Abstract
Progressive inherited retinal degenerative disorders (PIRDDs) are the leading cause of blindness in developed countries, with AMD and RP constituting the majority of PIRDDs. Currently, over 8 million Americans have PIRDDs, and that number is estimated to drastically increase by the end of this decade. Although a mutant protein is expressed starting early during retinal development in patients with PIRDDs, symptoms of retinal degeneration do not manifest until much later. Historically, research has focused on understanding the role a mutation has in the function of a protein and what role the mutant protein has in the disease process. However, it remains unknown why the disease, irrespective of the mutation, manifests clinically much later in life, while cellular indicators of disease (e.g., accumulation of toxic protein products and cell death) occur throughout early and middle life. Herein, we propose that there exists a time point at which the degenerative process is accelerated, leading to the appearance of clinical symptoms. This point is defined by structural disruptions of the extracellular matrix (ECM). Death of a critical number of ECM-maintaining mutant protein–expressing retinal cells contributes to that break point in the degenerative process. Therefore, it is important to understand the changes occurring at the ECM during PIRDDs and to take that into account when therapeutic approaches are designed.
Keywords: retinal degeneration, extracellular matrix, aging
The extracellular matrix (ECM) of any tissue changes with age and the retina is no exception. Age-dependent visual changes are well described in the literature. However, role of retinal ECM changes in visual capabilities have not been well studied. Even less studied is the role of retinal ECM changes in progressive retinal degenerative diseases, especially in cases when the mutant protein is not a member of the ECM. This article focuses the attention on the role of retinal ECM in visual function in normal aging and during progressive retinal degenerative changes.
Introduction
Unless there are mutations in gene regulatory sequences, mutant proteins are expressed at the scheduled onset of their normal counterparts. For example, mutant rhodopsin exhibits the same temporal expression pattern as its normal counterpart (e.g., see the article by Naash et al.1). However, in spite of the fact that this mutant rhodopsin is expressed early during retinal development, most rhodopsin-associated diseases manifest many decades into life (e.g., see the article by Shastry2). Importantly, this phenomenon is by no means unique to rhodopsin, occurring with almost all genetically associated age-related diseases. Hence, a critical unanswered question is why, with a mutant protein expressed so early, does the disease manifest so late? It has been proposed that the delayed manifestation is the result of protective effects afforded by neuroprotective factors.3 Although neurotrophic factors are present in the retina and may be upregulated or activated in response to injurious effects of expression of a mutant protein, it is not clear why their role is diminished with age, resulting in a sudden onset of a phenotype in midlife. Herein, we offer a view that explains this sudden manifestation of a phenotype in patients with progressive inherited retinal degenerative disorders (PIRDDs). We further explore the impact this explanation has on the development of gene and cell therapy for the treatment of PIRDDs.
Retinal Extracellular Matrix
The extracellular matrix (ECM) is composed of a variety of proteins and complex carbohydrates that are secreted locally and assembled into a well-organized meshwork that is in close association with the surrounding cells. The main components of the ECM are proteoglycans (heparan sulfate, chondroitin sulfate, and keratin sulfate), nonproteoglycan polysaccharides (hyaluronic acid), fibers (collagen and elastin), and other proteins such as fibronectin, fibrillin, lamnins, and fibulins.4 Minor components of the ECM include growth factors, immune mediators such as complement system components (e.g., complement factor H and vitronectin) and extracellular proteases (e.g., matrix metalloproteinases [MMPs]), and their regulators that allow the restructuring of the ECM (for review, see the article by Mecham5).
In the retina, the ECM is divided into two separate entities, the interphotoreceptor matrix (IPM), which is the extracellular material that fills the subretinal space, and the retinal ECM, which is composed of the rest of the extracellular areas outside the IPM. The sources of IPM proteins are the retina and the RPE.6 For simplicity, henceforth the use of the abbreviation ECM covers both.
Multiple factors contribute to the character of the ECM in a given tissue. First, variation in the relative amounts and organization of both proteins and carbohydrates creates substantial ECM diversity. Second, the organization of the cytoskeleton in the surrounding cells influences the shape and structure of the ECM. The type and distribution of connections between the ECM and the cytoskeleton, mediated by transmembrane integrin receptors (e.g., see the article by Gardiner7), can vary from tissue to tissue. Third, signaling between the ECM and the adjacent cells regulates the development and maintenance of blood vessels, hence influencing the flux of oxygen and nutrients to the cells within the ECM.8 All these factors can be fine-tuned to meet the demands of the environment, specifically providing the cells with the ability to move and respond, while preserving the tissue integrity.
Retinal Aging: A Role of the ECM?
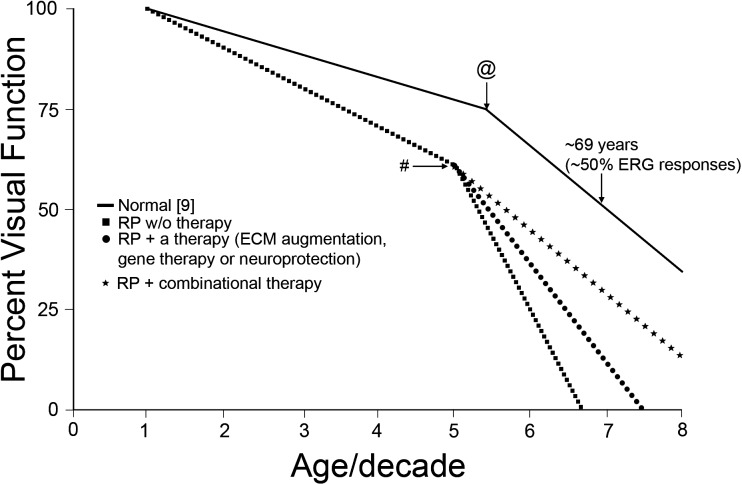
Changes to the normal (i.e., nondiseased) retina over time have been well studied, and two commonly observed phenomena are worth discussing. The first is that changes in retinal function often do not correlate with changes in photoreceptor or neuronal cell number, and the second is that changes in retinal function are not constant throughout life. For example, Birch and Anderson9 showed that ERG amplitudes in the healthy human population gradually declined with age up to 55 years and then rapidly declined afterward until age 69 years (Figure), when rod responses dropped to half those of young adult cohorts (age range, 15–24 years). Cone responses followed closely, with slow declines in function up to age 55 years, followed by severe drops thereafter, with only 50% ERG amplitude compared with that of young adults by age 70 years.
Figure.
Changes in ERG responses as a function of age (solid line). As humans age, there is a gradual decline in ERG responses until age 55 years, followed by rapid decline until ERG responses drop to approximately 50% of that of young cohorts by age 69 years for rods and by age 70 years for cones.9 Shown are hypothetical ERG responses from patients with PIRDDs as a function of age, without any therapeutic interventions (squares); with one form of therapy such as gene, cell, or neurotrophic therapy (circles); or with combinational therapy (stars). @, Age-dependent critical point; #, age when PIRDDs-associated changes become prominent. This can shift depending upon the rate of degeneration.
However, in general this pattern of “slow early” then “fast late” functional change is not reflected in photoreceptor cell loss. Gao and Hollyfield10 analyzed human retinas from donors without any history of eye disease ranging from the second to the ninth decades of life. They showed that rod loss (15%) in the equator was pronounced during the second to the fourth decades of life, followed by only a gradual decline thereafter. By the ninth decade, rod density had declined to 32%. Cones, however, were reduced by 6.7% by the fourth decade and by 23% by the ninth decade. Notably, evaluation of RPE cell populations with age showed a uniform rate of cell loss with age, but the ratio of rods to RPE did not significantly change at different ages. Analysis of the foveal cones showed that no significant cell loss occurred with age.10 Based on data by these authors, an estimate of less than 24% of rod cell loss would have occurred by age 69 years. These changes cannot account for the 50% loss in rod maximum responses at age 69 years observed by Birch and Anderson.9
A similar disconnect between cellular loss and functional loss is observed in the murine retina. Analysis of the effect of age on the cellular composition of the C57BL/6 mouse retina showed that the overall thickness of the retina decreased by approximately 15% in older mice (age range, 24–28 months) compared with younger mice (age range, 3–5 months).11 However, there was an increase in total retinal area that offsets the reduction in thickness; therefore, no change was observed in total retinal volume.11 Similarly, we previously observed no thinning in the outer nuclear layer in 12-month-old C57BL/6 mice (compared with 2-month-old animals), but we saw a significant drop in maximum scotopic a-wave (approximately a 33% decrease), scotopic b-wave (approximately a 33% decrease), and photopic b-wave (approximately a 42% decrease) amplitudes from age 2 to 12 months.12 As in humans, this decrease is not evenly distributed throughout life: we observed no changes in scotopic or photopic ERG values from age 1 to 5 months, but we saw dramatic decreases from age 5 to 10 months and age 10 to 18 months. A similar phenomenon was observed in another study13 on aging that used the B6D2F1/J strain of mice. The authors observed approximately a 20% reduction in rod photoreceptor number and approximately a 10% decrease in outer segment length by age 2½ years (compared with 4-month-old controls13) but saw approximately a 50% reduction in scotopic a-wave and b-wave amplitudes. The modest structural degeneration in these mice may be due to their origin: B6D2F1/J are F1 hybrids obtained by mating C57BL/6 females to DBA/2J males.13 The DBA/2J mice exhibit well-characterized progressive glaucoma in which elevated IOP and consequent late-onset retinal degeneration are caused by iris atrophy or iris pigment dispersion (The Jackson Laboratory, Bar Harbor, ME [http://jaxmice.jax.org/strain/000671.html]), and it is not clear to what extent these phenotypes may exist in the B6D2F1/J line. What is clear, however, is that in multiple lines it has been demonstrated that functional losses exceed losses in retinal cellularity. To assess the role of the ECM in these age-related changes, two questions must be asked: first, does the ECM change over time, and second, can changes in the ECM affect retinal function? The answer to both questions is, unequivocally, yes.
Changes in the ECM during the aging process are well documented,14 with the most obvious being the appearance of facial wrinkles. However, aging-related ECM changes are not limited to the skin but occur in all organs, including the eye. One example of ocular-associated ECM changes is found in people in their fifth decade of life, who routinely attest to changes in their visual capabilities and the need for higher intensities of light to see fine print. These clinical signs are brought about by the onset of presbyopia, a vision condition in which the lens loses its flexibility, making it difficult to focus on close objects (e.g., see the article by Truscott15). Another age-dependent ocular change involves the separation of the vitreous from the retina and changes in the physical properties of the vitreous,16 a mostly acellular extracellular structure that fills the space between the lens and the retina. Importantly, ocular ECM changes are not limited to the vitreous but extend to the retinal ECM. For example, atomic force microscopy investigations have shown that the retinal inner limiting membrane (a basement membrane composed of ECM materials) significantly increases in stiffness and thickness as the retina ages.17 To look from another approach, transcriptome analysis investigations of the retina and choroid has shown that an accelerated-aging rat model exhibits substantial changes in expression of ECM genes and ECM turnover genes.18 Other age-related ECM changes can be brought about by oxidative stress, as well as increased proteolysis of the ECM,19 variations in the contributions of ECM components,20 or even changes in the posttranslational modifications of ECM proteins (e.g., see the article by Bartling et al.21).
Evidence also exists in favor of the idea that alterations in the ECM can contribute to changes in retinal function. Because the ECM is such a complex entity, there is no way to simply eliminate it and assess retinal function as one would commonly do to assess the role of a single gene in tissue function. However, recent analysis has shown that alteration of a broad swath of ECM proteins significantly affects retinal function. Sulfated proteins, which are all secreted, are components of the ECM. We have observed that mice that lack the enzymes required to perform tyrosine sulfation (TPST1 and TPST2) exhibit drastic reductions in scotopic and photopic ERG responses.22,23 Importantly, in wild-type mice transcript levels of the two sulfation enzymes (TPST1 and TPST2) decrease dramatically from age 2 to 11 months, suggesting that sulfation-associated changes in the ECM are a normal part of aging in the murine eye.
Combined, these observations suggest that age-related changes in the ECM may contribute to changes in vision observed with aging. Although the mechanism connecting ECM changes to functional changes is poorly understood, it is likely tied to the role of the ECM in supporting the organization of retinal cells. For example, although retinal synapses form in the TPST1 and TPST2 double knockout model of ECM abnormalities, we observed subtle defects in the organization and density of these synapses.23 Similarly, although photoreceptor outer segments form, their structure is highly abnormal, likely due to a lack of proper ECM support.23 In the aged C57BL/6 retina, mild defects in the density and distribution of synapses are observed.11 However, while these subtle structural issues may contribute to the role of the ECM in retinal function, the magnitude of the defects observed suggests that other, nonstructural, changes also have a role. These may include, for example, changes in nutrient delivery or removal, oxidative stress, and immune response, all of which require a properly functioning ECM.
ECM Changes During PIRDDs
PIRDDs are caused by mutations in over 200 genes (RETNET; https://sph.uth.edu/retnet/ [in the public domain]), including some genes that code for ECM-specific proteins. Retinitis pigmentosa and AMD constitute the bulk of PIRDDs. Currently, over 8 million Americans have PIRDDs, and that number is estimated to drastically increase by the end of this decade.
Thus far, most research has focused on understanding the mechanistic link between the mutant protein and the disease phenotype. However, it remains unknown why the disease suddenly manifests late in life even though the mutant protein is expressed (and some cell loss is observed) throughout early and middle life (e.g., see the article by Sandberg et al.24). We propose that the time point when the degenerative process is accelerated and clinical symptoms begin to manifest is defined by structural disruptions of the ECM, which are tolerated during the early stages of PIRDDs. Although ECM changes occur as a normal part of aging, they are exacerbated in cases of PIRDDS, and the phenotype suddenly develops (Figure) as a result of two events. First, the progressive drop in cell number due to the underlying PIRDD means that fewer cells are contributing to the ECM. Second, because of the declining number of cells, there will be a reduced overall number of integrin receptors and hence fewer attachment points between the ECM and the cells. Together, these changes will reach a critical point, leading to increased spacing between cells and reduced ECM material that is required for proper retinal support. This in turn will alter the delivery of oxygen, nutrients, and growth factors to the cells from the surrounding choroidal or retinal blood supplies.8 Furthermore, these changes may also influence the shuttling of retinoids between the retina and the RPE and may also limit the delivery of 11-cis retinal to the photoreceptors, thus altering the rate of regeneration of photosensitive rhodopsin. In fact, the change in the delivery of 11-cis retinal to the photoreceptors was proposed as a mechanism for the decline in visual capabilities during aging.25 The reorganization of the ECM during the degenerative process may also influence MMP activity because levels of MMPs can be modulated by cell-cell and cell-ECM interactions.26 All these factors combined make assessing changes, particularly age-related changes, in the ECM during the development of PIRDDs critical for our understanding of the disease process and suggest that significantly more research effort should be directed toward this goal. Furthermore, understanding that the ECM has a significant role in the PIRDDs disease process severely impacts the design and implementation of therapeutic approaches for PIRDDs treatments.
The Role of the ECM in Gene Therapy for PIRDDs
There are three ways in which the ECM should be considered in terms of retinal gene therapy. The first and simplest is that if defects in an ECM gene are the underlying cause of the PIRDDs (e.g., the fibulin-3 gene, which is associated with malattia leventinese and AMD27,28), then that gene may be a standard gene therapy target. This could include straightforward gene supplementation therapy or, if necessary, knockdown of mutant alleles and simultaneous delivery of the wild-type allele. Considerations for this approach would be largely the same as for any other genetic therapy and are extensively reviewed elsewhere (e.g., see the article by Conley and Naash29). One specific issue worth mentioning is the capacity of the delivery vehicle, which can vary widely. The ECM proteins are often very large, so a high-capacity delivery vehicle (e.g., lentivirus30 or compacted DNA nanoparticles31,32) would likely be needed.
The second consideration is that of the role of the ECM in modulating penetration of retinal gene therapies and is more widely applicable to a variety of PIRDDs. Successful gene therapy of any kind requires that the packaged DNA reach and penetrate the cells of interest. Because the delivery is usually to a highly restricted area (i.e., a single site of injection), the process of the distribution of the therapy through the ECM can be a critical factor, ultimately influencing therapeutic efficacy. This issue is of great import in the retina. Thus far, optimal targeting of photoreceptors (a key PIRDDs disease target) has been achieved after subretinal injection with only low gene transduction after intravitreal delivery, a phenomenon attributed to low penetration of the inner limiting membrane and other retinal ECM. The problem is not limited to the inner limiting membrane, however: the IPM has also been shown to impede the efficiency of the distribution and uptake of subretinally delivered gene delivery vectors33 (in this case, lentiviral vectors, although the phenomenon likely extends to other delivery vehicles). Importantly, the retinal detachment and long-term sequelae associated with subretinal injection make optimizing intravitreal delivery a critical clinical goal.
There are two practical options available to overcome the issue of ECM penetration. The first is that enzymes that transiently disrupt the ECM can be codelivered with the gene therapy. Proof of principle demonstrating the efficacy of this approach has been demonstrated after intravitreal34 and subretinal33 delivery. However, it is not yet established how the decline in retinal ECM health during PIRDDs influences the resolution of the detachment that results from the subretinal injections of the therapeutic agents, so inducing further disruption of the ECM may not be clinically wise. In any case, implementation of this approach would definitely require careful assessment of the recovery of the ECM after treatment. The second option is that careful study of the PIRDDs disease pathology may identify a time point at which ECM changes that favor enhanced penetration of genetic therapies occur as part of the natural history of the disease. In this case, precise timing of the delivery of the genetic cargo may result in improved efficacy. In order for either of these two approaches to be effective, characterization of the ECM will need to be included as a routine part of study of the PIRDDs disease process.
The third and potentially most relevant intersection between the ECM and retinal gene therapy is the idea that overall modulation of the ECM could promote retinal health and retard the disease process. For example, if a certain matrix component is identified to be routinely downregulated in PIRDDs (i.e., because the cells that secrete that component are lost due to an unrelated genetic defect), then gene supplementation with that ECM component or knockdown of ECM proteases that degrade it could, by preserving the ECM, significantly improve phenotypic outcomes. Although it would not correct the underlying genetic defect, this approach has the advantage of being applicable to a variety of PIRDDs (rather than requiring a different genetic therapy for each disease gene), and even to nonmonogenic degenerative conditions such as AMD and glaucoma. This approach has already been tested for disease conditions in which the role of the ECM has been more extensively studied. For example, lentiviral delivery of small interfering RNAs (siRNAs) against tissue inhibitor of metalloproteinase-1 (TIMP-1) to an in vitro keloid model of abnormal scarring has been shown to increase degradation of collagen (due to decreased turnover of MMPs by TIMP-1), an ECM component involved in keloid formation.35 Initial explorations of this idea in the eye are under way as well, specifically in models of diabetic retinopathy (DR). The role of the ECM in the abnormal neovascularization associated with DR is better explored than the role of the ECM in PIRDDs, thus illuminating potential ECM drug targets such as connective tissue growth factor (CTGF).36 A secreted protein, CTGF induces ECM production and angiogenesis during DR, and intravitreal delivery of siRNA targeted to CTGF was shown to result in decreased expression of a specific subset of ECM proteins.37 Subsequent results to evaluate the therapeutic impact of this ECM modulation (e.g., to determine whether neovascularization or DR progression is affected) will be eagerly awaited. Certainly, general modulation of the ECM as a target for gene therapy in the case of PIRDDs will require extensive studies to determine the classes of ECM proteins in the retina and their regulation during health and disease.
The ECM and Cell Therapy
Cell therapy is currently a highly researched topic for the treatment of PIRDDs, due to its theoretical ability to replace retinal cells lost to the degenerative process (an outcome not achieved by traditional gene therapy). Effective retinal cell therapy is biologically complicated and requires the delivery and distribution of progenitor cells to the target area, integration of those cells into the retinal layers, differentiation into the target cell type, and formation of correct synaptic connections. Optimizing these factors is now the subject of extensive research (reviewed by West et al.38 and Boucherie et al.39). However, broadly speaking, the role of the ECM in the effectiveness of cell therapy is grossly similar to that described above for gene therapy. First, replenishment of the degenerating retina with healthy neurons via effective cell therapy can be used to directly replace lost ECM and ECM regulators (by delivering cells that will secrete ECM components), thus promoting all of the positive benefits conferred by the ECM in the healthy retina.
In addition, the status of the ECM and the extracellular environment in general may have a significant role in the efficacy of cell transplantation and specifically in the efficiency of integration of transplanted cells into the appropriate retinal layer. For example, CD44 and neurocan, two ECM components found in the retina, have been shown to hamper cell migration.40 Targeting the extracellular environment can be an effective strategy to improve integration of cells. It has been shown that disruption of the outer limiting membrane (which is not composed of the ECM per se but rather comprises tight junctions between photoreceptors and Müller glia) results in increased integration of transferred retinal progenitor cells.41 A similar effect can be seen by modulating the ECM specifically. The codelivery of poly(lactic-co-glycolic acid) microspheres containing the matrix degrading enzyme MMP-2 along with retinal progenitor cells to the subretinal space of rhodopsin knockout mice resulted in enhanced cell integration into the retina (compared with the delivery of cells alone).40 Other groups have reported similar outcomes when using MMP-2 stimulants42 or chondroitinase43 (to degrade chondroitin sulfate proteoglycans).
Similarly, the inner limiting membrane is a barrier to integration of intravitreally delivered stem cells. Mechanical peeling of the inner limiting membrane in retinal explants was shown to enhance penetration of mesenchymal stem cells into the retina.43 However, this result was not recapitulated by the delivery of collagenase (which digests ECM components in the inner limiting membrane), suggesting that cellular components of the inner limiting membrane formed by the Müller endfeet may be more of a barrier than the basement membrane itself.44 The practical outcomes of these studies are similar to those for gene therapy, namely, that (1) disruption of the ECM and extracellular environment may effectively increase cell integration but may not be clinically wise and (2) that timed delivery of transplanted cells to coincide with existing ECM defects (resulting from the progression of the degenerative process in PIRDDs) may enhance integration of transplanted retinal cells.
Combinatorial Therapy
The ultimate goal of cell-based therapy is to introduce a sufficient number of cells into the retina that develop into fully functioning neurons complete with proper cell-cell junctions, synapses, and outer segments to initiate light-induced signal transduction. However, we are a long way from achieving this goal. In the meantime, the full accomplishment of that goal may not be necessary if a combinatorial therapy approach is utilized. If cell-based therapy can be advanced to the point where transferred cells can integrate into the retina and survive long term, they may have beneficial effects (in terms of supporting retinal structure and the ECM) even without forming synapses or mediating visual transduction. In this case, it is possible that they may be sufficient to prevent the late-onset rapid decline in visual capabilities so often seen in PIRDDs (Figure). However, because the native cells harboring the mutant protein would continue to degenerate, the full potential of this approach can only be realized in combination with gene therapy to supplement or correct the underlying genetic defect.
Neuroprotection in PIRDDs
Since the initial proof-of-concept experiment showing that the injection of basic fibroblast growth factor into the eyes of Royal College of Surgeons rats leads to preservation of photoreceptors,45 a multitude of studies have shown that administration of neurotrophic factors, soluble components of the ECM, restores vision in animal models of PIRDDs (for review, see the article by Wen et al.46). However, the issue of specificity complicates the use of trophic factors for the treatment of PIRDDs. For example, neurotrophin-3, a neurotrophic factor that protected albino rats and overexpressing transgenic mice from light damage, failed to change the course of degeneration in four animal models of PIRDDs.47
A variety of neurotrophic factors have been tested for the treatment and prevention of various forms of retinal degeneration, including the following: glial cell line–derived neurotrophic factor (e.g., see the articles by Buch et al.48 and Ward et al.49), ciliary neurotrophic factor (CNTF) (e.g., see the article by Gregory-Evans et al.50), pigment epithelia–derived factor (e.g., see the article by Jiang et al.51), and brain-derived neurotrophic factor (e.g., see the articles by Chen and Weber,52 Di et al.,53 and Ko et al.54). Initially showing the most promising results, CNTF was used in phase 1 clinical trials, with encouraging outcomes.55 However, other studies56,57 have shown that, while CNTF mediates neuroprotection (i.e., prevents retinal cell death), it also caused decreases in ERG function, making careful examination critical.
Since neurotrophic factors cannot pass through the blood-retinal barrier, therapeutic application usually involves the delivery into the vitreous directly. As a result, the distribution of the treatment is influenced by the health of retinal ECM more so than gene-based and cell-based therapies because the efficacy of neurotrophic therapy requires other ECM components. For example, CNTF-induced astrocyte development relies on CNTF-mediated interactions with ECM components, although the specific identity of those components is unknown.58
Conclusions
We propose herein that changes in the ECM during the degenerative process (due to both aging and death of cells expressing a mutant protein) confound the phenotype observed in patients with PIRDDs and can accelerate the degenerative process after a certain time point. This time point is likely to shift depending upon the rate of the degenerative process, which is largely dependent upon the type of mutation or the mutant protein. We also propose several genetic and cell-based therapeutic approaches for PIRDDs that rely on modification of the ECM. A core thread unifying all these ideas is the observation that basic research studies and preclinical therapy studies should pay substantial attention to the character, function, and role of the ECM in retinal homeostasis and disease.
Acknowledgments
Disclosure: M.R. Al-Ubaidi, None; M.I. Naash, None; S.M. Conley, None
References
- 1. Naash MI, Hollyfield JG, Al-Ubaidi MR, Baehr W. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proc Natl Acad Sci U S A. 1993; 90: 5499–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shastry BS. Retinitis pigmentosa and related disorders: phenotype of rhodopsin and peripherin/rds mutations. Am J Med Genet. 1994; 52: 467–474. [DOI] [PubMed] [Google Scholar]
- 3. Roth S. Endogenous neuroprotection in the retina. Brain Res Bull. 2004; 62: 461–466. [DOI] [PubMed] [Google Scholar]
- 4. Hubmacher D, Apte SS. The biology of the extracellular matrix: novel insights. Curr Opin Rheumatol. 2013; 25: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mecham RP. Overview of extracellular matrix. Curr Protoc Cell Biol. 2012; chap 10:unit 10.1. [DOI] [PubMed] [Google Scholar]
- 6. Kanan Y, Hoffhines A, Rauhauser A, Murray A, Al-Ubaidi MR. Protein tyrosine-O-sulfation in the retina. Exp Eye Res. 2009; 89: 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gardiner NJ. Integrins and the extracellular matrix: key mediators of development and regeneration of the sensory nervous system. Dev Neurobiol. 2011; 71: 1054–1072. [DOI] [PubMed] [Google Scholar]
- 8. Rhodes JM, Simons M. The extracellular matrix and blood vessel formation: not just a scaffold. J Cell Mol Med. 2007; 11: 176–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Birch DG, Anderson JL. Standardized full-field electroretinography: normal values and their variation with age. Arch Ophthalmol. 1992; 110: 1571–1576. [DOI] [PubMed] [Google Scholar]
- 10. Gao H, Hollyfield JG. Aging of the human retina: differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992; 33: 1–17. [PubMed] [Google Scholar]
- 11. Samuel MA, Zhang Y, Meister M, Sanes JR. Age-related alterations in neurons of the mouse retina. J Neurosci. 2011; 31: 16033–16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li C, Cheng M, Yang H, Peachey NS, Naash MI. Age-related changes in the mouse outer retina. Optom Vis Sci. 2001; 78: 425–430. [DOI] [PubMed] [Google Scholar]
- 13. Kolesnikov AV, Fan J, Crouch RK, Kefalov VJ. Age-related deterioration of rod vision in mice. J Neurosci. 2010; 30: 11222–11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sprenger CC, Plymate SR, Reed MJ. Aging-related alterations in the extracellular matrix modulate the microenvironment and influence tumor progression. Int J Cancer. 2010; 127: 2739–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Truscott RJ. Presbyopia: emerging from a blur towards an understanding of the molecular basis for this most common eye condition. Exp Eye Res. 2009; 88: 241–247. [DOI] [PubMed] [Google Scholar]
- 16. de Smet MD. Gad Elkareem AM, Zwinderman AH. The vitreous, the retinal interface in ocular health and disease. Ophthalmologica. 2013; 230: 165–178. [DOI] [PubMed] [Google Scholar]
- 17. Candiello J, Cole GJ, Halfter W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010; 29: 402–410. [DOI] [PubMed] [Google Scholar]
- 18. Kozhevnikova OS, Korbolina EE, Ershov NI, Kolosova NG. Rat retinal transcriptome: effects of aging and AMD-like retinopathy. Cell Cycle. 2013; 12: 1745–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Macgregor AM, Eberhart CG, Fraig M, Lu J, Halushka MK. Tissue inhibitor of matrix metalloproteinase-3 levels in the extracellular matrix of lung, kidney, and eye increase with age. J Histochem Cytochem. 2009; 57: 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sicari BM, Johnson SA, Siu BF, et al. The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials. 2012; 33: 5524–5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bartling B, Desole M, Rohrbach S, Silber RE, Simm A. Age-associated changes of extracellular matrix collagen impair lung cancer cell migration. FASEB J. 2009; 23: 1510–1520. [DOI] [PubMed] [Google Scholar]
- 22. Sherry DM, Kanan Y, Hamilton R, et al. Differential developmental deficits in retinal function in the absence of either protein tyrosine sulfotransferase-1 or -2. PLoS One. 2012; 7: e39702 Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3382163/. Accessed November 24, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sherry DM, Murray AR, Kanan Y, et al. Lack of protein-tyrosine sulfation disrupts photoreceptor outer segment morphogenesis, retinal function and retinal anatomy. Eur J Neurosci. 2010; 32: 1461–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sandberg MA, Rosner B, Weigel-DiFranco C, Dryja TP, Berson EL. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Invest Ophthalmol Vis Sci. 2007; 48: 1298–1304. [DOI] [PubMed] [Google Scholar]
- 25. Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002; 1: 381–396. [DOI] [PubMed] [Google Scholar]
- 26. Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011; 41: 271–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bessant DA, Ali RR, Bhattacharya SS. Molecular genetics and prospects for therapy of the inherited retinal dystrophies. Curr Opin Genet Dev. 2001; 11: 307–316. [DOI] [PubMed] [Google Scholar]
- 28. Marmorstein L. Association of EFEMP1 with malattia leventinese and age-related macular degeneration: a mini-review. Ophthalmic Genet. 2004; 25: 219–226. [DOI] [PubMed] [Google Scholar]
- 29. Conley SM, Naash MI. Nanoparticles for retinal gene therapy. Prog Retin Eye Res. 2010; 29: 376–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kong J, Kim SR, Binley K, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008; 15: 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han Z, Conley SM, Makkia RS, Cooper MJ, Naash MI. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J Clin Invest. 2012; 122: 3221–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fink TL, Klepcyk PJ, Oette SM, et al. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther. 2006; 13: 1048–1051. [DOI] [PubMed] [Google Scholar]
- 33. Grüter O, Kostic C, Crippa SV, et al. Lentiviral vector-mediated gene transfer in adult mouse photoreceptors is impaired by the presence of a physical barrier. Gene Ther. 2005; 12: 942–947. [DOI] [PubMed] [Google Scholar]
- 34. Cehajic-Kapetanovic J, Le Goff MM, Allen A, Lucas RJ, Bishop PN. Glycosidic enzymes enhance retinal transduction following intravitreal delivery of AAV2. Mol Vis. 2011; 17: 1771–1783. [PMC free article] [PubMed] [Google Scholar]
- 35. Aoki M, Miyake K, Ogawa R, et al. siRNA knockdown of tissue inhibitor of metalloproteinase-1 in keloid fibroblasts leads to degradation of collagen type I [ published online ahead of print September 16, 2013]. J Invest Dermatol. doi:10.1038/jid.2013.396. [DOI] [PubMed] [Google Scholar]
- 36. Yan L, Chaqour B. Cysteine-rich protein 61 (CCN1) and connective tissue growth factor (CCN2) at the crosshairs of ocular neovascular and fibrovascular disease therapy [ published online ahead of print June 7, 2013]. J Cell Commun Signal. doi:10.1007/s12079-013-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Winkler JL, Kedees MH, Guz Y, Teitelman G. Inhibition of connective tissue growth factor by small interfering ribonucleic acid prevents increase in extracellular matrix molecules in a rodent model of diabetic retinopathy. Mol Vis. 2012; 18: 874–886. [PMC free article] [PubMed] [Google Scholar]
- 38. West EL, Pearson RA, MacLaren RE, Sowden JC, Ali RR. Cell transplantation strategies for retinal repair. Prog Brain Res. 2009; 175: 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boucherie C, Sowden JC, Ali RR. Induced pluripotent stem cell technology for generating photoreceptors. Regen Med. 2011; 6: 469–479. [DOI] [PubMed] [Google Scholar]
- 40. Yao J, Tucker BA, Zhang X, Checa-Casalengua P, Herrero-Vanrell R, Young MJ. Robust cell integration from co-transplantation of biodegradable MMP2-PLGA microspheres with retinal progenitor cells. Biomaterials. 2011; 32: 1041–1050. [DOI] [PubMed] [Google Scholar]
- 41. West EL, Pearson RA, Tschernutter M, Sowden JC, MacLaren RE, Ali RR. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res. 2008; 86: 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suzuki T, Mandai M, Akimoto M, Yoshimura N, Takahashi M. The simultaneous treatment of MMP-2 stimulants in retinal transplantation enhances grafted cell migration into the host retina. Stem Cells. 2006; 24: 2406–2411. [DOI] [PubMed] [Google Scholar]
- 43. Singhal S, Lawrence JM, Bhatia B, et al. Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted Müller stem cells into degenerating retina. Stem Cells. 2008; 26: 1074–1082. [DOI] [PubMed] [Google Scholar]
- 44. Johnson TV, Martin KR. Development and characterization of an adult retinal explant organotypic tissue culture system as an in vitro intraocular stem cell transplantation model. Invest Ophthalmol Vis Sci. 2008; 49: 3503–3512. [DOI] [PubMed] [Google Scholar]
- 45. Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci. 1992; 12: 3554–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wen R, Tao W, Li Y, Sieving PA. CNTF. and retina. Prog Retin Eye Res. 2012; 31: 136–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. LaVail MM. Survival factors for treatment of retinal degenerative disorders: preclinical gains and issues for translation into clinical studies. Retina. 2005; 25 (suppl): S25–S26. [DOI] [PubMed] [Google Scholar]
- 48. Buch PK, MacLaren RE, Durán Y, et al. In contrast to AAV-mediated Cntf expression, AAV-mediated Gdnf expression enhances gene replacement therapy in rodent models of retinal degeneration. Mol Ther. 2006; 14: 700–709. [DOI] [PubMed] [Google Scholar]
- 49. Ward MS, Khoobehi A, Lavik EB, Langer R, Young MJ. Neuroprotection of retinal ganglion cells in DBA/2J mice with GDNF-loaded biodegradable microspheres. J Pharm Sci. 2007; 96: 558–568. [DOI] [PubMed] [Google Scholar]
- 50. Gregory-Evans K, Chang F, Hodges MD, Gregory-Evans CY. Ex vivo gene therapy using intravitreal injection of GDNF-secreting mouse embryonic stem cells in a rat model of retinal degeneration. Mol Vis. 2009; 15: 962–973. [PMC free article] [PubMed] [Google Scholar]
- 51. Jiang C, Moore MJ, Zhang X, Klassen H, Langer R, Young M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol Vis. 2007; 13: 1783–1792. [PubMed] [Google Scholar]
- 52. Chen H, Weber AJ. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest Ophthalmol Vis Sci. 2001; 42: 966–974. [PubMed] [Google Scholar]
- 53. Di PA, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Müller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998; 95: 3978–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ko ML, Hu DN, Ritch R, Sharma SC. The combined effect of brain-derived neurotrophic factor and a free radical scavenger in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000; 41: 2967–2971. [PubMed] [Google Scholar]
- 55. Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006; 103: 3896–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bok D, Yasumura D, Matthes MT, et al. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res. 2002; 74: 719–735. [DOI] [PubMed] [Google Scholar]
- 57. Liang FQ, Aleman TS, Dejneka NS, et al. Long-term protection of retinal structure but not function using RAAV.CNTF in animal models of retinitis pigmentosa. Mol Ther. 2001; 4: 461–472. [DOI] [PubMed] [Google Scholar]
- 58. Lillien LE, Sendtner M, Raff MC. Extracellular matrix–associated molecules collaborate with ciliary neurotrophic factor to induce type-2 astrocyte development. J Cell Biol. 1990; 111: 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]