Abstract
Background
Eosinophils accumulate at the site of allergic inflammation and are critical effector cells in allergic diseases. Recent studies have also suggested a role for eosinophils in the resolution of inflammation.
Objective
To determine the role of eosinophils in the resolution phase of the response to repeated allergen challenge.
Methods
Eosinophil-deficient (PHIL) and wild-type (WT) littermates were sensitized and challenged to ovalbumin (OVA) 7 or 11 times. Airway inflammation, airway hyperresponsiveness (AHR) to inhaled methacholine, bronchoalveolar lavage (BAL) cytokine levels, and lung histology were monitored. Intracellular cytokine levels in BAL leukocytes were analyzed by flow cytometry. Groups of OVA-sensitized PHIL mice received bone marrow from WT or IL-10−/− donors, 30 days prior to OVA challenge.
Results
PHIL and WT mice developed similar levels of AHR and numbers of leukocytes and cytokine levels in BAL fluid following OVA sensitization and 7 airway challenges; no eosinophils were detected in the PHIL mice. Unlike WT mice, sensitized PHIL mice maintained AHR, lung inflammation, and increased levels of IL-4, IL-5, and IL-13 in BAL fluid following 11 challenges whereas IL-10 and TGF-β were decreased. Restoration of eosinophil numbers following injection of bone marrow from WT but not IL-10-deficient mice restored levels of IL-10 and TGF-β in BAL fluid as well as suppression of AHR and inflammation. Intracellular staining of BAL leukocytes revealed the capacity of eosinophils to produce IL-10.
Conclusions
Following repeated allergen challenge, eosinophils appeared not essential for development of AHR and lung inflammation but contributed to the resolution of AHR and inflammation by producing IL-10.
Keywords: Eosinophils, resolution of inflammation, IL-10
Introduction
Asthma is the most common chronic respiratory condition in Western countries. Despite advances in asthma treatment strategies, disease prevalence, severity, and morbidity remain high, particularly among certain ethnic groups (1). A number of clinical and experimental studies have addressed the underlying mechanisms of the disease in order to identify novel therapeutic targets. The most widely accepted mechanistic theory is that asthma is a Th2-type cell-mediated airway inflammatory disease where production of allergen-specific IgE, accumulation of eosinophils at airway inflammatory sites and in peripheral blood, and increases in IL-4, IL-5 and IL-13 levels have been linked to the pathophysiology of the disease (2–4).
In this thinking, eosinophils play a central role, identified as major effector cells in large part because of the numbers that are detected in the airways and lung parenchyma and their ability to secrete a wide array of proinflammatory cytokines including IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, IL-16, IL-18, and transforming growth factor (TGF)-α/β, chemokines (RANTES and eotaxin-1), lipid mediators (platelet-activating factor and leukotriene C4 (LTC4)) and four cationic proteins, major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil peroxidase (EPO), and eosinophil-derived neurotoxin (EDN) (5–7). Nevertheless, the specific role of eosinophils in asthma has been controversial as dissociations between numbers of eosinophils in the airways and lung function have been observed in several clinical and experimental studies. To this point, the early failures of anti-IL-5 to modify lung disease despite significant reductions in airway and peripheral blood eosinophil numbers triggered a re-examination of the role of eosinophils in asthma (8–15). In animal models of asthma, eosinophils have been intensively investigated in terms of the development of airway inflammation, airway hyperresponsiveness (AHR) and airway remodeling. Initially, studies in mice depleted of eosinophils (16–20) or rendered eosinophil-deficient in the absence of IL-5 (21) demonstrated a failure to develop lung allergic responses. Subsequently, genetically-manipulated, eosinophil-deficient mouse strains were generated, including GATA1-deficient (22–25) and an eosinophil-deficient strain created through eosinophil peroxidase-diphtheria toxin A targeting (PHIL) (26–28). However, when the role of eosinophils in the development of AHR and airway inflammation was examined in these novel strains, the results were contradictory; GATA1-deficient mice developed AHR similar to wild-type (WT) controls, whereas PHIL mice failed to develop AHR. Some of the discrepancies may have been strain-dependent (23). Specific depletion of eosinophil granule-specific proteins had little impact on the development of AHR (29, 30) and eosinophils appeared dispensable in the development of airway remodeling and AHR following repeated allergen challenge (25). Functionally, the role of eosinophils in airway remodeling may be more important than effects on lung function (22). With increased attention on mechanisms resulting in resolution of inflammation, eosinophil-derived anti-inflammatory mediator generation has been highlighted (31, 32).
In earlier studies, we noted that repeated allergen challenge of sensitized mice was associated with a decline in AHR, even at time points where airway eosinophilia was sustained (33, 34). At these time points, increased levels of IL-10 were detected in bronchoalveolar lavage (BAL) fluid (34). In the present study, we investigated the role of eosinophils in both the development and resolution phases of allergen-induced airway inflammation and AHR using a repetitive allergen challenge model in both WT and eosinophil-deficient mice. Under these conditions, a role for eosinophils could not be demonstrated in the development phase but eosinophils were essential to resolution of AHR and airway inflammation through their ability to produce the anti-inflammatory cytokine, IL-10.
Methods
Animals
Eosinophil peroxidase-diphtheria toxin A transgenic mice (PHIL) (C57BL/6 background) (26) were bred at National Jewish Health. Female PHIL mice were mated with male C57BL/6 mice purchased from Jackson Laboratories (Bar Harbor, ME). The genotype of PHIL mice and their WT littermates were confirmed by PCR analysis on tail DNA (26). IL-10-deficient (IL-10−/−) mice (B6.129P2-Il10tm1Cgn/J) were purchased from Jackson Laboratories. All mice were housed under specific pathogen-free conditions and maintained on an ovalbumin (OVA)-free diet at National Jewish Health. All experimental animals used in this study were under a protocol approved by the Institutional Animal Care and Use Committee of National Jewish Health.
Sensitization and repetitive airway challenge to OVA
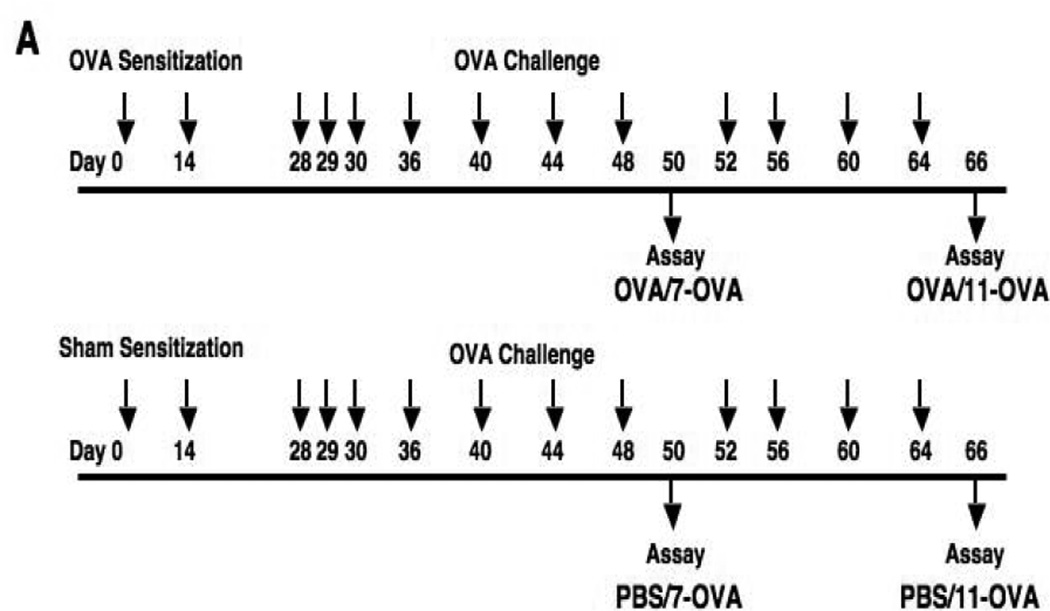
Sensitization and repetitive airway challenges were carried out as described previously (34). Briefly, six week old female WT littermates and PHIL mice were sensitized by intraperitoneal injection of 20 µg of OVA (Fisher Scientific, Pittsburgh, PA) emulsified in 2.25 mg of alum (Imject® Alum; Thermo Scientific Pierce Protein Research Products, Rockford, IL) or saline in a total volume of 100 µl on days 0 and 14. On days 28–30, followed by 2 times a week for 2 weeks (total 7 challenges; OVA/OVA-7) or 2 times a week for 4 weeks (total 11 challenges; OVA/OVA-11), mice were challenged with aerosolized OVA (1% w/v in saline for 20 minutes) (Fig. 1A). Sham-sensitized but OVA challenged mice served as controls.
Figure 1.
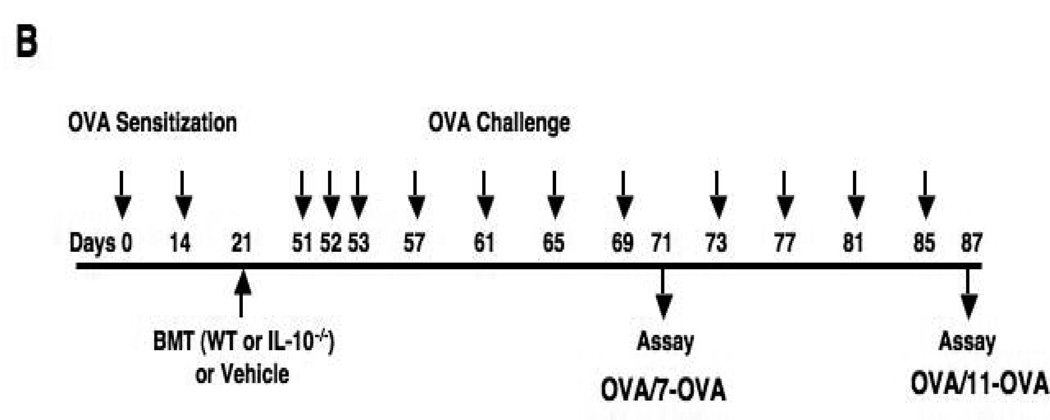
Experimental protocol for sensitization and challenge with OVA. (A) PHIL mice and WT littermates were sensitized to OVA on days 0 and 14 followed by aerosolized OVA challenges 7 times (OVA/OVA-7) or 11 times (OVA/OVA-11). Control mice received sham sensitization (PBS/OVA-7 and PBS/OVA-11). (B) Mice were sensitized with OVA in the same manner as described above. One week after the last sensitization, mice received bone marrow cells (5×106) from WT or IL-10−/− mice. OVA challenges were initiated 30 days following bone marrow cell transfer. Controls received vehicle only.
Measurement of airway responsiveness
Airway responsiveness to inhaled aerosolized methacholine (MCh; Sigma-Aldrich) was assessed 48 hrs following the last challenge (35, 36). Mice were anesthetized with 200 mg/kg of pentobarbital and ventilated with 160 breaths/min and a tidal volume of 0.15 mL and 2 cmH2O positive end-expiratory pressure (SN-480-7, SHINANO manufacturing CO., Ltd., Japan) through an intratracheal tube. Aerosolized MCh (0, 12.5, 25, 50, and 100 mg/ml in saline) was administered to mice for 10 seconds with a tidal volume of 0.45 mL and frequency of 60 breaths/min through bypass tubing via an ultrasonic nebulizer (model 5500D, DeVilbiss Healthcare LLC, Somerset, PA) placed between the expiratory port of the ventilator and the four-way connector. Airway responsiveness was measured as the change in lung resistance (RL) after exposure to increased concentrations of aerosolized MCh. RL was continuously monitored for up to 3 minutes after aerosolized MCh exposure and maximum values of RL were taken and expressed as the percent change from baseline following saline aerosol. Baseline values (saline) for RL were not significantly different among the groups.
Bronchoalveolar lavage
Immediately after assessment of AHR, lungs were lavaged one time with 1 ml of Hanks’ balanced salt solution (HBSS) through the tracheal tube. Recovered BAL fluid supernatants were stored at −80°C. Total leukocyte numbers in BAL fluid were counted using a hemocytometer and differential cell counts were performed by counting at least 200 cells on HEMA 3® stained (Fisher Scientific Company L.L.C., Middletown, VA) cytospin slides (Thermo Shandon Cytospin 3 Cytocentrifuge; Thermo Fisher Scientific, Pittsburgh, PA) using standard hematologic procedures in a blinded fashion.
Measurement of cytokines
IL-4, IL-5, IL-10, IL-12p70, IL-13, TGF-β, and IFN-γ were measured by ELISA (eBioscience, San Diego, CA) according to the manufacturer’s directions.
Lung histopathology and morphometric analyses
After BAL was recovered, lungs were removed and fixed in 10% (w/v) neutralized buffered formalin (pH 7.4). Lung tissues were embedded in paraffin and 5-µm thick sections cut. Mucus containing goblet cells were detected by staining with periodic acid-Schiff (PAS). Histologic analyses were performed in a blinded manner by light microscopy linked to an image capture system (BX51 microscope, DP72 digital camera, and QC-capture image capture software version 2.68, Quad-Cities Online, Moline, IL). Quantitative morphometry analyses were performed using Image J 1.47h (the U.S. National Institutes of Health (http://rsb.info.nih.gov/ij/)). Numbers of PAS-positive goblet cells were determined only in cross-sectional areas of the airway wall. Six to eight different fields per slide in four to six samples from each group of mice were examined in a blinded manner.
Injection of bone marrow cells
To reconstitute eosinophils in PHIL mice, suspensions of bone marrow cells were obtained from WT or IL-10−/− mice and injected (5×106 cells in 200 µL of HBSS) via the lateral tail vein on day 21. Thirty days later, mice were challenged to OVA on 3 consecutive days followed by 2 times a week for 2 or 4 weeks (Fig. 1B).
Intracellular cytokine staining
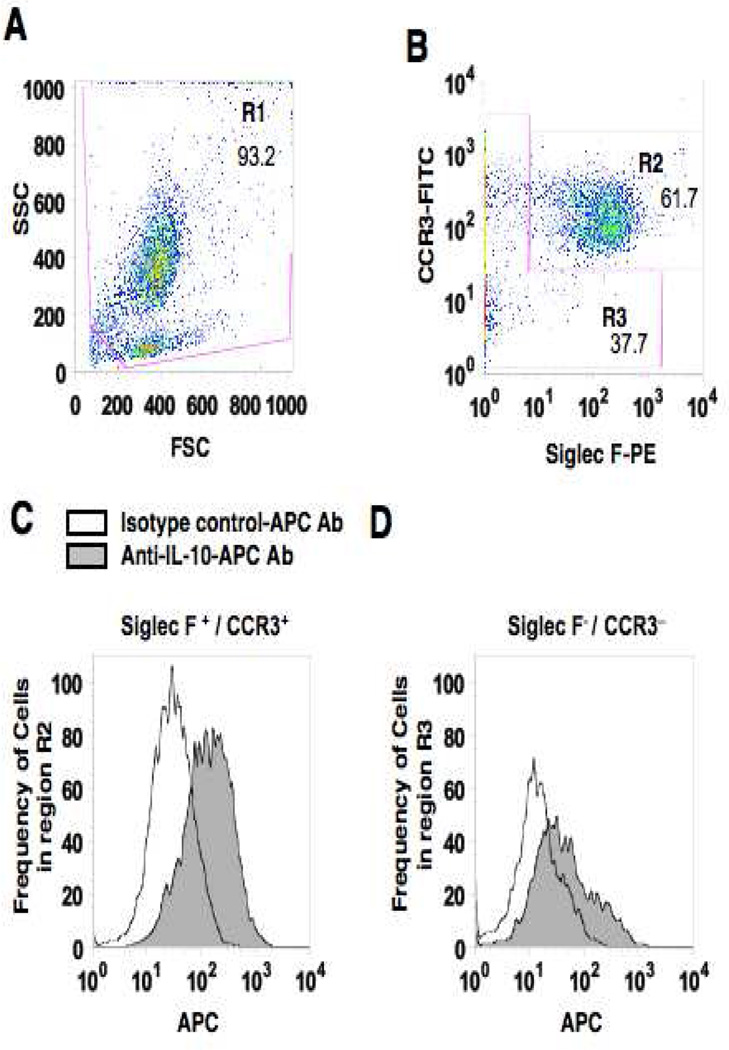
Intracellular IL-10 staining of eosinophils from BAL fluid of mice challenged 7 times was carried out. BAL fluid leukocytes were stimulated for 8 hrs with phorbol 12-myristate 13-acetate (PMA) (50 ng/ml) and ionomycin (1 µM) in the presence of brefeldin A (10 µg/mL). After stimulation, FcγII/III receptors were blocked with anti-mouse CD16/CD32 antibody (clone 2.4G2, BD Biosciences, San Jose, CA) and stained with anti-CCR3-FITC (R&D Systems, Minneapolis, MN) and anti-Siglec F-PE (BD Biosciences, San Jose, CA) for 15 minutes at 4°C to identify eosinophils. Cells were then fixed in 4% paraformaldehyde in 0.1 M PBS (pH 7.4) for 15 minutes at 4°C, permeabilized with 0.1% saponin for 10 minutes at 4°C, and stained with anti-IL-10-APC (eBioscience, San Diego, CA) or isotype control antibody (rat IgG2b κ ; eBioscience) for 30 minutes at 4°C. Cells were analyzed by flow cytometry (FACSCalibur and CellQuest Pro ver. 5.2.1, BD Biosciences). Live cells were gated based on forward vs. side scatter profiles (Fig. 4A, region R1). CCR3− and Siglec F-double positive cells were identified as eosinophils (Fig. 4B, region R2). Cells from this region were confirmed as more than 99% eosinophils by cell sorting and morphology (data not shown). To analyze cytoplasmic IL-10 expression in eosinophils, (CCR3+/Siglec F+ cells), and other cell (non-eosinophil) populations (Fig. 4B, region R3), 10,000 total events were acquired and each group compared by mean fluorescence intensity (MFI) using Flo-Jo software (Tree Star Inc., Ashland, OR).
Figure 4.
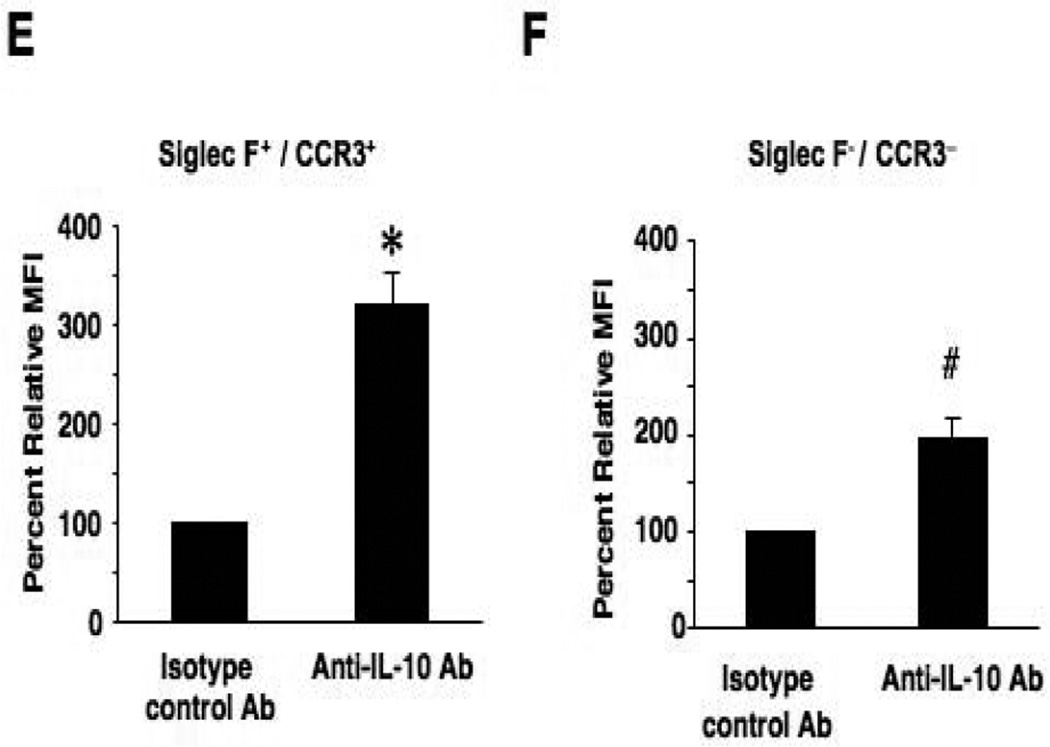
Intracellular IL-10 staining of airway eosinophils. BAL leukocytes from OVA sensitized and 7-challenged WT mice were activated with PMA/ionomysin and then stained with anti-mouse CCR3-FITC, anti-mouse Siglec-F-PE and anti-mouse IL-10-APC or APC-conjugated isotype control antibody. To identify the source of IL-10 in BAL fluid, live cells were first gated (region 1, R1) on forward scatter (FSC) and side scatter (SSC) (panel A). Cells in R1 were separated into Siglec-F- and CCR3-double-positive cells (R2) or negative cells (R3) (panel B). IL-10-positive cells in R2 or R3 were expressed as histograms (panel C and D) and the values for MFI were analyzed and expressed as the ratio to MFIs of isotype control staining in each sample (panels E and F). n=6. *p<0.001 and #p<0.01 vs. isotype control antibody group.
Statistical analyses
All values were expressed as means±SEM. The AHR values were analyzed with repeated measures two-way ANOVA followed by Bonferroni correction as a post-hoc test. The MFI values from intracellular IL-10 staining of eosinophils were analyzed using paired t test. All other values were analyzed with one-way ANOVA followed by Bonferroni correction. Statistical analyses were performed using GraphPad Prism 6.0b (GraphPad Software Inc., La Jolla, CA). A p value <0.05, in a two-tailed t test, was considered statistically significant.
Results
AHR and airway inflammation in eosinophil-deficient mice following repeated OVA challenge
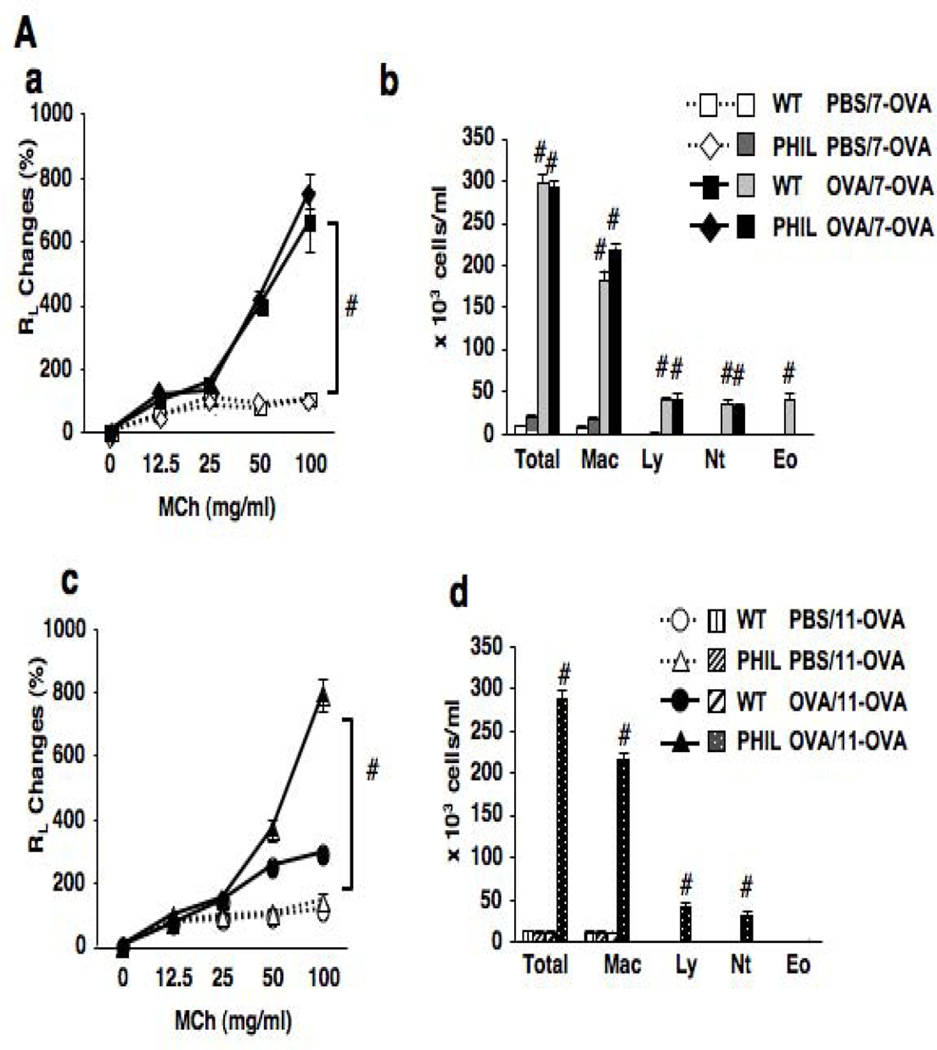
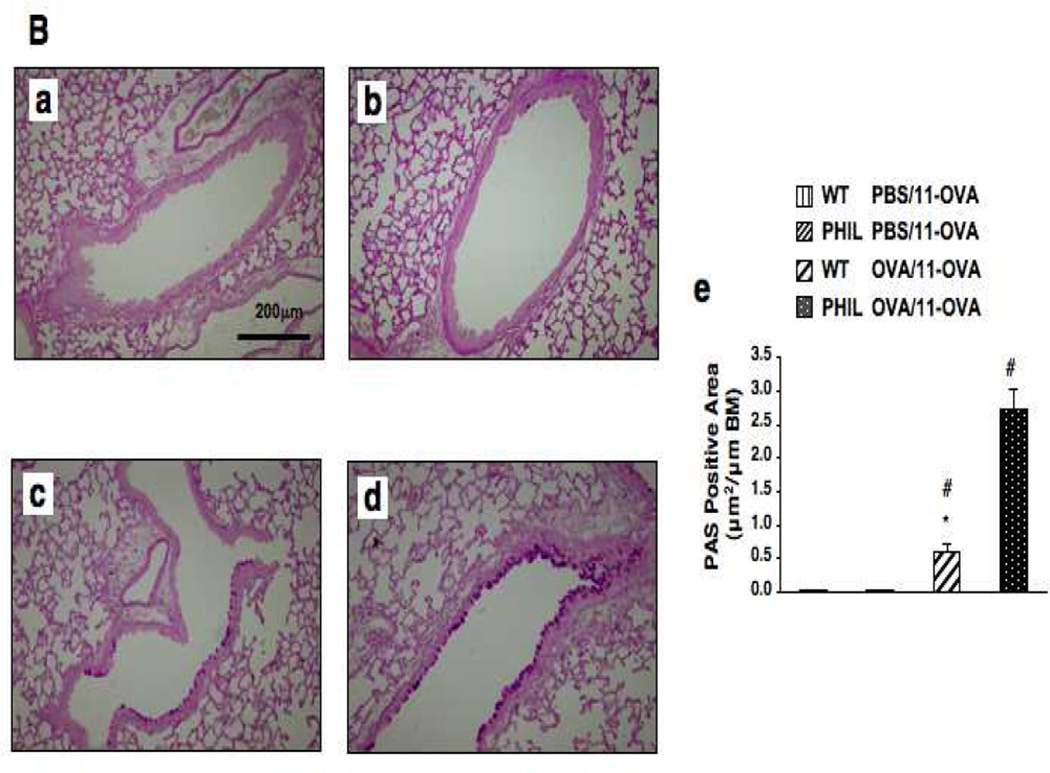
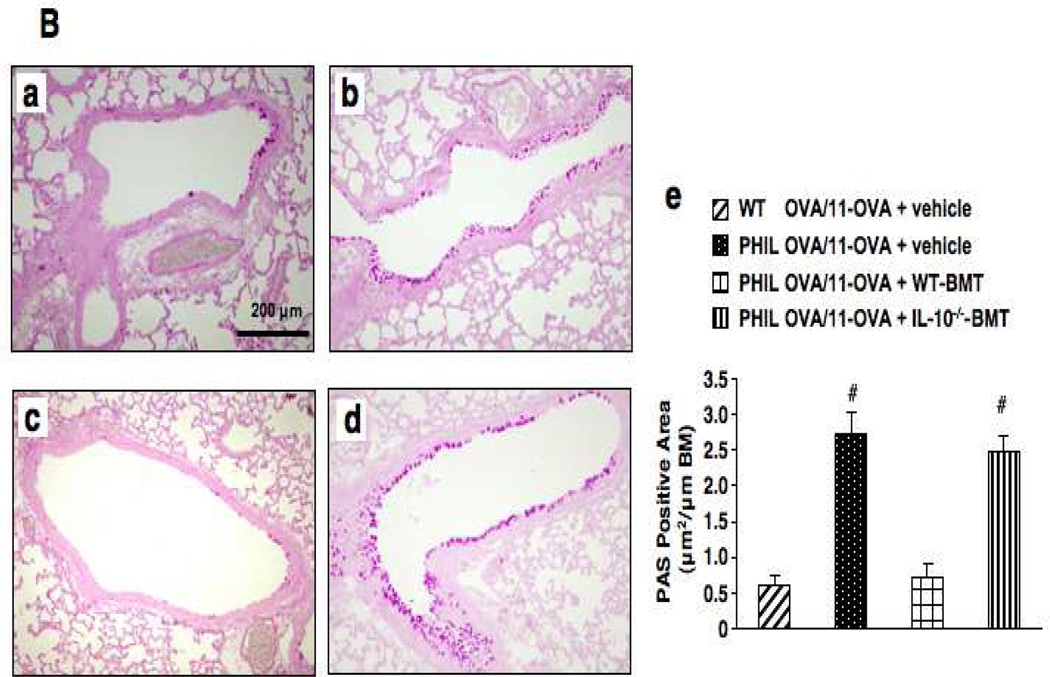
Following 7 OVA challenges, OVA-sensitized PHIL and WT mice developed comparable levels of AHR to inhaled MCh (Fig. 2A-a). Airway inflammatory responses, numbers of neutrophils, lymphocytes and macrophages, were similar in both strains except for the absence of eosinophils in the BAL fluid of PHIL mice (Fig. 2A-b). When mice received additional OVA challenges (11-OVA), AHR and BAL cell compositions returned to baseline levels in WT mice, similar to sham-sensitized controls. In contrast, AHR and airway inflammation in OVA-sensitized PHIL mice remained high following 11 challenges (Figs. 2A-c and 2A-d). PAS staining of lung sections revealed a marked increase in goblet cell numbers in PHIL compared to WT mice following 11 challenges (Fig. 2B).
Figure 2.
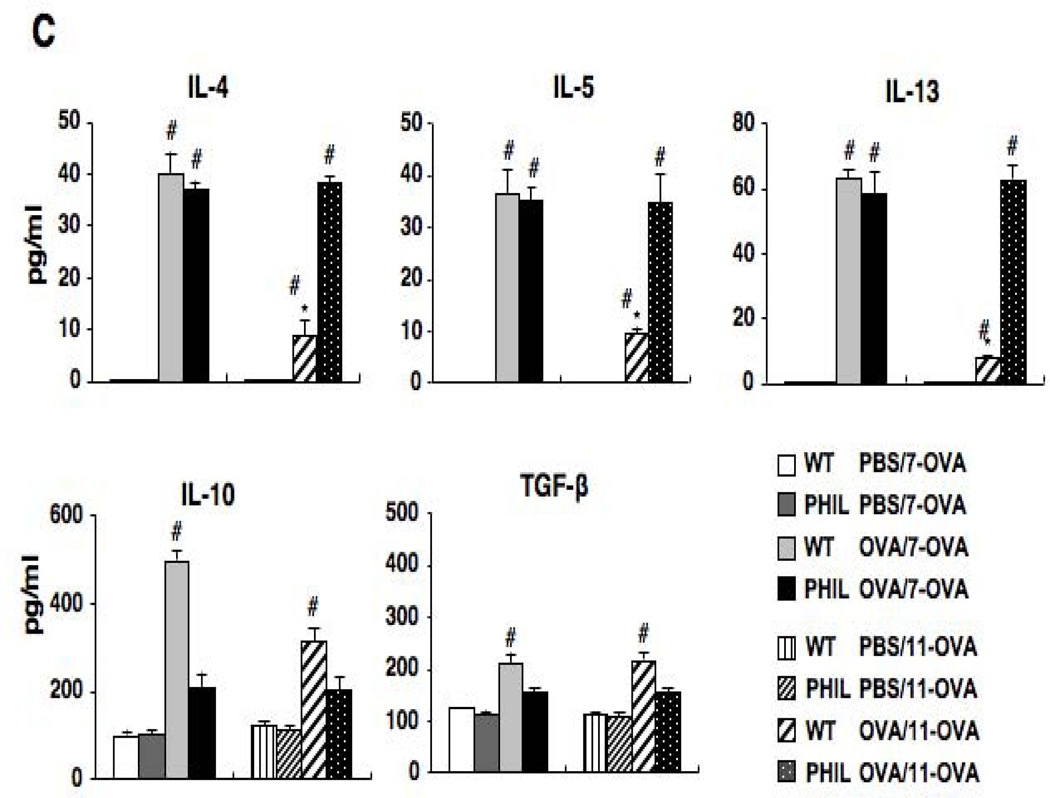
Changes in airway function and inflammation following repeated OVA challenges in WT and PHIL mice. (A) (a) Changes in lung resistance (RL) following 7 OVA challenges. (b) BAL cell composition. (c) Airway responsiveness following 11 OVA challenges. (d) BAL cell composition. n=6–9. #p<0.05 vs. PBS/OVA-7 or PBS/OVA-11 groups. (B) Goblet cell metaplasia. Representative photomicrographs of lung tissue from (a) WT mice following sham sensitization and 11 OVA challenges, (b) PHIL mice following sham sensitization and 11 OVA challenges, (c) WT mice following sensitization and 11 OVA challenges. (d) PHIL mice following sensitization and 11 OVA challenges. (e) Quantitation of PAS-positive areas along the airways. (C) BAL cytokine levels in WT and PHIL mice. BAL fluid samples were collected 48 hrs after 7 or 11 OVA challenges. n=9 in all experiments except panel B-e (n=6). Mac; macrophages, Ly; lymphocytes, Eo; eosinophils, Nt; neutrophils. #p<0.05 vs. PBS/OVA-7 and PBS/OVA-11. *p<0.05 vs. PHIL OVA/OVA-11.
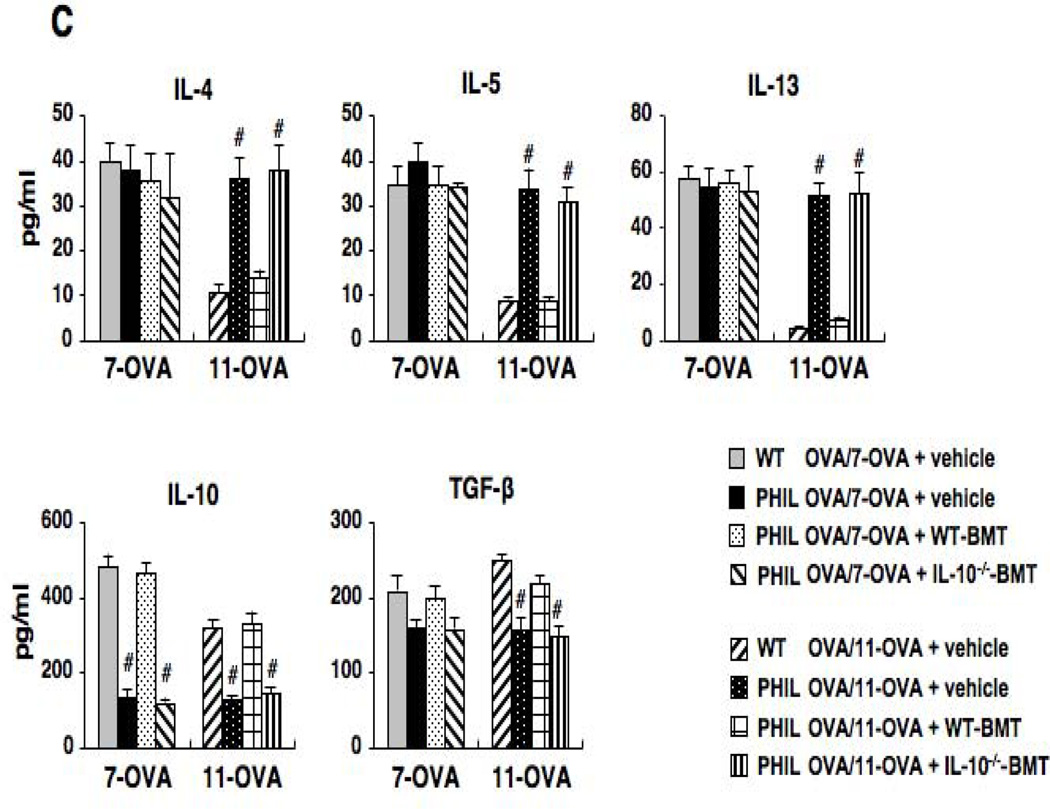
Sensitization and 7 OVA challenges resulted in increased levels of IL-4, IL-5, and IL-13 in the BAL fluid of both PHIL and WT mice (Fig. 2C). In the lung tissue of these mice, comparable numbers of IL-4-, IL-5- or IL-13-producing CD4+ cells were observed (Fig. E1). When mice were exposed to 11 OVA challenges, levels of these cytokines were markedly decreased in OVA-sensitized WT mice whereas PHIL mice maintained the elevated BAL Th2 cytokine levels. In WT mice, levels of IL-10 in BAL fluid were increased following sensitization and 7 challenges, and these levels were further increased following 11 challenges. BAL levels of TGF-β were also increased following repeated OVA challenges in OVA-sensitized WT mice. Unlike WT mice, OVA-sensitized PHIL mice showed little increase in IL-10 or TGF-β levels following 7 or 11 OVA challenges, similar to the sham-sensitized group.
AHR and airway inflammation in PHIL mice following restoration of eosinophil numbers
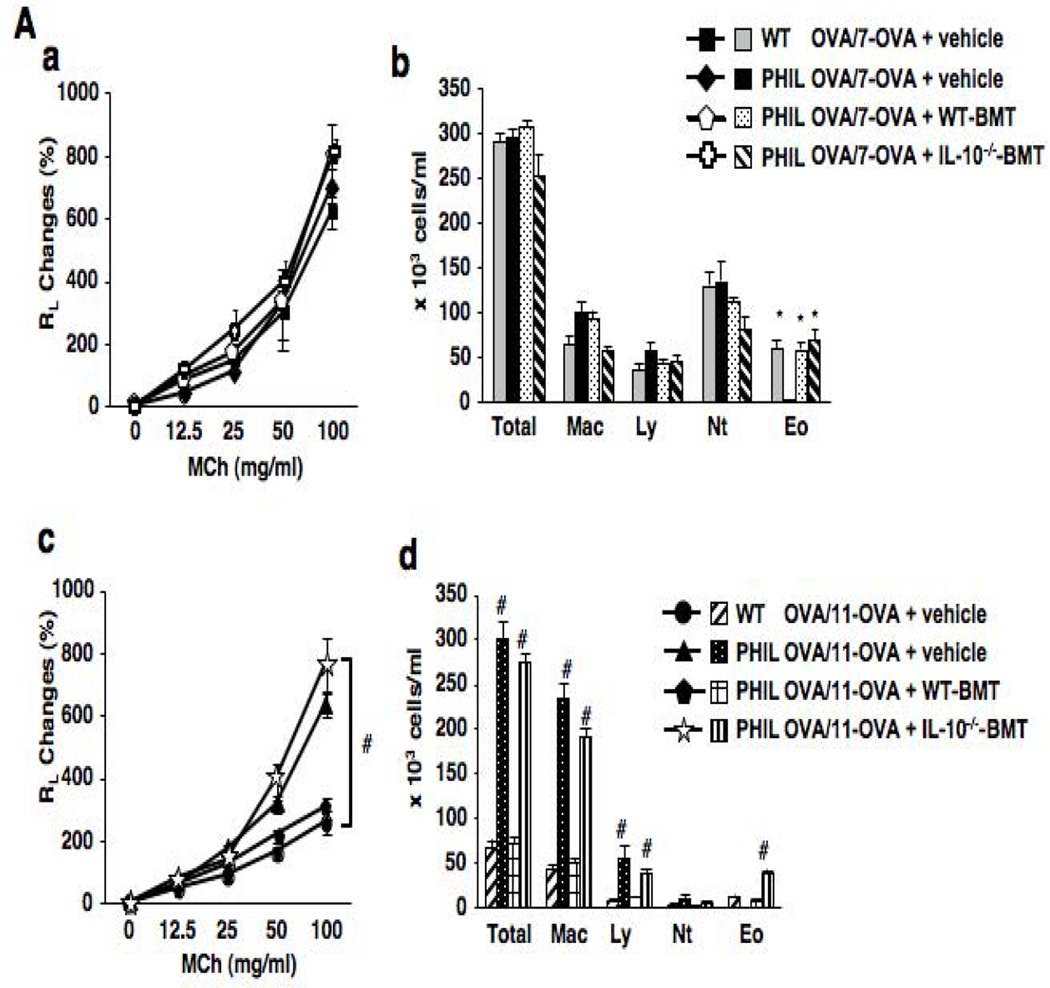
To define whether restoration of eosinophils in the deficient mice was associated with resolution of AHR and airway inflammation, PHIL mice received bone marrow cells from either WT or IL-10−/− mice and subjected to repeated allergen challenges. A total of 5×106 bone marrow cells were injected intravenously 30 days prior to 7 or 11 OVA challenges of sensitized mice. As eosinophils may serve as antigen-presenting cells (37), bone marrow cell transfer to restore eosinophils was carried out after sensitization and prior to challenge to avoid any modulation of the sensitization phase to allergen. Prior to transfer of bone marrow cells, physiological characteristics of the eosinophils such as levels of eosinophil peroxidase in eosinophils from WT vs. IL-10−/− recipient mice were shown to be comparable (Fig. E2). As shown in Figures 3A-a and 3A-b, PHIL mice which received bone marrow cells from WT or IL-10−/− mice following OVA sensitization and prior to 7 OVA challenges restored both BAL eosinophilia with similar numbers of eosinophils in BAL fluid, and AHR as the responses detected in WT mice after 7 OVA challenges. Similarly, the localization of eosinophils in the tissue and their numbers were also comparable in all recipient mice (Fig. E3). When OVA-sensitized PHIL mice received bone marrow cells from WT mice followed by 11 OVA challenges, AHR and numbers of airway inflammatory cells including eosinophils decreased to levels seen in similarly challenged WT mice (Fig. 3A-c). However, when IL-10−/− mice served as bone marrow donors, AHR and airway inflammation in PHIL recipients after 11 challenges remained high. As shown in Figure 3A-d, total cell numbers in BAL fluid were decreased in PHIL recipients of WT bone marrow following 11 OVA challenges whereas PHIL recipients of IL-10−/− bone marrow cells exhibited increased numbers of total cells including eosinophils in BAL fluid. Sham-sensitized PHIL recipients of WT or IL-10−/− bone marrow cells did not exhibit AHR or airway inflammation (data not shown).
Figure 3.
Consequences of eosinophil restoration following transfer of bone marrow cells. (A) (a) Lung resistance following bone marrow cell transfer and 7 OVA challenges. (b) BAL cell composition. (c) Lung resistance following bone marrow cell transfer and 11 OVA challenges. (d) BAL cell composition after 11 OVA challenges. (B) Goblet cell metaplasia. Representative photomicrographs in the lung tissue from (a) vehicle-treated WT mice after sensitization and 11 OVA challenges, (b) vehicle-treated PHIL mice after sensitization and 11 OVA challenges, (c) PHIL mice which received bone marrow cells from WT mice after sensitization and 11 OVA challenges, (d) PHIL mice which received bone marrow cells from IL-10−/− mice after sensitization and 11 OVA challenges, and (e) Quantitation of PAS-positive areas along the airways. (C) BAL fluid cytokine levels following bone marrow cell transfer and 11 OVA challenges. n=9 in all experiments except panel B-e (n=6). *p<0.05 vs. PHIL OVA/OVA-7+vehicle group, #p<0.05 vs. WT OVA/OVA-11+vehicle and PHIL OVA/OVA-11+ WT-BM.
In parallel, PAS staining of lung tissue sections from mice sensitized and challenged to OVA 11 times revealed decreased goblet cell metaplasia in PHIL recipients of bone marrow cells from WT mice, whereas PHIL recipients of cells from IL-10−/− mice developed goblet cell metaplasia to similar levels seen in untreated PHIL recipients (Fig. 3B).
Analyses of BAL cytokine levels demonstrated that PHIL recipients of bone marrow cells from WT mice and exposed to 11 OVA challenges exhibited decreased levels of IL-4, IL-5, and IL-13 (Fig. 3C). However, when PHIL mice received cells from IL-10−/− mice, Th2 cytokine levels in BAL fluid were increased to levels similar to non-restored PHIL mice, and, in contrast to recipients of WT bone marrow, in recipients of IL-10−/− cells, IL-10 and TGF-β levels remained low.
IL-10 production by eosinophils
These results suggested a correlation between numbers of eosinophils and the ability to reduce AHR and airway inflammation following repeated allergen challenge and an inverse correlation with levels of IL-10 and TGF-β in BAL fluid. Further, the results seen following injection of IL-10−/− bone marrow cells suggested the importance of eosinophils as a source of IL-10 in the resolution of AHR. To determine whether airway eosinophils were a source of IL-10 in the airways of mice exposed to repeated allergen challenges, BAL leukocytes from WT mice exposed to 7 OVA challenges were analyzed by intracellular cytokine staining for IL-10. As shown in Figure 4, BAL cells expressing both Siglec-F and CCR3 were identified as IL-10-positive with a marked increase in MFI compared to staining with isotype antibody controls or the gated non-eosinophil population (Fig. 4E). Other cell types, which were negative for Siglec-F or CCR3 expression, were also positive for IL-10, but at lower levels compared to eosinophils (Fig. 4F). This capacity for IL-10 production by eosinophils was also observed in ex vivo bone marrow-derived eosinophils (Fig. E4). That is, eosinophils derived from bone marrow cells of WT mice were shown to be a potent IL-10 producer whereas expected eosinophils derived from IL10−/− mice did not produce IL-10. In contrast, eosinophils derived from these two strains of mice (i.e., WT vs. IL-10−/−) showed comparable eosinophil peroxidase production levels.
Discussion
For several decades, eosinophils have been considered important contributors to the features of asthma based on increased numbers in peripheral blood and accumulation in the airways. Their role in asthma pathogenesis was similarly supported by the capacity to synthesize and release a variety of chemical mediators, cationic proteins, and cytokines (5, 6). Some clinical studies suggested correlations between airway eosinophilia and asthma severity (38, 39). In animal studies, depletion of eosinophils by genetic manipulation (IL-5 knockout mice (21) and congenitally eosinophil-deficient mouse models (PHIL (26) and ΔdblGATA (22)) or use of biologics (anti-IL-5) monoclonal antibody treatment (40) and anti-CCR3 monoclonal antibody depletion (16) was also associated with prevention of development of AHR, at least in the acute stage. However, despite the abundance of support for eosinophil-mediated asthma-like features, a number of inconsistencies emerged questioning the role of eosinophils in asthmatics or experimental models of asthma. Several reports demonstrated a dissociation of airway eosinophilia, airway function, and MCh responsiveness in the majority of asthmatic patients (15, 41) as well as in experimental systems (see for example (21) vs. (42)). Subsequent discrepancies of results between various laboratories using eosinophil-deficient mice (e.g., (26) vs. (22) vs. (43)) has suggested that differences in environmental cues (animal facility exposures and/or the use of different allergens) may also contribute to a diversity of phenotypes associated with these strains. Indeed, the studies presented here and observations with several eosinophil-deficient strains of mice (PHIL, ΔdblGATA, MBP-1−/−/EPX−/− (44)) support this diversity of immune responses and inflammation occurring in the absence of eosinophils. Further, in clinical studies, administration of anti-IL-5 successfully depleted eosinophils in blood and airways, but, without impacting lung function or MCh responsiveness in the majority of patients (8–14).
In a model of repeated allergen challenge (34), we noted that numbers of eosinophils remained high even after AHR returned to baseline levels. Sustained eosinophilia during the resolution phase was also demonstrated in airways of asthmatics following segmental allergen challenge (45). Hypothesizing that eosinophils may be at the root of this resolution of altered airway function, we approached this question using PHIL mice, a strain previously shown to fail to develop AHR, lung inflammation, or increases in Th2 cytokine levels in response to acute allergen challenge while maintained in a barrier facility (26). Using a 7 OVA challenge protocol, sensitized PHIL mice from a conventional mouse facility developed the full spectrum of allergic responses seen in WT mice, AHR to inhaled MCh, airway inflammation, goblet cell metaplasia, and increased Th2 cytokine levels, but without airway eosinophilia. However, following 4 additional challenges (11 challenges total), lung dysfunction and airway inflammation returned to baseline levels in WT mice, whereas AHR, the presence of airway lymphocytes and neutrophils, goblet cell metaplasia, and elevated Th2 cytokine levels persisted in the PHIL mice. The consequences of transfer of WT bone marrow into PHIL mice were examined to determine whether these airway response differences were the result of the presence vs. absence of eosinophils. Transfer of WT or IL-10−/− bone marrow cells into PHIL mice after sensitization but before challenge fully restored airway eosinophilia following 7 challenges. When PHIL mice were challenged 11 times following sensitization and WT bone marrow cell transfer, AHR, numbers of BAL leukocytes, including eosinophils, goblet cell metaplasia, and Th2 cytokine levels were decreased to the levels seen in WT mice. In contrast, despite 11 OVA challenges, when IL-10−/− mice served as bone marrow cell donors for PHIL recipient mice, AHR, numbers of BAL leukocytes, including eosinophils, goblet cell metaplasia, and Th2 cytokine levels remained similar to non-reconstituted PHIL mice. Of note, in these mice airway eosinophilia was sustained but the levels of IL-10 and TGF-β in BAL fluid remained low suggesting that the levels of these cytokines and the numbers of WT but not IL-10−/− eosinophils were linked, as was the resolution phase following repeated allergen challenge. This may explain the sustained levels of Th2 cytokines and eosinophils in the airways. Neutrophils, which were observed in the airways following 7- or 11-OVA challenges, may play a role in the development of AHR, as demonstrated in a recent study with inducible PHIL (46). BAL leukocytes obtained from sensitized WT mice after 7 challenges were stimulated with PMA/ionomycin and analyzed for IL-10 production to determine if eosinophils were a source of IL-10 under these conditions. Gating on Siglec-F- and CCR3-positive cells, eosinophils were shown to be capable of IL-10 production. This was also confirmed in bone marrow-derived cultured eosinophils. Following stimulation with PMA/ionomycin, these eosinophils produced high amounts of IL-10. Although culture conditions differ for different T cell types, the amounts of IL-10 produced by eosinophils appeared much higher than levels produced from naturally occurring T regulatory cells as shown in our previous study (47) and was not less than levels reported from Th1 cells (48). Nakajima et al. showed that human eosinophils produced IL-10 spontaneously (49) and Kayaba et al. also demonstrated that both human and mouse eosinophils secreted IL-10 following stimulation with IgE (50). Colavita et. al. demonstrated increased IL-10 levels in eosinophils from BAL fluid of asthmatics after a segmental allergen challenge, and more recently, Spencer et al. demonstrated that human eosinophils produce IL-10 following stimulation with IL-4 or 1L-12 (51, 52).
IL-10 is a potent anti-inflammatory cytokine with important functions in preventing autoimmune and allergic inflammatory responses (53, 54). IL-10 is known to be a major anti-inflammatory cytokine capable of controlling diverse immune responses by downregulating important functions of monocytes, macrophages and dendritic cells such as phagocytosis, production of cytokines, expression of co-stimulating accessory molecules, and the processing and presentation of antigen (55, 56). One mechanism regulating inflammatory responses may be by IL-10 suppression of cytokine production through upregulation of suppressor of cytokine signaling-3 (57).
Our previous studies and those of others demonstrated that IL-10 downregulated the function of dendritic cells and prevented development of allergen-induced airway inflammation and AHR (55, 58). Together with the evidence that adoptive transfer of IL-10 treated bone marrow-derived dendritic cells into mice following sensitization and repetitive allergen challenge prevented AHR and airway inflammation (55), we hypothesized that eosinophils played a role in the resolution phase of allergen-induced AHR and airway inflammation through upregulation of IL-10 in the airways. In eosinophil-deficient PHIL mice, restoration of eosinophils following bone marrow cell transfer from WT but not IL-10−/− mice restored suppression of AHR and airway inflammation. IL-10 is expressed by a number of cells of the adaptive immune system, including different T cell subsets and B cells as well as cells of the innate immune system (47, 55, 56). As the bone marrow cell inoculum contained all hematopoietic cell types and could have populated cell lineages in addition to eosinophils, transferred cells capable of IL-10 production other than eosinophils might have played a role in the suppressive phase. However, as the only deficiency described in PHIL mice appeared restricted to eosinophils (26), we assumed that eosinophils and eosinophil-derived IL-10 were central to the resolution of AHR and airway inflammation following repeated allergen challenge under these conditions.
The role of IL-10 in the resolution phase may be indirect. One possibility is that it is essential in the induction of TGF-β. In the bone marrow transfer experiments, donor cells from IL-10−/− mice not only failed to restore IL-10 levels in recipients but levels of TGF-β also remained low. TGF-β has been implicated in the regulation of T cell responses through conversion of CD25− T cells to regulatory CD25+ Treg cells by induction of FoxP3 and inhibition of T cell expansion (59, 60). Administration of TGF-β directly suppressed development of lung allergic responses (47). As the IL-10 secreted by eosinophils may trigger other types of regulatory cells such as naturally-occurring T regulatory cells to induce complete resolution of allergen-induced airway inflammation and AHR, future studies will determine the downstream consequences of eosinophil-derived IL-10 in this model.
In this study, we demonstrated that eosinophils play an important role in the resolution phase of allergen-induced airway inflammation and AHR seen following repeated allergen challenge through production of IL-10. A number of stimuli including cytokines, chemokines, and immunoglobulins have been demonstrated to activate eosinophils to release granule-associated proteins, reactive oxygen species, cytokines, and chemokines, although different stimuli may be involved in the activation of mouse compared to human eosinophils (61). Currently, airway eosinophils remain a major therapeutic target in asthma (62), although bimodal effector functions of eosinophils are increasingly being recognized (31, 63). Based on the data from different sources, it also appears that the role and contributions of eosinophils differ markedly when examined in an acute challenge model compared to more chronic models of allergen exposure. Unlike experimental models in mice where distinct stages may be controlled, studies in asthmatics are more difficult. With the advent of biologics (anti-IL-5), it may be possible to dissect the influence of eosinophils at different stages of the human disease. Collectively, our results highlight the complexity of the role eosinophils may play in asthmatics and in experimental models of asthma. Moreover, these data suggest that the predominant therapeutic strategy in asthma (administration of corticosteroids), which targets eosinophils, may need to be reevaluated under certain conditions.
Supplementary Material
Acknowledgments
We thank Diana Nabighian for assistance in the preparation of this manuscript.
Grant Support: This work was supported by NIH grants HL-58723 (to NAL), HL-065228 (to JJL), and AI-77609 and HL-36577 (to EWG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH.
Abbreviations
- AHR
Airway hyperresponsiveness
- BAL
Bronchoalveolar lavage
- ECP
Eosinophil cationic protein
- EDN
Eosinophil-driven neurotoxin
- EPO
Eosinophil peroxidase
- HBSS
Hank’s balanced salt solution
- LTC4
Leukotriene C4
- MBP
Major basic protein
- MCh
Methacholine
- MFI
Mean fluorescence intensity
- OVA
Ovalbumin
- PAS
Periodic acid-Schiff
- PHIL
Eosinophil-deficient
- RL
Lung resistance
- TGF
Transforming growth factor
- WT
Wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Implications
Targeting eosinophils, beneficial in some circumstances, may have deleterious effects under chronic allergen challenge.
References
- 1.Bousquet J, Mantzouranis E, Cruz AA, Ait-Khaled N, Baena-Cagnani, Bleecker ER, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126:926–938. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immunol. 2003;111:227–242. doi: 10.1067/mai.2003.139. quiz 243. [DOI] [PubMed] [Google Scholar]
- 3.Wills-Karp M, Karp CL. Biomedicine. Eosinophils in asthma: remodeling a tangled tale. Science. 2004;305:1726–1729. doi: 10.1126/science.1104134. [DOI] [PubMed] [Google Scholar]
- 4.Kallinich T, Beier KC, Wahn U, Stock P, Hamelmann E. T-cell co-stimulatory molecules: their role in allergic immune reactions. Eur Respir J. 2007;29:1246–1255. doi: 10.1183/09031936.00094306. [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120:3882–3890. doi: 10.1182/blood-2012-06-330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Büttner C, Lun A, Splettstoesser T, Kunkel G, Renz H. Monoclonal anti-interleukin-5 treatment suppresses eosinophil but not T-cell functions. Eur Respir J. 2003;21:799–803. doi: 10.1183/09031936.03.00027302. [DOI] [PubMed] [Google Scholar]
- 9.Leckie MJ. Anti-interleukin-5 monoclonal antibodies: preclinical and clinical evidence in asthma models. Am J Respir Med. 2003;2:245–259. doi: 10.1007/BF03256653. [DOI] [PubMed] [Google Scholar]
- 10.Flood-Page P, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 11.Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176:1062–1071. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 12.Menzies-Gow A, Flood-Page P, Sehmi R, Burman J, Hamid Q, Robinson DS, et al. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003;111:714–719. doi: 10.1067/mai.2003.1382. [DOI] [PubMed] [Google Scholar]
- 13.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phipps S, Flood-Page P, Menzies-Gow A, Ong YE, Kay AB. Intravenous anti-IL-5 monoclonal antibody reduces eosinophils and tenascin deposition in allergen-challenged human atopic skin. J Invest Dermatol. 2004;122:1406–1412. doi: 10.1111/j.0022-202X.2004.22619.x. [DOI] [PubMed] [Google Scholar]
- 15.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 16.Justice JP, Borchers MT, Crosby JR, Hines EM, Shen HH, Ochkur SI, et al. Ablation of eosinophils leads to a reduction of allergen-induced pulmonary pathology. Am J Physiol Lung Cell Mol Physiol. 2003;284:L169–L178. doi: 10.1152/ajplung.00260.2002. [DOI] [PubMed] [Google Scholar]
- 17.Hamelmann E, Cieslewicz G, Schwarze J, Ishizuka T, Joetham A, Heusser C, et al. Anti-interleukin 5 but not anti-IgE prevents airway inflammation and airway hyperresponsiveness. Am J Respir Crit Care Med. 1999;160:934–941. doi: 10.1164/ajrccm.160.3.9806029. [DOI] [PubMed] [Google Scholar]
- 18.Hamelmann E, Gelfand EW. Role of IL-5 in the development of allergen-induced airway hyperresponsiveness. Int Arch Allergy Immunol. 1999;120:8–16. doi: 10.1159/000024215. [DOI] [PubMed] [Google Scholar]
- 19.Hamelmann E, Gelfand EW. IL-5-induced airway eosinophilia--the key to asthma? Immunol Rev. 2001;179:182–191. doi: 10.1034/j.1600-065x.2001.790118.x. [DOI] [PubMed] [Google Scholar]
- 20.Hamelmann E, Oshiba A, Loader J, Larsen GL, Gleich G, Lee J, et al. Antiinterleukin-5 antibody prevents airway hyperresponsiveness in a murine model of airway sensitization. Am J Respir Crit Care Med. 1997;155:819–825. doi: 10.1164/ajrccm.155.3.9117011. [DOI] [PubMed] [Google Scholar]
- 21.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 23.Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, et al. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med. 2008;205:1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohtomo T, Kaminuma O, Yamada J, Kitamura N, Abe A, Kobayashi N, et al. Eosinophils are required for the induction of bronchial hyperresponsiveness in a Th transfer model of BALB/c background. Int Arch Allergy Immunol. 2010;152:79–82. doi: 10.1159/000312130. [DOI] [PubMed] [Google Scholar]
- 25.Fattouh R, Al-Garawi A, Fattouh M, Arias K, Walker TD, Goncharova S, et al. Eosinophils are dispensable for allergic remodeling and immunity in a model of house dust mite-induced airway disease. Am J Respir Crit Care Med. 2011;183:179–188. doi: 10.1164/rccm.200905-0736OC. [DOI] [PubMed] [Google Scholar]
- 26.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J Immunol. 2011;187:6059–6068. doi: 10.4049/jimmunol.1102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denzler KL, Farmer SC, Crosby JR, Borchers MT, Cieslewicz G, Larson KA, et al. Eosinophil major basic protein-1 does not contribute to allergen-induced airway pathologies in mouse models of asthma. J Immunol. 2000;165:5509–5517. doi: 10.4049/jimmunol.165.10.5509. [DOI] [PubMed] [Google Scholar]
- 30.Denzler KL, Borchers MT, Crosby JR, Cieslewicz G, Hines EM, Justice JP, et al. Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J Immunol. 2001;167:1672–1682. doi: 10.4049/jimmunol.167.3.1672. [DOI] [PubMed] [Google Scholar]
- 31.Arita M. Mediator lipidomics in acute inflammation and resolution. J Biochem. 2012;152:313–319. doi: 10.1093/jb/mvs092. [DOI] [PubMed] [Google Scholar]
- 32.Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R, et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J Allergy Clin Immunol. 2013;131:353–360. doi: 10.1016/j.jaci.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 33.Cui ZH, Joetham A, Aydintug MK, Hahn YS, Born WK, Gelfand EW. Reversal of allergic airway hyperreactivity after long-term allergen challenge depends on gamma/delta T cells. Am J Respir Crit Care Med. 2003;168:1324–1332. doi: 10.1164/rccm.200305-634OC. [DOI] [PubMed] [Google Scholar]
- 34.Koya T, Kodama T, Takeda K, Miyahara N, Yang ES, Taube C, et al. Importance of myeloid dendritic cells in persistent airway disease after repeated allergen exposure. Am J Respir Crit Care Med. 2006;173:42–55. doi: 10.1164/rccm.200505-783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, et al. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med. 1997;186:449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeda K, Dow SW, Miyahara N, Kodama T, Koya T, Taube C, et al. Vaccine-induced CD8+ T cell-dependent suppression of airway hyperresponsiveness and inflammation. J Immunol. 2009;183:181–190. doi: 10.4049/jimmunol.0803967. [DOI] [PubMed] [Google Scholar]
- 37.Shi HZ. Eosinophils function as antigen-presenting cells. J Leukoc Biol. 2004;76:520–527. doi: 10.1189/jlb.0404228. [DOI] [PubMed] [Google Scholar]
- 38.Niimi A, Amitani R, Suzuki K, Tanaka E, Murayama T, Kuze F. Serum eosinophil cationic protein as a marker of eosinophilic inflammation in asthma. Clin Exp Allergy. 1998;28:233–240. doi: 10.1046/j.1365-2222.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 39.Di Franco A, Bartoli ML, Carnevali S, Cianchetti S, Bacci E, Dente FL, et al. Analysis of sputum cell counts during spontaneous moderate exacerbations of asthma in comparison to the stable phase. J Asthma. 2003;40:155–162. doi: 10.1081/jas-120017986. [DOI] [PubMed] [Google Scholar]
- 40.Hamelmann E, Oshiba A, Schwarze J, Bradley K, Loader J, Larsen GL, et al. Allergen-specific IgE and IL-5 are essential for the development of airway hyperrresponsiveness. Am J Respir Cell Molec Biol. 1997;16:674–682. doi: 10.1165/ajrcmb.16.6.9191469. [DOI] [PubMed] [Google Scholar]
- 41.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. New Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hogan SP, Matthaei KI, Young JM, Koskinen A, Young IG, Foster PS. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J Immunol. 1998;161:1501–1509. [PubMed] [Google Scholar]
- 43.Botelho FM, Llop-Guevara A, Trimble NJ, Nikota JK, Bauer CM, Lambert KN, Kianpour S, Jordana M, Stampfli MR. Cigarette smoke differentially affects eosinophilia and remodeling in a model of house dust mite asthma. Am J Resp Cell Molec Biol. 2011;45:753–760. doi: 10.1165/rcmb.2010-0404OC. [DOI] [PubMed] [Google Scholar]
- 44.Doyle AD, Jacobsen EA, Ochkur SI, McGarry MP, Shim KG, Nguyen DTC, et al. Expression of the secondary granule proteins major basic protein (MBP)-1 and eosinophil peroxidase (EPX) is required for eosinophilopoiesis in mice. Blood Cells. 2013;122:781–790. doi: 10.1182/blood-2013-01-473405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaver JR, Zangrilli JG, Cho SK, Cirelli RA, Pollice M, Hastie AT, et al. Kinetics of the development and recovery of the lung from IgE-mediated inflammation: Dissociation of pulmonary eosinophilia, lung injury, and eosinophil-active cytokines. Am J Respir Crit Care Med. 1997;155:442–448. doi: 10.1164/ajrccm.155.2.9032176. [DOI] [PubMed] [Google Scholar]
- 46.Jacobsen EA, Lesuer WE, Willetts L, Zellner KR, Mazzolini K, Antonios N, Beck B, Protheroe C, Ochkur SI, Colbert D, Lacy P, Moqbel R, Appleton J, Lee NA, Lee JJ. Eosinophil activities modulate the immune/inflammatory character of allergic respiratory responses in mice. Allergy. 2014;69:315–327. doi: 10.1111/all.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, et al. Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-beta. J Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 48.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakajima H, Gleich GJ, Kita H. Constitutive production of IL-4 and IL-10 and stimulated production of IL-8 by normal peripheral blood eosinophils. J Immunol. 1996;156:4859–4866. [PubMed] [Google Scholar]
- 50.Kayaba H, Dombrowicz D, Woerly G, Papin JP, Loiseau S, Capron M. Human eosinophils and human high affinity IgE receptor transgenic mouse eosinophils express low levels of high affinity IgE receptor, but release IL-10 upon receptor activation. J Immunol. 2001;167:995–1003. doi: 10.4049/jimmunol.167.2.995. [DOI] [PubMed] [Google Scholar]
- 51.Colavita AM, Hastie AT, Musani AI, Pascual RM, Reinach AJ, Lustine HT, Galati SA, Zangrilli JG, Fish JE, Peters SP. Kinetics of IL-10 production after segmental antigen challenge of atopic asthmatic subjects. J Allergy Clin Immunol. 2000;106:880–886. doi: 10.1067/mai.2000.110475. [DOI] [PubMed] [Google Scholar]
- 52.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu CL, Ye YL, Lee YL, Chiang BL. Effects of overexpression of IL-10, IL-12, TGF-beta and IL-4 on allergen induced change in bronchial responsiveness. Respir Res. 2006;7:72–75. doi: 10.1186/1465-9921-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kosaka S, Tamauchi H, Terashima M, Maruyama H, Habu S, Kitasato H. IL-10 controls Th2-type cytokine production and eosinophil infiltration in a mouse model of allergic airway inflammation. Immunobiology. 2011;216:811–820. doi: 10.1016/j.imbio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Koya T, Matsuda H, Takeda K, Matsubara S, Miyahara N, Balhorn A, et al. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J Allergy Clin Immunol. 2007;119:1241–1250. doi: 10.1016/j.jaci.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 56.Banchereau J, Pascual V, O'Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13:925–931. doi: 10.1038/ni.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogawa Y, Duru EA, Ameredes BT. Role of IL-10 in the resolution of airway inflammation. Curr Mol Med. 2008;8:437–445. doi: 10.2174/156652408785160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nayyar A, Dawicki W, Huang H, Lu M, Zhang X, Gordon JR. Induction of prolonged asthma tolerance by IL-10-differentiated dendritic cells: differential impact on airway hyperresponsiveness and the Th2 immunoinflammatory response. J Immunol. 2012;189:72–79. doi: 10.4049/jimmunol.1103286. [DOI] [PubMed] [Google Scholar]
- 59.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Chen D, et al. TGF-beta induces Foxp3+ T-regulatory cells from CD4+ CD25− precursors. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 61.Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, et al. Human versus mouse eosinophils: That which we call an eosinophil, by any other name would stain as red. J Allergy Clin Immunol. 2012;130:572–584. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, Busse WW, Wenzel S, Wu Y, Datta V, Kolbeck R, Molfino NA. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132:1086–1096. doi: 10.1016/j.jaci.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Frontiers Immunol. 2012;3:270. doi: 10.3389/fimmu.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ashino S, Takeda K, Li H, Taylor V, Joetham A, Pine PR, Gelfand EW. Janus kinase 1/3 signaling pathways are key initiators of TH2 differentiation and lung allergic responses. J Allergy Clin Immunol. 2014 Apr;133(4):1162–1174. doi: 10.1016/j.jaci.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borchers MT, Ansay T, DeSalle R, Daugherty BL, Shen H, Metzger M, Lee NA, Lee JJ. In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration. J Leukoc Biol. 2002;71:1033–1041. [PubMed] [Google Scholar]
- 66.Ochkur SI, Kim JD, Protheroe CA, Colbert D, Moqbel R, Lacy P, Lee JJ, Lee NA. The development of a sensitive and specific ELISA for mouse eosinophil peroxidase: assessment of eosinophil degranulation ex vivo and in models of human disease. J Immunol Methods. 2012;375:138–147. doi: 10.1016/j.jim.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang M, Ramirez J, Han J, Jia Y, Domenico J, Seibold MA, Hagman JR, Gelfand EW. The steroidogenic enzyme Cyp11a1 is essential for development of peanut-induced intestinal anaphylaxis. J Allergy Clin Immunol. 2013;132:1174–1183. doi: 10.1016/j.jaci.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dyer KD, Garcia-Crespo KE, Percopo CM, Sturm EM, Rosenberg HF. Protocols for identifying, enumerating, and assessing mouse eosinophils. Methods Mol Biol. 2013;1032:59–77. doi: 10.1007/978-1-62703-496-8_5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.