Abstract
Encapsulating peritoneal sclerosis (EPS) is a rare but severe complication of peritoneal dialysis (PD) characterized by extensive fibrosis of the peritoneum. Changes in peritoneal water transport may precede EPS, but the mechanisms and potential predictive value of that transport defect are unknown. Among 234 patients with ESRD who initiated PD at our institution over a 20-year period, 7 subsequently developed EPS. We evaluated changes in peritoneal transport over time on PD in these 7 patients and in 28 matched controls using 3.86% glucose peritoneal equilibration tests. Compared with long-term PD controls, patients with EPS showed early loss of ultrafiltration capacity and sodium sieving before the onset of overt EPS. Multivariate analysis revealed that loss of sodium sieving was the most powerful predictor of EPS. Compared with long-term PD control and uremic peritoneum, EPS peritoneum showed thicker submesothelial fibrosis, with increased collagen density and a greater amount of thick collagen fibers. Reduced osmotic conductance strongly correlated with the degree of peritoneal fibrosis, but not with vasculopathy. Peritoneal fibrosis was paralleled by an excessive upregulation of vascular endothelial growth factor and endothelial nitric oxide synthase, but the expression of endothelial aquaporin-1 water channels was unaltered. Our findings suggest that an early and disproportionate reduction in osmotic conductance during the course of PD is an independent predictor of EPS. This functional change is linked to specific alterations of the collagen matrix in the peritoneal membrane of patients with EPS, thereby validating the serial three-pore membrane/fiber matrix and distributed models of peritoneal transport.
Keywords: peritoneal dialysis, water channels, ultrafiltration, vascular endothelial growth factor
Peritoneal dialysis (PD) is a technique of RRT used by approximately 200,000 patients with ESRD worldwide, a prevalence that is rapidly growing in many parts of the world.1,2 Functional alterations of the peritoneal membrane constitute an obstacle to long-term PD, because they are associated with technical failure and increased morbidity and mortality.3–5 Prolonged exposure of the peritoneal membrane to nonphysiologic dialysis solutions leads to vascular proliferation, vasculopathy, and peritoneal fibrosis, with ensuing loss of ultrafiltration (UF) capacity resulting from enlarged vascular surface area, faster peritoneal solute transport rate, and early dissipation of the osmotic gradient.3,4,6–9
Encapsulating peritoneal sclerosis (EPS) is an exaggerated fibrogenic response of the peritoneal membrane, leading to encapsulation of the bowels and intestinal obstruction. This entity constitutes the most severe complication of PD (for review, see Korte et al.10). Although EPS affects a minority (0.9%–3.5%) of patients on long-term PD, it has a mortality rate reaching 50%, resulting from intestinal occlusion, malnutrition, and sepsis.10 EPS is probably a multifactorial disease, in which PD duration represents an important risk factor.11–13 Other conditions that have been inconsistently associated with EPS include cumulative glucose exposure, younger age, peritonitis episodes, β-blockers, and kidney transplantation.11–13 Surprisingly, symptoms of EPS occur after PD withdrawal in most cases.11–13
There are currently no definitive criteria that enable the detection of early stages of EPS. Arguably, a better understanding of the early phase of the disease may help in identifying patients at risk and developing prevention strategies. Preliminary studies suggested that a progressive loss of UF capacity often precedes EPS development.14,15 However, these studies were limited by small numbers, the absence of appropriate matching with long-term PD controls, and/or a lack of evaluation of free-water transport. In addition, the mechanisms of impaired water transport in patients with EPS have not been investigated and remain unelucidated.
In this study, we took advantage of a large cohort of incident PD patients who underwent a systematic functional evaluation of the peritoneal membrane at baseline and during the course of PD, and of a unique collection of peritoneal biopsies to evaluate the specific changes in transport parameters and molecular components of the peritoneal membrane of patients with EPS versus appropriate controls. For the first time, we provide evidence linking the development of a specific fibrotic reaction with an early loss of water transport in the peritoneal membrane of patients with EPS.
Results
Early Loss in UF and Sodium Sieving Predicts EPS
We first assessed the evolution of peritoneal transport parameters in PD patients who subsequently developed EPS, compared with PD controls without EPS, matched in a 1:4 ratio for PD duration and sex. Peritoneal function was assessed yearly, with a modified 3.86% glucose-based peritoneal equilibration test (PET). We identified 7 cases of EPS among the 234 ESRD patients who initiated PD at our institution between January 1, 1994 and December 31, 2013, representing an overall incidence of 3.0%. Demographic and clinical features of patients with EPS are presented in Table 1; mean age at PD onset was 47.5±3.6 years, the sex ratio was 5 men/2 women, and mean PD exposure was 57.8±7.6 months. EPS occurred after PD withdrawal in 5 of 7 patients.
Table 1.
Demographic and clinical characteristics of patients with EPS
| Age at PD Onset (yr) | Sex | Renal Disease | PD Duration (mo) | RRT after PD | Time from PD Withdrawal to EPS (mo) | Recurrent Bowel Occlusion | Symptoms at Diagnosis | CT Diagnosis | Surgical Diagnosis | Outcome after EPS | Time from EPS Diagnosis to Death (mo) | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 52 | M | MPGN | 102 | TP | 1.8 | + | FNVP | + | + | Death | 82 | Bowel occlusion |
| 57 | M | Diabetic | 60 | HD (UFF) | 25.0 | + | FNVP, bloody ascites | + | NA | Death | 6.6 | Septic shock (peripheral arteritis) |
| 39 | M | Renal dysplasia | 59 | HD (UFF) | 2.8 | + | FNVP bloody ascites | + | + | Death | 36.4 | Septic shock (endocarditis) |
| 62 | M | Vascular | 55 | TP | 2.5 | + | FNVP | + | + | Alive, functioning transplant | NA | NA |
| 34 | F | Undetermined | 39 | HD (UFF and EPS) | 0.0 | + | NVP | + | + | Death | 23.5 | Bowel perforation |
| 45 | F | GN | 34 | HD (umbilical hernia) | 49.6 | + | NVP | + | + | Death | 3.0 | Undetermined |
| 42 | M | Undetermined | 57 | TP (UFF and EPS) | −2.8 | + | NVP | + | NA | Alive, functioning transplant | NA | NA |
M, male; F, female; MPGN, membranoproliferative GN; TP, transplantation; HD, hemodialysis; UFF, ultrafiltration failure; +, positive; F, fever; N, nausea; V, vomiting; P, pain; CT, computed tomography; −, negative; NA, not applicable.
The comparison of baseline demographic and clinical data between cases and controls is shown in Table 2. The matching for PD duration and sex was effective. The net UF, sodium sieving, and small solute transport parameters at baseline were similar in both groups. There were no significant differences in terms of PD modality, glucose exposure, and peritonitis rates.
Table 2.
Clinical, functional, and treatment characteristics of patients with EPS and long-term PD controls
| Characteristic | Patients with EPS (n=7) | Controls (n=28) | P Value |
|---|---|---|---|
| Men | 71 | 71 | 1.00 |
| PD duration, mo | 57.8±7.6 | 55.9±4.2 | 0.84 |
| Age at PD start, yr | 47.5±3.6 | 56.9±3.4 | 0.09 |
| Underlying nephropathy | 0.24 | ||
| GN | 43 | 32 | |
| Vascular nephropathy | 14 | 21 | |
| Chronic pyelonephritis | 0 | 21 | |
| Genetic disease | 14 | 21 | |
| Other or undetermined | 29 | 4 | |
| Charlson comorbidity index | 4.9±1.0 | 4.6±0.4 | 0.85 |
| Diabetes, n | 2 | 4 | 0.17 |
| Residual diuresis, ml/d | 1083±159 | 1021±130 | 0.78 |
| Body mass index, kg/m2 | 22.9±1.1 | 22.3±0.6 | 0.67 |
| Medications | |||
| ACE inhibitors and/or ARBs | 57 | 63 | 0.78 |
| Statins | 43 | 29 | 0.10 |
| β-blockers | 29 | 14 | 0.58 |
| Baseline adequacy | |||
| Peritoneal Kt/V | 1.51±0.22 | 1.46±0.07 | 0.84 |
| Residual Kt/V | 0.97±0.16 | 0.80±0.09 | 0.42 |
| Total Kt/V | 2.48±0.15 | 2.23±0.09 | 0.20 |
| Baseline functional parameters | |||
| Net UF, ml | 743±124 | 648±57 | 0.54 |
| D/P creatinine, 4 h | 0.75±0.05 | 0.74±0.05 | 0.96 |
| Sodium sieving | 0.05±0.01 | 0.05±0.01 | 0.44 |
| PD modality, % APD | 100 | 68 | 0.11 |
| Glucose and icodextrin exposure | |||
| Mean annual glucose exposure, kg | 122±9 | 110±7 | 0.20 |
| Mean annual icodextrin exposure, kg | 51±4 | 47±3 | 0.43 |
| Peritonitis | |||
| Patients with peritonitis | 71 | 71 | 1.00 |
| No. of peritonitis per patient | 1.4±0.4 | 1.3±0.2 | 0.84 |
| Peritonitis rate, patient−1×year−1 | 0.36±0.11 | 0.30±0.05 | 0.66 |
| Patients with GNR peritonitis | 14 | 43 | 0.12 |
Data are presented as the mean±SEM or percentage, unless otherwise indicated. ACE, angiotensin converting enzyme; ARB, angiotensin 2 receptor antagonist; D/P, dialysate-over-plasma; APD, automated peritoneal dialysis; GNR, Gram-negative rod.
The longitudinal evolution of peritoneal transport parameters is shown in Figure 1. A total of 147 PETs were analyzed, and the mean number of PETs per patient was 4.3±0.5 and 4.0±0.3 in the EPS and control groups, respectively. Patients who subsequently developed EPS showed a progressive decline in water transport, with significantly lower net UF values during the last 2 years on PD compared with matched controls (Figure 1A). This loss of UF capacity was associated with an increased solute transport (Figure 1B). Importantly, increased solute transport did not fully account for the disproportionate loss of water transport, suggesting an uncoupling between water and solute transport in the EPS peritoneal membrane (Figure 1C). In parallel, sodium sieving—a reliable indicator of free-water transport and osmotic conductance—progressively decreased in the EPS group with time on PD, and this decline was faster in EPS than in controls (annual loss in sodium sieving −0.011±0.001 versus −0.003±0.001 in EPS versus control patients, respectively; P=0.01) (Figure 1, D and E). The reduced osmotic conductance observed in patients with EPS during the last 2 years on PD was associated with higher glucose exposure and a trend toward better preserved residual renal function in the same period compared with PD controls (Supplemental Figure 1).
Figure 1.
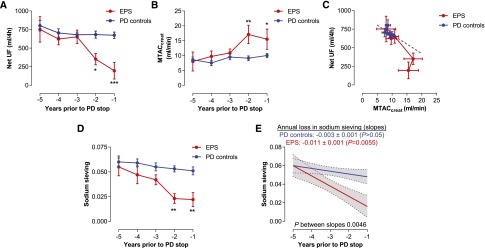
Early loss in UF capacity and sodium sieving in patients with EPS. (A) Patients who will develop EPS (red) have a premature loss in UF capacity, approximately 2 years before PD withdrawal, compared with PD controls (blue) matched in a 4:1 ratio for PD duration and sex. Control patients show a trend to lower UF with time on PD. (B) Small solute transport, evaluated by MTAC creatinine, tends to increase in PD controls but is significantly higher with time on PD in patients with EPS. (C) The loss of UF capacity is disproportionate to the increased MTAC creatinine in patients with EPS, suggestive of an uncoupling between peritoneal fluid and solute transport. The blue line corresponds to the projection of linear regression of net UF in control patients. (D) The sodium sieving significantly decreases with time on PD in patients with EPS, supporting the development of a low osmotic conductance to glucose. (E) The decline in sodium sieving over time on PD is more severe in patients with EPS compared with controls (slope −0.011±0.001 versus −0.003±0.001 in patients with EPS and controls, respectively; P=0.01). Longitudinal follow-up of membrane function was performed with annual modified 3.86% glucose-based dialysate. Data are presented as the mean±SEM (A–D) or mean±95% confidence interval (E). n=7 patients with EPS versus n=28 PD duration–matched and sex-matched controls. *P<0.05; **P<0.01; ***P<0.001. MTAC, mass transfer area coefficient.
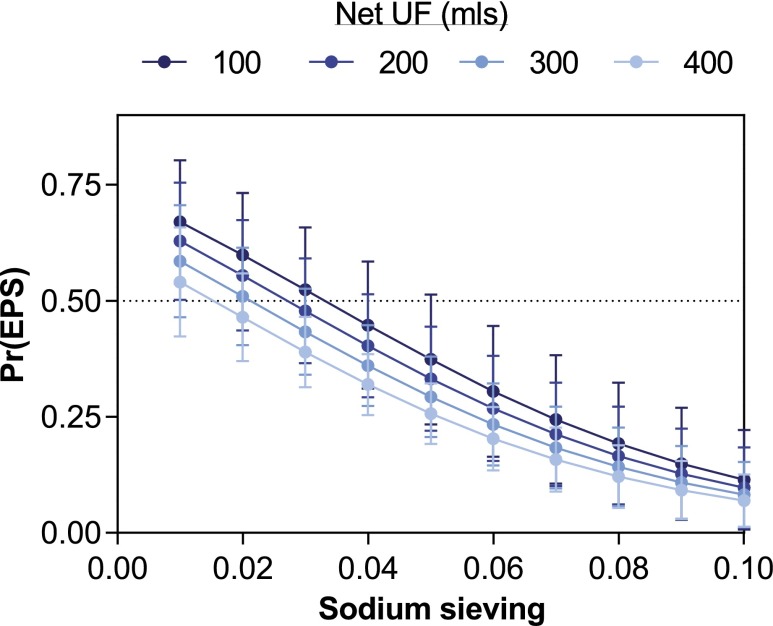
We next performed a multivariate analysis to test the predictive value of low sodium sieving values during the course of PD for subsequent development of EPS (Table 3). In this cohort, a first model (without considering sodium sieving values) identified younger age at dialysis onset, longer PD duration, lower residual kidney function, β-blocker use, and peritonitis rate as risk factors associated with EPS (Table 3). Among all potential risk factors integrated in this model, glucose exposure was the only variable that was not independently associated with the disease. Adding sodium sieving to the model showed that impaired free-water transport is a powerful and independent risk factor associated with EPS, outperforming other variables (Table 3). Logistic regression analysis showed a gradual increase in the risk of EPS with declining sodium sieving during the course of PD. In this cohort of long-term PD patients, the risk of EPS is negligible for patients with preserved UF capacity and sodium sieving during the whole course of PD, but increases >25% for sodium sieving values ≤0.04, and to >50% for values ≤0.02. This risk is even greater for patients developing UF failure during the course of PD (Figure 2).
Table 3.
Multivariate analysis performed using a logistic regression random-effects model and including potential risk factors for EPS, alone (model 1) or in association with the mean value of sodium sieving during the 2 last years of PD (model 2)
| Characteristic | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Coefficient (95% Confidence Interval) | P Value | Coefficient (95% Confidence Interval) | P Value | |
| Sex | 11.5 (−13.1 to 36.0) | 0.36 | 2.4 (−1.8 to 6.7) | 0.27 |
| Age at PD start | −1.15 (−1.7 to −0.6) | <0.001 | −0.1 (−0.2 to 0.05) | 0.21 |
| PD duration | 0.6 (0.2 to 1.0) | 0.003 | 7.10−4 (−0.07 to 0.08) | 0.99 |
| Residual Kt/V | 33.6 (11.2 to 56.0) | 0.003 | −0.3 (−4.45 to 3.8) | 0.87 |
| β-blocker use | 53.1 (27.7 to 78.5) | <0.001 | 4.2 (−1.1 to 9.5) | 0.12 |
| Peritonitis rate | 20.8 (7.7 to 33.9) | 0.002 | 0.4 (−2.8 to 3.6) | 0.24 |
| Annual glucose exposure | −9.4×10−5 (−3.1×10−4 to 1.2×10−4) | 0.39 | 1.0×10−5 (−3.5×10−5 to 5.8×10−5) | 0.46 |
| Sodium sieving | −186.7 (−340.4 to −33.0) | 0.02 | ||
Figure 2.
Low sodium sieving and net ultrafiltration during PD are predictive of the risk of EPS. The risk of EPS, estimated by logistic regression analysis, progressively increases with lower values of sodium sieving and impaired UF capacity. In this cohort of long-term PD patients, the combination of UF failure (<400 ml during the 4-hour 3.86% modified PET) with a sodium sieving <0.03 is associated with a risk of EPS >50%. All values of sodium sieving and net UF obtained during the course of PD are included in the logistic regression analysis. n=7 patients with EPS versus n=28 long-term PD controls. Pr(EPS), probability of developing EPS.
Altogether, these analyses demonstrate that UF capacity is impaired in patients with EPS before disease onset, resulting from an uncoupling with solute transport. Furthermore, there is a significant decline in sodium sieving in patients with EPS compared with matched long-term PD controls. Multivariate analysis demonstrated that impairment in osmotic conductance to glucose during the course of PD is the most powerful indicator for the development of this rare complication and can help in identifying patients at risk.
Expression of Aquaporin 1, Endothelial Nitric Oxide Synthase, and Vascular Endothelial Growth Factor in the EPS Peritoneum
Because aquaporin 1 (AQP1) water channels mediate free-water transport and sodium sieving during PD,16 we evaluated their expression in the peritoneal membrane of patients with EPS and long-term PD controls (Figure 3). Clinical and functional characteristics of patients with EPS and PD controls from whom peritoneal biopsies were analyzed are provided in Supplemental Table 1. The duration of PD exposure (62.4±9.3 and 62.8±9.2 months in controls and patients with EPS, respectively; P=0.98) and the number of peritonitis episodes (1.5±0.4 and 1.4±0.4 in EPS and long-term PD, respectively; P=0.91) were similar in both groups. Parameters of peritoneal water and solute transport were comparable to those of the case-control study cohort.
Figure 3.
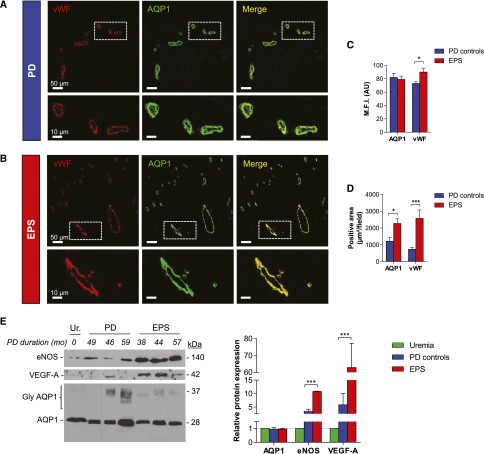
Expression of AQP1 in the peritoneum of patients with EPS. (A and B) Representative pictures of double immunostaining with anti-AQP1 (green channel) and anti-vWf (red channel) antibodies viewed under confocal fluorescence microscopy in peritoneal sections from a patient with EPS (A) and a long-term PD control (B). AQP1 is expressed in endothelial cells lining the capillaries and small vessels in the peritoneal membrane, both in the patient with EPS with impaired water transport (net UF=190 ml, and sodium sieving=0.02, during a 4-hour modified 3.86% glucose base PET) and in the long-term PD control (net UF 675 ml and sodium sieving 0.04). (C) MFI in AQP1-positive and vWf-positive areas in peritoneal sections of PD controls (blue) and patients with EPS (red). In contrast with the stable fluorescent intensity of AQP1, the endothelial marker vWf is significantly upregulated in peritoneal vessels from patients with EPS compared with PD controls. (D) Increase in both AQP1-positive and vWf-positive areas in the peritoneal membrane of patients with EPS compared with controls, suggesting vascular proliferation. n=7 long-term PD controls, and n=7 patients with EPS. (E) Representative immunoblot images and quantitative data showing levels of eNOS, VEGF-A, and AQP1 in the peritoneum of three patients with EPS, three PD duration–matched patients, and one uremic patient. PD controls and patients with EPS show a significant upregulation of eNOS (3- and 11-fold, respectively) and VEGF-A (6- and 63-fold, respectively) in their peritoneal membrane, whereas the expression of AQP1 remains unchanged. Twenty micrograms of proteins are loaded in each lane. Molecular mass (in kilodaltons) is indicated on the right side of the blot. PD duration (months) is indicated in italics. MFI, mean fluorescence intensity; A.U., arbitrary units; gly AQP1 and AQP1, glycosylated and unglycosylated isoforms of AQP1. *P<0.05; ***P<0.001. Bar, 50 µm in A and B (low magnification); 10 µm in A and B (detail). Original magnification, ×20.
Confocal immunofluorescence microscopy showed similar patterns of expression and density of AQP1 in the endothelium lining peritoneal capillaries in patients with EPS and controls (mean fluorescence intensity in AQP1-positive areas: 79±5 arbitrary units [AU] versus 82±6 AU in EPS and PD controls, respectively; P=0.71) (Figure 3, A–C). By contrast, the expression of the vWf, used as endothelial marker, was significantly upregulated in samples from patients with EPS compared with controls (mean fluorescence intensity in vWf-positive areas: 90±5 AU versus 73±3 AU in patients with EPS and PD controls, respectively; P=0.01) (Figure 3, A–C). vWf-stained area was also significantly increased in the peritoneal membrane of patients with EPS compared with PD controls (P=0.01), together with a parallel increase in AQP1-positive area (Figure 3D).
Immunoblotting (Figure 3E) confirmed a virtually unchanged expression of AQP1 in the peritoneal membrane of patients with EPS, contrasting with the increased expression of the endothelial nitric oxide synthase (eNOS) compared with PD duration–matched controls and uremic patients (relative AQP1 expression: 0.96±0.06, 0.94±0.12, and 1.00 [reference value], respectively; P=0.99; and relative eNOS expression: 10.8±0.1, 3.3±0.9, and 1.0 [reference value], in the peritoneal membrane of EPS, PD, and uremic patients, respectively; P<0.001) (Figure 3E). The upregulation of eNOS in the peritoneum of patients with EPS was paralleled by an increased expression of the vascular endothelial growth factor (VEGF)-A (relative VEGF-A expression 62.8±14.4, 5.8±4.2, and 1.0 [reference value] in EPS, PD, and uremic peritoneum, respectively; P=0.003) (Figure 3E).
Overall, these data demonstrate that the impaired UF capacity and free-water transport observed in patients with EPS was not associated with an alteration in the expression of the AQP1 water channels, but was associated with a significant upregulation of eNOS and VEGF.
The Peritoneal Membrane of Patients with EPS Undergoes Severe Angiogenesis and Submesothelial Fibrosis
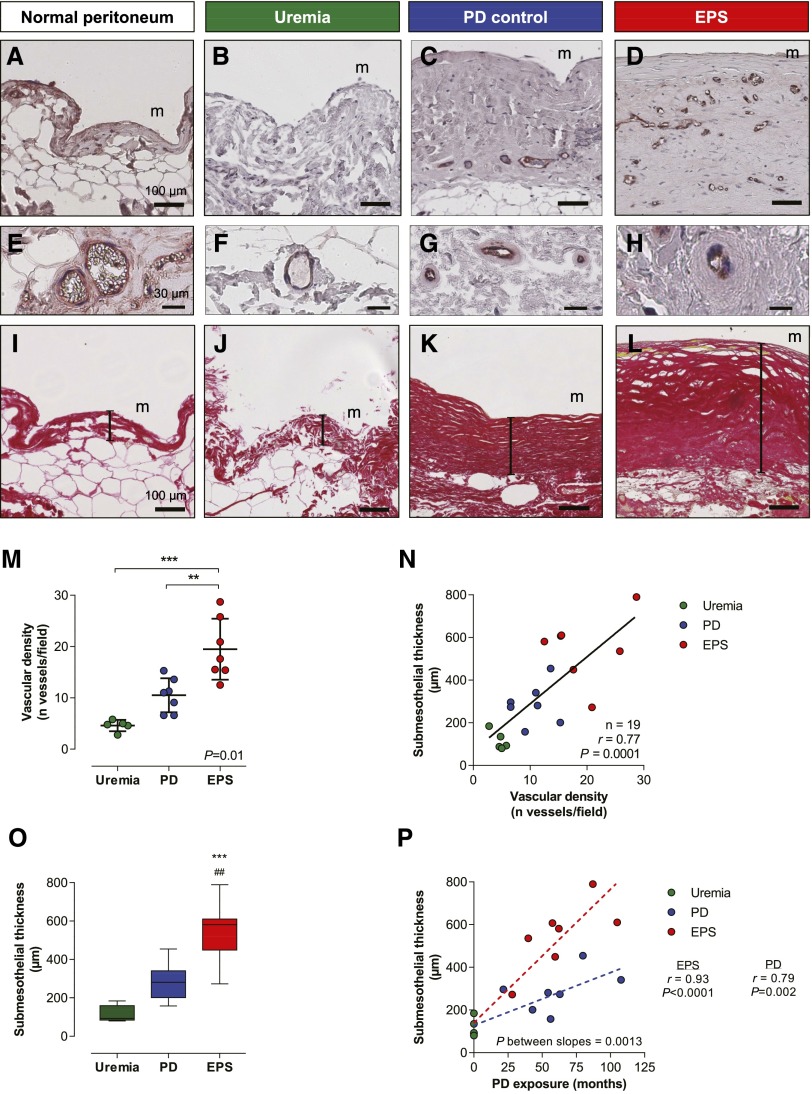
Because both eNOS and VEGF promote angiogenesis and vascular permeability,17 participating to fibrinogen leakage and stroma generation, we next evaluated the degree of angiogenesis and fibrosis in the peritoneal membrane of uremic, long-term PD, and EPS patients (Figure 4). Vascular density and submesothelial thickness were evaluated by light microscopy, in vWf– or picrosirius red–stained peritoneal sections, respectively, and quantified in each group. Compared with patients with uremia (Figure 4, B, F, and J), exposure to PD led to vascular proliferation and peritoneal fibrosis in the submesothelial area (Figure 4, C, G, and K), both processes progressing in parallel in the membrane (Figure 4, M–O). For a same PD duration, patients with EPS had increased vascular densities and submesothelial thickness compared with controls (vascular density: 19.5±2.1 versus 10.5±1.2 vessels/field, P<0.01; submesothelial thickness: 549±56 versus 287±34 µm in patients with EPS versus PD controls, respectively; P<0.001), with slopes of fibrosis progression that were significantly higher in the former group (Figure 4P; P=0.001). Signs of vasculopathy also increased with time on PD but to a same extent in patients with and without EPS (lumen-to-vessel ratio of postcapillary venules decreases from 0.72±0.03 in uremic patients, to 0.51±0.07 and 0.45±0.06 in PD controls and patients with EPS patients; P=0.57) (Figure 4, E–H, Supplemental Figure 2).
Figure 4.
The EPS peritoneum is characterized by an excessive vascular and fibrotic response to PD. (A–D) Immunostaining for vWf in normal (A), uremic (B), control PD (C), and EPS (D) peritoneum shows a progressive vascular proliferation from A to D. (E–H) Representative sections of peritoneum with evaluation of the degree of vasculopathy in the postcapillary venules stained for vWf in normal (E), uremic (F), control PD (G), and EPS (H) peritoneum. Long-term exposure to PD and EPS both associate with a thickening of the capillary wall in venules with a 25- to 50-µm diameter. (I–L) Representative sections of parietal peritoneum from normal (I), uremic (J), control PD (K), and EPS (L) peritoneum stained with picrosirius red. Submesothelial thickness, represented by the vertical bar drawn between the mesothelial surface and the upper limit of the adipose tissue, significantly increases from I to L. (M–O) Peritoneal vascular proliferation and fibrosis are more severe in patients with EPS compared with PD controls matched for the same PD duration. (P) Submesothelial fibrosis correlates with time on PD in both groups, but correlation is clearly steeper in patients with EPS compared with PD patients with no EPS. Peritoneal samples are from five uremic patients, seven long-term PD patients without EPS, and seven patients with EPS. Circles represent individuals (M, N, and P; green circles for uremic, blue for PD controls, and red for patients with EPS). Box and whiskers (minimum to maximum) are represented in C. ##P<0.01 versus PD; **P<0.01; ***P<0.001 versus uremia. m, mesothelium. Bar, 100 µm in A–D and I–L. Original magnification, ×20 in A–D and I–L (top); ×40 in A–D and I–L (bottom).
These data demonstrate that patients with EPS are characterized by an excessive angiogenic and fibrotic response to PD exposure in the peritoneal membrane, causing severe peritoneal remodeling and scarring.
Specific Changes in Collagen Density and Quality in the Peritoneum of Patients with EPS
To test whether EPS was associated with qualitative changes in the peritoneal interstitium, we next assessed peritoneal collagen density and structure using picrosirius red polarization microscopy. This methodology allows the detection and structural analysis of collagen content based on its anisotropic molecular organization and birefringence under polarized light18,19 (Figure 5). These analyses revealed a 1.8- and 2.7-fold increase in the density of collagen fibers in the peritoneum of patients with EPS compared with control PD and uremic patients, respectively (collagen volume fraction: 40.9%±2.9%, 22.3%±1.5%, and 14.9%±0.7% in EPS, PD, and uremic patients, respectively; P<0.001) (Figure 5, A–D). The increased density was paralleled by a change in the structure of collagen bundles in the submesothelial area, with an accumulation of thicker, red-orange fibers in the EPS peritoneum, whereas the relative amount of thinner, green-yellow fibers remained unchanged (relative increase in red-positive area in the submesothelial area: 2.2±0.2, 1.5±0.2, and 1.0±0.1 [reference value], P=0.002; relative increase in green-positive area: 0.8±0.2, 1.0±0.1, and 1.0±0.05 [reference value], P=0.59, in the submesothelial area of EPS, PD, and uremic patients, respectively) (Figure 5, E–K). These data demonstrate specific qualitative changes in the fibrotic interstitium of patients with EPS, with increased collagen density and more abundant thick collagen fibers.
Figure 5.
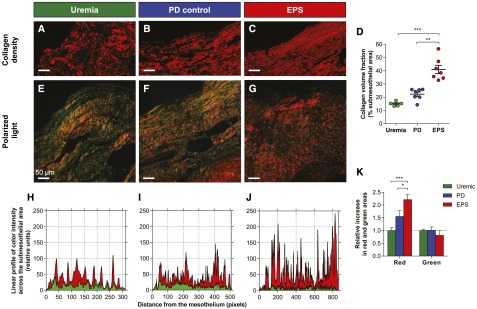
Changes in collagen density and quality in the peritoneal interstitium of patients with EPS. (A–C) Representative sections of the parietal peritoneum from uremic (A), PD (B), and EPS (C) patients stained with picrosirius red and visualized under circularly polarized light. Stained tissue (red mask) indicates collagen fibers in the submesothelial area. (D) Collagen volume fraction from picrosirius red–stained peritoneum as percentage of submesothelial area from uremic (green circles), PD (blue), and EPS (red) patients. (E–G) Representative sections of the peritoneum from uremic (E), PD (F), and EPS (G) patients stained with picrosirius red, visualized under circularly polarized light. Thick collagen fibers appear red-orange and thinner ones are green-yellow. (H–J) Profile of red and green intensities along a line perpendicular to the mesothelial surface, from the mesothelium to the adipose tissue in peritoneal biopsies from uremic (H), PD (I), and EPS (J) patients. The x axis represents the distance from the mesothelial surface, in pixels. (K) Relative increase in the red- and green-positive areas in the submesothelium of uremic (reference), PD, and EPS patients. The relative proportion of red, thicker, collagen fibers increases in the EPS submesothelial area compared with uremic and PD controls. Data are the mean±SEM. n=5, n=7, and n=7 in uremic, long-term PD, and EPS peritoneum, respectively. *P<0.05; **P<0.01; ***P<0.001. Bar, 50 µm. Original magnification, ×20.
Fibrotic Changes in the Peritoneum of Patients with EPS Associate with Impaired Water But Not Solute Transport
Finally, we tested whether the morphologic changes observed in the peritoneal membrane of patients with EPS are involved in the early loss of osmotic conductance to glucose that characterizes these patients. Indeed, the three-pore model suggests that an expanded fibrotic interstitium constitutes an additional barrier to the transport of water and solute between peritoneal capillaries and the dialysate.20 Accordingly, peritoneal remodeling could impair UF and free-water transport despite the fact that the capillary αc (reflecting the relative capillary density of AQP1) is kept unchanged.20 We therefore evaluated the correlation between water transport and structural changes in the peritoneal interstitium (Figure 6). Both net UF and sodium sieving were inversely closely correlated with submesothelial thickness and with collagen volume fraction in the submesothelial area (Figure 6, A, B, D, and E, Table 4). By contrast, peritoneal fibrosis was not associated with impaired peritoneal solute transport, estimated by the dialysate-over-plasma creatinine ratio (Figure 6C, Table 4) and by the glucose disappearance rate (data not shown). Taken together, these data give new insights into the mechanism of low osmotic glucose conductance and impaired UF capacity in EPS. They support the hypothesis that a fibrotic interstitium restricts water transport, in line with the serial three-pore membrane/fiber matrix model (Figure 6F).
Figure 6.
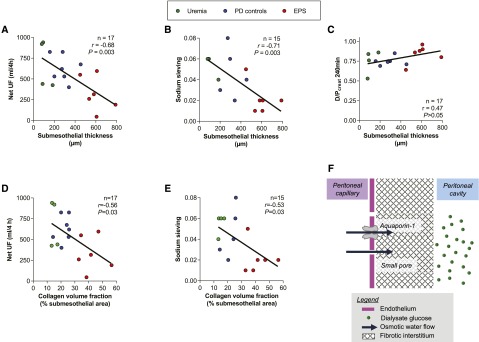
Fibrotic changes in the EPS peritoneum associate with impaired water but not solute transport. (A–C) Correlation between net UF (A), sodium sieving (B), and dialysate-over-plasma creatinine (D/P) ratio at 4 hours (C), and submesothelial thickness in PD patients. (D and E) Correlation between net UF (D) and sodium sieving (E), and collagen volume fraction in the submesothelial area. (A–E) Circles represent individuals; green circles for uremic patients, blue for PD patients, and red for EPS patients. The number of observations, r Pearson coefficients, and P values are shown in each panel. Peritoneal transport parameters are obtained using a modified 4-hour 3.86% glucose-based dialysate PET. (F) The serial three-pore membrane/fiber matrix model (adapted from 20). Interstitial fibrosis acts as a second resistance, in series with the capillary wall, and markedly reduces the UF coefficient of the membrane, contributing to the loss of sodium sieving and free-water transport, despite the fact that the capillary αc (i.e., AQP1 density) remains unchanged.
Table 4.
Relationship between parameters of peritoneal transport and structural changes in the peritoneum of uremic, long-term PD, and EPS patients
| Parameter | Pearson Coefficient (r) | P Value |
|---|---|---|
| Submesothelial thickness versus | ||
| Net UF | −0.68 | 0.003 |
| Sodium sieving | −0.71 | 0.003 |
| D/P creatinine 240 min | 0.47 | 0.06 |
| Collagen volume fraction in the submesothelial area versus | ||
| Net UF | −0.56 | 0.03 |
| Sodium sieving | −0.53 | 0.03 |
| D/P creatinine 240 min | 0.41 | 0.11 |
D/P, dialysate-over-plasma.
Discussion
In this study, we combine clinical, molecular, and modeling data to demonstrate that the reduction in osmotic conductance to glucose in PD patients is a predictive feature of EPS, and that reduced osmotic water transport is directly related to the degree of interstitial fibrosis and to changes in collagen content and structure in the EPS peritoneal membrane.
The availability of serial testing of peritoneal transport in a large cohort of incident PD patients allowed us to demonstrate that patients who will develop EPS show an early loss of UF capacity, due to a progressive impairment in osmotic conductance of the peritoneal membrane, compared with PD duration- and sex-matched long-term PD patients. The loss of UF capacity in patients predisposed to EPS is disproportionate to the increase in small solute transport, revealing an uncoupling between peritoneal water and solute transport. This uncoupling suggests that increased small solute transport with earlier absorption of dialysate glucose—usually seen as the main cause of UF failure—is not sufficient to explain impaired UF capacity in patients with EPS. These results are in line with those from an uncontrolled Dutch cohort showing that patients with EPS had a progressive decline in UF capacity over 4 years and an inverse U-shaped trend in small solute transport.21 The use of time-matched controls in a UK cohort showed a clear difference in UF capacity between patients with EPS and controls at least 2 years before PD withdrawal.14 However, the latter study, based on 2.27% glucose PET, could not assess free-water transport. By contrast, we systematically used 3.86% glucose, which provides a more accurate evaluation of UF capacity, as well as sodium sieving, a surrogate for free-water transport.22 Although most patients on long-term PD have a trend toward decreased sodium sieving during the course of PD,22 we show here that the decline is significantly faster in patients with EPS. The importance of this functional loss is substantiated by the multivariate analysis, which identifies for the first time the loss of sodium sieving as a powerful and independent predictor of EPS.
In addition to free-water transport across AQP1, sodium sieving also provides information on the maintenance of the osmotic gradient and properties of the membrane that affect osmotic conductance. In line with this, our data demonstrate that the early changes in water transport capacity reflect significant structural and molecular alterations in the peritoneal membrane of patients who subsequently developed EPS. Importantly, we show that the EPS peritoneum is characterized by an excessive fibrogenic response, with specific changes in collagen density and structure. In agreement with previous observations,23–26 we show a severe thickening of the submesothelial layer, as well as vascular proliferation in the EPS peritoneum. These changes are clearly excessive in EPS: after an average of 62 months of PD exposure, patients with EPS had a 4.7- and a 1.9-fold increase in the thickness of the submesothelial area compared with uremic and control PD patients, respectively. Using polarized light microscopy, we could then substantiate qualitative changes in the fibrotic interstitium of patients with EPS, with an increased collagen density (+174% and +84% compared with the uremic and control long-term PD peritoneum, respectively), and accumulation of thicker collagen fibers (2.2- and 1.4-fold increase compared with uremic and control long-term PD, respectively). To our knowledge, this is the first time that such qualitative changes are evidenced in the peritoneum of patients with EPS. Of note, similar changes in collagen content and optical properties have been evidenced as part of the scarring process after myocardial infarction.18,19,27
The data presented here show that fibrotic changes in the peritoneum of patients on long-term PD closely correlate with impaired water transport across the membrane, thereby confirming the hypothesis that structural changes in the peritoneal interstitium account for the excessive reduction in osmotic conductance.28 The interstitium, which is mainly composed of collagen and glycosaminoglycans, constitutes a porous matrix across which interstitial fluid flows. Accumulation of collagen fibers leads to a decreased hydraulic conductance, depending on the volume occupied by collagen fibrils.29 The effect of interstitial collagen accumulation on water flow has been demonstrated in vitro both in normal interstitia from various organs29 and in conditions of fibrosis.30 Reduced osmotic conductance by the fractional volume occupied by collagen fibrils results from three mechanisms: (1) exclusion of glycosaminoglycans from the intrafibrillar space, thereby increasing their extrafibrillar concentration; (2) reduction of the mean hydraulic radius in the interstitium, leading to a reduced area for flow; and (3) tortuosity, which increases the path length for flow.29 Our results show that increased collagen deposition and concentration in the peritoneal interstitium restricts water transport in vivo, leading to UF failure and loss of free-water transport in patients with EPS. These observations validate the serial three-pore membrane/fiber matrix model proposed by Rippe and colleagues.20 In this model, fibrotic alterations in the peritoneal interstitium have been suggested to constitute a second barrier outside the capillaries, restricting osmotic water transport without affecting small solute transport. Such an uncoupling between water and solute transport can be explained by the dependence of water filtration to the fourth power of the interstitial radius pores (r4) according to Poiseuille’s law, whereas solute diffusion is proportional to the surface of pores (r2).20 As a result, a reduction of interstitial pore radius by increased collagen density and thicker collagen fibers will thus predominantly affect water transport, whereas its slight theoretical restriction on solute transport will be counterbalanced by vascular proliferation and larger effective vascular surface area. Simulations from the serial three-pore membrane/fiber matrix model showed that the sodium sieving is progressively reduced when interstitial fibrosis and the vascular surface area both progress in parallel, despite the fact that the relative capillary density of aquaporins is kept unchanged.20 Alternatively, one could postulate that the thickening of the submesothelial area limits the penetration of glucose around peritoneal capillaries, thereby reducing the osmotic gradient across the capillary wall. Studies using 14C-EDTA (molecular radius, 0.48 nm) have shown an inverse relationship between peritoneal tissue concentration of small substances (urea, creatinine, and glucose) and the distance from the peritoneum, suggesting a distributed model for peritoneal transport.31 In this model, severe peritoneal fibrosis is expected to alter the dextrose concentration profile,32 thereby reducing the osmotic gradient and AQP1-mediated water flow across the capillary wall.
We also observed a significant upregulation in VEGF, eNOS, and vWf in the peritoneum of patients with EPS and, to a lesser extent, in long-term PD patients with no EPS. In the latter group, the results were concordant with previous data indicating a 3-fold increase in eNOS as well as the induction of VEGF expression in peritoneal biopsies from long-term PD patients compared with uremic patients.33 These molecular changes suggest a tight association among inflammation, angiogenesis, vascular permeability, and fibrosis in the peritoneal membrane of patients with EPS.33–43 In many organs, it has been established that perpetuation of tissue damage contributes to a chronic wound-healing response with continued tissue repair, regeneration and remodeling that ultimately leads to tissue fibrosis.34 In turn, fibrotic tissues—including the peritoneum35—become hypoxic when the expanding tissue remodeling outstrips its vascular supply. Hypoxia then potentiates and prolongs fibrogenesis and angiogenesis, through hypoxia-inducible factor 1α and VEGF.35 eNOS is a downstream effector of VEGF, and has been shown to promote angiogenesis in ischemic tissues36 and in the peritoneal membrane.37 Our data show that EPS is associated with VEGF and eNOS upregulation, either as residua of an early inflammatory phase or secondary to tissue hypoxia due to fibrosis.
The fact that impaired free-water transport is not associated with altered expression or location of AQP1 in EPS is surprising at first sight. AQP1 is the molecular counterpart of ultrasmall pore, accounting for half the UF and for the sodium sieving when hypertonic glucose is used as osmotic agent.44,45 The loss of AQP1-mediated free-water transport has been suggested as a potential cause of UF failure in long-term PD patients.46,47 Admittedly, this study does not allow ruling out a dysfunction or biochemical modification of AQP1. However, free-water transport estimated by the sodium sieving can be affected not only by the availability of functional AQP1 water channels but also by any mechanism of membrane obstruction, such as severe peritoneal fibrosis.48 This study is in line with predictions of the three-pore membrane/fiber matrix and distributed models, by showing that the vascular density of water channels is kept unchanged in patients with EPS, and by pointing toward excessive peritoneal fibrosis as the most important barrier to fluid flow in these patients.
In summary, reduced osmotic conductance during the course of PD was identified as a powerful and independent predictor of EPS. This functional change is linked to specific alterations of the collagen matrix in the peritoneal membrane of patients with EPS, rather than changes in the degree of vasculopathy or in the expression of water channels. Our multilevel data, based on transport, structural, and expression studies, thus clarify the cause of the excessive reduction in osmotic conductance in patients with EPS.
Concise Methods
Patients with EPS and Peritoneal Transport Analyses
In agreement with EPS diagnosis criteria,13,49,50 EPS cases were defined as PD patients who presented with recurrent, partial or total, intestinal occlusion, with a definitive EPS diagnosis confirmed by computed tomography and/or laparotomy.
Peritoneal function was assessed yearly, with a modified 3.86% glucose-based PET,22,51 which has the advantages over the conventional 2.27% glucose-based PET52 to assess sodium sieving22 and allow the diagnosis of UF failure (net UF <400 ml at the end of the 4-hour dwell with 3.86% glucose, as defined by the International Society for Peritoneal Dialysis53). Sodium sieving was shown to be a reliable—although indirect—indicator of free-water transport,16 which can be also affected by the diffusivity of small solutes and the overall effect of the reduced ultrafiltration rate per se.48 All values of sodium sieving were corrected for the rate of sodium diffusion. The PET was part of the normal procedure of care for PD patients.
Peritoneal Sampling and Processing
Paraumbilical biopsy samples of human parietal peritoneum were obtained from uremic patients at the time of catheter insertion, and from PD patients at the time of catheter removal because of renal transplantation or transfer to hemodialysis as part of a routine protocol in our center, and were processed as previously described.33 The time between last PET and peritoneal biopsy was similar in patients with EPS and controls (9.1±2.1 versus 7.5±1.6 months in patients with EPS and controls; P=0.61). Informed consent was obtained from patients. The use of human biopsy samples was approved by the Ethical Review Board of Saint-Luc University Clinics.
Immunoblotting, Immunohistochemistry, and Immunofluorescence
SDS-PAGE and immunoblotting, immunoperoxidase staining on human peritoneum sections and immunofluorescence were performed as previously described.33,54,55 We used the following antibodies: mouse monoclonal anti-human eNOS (Transduction Laboratories, Lexington, KY), purified rabbit anti-vWf (DAKO, Glostrup, Denmark), monoclonal anti-human VEGF (Santa Cruz Biotechnology, Santa Cruz, CA), and polyclonal rabbit anti-human AQP1 (Chemicon International, Temecula, CA).
Image Analyses
Information on the criteria used for adequacy of each peritoneal specimen, the assessment of peritoneal fibrosis, vasculopathy, vascular proliferation, collagen volume fraction, and collagen structure is available in the Supplemental Methods. Image analysis was performed using ImageJ software (1.47v).
Statistical Analyses
Data are presented as the mean±SEM. Comparisons between the means from different groups were performed using unpaired t tests, Fisher’s exact test, or one-way ANOVA, followed by Bonferroni’s multiple comparison tests, as appropriate. Trends were assessed by linear ordinary least-squares regression. Multivariate analysis was performed using a logistic regression random-effects model and included potential risk factors for EPS (age at PD start, PD duration, peritonitis rate, β-blocker use, residual renal function, glucose exposure) in a first model. In a second model, the mean value of sodium sieving during the last 2 years of PD was added to these variables. Probabilities of EPS in function of sodium sieving and UF levels were estimated by multivariate logistic regression. All analyses were performed by GraphPad Prism (version 6.01) or Stata (version 12) software. The significance level is indicated in each figure.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank all of the members of the nursing team of the home dialysis unit for performing PETs; Samira Azarzar, Yvette Cnops, Sebastien Druart, and Chantal Fregimilicka for expert technical assistance; Drs. Michel Mourad, Jacques Malaise, Tom Darius for peritoneal biopsy sampling at the time of catheter insertion or withdrawal; and Dr. Jean-Luc Balligand for fruitful discussions.
This work was supported in part by the Saint-Luc Foundation (to J.M.), the Horlait-Dapsens Foundation (to J.M.), the French Speaking Society of Dialysis (to J.M.), Baxter Healthcare (Extramural Grant to J.M. and O.D.), and the National Fund for Scientific Research (to J.M. and O.D.). A.S. was supported by a Merit Scholarship Program Fellowship of the Islamic Development Bank (Kingdom of Saudi Arabia).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014090939/-/DCSupplemental.
References
- 1.Jain AK, Blake P, Cordy P, Garg AX: Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 23: 533–544, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E: Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 171: 110–118, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Davies SJ, Phillips L, Naish PF, Russell GI: Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol 12: 1046–1051, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Devuyst O, Margetts PJ, Topley N: The pathophysiology of the peritoneal membrane. J Am Soc Nephrol 21: 1077–1085, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Devuyst O, van Westrhenen R, Topley N: Longterm peritoneal dialysis patients. In: Nolph and Gokal’s Textbook of Peritoneal Dialysis, edited by Khanna R, Krediet RT, 3rd Ed., New York, Springer, 2009, pp 757–780 [Google Scholar]
- 6.Davies SJ, Phillips L, Griffiths AM, Russell LH, Naish PF, Russell GI: What really happens to people on long-term peritoneal dialysis? Kidney Int 54: 2207–2217, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, Mackenzie RK, Williams GT, Peritoneal Biopsy Study Group : Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 13: 470–479, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Williams JD, Craig KJ, von Ruhland C, Topley N, Williams GT, Biopsy Registry Study Group : The natural course of peritoneal membrane biology during peritoneal dialysis. Kidney Int Suppl 88: S43–S49, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Honda K, Hamada C, Nakayama M, Miyazaki M, Sherif AM, Harada T, Hirano H, Peritoneal Biopsy Study Group of the Japanese Society for Peritoneal Dialysis : Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: A quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol 3: 720–728, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korte MR, Sampimon DE, Betjes MG, Krediet RT: Encapsulating peritoneal sclerosis: the state of affairs. Nat Rev Nephrol 7: 528–538, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Brown MC, Simpson K, Kerssens JJ, Mactier RA, Scottish Renal Registry : Encapsulating peritoneal sclerosis in the new millennium: A national cohort study. Clin J Am Soc Nephrol 4: 1222–1229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson DW, Cho Y, Livingston BE, Hawley CM, McDonald SP, Brown FG, Rosman JB, Bannister KM, Wiggins KJ: Encapsulating peritoneal sclerosis: Incidence, predictors, and outcomes. Kidney Int 77: 904–912, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Korte MR, Sampimon DE, Lingsma HF, Fieren MW, Looman CW, Zietse R, Weimar W, Betjes MG, Dutch Multicenter EPS Study : Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS study. Perit Dial Int 31: 269–278, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Lambie ML, John B, Mushahar L, Huckvale C, Davies SJ: The peritoneal osmotic conductance is low well before the diagnosis of encapsulating peritoneal sclerosis is made. Kidney Int 78: 611–618, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Sampimon DE, Coester AM, Struijk DG, Krediet RT: The time course of peritoneal transport parameters in peritoneal dialysis patients who develop encapsulating peritoneal sclerosis. Nephrol Dial Transplant 26: 291–298, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Ni J, Verbavatz JM, Rippe A, Boisdé I, Moulin P, Rippe B, Verkman AS, Devuyst O: Aquaporin-1 plays an essential role in water permeability and ultrafiltration during peritoneal dialysis. Kidney Int 69: 1518–1525, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC: Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 100: 3131–3139, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whittaker P, Boughner DR, Kloner RA: Analysis of healing after myocardial infarction using polarized light microscopy. Am J Pathol 134: 879–893, 1989 [PMC free article] [PubMed] [Google Scholar]
- 19.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT: Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106: 55–62, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rippe B, Venturoli D: Simulations of osmotic ultrafiltration failure in CAPD using a serial three-pore membrane/fiber matrix model. Am J Physiol Renal Physiol 292: F1035–F1043, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Sampimon DE, Coester AM, Struijk DG, Krediet RT: Time course of peritoneal transport parameters in peritoneal dialysis patients who develop peritoneal sclerosis. Adv Perit Dial 23: 107–111, 2007 [PubMed] [Google Scholar]
- 22.La Milia V, Pozzoni P, Virga G, Crepaldi M, Del Vecchio L, Andrulli S, Locatelli F: Peritoneal transport assessment by peritoneal equilibration test with 3.86% glucose: A long-term prospective evaluation. Kidney Int 69: 927–933, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Mateijsen MA, van der Wal AC, Hendriks PM, Zweers MM, Mulder J, Struijk DG, Krediet RT: Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int 19: 517–525, 1999 [PubMed] [Google Scholar]
- 24.Honda K, Nitta K, Horita S, Tsukada M, Itabashi M, Nihei H, Akiba T, Oda H: Histologic criteria for diagnosing encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. Adv Perit Dial 19: 169–175, 2003 [PubMed] [Google Scholar]
- 25.Braun N, Fritz P, Ulmer C, Latus J, Kimmel M, Biegger D, Ott G, Reimold F, Thon KP, Dippon J, Segerer S, Alscher MD: Histological criteria for encapsulating peritoneal sclerosis - a standardized approach. PLoS ONE 7: e48647, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plum J, Hermann S, Fusshöller A, Schoenicke G, Donner A, Röhrborn A, Grabensee B: Peritoneal sclerosis in peritoneal dialysis patients related to dialysis settings and peritoneal transport properties. Kidney Int Suppl 78: S42–S47, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Whittaker P, Kloner RA, Boughner DR, Pickering JG: Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol 89: 397–410, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Davies SJ: Longitudinal relationship between solute transport and ultrafiltration capacity in peritoneal dialysis patients. Kidney Int 66: 2437–2445, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Levick JR: Flow through interstitium and other fibrous matrices. Q J Exp Physiol 72: 409–437, 1987 [DOI] [PubMed] [Google Scholar]
- 30.Ng CP, Hinz B, Swartz MA: Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci 118: 4731–4739, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Flessner MF, Dedrick RL, Schultz JS: A distributed model of peritoneal-plasma transport: Theoretical considerations. Am J Physiol 246: R597–R607, 1984 [DOI] [PubMed] [Google Scholar]
- 32.Flessner MF: Peritoneal transport physiology: Insights from basic research. J Am Soc Nephrol 2: 122–135, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Combet S, Miyata T, Moulin P, Pouthier D, Goffin E, Devuyst O: Vascular proliferation and enhanced expression of endothelial nitric oxide synthase in human peritoneum exposed to long-term peritoneal dialysis. J Am Soc Nephrol 11: 717–728, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Wynn TA, Ramalingam TR: Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med 18: 1028–1040, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekiguchi Y, Zhang J, Patterson S, Liu L, Hamada C, Tomino Y, Margetts PJ: Rapamycin inhibits transforming growth factor β-induced peritoneal angiogenesis by blocking the secondary hypoxic response. J Cell Mol Med 16: 1934–1945, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM: Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest 101: 2567–2578, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni J, Moulin P, Gianello P, Feron O, Balligand JL, Devuyst O: Mice that lack endothelial nitric oxide synthase are protected against functional and structural modifications induced by acute peritonitis. J Am Soc Nephrol 14: 3205–3216, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A: Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Carmeliet P, Jain RK: Molecular mechanisms and clinical applications of angiogenesis. Nature 473: 298–307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK: Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A 98: 2604–2609, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenting PJ, Casari C, Christophe OD, Denis CV: von Willebrand factor: The old, the new and the unknown. J Thromb Haemost 10: 2428–2437, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Mojiri A, Nakhaii-Nejad M, Phan WL, Kulak S, Radziwon-Balicka A, Jurasz P, Michelakis E, Jahroudi N: Hypoxia results in upregulation and de novo activation of von Willebrand factor expression in lung endothelial cells. Arterioscler Thromb Vasc Biol 33: 1329–1338, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Vischer UM: von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J Thromb Haemost 4: 1186–1193, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Devuyst O, Rippe B: Water transport across the peritoneal membrane. Kidney Int 85: 750–758, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Rippe B, Stelin G, Haraldsson B: Computer simulations of peritoneal fluid transport in CAPD. Kidney Int 40: 315–325, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Goffin E, Combet S, Jamar F, Cosyns JP, Devuyst O: Expression of aquaporin-1 in a long-term peritoneal dialysis patient with impaired transcellular water transport. Am J Kidney Dis 33: 383–388, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Smit W, Schouten N, van den Berg N, Langedijk MJ, Struijk DG, Krediet RT, Netherlands Ultrafiltration Failure Study Group : Analysis of the prevalence and causes of ultrafiltration failure during long-term peritoneal dialysis: A cross-sectional study. Perit Dial Int 24: 562–570, 2004 [PubMed] [Google Scholar]
- 48.Rippe B, de Arteaga J, Venturoli D: Aquaporins are unlikely to be affected in marked ultrafiltration failure: Results from a computer simulation. Perit Dial Int 21[Suppl 3]: S30–S34, 2001 [PubMed] [Google Scholar]
- 49.Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG, International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis : Encapsulating peritoneal sclerosis: Definition, etiology, diagnosis, and treatment. Perit Dial Int 20[Suppl 4]: S43–S55, 2000 [PubMed] [Google Scholar]
- 50.Summers AM, Abrahams AC, Alscher MD, Betjes M, Boeschoten EW, Braun N, Brenchley PE, Davies S, Dunn L, Engelsman L, Fieren M, Garosi G, Goffin E, Heuveling L, Korte M, Lindholm B, Rutherford P, Struijk D, Verduijn M, Verger C, Westerhuis R: A collaborative approach to understanding EPS: The European perspective. Perit Dial Int 31: 245–248, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Clerbaux G, Francart J, Wallemacq P, Robert A, Goffin E: Evaluation of peritoneal transport properties at onset of peritoneal dialysis and longitudinal follow-up. Nephrol Dial Transplant 21: 1032–1039, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Twardowski ZJ, Nolph KD, Khanna R, Prowant BF, Ryan LP, Moore HL, Nielsen MP: Peritoneal equilibration test. Perit Dial Bull 7: 138–147, 1987 [Google Scholar]
- 53.Mujais S, Nolph K, Gokal R, Blake P, Burkart J, Coles G, Kawaguchi Y, Kawanishi H, Korbet S, Krediet R, Lindholm B, Oreopoulos D, Rippe B, Selgas R, International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis : Evaluation and management of ultrafiltration problems in peritoneal dialysis. Perit Dial Int 20[Suppl 4]: S5–S21, 2000 [PubMed] [Google Scholar]
- 54.Devuyst O, Nielsen S, Cosyns JP, Smith BL, Agre P, Squifflet JP, Pouthier D, Goffin E: Aquaporin-1 and endothelial nitric oxide synthase expression in capillary endothelia of human peritoneum. Am J Physiol 275: H234–H242, 1998 [DOI] [PubMed] [Google Scholar]
- 55.de Arteaga J, Ledesma F, Garay G, Chiurchiu C, de la Fuente J, Douthat W, Massari P, Terryn S, Devuyst O: High-dose steroid treatment increases free water transport in peritoneal dialysis patients. Nephrol Dial Transplant 26: 4142–4145, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.