Abstract
Background
We report a cluster-randomised trial of a home-based counselling strategy, designed for large-scale implementation, in a population of 1.2 million people in rural southern Tanzania. We hypothesised that the strategy would improve neonatal survival by around 15%.
Methods and Findings
In 2010 we trained 824 female volunteers to make three home visits to women and their families during pregnancy and two visits to them in the first few days of the infant’s life in 65 wards, selected randomly from all 132 wards in six districts in Mtwara and Lindi regions, constituting typical rural areas in Southern Tanzania. The remaining wards were comparison areas. Participants were not blinded to the intervention. The primary analysis was an intention-to-treat analysis comparing the neonatal mortality (day 0–27) per 1,000 live births in intervention and comparison wards based on a representative survey in 185,000 households in 2013 with a response rate of 90%. We included 24,381 and 23,307 live births between July 2010 and June 2013 and 7,823 and 7,555 live births in the last year in intervention and comparison wards, respectively. We also compared changes in neonatal mortality and newborn care practices in intervention and comparison wards using baseline census data from 2007 including 225,000 households and 22,243 births in five of the six intervention districts. Amongst the 7,823 women with a live birth in the year prior to survey in intervention wards, 59% and 41% received at least one volunteer visit during pregnancy and postpartum, respectively. Neonatal mortality reduced from 35.0 to 30.5 deaths per 1,000 live births between 2007 and 2013 in the five districts, respectively. There was no evidence of an impact of the intervention on neonatal survival (odds ratio [OR] 1.1, 95% confidence interval [CI] 0.9–1.2, p = 0.339). Newborn care practices reported by mothers were better in intervention than in comparison wards, including immediate breastfeeding (42% of 7,287 versus 35% of 7,008, OR 1.4, CI 1.3–1.6, p < 0.001), feeding only breast milk for the first 3 d (90% of 7,557 versus 79% of 7,307, OR 2.2, 95% CI 1.8–2.7, p < 0.001), and clean hands for home delivery (92% of 1,351 versus 88% of 1,799, OR 1.5, 95% CI 1.0–2.3, p = 0.033). Facility delivery improved dramatically in both groups from 41% of 22,243 in 2007 and was 82% of 7,820 versus 75% of 7,553 (OR 1.5, 95% CI 1.2–2.0, p = 0.002) in intervention and comparison wards in 2013. Methodological limitations include our inability to rule out some degree of leakage of the intervention into the comparison areas and response bias for newborn care behaviours.
Conclusion
Neonatal mortality remained high despite better care practices and childbirth in facilities becoming common. Public health action to improve neonatal survival in this setting should include a focus on improving the quality of facility-based childbirth care.
Trial Registration
ClinicalTrials.gov NCT01022788
Editors' Summary
Background
In 1990, 12 million children—most of them living in resource-limited countries—died before their fifth birthday. Faced with this largely avoidable loss of young lives, in 2000, world leaders set a target of reducing under-five mortality (deaths) to one-third of its 1990 level by 2015 as Millennium Development Goal 4 (MDG4); this goal, together with seven others, aims to eradicate extreme poverty globally. Progress towards reducing under-five mortality has been good. However, MDG4 has not been met because of slow progress in reducing neonatal mortality—death during the first 28 days of life. In sub-Saharan Africa, for example, mortality during the first five years of life fell by 47% between 1990 and 2012, whereas neonatal mortality fell by only 28%. Consequently, neonatal deaths now account for a greater proportion of global child deaths than in 1990, and every year there are still 3 million neonatal deaths.
Why Was This Study Done?
Experts estimate that advising mothers during pregnancy and in the days following delivery (the early postpartum period) about good delivery and newborn care practices could prevent a quarter of neonatal deaths. WHO and the United Nations Children's Fund (UNICEF) therefore recommend that all mothers in areas with high neonatal mortality should receive two home visits during the early postpartum period to assess the newborn’s health and to counsel mothers on newborn care practices. In this cluster randomized trial, the researchers investigate the effect on neonatal care and survival in rural southern Tanzania, a low-resource setting, of a volunteer-led home-based counseling strategy that meets these recommendations. The neonatal mortality rate (NMR) has declined markedly in recent years in Tanzania but varies widely across the country. A cluster randomized trial randomly assigns groups of people (here, women living in different “wards” consisting of three or four villages) to receive alternative interventions and compares outcomes in the differently treated “clusters.”
What Did the Researchers Do and Find?
The researchers estimated baseline neonatal mortality in five rural districts in southern Tanzania (35 deaths per 1,000 live births) from a 2007 census. In early 2010, they trained 824 female volunteers to deliver key counseling messages (including messages about handwashing with soap before delivery and early and exclusive breast feeding) and supporting messages such as the importance of childbirth in a health care facility. The volunteers were asked to make three home visits during pregnancy and two home visits shortly after delivery to women living in 65 intervention wards in six rural Tanzanian districts (a district not included in the baseline census was included in the trial); 67 comparison wards did not receive the intervention. A 2013 survey of households indicated that, in the intervention wards, 59% of women received at least one volunteer visit during pregnancy and 41% received at least one visit postpartum. Neonatal mortality reduced from 35 to 31 and 30 deaths per 1,000 live births in the intervention and comparison wards, respectively, in the five districts with baseline data. Newborn care practices reported by mothers were better in the intervention wards than in the comparison wards. For example, 42% and 35% of the women in the intervention and comparison wards, respectively, reported immediate breast feeding. Finally, childbirth in a health care facility increased from 41% in 2007 to 82% and 75% in 2013 in the intervention and comparison wards, respectively.
What Do These Findings Mean?
These findings provide no evidence for an effect on neonatal survival of the home-based counseling strategy tested here even though the intervention improved newborn care practices. Notably, although many of the women in the intervention wards received antenatal visits from the trained volunteers, fewer women received early postpartum visits, a finding that could be partly responsible for the lack of effectiveness of the intervention. The accuracy of these findings may be limited by certain aspects of the trial design. For example, some of the women in the comparison areas may have inadvertently received part of the intervention (“leakage”). In addition, newborn care practises improved much in both, intervention and comparison areas, included a doubling of facility delivery. Nevertheless, the lack of an effect of the intervention on neonatal mortality despite a moderate effect on newborn care behaviours throws some doubt on the existing evidence base in support of home-based counselling as an intervention to reduce neonatal mortality. Finally, the finding that childbirth in a health care facility increased during the study period without a concomitant reduction in neonatal mortality highlights the need to improve the quality of facility-based childbirth care in rural southern Tanzania and, possibly, in other resource-limited settings.
Additional Information
This list of resources contains links that can be accessed when viewing the PDF on a device or via the online version of the article at http://dx.doi.org/10.1371/journal.pmed.1001881.
WHO provides information on global efforts to reduce child mortality and on ending preventable neonatal deaths (available in several languages); its 2009 recommendations, "Home Visits for the Newborn Child. A Strategy to Improve Survival," are available
UNICEF works for children’s rights, survival, development, and protection around the world; it provides information about MDG4 and about child health in Tanzania; its website provides detailed statistics about child health; its Committing to Child Survival: A Promise Renewed—Progress Report 2014 addresses neonatal mortality
The Millennium Development Goals 2015 Report is available
The Healthy Newborn Network is an online community of more than 80 partner organizations that addresses critical knowledge gaps in newborn health
More information about the trial described here is available
Introduction
Every year, 3 million babies around the world die during their first 28 d of life. Despite major improvements in child survival in the past decade, neonatal mortality has declined slowly. In sub-Saharan Africa between 1990 and 2012, mortality during the first 5 y of life fell by 47%, from 177 to 98 deaths per 1,000 live births, but mortality in the first 28 d of life, which is the newborn or neonatal period, fell by only 28%, from 45 to 32 deaths per 1,000 live births. Around 44% of all child deaths now occur in the first 4 wk of life [1]. Millennium Development Goal 4—to reduce child mortality by two-thirds between 1990 and 2015—will not be reached without accelerated progress in reducing neonatal mortality.
The recent Lancet Every Newborn series supports community-based strategies to improve intervention coverage and reduce inequities [2]. In 2005, the Lancet Neonatal Survival series estimated that 12%–26% of neonatal deaths could be prevented by universal outreach and family-community care during the antepartum, peripartum, and postpartum period by promoting uptake of care and evidence-based newborn practices such as early and exclusive breastfeeding, thermal care, and clean cord care, among others [3]. In 2009, after trials in Asia showed dramatic effects of home-based counselling on neonatal survival [4–7], WHO and the United Nations Children's Fund (UNICEF) recommended two home visits in the early postpartum period in high-mortality settings to assess newborns and counsel mothers on newborn care practices [8]. The African evidence base for this strategy is limited to a single study [9].
In Tanzania, neonatal mortality has declined from around 29 to 21 deaths per 1,000 live births between 2005 and 2013 nationally [10], while wide variations in subnational estimates are described [11]. The health system has a pyramidal structure. Antenatal, intrapartum, and postpartum care is offered by a relatively dense network of primary and referral facilities [12]. The Tanzanian government is committed to the implementation and scale-up of a community health worker structure, making the effect of volunteer-based home counselling strategy on neonatal mortality of direct national relevance.
Here we report a cluster-randomised effectiveness trial of the effects of a volunteer-led, home-based counselling strategy—also called the Improving Newborn Survival in Southern Tanzania (INSIST) study—with three home visits in pregnancy and two in the first few days of life, on newborn care and neonatal survival in a population of over 1.2 million people.
Methods
Ethical Approval and Consent
The study was registered (www.clinicaltrials.gov NCT01022788) and approved by the review boards of Ifakara Health Institute, the Medical Research Coordinating Committee of the National Institute for Medical Research, Tanzania, Tanzania Commission for Science and Technology, and the London School of Hygiene and Tropical Medicine, United Kingdom. Written informed consent was sought from the household head. In 2013 we also sought written informed consent from interviewed women aged 18–49 y and assent from women aged 13–17 y.
Trial Design and Participants
This cluster randomised trial used wards (groups of three-to-four villages), as randomisation units, in a study area comprising all 132 wards in the six districts of Mtwara Rural, Newala, and Tandahimba in Mtwara region and Lindi Rural, Ruangwa, and Nachingwea in Lindi region. The intervention aimed to reach all pregnant women in intervention wards. Most residents were subsistence farmers living in small settlements (subvillages). Cashew nuts were the main cash crop, with fishing common along the coast. Most houses had mud walls and thatched roofs. A network of 200 dispensaries and health centres as well as six hospitals provided care of varying quality [13–15]. Almost 90% of women live within 5 km of primary facilities [16].
Home-Based Counselling Intervention
The home-based counselling strategy, branded Mtunze Mtoto Mchanga, which means “protect your newborn baby” in Swahili, was developed in 2008–2009. Formative work included a baseline household survey in 2007, qualitative enquiry including birth narratives and focus group discussions, and a rapid review of other volunteer programmes in the area. The strategy was designed in consultation with the Ministry of Health and members of the WHO, UNICEF, and professional organisations [17–20]. Key counselling messages were selected on the basis of the frequency of the behaviour in 2007 (Table 1), the feasibility of change, and the likely impact on survival on the basis of evidence published at the time [3,21]. They included hygiene during childbirth, early and exclusive breastfeeding, and extra care for low-birthweight babies, including skin-to-skin care (Table 2).
Table 1. Coverage of newborn care behaviours in the years prior to the 2007 and 2013 survey and changes between 2007 and 2013 restricted to the five districts with baseline data.
| Baseline Survey 2007 (results from 5 districts) | Impact Survey 2013 (results from 5/6 districts) | Percentage Point Change since Baseline (restricted to 5 districts) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Comparison | Odds ratio (OR) (95% confidence interval [CI]) | Intervention | Comparison | Difference | OR (95% CI) (results from 6 districts) | p-value | Intervention | Comparison | |||||
| N | % | N | % | N 5/6 districts | % 5/6 districts | N 5/6 districts | % 5/6 districts | |||||||
| Prepared soap for delivery * (home deliveries) | 6,641 | 84 | 6,508 | 84 | 1.0 (0.8–1.2) | 1,238/1,435 | 90/89 | 1,697/1,907 | 82/81 | 8% | 2.0 (1.5–2.5) | <0.001 | ↑6% | ↓2% |
| Prepared money for delivery * | N/A | N/A | N/A | N/A | N/A | 6,964/7,770 | 95/95 | 6,733/7,463 | 91/90 | 4% | 1.9 (1.5–2.3) | <0.001 | N/A | N/A |
| Had a plan in case of emergencies (home deliveries) ~ |
6,578 | 58 | 6,438 | 59 | 1.0 (09–1.1) | 1,224/1,415 | 71/70 | 1,676/1,882 | 64/63 | 7% | 1.4 (1.2–1.7) | <0.001 | ↑13% | ↑5% |
| Antenatal care (at least four times) * | 11,530 | 43 | 10,446 | 40 | 1.2 (1.0–1.4) | 6,981/7,789 | 47/47 | 6,781/7,519 | 43/43 | 4% | 1.2 (1.0–1.4) | 0.044 | ↑4% | ↑3% |
| Facility delivery * | 11,666 | 43 | 10,577 | 38 | 1.1 (0.8–1.6) | 7,005/7,820 | 82/82 | 6,813/7,553 | 75/75 | 7% | 1.5 (1.2–2.0) | 0.002 | ↑39% | ↑37% |
| Clean hands for home delivery ~ | 6,684 | 71 | 6,639 | 75 | 0.8 (0.6–1.1) | 1,168/1,351 | 93/92 | 1,598/1,799 | 89/88 | 6%/4% | 1.5 (1.0–2.3) | 0.033 | ↑22% | ↑14% |
| Baby immediately covered (<5 min after delivery) # | 11,662 | 27 | 10,569 | 27 | 1.0 (0.9–1.1) | 5,054/5,627 | 47/48 | 4,846/5,405 | 45/46 | 2% | 1.1 (1.0–1.2) | 0.057 | ↑20% | ↑18% |
| Baby not bathed before 6 h after birth # | 11,662 | 29 | 10,569 | 30 | 1.0 (0.7–1.3) | 6,377/7,083 | 92/91 | 6,177/6,799 | 82/80 | 10%/11% | 2.7 (2.1–3.4) | <0.001 | ↑63% | ↑52% |
| Breastfed within 1 h of birth # | 11,639 | 19 | 10,555 | 18 | 1.1 (0.9–1.2) | 6,562/7,287 | 42/42 | 6,346/7,008 | 34/35 | 8%/7% | 1.4 (1.3–1.6) | <0.001 | ↑23% | ↑16% |
|
Fed only breast milk in the first 3 d
after delivery # |
11,543 | 50 | 10,488 | 48 | 1.1 (0.8–1.4) | 6,801/7,557 | 90/90 | 6,316/7,307 | 79/79 | 11% | 2.2 (1.8–2.7) | <0.001 | ↑40% | ↑31% |
| Nothing put on the cord # | 11,404 | 72 | 10,335 | 72 | 1.0 (1.9–1.2) | 6,665/7,403 | 92/92 | 6,423/7,092 | 87/87 | 5% | 1.8 (1.5–2.1) | <0.001 | ↑20% | ↑15% |
| Babies born prematurely taken to hospital ^ | N/A | N/A | N/A | N/A | N/A | 213/251 | 34/31 | 203/236 | 31/30 | 3%/1% | 1.0 (0.6–1.9) | 0.920 | N/A | N/A |
| Practiced any skin-to-skin care for prematurely born babies^ | N/A | N/A | N/A | N/A | N/A | 213/248 | 43/42 | 203/233 | 37/36 | 6% | 1.3 (0.9–1.9) | 0.200 | N/A | N/A |
| Sick babies taken to health facility ¤ | N/A | N/A | N/A | N/A | N/A | 1,007/1,151 | 80/80 | 1,008/1,302 | 77/77 | 3% | 1.1 (0.9–1.4) | 0.255 | N/A | N/A |
Key behaviours marked in bold
* all women who reported a live birth
~ women who reported a live birth delivered at home
# women who reported a live birth with the baby surviving at least 3 d
^ live-born baby who the mother reported was born prematurely
¤ mother reported any sickness of her live-birth baby in the first month of life. N/A, not available
Table 2. Behaviours promoted in home-based counselling, adapted from [26].
| Visit | Timing | Key Behaviours | Additional Behaviours |
|---|---|---|---|
| 1 | As soon as pregnant woman identified | Information on importance of birth attendant washing hands and wearing gloves | Promotion of birth preparedness: facility delivery, saving money, clean cloths, soap, new blade for cutting and clean thread for tying cord, gloves for birth attendant |
| 2 | 4 wk after visit 1 | Promotion of early and exclusive breastfeeding | Promotion of birth preparedness (as in visit 1) |
| 3 | At the beginning of the 9th mo of gestation | Reinforcing early and exclusive breastfeeding practices, including breastfeeding position. In case of home birth, reinforcing the following: birth attendant should wash hands and wear gloves, identification of low-birth-weight babies using foot size as a proxy, immediate referral for very small or premature babies and those who do not cry, and skin-to-skin care for small babies. | Promotion of birth preparedness (as in visit 1); information on the importance of thermal care: immediate drying and wrapping and delayed bathing; information on danger signs in newborns. In case of home birth, the cord should be cut with a clean blade and tied with a clean thread. |
| 4 | Day of delivery | For home and facility births: observe and counsel on breastfeeding and remind women to practice exclusive breastfeeding. In case of home birth: identify low-birth-weight babies using foot size as a proxy, immediate referral for very small or premature babies, and skin-to-skin care for small babies | Check on thermal care and knowledge of danger signs and reinforce putting nothing on the cord |
| 5 | 3rd d after delivery | Observe and counsel on breastfeeding and remind about exclusive breastfeeding | Reinforce putting nothing on the cord |
| Extra visits for small babies: | |||
| First extra visit | Day after visit 5 | Promotion of skin-to-skin care until the baby does not want to be carried skin to skin | |
| Second extra visit | Day after visit 6 | Promotion of skin-to-skin care until the baby does not want to be carried skin to skin | |
Because weighing scales were unlikely to be sustainable, we developed a screening tool using newborn foot size as a proxy for birth weight so that volunteers could identify low birthweight or premature babies born at home [22]. Home-based treatment of sepsis was not included in the advice of key national stakeholders, who felt it would be neither feasible nor necessary given the relatively dense network of primary facilities. Supporting messages included advice and information on childbirth in health facilities, birth preparedness, thermal care (immediate drying and wrapping and delayed bathing), cord cutting with a clean blade, tying with clean thread, dry cord care, and danger signs for sick newborns [23]. We hypothesised that improved hygiene during childbirth, early and exclusive breastfeeding, and better thermal care for low-birth-weight babies would lead to a measurable reduction in neonatal mortality [19]. Facility-based work to improve the quality of care in pregnancy and childbirth was implemented in only 24 of 200 facilities because of financial and human resource constraints, half in intervention and half in comparison areas [23]. Women in intervention and comparison groups received standard facility-based health care throughout the study.
During January–June 2010, 824 female volunteers were trained, two from each village. They were selected by their communities and trained for 6 d by council health management teams, who were trained in turn by regional health teams. Although some had experience of volunteering, those involved in other volunteer programme at the time were not eligible to apply. The volunteers were trained to visit women and their families three times in pregnancy and twice in the early postpartum period. We chose to emphasise counselling during pregnancy through three pregnancy visits rather than two because a 2009 field visit to a similar study in Ghana [24] suggested that high coverage of an early postnatal visit would be challenging.
The strategy was designed for large-scale implementation using existing community governance structures and the health system. Supervision and support was provided by local leaders known as village executive officers, as well as local health facility staff once a month [25]. Every 3–4 mo, volunteers met with supervisors and district health staff at a ward-level review meeting. In each ward, ten review meetings were held between June 2010 and July 2013. These meetings gave important opportunities to collect, summarise, and provide feedback on internal monitoring data on coverage of home visits for the volunteers, to build their skills and knowledge beyond the initial training, and to fine-tune the intervention. For example, given emerging findings on high facility delivery rates, we introduced birth notification slips for facility-based staff to give to mothers to inform the volunteers about the need for a home visit following birth in a health facility.
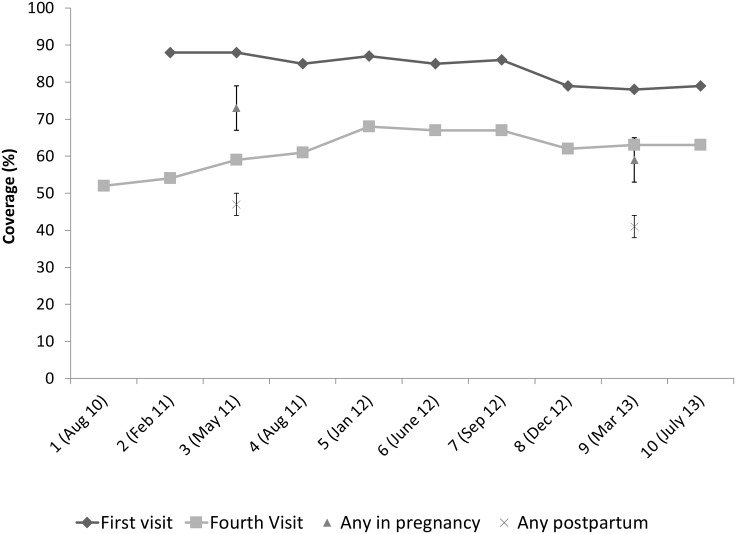
We monitored volunteer visits recorded during quarterly review meetings and calculated coverage using expected births as a denominator; the expected births were calculated based on population data from 2007 and using a birth rate of 39 per 1,000 live births. These internal monitoring data suggested that of the 64,932 expected women with a live birth, approximately 80% in intervention areas had been visited by a volunteer in pregnancy and around 60% had been visited in the early newborn period (Fig 1, S1 Data). We did a follow-up survey including 5,000 households in 2011 to estimate implementation strength and changes in behaviour in intervention compared to comparison wards, to justify funding both for implementation until mid-2013 and for the 2013 end-line mortality survey. We saw improved newborn care behaviours such as the baby being bathed at least 6 h after birth (OR 2.0; 95% CI 1.2–3.4 comparing intervention and comparison areas) and exclusive breastfeeding for the first 3 d (OR 1.9; 95% CI 1.3–2.9) [26]. As a result, the international technical advisory board recommended that the impact evaluation was warranted.
Fig 1. Estimated coverage of volunteer home visits from internal monitoring and household surveys.
~ Internal monitoring information (first and fourth visits) refers to information from the volunteers’ workbooks, which were collected throughout the study on a quarterly basis. The number of quarterly review meetings and the median month of data collection are given. * Household survey data include the adequacy survey done in 2011 (based on 257 women with a live birth in the year prior the survey [26]) and the impact evaluation household survey in 2013 (based on 7,823 women with a live birth in the year prior the survey).
Outcomes
The primary outcome was the all-cause neonatal mortality rate (NMR) per 1,000 live births, defined as the proportion of all live births who died in the first 28 d of life (days 0–27), for children born between 1 July 2010 and 30 June 2013. Other mortality outcomes were days 1–27 mortality. The restriction to babies who survived the first 24 h after birth (day 0) was to exclude deaths due to intrapartum-related complications including asphyxia, for which the intervention was not expected to have a major impact.
The key behaviour (secondary) indicators were breastfeeding within an hour of delivery, prior handwashing with soap or use of gloves for those attending home deliveries, and exclusive breastfeeding for the first 3 d after birth. Other behaviour outcomes were skilled attendance at childbirth, birth preparedness, immediate drying and covering of the baby, clean cord care, delayed bathing, and identification and extra care for small babies, including skin-to-skin care for small babies and referral to hospital for very small babies. A data and safety monitoring board reviewed the study procedures and participant safety.
Assessment of the Outcomes
Baseline neonatal mortality and newborn care practices were assessed through a survey of all households in five of the six study districts in 2007 (excluding Mtwara Rural) as part of a study assessing the effect of intermittent preventive treatment of malaria in infants [18,27]. In 2013 we did a representative household survey, including 185,000 households from all 132 wards. The primary sampling unit was the subvillage with a median of 100 households. As the study was randomised by ward and ward population varied from 1,000 to 22,000 people, we limited the sampling fraction in larger wards. We selected all subvillages in 56 smaller wards with 1,800 households or less and 20 subvillages from the 76 larger wards, chosen with probability proportional to size of the subvillage. Because of civil unrest, we reduced the sampling fraction to 11 subvillages in each ward of the Mtwara Rural district. Within the selected subvillages, we included all households if the local leaders estimated there were fewer than 130 households, which was the number that a single team of interviewers could manage in 1 d. For larger subvillages, we used segmentation to limit the sample to a maximum of 131 households. The survey sampled an estimated average of 94 households per subvillage.
With this sample size, we estimated that we would have complete data for at least 100 live births per ward per year in the 3 y leading to the survey, which would give 80% power to detect a 15% effect on neonatal mortality using a two-sided test at the 5% significance level assuming a coefficient of variation in ward mortality rates (SD/mean) of 0.21 and neonatal mortality of 34 per 1,000 in the comparison wards [28].
Data collection
In 2007 and 2013, data were collected by 22 and 20 teams, respectively, each with seven interviewers, a supervisor, a mapper/sensitiser, and a driver. An initial household listing module included the geographic location and the household head’s name. A household was defined as a group of people who live and eat together. If a household head refused to participate, replacement households were not approached. A household module included information on all members of the household, their dates of birth, education, and occupation; ethnic group of the household head; and asset ownership and housing characteristics as proxies of socioeconomic status. In a separate module, all resident, consenting women aged 18–49 y and assenting women aged 13–17 y were asked about live births in the 3 y prior to the survey, whether the child was still alive, and dates of all demographic events. For live births in the year before the survey, we asked about care in pregnancy, childbirth, and postpartum and about home counselling visits. We did not collect information on stillbirths.
Data collection and recording
In 2007 and 2013, all data were entered at the point of collection using personal digital assistants (PDAs, HP iPAQ HX2490 v6.1) programmed to allow internal range and consistency checks [29]. At the end of each module, each day, and once a week, data were backed up on electronic storage media. Quality control measures included accompanied interviews, random repeat interviews with follow-up action for discrepancies, supervisor visits to reportedly empty households, and daily reconciliation of handwritten summaries with computer-generated summaries before leaving the subvillage. The weekly reports summarised interviewer and team performance.
Randomization and Masking
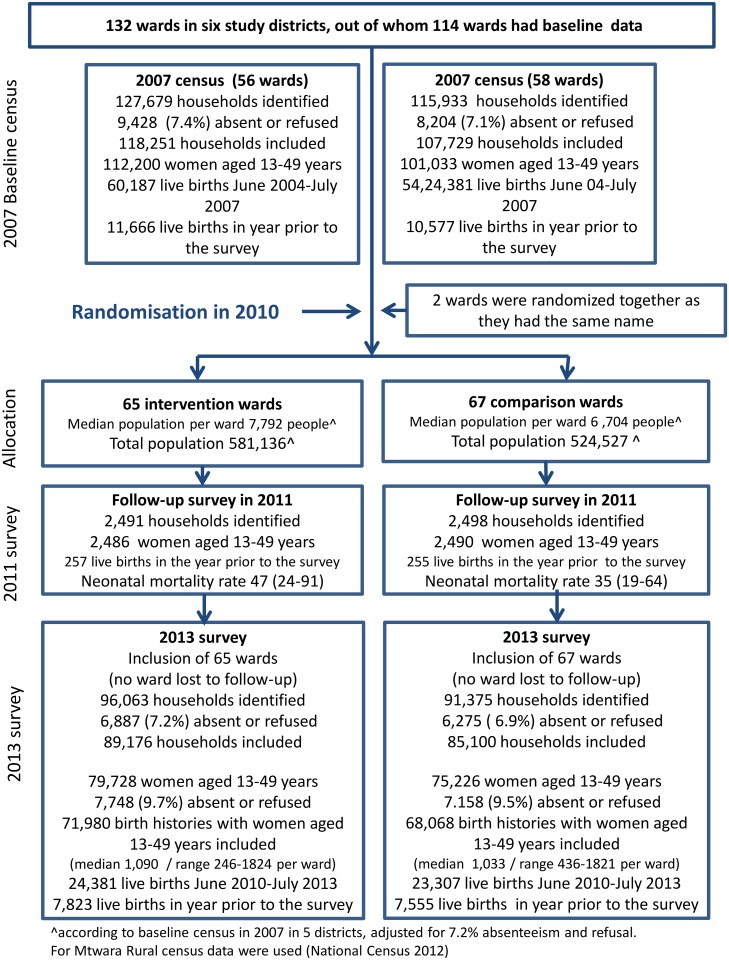
In 2009, 65 wards were randomised to the intervention, and 67 wards comprised comparison areas. Two wards with the same name were inadvertently randomised as one to the comparison group: they were analysed as two separate wards. To maximize balance between the two groups in the five districts with baseline data, we used implicit stratification with respect to district, division (an administrative structure between districts and wards), baseline NMR, and population. For the district without baseline data, we used implicit stratification by division [26]. Randomization was performed by JS using STATA. There were no exclusion criteria for clusters, households, or women. All wards agreed to participate, and volunteers were recruited from all intervention area villages. Consent to participate in the intervention was not formally sought from pregnant women, but they were free to refuse volunteer visits. Community members and health staff were not masked. The survey team was unaware of cluster allocation. The data analyst was masked to the cluster allocation until data cleaning was complete and a copy of the data lodged with the data and safety monitoring board.
Analytical Methods
We used an intention-to-treat analysis, comparing children born to women in intervention and comparison wards according to a predefined analytical plan. We used random-intercept effects logistic regression using the xtmelogit command provided in Stata (Stata/IC Version 12.1), specifying the ward and subvillage level to account for the randomisation unit (ward level) and the clustered nature of the sample survey (subvillage level) [30].
The primary analysis was based on end-line data from all six districts. We computed odds ratios (OR) with 95% confidence intervals (CIs). We also estimated absolute risk differences for mortality.
We did secondary analyses of mortality for (1) the time periods of 2010–2011, 2011–2012, and 2012–2013 to investigate whether any effect changed over time; (2) babies surviving the first day of life (days 1–27); (3) facility and home births; (4) singletons; and (5) restricting the analysis to intervention wards with high home visit coverage, compared to all comparison wards. The analytical plan was reviewed and approved by the DSMB before data sets were “locked.” We repeated the analysis restricted to five districts and, as reviewers suggested, adjusted for baseline (2007) neonatal mortality, population size, and division. Based on reviewer advice, we also report a “per protocol” analysis in the web annex, although such results are likely to be biased (S2 Text). We estimated the effect of the intervention on newborn care behaviours using OR and percentage-point differences for newborn care practices.
We did not impute data for the district of Mtwara Rural, for which no baseline data were available. Multiple imputation would assume the information was missing at random. However, the Mtwara Rural district differs in numerous ways from the other five districts included in the study, for example, by not having a hospital. We also observed differences in poverty status and ethnic background.
We updated a meta-analysis conducted by Kirkwood and colleagues [9]. We searched PubMed using the terms (neonatal OR newborn) AND mortality AND trial and included those studies that examined the effect of community volunteers providing home visits during pregnancy or postpartum. No trials examining the effect of home visits on neonatal mortality other than those included in the 2013 systematic review of Kirkwood and colleagues [9] were retrieved. This included four proof-of-principle studies done in Southeast Asian countries [4–7] and a further four studies in a programme setting [9,31–33]. As most of the included studies presented risk ratios (RRs) and not ORs, we calculated the RR for NMR for our study using the margins from the random effects logistic model (marginal standardisation method) and estimated the CI via the delta technique [34]. As there was significant heterogeneity (>50%) between studies, we report the pooled result from a random effects meta-analysis.
Results
The baseline survey in 2007 included all 243,612 households in five districts (except Mtwara Rural); 17,632 (7%) of the household heads were not present or refused participation. We interviewed 193,867 (91%) of 213,233 identified women of reproductive age (13–49 y); 22,243 had a live birth in the year prior to the survey (Fig 2, Table 3).
Fig 2. Trial profile.
Table 3. Characteristics of respondents of intervention and comparison wards in the 2007 and 2013 surveys.
The 2007 survey included the five districts of Lindi Rural, Ruangwa, Nachingwea, Newala, and Tandahimba. The 2013 survey additionally included the Mtwara Rural district.
| Intervention Wards 2007/2013 n = 127,679/96,063 | Comparison Wards 2007/2013 N = 115,933/91,375 | Percentage Point Difference 2007/2013 | |||
|---|---|---|---|---|---|
| n | % | N | % | % | |
| Household head present | 119,222/90,180 | 94/94 | 108,567/86,074 | 94/94 | 0/0 |
| Household head agreed to participate | 118,251/89,176 | 99/99 | 107,729/85,100 | 99/99 | 0/0 |
| Region | |||||
| Lindi Region | 65,989/45,655 | 56/51 | 60,776/43,165 | 56/51 | 0/0 |
| Mtwara Region | 52,262/43,521 | 44/48 | 46,953/41,935 | 44/49 | 0/-1 |
| Ethnic group | |||||
| Makonde | 63,259/49,546 | 54/56 | 60,836/49,730 | 56/58 | -2/-2 |
| Other | 54,992/39,630 | 47/44 | 46,893/35,370 | 44/42 | +3/+2 |
| Wealth quintiles (assets) | |||||
| Most poor | 21,900/15,999 | 19/18 | 20,846/16,565 | 19/20 | 0/-2 |
| Very poor | 19,855/17,374 | 17/20 | 18,385/16,856 | 17/20 | 0/0 |
| Poor | 24,597/16,891 | 21/19 | 18,178/15,956 | 21/19 | 0/0 |
| Less poor | 22,402/17,075 | 19/19 | 20,906/16,146 | 19/19 | 0/0 |
| Least poor | 23,008/18,723 | 20/21 | 20,078/16,567 | 19/20 | 1/1 |
| Missing | 6,489/3,114 | 6/4 | 5,336/3,010 | 5/4 | 1/0 |
| Maternal education | |||||
| 0–6 y of education | 51,805/28,641 | 46/36 | 46,310/27,032 | 46/36 | 0/0 |
| Completed primary education | 59,609/50,523 | 53/63 | 53,993/47,698 | 53/63 | 0/0 |
| Missing | 786/564 | 1/1 | 730/496 | 1/1 | 0/0 |
| Maternal occupation | |||||
| Farming | 88,102/65,089 | 79/82 | 80,280/62,396 | 80/83 | -1/-1 |
| Other | 4,721/3,785 | 4/5 | 3,751/2,924 | 4/4 | 0/1 |
| Missing | 19,377/10,854 | 17/14 | 17,002/9,906 | 17/13 | 0/0 |
No ward was lost to follow-up, and the data and safety monitoring board reported no safety concerns. In 2011, we did a follow-up survey in 5,000 households and estimated improved newborn care behaviours such as baby bathed at least 6 h after birth (OR 2.0; 95% CI 1.2–3.4 comparing intervention and comparison area) and exclusive breastfeeding for the first 3 d (OR 1.9; 95% CI 1.3–2.9) [26].
In 2013, six of 2,193 sampled subvillages refused to participate. A total of 187,438 households were visited between 1 July and 28 October 2013 (Fig 2, Table 3). In 11,184 (6%) households, no one was present; 1,978 (1%) refused to participate. We identified 154,954 women aged 13–49, of whom 140,048 (90%) agreed to be interviewed, 71,980 in the intervention wards and 68,068 in the comparison wards. These women reported 24,381 and 23,307 live births in the 3 y before the survey in intervention and comparison wards, respectively. Households and women were similar with regard to sociodemographic factors in the baseline and end-line surveys.
Implementation strength
Reported coverage of at least one home-based counselling visit using the Mtunze job aids in pregnancy and postpartum was 59% (4,601 women) and 41% (3,216), respectively, in intervention areas compared with 4% (411 women) and 3% (259) in comparison areas (Table 4, Fig 1). Only 934 women (15%) in the intervention group and 51 (1%) in the comparison group reported a visit within 2 d postpartum after facility delivery (OR 21.7; 95% CI 14.6–32.2). Only 409 (5%) and 43 (1%) women reported receiving the full home visit schedule of three visits in pregnancy and two visits postpartum in intervention and comparison areas, respectively.
Table 4. Home counselling visit coverage reported by women with a live birth in the year prior to the 2013 survey.
| Intervention Wards | Comparison Wards | OR (95% CI) | p | Percentage Point Difference | |||
|---|---|---|---|---|---|---|---|
| n/N = 7,823 | % | n/N = 7,555 | % | % | |||
| Home-based Counselling | |||||||
| Women received a counselling visit during pregnancy* | 4,601 | 59 | 411 | 4 | 41.5 (31.0–55.7) | <0.001 | 55 |
| Women received a postpartum counselling visit* | 3,216 | 41 | 259 | 3 | 28.6 (21.3–38.4) | <0.001 | 38 |
| Women received a counselling visit* within 2 d of home delivery | 275 | 19 | 7 | 0 | 75.0 (33.8–166.4) | <0.001 | 19 |
| Women received a counselling visit within 2 d of facility delivery* | 934 | 15 | 51 | 1 | 21.7 (14.6–32.2) | <0.001 | 14 |
| Women received three visits in pregnancy and two postpartum, first within 2 d* | 409 | 5 | 43 | 1 | 10.1 (7.1–15.8) | <0.001 | 4 |
* Volunteer who used the Mtunze counselling card or doll during the visit and where a card was left with the family
Primary mortality results
There was no evidence of an impact of the intervention on neonatal survival (31.6 versus 29.9 deaths per 1,000 live births in intervention and comparison wards, OR 1.1, 95% CI 0.9–1.2, p = 0.339) in the six districts (Table 5). Neonatal mortality reduced from 35.1 to 31.0 deaths per 1,000 live births in the intervention wards and from 34.9 to 30.0 in the comparison wards between 2007 and 2013 in the five districts, respectively. Analysis adjusted for baseline neonatal mortality, population, and division, restricted to the five districts when this was available, gave similar results (OR 1.0, 95% CI 0.9–1.2, p = 0.779). Neonatal mortality in the five districts where baseline data were available declined at 2% per year on average, from 35.0 (95% CI 33.5–36.5) to 30.8 per 1,000 live births (95% CI 29.3–32.5), a drop of 13% in 6 y.
Table 5. Primary analysis: Newborn mortality in intervention areas compared to comparison areas in 2007 and 2013 and adjusted for baseline.
| Neonatal Mortality Rate per 1,000 Live Births | 2007 (5 districts) | 2013 (5/6 districts) | ||||||
|---|---|---|---|---|---|---|---|---|
| Intervention wards | Comparison wards | OR~ (95% CI) | p | Intervention wards | Comparison wards | OR~ (95% CI) | p | |
| Live births | 33,553 | 30,603 | 21,898 /24,381 | 21,085/23,307 | ||||
| Deaths, days 0–27 | 1,140 | 1,035 | 661/749 | 616/679 | ||||
| Neonatal mortality rate per 1,000 live births (95% CI) | 35.1 (33.1–37.2) | 34.9 (32.8–37.1) | 1.0 (0.9–1.1) | 0.830 | 31.0 (28.8–33.5)/ 31.6 (29.5–34.0) | 30.0 (27.7–32.5)/ 29.9 (27.8–32.3) | 1.0 (0.9–1.2)/1.1 (0.9–1.2) | 0.547/0.339 |
| Effect adjusted for baseline mortality and population size within the wards and division | 1.0 (0.9–1.2) | 0.779 | ||||||
~We used multilevel logistic regression to compute ORs specifying the ward and subvillage level. The intracluster correlation coefficients were 5.6% (95% CI 3.5%–8.8%) for the subvillage nested within the ward. The respective value for the ward level was 0.5% (95% CI 0.1%–2.7%).
Secondary mortality outcomes
There was no evidence of any difference in neonatal mortality between intervention and comparison groups of babies who survived the day of birth or for singleton babies (Table 6). The OR of dying in intervention compared with comparison wards was 1.1 (95% CI 0.9–1.3, p = 0.323) for babies who survived the day of birth and 1.1 (95% CI 0.9–1.2, p = 0.473) for the subgroup of singleton babies. Half of the neonatal deaths were on the first day of life (703 of 1,428, 49%), with no difference between intervention and comparison groups. We observed no difference in neonatal mortality between intervention and comparison areas when analysing the 3 y separately (2010–2011, 2011–2012, and 2012–2013) and thus no indication of a time trend.
Table 6. Secondary analysis, neonatal mortality in subgroups by intervention status, 2013 survey.
| Neonatal Mortality Rate per 1,000 Live Births | Intervention Wards | Comparison Wards | Odds Ratio (95% CI) | p | Rate Difference |
|---|---|---|---|---|---|
| All babies, post day 0 (day of birth) | |||||
| Live births | 23,966 | 22,922 | |||
| Deaths, days 1–27 | 334 | 294 | |||
| NMR per 1,000 live births (95% CI) | 14.1 (12.7–15.7) | 13.0 (11.6–14.5) | 1.1 (0.9–1.3) | 0.323 | 1 |
| Single births | |||||
| Live births | 23,760 | 22,714 | |||
| Deaths, days 0–27 | 665 | 611 | |||
| NMR per 1,000 live births (95% CI) | 28.8 (26.6–31.0) | 27.6 (25.5–29.9) | 1.1 (0.9–1.2) | 0.473 | 1 |
| Births in the year prior to the survey (July 2012–June 2013) | |||||
| Live births | 8,148 | 7,877 | |||
| Deaths, days 0–27 | 293 | 274 | |||
| NMR per 1,000 live births (95% CI) | 37.3 (33.3–41.8) | 36.0 (32.0–40.5) | 1.0 (0.9–1.2) | 0.674 | 1 |
| Births in the 2 y prior to the survey (July 2011–June 2012) | |||||
| Live births | 8,841 | 8,286 | |||
| Deaths, days 0–27 | 267 | 240 | |||
| NMR per 1,000 live births (95% CI) | 31.0 (27.5–35.0) | 29.8 (26.2–33.8) | 1.04 (0.9–1.3) | 0.645 | 1 |
| Births in the 3 y prior to the survey (Jul 2010–June 2011) | |||||
| Live births | 7,392 | 7,144 | |||
| Deaths, days 0–27 | 189 | 165 | |||
| NMR per 1,000 live births (95% CI) | 26.2 (22.7–30.2) | 23.6 (20.3–27.5) | 1.11 (0.9–1.4) | 0.348 | 2 |
| Analysis restricted to live births in the year prior to the survey with information on place of birth | |||||
| Live births in health facilities (any type) | |||||
| Live births | 5,309 | 4,686 | |||
| Deaths, days 0–27 | 186 | 153 | |||
| NMR per 1,000 live births (95% CI) | 32.2 (27.7–37.2) | 29.9 (25.5–35.0) | 1.1 (0.9–1.4) | 0.510 | 2 |
| Live births at home | |||||
| Live births | 1,240 | 1,654 | |||
| Deaths, days 0–27 | 38 | 57 | |||
| NMR per 1,000 live births (95% CI) | 31.7 (23.1–43.6) | 35.7 (27.5–46.2) | 0.9 (0.6–1.3) | 0.569 | -4 |
| Restriction to intervention wards with coverage > 70% home visits * (all comparison wards included) | |||||
| Live births | 3,082 | 6,342 | |||
| Deaths, days 0–27 | 98 | 210 | |||
| NMR per 1,000 live births (95% CI) | 32.8 (26.9–40.0) | 34.2 (29.9–39.2) | 1.0 (0.8–1.4) | 0.749 | -1 |
| Restriction to intervention wards with coverage > 80% home visits * (all comparison wards included) | |||||
| Live births | 1,095 | 6,342 | |||
| Deaths, days 0–27 | 36 | 210 | |||
| NMR per 1,000 live births (95% CI) | 34.0 (24.5–47.1) | 34.2 (29.9–39.2) | 1.0 (0.7–1.4) | 0.958 | 0 |
* Volunteer who used the Mtunze counselling card or doll during the visit
# Volunteer visit in pregnancy and postpartum in which a Mtunze counselling card or doll was used during the visit
We found no evidence of any difference in neonatal mortality in babies born at home in intervention compared to comparison wards in the year prior to the survey (OR 0.9, 95% CI 0.6–1.3, p = 0.569) or in babies born in wards where the intervention had at least 70% coverage of at least one volunteer visit compared to comparison wards (OR 1.0, 95% CI 0.8–1.4, p = 0.749).
Newborn care behaviours (secondary outcomes)
There was strong evidence that the intervention improved coverage of key newborn care behaviours (Table 1) in the six districts. More women delivering at home had a birth attendant with clean hands (92% of 1,351 compared to 88% of 1,799; OR 1.5 95% CI 1.0–2.3, p = 0.033) comparing intervention and comparison wards of all six districts. More women reported breastfeeding within 1 h of birth (42% of 7,287 compared to 35% of 7,008; OR 1.4 95% CI 1.3–1.6, p < 0.001) and breastfeeding their babies exclusively for the first 3 d (90% of 7,557 compared to 79% of 7,307; OR 2.2, 95% CI 1.8–2.7, p < 0.001).
More women had emergency plans in case of home delivery (70% of 1,415 in intervention compared to 63% of 1,882 in the comparison areas; OR 1.4 95% CI 1.2–1.7, p < 0.001). More women reported delaying bathing their baby for six or more hours after birth (91% of 7,083 in intervention compared to 80% of 6,799 in comparison area; OR 2.7, 95% CI 2.1–3.4, p < 0.001). The proportion of sick babies taken to a health facility was similar in the two groups (80% of 1,151 and 77% of 1,302 in intervention and comparison groups, OR 1.1, 95% CI 0.9–1.4, p = 0.255). Coverage rates were virtually identical when restricting the analysis to five districts.
Comparison of key newborn care behaviours between 2007 and 2013 in intervention and comparison areas, for the five districts with baseline data, indicated relative large increases between 2007 and 2013 for clean hands for home delivery (22% compared to 14% increase in intervention and comparison wards, respectively), breastfeeding within 1 h (23% compared to 16% increase), and exclusive breastfeeding (40% compared to 31% increase).
Delivery in a health facility was 41% of 22,243 in 2007 and increased to 82% of 7,820 in the intervention area and 75% of 7,553 in the comparison area, a 39% and 37% point increase between 2007 and 2013 in intervention and comparison areas, respectively. Half of the facility births in 2013 took place in a hospital (5,937; 39%), and the other half in primary facilities: health centres (1,379; 9%) and dispensaries (4,715; 31%). In 2007, only 11% of 22,243 mothers delivered in primary facilities, and 29% in a hospital.
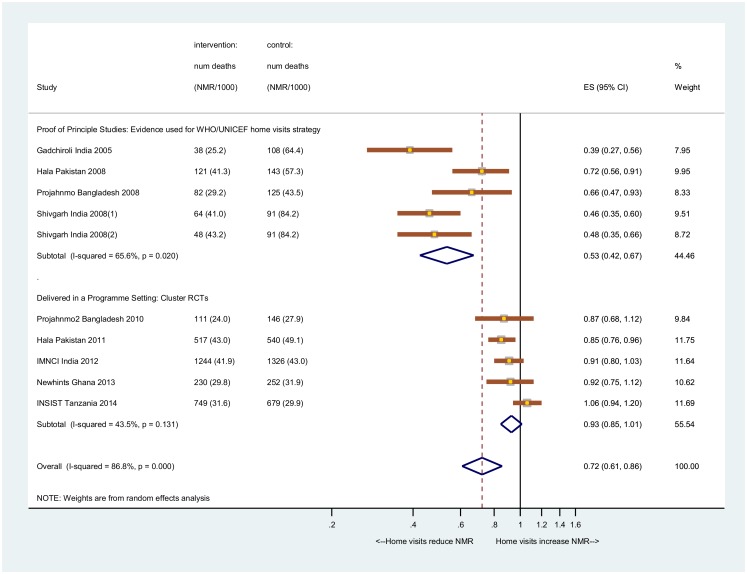
The updated meta-analysis showed no evidence that antepartum and postpartum home visits have an effect on neonatal survival in programme settings, based on three trials from Southeast Asia and two trials from sub-Saharan Africa: ours and Newhints, Ghana [9] (risk ratio of 7%; 95% CI -1%–15%) (test for heterogeneity p = 0.131, I^2 89%; 95% CI 78–92) (Fig 3).
Fig 3. Meta-analysis of the effect of home visits on NMR.
Data are the number of deaths (newborn mortality rate per 1,000 live births). Proof-of-principle studies: Gadchiroli, India, 2005 [4]; Hala, Pakistan [7]; Projahnmo, Bangladesh, 2008 [6]; Shivgarh, India, 2008 [5]; Projahnmo-2, Bangladesh [33]; Hala, Pakistan [31], Integrated Management of Neonatal and Childhood Illnesses, India [32]; Newhints, Ghana [9]; and Improving Newborn Survival in Southern Tanzania (INSIST). Shivgarh-1 = home visits only. Shivgarh-2 = home visits plus thermospot.
Discussion
This large cluster-randomised controlled trial in southern Tanzania assessing the effect of a home-based counselling strategy in pregnancy and postpartum found no evidence of an effect on neonatal survival. Neonatal mortality decreased from 35 to 31 deaths per 1,000 live births between 2004–2007 and 2010–2013. The intervention resulted in improved newborn care practices, particularly for exclusive breastfeeding in the first 3 d and delaying bathing, each of which improved by at least ten percentage points. Large secular increases in facility delivery from 41% in 2007 to 79% in 2013 were observed. Remarkably, almost 3 y after a 6-d volunteer training course given by district health teams, around 60% of pregnant women received a home visit by a volunteer supported through existing community structures [25]. However, early postpartum visits remained very low.
The lack of an impact on survival was unexpected, given the 12% reduction in neonatal mortality for a home visit strategy suggested by a recent systematic review of programmatic trials [9] and the 25% reduction proposed in the work of Lassi and Bhutta, which included studies evaluating community-based interventions [35]. Reasons could include low postpartum visit coverage. There was often a delay in volunteers receiving information about a delivery, and the distance to reach the woman’s home was another main barrier. Another reason for the lack of effect on mortality might be the relatively high coverage of recommended newborn care behaviours, including antenatal care and facility delivery. This context contrasts with that of Asian trials in which home-based counselling strategies had the most dramatic effects on neonatal mortality and both facility-based antepartum care and facility births were at a low level (S1 Table) [31–33].
In addition, our home-based approach did not include any clinical component such as identification and referral of sick newborns or antibiotic therapy. However, the increase in facility delivery alone should have had a large impact on mortality over time if common assumptions that this leads to improved care hold true [36]. Bhandari et al. reported an effect of home-based care on neonatal mortality in home births but not in women who delivered in a health facility [32]. Further, almost half of the deaths in our study were on the day of birth and likely due to intrapartum-related and preterm birth complications [37]. The potential for home visits to prevent such deaths is limited, but even after excluding these first-day deaths, we found no evidence of a survival impact. Our study did not include morbidity data [38] or information on the quality of intrapartum care, for which severe limitations are reported from elsewhere in Tanzania [39].
Our study has several limitations. First, the effects of the intervention may have reached comparison areas, giving an underestimate of the effectiveness of the intervention. Some volunteers may have visited women in comparison areas living close to ward boundaries or with family ties, because as the due date approaches some women stay with relatives. In addition, other groups supported home visit programmes with similar messages in small parts of the study area. The main focus of our facility-based quality improvement work was promotion of facility-based childbirth: this was implemented in 24 of 200 facilities (all of those in the Ruangwa district, with a further four in Mtwara Rural), with a balance between intervention and comparison wards. Second, recall of care practices and neonatal mortality could be prone to error. To mitigate the effects of this, information on care practices was only collected for live births in the year prior to the survey, and all analysis was based on an intention-to-treat approach. Thirdly, we did not collect information on stillbirths: misclassification of early neonatal deaths as stillbirths and vice versa is common. Fourthly, it was not feasible to mask participants. The intervention itself might have led to reporting bias, with women in the intervention groups reporting mortality or care practices differently than those in the comparison group.
The trial took place in a rapidly changing and dynamic environment where facility delivery almost doubled within 6 y from 41% at baseline in 2007 [18]. Although the intervention might have played some role in the increase, it is likely that other contextual factors were also important: just 1 y after implementation started, 65% of the comparison area births were in a health facility [26]. In part the increase could be due to improved communication and transport: less than 10% of households had a mobile phone in 2007 compared to 48% in 2013. The availability of motorcycle transport also increased substantially, and the focused national antenatal care programme promoting facility delivery might have contributed to the shift. Lastly, facility-based quality improvement programmes emphasising facility delivery, including our own in 24 facilities, have been ongoing in the study area, as well as focal home counselling work by different development agencies [40].
Our study illustrates some of the challenges of undertaking effectiveness evaluations of an intervention designed for scale in a rapidly changing context. Firstly, despite a robust, cluster-randomised design, we found strong evidence of behaviour change in the comparison group and cannot rule out that this is due in part to our intervention. Secondly, we found that although home visits in pregnancy were common, only 15% of women who delivered in a facility had a postnatal visit within 2 d of childbirth. Although disappointing, this result illustrates the importance of studying effectiveness: a large-scale programme in a similar context is unlikely to achieve high coverage of early postnatal visits. In addition, our effectiveness trial faced similar problems to other recent large-scale evaluations in terms of being overridden by health systems improvements [41], supporting the call by Victora and colleagues for more investments in national evaluation platforms [42].
Our results from the meta-analysis including five studies in programme settings found no evidence of a reduction of neonatal mortality (7% CI -1%–15%), which is at odds with the 45% impact (95% CI 37%–52%) for the proof-of-principle trials, all done in settings with limited access to facility-based health care services and high neonatal mortality (>45 deaths per 1,000 live births) [43]. Our updated estimate is also at odds with the estimated effect of 12% (95% CI 5%–18%) previously provided by Kirkwood and colleagues [9].
In a separate systematic review that examined intervention packages including home visits rather than home visits alone, the authors found insufficient evidence to draw any conclusion on the effect of postnatal home visits on neonatal mortality, reporting that any effect might depend on the context and the extent to which home visits can complement or replace facility-based newborn care [44]. Our results are in line with this conclusion. In a Cochrane review, Lassi and Bhutta discussed that the education, training, and support of community health workers differs between the studies, which might explain some of the differences observed in the effect of the interventions [35].
Despite moderate increases in newborn care behaviours associated with the intervention, neonatal mortality was similar in intervention and comparison areas, questioning the evidence base in support of home-based counselling [3,9]. Factors affecting intervention success include overall levels of recommended care practices, the extent to which women and families use facilities for preventive and curative care, NMRs, and the quality of care provided in facilities [45,46], suggesting a need for better knowledge on why and how interventions work and under which conditions they might achieve greater mortality declines before recommending them for wider implementation [47].
Our findings also give a stark reminder that demand and supply side strengthening should go hand in hand. The moderate decline in neonatal mortality contrasted with substantial improvements in newborn care practices, suggesting that improvement in the quality of facility care is of highest relevance in this setting. Our results thus support the recent shift to prioritize improvement in quality of facility-based care in Tanzania [48] and internationally [49].
Supporting Information
(XLSX)
(DOCX)
(PDF)
(DOCX)
(DOCX)
Acknowledgments
We are grateful to the community volunteers, the regional and district health management teams, the survey teams, and the staff of the Ifakara Health Institute who have supported this study since its inception in 2007. Thanks also to key national stakeholders Dr. Neema Rusibamayila, Dr. Georgina Msemo, Dr. Asia Hussein, Dr. Theopista John, and Dr. Rodrick Kisenge; to the International Technical Advisory Group, Prof. Joy Lawn, Prof. Lynn Freedman, Dr. Rajiv Bahl, and Dr. Leslie Mgalula; and to the Data and Safety Monitoring Board, Prof. Stefan Peterson, Dr. Sisti Moshi, and Dr. Maria Quigley.
Abbreviations
- CI
confidence interval
- INSIST
Improving Newborn Survival in Southern Tanzania
- NMR
neonatal mortality rate
- OR
odds ratio
- PDA
personal digital assistant
- RR
risk ratio
- UNICEF
United Nations Children's Fund
Data Availability
The data are available on the repository of Ikakara Health Institute http://data.ihi.or.tz/index.php/catalog/16.
Funding Statement
This study received funding from the Bill & Melinda Gates Foundation through Saving Newborn Lives (Save the Children, Grant number: 84050124 / 235 http://www.savethechildren.org/site/c.8rKLIXMGIpI4E/b.6234293/k.7FC1/Newborn_Health.htm#SNL). The study also received funding from UNICEF Tanzania, the Batchworth Trust, and the Laerdal Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. UN Inter-agency Group for Child Mortality Estimation (2013) Levels & Trends in Child Mortality Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation. New York. [Google Scholar]
- 2. Bhutta ZA, Das JK, Bahl R, Lawn JE, Salam RA, Paul VK, et al. (2014) Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? The Lancet 384: 347–370. [DOI] [PubMed] [Google Scholar]
- 3. Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L (2005) Evidence-based, cost-effective interventions: how many newborn babies can we save? The Lancet 365: 977–988. [DOI] [PubMed] [Google Scholar]
- 4. Bang A, Reddy H, Deshmukh M, Baitule S, Bang R (2005) Neonatal and Infant Mortality in the Ten Years (1993 to 2003) of the Gadchiroli Field Trial: Effect of Home-Based Neonatal Care. J Perinatol 25: S92–S107. [DOI] [PubMed] [Google Scholar]
- 5. Kumar V, Mohanty S, Kumar A, Misra RP, Santosham M, Awasthi S, et al. (2008) Effect of community-based behaviour change management on neonatal mortality in Shivgarh, Uttar Pradesh, India: a cluster-randomised controlled trial. The Lancet 372: 1151–1162. [DOI] [PubMed] [Google Scholar]
- 6. Baqui AH, El-Arifeen S, Darmstadt GL, Ahmed S, Williams EK, Seraji HR, et al. (2008) Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. The Lancet 371: 1936–1944. [DOI] [PubMed] [Google Scholar]
- 7. Bhutta ZA, Memon ZA, Soofi S, Salat MS, Cousens S, Martines J (2008) Implementing community-based perinatal care: results from a pilot study in rural Pakistan. Bulletin of the World Health Organization 86: 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO, & UNICEF (2009) Home visits for newborn child:a strategy to improve survival Geneva: WHO and UNICEF joint statement; www.who.int. [PubMed] [Google Scholar]
- 9. Kirkwood BR, Manu A, ten Asbroek AHA, Soremekun S, Weobong B, Gyan T, et al. (2013) Effect of the Newhints home-visits intervention on neonatal mortality rate and care practices in Ghana: a cluster randomised controlled trial. The Lancet 381: 2184–2192. [DOI] [PubMed] [Google Scholar]
- 10. UN Inter-agency Group for Child Mortality Estimation (2014) Child Mortality Estimates. In: UN Inter-agency Group for Child Mortality Estimation, editor. www.childmortality.org. [Google Scholar]
- 11. Armstrong CE, Magoma M, Ronsmans C Magnitude of maternal and neonatal mortality in Tanzania: A systematic review. International Journal of Gynecology & Obstetrics. [DOI] [PubMed] [Google Scholar]
- 12. National Bureau of Statistics (NBS) Tanzania, Macro International Inc (2007) Tanzania Service Provision Assessment Survey 2006. DSM, Tanzania. [Google Scholar]
- 13. Hanson C, Ronsmans C, Penfold S, Maokola W, Manzi F, Jaribu J, et al. (2013) Health system support for childbirth care in Southern Tanzania: results from a health facility census BMC Research Notes 6: 435 10.1186/1756-0500-6-435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Penfold S, Shamba D, Hanson C, Jaribu J, Manzi F, Marchant T, et al. (2013) Staff experiences of providing maternity services in rural southern Tanzania—a focus on equipment, drug and supply issues. BMC Health Services Research 13: 61 10.1186/1472-6963-13-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanson C, Cox J, Mbaruku G, Manzi F, Gabrysch S, Schellenberg D, et al. (2015) Maternal mortality and distance to facility-based obstetric care in rural southern Tanzania: a secondary analysis of cross-sectional census data in 226 000 households. The Lancet Global Health 3: e387–e395. 10.1016/S2214-109X(15)00048-0 [DOI] [PubMed] [Google Scholar]
- 16. Hanson C (April 2013) The epidemiology of maternal mortality in southern Tanzania. London, UK, http://researchonline.lshtm.ac.uk/1012993/: London School of Hygiene and Tropical Medicine. [Google Scholar]
- 17. Shamba D, Schellenberg J, Penfold S, Mashasi I, Mrisho M, Manzi F, et al. (2013) Clean Home-delivery in Rural Southern Tanzania: Barriers, Influencers, and Facilitators. J Health Popul Nutr 1: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Penfold S, Hill Z, Mrisho M, Manzi F, Tanner M, Mshinda H, et al. (2010) A Large Cross-Sectional Community-Based Study of Newborn Care Practices in Southern Tanzania. PLoS ONE 5: e15593 10.1371/journal.pone.0015593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hill Z, Jaribu J, Mashasi I, Mrisho M, Penfold S, Sagga R, et al. (2009) INSIST. Report on Formative Research for the Community Intervention. Dar-es-Salaam, 8–12 March: Ifakara Health Institute. [Google Scholar]
- 20. Shamba D, Schellenberg J, Hildon ZJ-L, Mashasi I, Penfold S, Tanner M, et al. (2014) Thermal care for newborn babies in rural southern Tanzania: a mixed-method study of barriers, facilitators and potential for behaviour change. BMC Pregnancy and Childbirth 14: 267 10.1186/1471-2393-14-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haws RA, Thomas AL, Bhutta ZA, Darmstadt GL (2007) Impact of packaged interventions on neonatal health: a review of the evidence. Health Policy and Planning 22: 193–215. [DOI] [PubMed] [Google Scholar]
- 22. Marchant T, Jaribu J, Penfold S, Tanner M, Schellenberg J (2010) Measuring newborn foot length to identify small babies in need of extra care: a cross sectional hospital based study with community follow-up in Tanzania. BMC Public Health 10: 624 10.1186/1471-2458-10-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borghi J, Cousens S, Hamisi Y, Hanson C, Jaribu J, Manzi F, et al. (2013) STUDY PROTOCOL: Improving newborn survival in rural southern Tanzania: a cluster-randomised trial to evaluate the impact of a scaleable package of interventions at community level with health system strengthening. www.researchonline.lshtm.ac.uk. [Google Scholar]
- 24. Kirkwood B, Manu A, Tawiah-Agyemang C, ten Asbroek G, Gyan T, Weobong B, et al. (2013) NEWHINTS cluster randomised trial to evaluate the impact on neonatal mortality in rural Ghana of routine home visits to provide a package of essential newborn care interventions in the third trimester of pregnancy and the first week of life: trial protocol. Trials 11: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mkumbo E, Hanson C, Penfold S, Manzi F, Schellenberg J (2014) Innovation in supervision and support of community health volunteers: experience from a six-district newborn survival study in rural southern Tanzania. International Health. [DOI] [PubMed] [Google Scholar]
- 26. Penfold S, Manzi F, Mkumbo E, Temu S, Jaribu J, Shamba D, et al. (2014) Effect of home-based counselling on newborn care practices in southern Tanzania one year after implementation: a cluster-randomised controlled trial. BMC Pediatrics 14: 187 10.1186/1471-2431-14-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schellenberg J, Maokola W, Shirima K, Manzi F, Mrisho M, Mushi A, et al. (2011) Cluster-randomized study of intermittent preventive treatment for malaria in infants (IPTi) in southern Tanzania: evaluation of impact on survival. Malaria Journal 10: 387 10.1186/1475-2875-10-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hayes R, Bennett S (1999) Simple sample size calculation for cluster-randomized trials. International Journal of Epidemiology 28. [DOI] [PubMed] [Google Scholar]
- 29. Shirima K, Mukasa O, Schellenberg J, Manzi F, John D, Mushi A, et al. (2007) The use of personal digital assistants for data entry at the point of collection in a large household survey in southern Tanzania. Emerging Themes in Epidemiology 4: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stata Statistical Software (2012) Release 12.0. Texas: Stata Corp LP,. [Google Scholar]
- 31. Bhutta ZA, Soofi S, Cousens S, Mohammad S, Memon ZA, Ali I, et al. (2011) Improvement of perinatal and newborn care in rural Pakistan through community-based strategies: a cluster-randomised effectiveness trial The Lancet 377: 403–412. [DOI] [PubMed] [Google Scholar]
- 32. Bhandari N, Mazumder S, Taneja S, Sommerfelt H, Strand TA (2012) Effect of implementation of Integrated Management of Neonatal and Childhood Illness (IMNCI) programme on neonatal and infant mortality: cluster randomised controlled trial. BMJ 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Darmstadt G, Choi Y, Arifeen S, Bari S, Rahman S, Mannan I, et al. (2010) Evaluation of a cluster-randomized controlled trial of a package of community-based maternal and newborn interventions in Mirzapur, Bangladesh. PLoS ONE 5: e9696 10.1371/journal.pone.0009696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Localio AR, Margolis DJ, Berlin JA (2007) Relative risks and confidence intervals were easily computed indirectly from multivariable logistic regression. Journal of Clinical Epidemiology 60: 874–882. [DOI] [PubMed] [Google Scholar]
- 35. Lassi ZS, Bhutta Z (2015) Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 10.1002/14651858.ED000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Friberg IK, Bhutta ZA, Darmstadt GL, Bang A, Cousens S, Baqui AH, et al. (2010) Comparing modelled predictions of neonatal mortality impacts using LiST with observed results of community-based intervention trials in South Asia. International Journal of Epidemiology 39: i11–i20. 10.1093/ije/dyq017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mrisho M, Schellenberg D, Manzi F, Tanner M, Shirima K, Msambichaka B, et al. (2012) Neonatal Deaths in Rural Southern Tanzania: Care-Seeking and Causes of Death. ISRN Pediatrics 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mazumder S, Taneja S, Bahl R, Mohan P, Strand TA, Sommerfelt H, et al. (2014) Effect of implementation of Integrated Management of Neonatal and Childhood Illness programme on treatment seeking practices for morbidities in infants: cluster randomised trial. BMJ 349: g4988 10.1136/bmj.g4988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sorensen BL, Elsass P, Nielsen BB, Massawe S, Nyakina J, Rasch V (2010) Substandard emergency obstetric care—a confidential enquiry into maternal deaths at a regional hospital in Tanzania. Tropical Medicine & International Health 15: 894–900. [DOI] [PubMed] [Google Scholar]
- 40. Hanson C, Waiswa P, Marchant T, Marx M, Manzi F, Mbaruku G, et al. (2014) Expanded Quality Management Using Information Power (EQUIP): protocol for a quasi-experimental study to improve maternal and newborn health in Tanzania and Uganda. Implementation Science 9: 41 10.1186/1748-5908-9-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arifeen SE, Hoque DME, Akter T, Rahman M, Hoque ME, Begum K, et al. (2009) Effect of the Integrated Management of Childhood Illness strategy on childhood mortality and nutrition in a rural area in Bangladesh: a cluster randomised trial. The Lancet 374: 393–403. [DOI] [PubMed] [Google Scholar]
- 42. Victora CG, Black RE, Boerma JT, Bryce J (2011) Measuring impact in the Millennium Development Goal era and beyond: a new approach to large-scale effectiveness evaluations. The Lancet 377: 85–95. [DOI] [PubMed] [Google Scholar]
- 43. Gogia S, Sachdev H (2010) Home visits by community health workers to prevent neonatal deaths in developing countries: a systematic review. Bull World Health Organ 88: 658–666. 10.2471/BLT.09.069369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yonemoto N, Dowswell T, Nagai S, Mori R (2013) Schedules for home visits in the early postpartum period. Cochrane Database of Systematic Reviews 2013, Issue 7 Art No: CD009326 [DOI] [PubMed] [Google Scholar]
- 45. Pasha O, McClure E, Wright L, Saleem S, Goudar S, Chomba E, et al. (2013) A combined community- and facility-based approach to improve pregnancy outcomes in low-resource settings: a Global Network cluster randomized trial. BMC Medicine 11: 215 10.1186/1741-7015-11-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. (2013) Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. The Lancet 381: 1747–1755. [DOI] [PubMed] [Google Scholar]
- 47. Victora CG, Barros FC (2013) Participatory women's groups: ready for prime time? The Lancet 381: 1693–1694. [DOI] [PubMed] [Google Scholar]
- 48.The World Bank (2015) Big Results Now for Health. documents.worldbank.org.
- 49. Tunçalp Ӧ, Were WM, MacLennan C, Oladapo OT, Gülmezoglu AM, Bahl R, et al. (2015) Quality of care for pregnant women and newborns—the WHO vision. BJOG: An International Journal of Obstetrics & Gynaecology 122: 1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
(PDF)
(DOCX)
(DOCX)
Data Availability Statement
The data are available on the repository of Ikakara Health Institute http://data.ihi.or.tz/index.php/catalog/16.