Abstract
Background
Gene-environment interactions mediate through the placenta and shape the fetal brain development. Between the environmental determinants of the fetal brain, maternal psychosocial stress in pregnancy has been shown to negatively influence the infant temperament development. This in turn may have adverse consequences on the infant neurodevelopment extending throughout the entire life-span. However little is known about the underlying biological mechanisms of the effects of maternal psychosocial stress in pregnancy on infant temperament. Environmental stressors such as maternal psychosocial stress in pregnancy activate the stress response cascade that in turn drives the increase in the cellular energy demand of vital organs with high metabolic rates such as, in pregnancy, the placenta. Key players of the stress response cascade are the mitochondria.
Results
Here, we tested the expression of all 13 protein-coding genes encoded by the mitochondria in 108 placenta samples from the Stress in Pregnancy birth cohort, a study that aims at determining the influence of in utero exposure to maternal psychosocial stress in pregnancy on infant temperament. We showed that the expression of the protein-coding mitochondrial-encoded gene MT-ND2 was positively associated with indices of maternal psychosocial stress in pregnancy including Prenatal Perceived Stress (β = 0.259; p-regression = 0.004; r2-regression = 0.120), State Anxiety (β = 0.218; p-regression = 0.003; r2-regression = 0.153), Trait Anxiety (β = 0.262; p-regression = 0.003; r2-regression = 0.129) and Pregnancy Anxiety Total (β = 0.208; p-regression = 0.010; r2-regression = 0.103). In the meantime MT-ND2 was negatively associated with the infant temperament indices of Activity Level (β = -0.257; p-regression = 0.008; r2-regression = 0.165) and Smile and Laughter (β = -0.286; p-regression = 0.036; r2-regression = 0.082). Additionally, MT-ND6 was associated with the maternal psychosocial stress in pregnancy index of Prenatal Perceived Stress (β = -0.231; p-regression = 0.004; r2-regression = 0.120), while MT-CO2 was associated with the maternal psychosocial stress in pregnancy indices of State Anxiety (β = 0.206; p-regression = 0.003; r2-regression = 0.153) and Trait Anxiety (β = 0.205; p-regression = 0.003; r2-regression = 0.129).
Conclusions
Our data support the role of mitochondria in responding to maternal psychosocial stress in pregnancy, as assessed in placenta, while also suggesting an important role for the mitochondria in the infant temperament development.
Introduction
Gene-environment interactions are considered more powerful in determining the phenotype as they occur at earlier stages of development triggering both the increase in incidence and the earlier onset of many developmental disorders, including infant temperament disorders [1, 2, 3]. The period of intrauterine development is probably the most critical that can influence infant temperament and neurodevelopment affecting the offspring throughout the entire life [4, 5, 6, 7, 8].
Maternal psychosocial stress in pregnancy (MPSP) is considered a powerful environmental determinant of the infant temperament [9, 10, 11], however little is known on the mechanistic basis of this phenomenon.
MPSP, like any other stress stimulus, is responsible for activating the cascade of events leading to increase in the cellular energy demand and altered calcium (Ca2+) homeostasis [12, 13]. In the brain, psychosocial stress increases energy demand by a mechanism referred to as "cerebral insulin suppression". This mechanism limits glucose flux into peripheral tissue to enhance cerebral glucose supply thus affecting the metabolism of many other organs as, particularly, those with high metabolic rates [14, 15, 16, 17].
Mitochondria play a key role in the stress response [13, 18]. Mitochondria act by both providing the cellular energy needed for the stress response by means of the oxidative phosphorylation (OXPHOS) machinery [12] and by actively controlling Ca2+ homeostasis, in cooperation with the endoplasmic reticulum [19, 20], thermogenesis, reactive oxygen species generation and apoptosis among others. [21]. Mitochondria are thus among the first responders to stressors [22]. Consequently, modifications of the mitochondrial activity are considered as key events in both acute and chronic homeostatic imbalance [23]. At the same time many neurodevelopmental disorders have been linked to modifications of the mitochondrial activity [21].
Mitochondria are often referred to as the “powerhouse of the cell”, as they produce over 80% of the energy needed to carry out housekeeping and specialized cellular functions [24]. The role of mitochondria is thus more important in vital organs with high metabolic rates and peculiar homeostatic requirements like the brain and muscles and, in pregnancy, the placenta [25, 26].
The placenta is a very dynamic organ with high metabolic rate and tightly regulated homeostatic requirements, where a continuum of phenotypic and morphological changes takes place over the course of gestation [27]. Placental oxygen consumption is second only to that of the fetal brain [28]. By 22 weeks of gestation, the mean placental weight is one third of that of the fetus while the mid-gestation fetus consumes only 25% of the oxygen required by the placenta [29]. The placenta has also been shown, like for the brain, to mainly use glucose to support its high pace metabolism [30, 31]. The placenta acts like the effector of the intrauterine environment to drive embryonic development [32, 33].
Concomitantly, different studies have shown that the fetal brain development is deeply intertwined with that of the placenta [27, 34, 35, 36] that support serotoninergic neuron differentiation by providing serotonin to the developing brain up to the fourteenth week of gestation [37, 38, 39]. Serotonin plays a major role in a variety of cognitive functions [38, 39] and participate in the developmental determination of psychological traits including an increased impulsivity, lower levels of response to inhibition and sensation seeking in toddlers [40].
The placenta also shares the genetic and epigenetic profile of the developing fetus as it originates from the extraembryonic cell layer of the blastocyst [41]. This status affords a unique opportunity of exploring the association of the mitochondria gene expression profile with fetal growth and development by using placental tissue.
In this study we showed that MPSP modifies the mitochondrial gene expression profile in the placenta. We also showed that modifications of placental mitochondria gene expression profiles correlate with the infant temperament development. We conducted our investigation using placenta samples collected by the Stress in Pregnancy (SIP) Study, a birth cohort generated in the New York City metropolitan area that aims at investigating the influence of MPSP on infant temperament.
Material and Methods
Study Population
The SIP Study enrolls subjects at Icahn School of Medicine at Mount Sinai, New York Hospital of Queens and Queens College. The SIP Study examines the influence of in utero exposure to MPSP on infant temperament.
MPSP is determined through self-administered questionnaires [42, 43, 44, 45] at the 2nd and 3rd trimesters. MPSP questionnaires are aimed at assessing: objective and subjective levels of stressful experiences, feelings and thoughts during pregnancy, perceived stress, prenatal and perinatal post-traumatic stress symptoms, depressive symptoms and stressful life events.
Questionnaires include: 1) the Perceived Stress Scale (PSS-14) [46], a well-validated 14-item scale instrument that asks about the mother’s feelings and thoughts during the last month (during pregnancy); 2) the Pregnancy Related Anxieties Questionnaire (PRAQ)-Revised [44, 47] which measures five common pregnancy fears subscales (giving birth, bearing a physically or mentally handicapped child, changes and disillusion in partner relationship, changes and concerns about one's mental well-being and the mother-child relationship); 3) the Perinatal PTSD Questionnaire (PPQ) [42, 43] that, with 14 items, measures the severity of maternal PTSD symptoms during prenatal and perinatal periods; and 4) the State-Trait Anxiety Inventory for Adults (STAI) [45] that, with 40 items, measures the temporary condition of “State Anxiety” and long-standing quality of “Trait Anxiety”.
Parental psychopathology is assessed using three detection strategies: 1) direct observation by professionals using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), used to determine DSM-IV Axis I disorders (major mental disorders) [48]; 2) clinical chart analysis; and 3) self-report by using the Family History Screening [49].
Infant temperament at 6 months of age is assessed by the Infant Behavior Questionnaire Revised (IBQ-R) administered to mothers [50, 51]. IBQ-R items were rationally derived from six constructs (Activity Level, Smile and Laughter, Fear, Distress to Limitations, Duration of Orientation, and Soothability) assessed by aggregating individual items across a range of contexts [52]. The 191 items on the IBQ-R ask parents to rate the frequency of specific temperament-related behaviors observed over the past week. In completing the IBQ-R, parents are asked to read each description of the infant’s behavior, indicating how often the infant engaged in various behaviors during the last week using a 7-point, Likert-type scale for frequency from never to always.
IBQ-R is validated for ages between 3 and 12 months. However it is generally accepted that reliably assessing infant temperament at younger ages is harder than at older ages. Moreover, the timing of the birth (gestational age at birth) could influence the maturity of the infant at earlier ages than older ages. Thus the SIP Study determined that 6 months of age is probably the earliest age for obtaining a reliable assessment.
Importantly, both the MPSP and infant temperament indices return continuous measurements, thus affording the chance of using linear, more informative, models for the analysis of the association of the expression profiles of the protein-coding mitochondrial-encoded genes with both MPSP and infant temperament outcomes.
For our study, summary scores (indices) elaborated from the MPSP and infant temperament questionnaires were used to test their association with the mitochondrial gene expression.
SIP also collects placental tissue at delivery from consented subjects. For this study, placenta samples from 108 subjects enrolled that successfully carried their pregnancies to term were used for the gene expression analysis. All subjects were consented as per the protocol approved by the Institutional Review Boards at Icahn School of Medicine at Mount Sinai, New York Hospital of Queens and Queens College. Informed written consent was obtained from the participants.
Placental Tissue Collection and RNA Isolation
Placentas were sampled by excising one full-thickness cylindrically shaped biopsy from each of the 4 placenta quadrants midway from the cord insertion and the placental rim, within 2 h from the delivery. Biopsies were initially processed by removing the maternal decidua and fetal membranes and abundantly washing the tissue in cold (4°C) sterile phosphate buffered saline (PBS). Biopsies were then blotted dry, snap-frozen in liquid nitrogen and store at -80°C.
RNA extraction was carried out by first grinding frozen tissue in liquid nitrogen-cooled mortar. Pulverized tissue was then processed for RNA extraction with RNeasy Plus Minikit (Qiagen—Valencia, CA, USA) and quantified with Nanodrop spectrophotometer (Thermo Electron North America—WI, USA). Aliquots of 1 μg of the total RNA extracted were converted into cDNA by using the iScript cDNA Synthesis Kit (BioRad—Hercules, CA, USA) for expression analysis following the manufacturer’s instructions. The rest of the sample was stored at -80°C. The iScript cDNA Synthesis Kit uses a random hexamer primer system to reliably amplify all transcripts in the extracted RNA.
Mitochondrial Gene Expression Analysis
We tested the expression of the 13 protein-coding mitochondrial-encoded genes listed in S1 Table. Primer sets for the gene expression experiment were designed and validated by: 1) BLASTing (www.blast.ncbi.nlm.nih.gov) the primers sequences against the whole human genome which allowed to determine that our primers did not match any RNA sequence listed by the “refseq” (www.ncbi.nlm.nih.gov/refseq/), “GenBank” (www.ncbi.nlm.nih.gov/genbank/) or UCSC (genome.ucsc.edu/cgi-bin/hgGateway) databases other that the specific mitochondrial genes; 2) testing by electrophoresis in agarose gel that the amplicons generated by the cDNA amplification did not show smears and that their length was within the expected size thus supporting the absence of spurious amplifications of different targets; and 3) verifying the consistency of the melting curves generate per each gene by the light-cycler across the 108 samples tested (see S1 Table for the complete list of primers and amplicons’ length). Primers were designed such to all properly work at the annealing temperature of 63°C in so allowing for thorough randomization of both samples and genes in each real-time PCR plate.
Gene expression was measured by standard real-time PCR in Roche 480 light cycler (Roche Diagnostics—Indianapolis, IN, USA). Cycling conditions for all genes were: 95.0°C for 1 min, followed by 35 cycles of 95.0°C for 30 sec, 63.0°C for 15 sec and 72.0°C for 30 sec. All reactions were run in triplicate and repeated if the standard deviation between the triplicate values was found >1 cycle. Expression values were normalized by first calculating the medians for the expression values of the 13 genes tested per each subject. The median of the subject medians was then calculated and the correction value was determined by subtracting the subject median to median of subject medians. The subject-specific correction value was then applied to each gene.
The expression of the housekeeping gene ACTB was used to validate the mitochondrial expression data by verifying that the variations observed in the mitochondrial-encoded gene expression was not due to technical artifacts. The lack of mitochondrial housekeeping genes together with the completely different nature of the transcription process carried out by the nuclear DNA and the mitochondrial DNA in fact warranted the use of a nuclear validated housekeeping gene to verify that the expression variation observed across the mitochondrial-encoded genes was not due to real-time PCR inter-plate variability.
Statistical Analysis
Statistical modeling was conducted by using stepwise multinomial linear and logistic regression models for, respectively, continuous and nominal outcomes. Normalized gene expression values were used as predictors while controlling for maternal ethnicity, age, marital status, education and welfare, delivery method, gestational age and infant gender. Standardized β values for the expression of mitochondrial-encoded genes significantly associated to the outcomes tested within logistic regression were obtained by applying the “Standardized Coefficients in Logistic Regression” method [53].
The component analysis was carried out by using hierarchical clustering and principal component analysis (PCA). Hierarchical clustering analysis on gene expression values was conducted by Ward Linkage to determine specific gene expression clusters. Dimensional stress analysis of the dataset to validate the clustering analysis was conducted by using multidimensional scaling (MDS). PCA was used to calculate summary scores for each of the expression cluster. PCA was also used to calculate summary scores among MPSP and infant temperament indices as well as infant birth measures.
The following non-parametric tests were used at different stages in the analysis:
1) Spearman's rho for bivariate correlation; 2) Wilcoxon rank-sum to compare mitochondrial gene expression between tertiles of significant MPSP and infant temperament indices; and 3) Kruskal-Wallis to determine the p-trend for mitochondrial gene expression across tertiles of significant MPSP and infant temperament indices.
We used PASW statistical software (version 20) (SPSS Inc.–Chicago, IL, USA) to carry out the statistical analysis.
Results
Mitochondrial Gene Expression in the SIP Study Birth Cohort
The population demographics and the characteristics of the variables analyzed are presented in Tables 1 and 2. The population in this study is a subset of the SIP Study birth cohort, a general description of which has been provided in the material and methods section. Briefly, the SIP Study aims at determining the influence of in utero exposure to MPSP on infant temperament.
Table 1. Population demographics and variables statistics: nominal.
| Variable | N. | % | Variable | N. | % |
|---|---|---|---|---|---|
| Mother | Obsessive Compulsive Disorder | ||||
| Ethnicity | No | 86 | 79.6 | ||
| Latina | 54 | 50.0 | Yes | 09 | 08.3 |
| Black | 35 | 32.4 | Not Available | 13 | 12.1 |
| Other (1) | 19 | 17.6 | Overweight | ||
| Marital Status | No | 57 | 52.8 | ||
| Married/Common Law | 29 | 26.9 | Yes | 51 | 47.2 |
| Single/Divorced/Separated/Widowed | 79 | 73.1 | Obesity | ||
| Education | No | 79 | 73.1 | ||
| Primary School | 36 | 33.3 | Yes | 29 | 26.9 |
| High School | 18 | 16.7 | Delivery Method | ||
| Some College | 36 | 33.3 | Vaginal | 73 | 67.6 |
| BA/Graduate Degree | 18 | 16.7 | C-Section | 35 | 32.4 |
| Welfare Status | Offspring | ||||
| No | 16 | 14.8 | Gender | ||
| Yes | 92 | 85.2 | Male | 60 | 55.6 |
| Female | 48 | 44.4 |
(1): Caucasian (white): N = 5 –% = 4.6.
Table 2. Population demographics and variables statistics: continuous.
| Variable | N. | Mean | St. Dev. | Min | Max |
|---|---|---|---|---|---|
| Mother | |||||
| Maternal Age (Years) | 108 | 27.36 | 5.83 | 17 | 44 |
| MPSP Indices | |||||
| Prenatal Perceived Stress | 108 | 37.05 | 7.33 | 23 | 56 |
| State Anxiety | 108 | 38.51 | 12.15 | 20 | 72 |
| Trait Anxiety | 108 | 38.73 | 11.08 | 20 | 64 |
| Pregnancy Anxiety Total | 108 | 6.06 | 2.33 | 3.00 | 12.83 |
| Offspring | |||||
| Infant Birth Measures and Temperament Indices | |||||
| Gestational Age at Birth (Days) | 108 | 274.26 | 17.16 | 167 | 291 |
| Birth Weight (g) | 108 | 3,217.09 | 630.32 | 560 | 4,450 |
| Birth Length (cm) | 108 | 49.80 | 3.69 | 29 | 57 |
| Head Circumference (cm) | 108 | 33.61 | 2.67 | 20.5 | 41.0 |
| Activity Level | 67 | 4.26 | 1.30 | 1.86 | 7.00 |
| Smile and Laughter | 67 | 5.48 | 1.48 | 1.14 | 7.00 |
| Mitochondrial Gene Expression (Ct Value) | |||||
| MT-ND1 | 108 | 15.05 | 2.00 | 14.79 | 29.84 |
| MT-ND2 | 108 | 17.64 | 2.27 | 14.35 | 30.24 |
| MT-CO1 | 108 | 17.16 | 2.93 | 14.04 | 32.52 |
| MT-CO2 | 108 | 16.52 | 2.13 | 13.03 | 29.95 |
| MT-ATP8 | 108 | 16.16 | 2.25 | 12.91 | 27.09 |
| MT-ATP6 | 108 | 17.50 | 2.78 | 13.94 | 27.56 |
| MT-CO3 | 108 | 17.68 | 2.48 | 14.21 | 28.10 |
| MT-ND3 | 108 | 17.23 | 1.83 | 14.33 | 22.26 |
| MT-ND4L | 108 | 19.38 | 3.37 | 14.10 | 30.03 |
| MT-ND4 | 108 | 18.62 | 3.43 | 14.90 | 28.37 |
| MT-ND5 | 108 | 18.72 | 2.76 | 14.50 | 24.06 |
| MT-ND6 | 108 | 17.06 | 2.16 | 13.70 | 21.25 |
| MT-CYB | 108 | 17.54 | 2.36 | 14.61 | 24.08 |
Data on MPSP indices and other variables were available across the whole cohort of 108 participants while data on the infant temperament indices were available on only 67 subjects. Not all newborns from the enrolled participants in fact reached age 6 months by the time the study was conducted and infant temperament data were not yet available on all infants.
We quantified the expression of all 13 protein-coding mitochondrial-encoded genes in 108 placenta samples from the SIP Study birth cohort and analyzed their association with MPSP and infant temperament indices. We also investigated additional outcomes, such as maternal psychopathology, maternal weight and infant birth measures, with known association with both high MPSP and pathologic infant temperament phenotypes.
By using multinomial linear and logistic regressions, significant associations with the gene expression were determined for: 1) the MPSP indices of Prenatal Perceived Stress, State Anxiety, Trait Anxiety and Pregnancy Anxiety Total; 2) the infant temperament indices of Activity Level and Smile and Laughter; 3) both maternal overweight and obesity; 4) all infant birth measures including birth weight, birth length and head circumference; and 5) the maternal psychopathology diagnosis of maternal obsessive compulsive disorder.
Significant associations between the mitochondrial gene expression and the MPSP and infant temperament indices listed above were found for genes MT-ND2, MT-ND6 and MT-CO2 (Table 3). MT-ND2, an OXPHOS Complex I gene was found positively associated with all significant MPSP indices and negatively with the infant temperament indices. Another OXPHOS Complex I gene, MT-ND6, was found negatively associated with the MPSP index of Prenatal Perceived Stress. MT-CO2, an OXPHOS Complex IV gene, was found positively associated with the MPSP indices of State and Trait Anxiety and with maternal overweight.
Table 3. Multinomial regression statistics for the correlation between mitochondrial gene expression and each of the analyzed outcomes from SIP.
| Outcome | Multinomial Regression | Mitochondrial Genes | |||||
|---|---|---|---|---|---|---|---|
| Area | Index | Type | p-value | r2 | Adj r2 | Item | β |
| MPSP | Prenatal Perceived Stress | Linear | 0.004 | 0.120 | 0.100 | MT-ND2 | 0.259 |
| MT-ND6 | -0.231 | ||||||
| State anxiety | Linear | 0.003 | 0.153 | 0.123 | MT-ND2 | 0.218 | |
| MT-CO2 | 0.206 | ||||||
| Trait anxiety | Linear | 0.003 | 0.129 | 0.109 | MT-ND2 | 0.262 | |
| MT-CO2 | 0.205 | ||||||
| Pregnancy Anxiety Total | Linear | 0.010 | 0.103 | 0.082 | MT-ND2 | 0.208 | |
| Psychopath | Obsessive Compulsive Disorder | Logistic | 0.022 | 0.135 | - | MT-CO3 | 0.048 |
| Maternal Weight | Overweight | Logistic | 0.001 | 0.191 | - | MT-CO2 | 0.162 |
| MT-ND6 | -0.145 | ||||||
| Obesity | Logistic | <0.001 | 0.320 | - | MT-ND1 | 0.131 | |
| MT-ATP6 | 0.103 | ||||||
| Birth Measures | Birth Weight | Linear | <0.001 | 0.546 | 0.519 | MT-CO3 | -0.164 |
| Birth Length | Linear | <0.001 | 0.597 | 0.583 | MT-ND5 | -0.192 | |
| Head Circumference | Linear | <0.001 | 0.367 | 0.337 | MT-ND5 | -0.187 | |
| MT-ATP8 | 0.183 | ||||||
| Infant | Activity Level | Linear | 0.008 | 0.165 | 0.134 | MT-ND2 | -0.257 |
| Temperament | Smile and Laughter | Linear | 0.036 | 0.082 | 0.064 | MT-ND2 | -0.286 |
- Regression p-values are reported as follows: 1) bold & underlined p < 0.01; 2) bold p < 0.05
- For the logistic regressions the Nagelkerke pseudo-r2 is reported
- Standardized β values for the logistic regressions have been calculated by using the “Standardized Coefficients in Logistic Regression” method [53].
The expression of other 5 protein-coding mitochondrial-encoded genes, MT-ND1, MT-ND5, MT-CO3, MT-ATP6, MT-ATP8, was found associated with the maternal psychopathology diagnosis of maternal obsessive compulsive disorder, maternal weight and with infant birth measures (Table 3).
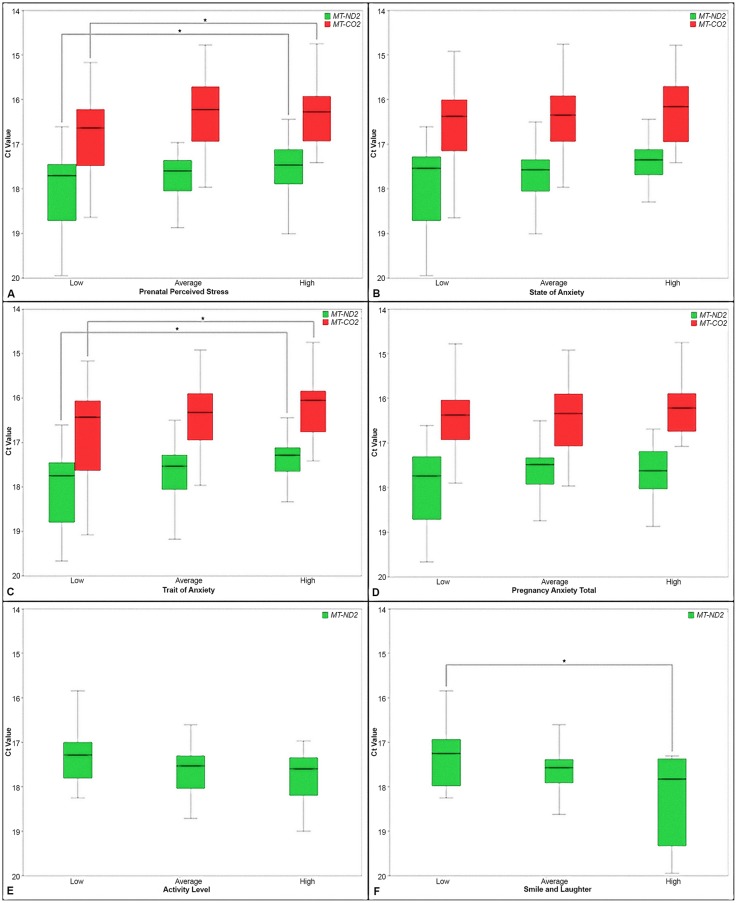
We further analyzed the association of the placental expression profiles of MT-ND2 and MT-CO2 with the significant MPSP indices of Table 3 by categorizing the MPSP indices into tertiles (Fig 1A–1D). Similarly we analyzed the placental expression profiles of MT-ND2 across tertiles of infant temperament indices (Fig 1E and 1F). MT-ND6 was not included in this analysis as it was found consistently non-significantly distributed across tertiles of MPSP indices.
Fig 1. Boxplots of the distribution of the expression of MT-ND2 and MT-CO2 across tertiles of MPSP and infant temperament indices.
A-D. MPSP indices. E-F. Infant temperament indices. In each graph “low”, “average” and “high” represent the tertiles of MPSP and infant temperament indices. The star symbol and the bracketing lines represent the tertiles for which significant (p < 0.05) differences in gene expression have been detected.
Significant upregulation of the gene expression was determined between the low (tertile) and high (tertile) of the MPSP indices of Prenatal Perceived Stress and Trait Anxiety for both MT-ND2 (p = 0.016 for Prenatal Perceived Stress and p = 0.019 for Trait Anxiety) and MT-CO2 (p = 0.042 for Prenatal Perceived Stress and p = 0.039 for Trait Anxiety). Overall the expression of both MT-ND2 and MT-CO2 showed a consistent trend toward an upregulation across tertiles of MPSP indices (Fig 1A–1D). Trend analysis was also conducted to determine the significance of the expression upregulation of MT-ND2 and MT-CO2 across tertiles of MPSP indices. Significant p-values were determined only for MT-ND2 relatively to the Prenatal Perceive Stress (p = 0.017) and State Anxiety (p = 0.031). Borderline significant p-values were determined for MT-ND2 for the other MPSP indices as, respectively, Trait Anxiety (p = 0.068) and Pregnancy Anxiety Total (p = 0.059).
MT-ND2 on the other hand showed a consistent trend to dowregulation across tertiles of infant temperament indices (Fig 1E and 1F). Statistically significant dowregulation of MT-ND2 was determined between the low (tertile) and high (tertile) of the Smile and Laughter index (Fig 1E and 1F). A significant p-value for downregulation of MT-ND2 expression across infant temperament tertiles was determined for only Smile and Laughter (p = 0.048).
Component Analyses
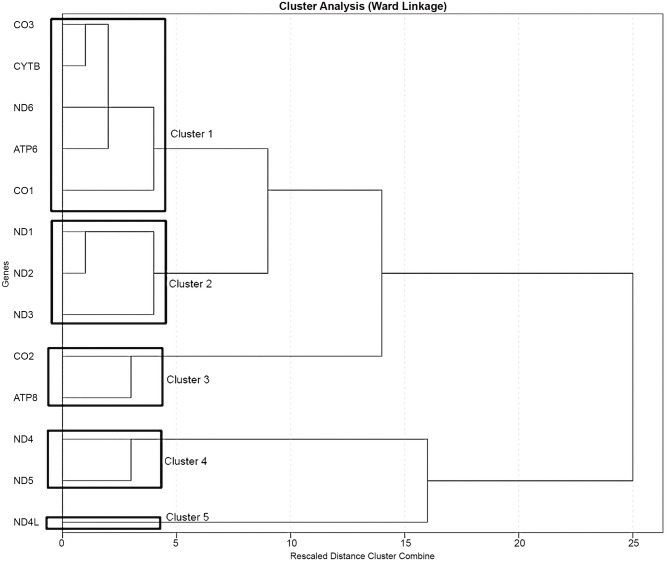
As suggested by the role that protein-coding mitochondrial-encoded genes play in the OXPHOS, the expression profiles of the mitochondrial-encoded genes tested showed, for several genes, a relevant degree of colinearity as determined by the high (rho > |0.4|) and significant (p < 0.01) bivariate correlation between samples (S2 Table). To address the effect of colinearity on the significance of our findings we first conducted a clustering analysis that revealed the existence of 5 expression clusters (Fig 2). The expression clusters were then validated by dimensional stress analysis conducted by using multidimensional scaling showing that the expression of the mitochondrial genes can efficiently be fit into a 5 dimension space (e.g. 5 clusters) without imposing an excessive degree of stress to the dataset (S1 Fig). Finally we calculated a summary score for each expression cluster by using principal component analysis (PCA). The efficacy of this approach was further supported by the low bivariate correlation found between the 5 summary scores (S3 Table). Similarly we calculated PCA summary scores for MPSP and infant temperament indices as well as maternal weight (including overweight and obesity) and infant birth measures (including birth weight, birth length and head circumference).
Fig 2. Mitochondrial Expression Clusters.
The graph represents the five expression clusters identified for the expression of the 13 protein-coding mitochondrial-encoded genes. The expression clusters have been determined by Ward Linkage. The cutoff level used to generate the clusters in the clustering tree have been chosen accordingly to the multidimensional scaling analysis (see S1 Fig) that showed that the expression data can efficiently be fit into a 5-dimensional space without imposing an excessive degree of stress to the dataset.
We finally rerun the regression models using the same covariates as above and the PCA summary scores for each expression cluster in place of the individual gene expression values, as predictors, and the PCA summary scores as outcomes in place of the individual outcome indices/values. The results of this test strongly support the validity of our original approach (Table 4). The PCA summary score for the MPSP indices was found positively associated with expression clusters 2 and 3 which include, respectively, genes MT-ND2 and MT-CO2 that were previously found positively associated with the individual MPSP indices. A negative non-significant association was detected between expression cluster 2 and the PCA summary score for the infant temperament indices, partially supporting the negative correlation described between the individual infant temperament indices and the expression of MT-ND2.
Table 4. Multinomial regression statistics for the correlation between the PCA summary scores for the five mitochondrial gene expression clusters and the PCA summary scores for MPSP and infant temperament indices, maternal weight class and infant birth measures.
| Index | Multinomial Regression | Expression Clusters | ||||
|---|---|---|---|---|---|---|
| Type | p-value | r2 | Adj r2 | Item | β | |
| MPSP | Linear | 0.001 | 0.166 | 0.136 | Cluster 2 | 0.228 |
| Cluster 3 | 0.218 | |||||
| Maternal Weight | Logistic | 0.006 | 0.169 | - | ||
| - Normal vs Overweight | Cluster 2 | 0.107 | ||||
| - Normal vs Obese | Cluster 2 | 0.145 | ||||
| Birth Measures | Linear | <0.001 | 0.579 | 0.559 | Cluster 4 | -0.198 |
| Infant Temperament | Linear | 0.277 | 0.311 | 0.064 | Cluster 2 | -0.278 |
- Regression p-values are reported as follows: 1) bold & underlined p < 0.01; 2) bold p < 0.05; 3) regular p ≥ 0.05
- For the logistic regressions the Nagelkerke pseudo-r squared is reported
- Standardized β values for the logistic regressions have been calculated by using the “Standardized Coefficients in Logistic Regression” method [53].
Additionally expression cluster 2 was positively associated with the PCA summary score for maternal weight. Expression cluster 2 includes gene MT-ND1 which was the gene with the most significant (positive) association with maternal obesity. Expression cluster 4 was instead found negatively associated with the PCA summary score for infant birth measures supporting the association previously found for MT-ND5, the only gene associated with two birth measures (birth length and head circumference).
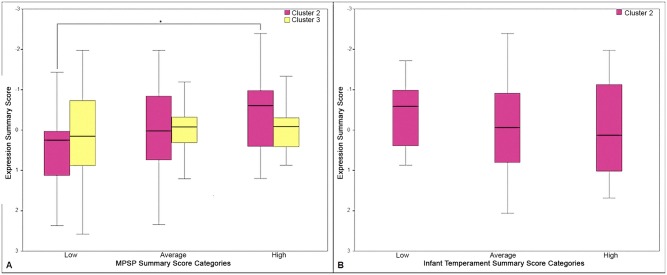
In line with our prior analysis, we tested the placental expression profile of expression clusters 2 and 3 across tertiles of the PCA summary score for the MPSP indices and the placental expression profile of expression cluster 2 across PCA summary score for the infant temperament indices (Fig 3). A statistically significant upregulation of the score for the expression cluster 2 was determined between the low (tertile) and the high (tertile) of the PCA summary score for the MPSP indices. This finding indicates that the genes of this expression cluster (see Fig 2 for reference) follow a similar expression trend. These data are partially supported by a borderline significant p-value for trend determined for expression cluster 2 across the tertiles of PCA summary score for the MPSP indices (p = 0.079). Expression cluster 3 showed a similar but less marked and non-significant expression trend (Fig 3A).
Fig 3. Boxplots representing the distribution of the expression clusters 2 and 3 across tertiles of PCA summary score for MPSP and infant temperament indices.
A. PCA summary score for MPSP indices. B. PCA summary score for infant temperament indices. In each graph “low”, “average” and “high” represent the tertiles of the PCA summary score for MPSP and infant temperament indices. The star symbol and the bracketing lines represent the tertiles for which significant (p < 0.05) differences in gene expression have been detected.
A non-significant downregulation of the score for the expression cluster 2 was observed across tertiles of PCA summary score for the infant temperament indices (Fig 3B).
Mitochondrial Gene Expression and the Expression of Corticotropin and Glucocorticoid Receptors in Placenta
In a previous investigation on a subset of 50 among the 108 samples used here [54], we found limited evidence of the association between the expression of CRHR1, CRHR2 and NR3C1, three key hormonal receptor of the HPA (Hypothalamic-Pituitary-Adrenal) axis, and both MPSP and infant temperament indices. The corticotropin-releasing hormone (CRH) receptors CRHR1 and CRHR2 and the glucocorticoids’ (GHs) receptor NR3C1 regulate the HPA axis, a fundamental mediator of the stress response and a modulator of the mitochondrial activity [55].
We tested the interaction between the HPA axis and the mitochondrial gene expression in determining the tested outcomes by modeling each outcome within linear and logistic regressions. We used as predictors the same covariates as in the previous analyses together with the PCA summary scores for each mitochondrial expression cluster and the normalized expression values for CRHR1, CRHR2 and NR3C1. The only association with the outcomes was found for CRHR1 that was positively associated with the MPSP index of State Anxiety together, as previously determined, with mitochondrial expression cluster 2 (S4 Table).
Noticeably, we also found a high degree of significant negative bivariate correlation between the expression of NR3C1 and MT-CO1 (S5 Table).
Discussion
In this study we explored the role of the mitochondrial gene expression in responding to MPSP, a powerful environmental determinant of fetal brain development [3, 9, 10, 11], affecting infant temperament. By using placenta samples from the SIP Study birth cohort we determined that the expression of 3 of the 13 protein-coding mitochondrial-encoded genes of the OXPHOS, MT-ND2, MT-ND6, MT-CO2, is correlated to both MPSP and infant temperament indices. The expression of other 5 protein-coding mitochondrial-encoded genes was found associated with maternal and infant outcomes associated with high MPSP and pathologic infant temperament phenotypes.
MPSP predicts considerable portions of the variance in infant neurodevelopment leading to lower mental development scores and irritable temperament [9, 56, 57, 58]. This scenario theoretically confers an evolutionary advantage to the progeny [59, 60, 61] as the increased levels of anxiety in the offspring of mothers experiencing high MPSP may in fact be explained by increased vigilance and alertness to danger [62]. These elevated rates of anxiety, often accompanied by increased aggression, may thus direct a process of adaptation leading to a higher sensibility to adverse environments, higher drive in exploring new environments and fight possible predators and lower proneness to engage in developing deep relationships with other individuals [63, 64, 65].
MPSP indices that were found associated with the expression profiles of mitochondrial genes in this study are representative of objective and subjective levels of stressful experiences, feelings and thoughts during pregnancy and perceived stress [42, 43, 44, 46, 47, 66]. Infant temperament indices significantly associated with the expression profiles of mitochondrial genes belong to the temperament developmental factors of Surgency/Extraversion (Activity Level) and Effortful Control (Smile and Laughter). High scores for indices grouped under these temperament developmental factors are representative of the infant temperament phenotypes of, respectively, anxiety [67, 68, 69] and aggression [70, 71]. While it is beyond the scope of the current study, it is worth to additionally report that influences of postnatal psychosocial rearing environment on infant temperament at 6 months of age, as especially maternal postpartum depression, have been found not to affect infant temperament indices as determined by the IBQ-R in the SIP Study birth cohort (data not shown).
Stress stimuli like MPSP set off the physiologic stress response involving mitochondria [12, 13, 18]. Mitochondria code for 13 proteins of the OXPHOS system [24]. Eleven of these 13 proteins represent the building blocks of 3 OXPHOS protein complexes, the NADH dehydrogenase (Complex I), the cytochrome c reductase (Complex III) and the cytochrome c oxidase (Complex IV), that, together with the entirely nuclear-encoded succinate dehydrogenase (Complex II), form the electron transport chain (ETC). Two additional mitochondrial proteins regulate the activity of the ATP synthase (Complex V).
In our study the expression of OXPHOS Complex I gene MT-ND2 showed the strongest association with both MPSP and infant temperament indices. Interestingly MPSP, which is known to negatively affect infant temperament, in this study was associated with an increase on the expression of MT-ND2 that then shows a negative association with the infant temperament indices. MT-ND6, another member of the OXPHOS Complex I, shows instead a decreased expression even if limited to the MPSP index of Prenatal Perceived Stress.
OXPHOS Complex I, the initiator of the mitochondrial ETC [24], is a large multiprotein complex arranged in three modules known as N, Q and P. The P module is made out of a proximal and distal portion [72, 73] organized into a transmembrane proton pump that can work uncoupled from the ETC [73]. Genes MT-ND1, MT-ND2, MT-ND3 and MT-ND4L belong to the proximal portion of the P module while MT-ND4 and MT-ND5 belong to the distal portion [72]. MT-ND6 instead belongs to the P-Q bridging region of the Q module [72, 74]. Interestingly, in our study we found that MT-ND1, MT-ND2 and MT-ND3 expression profiles group together in expression cluster 2, while MT-ND4 and MT-ND5 form expression cluster 4 in so mirroring the P module organization. MT-ND6 separately clusters with other genes to form expression cluster 1. The parallel between the cluster distribution of Complex I genes and the topology of the P module substantially adds confidence to the results of the clustering analysis.
Complex I alone contributes for 40% to the generation of the mitochondrial proton-motive force utilized for ATP synthesis and transport processes and it is a major contributor to reactive oxygen species production in the cell [75]. Complex I deficiency is also the most frequent defects of mitochondrial energy metabolism [76] which have been linked to a wide range of neurodevelopmental and neurodegenerative disorders [75].
Complex I is thus likely a key player of the response to stressors such as MPSP. Such response entails the activation of the OXPHOS to respond to the increased energy demand [12, 13] for actively regulating the Ca2+ cellular homeostasis [12, 13, 37]. Ca2+ in turns cumulates in the mitochondria, increases the OXPHOS rate and ATP production and act as a regulator of the secretion of many neurotransmitters such as serotonin [37]. Serotonin regulates neurodevelopmental processes through maternal-fetal interactions that have long-term mental health implications [37]. Importantly, the placenta supplies serotonin to the developing brain to support neuronal differentiation [37].
As the P module evolved from passive bacterial kation antiporter [77], our data are in agreement with the literature reporting a partial uncoupling of the P module of Complex I with the ETC in response to the increased mitochondrial concentration of Ca2+ [73, 78]. Stress-driven Ca2+ sequestration from the cytoplasm into the mitochondria however results in decreased serotonin release that may alter fetal development [37]. Accordingly MT-ND2 expression profile was found negatively associated with infant temperament.
The high rates of ATP production in response to the increased energy demand brought about by MPSP may also explain the expression pattern of Complex IV genes. Complex IV is the last component of the ETC catalyzing the conversion of oxygen, the final acceptor of the electrons of the ETC, into water [24]. MT-CO2 positive expression association with the MPSP indices of State and Trait Anxiety may be linked to a higher OXPHOS activity. The positive correlation between MT-CO3 expression and maternal obsessive compulsive disorder lend further support to this hypothesis. Maternal obsessive compulsive disorder in our dataset is in fact significantly associated with each of the MPSP indices (S6 Table) in agreement with the existing literature [79, 80, 81, 82].
The high incidence of overweight (~47%) and obesity (~27%) (Table 1) within the SIP population analyzed here add an additional workload on mitochondria. Alterations of the mitochondrial activity have in fact been reported in placentas from women with elevated BMI [83]. Particularly, a large number of studies show that obesity leads to dissipations of the mitochondrial membrane potential [84, 85, 86]. The positive correlation between overweight and MT-CO2 expression and between obesity and MT-ND1 and MT-ATP6 expression that we determined in the SIP cohort placentas can therefore be attributable to the attempt by mitochondria to respond to the MPSP effect while also battling the effect brought about by obesity.
In our investigation we found several examples of increased vs decreased mitochondrial gene expression (e.g. MT-ND2 and MT-ND6) in association with the MPSP and infant temperament indices (Table 3). Accordingly we also determined the existence of significant negative bivariate correlation between the expression profiles of mitochondrial genes (S2 Table). However, mitochondrial genes are transcribed into polycistronic RNAs, one per each mitochondrial DNA strand. These transcripts are then cleaved and processed for translation into proteins. Such transcription method would speak for a coordinated regulation of the expression of all mitochondrial genes returning a homogenous expression profile and high positive correlation scores between genes. However mechanistic studies have shown that the mitochondrial polycistronic RNAs, before translation, undergo differential cleavage/degradation in order to respond to specific physiologic needs that accordingly modulate the expression of only some mitochondrial genes [87]. Additionally several studies reported that the expression of individual mitochondrial genes undergo to differential regulation [18, 88], supporting the findings of our study. These data are also in agreement with the role that mitochondrial-encoded genes play within their OXPHOS complexes. Mitochondrial genes of complexes I, III and IV in fact code for the catalytic components of their respective complexes [72, 89] and their expression profiles are differentially related to each other and unrelated to the chaperon-like nuclear-encoded subunits of the same complexes.
While partial and preliminary, our interpretation is further supported by the integrated analysis with the expression data on CRH and GHs receptors (GRs). As expected, CRH receptors’ expression was found associated with MPSP indices in conjunction with mitochondrial genes of the P module of Complex I. Concurrently a significant correlation was found between the GRs and the expression of the complex IV gene MT-CO1, supporting the findings that showed that GRs participate in the modulation of the mitochondrial activity [55]. Mitochondria in fact carry 4 glucocorticoid response elements within the MT-CO1 gene [90] and the activated NR3C1 has been shown to bind all four of them [91].
These data support the role that HPA axis plays in modulating the energy production through hormonal feedback cycles. Our data also suggest that the HPA axis activation may be a more transient phenomenon that participates in determining long lasting changes that reflect in the association between the mitochondrial expression and infant temperament indices and birth measures.
Overall this study highlights the importance of placental mitochondria gene expression in responding to MPSP while suggesting a possible role of placental mitochondria gene expression in affecting infant temperament. Additional data are however needed to better describe the mitochondrial response to stressors like MPSP. The increased sample size of the cohort would be of great benefit to support the validity of these findings in future investigations while also allowing for the study of the role of the placental mitochondria gene expression in mediating/moderating the effects of MPSP on infant temperament. The analysis of the mitochondrial metabolism by means of enzymatic assays targeting Complex I and IV activity rates would also provide important information of the mitochondrial functioning.
Supporting Information
The graph shows that the expression of the mitochondrial genes can efficiently be fit into a 5 dimension space (e.g. 5 clusters) without imposing an excessive degree of stress to the dataset.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Venture Capital Research Funding Program of the Mount Sinai Children’s Environmental Health Center, the Queens College, CUNY Research Enhancement Grant, and NIMH grants K01 MH080062 and ARRA supplement K01 MH080062S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Del Giudice M. Fetal programming by maternal stress: Insights from a conflict perspective. Psychoneuroendocrinology. 2012;37(10):1614–29. 10.1016/j.psyneuen.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 2. Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293(5532):1089–93. [DOI] [PubMed] [Google Scholar]
- 3. Santos F, Dean W. Epigenetic reprogramming during early development in mammals. Reproduction. 2004;127(6):643–51. [DOI] [PubMed] [Google Scholar]
- 4. Austin MP, Hadzi-Pavlovic D, Leader L, Saint K, Parker G. Maternal trait anxiety, depression and life event stress in pregnancy: relationships with infant temperament. Early Hum Dev. 2005;81(2):183–90. [DOI] [PubMed] [Google Scholar]
- 5. Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68(4):314–9. 10.1016/j.biopsych.2010.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brand SR, Engel SM, Canfield RL, Yehuda R. The effect of maternal PTSD following in utero trauma exposure on behavior and temperament in the 9-month-old infant. Ann N Y Acad Sci. 2006;1071:454–8. [DOI] [PubMed] [Google Scholar]
- 7. Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, et al. Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC psychiatry. 2008;8:71 10.1186/1471-244X-8-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu P, Sun MS, Hao JH, Chen YJ, Jiang XM, Tao RX, et al. Does prenatal maternal stress impair cognitive development and alter temperament characteristics in toddlers with healthy birth outcomes? Dev Med Child Neurol. 2014;56(3):283–9. 10.1111/dmcn.12378 [DOI] [PubMed] [Google Scholar]
- 9. Beijers R, Jansen J, Riksen-Walraven M, de Weerth C. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126(2):e401–9. 10.1542/peds.2009-3226 [DOI] [PubMed] [Google Scholar]
- 10. Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2011;52(2):119–29. 10.1111/j.1469-7610.2010.02314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32(6):1073–86. 10.1016/j.neubiorev.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 12. Goldenthal MJ, Marin-Garcia J. Mitochondrial signaling pathways: a receiver/integrator organelle. Molecular and cellular biochemistry. 2004;262(1–2):1–16. [DOI] [PubMed] [Google Scholar]
- 13. Manoli I, Alesci S, Blackman MR, Su YA, Rennert OM, Chrousos GP. Mitochondria as key components of the stress response. Trends in endocrinology and metabolism: TEM. 2007;18(5):190–8. [DOI] [PubMed] [Google Scholar]
- 14.Acton QA Ed. Advances in Overweight Research and Treatment: 2012 Edition: ScholarlyBrief: ScholarlyEditions; 2012.
- 15. Heininger K. The Cerebral Glucose-Fatty Acid Cycle: Evolutionary Roots, Regulation and (Patho)physiological Importance In: Dwyer DS Ed. Glucose Metabolism in the Brain. Elsevier Science; 2002:103–58. [DOI] [PubMed] [Google Scholar]
- 16. Kubera B, Hubold C, Otte S, Lindenberg AS, Zeiss I, Krause R, et al. Rise in plasma lactate concentrations with psychosocial stress: a possible sign of cerebral energy demand. Obesity facts. 2012;5(3):384–92. 10.1159/000339958 [DOI] [PubMed] [Google Scholar]
- 17. Peters A, Kubera B, Hubold C, Langemann D. The selfish brain: stress and eating behavior. Frontiers in neuroscience. 2011;5:74 10.3389/fnins.2011.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dalwadi DA, Uht RM. Hypothalamic and amygdalar cell lines differ markedly in mitochondrial rather than nuclear encoded gene expression. BMC Genomics. 2013;14:413 10.1186/1471-2164-14-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujimoto M, Hayashi T. New insights into the role of mitochondria-associated endoplasmic reticulum membrane. International review of cell and molecular biology. 2011;292:73–117. 10.1016/B978-0-12-386033-0.00002-5 [DOI] [PubMed] [Google Scholar]
- 20. Raturi A, Simmen T. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM). Biochim Biophys Acta. 2013;1833(1):213 10.1016/j.bbamcr.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 21. Scatena R, Bottoni P, Giardina B Eds. Advances in Mitochondrial Medicine. Adv Exp Med Biol. 2012;942:1–461. [DOI] [PubMed] [Google Scholar]
- 22. Adzic M, Djordjevic A, Demonacos C, Krstic-Demonacos M, Radojcic MB. The role of phosphorylated glucocorticoid receptor in mitochondrial functions and apoptotic signalling in brain tissue of stressed Wistar rats. The international journal of biochemistry & cell biology. 2009;41(11):2181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the 'gluc' back in glucocorticoids. Nature reviews Endocrinology. 2014;10(5):303–10. 10.1038/nrendo.2014.22 [DOI] [PubMed] [Google Scholar]
- 24. Papa S, Martino PL, Capitanio G, Gaballo A, De Rasmo D, Signorile A, et al. The oxidative phosphorylation system in mammalian mitochondria. Advances in experimental medicine and biology. 2012;942:3–37. 10.1007/978-94-007-2869-1_1 [DOI] [PubMed] [Google Scholar]
- 25. Belkacemi L, Desai M, Nelson DM, Ross MG. Altered mitochondrial apoptotic pathway in placentas from undernourished rat gestations. American journal of physiology Regulatory, integrative and comparative physiology. 2011;301(6):R1599–615. 10.1152/ajpregu.00100.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayeur S, Lancel S, Theys N, Lukaszewski MA, Duban S, Bastide B, et al. Maternal calorie restriction modulates placental mitochondrial biogenesis and bioenergetic efficiency: involvement in feto-placental growth defects in rats. Am J Physiol Endocrinol Metab. 2012. [DOI] [PubMed] [Google Scholar]
- 27. Hu D, Cross JC. Development and function of trophoblast giant cells in the rodent placenta. Int J Dev Biol. 2010;54(2–3):341–54. 10.1387/ijdb.082768dh [DOI] [PubMed] [Google Scholar]
- 28. Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92(1):35 [DOI] [PubMed] [Google Scholar]
- 29. Molina RD, Meschia G, Wilkening RB. Uterine blood flow, oxygen and glucose uptakes at mid-gestation in the sheep. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1990;195(3):379–85. [DOI] [PubMed] [Google Scholar]
- 30. Aldoreta PW, Hay WW. Metabolic Substrates for Fetal Energy Metabolism and Growth. Clinics in Perinat. 1995;22(1):15–37. [PubMed] [Google Scholar]
- 31. Martinez F, Milan R, Flores-Herrera O, Olvera-Sanchez S, Gomez-Chang E, Espinosa-Garcia MT. The Role of Mitochondria in Syncytiotrophoblast Cells: Bioenergetics and Steroidogenesis In: Zheng J Ed. Recent Advances in Research on the Human Placenta. InTech Open Access Publisher:. 2012. p. 397–428. [Google Scholar]
- 32. Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572(Pt 1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wright RO, Brunst KJ. Programming of respiratory health in childhood: influence of outdoor air pollution. Current opinion in pediatrics. 2013;25(2):232–9. 10.1097/MOP.0b013e32835e78cc [DOI] [PubMed] [Google Scholar]
- 34. Davies W, Isles AR, Wilkinson LS. Imprinted gene expression in the brain. Neurosci Biobehav Rev. 2005;29(3):421–30. [DOI] [PubMed] [Google Scholar]
- 35. Wilkinson LS, Davies W, Isles AR. Genomic imprinting effects on brain development and function. Nat Rev Neurosci. 2007;8(11):832–43. [DOI] [PubMed] [Google Scholar]
- 36. Zeltser LM, Leibel RL. Roles of the placenta in fetal brain development. Proc Natl Acad Sci U S A. 2011;108(38):15667–8. 10.1073/pnas.1112239108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472(7343):347–50. 10.1038/nature09972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67(1):53–83. [DOI] [PubMed] [Google Scholar]
- 39. Turlejski K. Evolutionary ancient roles of serotonin: long-lasting regulation of activity and development. Acta neurobiologiae experimentalis. 1996;56(2):619–36. [DOI] [PubMed] [Google Scholar]
- 40. Holmboe K, Nemoda Z, Fearon RM, Csibra G, Sasvari-Szekely M, Johnson MH. Polymorphisms in dopamine system genes are associated with individual differences in attention in infancy. Developmental psychology. 2010;46(2):404–16. 10.1037/a0018180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolpert L, Tickle C, Lawrence P, Meyerowitz E, Robertson E, Smith J, et al. Principles of Development- 4th Edition. New York, NY—USA: Oxford University Press; 2010. [Google Scholar]
- 42. Callahan JL, Hynan MT. Identifying mothers at risk for postnatal emotional distress: further evidence for the validity of the perinatal posttraumatic stress disorder questionnaire. J Perinatol. 2002;22(6):448–54. [DOI] [PubMed] [Google Scholar]
- 43. DeMier RL, Hynan MT, Hatfield RF, Varner MW, Harris HB, Manniello RL. A measurement model of perinatal stressors: identifying risk for postnatal emotional distress in mothers of high-risk infants. J Clin Psychol. 2000;56(1):89–100. [DOI] [PubMed] [Google Scholar]
- 44. Huizink AC, Mulder EJ, Robles de Medina PG, Visser GH, Buitelaar JK. Is pregnancy anxiety a distinctive syndrome? Early Hum Dev. 2004;79(2):81–91. [DOI] [PubMed] [Google Scholar]
- 45. Spielberger CD. State-Trait Anxiety Inventory: Bibliography (2nd ed.). Palo Alto, CA—USA: Consulting Psychologists Press; 1989. [Google Scholar]
- 46. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 47. van den Bergh B. The influence of maternal emotions during pregnancy on fetal and neonatal behavior. Pre Peri Psychol J. 1990;5:119–30. [Google Scholar]
- 48. First MB, Williams JBW, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT). New York (NY), USA: New York: Biometrics Research, New York State Psychiatric Institute; 2007. [Google Scholar]
- 49. Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57(7):675–82. [DOI] [PubMed] [Google Scholar]
- 50. Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Inf Behav Dev. 2003;26(1):64–86. [Google Scholar]
- 51. Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: origins and outcomes. J Pers Soc Psychol. 2000;78(1):122–35. [DOI] [PubMed] [Google Scholar]
- 52. Goldsmith HH, Campos JJ. The structure of temperamental fear and pleasure in infants: a psychometric perspective. Child Dev. 1990;61(6):1944–64. [PubMed] [Google Scholar]
- 53.King JE. Standardized Coefficients in Logistic Regression. Baylor College of Medicine. 2007 [Internet]. Available: www.ccitonline.org/jking/homepage/standardized_paper.doc.
- 54. Chen J, Li Q, Rialdi A, Mystal E, Ly J, Finik J, et al. Influences of Maternal Stress during Pregnancy on the Epi/genome: Comparison of Placenta and Umbilical Cord Blood. Depress Anxiety. 2014;3(2):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci U S A. 2009;106(9):3543–8. 10.1073/pnas.0812671106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81(1):131–48. 10.1111/j.1467-8624.2009.01385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nomura Y, Halperin JM, Newcorn JH, Davey C, Fifer WP, Savitz DA, et al. The risk for impaired learning-related abilities in childhood and educational attainment among adults born near-term. J Pediatr Psychol. 2009;34(4):406–18. 10.1093/jpepsy/jsn092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nomura Y, Rajendran K, Brooks-Gunn J, Newcorn JH. Roles of perinatal problems on adolescent antisocial behaviors among children born after 33 completed weeks: a prospective investigation. J Child Psychol Psychiatry. 2008;49(10):1108–17. 10.1111/j.1469-7610.2008.01939.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Badcock C, Crespi B. Imbalanced genomic imprinting in brain development: an evolutionary basis for the aetiology of autism. J Evol Biol. 2006;19(4):1007–32. [DOI] [PubMed] [Google Scholar]
- 60. Badcock C, Crespi B. Battle of the sexes may set the brain. Nature. 2008;454(7208):1054–5. 10.1038/4541054a [DOI] [PubMed] [Google Scholar]
- 61. Crespi B, Badcock C. Psychosis and autism as diametrical disorders of the social brain. Behav Brain Sci. 2008;31(3):241–61; discussion 61–320. 10.1017/S0140525X08004214 [DOI] [PubMed] [Google Scholar]
- 62. Bergman K, Sarkar P, O'Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1454–63. [DOI] [PubMed] [Google Scholar]
- 63. Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J Am Acad Child Adolesc Psychiatry. 2008;47(9):1063–72. 10.1097/CHI.0b013e31817eec80 [DOI] [PubMed] [Google Scholar]
- 64. van den Bergh BR, Mennes M, Oosterlaan J, Stevens V, Stiers P, Marcoen A, et al. High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neurosci Biobehav Rev. 2005;29(2):259–69. [DOI] [PubMed] [Google Scholar]
- 65. van den Bergh BR, Mennes M, Stevens V, van der Meere J, Borger N, Stiers P, et al. ADHD deficit as measured in adolescent boys with a continuous performance task is related to antenatal maternal anxiety. Pediatr Res. 2006;59(1):78–82. [DOI] [PubMed] [Google Scholar]
- 66. Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157:288–90. [DOI] [PubMed] [Google Scholar]
- 67. Buss KA, Dennis TA, Brooker RJ, Sippel LM. An ERP study of conflict monitoring in 4-8-year old children: associations with temperament. Developmental cognitive neuroscience. 2011;1(2):131–40. 10.1016/j.dcn.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ferguson E, Semper H, Yates J, Fitzgerald JE, Skatova A, James D. The 'dark side' and 'bright side' of personality: when too much conscientiousness and too little anxiety are detrimental with respect to the acquisition of medical knowledge and skill. PLoS One. 2014;9(2):e88606 10.1371/journal.pone.0088606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hagekull B, Bohlin G. Early temperament and attachment as predictors of the Five Factor Model of personality. Attachment & human development. 2003;5(1):2–18. [DOI] [PubMed] [Google Scholar]
- 70. Choe DE, Lane JD, Grabell AS, Olson SL. Developmental precursors of young school-age children's hostile attribution bias. Developmental psychology. 2013;49(12):2245–56. 10.1037/a0032293 [DOI] [PubMed] [Google Scholar]
- 71. Minamoto T, Osaka M, Yaoi K, Osaka N. Extrapunitive and intropunitive individuals activate different parts of the prefrontal cortex under an ego-blocking frustration. PLoS One. 2014;9(1):e86036 10.1371/journal.pone.0086036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hunte C, Zickermann V, Brandt U. Functional modules and structural basis of conformational coupling in mitochondrial complex I. Science. 2010;329(5990):448–51. 10.1126/science.1191046 [DOI] [PubMed] [Google Scholar]
- 73. Mimaki M, Wang X, McKenzie M, Thorburn DR, Ryan MT. Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta. 2012;1817(6):851–62. 10.1016/j.bbabio.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 74. Ugalde C, Vogel R, Huijbens R, Van Den Heuvel B, Smeitink J, Nijtmans L. Human mitochondrial complex I assembles through the combination of evolutionary conserved modules: a framework to interpret complex I deficiencies. Hum Mol Genet. 2004;13(20):2461 [DOI] [PubMed] [Google Scholar]
- 75. Petruzzella V, Sardanelli AM, Scacco S, Panelli D, Papa F, Trentadue R, et al. Dysfunction of mitochondrial respiratory chain complex I in neurological disorders: genetics and pathogenetic mechanisms. Advances in experimental medicine and biology. 2012;942:371–84. 10.1007/978-94-007-2869-1_17 [DOI] [PubMed] [Google Scholar]
- 76. Smeitink J, van den Heuvel L, DiMauro S. The genetics and pathology of oxidative phosphorylation. Nat Rev Genet. 2001;2(5):342–52. [DOI] [PubMed] [Google Scholar]
- 77. Lazarou M, Thorburn DR, Ryan MT, McKenzie M. Assembly of mitochondrial complex I and defects in disease. Biochim Biophys Acta. 2009;1793(1):78–88. 10.1016/j.bbamcr.2008.04.015 [DOI] [PubMed] [Google Scholar]
- 78. Mathiesen C, Hagerhall C. The 'antiporter module' of respiratory chain complex I includes the MrpC/NuoK subunit—a revision of the modular evolution scheme. FEBS letters. 2003;549(1–3):7–13. [DOI] [PubMed] [Google Scholar]
- 79. Gureje O, Oladeji B, Hwang I, Chiu WT, Kessler RC, Sampson NA, et al. Parental psychopathology and the risk of suicidal behavior in their offspring: results from the World Mental Health surveys. Mol Psychiatry. 2011;16(12):1221–33. 10.1038/mp.2010.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hirshfeld-Becker DR, Micco JA, Henin A, Petty C, Faraone SV, Mazursky H, et al. Psychopathology in adolescent offspring of parents with panic disorder, major depression, or both: a 10-year follow-up. Am J Psychiatry. 2012;169(11):1175–84. [DOI] [PubMed] [Google Scholar]
- 81. Vera J, Granero R, Ezpeleta L. Father's and mother's perceptions of parenting styles as mediators of the effects of parental psychopathology on antisocial behavior in outpatient children and adolescents. Child psychiatry and human development. 2012;43(3):376–92. 10.1007/s10578-011-0272-z [DOI] [PubMed] [Google Scholar]
- 82. Vidair HB, Fichter CN, Kunkle KL, Boccia AS. Targeting parental psychopathology in child anxiety. Child Adolesc Psychiatr Clin N Am. 2012;21(3):669–89. 10.1016/j.chc.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 83. Oliva K, Barker G, Riley C, Bailey MJ, Permezel M, Rice GE, et al. The effect of pre-existing maternal obesity on the placental proteome: two-dimensional difference gel electrophoresis coupled with mass spectrometry. Journal of molecular endocrinology. 2012;48(2):139–49. 10.1530/JME-11-0123 [DOI] [PubMed] [Google Scholar]
- 84. Azzu V, Jastroch M, Divakaruni AS, Brand MD. The regulation and turnover of mitochondrial uncoupling proteins. Biochim Biophys Acta. 2010;1797(6–7):785–91. 10.1016/j.bbabio.2010.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Echtay KS. Mitochondrial uncoupling proteins—what is their physiological role? Free radical biology & medicine. 2007;43(10):1351–71. [DOI] [PubMed] [Google Scholar]
- 86. Sluse FE. Uncoupling proteins: molecular, functional, regulatory, physiological and pathological aspects. Advances in experimental medicine and biology. 2012;942:137–56. 10.1007/978-94-007-2869-1_6 [DOI] [PubMed] [Google Scholar]
- 87. Nicholls TJ, Rorbach J, Minczuk M. Mitochondria: Mitochondrial RNA metabolism and human disease. The international journal of biochemistry & cell biology. 2013. [DOI] [PubMed] [Google Scholar]
- 88. Reinecke F, Smeitink JA, van der Westhuizen FH. OXPHOS gene expression and control in mitochondrial disorders. Biochim Biophys Acta. 2009;1792(12):1113–21. 10.1016/j.bbadis.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 89. Lazarou M, Smith SM, Thorburn DR, Ryan MT, McKenzie M. Assembly of nuclear DNA-encoded subunits into mitochondrial complex IV, and their preferential integration into supercomplex forms in patient mitochondria. The FEBS journal. 2009;276(22):6701–13. 10.1111/j.1742-4658.2009.07384.x [DOI] [PubMed] [Google Scholar]
- 90. Demonacos C, Djordjevic-Markovic R, Tsawdaroglou N, Sekeris CE. The mitochondrion as a primary site of action of glucocorticoids: the interaction of the glucocorticoid receptor with mitochondrial DNA sequences showing partial similarity to the nuclear glucocorticoid responsive elements. J Steroid Biochem Mol Biol. 1995;55(1):43–55. [DOI] [PubMed] [Google Scholar]
- 91. Berdanier CD. Mitochondrial gene expression: influence of nutrients and hormones. Exp Biol Med (Maywood). 2006;231(10):1593–601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The graph shows that the expression of the mitochondrial genes can efficiently be fit into a 5 dimension space (e.g. 5 clusters) without imposing an excessive degree of stress to the dataset.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.