Abstract
Patients with CKD often progress to ESRD and develop cardiovascular disease (CVD), yet available therapies only modestly improve clinical outcomes. Observational studies report independent associations between elevated serum phosphate and fibroblast growth factor 23 (FGF23) levels and risks of ESRD, CVD, and death. Phosphate excess induces arterial calcification, and although elevated FGF23 helps maintain serum phosphate levels in the normal range in CKD, it may contribute mechanistically to left ventricular hypertrophy (LVH). Consistent epidemiologic and experimental findings suggest the need to test therapeutic approaches that lower phosphate and FGF23 in CKD. Dietary phosphate absorption is one modifiable determinant of serum phosphate and FGF23 levels. Limited data from pilot studies in patients with CKD stages 3–4 suggest that phosphate binders, low phosphate diets, or vitamin B3 derivatives, such as niacin or nicotinamide, may reduce dietary phosphate absorption and serum phosphate and FGF23 levels. This review summarizes current knowledge regarding the deleterious systemic effects of phosphate and FGF23 excess, identifies questions that must be addressed before advancing to a full-scale clinical outcomes trial, and presents a novel therapeutic approach to lower serum phosphate and FGF23 levels that will be tested in the COMBINE Study: The CKD Optimal Management With BInders and NicotinamidE study.
Keywords: phosphate uptake, phosphate binders, chronic kidney disease
CKD is associated with impaired physical and cognitive function, and it shortens lifespan and consumes healthcare resources.1–3 Evidence from clinical trials of angiotensin-converting enzyme inhibitors and statins supports the importance of targeting glomerular hemodynamics and dyslipidemia in CKD.4,5 Despite increasing utilization of these proven protective interventions, the frequency of adverse clinical events in patients with CKD remains high.6 At the 2011 National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Workshop on Reducing the Impact of CKD: Opportunities for Randomized Clinical Trials, phosphate and fibroblast growth factor 23 (FGF23) excess were cited as leading candidates to test in a future full-scale randomized clinical trial aimed at improving outcomes in CKD. However, workshop participants identified major knowledge gaps regarding efficacy assessments, optimal interventions and appropriate outcomes. Scientists called for additional pilot studies to generate high-quality data that could address these gaps before embarking on a long-term, costly outcomes trial targeting phosphate and FGF23 excess in CKD (Figure 1). The purpose of this review is to present data supporting phosphate and FGF23 excess as potential modifiable targets in CKD, provide an overview of prior studies that aimed to lower phosphate and FGF23 levels in CKD, and outline a development plan that will address critical unanswered questions. Within this context, we briefly describe the justification, hypotheses, and design features of the NIDDK-funded the CKD Optimal Management With BInders and NicotinamidE (COMBINE) Study and how its completion will inform the design of a full-scale trial (Supplemental Material).
Figure 1.
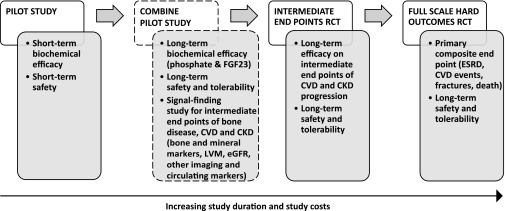
Multi-step development of phosphate and FGF23 reduction strategies in CKD. Schematic representation of the multi-step process for testing the utility of phosphate and FGF23 reduction strategies in CKD. The COMBINE (CKD Optimal Management with Binders and Nicotinamide) study and its objectives are represented by the boxes with dashed outlines.
CKD and Risks of Adverse Outcomes
CKD is a strong independent risk factor for ESRD, cardiovascular disease (CVD), fractures, and death.2,7–11 While progression to ESRD is common in younger CKD patients,7,8 CVD and death prevail as more likely complications in older individuals and those with diabetes.10,11 Clinical guidelines acknowledge the interrelationships between CKD and CVD, and recommend that all patients with CKD be viewed as high risk for CVD.12 At the same time, the guidelines cite the dearth of interventions proven to reduce CVD risk in CKD patients and emphasize the need for novel therapeutic approaches.12
Elevated Phosphate and FGF23 as Potential Therapeutic Targets in CKD
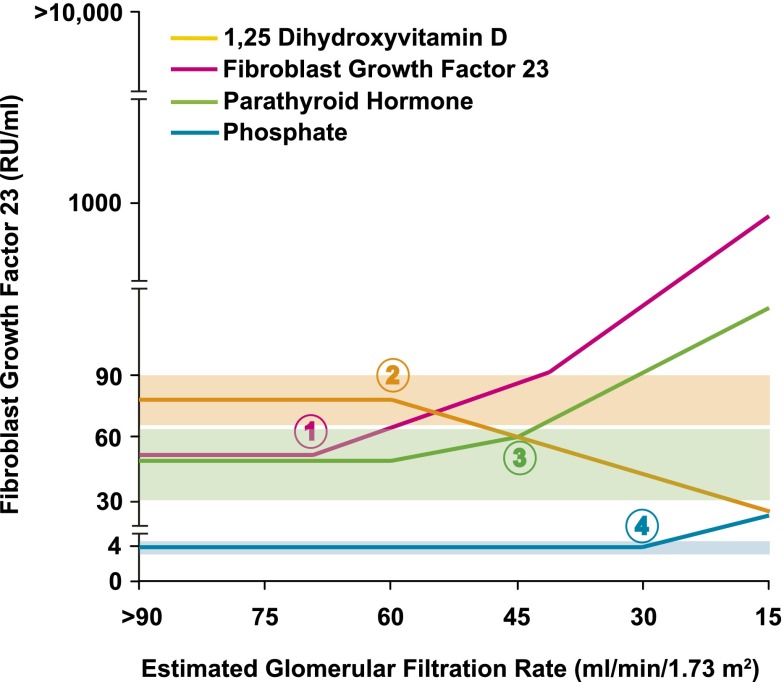
The typical biochemical phenotype of altered mineral metabolism in CKD stages 2–4 includes early and progressive FGF23 elevation, decline in calcitriol, increase in parathyroid hormone, and gradual rise in phosphate levels within the normal range (Figure 2).13 Emerging data suggest that phosphate and FGF23 excess in CKD may serve as novel modifiable targets. In addition to epidemiologic studies linking higher serum phosphate and FGF23 levels to ESRD, CVD, and death,14–21 experimental studies implicate high phosphate in the pathogenesis of vascular calcification and high FGF23 as a potential mechanistic contributor to the development of left ventricular hypertrophy (LVH) in CKD.22,23 Meta-analyses and pharmaco-epidemiologic studies support the association of phosphate excess with mortality in CKD.24,25 Thus, multiple lines of evidence (reviewed in detail in Scialla and Wolf26) support the need to conduct a randomized controlled trial of therapeutic approaches that lower phosphate and FGF23 to determine their impact on hard outcomes in patients with CKD.
Figure 2.
Biochemical phenotype of disordered mineral metabolism in CKD. The graph summarizes evolution of abnormal mineral metabolism along the spectrum of CKD. Depicted values are based on published literature. The x-axis represents glomerular filtration. The y-axis represents circulating levels of individual analytes with temporal changes in and normal ranges of FGF23 shown in red, 1,25 dihydroxyvitamin D (1,25D) shown in purple, parathyroid hormone (PTH) shown in green, and phosphate shown in blue. Elevated FGF23 is the earliest alteration in mineral metabolism in CKD (1). Elevations in FGF23 levels cause the early decline in 1,25D levels (2) that leads to secondary hyperparathyroidism (3). All of these changes occur prior to elevations in serum phosphate levels (4). This figure is reproduced from Wolf,102 with permission from the American Society of Nephrology. Copyright © [2010] the American Society of Nephrology. All rights reserved.
Testing Strategies to Reduce Phosphate and FGF23 Levels in CKD
Clinical guidelines do not recommend phosphate- or FGF23-lowering treatments for normophosphatemic patients with CKD stages 3–4.27,28 Therefore, definitive demonstration of the benefits of phosphate and FGF23 reduction in this population, which numbers in the millions in the United States, will have major implications on clinical practice and public health. Only a large randomized controlled clinical outcomes trial can generate such convincing evidence. Although several phosphate- and FGF23-lowering approaches have been tested in small short-term studies,29–32 additional pilot studies are needed to address major knowledge gaps and to inform the design of the full-scale trial (Figure 1). First, we need to evaluate the phosphate and FGF23 response to interventions aimed at lowering dietary phosphate absorption in long-term interventional studies of patients with CKD. This information is necessary as the full-scale trial is likely to be long, perhaps up to 5 years. Second, we must define which specific interventions most safely and sustainably lower phosphate and FGF23 levels in patients with CKD, and which interventions provide good long-term tolerability. The most efficacious and well-tolerated approach will be the ideal candidate to advance to the full-scale trial. Third, pilot studies should demonstrate that the chosen phosphate- and FGF23-lowering intervention has beneficial effects on intermediate endpoints of CKD complications. Such subclinical surrogates might include LVH, decline in eGFR, or development of secondary hyperparathyroidism. These proof-of-concept data would strengthen the justification for the definitive trial and would facilitate the choice of hard endpoints for the trial, which would likely include CVD events, ESRD, fractures, mortality, or a composite of several of these. By addressing important unanswered questions related to efficacy assessments, optimal interventions, and appropriate intermediate endpoints, completion of this multi-step development process would support the considerable investment required for a full-scale intervention trial (Figure 1).
Targeting Dietary Phosphate Absorption to Modify Phosphate and FGF23 Excess
Dietary phosphate absorption is one modifiable target that may influence serum phosphate and FGF23 levels. In physiologic studies of healthy humans, circulating FGF23 levels rose after 3–5 days of dietary phosphate loading in the absence of a significant increase in serum phosphate.33–35 However, when dietary phosphate loading follows a period of dietary phosphate depletion, serum phosphate levels rise modestly.34,36,37 In contrast, several days of exposure to very low phosphate diets combined with dietary phosphate binders lower both fasting levels of serum phosphate34,37 and FGF23.34 Taken together, the data from healthy volunteers demonstrate that aggressively reducing dietary phosphate absorption with multiple modalities can lower serum phosphate and FGF23 levels.
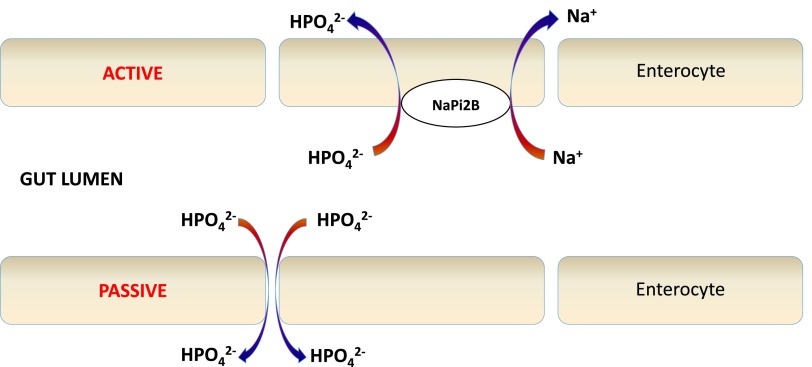
The dual contributions of passive paracellular diffusion and active cell-mediated transport to intestinal phosphate absorption can be leveraged in the design of synergistic serum phosphate- and FGF23-lowering interventions (Figure 3). High luminal phosphate concentration gradient drives passive paracellular diffusion through tight junctions across the intestinal mucosa. Active transport of phosphate occurs via sodium phosphate (NPT2b) co-transporters located on the luminal surface of enterocytes, and is induced by dietary phosphate depletion and calcitriol.38,39 Therefore, low dietary phosphate, luminal binding of dietary phosphate by a phosphate binder, and NPT2b blockade in combination may yield maximal reduction of dietary phosphate absorption.
Figure 3.
Dietary phosphate absorption in the small intestine. Intestinal absorption of dietary phosphate may occur by passive paracellular diffusion via tight junctions and by active transcellular transport via NPT2b, the major sodium phosphate co-transporter in the small intestine. Low phosphate diets and phosphate binders reduce luminal phosphate concentration, which may upregulate NPT2b-dependent dietary phosphate absorption. Because nicotinamide reduces NPT2b expression, use of this agent in combination with low phosphate diets and phosphate binders may maximize reductions in dietary phosphate absorption.
Expected Biochemical Response to Reduction of Dietary Phosphate Absorption
Several biochemical parameters can be used to identify which intervention most potently blocks dietary phosphate absorption. The characteristics of each have important implications for study design.
Urinary Phosphate Excretion
Under steady-state conditions, 24-hour urinary phosphate excretion matches daily dietary phosphate absorption, which is estimated to be approximately 70%–80% of daily dietary phosphate intake.40,41 Interventions that substantially lower dietary phosphate absorption typically30,42,43 but not always31,44 result in reductions in 24-hour urinary phosphate excretion, which may be detected even while serum phosphate levels remain unchanged. Inability to collect complete 24-hour urine collections or waning compliance with the interventions may complicate interpretation of 24-hour urinary phosphate data.
Serum Phosphate
Because serum phosphate levels typically rise very slowly within the normal range as CKD progresses, overt hyperphosphatemia is uncommon among patients with CKD whose eGFR is >30 ml/min per 1.73 m2.13 Given the ability of CKD patients to maintain serum phosphate within the normal range, it is not surprising that single interventions aimed at lowering dietary phosphate absorption, such as use of phosphate binders alone, have minimal, if any, effects on serum phosphate levels.45–47 Furthermore, serum phosphate levels are at their nadir in the morning and peak in the afternoon, when the largest differences in serum phosphate levels across a spectrum of dietary phosphate loads are noted.48,49 Because the normal diurnal rhythm in serum phosphate levels is preserved in CKD,48–51 studies that only measured morning fasting serum phosphate levels may have missed meaningful changes that might have been detected if phosphate was measured later in the day. Therefore, measurements in the afternoon are likely to be more sensitive at detecting changes in serum phosphate levels following interventions targeting dietary phosphate absorption.
Serum FGF23
Elevated FGF23 is the earliest and most common manifestation of disordered mineral metabolism in CKD.13 In contrast to serum phosphate, FGF23 levels do not vary substantially within individuals throughout the day, in relation to prandial status or over the course of weeks to months.51–53 Because FGF23 regulates serum phosphate levels through its endocrine effects on the kidney, gut, and parathyroid glands, FGF23 is a promising biomarker to detect responses to interventions aimed at lowering phosphate absorption. Interventional studies in healthy individuals33–35 and patients with CKD29–31,45,49,54–60 and experimental studies in animals39,61,62 treated with phosphate binders, nicotinamide, and low phosphate diets support FGF23 as a useful biomarker of phosphate-targeting interventions. In many, but not all, of these studies, serum phosphate was unchanged in response to interventions, but within days to weeks, FGF23 levels were reduced and found to correlate with lower urinary phosphate excretion. In these settings, FGF23 may have declined because less FGF23 was required to dispose of the reduced phosphate load, while maintaining normal serum phosphate levels.
Existing Therapeutic Modalities to Lower Phosphate and FGF23
The most effective and safe interventions to carry forward into a full-scale trial remain to be determined. Limited evidence from prior studies in patients with CKD supports the hypothesis that maximal reduction in dietary phosphate absorption may reduce serum phosphate and FGF23 levels, but findings have been inconsistent, highlighting the need for more research (Table 1).
Table 1.
Selected controlled studies of interventions aimed at phosphate and FGF23 reduction in patients with CKD
| Study | N | Mean GFR | Duration | Intervention | Summary of biochemical effects |
|---|---|---|---|---|---|
| Oliveira et al.30 | 40 | 35 | 6 weeks | Sevelamer hydrochloride | Compared with calcium acetate, sevelamer carbonate treatment led to: |
| Calcium acetate | • modest reduction in 24-hr urine phosphate | ||||
| • no change in serum phosphate levels | |||||
| • 40% reduction in FGF23 levels | |||||
| Isakova et al.31 | 39 | 38 | 12 weeks | 900 mg phosphate diet–lanthanum carbonate | Compared with ad libitum diet–placebo, 900 mg phosphate diet–lanthanum carbonate treatment led to: |
| Ad libitum diet–placebo | • no change in 24-hr urine phosphate | ||||
| • no change in serum phosphate levels | |||||
| • 35% reduction in FGF23 levels | |||||
| Block et al.45 | 145 | 31 | 36 weeks | Phosphate bindersa | Compared with placebo, phosphate binder treatment led to: |
| Placebo | • 22% reduction in 24-hr urine phosphate | ||||
| • reduction in serum phosphate levels from a mean of 4.2–3.9 mg/dl | |||||
| • no reduction in C-terminal FGF23 levels | |||||
| Intact FGF23 levels reduced with sevelamer carbonate, but not with calcium acetate or with lanthanum carbonate | |||||
| Intact FGF23 levels increased with calcium acetate treatment | |||||
| Block et al.57 | 141 | 24 | 12 weeks | Ferric citrate | Compared with placebo, ferric citrate treatment led to: |
| Placebo | • 39% reduction in 24-hr urine phosphate | ||||
| • reduction in serum phosphate levels from a mean of 4.5–3.9 mg/dl | |||||
| • reduction in FGF23 levels from a median of 159–105 pg/ml | |||||
| Ix, Rao et al.32,60 | 261 | 52 | 24 weeks | Niacin | Compared with placebo, niacin treatment led to: |
| Placebo | • no data on 24-hr urine phosphate | ||||
| • reduction in serum phosphate levels | |||||
| • 11% reduction in FGF23 levels | |||||
| Chue et al.89 | 109 | 50 | 36 weeks | Sevelamer carbonate | Compared with placebo, sevelamer carbonate treatment led to: |
| Placebo | • no change in 24-hr urine phosphate | ||||
| • no change in serum phosphate levels | |||||
| • no change in FGF23 levels | |||||
| Reduction in FGF23 levels among individuals compliant with active therapy | |||||
| Seifert et al.47 | 38 | 46 | 48 weeks | Lanthanum carbonate | Compared with placebo, lanthanum carbonate treatment led to: |
| Placebo | • no change in 24-hr urine phosphate | ||||
| • no change in serum phosphate levels | |||||
| • no change in FGF23 levels | |||||
| Moe et al.29 | 9 | 32 | 1 week | Vegetarian diet | Compared with meat diet, vegetarian diet in this crossover study led to: |
| Meat diet | • Reduction in 24-hr urine phosphate from a mean of 778–416 mg/day | ||||
| • Reduction in serum phosphate levels from a mean of 3.5–3.2 mg/dl | |||||
| • Reduction in FGF23 levels from a mean of 84–61 pg/ml | |||||
| Diorio et al.58 | 32 | 30 | 1 week | Very low protein diet | Compared with low protein diet, very low protein diet in this crossover study led to: |
| Low protein diet | • 34% reduction in 24-hr urine phosphate | ||||
| • 12% reduction in serum phosphate levels | |||||
| • 34% reduction in FGF23 levels |
Active therapy included calcium acetate, sevelamer hydrochloride, and lanthanum carbonate.
Phosphate Binders
Phosphate binders are the mainstay in the management of hyperphosphatemic patients undergoing dialysis because they effectively reduce dietary phosphate absorption.63,64 In dose-finding studies performed in healthy volunteers, phosphate binders potently reduce urinary phosphate excretion, while maintaining serum phosphate levels within the normal range.65,66 Similar findings have been observed in normophosphatemic patients with CKD stages 3−4 in whom phosphate binders, but not placebo, reduce 24-hour urine phosphate by 20%–50%.30,42,45,67 The effects of phosphate binders on levels of FGF23 in patients with CKD are not consistent. Several studies suggest that non-calcium-based phosphate binders lower FGF23 levels in this population by 30%–40%,30,31,45,54–57 whereas calcium-based binders do not,22,38 likely because calcium is a secondary stimulus for FGF23 production.68,69 Taken together, the effect of phosphate binders on serum phosphate and FGF23 levels is relatively small. Insights from elegant experimental studies described below suggest that the potency of phosphate binders may, in part, be offset by the contribution of active transport to total intestinal phosphate absorption.62,70,71
Nicotinamide
Nicotinamide (vitamin B3, also called niacinamide) reduces intestinal NPT2b expression.62,72 This is thought to be one of the mechanisms by which nicotinamide and its derivatives reduce dietary phosphate absorption73 and lower serum phosphate levels in animals62 and in patients with ESRD.74–77 Comparable data have also emerged from pooled analyses of two trials of dyslipidemic patients with CKD stage 3 treated for 24 weeks with niacin or matched placebo. Active treatment resulted in a significant and sustained decrease in the fasting levels of serum phosphate (between-groups change in phosphate level = −0.42 mg/dl; 95% confidence interval, −0.52 to −0.33).32 The same intervention also resulted in an approximately 11% decline in FGF23 levels in the niacin-treated group compared with placebo.60 The efficacy of blocking dietary phosphate absorption on biochemical and even cardiovascular endpoints in CKD is also supported by results from recent experimental studies. When an inhibitor of intestinal sodium-hydrogen exchanger-3 that also blocked dietary phosphate absorption78 was administered to rats with CKD, it decreased urinary phosphate excretion, reduced serum phosphate and FGF23 levels, attenuated vascular calcification, and reduced heart mass.79
Nicotinamide may also promote phosphaturia. Intra-peritoneal delivery of nicotinamide increased urinary phosphate excretion without changing urinary excretion of creatinine, potassium, sodium, calcium, or urinary flow rates in rats.80 Similar observations were made in parathyroidectomized rats, suggesting a PTH-independent effect.80,81 Further evidence in support of nicotinamide’s phosphaturic effect comes from studies of rats with normal kidney function treated for 4 days with niceritrol, a nicotinic acid derivative.73 In the treated animals compared with controls, urinary phosphate excretion was not decreased despite a significant increase in stool phosphate content. This observation suggests that, in addition to blocking active phosphate transport in the gut, niceritrol and other nicotinic acid derivatives may induce a renal phosphate leak, perhaps by reducing the levels of renal sodium phosphate co-transporters (NPT2a and NPT2c).82 These dual actions may explain the sustained hypophosphatemic effects of niacin in patients with CKD stage 332 and were also recently implicated as the causal mechanisms for hepatectomy-related hypophosphatemia, in which renal and intestinal sodium phosphate transport were decreased due to increased levels of nicotinic acid derivatives.82
Experimental studies have shown that exposure to phosphate-depleted diets or phosphate binders upregulates NPT2b expression, and leads to enhanced dietary phosphate absorption when dietary phosphate loading is reinstated.39,62,70 This is consistent with the observations from human studies that demonstrate spikes in serum phosphate with dietary phosphate loading only after a period of dietary phosphate depletion,29,31,32 which is presumably due to a compensatory increase in active intestinal phosphate transport in the setting of low luminal dietary phosphate. These data suggest that attempts to reduce dietary phosphate absorption using phosphate binders alone may be limited by NPT2b upregulation and resultant increases in dietary phosphate absorption at times when phosphate binders are not present in the intestinal lumen. By blocking intestinal active transport, addition of nicotinamide to binder regimens may therefore synergistically maximize reduction of dietary phosphate absorption. Furthermore, nicotinamide’s phosphaturic effect may also contribute to greater reduction in serum phosphate and FGF23 levels in CKD when phosphate binders are used in combination with blockade of sodium phosphate transporters in the gut and kidney. Proof-of-concept data in support of this hypothesis come from experimental studies of NPT2b-deficient uremic mice.39 Compared with wild-type uremic mice, NPT2b-deficient uremic animals had lower phosphate and FGF23 levels, and treatment with sevelamer carbonate further reduced serum phosphate and FGF23 levels. In contrast, the tested sevelamer dose did not induce significant reductions in serum phosphate in the wild-type uremic mice, although there was a trend for a decline in FGF23 levels. Human studies are under way to test the possibility of synergistic or additive benefit from phosphate binders combined with nicotinamide in CKD. Importantly, unlike the lipid-lowering drug niacin, which contains both nicotinamide and nicotinic acid, nicotinamide does not cause flushing and is thought to be less likely to cause other adverse effects related to the nicotinic acid moiety, including liver test abnormalities, hyperuricemia, and insulin resistance.83
Dietary Phosphate Restriction
Dietary interventions that reduce phosphate intake or modify its dietary sources, and hence bioavailability, can lower serum phosphate and FGF23.29,58,59 Sources of dietary phosphate in order of increasing bioavailability are organic phosphate from plants and legumes, organic phosphate from animal and dairy sources, and inorganic phosphates that are used by the food industry as additives to enhance flavor, appearance, and shelf life of processed meats, cheeses, baked goods, and beverages.84–86 Consistent with the bioavailability data, serum and urinary phosphate and FGF23 were significantly lower in uremic rats fed a grain-based versus a meat-based diet.87 Similar findings were reported in a crossover study of patients with CKD stages 3–4 who were fed grain-based and meat-based diets that contained identical total phosphate content.29 Compared with a meat-based diet, a grain-based diet led to a 27% decrease in FGF23 and a 9% reduction in serum phosphate (Table 1).29
Another approach to reduce phosphate consumption is to target inorganic phosphate additives. In a randomized trial of hyperphosphatemic ESRD patients, educating patients how to read food labels and to select alternative items when they detected a phosphate-based additive on the label was followed by a significant reduction in serum phosphate compared with no counseling.88 These data suggest that substituting high phosphate bioavailability foods with lower bioavailability alternatives will reduce net phosphate absorption. This approach may facilitate the efficacy of other phosphate- and FGF23-lowering interventions, as has been shown in a pilot study that tested the combination of phosphate binder therapy with dietary phosphate reduction in patients with CKD stages 3–4.31
Possible Intermediate Endpoints
In addition to demonstrating that the chosen interventions aimed at reducing dietary phosphate absorption safely lower phosphate and FGF23, a key step will be to test whether the candidate therapies also have beneficial systemic effects. Prior studies attempted to test the hypothesis that phosphate- and FGF23-lowering interventions would improve intermediate CVD endpoints, including LVH, vascular dysfunction, and vascular calcification.45,47,55,89 However, no pilot study has tested whether the combination of phosphate binders and nicotinamide will have beneficial effects on markers of bone and mineral metabolism and on intermediate endpoints of CVD and CKD progression in patients with CKD stages 3–4. The results from such a study will help identify which of the tested surrogate markers should be advanced as the primary endpoints to a larger randomized study to test the utility of phosphate and FGF23 lowering on intermediate endpoints of CVD, renal, and skeletal risks (Figure 1). Taken together, the findings from these studies will be critical to define appropriate hard endpoints for the phase III trial (Figure 1). These may include some combination of congestive heart failure, other CVD events, CVD hospitalizations, CKD progression, ESRD, fractures, and mortality. Below, we provide justification for some of the intermediate endpoints that will be evaluated in the COMBINE study (Table 2).
Table 2.
The COMBINE Study Hypotheses
| Primary hypotheses |
| 1. Compared with placebo, monotherapy with nicotinamide, monotherapy with lanthanum carbonate, and combined nicotinamide and lanthanum carbonate therapy will lower serum phosphate and FGF23 levels |
| 2. Combined treatment with nicotinamide and lanthanum carbonate will be safe, well tolerated, and acceptable to CKD participants, demonstrating feasibility of long-term compliance in a subsequent phase 3 clinical trial |
| Secondary hypotheses |
| 1. Combined active therapy with nicotinamide and lanthanum carbonate will lower serum phosphate and FGF23 levels more than nicotinamide alone or lanthanum carbonate alone |
| 2. Compared with placebo, active therapy with nicotinamide and lanthanum carbonate will blunt the slope of PTH rise, attenuate the decline of calcitriol and klotho levels, and improve bone turnover markers, P1NP and Trap-5b |
| 3. Compared with placebo, active therapy with nicotinamide and lanthanum carbonate will reduce or blunt the increase in LV mass index; attenuate LV diastolic dysfunction, indicated by increased LV end diastolic volume and decreased left atrial volume (all measures assessed by gadolinium-free cardiac MRI); and reduce levels of biomarkers that are associated with CVD: BNP, troponin T, cholesterol, and ADMA |
| 4. Compared with placebo, active therapy with nicotinamide and lanthanum carbonate will blunt the slope of decline in GFR and the rise in proteinuria and the inflammatory markers, CRP and IL-6, improve intra-renal oxygenation and stabilize or reduce progression of renal fibrosis, as assessed by gadolinium-free renal BOLD MRI and diffusion-weighted MRI |
P1NP, N terminal propeptide of Type 1 procollagen; Trap-5b, tartrate-resistant acid phosphatase; LV, left ventricular; BNP, brain natriuretic peptide; ADMA, asymmetric dimethyl arginine; GFR, glomerular filtration rate; CRP, C-reactive protein; IL-6, interleukin 6; BOLD, blood oxygenation level dependent.
Imaging Biomarkers of CVD Risk
LVH is a common pattern of CVD in CKD and is an established risk factor for CVD events and cardiovascular mortality.90 High FGF23 and serum phosphate levels are associated with elevated left ventricular mass.23,46 Cardiac magnetic resonance imaging (MRI) provides the most precise measurements of left ventricular mass and chamber geometry,91 and is especially useful for detecting small changes over time given its high resolution, reproducibility, and low variability.92 In addition to structural assessments, cardiac MRI yields important functional measures that can identify diastolic dysfunction, such as left ventricular end diastolic volume and left atrial volume. The latter are critical in relatively shorter-term studies when beneficial changes in cardiac function are likely to antedate architectural changes in cardiac structure.
Imaging Biomarkers of CKD Progression
Phosphate and FGF23 excess have been linked to accelerated CKD progression,19 but whether these represent causal effects is unknown. Renal fibrosis is a final common pathway for CKD progression and is a key determinant of disease severity.93,94 Renal hypoxia, an important determinant of acute kidney injury,95 has also been implicated in the pathogenesis of renal fibrosis in CKD.96,97 Gadolinium-free diffusion-weighted MRI may quantify renal fibrosis and blood oxygenation level dependent MRI can evaluate intra-renal oxygenation.98–100 These novel methods have not yet been applied to interventional studies designed to lower phosphate and FGF23 levels in patients with CKD.
Circulating Biomarkers of Bone and Mineral Metabolism
Phosphate and FGF23 excess play a central role in the pathogenesis of disordered bone and mineral metabolism in CKD.13,101 However, human interventional data directly implicating phosphate and FGF23 excess in the development of secondary hyperparathyroidism, calcitriol deficiency, and abnormal bone metabolism are lacking. Given that serum phosphate and FGF23 levels would be expected to rise over time in untreated patients with progressive CKD,19 a placebo-controlled randomized study aimed at phosphate and FGF23 reduction will permit this important hypothesis to be tested by serial measurement of biochemical markers, including PTH, klotho, calcitriol, and bone turnover markers (Table 2).
Brief Overview of the COMBINE Study
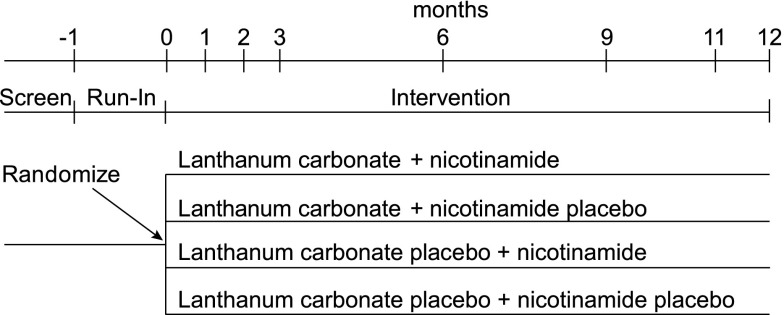
In order to advance testing of promising therapeutic targets in CKD, the NIDDK convened a consortium of clinical centers and a data coordinating center funded by a cooperative agreement (U01; RFA-DK-12–016) to design and conduct pilot studies that would inform the design of a full-scale randomized clinical trial with hard outcomes in CKD. Within this consortium, the COMBINE Study will test the hypothesis that use of nicotinamide combined with lanthanum carbonate on a background of reduced dietary phosphate intake safely reduces serum phosphate and FGF23 levels over 12 months in 200 patients with stages 3–4 CKD. Tables 2 and 3 and Figure 4 display the main features of the COMBINE study. During the first month following randomization, the dose of nicotinamide will be 750 mg once daily and the dose of lanthanum carbonate will be 500 mg three times daily with meals. After the first month, the dose of nicotinamide will be increased to 750 mg twice daily and the dose of lanthanum carbonate will be increased to 1000 mg three times daily with meals. All participants will receive information on how to reduce dietary phosphate intake. Study visits will take place in the afternoon to ensure that serum phosphate levels are measured at a time when the maximal differences between the intervention arms could be detected. Serial 24-hour urine collections will also be obtained to assess impact on dietary phosphate absorption. Pill counts and questionnaires will be used to assess compliance. Tolerability will be summarized as the percentage of persons who come off study drug, and safety will be defined by the number and percentage of the participants who report at least one adverse event. In addition to testing the primary endpoints of safety and biochemical efficacy, there will be longitudinal assessments of circulating biomarkers of bone and mineral metabolism and circulating and MRI imaging intermediate measures of CVD and renal risks at baseline and at 12 months post-randomization (Table 2). By addressing unanswered questions regarding the biochemical response, optimal interventions and intermediate endpoints, the results of the COMBINE study will provide important information to inform the design of a future full-scale outcomes trial. Enrollment began in March 2015, and results are anticipated by 2018.
Table 3.
The COMBINE Study Inclusion and Exclusion Criteria
| Key inclusion criteria |
| Patients with eGFR 20–45 ml/min/1.73 m2 |
| Age 18–85 years |
| Serum phosphate≥2.8 mg/dl |
| Platelet count≥125,000/mm3 |
| Key exclusion criteria |
| History of allergic reaction to nicotinamide, or lanthanum carbonate |
| Liver disease |
| Elevated creatine kinase concentrations (>2 times the upper limit of normal) |
| Major hemorrhagic event within the past 6 months requiring in-patient admission |
| Blood or platelet transfusion within the past 6 months |
| Secondary hyperparathyroidism (parathyroid hormone >5 times the upper limit of normal) |
| Malabsorption |
| Anemia (hemoglobin<9.0 g/dl) |
| Serum albumin<2.5 mg/dl |
| Anticipated initiation of dialysis or kidney transplantation within 12 months |
| Use of immunosuppressive medications |
| Recent (within 14 days) initiation or change in dose of active vitamin D or its analogs |
| Recent treatment (within 14 days) with phosphate binder or niacin/nicotinamide |
| Current participation in another clinical trial or other interventional research |
| Currently taking investigational drugs |
| Institutionalized individuals |
| Malignancy requiring therapy within 2 years |
| Pregnancy or planning to become pregnant or currently breast-feeding |
| Life expectancy<12 months |
| Hospitalization within 30 days of screening |
Figure 4.
The COMBINE study schema. The COMBINE study is a randomized, double-blind, placebo-controlled, 12-month study of 200 CKD stages 3–4 patients that will test the hypothesis that nicotinamide and lanthanum carbonate will safely lower serum phosphate and FGF23 levels compared with placebo.
Disclosures
T.I. has received honoraria from Bayer and consulting fees from Guidepoint Global and Daiichi Sankyo. J.H.I. has consulted for AstraZeneca. S.M.S. has received research support, honoraria, or consultant fees from Amgen, Inc., Shire, NPS, OPKO, Fresenius, and Vifor. M.W. has received research support, honoraria, or consultant fees from Shire, Keryx, Shield, Sanofi, Luitpold, OPKO, Panion, and Pfizer. G.A.B. has received research support, honoraria, or consultant fees from Keryx, Ardelyx, Shield, AstraZeneca, and Amgen, Inc.
Supplementary Material
Acknowledgments
T.I. is supported by grants K23-DK081673 and R01-DK102438 from the National Institute of Diabetes and Digestive and Kidney Diseases and by the Carl W. Gottschalk Research Scholar Grant from the American Society of Nephrology Foundation for Kidney Research.
K.L.R. is supported by the VA Career Development Award (IK2CX000537) and the Harold Amos Medical Faculty Development Award from the Robert Wood Johnson Foundation.
The COMBINE study is supported by grants U01-DK099877, U01-DK097093, U01-DK099930, U01-DK099933, U01-DK099924, and R01-DK102438 from the National Institute of Diabetes and Digestive and Kidney Diseases.
The authors would like to thank Orlando Gutiérrez, MD, MMSc, for his assistance with Figure 3.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015020117/-/DCSupplemental.
References
- 1.Weiner DE, Seliger SL: Cognitive and physical function in chronic kidney disease. Curr Opin Nephrol Hypertens 23: 291–297, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D: Medical costs of CKD in the Medicare population. J Am Soc Nephrol 24: 1478–1483, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group : The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R, SHARP Investigators : The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377: 2181–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J: Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305: 2532–2539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin A, Djurdjev O, Beaulieu M, Er L: Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis 52: 661–671, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Landray MJ, Emberson JR, Blackwell L, Dasgupta T, Zakeri R, Morgan MD, Ferro CJ, Vickery S, Ayrton P, Nair D, Dalton RN, Lamb EJ, Baigent C, Townend JN, Wheeler DC: Prediction of ESRD and death among people with CKD: the Chronic Renal Impairment in Birmingham (CRIB) prospective cohort study. Am J Kidney Dis 56: 1082–1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nickolas TL, McMahon DJ, Shane E: Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 17: 3223–3232, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, UKPDS GROUP : Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63: 225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, Seliger S, Siscovick D, Newman AB, Fried L: Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med 26: 379–385, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidney Disease. Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 3: S1–S150, 2013 [Google Scholar]
- 13.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Norris KC, Greene T, Kopple J, Lea J, Lewis J, Lipkowitz M, Miller P, Richardson A, Rostand S, Wang X, Appel LJ: Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 17: 2928–2936, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR: Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 20: 381–387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, HOST Investigators : FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M: Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol 24: 125–135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scialla JJ, Lau WL, Reilly MP, Isakova T, Yang HY, Crouthamel MH, Chavkin NW, Rahman M, Wahl P, Amaral AP, Hamano T, Master SR, Nessel L, Chai B, Xie D, Kallem RR, Chen J, Lash JP, Kusek JW, Budoff MJ, Giachelli CM, Wolf M, Chronic Renal Insufficiency Cohort Study Investigators : Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int 83: 1159–1168, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25: 349–360, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM: Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87: E10–E17, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF: Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 305: 1119–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Kovesdy CP, Kuchmak O, Lu JL, Kalantar-Zadeh K: Outcomes associated with phosphorus binders in men with non-dialysis-dependent CKD. Am J Kidney Dis 56: 842–851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scialla JJ, Wolf M: Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol 10: 268–278, 2014 [DOI] [PubMed] [Google Scholar]
- 27.National Kidney Foundation : K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 28.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR: Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 6: 257–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moysés RM: Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol 5: 286–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isakova T, Barchi-Chung A, Enfield G, Smith K, Vargas G, Houston J, Xie H, Wahl P, Schiavenato E, Dosch A, Gutiérrez OM, Diego J, Lenz O, Contreras G, Mendez A, Weiner RB, Wolf M: Effects of dietary phosphate restriction and phosphate binders on FGF23 levels in CKD. Clin J Am Soc Nephrol 8: 1009–1018, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ix JH, Ganjoo P, Tipping D, Tershakovec AM, Bostom AG: Sustained hypophosphatemic effect of once-daily niacin/laropiprant in dyslipidemic CKD stage 3 patients. Am J Kidney Dis 57: 963–965, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Antoniucci DM, Yamashita T, Portale AA: Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 91: 3144–3149, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Burnett SM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS: Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21: 1187–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Ferrari SL, Bonjour J-P, Rizzoli R: Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90: 1519–1524, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Dominguez JH, Gray RW, Lemann J, Jr: Dietary phosphate deprivation in women and men: effects on mineral and acid balances, parathyroid hormone and the metabolism of 25-OH-vitamin D. J Clin Endocrinol Metab 43: 1056–1068, 1976 [DOI] [PubMed] [Google Scholar]
- 37.Portale AA, Halloran BP, Murphy MM, Morris RC, Jr: Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans. J Clin Invest 77: 7–12, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radanovic T, Wagner CA, Murer H, Biber J: Regulation of intestinal phosphate transport. I. Segmental expression and adaptation to low-P(i) diet of the type IIb Na(+)-P(i) cotransporter in mouse small intestine. Am J Physiol Gastrointest Liver Physiol 288: G496–G500, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Schiavi SC, Tang W, Bracken C, O’Brien SP, Song W, Boulanger J, Ryan S, Phillips L, Liu S, Arbeeny C, Ledbetter S, Sabbagh Y: Npt2b deletion attenuates hyperphosphatemia associated with CKD. J Am Soc Nephrol 23: 1691–1700, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez JA, Emmett M, White MG, Fathi N, Santa Ana CA, Morawski SG, Fordtran JS: The absorption of dietary phosphorus and calcium in hemodialysis patients. Kidney Int 30: 753–759, 1986 [DOI] [PubMed] [Google Scholar]
- 41.Hruska KA, Mathew S, Lund R, Qiu P, Pratt R: Hyperphosphatemia of chronic kidney disease. Kidney Int 74: 148–157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isakova T, Gutiérrez OM, Smith K, Epstein M, Keating LK, Jüppner H, Wolf M: Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant 26: 584–591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sprague SM, Abboud H, Qiu P, Dauphin M, Zhang P, Finn W: Lanthanum carbonate reduces phosphorus burden in patients with CKD stages 3 and 4: a randomized trial. Clin J Am Soc Nephrol 4: 178–185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE: The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 72: 1255–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chue CD, Edwards NC, Moody WE, Steeds RP, Townend JN, Ferro CJ: Serum phosphate is associated with left ventricular mass in patients with chronic kidney disease: a cardiac magnetic resonance study. Heart 98: 219–224, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Seifert ME, de las Fuentes L, Rothstein M, Dietzen DJ, Bierhals AJ, Cheng SC, Ross W, Windus D, Dávila-Román VG, Hruska KA: Effects of phosphate binder therapy on vascular stiffness in early-stage chronic kidney disease. Am J Nephrol 38: 158–167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Portale AA, Halloran BP, Morris RC, Jr: Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest 80: 1147–1154, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ix JH, Anderson CA, Smits G, Persky MS, Block GA: Effect of dietary phosphate intake on the circadian rhythm of serum phosphate concentrations in chronic kidney disease: a crossover study. Am J Clin Nutr 100: 1392–1397, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calvo MS, Kumar R, Heath H, 3rd: Elevated secretion and action of serum parathyroid hormone in young adults consuming high phosphorus, low calcium diets assembled from common foods. J Clin Endocrinol Metab 66: 823–829, 1988 [DOI] [PubMed] [Google Scholar]
- 51.Isakova T, Xie H, Barchi-Chung A, Smith K, Sowden N, Epstein M, Collerone G, Keating L, Jüppner H, Wolf M: Daily variability in mineral metabolites in CKD and effects of dietary calcium and calcitriol. Clin J Am Soc Nephrol 7: 820–828, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isakova T, Gutierrez O, Shah A, Castaldo L, Holmes J, Lee H, Wolf M: Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol 19: 615–623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isakova T, Xie H, Barchi-Chung A, Vargas G, Sowden N, Houston J, Wahl P, Lundquist A, Epstein M, Smith K, Contreras G, Ortega L, Lenz O, Briones P, Egbert P, Ikizler TA, Jueppner H, Wolf M: Fibroblast growth factor 23 in patients undergoing peritoneal dialysis. Clin J Am Soc Nephrol 6: 2688–2695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khosla N, Fettman S, Gerseny H, Sprague SM: Impact of lanthanum carbonate on FGF23 in chronic kidney disease stages 3-5. [Abstract] J Am Soc Nephrol 23: 312A, 2012 [Google Scholar]
- 55.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Mallamaci F, Zoccali C: Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis 59: 177–185, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Vlassara H, Uribarri J, Cai W, Goodman S, Pyzik R, Post J, Grosjean F, Woodward M, Striker GE: Effects of sevelamer on HbA1c, inflammation, and advanced glycation end products in diabetic kidney disease. Clin J Am Soc Nephrol 7: 934–942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Block GA, Fishbane S, Rodriguez M, Smits G, Shemesh S, Pergola PE, Wolf M, Chertow GM: A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD stages 3-5. Am J Kidney Dis 65: 728–736, 2014 [DOI] [PubMed] [Google Scholar]
- 58.Di Iorio B, Di Micco L, Torraca S, Sirico ML, Russo L, Pota A, Mirenghi F, Russo D: Acute effects of very-low-protein diet on FGF23 levels: a randomized study. Clin J Am Soc Nephrol 7: 581–587, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Sigrist M, Tang M, Beaulieu M, Espino-Hernandez G, Er L, Djurdjev O, Levin A: Responsiveness of FGF-23 and mineral metabolism to altered dietary phosphate intake in chronic kidney disease (CKD): results of a randomized trial. Nephrol Dial Transplant 28: 161–169, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Rao M, Steffes M, Bostom A, Ix JH: Effect of niacin on FGF23 concentration in chronic kidney disease. Am J Nephrol 39: 484–490, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reilly AM, Gray AK, Moe SM, Ichikawa S: Nicotinamide treatment in a murine model of familial tumoral calcinosis reduces serum Fgf23 and raises heart calcium. Bone 67: 139–144, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabbagh Y, O’Brien SP, Song W, Boulanger JH, Stockmann A, Arbeeny C, Schiavi SC: Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol 20: 2348–2358, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daugirdas JT, Finn WF, Emmett M, Chertow GM, Frequent Hemodialysis Network Trial Group : The phosphate binder equivalent dose. Semin Dial 24: 41–49, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Tonelli M, Pannu N, Manns B: Oral phosphate binders in patients with kidney failure. N Engl J Med 362: 1312–1324, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Burke SK, Slatopolsky EA, Goldberg DI: RenaGel, a novel calcium- and aluminium-free phosphate binder, inhibits phosphate absorption in normal volunteers. Nephrol Dial Transplant 12: 1640–1644, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Pennick M, Poole L, Dennis K, Smyth M: Lanthanum carbonate reduces urine phosphorus excretion: evidence of high-capacity phosphate binding. Ren Fail 34: 263–270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, Peacock M: Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int 83: 959–966, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T: Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 289: F1088–F1095, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez-Ortiz ME, Lopez I, Muñoz-Castañeda JR, Martinez-Moreno JM, Ramírez AP, Pineda C, Canalejo A, Jaeger P, Aguilera-Tejero E, Rodriguez M, Felsenfeld A, Almaden Y: Calcium deficiency reduces circulating levels of FGF23. J Am Soc Nephrol 23: 1190–1197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giral H, Caldas Y, Sutherland E, Wilson P, Breusegem S, Barry N, Blaine J, Jiang T, Wang XX, Levi M: Regulation of rat intestinal Na-dependent phosphate transporters by dietary phosphate. Am J Physiol Renal Physiol 297: F1466–F1475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marks J, Churchill LJ, Srai SK, Biber J, Murer H, Jaeger P, Debnam ES, Unwin RJ, Epithelial T, Epithelial Transport and Cell Biology Group : Intestinal phosphate absorption in a model of chronic renal failure. Kidney Int 72: 166–173, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Katai K, Tanaka H, Tatsumi S, Fukunaga Y, Genjida K, Morita K, Kuboyama N, Suzuki T, Akiba T, Miyamoto K, Takeda E: Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant 14: 1195–1201, 1999 [DOI] [PubMed] [Google Scholar]
- 73.Kuboyama N, Watanabe Y, Yamaguchi M, Sato K, Suzuki T, Akiba T: Effects of niceritrol on faecal and urinary phosphate excretion in normal rats. Nephrol Dial Transplant 14: 610–614, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Cheng SC, Young DO, Huang Y, Delmez JA, Coyne DW: A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol 3: 1131–1138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shahbazian H, Zafar Mohtashami A, Ghorbani A, Abbaspour MR, Belladi Musavi SS, Hayati F, Lashkarara GR: Oral nicotinamide reduces serum phosphorus, increases HDL, and induces thrombocytopenia in hemodialysis patients: a double-blind randomized clinical trial. Nefrologia 31: 58–65, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Takahashi Y, Tanaka A, Nakamura T, Fukuwatari T, Shibata K, Shimada N, Ebihara I, Koide H: Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int 65: 1099–1104, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Young DO, Cheng SC, Delmez JA, Coyne DW: The effect of oral niacinamide on plasma phosphorus levels in peritoneal dialysis patients. Perit Dial Int 29: 562–567, 2009 [PubMed] [Google Scholar]
- 78.Rosenbaum DP, Johansson S, Carlsson B, Spencer AG, Stefansson BV, Knutsson M, Jacobs JW, Charmot D: Tenapanor, a minimally absorbed NHE3 inhibitor, reduces dietary phosphorus absorption in healthy volunteers [Abstract] J Am Soc Nephrol 25: 72A, 2014 [Google Scholar]
- 79.Labonté ED, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, He L, Dy E, Black D, Zhong Z, Langsetmo I, Spencer AG, Bell N, Deshpande D, Navre M, Lewis JG, Jacobs JW, Charmot D: Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 26: 1138–1149, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kempson SA, Colon-Otero G, Ou SY, Turner ST, Dousa TP: Possible role of nicotinamide adenine dinucleotide as an intracellular regulator of renal transport of phosphate in the rat. J Clin Invest 67: 1347–1360, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berndt TJ, Pfeifer JD, Knox FG, Kempson SA, Dousa TP: Nicotinamide restores phosphaturic effect of PTH and calcitonin in phosphate deprivation. Am J Physiol 242: F447–F452, 1982 [DOI] [PubMed] [Google Scholar]
- 82.Nomura K, Tatsumi S, Miyagawa A, Shiozaki Y, Sasaki S, Kaneko I, Ito M, Kido S, Segawa H, Sano M, Fukuwatari T, Shibata K, Miyamoto K: Hepatectomy-related hypophosphatemia: a novel phosphaturic factor in the liver-kidney axis. J Am Soc Nephrol 25: 761–772, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, Gale EA, European Nicotinamide Diabetes Intervention Trial Group : Safety of high-dose nicotinamide: a review. Diabetologia 43: 1337–1345, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Gutiérrez OM, Wolf M: Dietary phosphorus restriction in advanced chronic kidney disease: merits, challenges, and emerging strategies. Semin Dial 23: 401–406, 2010 [DOI] [PubMed] [Google Scholar]
- 85.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, Kovesdy CP, Bross R, Shinaberger CS, Noori N, Hirschberg R, Benner D, Nissenson AR, Kopple JD: Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 519–530, 2010 [DOI] [PubMed] [Google Scholar]
- 86.Uribarri J, Calvo MS: Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial 16: 186–188, 2003 [DOI] [PubMed] [Google Scholar]
- 87.Moe SM, Chen NX, Seifert MF, Sinders RM, Duan D, Chen X, Liang Y, Radcliff JS, White KE, Gattone VH, 2nd: A rat model of chronic kidney disease-mineral bone disorder. Kidney Int 75: 176–184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sullivan C, Sayre SS, Leon JB, Machekano R, Love TE, Porter D, Marbury M, Sehgal AR: Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: a randomized controlled trial. JAMA 301: 629–635, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Chue CD, Townend JN, Moody WE, Zehnder D, Wall NA, Harper L, Edwards NC, Steeds RP, Ferro CJ: Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol 24: 842–852, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O: Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 34: 125–134, 1999 [DOI] [PubMed] [Google Scholar]
- 91.Rathi VK, Biederman RW: Expanding role of cardiovascular magnetic resonance in left and right ventricular diastolic function. Heart Fail Clin 5: 421–435, vii, 2009 [DOI] [PubMed] [Google Scholar]
- 92.Shah SJ, Fonarow GC, Gheorghiade M, Lang RM. Phase II trials in heart failure: the role of cardiovascular imaging. Am Heart J 2011; 162:3–15 e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 94.Meguid El Nahas A, Bello AK: Chronic kidney disease: the global challenge. Lancet 365: 331–340, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Thadhani R, Pascual M, Bonventre JV: Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 96.Fine LG, Norman JT: Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int 74: 867–872, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Fine LG, Orphanides C, Norman JT: Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int Suppl 65: S74–S78, 1998 [PubMed] [Google Scholar]
- 98.Zhang JL, Morrell G, Rusinek H, Sigmund EE, Chandarana H, Lerman LO, Prasad PV, Niles D, Artz N, Fain S, Vivier PH, Cheung AK, Lee VS: New magnetic resonance imaging methods in nephrology. Kidney Int 85: 768–778, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inoue T, Kozawa E, Okada H, Inukai K, Watanabe S, Kikuta T, Watanabe Y, Takenaka T, Katayama S, Tanaka J, Suzuki H: Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol 22: 1429–1434, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prasad PV, Edelman RR, Epstein FH: Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation 94: 3271–3275, 1996 [DOI] [PubMed] [Google Scholar]
- 101.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T: Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78: 975–980, 2010 [DOI] [PubMed] [Google Scholar]
- 102.Wolf M: Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol 21: 1427–1435, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.