 hours before IRI (26 minutes of ischemia). Splenocytes from US-treated mice reduced plasma creatinine in a dose-dependent manner. n=4–5. ***P=0.04; *P<0.001.
Prior Ultrasound Treatment Reduces Severity of AKI in Sepsis
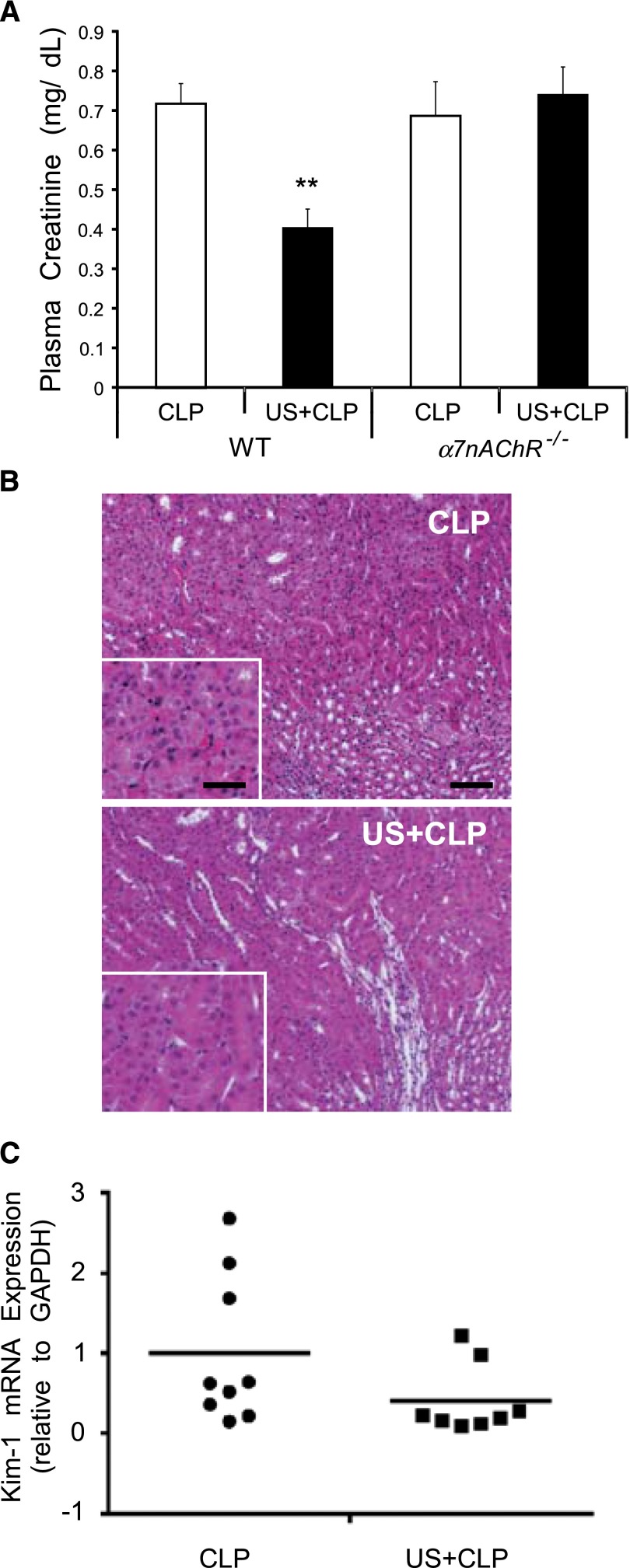
A critical issue in sepsis is the pathologic dysregulated response to bacterial infection leading to a hyperinflammatory cytokine storm response,18 and the lack of homeostasis from dysfunction of the neuroendocrine and immune systems leads to multiorgan dysfunction and early death.19 Using the cecal ligation puncture (CLP) model of sepsis,20 the rise in plasma creatinine (Figure 10A) and circulating cytokines (Table 2) in WT mice subjected to CLP was reduced significantly by pretreatment with ultrasound. Subtle histologic changes observed in kidneys of CLP mice, consistent with existing literature,21 were indistinguishable in CLP mice pretreated with ultrasound (Figure 10B). Furthermore, prior ultrasound treatment did not reduce plasma creatinine in α7nAChR–/– mice (Figure 10A), suggesting that, like our prior results with IRI, ultrasound protection from sepsis-induced AKI requires the cholinergic anti-inflammatory pathway. Ultrasound pretreatment reduced Kim-1 expression by approximately 25% (Figure 10C), but there was no significant difference from CLP alone (P=0.09). These results suggest that CLP induces marked functional changes and mild damage consistent with prior studies,22 and ultrasound blocks these effects in an α7nAChR-dependent manner.
Figure 10.
Prior ultrasound protects mice from AKI in a CLP model of sepsis but does not protect in α7nAChR–/– mice. Mice were exposed to ultrasound (US) 24 hours before CLP and were euthanized 24 hours after CLP. (A) US reduced plasma creatinine in WT mice but α7nAChR–/– mice were resistant to the protective effects. **P<0.001. (B) Hematoxylin and eosin staining of kidney sections from WT mice. Scale bar is 100 μm. (C) Kim-1 mRNA expression in WT kidney relative to glyceraldehyde 3-phosphate (GAPDH). P=0.09. n=8–9 from two experiments. Circles and squares refer to the 2 groups, CLP and US+CLP, respectively.
Table 2.
CLP-induced increases in plasma cytokine concentrations are reduced by prior US treatment
| Samples | Plasma Cytokine Concentrations (pg/ml) | ||||||
|---|---|---|---|---|---|---|---|
| IL-1α | IL-6 | IL-10 | LIF | MIP-1α | MIP-2 | RANTES | |
| CLP | 42±21 | 13198±7032 | 3343±4405 | 100±77 | 467±493 | 8889±7596 | 69±40 |
| US plus CLP | 14±9 | 1184±353 | 46±21 | 9±4 | 80±7 | 935±128 | 11±8 |
| P value | 0.031 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 |
Values are mean±SEM. RANTES, regulated upon activation, normal T cell expressed and secreted; US, ultrasound. n=4–5.
Discussion
We have shown that the spleen plays a critical role both in modulating the inflammatory response and subsequent kidney injury that occurs after IRI and in ultrasound-mediated protection from IRI. Our results suggest that ultrasound-mediated protection from IRI requires the splenic nerve and hematopoietic, but not parenchymal, α7nAChRs. Ultrasound altered the profile of circulating and renal inflammatory mediators after IRI and the response of splenocytes to proinflammatory stimulation. Transfer of splenocytes from ultrasound-treated mice to naïve mice was sufficient to reduce kidney IRI. Combined, these data support our ongoing hypothesis that ultrasound reduces ischemic AKI via stimulation of the splenic cholinergic anti-inflammatory pathway. In addition to IRI, ultrasound was protective in another model of AKI (i.e., CLP-induced sepsis).
Splenic Control of Inflammation and IRI
The spleen is a peripheral lymphoid organ that participates in innate and adaptive immunity through its unique organization. There are distinct areas of B and T cells, macrophages, dendritic cells, and stromal cells. The spleen removes older erythrocytes, bloodborne micro-organisms, and cellular debris from the circulation. Before the concept of the cholinergic anti-inflammatory pathway developed, the spleen had generally been considered a source of inflammatory mediators capable of propagating injury. Some work demonstrated a protective effect of prior splenectomy in animal models of organ injury.10 In contrast, others demonstrated that prior splenectomy had deleterious effects.8 Prophylactic treatments for AKI were ineffective in previously splenectomized animals,9 including the ultrasound treatment described here. Faubel's group demonstrated that splenectomy did not exacerbate AKI, but there was an increase in lung permeability.8 The severity of kidney injury may have precluded demonstrating an effect of splenectomy on AKI, which we uncovered by using a subthreshold IRI model. However, the authors found that splenic IL-10 production was increased in lymphocytes after kidney IRI, suggesting that the spleen contributed to the anti-inflammatory response and reduction of lung injury. Not all studies have demonstrated a protective effect in organ injury. In particular, splenectomy was associated with worse hepatic IRI; however, other factors may contribute, including reduction in pressure in the portal vein, which might increase the arterial blood supply to the liver, promoting hepatic recovery after IRI.10 Our results are consistent with studies demonstrating the potential role of the spleen in modulating the inflammatory response to organ injury. Given that the cholinergic anti-inflammatory pathway appears to be an intrinsic pathway that is initiated by the splenic nerve, we tested and confirmed that the deleterious effect of splenectomy could be recapitulated with splenic denervation alone. Therefore, our data suggest that the spleen performs an inherent anti-inflammatory function in AKI that is mediated by the splenic nerve.
Neural Control of Inflammation
The nervous and immune systems interact in complex ways to maintain homeostasis and respond to stress or injury.23 Clinical evidence of this interaction has been documented in times of stress and in athletes subjected to extreme conditions.24 Studies in animal models25–27 and human blood cells demonstrate a relationship between the autonomic nervous system and inflammation. Norepinephrine inhibits LPS-induced TNFα and IL-6 production through stimulation of β1-adrenergic receptors in human leukocytes,28 and epinephrine signaling through protein kinase A induces IL-10 synthesis in human mononuclear cells.29
Cholinergic Anti-Inflammatory Pathway
The spleen appears to be a critical site for the neural control of inflammation and provides a potential therapeutic target for immune-mediated diseases.30 Early work demonstrated that vagal nerve stimulation increased release of acetylcholine from the spleen.31,32 However, because choline acetyltransferase, the enzyme necessary to produce acetylcholine, had not been demonstrated in splenic nerve fibers, it was difficult to reconcile the finding of splenic production of acetylcholine in response to vagal stimulation.33 Seminal studies by Rosas-Ballina and colleagues determined that a subset of lymphocytes produced acetylcholine in response to vagal stimulation.34 Activation of the splenic nerve resulted in the release of norepinephrine that binds β-adrenergic receptors on nearby choline acetyltransferase–expressing splenic memory T cells (CD4+CD44highCD62Llow). Neurotransmitter control of neutrophil-mediated inflammation is achieved through acetylcholine-producing T cells34 and B cells.33 Electrical stimulation of splenic tissue, either ex vivo35 or in vivo via vagal nerve stimulation,13 reduces cytokine production in response to challenge with inflammatory stimuli.
In our prior study we hypothesized that ultrasound stimulated the cholinergic anti-inflammatory pathway and reduced kidney IRI.12 In this study we further strengthened the evidence for the involvement of the splenic cholinergic anti-inflammatory pathway by showing the dependence of ultrasound protection on the splenic nerve. We believe that the splenic nerve is the proximal target of ultrasound treatment, a hypothesis supported by observations that ultrasound alters frog peripheral nerve activity.36 Transcranial ultrasound–induced neuromodulation in rodents induces limb movement,37 thereby further suggesting a biomechanical effect of ultrasound. Although we have not shown a direct effect of ultrasound on splenic nerve activity, which would be technically difficult in the mouse, we used 6-OHDA, which selectively destroys catecholaminergic nerve terminals peripherally15 or when injected directly in brain regions,38,39 to denervate the spleen and interrupt the cholinergic anti-inflammatory signaling pathway. Chemical sympathectomy by systemic administration of 6-OHDA (i.p.) produced an 84% decrease in splenic norepinephrine40 and has been used in numerous studies, contributing to what is now a large body of evidence demonstrating an interaction between the sympathetic nervous system and immune function.41–43 In our studies, splenic denervation via splenic injections of 6-OHDA completely abolished the protective effect of ultrasound in IRI, strongly suggesting that ultrasound reduces IRI in a catecholaminergic-dependent and presumably splenic nerve–dependent manner. The immune modulatory effect of splenic chemical sympathectomy produced by systemic administration of 6-OHDA, increased splenocyte proliferation and cytokine production, was blocked by pretreating animals with desipramine, a norepinephrine reuptake inhibitor that blocks uptake of 6-OHDA into nerve terminals, suggesting that the effects of 6-OHDA were mediated neuronally.44 However neither the desipramine experiment nor our studies can exclude the possibility that 6-OHDA also depletes catecholamines from splenocytes (although their contribution to total norepinephrine is likely to be substantially smaller than nerve terminals). To our knowledge, there is no evidence of a direct effect of 6-OHDA on immune cells.
α7 Nicotinic Receptors in Inflammation
Nicotinic acetylcholine receptors belong to a family of ligand-gated pentameric ion channels. Acetylcholine binding to α7nAChRs can induce cellular effects through activation of canonical ion channel and calcium channel fluxes, increased activation of PI3K and phospholipase C–induced intracellular calcium release, or phosphorylation of STAT3 through Jak2,45 which may mediate the anti-inflammatory effects of α7nAChR stimulation.46 Nicotine, a nicotinic cholinergic agonist, inhibits endotoxin- and TNFα-induced HMGB1 release by preventing activation of the NF-κB pathway.47 Nicotine reduced the production of Th1 (TNFα and IFN-γ) and Th17 cytokines (IL-17, IL-17F, IL-21, and IL-22) but increased IL-4 production, thereby inducing a shift from Th1 cytokines to Th2 cytokines.48
Activation of α7nAChRs to reduce inflammation may be mediated through hematopoietic (T and B lymphocytes, macrophages, neutrophils, or dendritic cells) or nonhematopoietic cells.45 Previously we showed using both pharmacologic blockade and genetically α7nAChR-deficient mice that ultrasound-induced protection from AKI is dependent on α7nAChRs.12 In this study we used bone marrow chimeras to isolate α7nAChR deficiency to either bone marrow–derived cells or parenchymal cells and further showed that hematopoietic, but not parenchymal cell, α7nAChRs are responsible for mediating the protective effect of ultrasound, thereby supporting the original hypothesis that the cholinergic anti-inflammatory pathway culminates with the activation of α7nAChRs on leukocytes.
Ultrasound, Splenocytes, and Cytokines in IRI
Although we have shown how deficiencies (pharmacologic blockade and α7nAChR-deficient mice) in the cholinergic anti-inflammatory pathway prevent the protective effect of ultrasound, there has been little evidence linking these splenic processes to a specific cell type. Furthermore, the details of how these splenic processes interact with the end organ are still unclear. Here, we show that adoptive transfer of splenocytes isolated from ultrasound-treated mice conferred protection from IRI in the recipient mice, suggesting that ultrasound produced changes in splenocyte phenotype. Our flow cytometry analysis revealed that this does not appear to be caused by differences in splenocyte composition because the proportions of Ly6C+, CD3+, CD4+, and CD11b+ cells were similar in spleens from control and ultrasound-treated mice. It will be important to define splenocyte subsets and function after ultrasound treatment in vivo.
As a first step in exploring functional changes, we found that splenocytes isolated from ultrasound-treated mice responded differently to inflammatory signaling and produced more IL-6 in response to LPS incubation ex vivo. Increased IL-6 may seem paradoxical given our results that ultrasound pretreatment reduced circulating levels and kidney mRNA expression of IL-6 after IRI and the prevailing view that IL-6 has a proinflammatory role in various injury models, including kidney IRI.49 For example, we showed previously that IL-6 increased in kidney monocyte/macrophage populations after IRI.50 In humans, systemic IL-6 levels predict survival in dialysis patients.51,52 However, these roles may not apply in all cases because IL-6 can be pro- or anti-inflammatory.17 Furthermore, the complexity of the kidney and spleen microenvironment after ultrasound and IRI differs markedly from culture conditions of splenocytes examined ex vivo. Although future studies will be needed to understand our findings, they clearly demonstrate a functional change in splenocytes in response to ultrasound and in conjunction with prior work, also showing increased production of IL-6 and TNFα by splenocytes previously cultured with nicotine, further support the involvement of the cholinergic pathway in the protective effect of ultrasound.52 This does not preclude the possibility that other mechanisms are involved in the protective effect of transferred splenocytes.
In summary, our studies reveal mechanistic insight on the spleen-dependent protective effect of ultrasound and demonstrate that the spleen is capable of antagonizing inflammation and tissue injury in kidney IRI. Ultrasound-induced protection requires hematopoietic α7nAChRs and sympathetic innervation of the spleen, consistent with stimulation of the cholinergic anti-inflammatory pathway. Adoptive transfer of splenocytes from mice previously exposed to ultrasound is sufficient to protect recipient mice from kidney IRI, suggesting that ultrasound alone alters splenocyte function through mechanisms not yet identified and requiring additional investigation. These studies broaden the therapeutic potential for ultrasound in ischemic and sepsis-induced kidney injury. Given the possibility of off-target side effects of pharmacologic agents, the nonpharmacologic approach using therapeutic ultrasound within the spectrum of use currently approved for humans provides an attractive alternative therapy for this devastating disorder.
Concise Methods
Mice and Reagents
Male mice (8–12 weeks of age) were used for all experiments. Wild-type C57/Bl6 mice were purchased from the National Cancer Institute (Frederick, MD), and the α7nAChR–/– mice (B6.129S7-Chrna7tm1Bay/J) were obtained from The Jackson Laboratory (Bar Harbor, ME). The 6-OHDA (Sigma-Aldrich, St. Louis, MO) was used for denervation studies.
Ultrasound Application
All experiments were performed in accordance with the National Institutes of Health and Institutional Animal Care and Use Guidelines. The Animal Care and Use Committee of the University of Virginia approved all procedures and protocols. For ultrasound exposure, mice were anesthetized with an i.p. injection of a mixture of ketamine (90 mg/kg), xylazine (9 mg/kg), and atropine (0.18 mg/kg). Fur was shaved and removed using a depilatory. Mice were then placed on a modified microscope stage, which was positioned under an ultrasound transducer held in place with a ring clamp. Prewarmed ultrasound gel was then placed on the depilated skin for ultrasound application. Mouse body temperature was monitored via rectal probe (Fine Science Tools, Foster City, CA) and maintained at 36±0.5°C with a heating pad and heat lamp.
A clinical Sequoia 512 ultrasound machine with a 15L8w transducer (Acuson, Malvern, PA) was used for ultrasound application. Once the animal’s body temperature was stabilized, the left kidney was localized in real time using conventional B-mode imaging with a frequency of 14 MHz and an on-screen imaging mechanical index of 0.99. Cadence imaging began with a frequency of 7 MHz and an imaging mechanical index of 0.16. The ultrasound treatment consisted of ultrasound pulses administered with a bursting mechanical index of 1.2. Ultrasound pulses were 1 second in duration and were applied once every 6 seconds for 2 minutes (20 seconds of exposure in total per kidney). After 2 minutes, conventional B-mode imaging was used to localize the right kidney for ultrasound pulse application. Control animals underwent the same preparation procedures but were not exposed to ultrasound. Total ultrasound exposure was approximately 5 minutes, with small variations in the time required to stabilize body temperature and localize the kidneys. After ultrasound treatment, animals were allowed to recover from the anesthetic in a temperature-controlled incubator.
Surgical Procedures
IRI
Mice underwent renal IRI 24 hours after ultrasound or sham ultrasound treatments. Mice were anesthetized with an i.p. injection of ketamine (120 mg/kg), xylazine (12 mg/kg), and atropine (0.324 mg/kg). Bilateral renal IRI was performed through flank incisions by clamping the renal pedicles for 26 minutes. Severity of IRI was modulated by reducing the ischemic time from 26 (IRI) to 24 minutes (subIRI) in the splenectomy experiments only. The clamps were then removed, and the wound was sutured after restoration of blood flow to kidney (reperfusion) was visually observed. Sham-operated mice underwent the same procedure except that the renal pedicles were not clamped. For splenectomy, mice were anesthetized with an i.p. injection of ketamine (120 mg/kg), xylazine (12 mg/kg), and atropine (0.324 mg/kg). The splenic vessels were then ligated, and the spleen was removed through a small flank incision. Sham mice underwent the same procedure with the exception of splenic artery ligation and removal of the spleen. Chemical splenic denervation was performed by injecting 6-OHDA (60–120 µg, Sigma-Aldrich) or saline into the exteriorized spleen. Buprenorphine (0.15 mg/kg) was administered as a postoperative analgesic for both IRI and splenectomy. Sham and splenectomized mice were allowed to recover for 1 week before ultrasound and IRI procedures. With the exception of the survival studies, blood collection was performed 24 hours after reperfusion by retro-orbital puncture with light anesthetic, and mice were then euthanized by cervical dislocation.
CLP (Sepsis-Induced Kidney Injury)
We used the previously published modified CLP protocol with less severe sepsis for studying acute kidney injury.20,53 A longitudinal midline incision was made under anesthesia. The cecum was isolated and exteriorized by using blunt anatomic forceps, leaving the remainder of the small and large bowel within the peritoneal cavity. Before cecal perforation, the cecal contents were pushed gently toward the distal cecum, and at the time of cecal puncture, any trapped air or gas was gently aspirated. The cecum was perforated by two punctures side by side midway between the ligation and the tip of the cecum in a mesenteric-to-antimesenteric direction using a 25-gauge needle. After removing the needle, a small amount (droplet) of feces was extruded from both the mesenteric and antimesenteric penetration holes to ensure patency. In the sham-operated mice, the cecum was exposed, manipulated, and returned to the peritoneal cavity without being punctured. The cecum was relocated into the abdominal cavity, taking care to avoid spreading feces from the cecum onto the abdominal wall wound margins. After closing the abdominal wall and skin with sutures, prewarmed normal saline (0.5 ml/mouse) was injected subcutaneously. Mice were monitored every 6 hours.
Plasma Creatinine and Stereological Analysis of Tissue Morphology
Plasma was prepared by centrifuging heparinized blood at 7000 rpm for 5 minutes. Plasma creatinine (mg/dl) was determined via colorimetric analysis following the manufacturer’s protocol (Sigma-Aldrich). Kidneys were dissected, and the capsule was removed. A center transverse section was cut and placed in 4% paraformaldehyde/1.4% DL-lysine/0.2% sodium periodate in 0.1 M sodium phosphate buffer (pH 7.4) for 24 hours and then stored in 70% EtOH until paraffin embedding. Then 5 μm paraffin sections were cut and stained with hematoxylin and eosin.
The extent of acute tubular necrosis was assessed in an unbiased, systematic manner using design-based stereology to achieve statistically accurate random sampling of kidney sections and yielding the percentage of total area of the section occupied by injured tubules or fibrotic tissue. The investigator was blinded to the experimental identity of the sections. Sections were imaged using a Carl Zeiss Axio Imager Z1/Apotome Microscope fitted with motorized focus drives and motorized XYZ microscope stage and integrated to a workstation running Stereo Investigator software (MBF Bioscience, Williston, VT). The area fraction fractionator probe was used for stereological analysis of the fractional area of the section occupied by tubular necrosis. The following parameters were defined: counting frame (250×250 mm), sample grid (600×600 mm), and grid spacing (50 mm). These values were determined empirically such that adequate numbers of sample sites were visited and adequate numbers of markers (indicating injured tubules or extracellular deposition of collagen) were acquired, in keeping with accepted counting rules for stereology. Injured tubules were identified on the basis of the presence of cast formation, tubule dilation, and/or tubular epithelial denucleation.
Flow Cytometry and Immunofluorescence Microscopy
To determine splenocyte composition after ultrasound treatment, spleen single-cell suspensions were generated and stained with the following antibodies (clone, source): PE-Cy7–labeled anti-CD45 (30-F11; BioLegend, San Diego, CA), FITC-labeled anti-Ly6C (AL-21; BD Biosciences, San Jose, CA), Alexa-Fluor 647-labeled anti-CD3e (17A2; eBiosciences, San Diego, CA), V500-labeled anti-CD4 (RM4–5; BD Biosciences), and efluor 780-labeled anti-CD11b (M1/70; eBiosciences). Final antibody concentrations were 1 µg/ml. Live cell discrimination was performed by incubating cells with 7-AAD (50 ng/ml; Invitrogen) for 20 minutes. Flow cytometry data were acquired using BD FACSCalibur (BD Biosciences) with Cytek 8 color flow cytometry upgrade (Cytek Development, Fremont, CA) and analyzed with FlowJo software 9.0 (TreeStar, Ashland, OR).
For immunofluorescent localization of renal neutrophils, renal tissue was fixed in a 1% paraformaldehyde/1.4% DL-lysine/0.2% sodium periodate in 0.1 M sodium phosphate buffer solution (pH 7.4) for 24 hours, followed by 24–48 hour incubation in 30% sucrose solution for cryoprotection. Samples were then embedded in Tissue-TEK OCT embedding medium (Sakura Finetek, Torrance, CA) and stored at –80°C until sectioning. Then 5–10 µm thick sections were mounted, incubated with anti-CD16/CD32 to block Fc receptors; they were then stained with FITC-labeled anti-7/4 antibody (CL8993F, 7 μg/ml; CedarLane). Samples were covered with ProLong Gold antifade reagent with DAPI (Invitrogen) to label cell nuclei, coverslips were applied, and samples were visualized using a Carl Zeiss Axiovert 200M microscope with ApoTome imaging and AxioVision software (Carl Zeiss).
Bone Marrow Chimeras and Adoptive Transfer Studies
Chimeric mice were generated as described previously.54 Briefly, WT or α7nAChR–/– mice were lethally irradiated twice at 550 rad. Mice were then reconstituted with bone marrow (7–10×106) from either WT or α7nAChR–/– donor mice. Chimeric mice were housed in microisolators for 10 weeks before experimentation to allow complete reconstitution and were fed autoclaved food and water containing 5 mM sulfamethoxazole and 0.86 mM trimethoprim.
For the adoptive transfer of splenocytes, spleens were harvested from ultrasound- or sham-treated animals 24 hours after treatment. Single-cell suspensions were generated by passing whole spleen through 40 µm filters into 1% BSA/PBS solution. The cell pellet was collected by centrifugation and then treated with ACK red blood cell lysing buffer (Life Technologies). The samples were again centrifuged, the resulting cell pellet was diluted, and 2×105 or 1×107 cells were injected via tail vein 24 hours before IRI or transferred to tissue culture plates with RPMI, 10% FBS, 1% glutamine, 1% penicillin-streptomycin, and 1% β-mercaptoethanol for ex vivo stimulation.
Real-Time PCR and Cytokine Analysis
Renal mRNA was isolated after the ethanol-precipitation method, and RNA concentration was determined on the basis of spectrophometric determination of 260:280 ratio. cDNA was generated from the resultant tissue RNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) as described by the manufacturer. Resultant cDNA was then used to determine relative mRNA expression of IL-6, IL-1β, IL-10, MCP, MIP2 (CXCL2), CXCL1, Kim-1, and glyceraldehyde 3-phosphate dehydrogenase using the iTaq Universal SYBR Green Supermix (Bio-Rad). Primers used were as follows: IL-6 (fwd): ACG GCA AAT TCA ACG GCA CAG TCA, (rev): AAC GCA CTA GGT TTG CCG AGT AGA; CXCL1 (fwd): TGG CTG GGA TTC ACC TCA AGA ACA, (rev): TGT GGC TAT GAC TTC GGT TTG GGT; MCP1 (fwd): TCA CCT GCT GCT ACT CAT TCA CCA, (rev): TAC AGC TTC TTT GGG ACA CCT GCT; Kim-1 (fwd): ACA TAT CGT GGA ATC ACA ACG AC, (rev): ACT GCT CTT CTG ATA GGT GAC A; glyceraldehyde 3-phosphate dehydrogenase (fwd): ACG GCA AAT TCA ACG GCA CAG TCA, (rev): TGG GGG CAT CGG CAG AAG G; CXCL2/MIP2 (fwd): ACATCCCACCCACACAGTGAAAGA, (rev): TCCTTCCATGAAAGCCATCCGACT; IL1β (fwd): ATGACCTGTTCTTTGAAGTTGAC, (rev): GTGATACTGCCTGCCTGAAG; and IL-10: (fwd): TGCACTACCAAAGCCACAAAGCAG, (rev): TGCAGTTATTGTCTTCCCGGCTGT.
A panel of serum cytokines and chemokines from ultrasound and IRI experiments was assessed using Mouse Cytokine/Chemokine Magnetic Bead Multiplex Assay (EMD Millipore, MA) as described by the manufacturer. Plasma samples were analyzed as recommended by the manufacturer using a Luminex 100 IS system (UVA Flow Cytometry Core Facility). In the ex vivo stimulation studies, supernatant IL-6 was determined by ELISA following the manufacturer’s protocol (eBiosciences).
Statistical Analyses
All animal studies were conducted using a complete randomized design. Data were analyzed using one-way or two-way ANOVA, with a significant difference defined as P<0.05. Repeated experiments were analyzed as a randomized complete block design. Animals receiving sham treatments were included in the experiments for reference only and were not included in the statistical analysis. Means were compared by post hoc multiple comparison test (Tukey), and all values are presented as mean±SEM. The survival analysis was performed using log-rank Kaplan–Meier survival analysis. All statistical analyses were performed using SigmaPlot 11.0 software (Systat, Chicago, IL).
Disclosures
None.
Acknowledgments
This work was supported by the National Institutes of Health (grant nos. R01-DK085259, R01-DK062324, K01-DK091444 (A.B.), and T32-DK072922).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Okusa MD, Molitoris BA, Palevsky PM, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Faubel S, Kellum JA, Wald R, Chertow GM, Levin A, Waikar SS, Murray PT, Parikh CR, Shaw AD, Go AS, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu CY, Greene TH, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA: Design of clinical trials in acute kidney injury: a report from an NIDDK workshop--prevention trials. Clin J Am Soc Nephrol 7: 851–855, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Bonventre JV, Basile D, Liu KD, McKay D, Molitoris BA, Nath KA, Nickolas TL, Okusa MD, Palevsky PM, Schnellmann R, Rys-Sikora K, Kimmel PL, Star RA, Kidney Research National Dialogue (KRND) : AKI: A path forward. Clin J Am Soc Nephrol 8: 1606–1608, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosin DL, Okusa MD: Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol 22: 416–425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Hassoun HT, Santora R, Rabb H: Organ crosstalk: The role of the kidney. Curr Opin Crit Care 15: 481–487, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Faubel S: Acute kidney injury and multiple organ dysfunction syndrome. Minerva Urol Nefrol 61: 171–188, 2009 [PubMed] [Google Scholar]
- 7.Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS: Messengers without borders: Mediators of systemic inflammatory response in AKI. J Am Soc Nephrol 24: 529–536, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Andrés-Hernando A, Altmann C, Ahuja N, Lanaspa MA, Nemenoff R, He Z, Ishimoto T, Simpson PA, Weiser-Evans MC, Bacalja J, Faubel S: Splenectomy exacerbates lung injury after ischemic acute kidney injury in mice. Am J Physiol Renal Physiol 301: F907–F916, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leelahavanichkul A, Yasuda H, Doi K, Hu X, Zhou H, Yuen PS, Star RA: Methyl-2-acetamidoacrylate, an ethyl pyruvate analog, decreases sepsis-induced acute kidney injury in mice. Am J Physiol Renal Physiol 295: F1825–F1835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang H, Meng F, Li W, Tong L, Qiao H, Sun X: Splenectomy ameliorates acute multiple organ damage induced by liver warm ischemia reperfusion in rats. Surgery 141: 32–40, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Ajmo CT, Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR: The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res 86: 2227–2234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, Klibanov AL, Kalantari K, Rosin DL, Okusa MD: Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol 24: 1451–1460, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tracey KJ: Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117: 289–296, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ: Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A 105: 11008–11013, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brimijoin S, Molinoff PB: Effects of 6-hydroxydopamine on the activity of tyrosine hydroxylase and dopamine-β-hydroxylase in sympathetic ganglia of the rat. J Pharmacol Exp Ther 178: 417–424, 1971 [PubMed] [Google Scholar]
- 16.Sadis C, Teske G, Stokman G, Kubjak C, Claessen N, Moore F, Loi P, Diallo B, Barvais L, Goldman M, Florquin S, Le Moine A: Nicotine protects kidney from renal ischemia/reperfusion injury through the cholinergic anti-inflammatory pathway. PLoS One 2: e469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf J, Rose-John S, Garbers C: Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 70: 11–20, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Monneret G, Payen D: Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat Rev Immunol 13: 862–874, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deutschman CS, Tracey KJ: Sepsis: Current dogma and new perspectives. Immunity 40: 463–475, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Doi K, Leelahavanichkul A, Hu X, Sidransky KL, Zhou H, Qin Y, Eisner C, Schnermann J, Yuen PS, Star RA: Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome. Kidney Int 74: 1017–1025, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langenberg C, Bagshaw SM, May CN, Bellomo R: The histopathology of septic acute kidney injury: A systematic review. Crit Care 12: R38, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holly MK, Dear JW, Hu X, Schechter AN, Gladwin MT, Hewitt SM, Yuen PS, Star RA: Biomarker and drug-target discovery using proteomics in a new rat model of sepsis-induced acute renal failure. Kidney Int 70: 496–506, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinman L: Elaborate interactions between the immune and nervous systems. Nat Immunol 5: 575–581, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kox M, van Eijk LT, Zwaag J, van den Wildenberg J, Sweep FC, van der Hoeven JG, Pickkers P: Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc Natl Acad Sci U S A 111: 7379–7384, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matteoli G, Boeckxstaens GE: The vagal innervation of the gut and immune homeostasis. Gut 62: 1214–1222, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costes LM, Boeckxstaens GE, de Jonge WJ, Cailotto C: Neural networks in intestinal immunoregulation. Organogenesis 9: 216–223, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jänig W, Green PG: Acute inflammation in the joint: Its control by the sympathetic nervous system and by neuroendocrine systems. Auton Neurosci 182: 42–54, 2014 [DOI] [PubMed] [Google Scholar]
- 28.van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ: Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun 62: 2046–2050, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegmund B, Eigler A, Hartmann G, Hacker U, Endres S: Adrenaline enhances LPS-induced IL-10 synthesis: Evidence for protein kinase A-mediated pathway. Int J Immunopharmacol 20: 57–69, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Andersson U, Tracey KJ: Neural reflexes in inflammation and immunity. J Exp Med 209: 1057–1068, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandon KW, Rand MJ: Acetylcholine and the sympathetic innervation of the spleen. J Physiol 157: 18–32, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leaders FE, Dayrit C: The cholinergic component in the sympathetic innervation to the spleen. J Pharmacol Exp Ther 147: 145–152, 1965 [PubMed] [Google Scholar]
- 33.Reardon C, Duncan GS, Brüstle A, Brenner D, Tusche MW, Olofsson PS, Rosas-Ballina M, Tracey KJ, Mak TW: Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc Natl Acad Sci U S A 110: 1410–1415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ: Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334: 98–101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straub RH: Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol Sci 25: 640–646, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Tsui PH, Wang SH, Huang CC: In vitro effects of ultrasound with different energies on the conduction properties of neural tissue. Ultrasonics 43: 560–565, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Mehić E, Xu JM, Caler CJ, Coulson NK, Moritz CT, Mourad PD: Increased anatomical specificity of neuromodulation via modulated focused ultrasound. PLoS One 9: e86939, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ungerstedt U: 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol 5: 107–110, 1968 [DOI] [PubMed] [Google Scholar]
- 39.Kostrzewa RM, Jacobowitz DM: Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev 26: 199–288, 1974 [PubMed] [Google Scholar]
- 40.Wirth T, Westendorf AM, Bloemker D, Wildmann J, Engler H, Mollerus S, Wadwa M, Schäfer MK, Schedlowski M, del Rey A: The sympathetic nervous system modulates CD4(+)Foxp3(+) regulatory T cells via noradrenaline-dependent apoptosis in a murine model of lymphoproliferative disease. Brain Behav Immun 38: 100–110, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Bellinger DL, Lorton D: Autonomic regulation of cellular immune function. Auton Neurosci, 182: 15–41, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Bhowmick S, Singh A, Flavell RA, Clark RB, O’Rourke J, Cone RE: The sympathetic nervous system modulates CD4(+)FoxP3(+) regulatory T cells via a TGF-beta-dependent mechanism. J Leukoc Biol 86: 1275–1283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grebe KM, Hickman HD, Irvine KR, Takeda K, Bennink JR, Yewdell JW: Sympathetic nervous system control of anti-influenza CD8+ T cell responses. Proc Natl Acad Sci U S A 106: 5300–5305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kruszewska B, Felten SY, Moynihan JA: Alterations in cytokine and antibody production following chemical sympathectomy in two strains of mice. J Immunol 155: 4613–4620, 1995 [PubMed] [Google Scholar]
- 45.de Jonge WJ, Ulloa L: The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 151: 915–929, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiarandini DJ, Stefani E, Gerschenfeld HM: Ionic mechanisms of cholinergic excitation in molluscan neurons. Science 156: 1597–1599, 1967 [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L: Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 10: 1216–1221, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Nizri E, Irony-Tur-Sinai M, Lory O, Orr-Urtreger A, Lavi E, Brenner T: Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol 183: 6681–6688, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, Jeyarajah DR, Zhou XJ, Zhou H, Chiquett B, Nagami GT, Lu CY: Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol 16: 3315–3325, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr, Lobo PI, Okusa MD: The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526–1537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W, He J, Zhang F, Huang C, Wu Y, Han Y, Zhang W, Zhao Y: Prognostic role of C-reactive protein and interleukin-6 in dialysis patients: A systematic review and meta-analysis. J Nephrol 26: 243–253, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Cho Y, Johnson DW, Vesey DA, Hawley CM, Pascoe EM, Clarke M, Topley N: Baseline serum interleukin-6 predicts cardiovascular events in incident peritoneal dialysis patients [published online ahead of print April 7, 2014]. Perit Dial Int 10.3747/pdi.2013.00272 [DOI] [PMC free article] [PubMed]
- 53.Leelahavanichkul A, Huang Y, Hu X, Zhou H, Tsuji T, Chen R, Kopp JB, Schnermann J, Yuen PS, Star RA: Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing high mobility group box protein-1. Kidney Int 80: 1198–1211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Day Y-J, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD: Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest 112: 883–891, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]