Abstract
CKD is associated with higher risk of death, but details regarding differences in cause-specific death in CKD are unclear. We examined the leading causes of death among a non–dialysis-dependent CKD population using an electronic medical record-based CKD registry in a large healthcare system and the Ohio Department of Health mortality files. We included 33,478 white and 5042 black patients with CKD who resided in Ohio between January 2005 and September 2009 and had two measurements of eGFR<60 ml/min per 1.73 m2 obtained 90 days apart. Causes of death (before ESRD) were classified into cardiovascular, malignancy, and non-cardiovascular/non-malignancy diseases and non–disease-related causes. During a median follow-up of 2.3 years, 6661 of 38,520 patients (17%) with CKD died. Cardiovascular diseases (34.7%) and malignant neoplasms (31.8%) were the leading causes of death, with malignancy-related deaths more common among those with earlier stages of kidney disease. After adjusting for covariates, each 5 ml/min per 1.73 m2 decline in eGFR was associated with higher risk of death due to cardiovascular disease (hazard ratio [HR], 1.10; 95% confidence interval [95% CI], 1.08 to 1.12) and non-cardiovascular/non-malignancy diseases (HR, 1.12; 95% CI, 1.09 to 1.14) but not to malignancy. In the adjusted models, blacks had overall-mortality hazard ratios similar to those of whites but higher hazard ratios for cardiovascular deaths. Further studies to confirm these findings and explain the mechanisms for differences are warranted. In addition to lowering cardiovascular burden in CKD, efforts to target known risk factors for cancer at the population level are needed.
Keywords: chronic kidney disease, cardiovascular disease, malignancy, mortality
CKD burden continues to grow and more than 20 million Americans are estimated to have CKD with varying levels of severity.1 Several studies report that death prior to ESRD is much more common than progression to ESRD.2,3 Go et al. reported a graded association between lower eGFR and higher risk of death, cardiovascular events, and hospitalization.4 Subsequent studies have reported similar results of higher cardiovascular events and death, but cause-specific deaths in the non–dialysis-dependent CKD population are less well studied.5
The Center for Disease Control and Prevention (CDC) reports the leading causes of deaths in the general population every year by collecting cause-specific death from individual states. Diseases of the heart followed by malignancy are the leading causes of death in the general population.6 Yet, the CKD population has greater comorbid conditions and thus the data from the general population might not be applicable to those with CKD. It is well known that CKD patients have more cardiovascular disease and hospitalizations.4 Furthermore, patients with CKD are older and might sustain higher malignancy burden as cancer incidence increases with age. Few studies have reported higher incidence of various types of cancers among those with mild to moderate CKD.7 Thus, better understanding of the causes of death in CKD could help focus efforts that target prevention strategies or disease management in those with CKD. Further, racial differences in reaching ESRD and death have been reported, but details about cause-specific deaths among various races remain unclear in CKD.8–10
We used a large electronic medical record-based CKD registry and Ohio state death data to examine if the risk for all-cause mortality increases with lower levels of kidney function. Further, we also aimed to compare the risks for each type of death (cardiovascular, malignancy, and non-cardiovascular non-malignancy) based on the stage of kidney disease, age, gender, race, and diabetes among the CKD population followed in our health system.
Results
Patient characteristics
Our study population comprised 38,520 patients with CKD (Supplementary Figure 1). Mean age of the study population was 72.8±11.8 years with 43.7% being males and 13.1% blacks. Prevalence of diabetes, hypertension, malignancy, and coronary artery disease were 21.8%, 82.4%, 23.6%, and 19.4%, respectively. Mean eGFR was 46.5 ml/min per 1.73 m2 with serum albumin of 4.1 mg/dl. Angiotensin converting enzyme inhibitors/angiotensin receptor blockers and statins were used among 61.3% and 54.5% of study participants. Other details of the study population are outlined in Table 1. Characteristics of patients based on age >65 versus <65 years, gender, and race are outlined in Supplementary Table 1.
Table 1.
Characteristics of patients with CKD included in this analysis
| Variables | All (n=38,520) | eGFR 45–59 (n=24,639) | eGFR 30–44 (n=10,816) | eGFR<30 (n=3695) |
|---|---|---|---|---|
| Age (years) | 72.8 (11.8) | 72.1 (11.3) | 74.5 (11.8) | 72.5 (14.1) |
| Male gender (n, %) | 16818 (43.7) | 10796 (43.8) | 4393 (43.1) | 1629 (44.1) |
| Blacks (n, %) | 5042 (13.1) | 2932 (11.9) | 1368 (13.4) | 742 (20.1) |
| Diabetes (n, %) | 8385 (21.8) | 5116 (20.8) | 2309 (22.7) | 960 (26.0) |
| Malignancy (n, %) | 9100 (23.6) | 6174 (25.1) | 2234 (21.9) | 692 (18.7) |
| Hypertension (n, %) | 31742 (82.4) | 20766 (84.3) | 8117 (79.7) | 2859 (77.4) |
| Hyperlipidemia (n, %) | 29038 (75.4) | 18974 (77.0) | 7437 (73.0) | 2627 (71.1) |
| Coronary artery disease (n, %) | 7479 (19.4) | 4642 (18.8) | 2091 (20.5) | 746 (20.2) |
| Congestive heart failure (n, %) | 2812 (7.3) | 1435 (5.8) | 964 (9.5) | 413 (11.2) |
| Body mass index (kg/m2)a | 29.3 (6.5) | 29.4 (6.4) | 29.3 (6.6) | 29.3 (6.9) |
| BMI group (n, %) | ||||
| <18.5 kg/m2 | 460 (1.2) | 269 (1.1) | 136 (1.3) | 55 (1.5) |
| 18.5–24.9 kg/m2 | 8820 (22.9) | 5530 (22.4) | 2376 (23.3) | 914 (24.7) |
| 25–29.9 kg/m2 | 13099 (34.0) | 8578 (34.8) | 3392 (33.3) | 1129 (30.6) |
| ≥30 kg/m2 | 13971 (36.3) | 9156 (37.2) | 3569 (35.0) | 1246 (33.7) |
| Missing | 2170 (5.6) | 1106 (4.5) | 713 (7.0) | 351 (9.5) |
| Smoker (n, %) | ||||
| No | 28705 (74.5) | 19092 (77.5) | 7196 (70.6) | 2417 (65.4) |
| Yes | 2586 (6.7) | 1680 (6.8) | 654 (6.4) | 252 (6.8) |
| Missing | 7229 (18.8) | 3867 (15.7) | 2336 (22.9) | 1026 (27.8) |
| eGFR (ml/min per 1.73 m2) | 46.5 (11.1) | 53.4 (4.2) | 38.6 (4.2) | 22.2 (6.1) |
| Proteinuria (n, %) | 4861(26.5) | 2395(20.5) | 1438(30.1) | 1028(54.4) |
| Serum albumin (g/dl)a | 4.1 (0.5) | 4.1 (0.5) | 4.0 (0.5) | 3.9 (0.6) |
| Hemoglobin (g/dl)a | 12.8 (1.8) | 13.1 (1.7) | 12.5 (1.8) | 11.7 (1.8) |
| ACEI/ARB use (n, %) | 23620 (61.3) | 14715 (59.7) | 6553 (64.3) | 2352 (63.7) |
| Statins use (n, %) | 20984 (54.5) | 13536 (54.9) | 5481 (53.8) | 1967 (53.2) |
| Beta-blocker use (n, %) | 21198 (55.0) | 13084 (53.1) | 5866 (57.6) | 2248 (60.8) |
| Insurance (n, %) | ||||
| Medicare | 31364 (81.4) | 19851 (80.6) | 8542 (83.9) | 2971 (80.4) |
| Medicaid | 258 (0.7) | 127 (0.5) | 82 (0.8) | 49 (1.3) |
| Other | 5763 (15.0) | 3983 (16.2) | 1260 (12.4) | 520 (14.1) |
| Missing | 1135 (2.9) | 678 (2.8) | 302 (3.0) | 155 (4.2) |
Missing data for each of the following: BMI n=2170, proteinuria n=20,173; serum albumin n=7294, hemoglobin n=6258.
ACEI/ARB, Angiotensin converting enzyme inhibitors/angiotensin receptor blockers.
Unadjusted and age-adjusted mortality rates
During a median follow-up of 2.3 years (25th percentile 1.1, and 75th percentile 3.5), 6661 died. The unadjusted mortality rate was 76.1 per 1000 years of follow-up (95% confidence interval [95% CI], 64.9 to 87.3) with higher rates among those >65 years, males, blacks, and those with advanced stages of kidney disease (Supplementary Table 2). Table 2 provides age-adjusted overall and cause-specific mortality rates/1000 years based on the various categories of eGFR and urine albumin-to-creatinine ratio (UACR).
Table 2.
Age-adjusted mortality rates per 1000 years based on kidney function (eGFR and UACR)
| eGFR | UACR<10 | UACR 10-<30 | UACR 30–300 | UACR>300 |
|---|---|---|---|---|
| All-cause deaths | ||||
| 45–59 ml/min per 1.73 m2 | 24.6 (19.9, 30.5) | 29.0 (23.3, 36.1) | 50.1 (41.8, 59.9) | 82.2 (63.4, 106.6) |
| 30–44 ml/min per 1.73 m2 | 35.6 (26.1, 48.5) | 55.8 (43.3, 72.0) | 61.9 (49.9, 76.8) | 70.1 (52.1, 94.2) |
| <30 ml/min per 1.73 m2 | 54.4 (29.2,101.2) | 59.5 (36.8, 96.0) | 63.7 (44.0, 92.3) | 99.9 (72.3, 138.0) |
| Cardiovascular deaths | ||||
| 45–59 ml/min per 1.73 m2 | 7.8 (5.4, 11.4) | 10.8 (7.6, 15.3) | 20.2 (15.3, 26.7) | 35.3 (23.8, 52.4) |
| 30–44 ml/min per 1.73 m2 | 6.6 (3.3, 13.3) | 17.7 (11.4, 27.5) | 21.9 (15.4, 31.2) | 31.1 (20.0, 48.3) |
| <30 ml/min per 1.73 m2 | 20.5 (7.7, 55.0) | 29.5 (15.2, 57.1) | 22.4 (12.3, 41.0) | 33.9 (19.6, 58.5) |
| Malignancy-related deaths | ||||
| 45–59 ml/min per 1.73 m2 | 9.4 (6.6, 13.4) | 9.1 (6.1, 13.7) | 13.3 (9.3, 19.1) | 8.7 (3.9, 19.5) |
| 30–44 ml/min per 1.73 m2 | 12.3 (7.1, 21.3) | 15.2 (9.1, 25.3) | 13.2 (8.2, 21.4) | 6.5 (2.4, 17.3) |
| <30 ml/min per 1.73 m2 | 12.0 (3.0, 48.1) | 4.0 (0.6, 28.1) | 15.1 (6.7, 33.9) | 11.2 (4.2, 29.9) |
| Non-cardiovascular/non-malignancy deaths | ||||
| 45–59 ml/min per 1.73 m2 | 6.6 (4.4, 9.9) | 8.4 (5.7, 12.6) | 15.8 (11.5, 21.7) | 35.8 (24.2, 53.0) |
| 30–44 ml/min per 1.73 m2 | 17.0 (10.9, 26.5) | 22.8 (15.4, 33.9) | 25.4 (18.2, 35.5) | 31.6 (20.3, 49.0) |
| <30 ml/min per 1.73 m2 | 15.9 (5.1, 49.6) | 23.8 (11.3, 50.3) | 25.6 (14.4, 45.5) | 50.6 (32.2, 79.6) |
Kidney function and causes of death
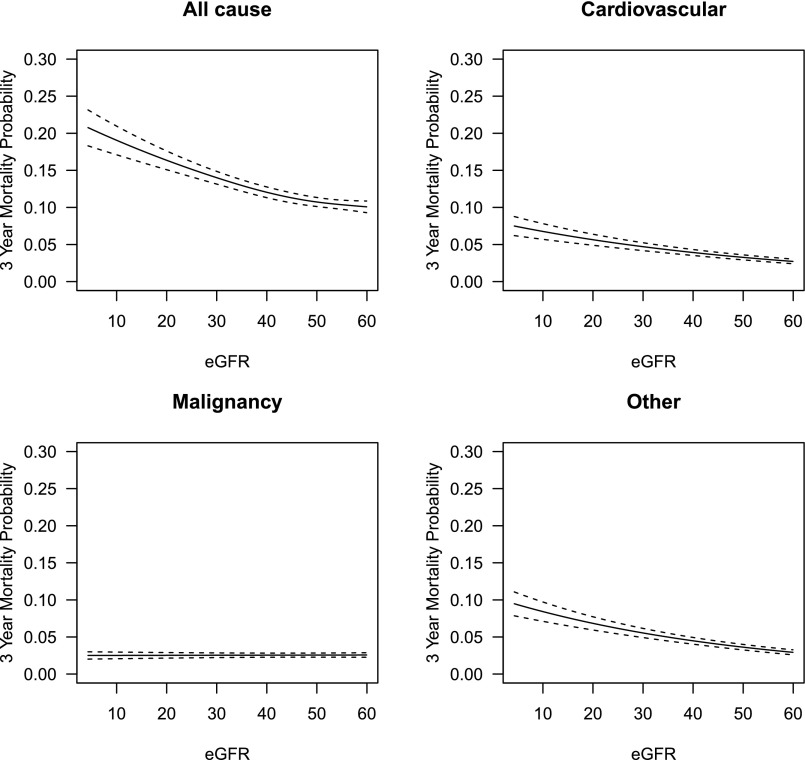
Overall, cardiovascular diseases (34.7%) and malignant neoplasms (31.8%) were the leading causes of death among our study population (Table 3). The proportion of deaths due to malignant neoplasms was higher among patients with eGFR 45–59 ml/min per 1.73 m2 (CKD Stage 3a) while cardiovascular disease and non-cardiovascular/non-malignancy related deaths were higher among patients with lower eGFR categories. Supplemental Table 3 outlines the various types of malignancy-related deaths. In the Cox proportional hazards model examining eGFR as a continuous measure, higher eGFR was associated with better survival (Figure 1), but there was a non-linear effect where the survival benefit associated with each unit increase was stronger at lower levels of eGFR. Each 5 ml/min per 1.73 m2 decline in eGFR was associated with higher risk due to cardiovascular disease (hazard ratio [HR] 1.11; 95% CI, 1.09 to 1.13), and non-cardiovascular/non-malignancy related deaths (HR 1.12; 95% CI, 1.10 to 1.14), but not malignancy-related deaths (HR 1.00; 95% CI, 0.98 to 1.02). Predicted three-year mortality risks due to various reasons are outlined in Figure 1. Similar results were noted in the categorical analysis based on the stages of CKD (Table 4). Associations between other covariates and cause-specific deaths are presented in Supplemental Table 4.
Table 3.
Cause-specific deaths among those with non–dialysis-dependent CKD
| Causes of death (n, %) | Overall (n=6661) | eGFR 45–59 (n=3308) | eGFR 30–44 (n=2261) | eGFR<30 (n=1092) |
|---|---|---|---|---|
| Cardiovascular diseases | 2311 (34.7) | 1017 (30.7) | 862 (38.1) | 432 (39.6) |
| Ischemic heart disease | 1227 (18.4) | 532 (16.1) | 457 (20.2) | 238 (21.8) |
| Heart failure | 163 (2.4) | 61 (1.8) | 75 (3.3) | 27 (2.5) |
| Cerebrovascular diseases | 255 (3.8) | 123 (3.7) | 94 (4.2) | 38 (3.5) |
| Other cardiovascular diseases | 666 (10.0) | 301 (9.1) | 236 (10.4) | 129 (11.8) |
| Malignant neoplasms | 2117 (31.8) | 1282 (38.8) | 616 (27.2) | 219 (20.1) |
| Non-cardiovascular/Non-malignancy | ||||
| Chronic lower respiratory disease | 316 (4.7) | 147 (4.4) | 109 (4.8) | 60 (5.5) |
| Diabetes mellitus | 228 (3.4) | 91 (2.8) | 71 (3.1) | 66 (6.0) |
| Nephritis, nephrotic syndrome and nephrosis | 125 (1.9) | 29 (0.9) | 37 (1.6) | 59 (5.4) |
| Alzheimer’s disease | 122 (1.8) | 58 (1.8) | 45 (2.0) | 19 (1.7) |
| Influenza and pneumonia | 113 (1.7) | 48 (1.5) | 43 (1.9) | 22 (2.0) |
| Septicemia | 95 (1.4) | 47 (1.4) | 33 (1.5) | 15 (1.4) |
| Chronic liver disease and cirrhosis | 66 (1.0) | 26 (0.8) | 27 (1.2) | 13 (1.2) |
| Pneumonitis due to solids and liquids | 50 (0.8) | 22 (0.7) | 18 (0.8) | 10 (0.9) |
| Parkinson's disease | 31 (0.5) | 19 (0.6) | 11 (0.5) | 1 (0.1) |
| All other diseases | 972 (14.6) | 464 (14.0) | 352 (15.6) | 156 (14.3) |
| Non-disease related deathsa | 115 (1.7) | 58 (1.8) | 37 (1.6) | 20 (1.8) |
For example: homicide, suicide, accidents.
Figure 1.
Predicted mortality due to all-cause, cardiovascular, malignancy, and other causes at three years in those with CKD.
Table 4.
Associations between age, gender, diabetes and kidney function, and all-cause and cause-specific death in non–dialysis-dependent kidney disease
| Number of events | HR (95% CI) adjusteda | |
|---|---|---|
| Age >65 years (n=29,501) versus <65 years (n=9019) | ||
| All-cause mortalityb | 6661 | 1.73 (1.60 to 1.87) |
| Deaths due to cardiovascular disease | 2311 | 2.45 (2.10 to 2.86) |
| Deaths due to malignancy | 2117 | 1.31 (1.15 to 1.48) |
| Non-cardiovascular/non-malignancy deathc | 2118 | 1.86 (1.62 to 2.14) |
| Males (n=16,818) versus females (n=21,702) | ||
| All-cause mortalityb | 6661 | 1.48 (1.41 to 1.56) |
| Deaths due to cardiovascular disease | 2311 | 1.56 (1.43 to 1.71) |
| Deaths due to malignancy | 2117 | 1.51 (1.38 to 1.65) |
| Non-cardiovascular/non-malignancy deathc | 2118 | 1.39 (1.27 to 1.53) |
| Patients with diabetes (n=8385) versus non-patients with diabetes (n=30,135) | ||
| All-cause mortalityb | 6661 | 1.03 (0.96 to 1.10) |
| Deaths due to cardiovascular disease | 2311 | 1.01 (0.90 to 1.13) |
| Deaths due to malignancy | 2117 | 0.80 (0.70 to 0.92) |
| Non-cardiovascular/non-malignancy deathsc | 2118 | 1.36 (1.21 to 1.53) |
| CKD Stage 3b (n=10,186) versus Stage 3a (n=24,639) | ||
| All-cause mortalityb | 5569 | 1.21 (1.14 to 1.27) |
| Deaths due to cardiovascular disease | 1879 | 1.35 (1.23 to 1.48 |
| Deaths due to malignancy | 1898 | 1.03 (0.93 to 1.14) |
| Non-cardiovascular/non-malignancy deathsc | 1697 | 1.29 (1.17 to 1.42) |
| CKD Stages 4 and 5 (n=3695) versus Stage 3a (n=24,639) | ||
| All-cause mortalityb | 4400 | 1.58 (1.46 to 1.70) |
| Deaths due to cardiovascular disease | 1449 | 1.85 (1.64 to 2.09) |
| Deaths due to malignancy | 1501 | 1.00 (0.86 to 1.16) |
| Non-cardiovascular/non-malignancy deathsc | 1372 | 1.93 (1.71 to 2.18) |
Model includes age, race gender, diabetes, hypertension, hyperlipidemia, BMI group, albumin and hemoglobin, malignancy, coronary artery disease, congestive heart failure, insurance, Angiotensin converting enzyme inhibitors/angiotensin receptor blockers, statin, beta blocker, smoking, and CKD stage for all models except one model where age was substituted for age >65 to present categorical effect. HR shown were pooled using MIanalyze from five data sets created using multiple imputations.
Includes all deaths related to non-disease causes.
This does not include non-disease cause-related deaths.
Cause-specific deaths for subgroups
Age >65 years versus age <65 years
In the Cox proportional hazards model, after adjusting for confounding variables, patients with CKD aged >65 years (versus age <65 years) had higher risk for all-cause deaths along with higher risk for deaths due to cardiovascular disease, malignancy, and non-cardiovascular/non-malignancy related deaths (Table 4).
Gender
After adjusting for covariates, males with CKD were at higher risk for overall deaths and had higher risk for deaths due to cardiovascular diseases, malignancy and non-cardiovascular/non-malignancy related deaths (Table 4).
Race
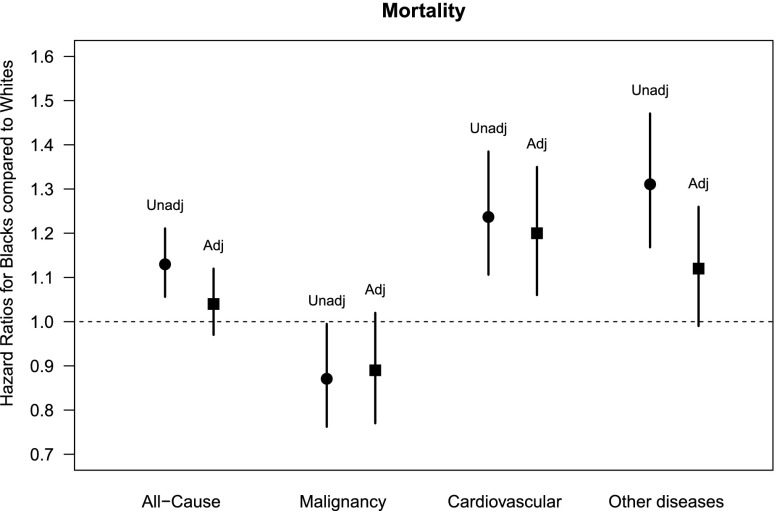
In the unadjusted model, blacks with CKD had higher risk of all-cause mortality and cardiovascular disease mortality, yet lower malignancy-related mortality compared with whites. However, after adjusting for covariates, blacks had similar risk for overall deaths and deaths due to malignancy but a higher risk of death due to cardiovascular diseases compared with whites (Figure 2).
Figure 2.
Risk for all-cause and major cause-specific deaths in blacks (versus whites).
Patients with diabetes versus non-patients with diabetes
After adjusting for covariates, patients with CKD and diabetes did not differ in their risk for all-cause deaths and cardiovascular deaths when compared with those without diabetes. However, patients with CKD and diabetes had lower risk for deaths due to malignancy and higher risk for non-cardiovascular/non-malignancy related deaths compared with those without diabetes (Table 4).
Sensitivity analyses
Results relating to additional sensitivity analyses are presented in the Supplemental methods/results section (Supplementary Tables 5 and 6).
Discussion
Presence of CKD is associated with higher risk for death.4,11,12 This report of cause-specific deaths among a large non–dialysis-dependent CKD population followed in our healthcare system documents that cardiovascular diseases and malignancy are the leading causes of death and accounted for two-thirds of the deaths, a proportion higher than that reported in the general population (heart disease: 24.2%; malignant deaths: 23.3%). After accounting for underlying comorbid conditions, a decrement in eGFR was associated with an increased risk for overall, cardiovascular, non-cardiovascular/non-malignancy related deaths but not with malignancy-related deaths. Higher proportions of deaths in those with eGFR 45–59 ml/min per 1.73 m2 were related to malignancy but cardiovascular deaths were the leading causes in those with lower eGFR categories. Even though blacks had similar risk as whites for all-cause mortality, they had higher risk for cardiovascular mortality and non-cardiovascular/non-malignancy related deaths.
Dialysis patients often die due to underlying cardiovascular causes, particularly sudden cardiac death.13 Using data from the National Health and Nutrition Examination Survey, Muntner et al. reported 68% higher risk for cardiovascular mortality with eGFR<70 ml/min per 1.73 m2 (versus >90 ml/min per 1.73 m2).14 A large meta-analysis of population-based cohorts reported increased risk for cardiovascular mortality with eGFR<60 ml/min per 1.73 m2.15,16 A secondary analysis of the Women’s Health Initiative (n=1315) showed that eGFR<60 ml/min per 1.73 m2 (versus >60 ml/min per 1.73 m2) was associated with an increased risk for cardiovascular mortality but not for non-cardiovascular mortality.17 Similarly, we noted higher cardiovascular mortality rates in those with lower levels of kidney function and among those aged >65 years and males. CKD is associated with higher prevalence of both traditional and non-traditional cardiovascular risk factors (decline in kidney function per se) and as such may explain the observed associations.18
Nonetheless, malignancy accounted for nearly a third of the deaths and was the leading cause of death among those with stage 3a CKD. Recent studies have shown that the age-adjusted incidence rate of cancer is higher among those with CKD.7,19 However, details about malignancy-related deaths are sparse, and a recent report from Taiwan indicated that patients with CKD had a higher mortality risk from liver, kidney, and urinary tract cancer.20 Our results also highlight the leading causes of malignancy-related deaths (Supplemental Table 3) among the CKD population followed in our healthcare system. Overall, these higher numbers of cancer-related deaths could be attributed to the fact that a large proportion of patients receiving care for cancer at our healthcare system had underlying CKD or develop CKD during the treatment course. However, it is also important to note that the malignancy-related deaths have increased in the general population and whether our data reflect this pattern warrants further studies.
Studies examining the associations between CKD, ESRD, and death among blacks and whites had conflicting findings.2,3,8,21 Weiner et al. reported that among those with CKD, blacks had relatively higher risk than whites (HR 1.76 versus 1.13) for all-cause mortality.22 However, similar to us, the Kidney Early Evaluation Program and the most recent meta-analysis of several cohort studies did not report higher risk for all-cause deaths for blacks.15,23,24 Differences between these studies could be explained by the study timing (more recent studies showing narrowing of life expectancy gaps between races) and the differences between the confounders adjusted for in these studies.25,26 While limited access to healthcare among blacks is reported to contribute to poor outcomes among blacks,27 the number of office visits per year (a proxy for healthcare access) for blacks was in fact higher in our study population (median number of visits 5.7 for blacks versus 5.2 for whites). Blacks with albuminuria had higher risk for incident cardiovascular events and whether such differences in urinary protein excretion contribute to this higher risk is unclear.28 Additionally, social determinants of health and other socio-economic factors likely could have played a role.29 Cumulatively, our results argue for further studies to elucidate mechanisms underlying these higher cardiovascular deaths and the need for better monitoring and management of cardiovascular risk factors among blacks with CKD.
Risk for overall and other major causes of death was higher in those aged >65 years and among males suggesting the need to pay particular attention to screening and risk management in these subgroups. Lack of higher risk for all-cause deaths and cardiovascular mortality, and the lower risk for malignancy in those with diabetes and CKD is somewhat surprising. Recent studies in the general population showed that mortality rates among treated patients with diabetes is declining and such a trend might explain our findings.30–32 The rates of deaths due to diabetes and respiratory diseases were higher in those with lower levels of kidney function. Given the smaller number of patients in individual subgroups, we could not conduct individual subgroup analysis. However, cumulatively, we noted higher risk for non-cardiovascular/non-malignancy related deaths with lower eGFR, highlighting the need for further studies.
The strengths of this study include a large CKD population which included those with two eGFR<60 ml/min per 1.73 m2 and censored those who progressed to dialysis from a validated CKD registry and the availability of cause-specific death data from the Ohio State Department of Health. Even though our study is restricted to a single healthcare system, it is unlikely that CKD patients who live in the State of Ohio are substantially different from CKD patients cared for in other healthcare systems in other parts of the country. However, further studies using national databases are needed to know if there are differences and whether these data are generalizable (as we excluded patients belonging to races other than whites and blacks) and comparable to those with eGFR≥60 ml/min per 1.73 m2. Proteinuria data were missing for about 50% of the study population; however, a sensitivity analysis restricting it to those with available urinary data showed similar findings. While we adjusted for several different confounding variables, we lacked details relating to screening procedures for cancer, treatment details for cancer, etc. It is also important to point out that we compared the risk for each type of death based on kidney disease and other characteristics. However, we did not examine the risk for underlying causes of death in various stages of kidney disease and based on other characteristics which could be examined in the future. Lastly, we obtained cause-specific deaths from the Ohio State Department of Health mortality files which provides data to the National Death Index for which there might be coding issues. However, these death indexes have been used by several studies in the past and are considered reliable.33–35 Our internal validation process of those who died within our healthcare system also showed the reliability of these data.
In summary, our study found that cardiovascular diseases and malignancy were the leading causes of death among this non–dialysis-dependent CKD followed in a large healthcare system. A lower level of kidney function was associated with higher risk for cardiovascular mortality and deaths due to non-cardiovascular/non-malignancy diseases. Furthermore, blacks with non–dialysis-dependent CKD had a higher risk of cardiovascular deaths compared with whites. Additional studies among other CKD populations are needed to confirm these findings, and explain the mechanisms for the observed cause-specific death differences.
Concise Methods
Patient population
We conducted an analysis using our preexisting electronic health record (EHR)-based CKD registry. The development and validation of it have been described in detail elsewhere.36 Briefly, patients who had at least one face-to-face outpatient encounter with a Cleveland Clinic healthcare provider and had two eGFR values <60 ml/min per 1.73 m2 more than 90 days apart between January 1, 2005, and September 15, 2009 were included. Patients aged <18 years old, and who were already diagnosed with ESRD needing dialysis or renal transplant were excluded. Patients who did not have ESRD prior to being included in the registry but subsequently developed ESRD or died during the study period are retained in the CKD registry. For this analysis, we included patients who met the following criteria: (a) had a valid social security number, (b) were residents of the State of Ohio, and (c) self-identified as either white or black (Supplementary Figure 1).
Kidney function
All serum creatinine measurements for the study population were performed in the same clinical laboratory by a Hitachi D 2400 Modular Chemistry Analyzer thereafter (Roche Diagnostics, Indianapolis, IN). CKD was classified into following stages: stage 3 CKD (eGFR 30–59 ml/min per 1.73 m2), stage 4 CKD (eGFR 15–29 ml/min per 1.73 m2), and stage 5 CKD (eGFR<15 ml/min per 1.73 m2). We calculated eGFR using the CKD-EPI equation.37 We further categorized stage 3 into CKD stage 3a (eGFR 45–59 ml/min per 1.73 m2) and stage 3b (eGFR 30–44 ml/min per 1.73 m2). To reflect clinical practice, patients who had a urine dipstick measurement, UACR, urine protein-to-creatinine ratio, and 24-hour urine studies were included to assess whether they had proteinuria or not. The following cut-offs were considered in determining whether someone had proteinuria: presence of ≥1+ proteinuria in dipstick studies, >30 mg/g in those who had UACR and urine protein-to-creatinine ratio studies, and >30 mg proteinuria in 24-hour studies.
Covariates
Demographic details (age, gender, race, insurance details) were extracted from the EHR. Comorbid conditions such as diabetes mellitus, hypertension, coronary artery disease, malignancy, congestive heart failure, and hyperlipidemia were defined using prespecified criteria and were validated in an earlier publication.36 These conditions existed prior to the second eGFR<60 ml/min per 1.73 m2. We also extracted relevant laboratory data (serum albumin and hemoglobin) from the EHR.
Ascertainment of death and its causes
We linked our CKD registry with the Social Security Death Index and Ohio Department of Health mortality data to obtain details about mortality rates and cause-specific mortality details. The underlying cause of death was coded according to the International Classification of Diseases, Tenth Revision. We grouped the underlying causes of death as per the National Center for Health Statistics for each coding system except for some changes as outlined below. We classified deaths into the following four major categories along with reporting data for individual causes: (a) cardiovascular deaths, (b) malignancy, (c) non-cardiovascular/non-malignancy related and (d) other non–disease-related deaths (such as homicide/suicide/accidents). We defined cardiovascular deaths as deaths due to diseases of the heart, essential hypertension, cerebrovascular disease, atherosclerosis or other diseases of the circulatory system (International Classification of Diseases, Tenth Revision codes I00–I78). We also categorized the cardiovascular deaths into following clinically meaningful subcategories: ischemic heart disease (I20–I25), heart failure (I50), cerebrovascular diseases (I60s) and all other cardiovascular disease (include all others from I00 to I78 except I20–I25, I50, and I60). We have merged our CKD registry with the US Renal Data System (USRDS) to obtain details about those who transitioned to dialysis (up to September 15, 2009). To ensure that analysis represents only non–dialysis-dependent CKD, we censored patients at the time of transitioning to dialysis. All patients who survived until September 15, 2009 (last date USRDS data available) were censored on that date for mortality analyses.
Statistical analysis
We compared baseline characteristics among patients in various stages using chi-squared and ANOVA tests for categorical and continuous variables, respectively. Unadjusted mortality rates (per 1000 years of follow-up) were calculated overall and for various stages of CKD separately. We used a Poisson model to estimate age-adjusted death rates across Kidney Disease: Improving Global Outcomes risk groups. We tabulated the leading causes of pre-ESRD death overall and within various eGFR categories. We evaluated the relationship between eGFR categories, age >65, race, gender, and diabetes with various pre-ESRD mortality outcomes. We inspected log log plots for violations of the proportional hazards assumption. We fit separate univariable Cox proportional hazards models for each cause of death outcome (all-cause deaths, deaths due to cardiovascular disease, malignancy, and non-cardiovascular, non-malignant deaths). We then fit multivariable models for each mortality outcome including the following covariates: eGFR group, age, race, gender, diabetes, hypertension, hyperlipidemia, BMI, malignancy, coronary artery disease, congestive heart failure, insurance, use of Angiotensin converting enzyme inhibitors/angiotensin receptor blockers, statins and beta blockers, albumin, hemoglobin, and smoking. Nineteen percent of patients were missing serum albumin data, 16% were missing hemoglobin data, 19% were missing smoking status, 6% were missing BMI, and 3% were missing insurance. We used multiple imputations (SAS proc MI) with the Markov Chain Monte Carlo method and a single chain to impute five data sets with complete continuous and binary covariate data in a first step, and then in a second step we imputed insurance group on each of the five data sets using discriminant function analysis. All Cox models were performed on each of the five imputed data sets, and parameter estimates were combined using SAS MIanalyze. We also fit similar adjusted models with patients classified into age >65 versus <65, because prior research has shown age-related differences among the general population.38 A separate analysis using eGFR as a continuous variable was also conducted. We tested the non-linearity of eGFR with restricted cubic splines. Methods relating to other additional sensitivity analyses are presented in the supplemental file (Supplementary methods and results).
All analyses were conducted using Unix SAS version 9.2 (SAS Institute, Inc., Cary, NC), and graphs were created using R 3.0.1 (The R Foundation for Statistical Computing, Vienna, Austria). The CKD registry and this study were approved by the Cleveland Clinic Institutional Review Board.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors wish to thank Welf Saupe, Vicky Konig, and John Sharp of Cleveland Clinic who helped in data extraction during the development of the registry.
Supported by grants R01-DK101500 (to S.D.N.), DK094112 (to J.V.N.), and 1K23-DK091363 (to S.E.J.). The authors have no relevant financial interest in the study. The creation of the CCF CKD registry was funded by an unrestricted grant from Amgen, Inc. to the Department of Nephrology and Hypertension Research and Education Fund.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Parts of this manuscript were presented as a poster at the annual American Society of Nephrology meeting held in Philadelphia, PA, November 11–16, 2014.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Not All Deaths in CKD Are from a Broken Heart,” on pages 2307–2308.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014101034/-/DCSupplemental.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, Stehman-Breen C, Gillen D, Bleyer AJ, Hirsch C, Siscovick D, Newman AB: Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 16: 3728–3735, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Center for Disease Control and Prevention : Deaths: Preliminary data for 2011. National Vital Statistics Reports. 61: 1–52, 2012 [PubMed] [Google Scholar]
- 7.Lowrance WT, Ordoñez J, Udaltsova N, Russo P, Go AS: CKD and the risk of incident cancer. J Am Soc Nephrol 25: 2327–2334, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alves TP, Wang X, Wright JT, Jr, Appel LJ, Greene T, Norris K, Lewis J, AASK Collaborative Research Group : Rate of ESRD exceeds mortality among African Americans with hypertensive nephrosclerosis. J Am Soc Nephrol 21: 1361–1369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 14: 2902–2907, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Derose SF, Rutkowski MP, Levin NW, Liu IL, Shi JM, Jacobsen SJ, Crooks PW: Incidence of end-stage renal disease and death among insured African Americans with chronic kidney disease. Kidney Int 76: 629–637, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX, Chronic kidney disease and mortality risk : a systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M, Alberta Kidney Disease Network : Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 13.United States Renal Data System : 2013. Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 14.Muntner P, He J, Hamm L, Loria C, Whelton PK: Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 13: 745–753, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Wen CP, Matsushita K, Coresh J, Iseki K, Islam M, Katz R, McClellan W, Peralta CA, Wang H, de Zeeuw D, Astor BC, Gansevoort RT, Levey AS, Levin A, Chronic Kidney Disease Prognosis Consortium : Relative risks of chronic kidney disease for mortality and end-stage renal disease across races are similar. Kidney Int 86: 819–827, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurth T, de Jong PE, Cook NR, Buring JE, Ridker PM: Kidney function and risk of cardiovascular disease and mortality in women: a prospective cohort study. BMJ 338: b2392, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ: The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis 51: 212–223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong G, Hayen A, Chapman JR, Webster AC, Wang JJ, Mitchell P, Craig JC: Association of CKD and cancer risk in older people. J Am Soc Nephrol 20: 1341–1350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weng PH, Hung KY, Huang HL, Chen JH, Sung PK, Huang KC: Cancer-specific mortality in chronic kidney disease: longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol 6: 1121–1128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS: Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 18: 2758–2765, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O’Hare AM: White/black racial differences in risk of end-stage renal disease and death. Am J Med 122: 672–678, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babayev R, Whaley-Connell A, Kshirsagar A, Klemmer P, Navaneethan S, Chen SC, Li S, McCullough PA, Bakris G, Bomback A, KEEP Investigators : Association of race and body mass index with ESRD and mortality in CKD stages 3-4: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 61: 404–412, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Masters RK, Hummer RA, Powers DA, Beck A, Lin SF, Finch BK: Long-term trends in adult mortality for U.S. Blacks and Whites: an examination of period- and cohort-based changes. Demography 51: 2047–2073, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith GL, Shlipak MG, Havranek EP, Masoudi FA, McClellan WM, Foody JM, Rathore SS, Krumholz HM: Race and renal impairment in heart failure: mortality in blacks versus whites. Circulation 111: 1270–1277, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Deswal A, Petersen NJ, Urbauer DL, Wright SM, Beyth R: Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am Heart J 152: 348–354, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Gutiérrez OM, Khodneva YA, Muntner P, Rizk DV, McClellan WM, Cushman M, Warnock DG, Safford MM, REGARDS Investigators : Association between urinary albumin excretion and coronary heart disease in black vs white adults. JAMA 310: 706–714, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powe NR: Let’s get serious about racial and ethnic disparities. J Am Soc Nephrol 19: 1271–1275, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Lind M, Garcia-Rodriguez LA, Booth GL, Cea-Soriano L, Shah BR, Ekeroth G, Lipscombe LL: Mortality trends in patients with and without diabetes in Ontario, Canada and the UK from 1996 to 2009: a population-based study. Diabetologia 56: 2601–2608, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Færch K, Carstensen B, Almdal TP, Jørgensen ME: Improved survival among patients with complicated type 2 diabetes in Denmark: a prospective study (2002-2010). J Clin Endocrinol Metab 99: E642–E646, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Romon I, Rey G, Mandereau-Bruno L, Weill A, Jougla E, Eschwège E, Simon D, Druet C, Fagot-Campagna A: The excess mortality related to cardiovascular diseases and cancer among adults pharmacologically treated for diabetes—the 2001-2006 ENTRED cohort. Diabet Med 31: 946–953, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC: Long-term mortality after gastric bypass surgery. N Engl J Med 357: 753–761, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Iso H, Jacobs DR, Jr, Goldman L: Accuracy of death certificate diagnosis of intracranial hemorrhage and nonhemorrhagic stroke. The Minnesota Heart Survey. Am J Epidemiol 132: 993–998, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Lee TA, Pickard AS, Au DH, Bartle B, Weiss KB: Risk for death associated with medications for recently diagnosed chronic obstructive pulmonary disease. Ann Intern Med 149: 380–390, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Navaneethan SD, Jolly SE, Schold JD, Arrigain S, Saupe W, Sharp J, Lyons J, Simon JF, Schreiber MJ, Jr, Jain A, Nally JV, Jr: Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol 6: 40–49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jolly SE, Burrows NR, Chen SC, Li S, Jurkovitz CT, Norris KC, Shlipak MG: Racial and ethnic differences in mortality among individuals with chronic kidney disease: results from the Kidney Early Evaluation Program (KEEP). Clin J Am Soc Nephrol 6: 1858–1865, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.