Abstract
The mechanisms by which alcohol drinking promotes addiction in humans and self-administration in rodents remain obscure, but it is well known that alcohol can enhance dopamine (DA) neurotransmission from neurons of the ventral tegmental area (VTA) and increase DA levels within the nucleus accumbens and prefrontal cortex. We recorded from identified DA neuronal cell bodies within ventral midbrain slices prepared from a transgenic mouse line (TH-GFP) using long-term stable extracellular recordings in a variety of locations and carefully mapped the responses to applied ethanol (EtOH). We identified a subset of DA neurons in the medial VTA located within the rostral linear and interfascicular nuclei that fired spontaneously and exhibited a concentration-dependent increase of firing frequency in response to EtOH, with some neurons responsive to as little as 20 mM EtOH. Many of these medial VTA DA neurons were also insensitive to the D2 receptor agonist quinpirole. In contrast, DA neurons in the lateral VTA (located within the parabrachial pigmented and paranigral nuclei) were either unresponsive or responded only to 100 mM EtOH. Typically, these lateral VTA DA cells had very slow firing rates, and all exhibited inhibition by quinpirole via D2 “autoreceptors”. VTA non-DA cells did not show any significant response to low levels of EtOH. These findings are consistent with evidence for heterogeneity among midbrain DA neurons and provide an anatomical and pharmacological distinction between DA neuron sub-populations that will facilitate future mechanistic studies on the actions of EtOH in the VTA.
Keywords: dopamine, VTA, tonic firing, ethanol, alcohol, addiction
INTRODUCTION
Alcohol is certainly the most ancient, and arguably the most widely abused substance in human society. The costs associated with alcoholism and alcohol use disorders are enormous, in terms of economic performance from lost productivity and the human suffering caused by alcohol-associated disease, death and family breakdown. Ethanol (EtOH) is readily self-administered by most mammalian species and is easily studied in rodent models (Crabbe et al., 2011; Carnicella et al., 2014), in which it provides an effective model for addiction, inducing a strong conditioned place preference (Melis et al., 2007) and robust drinking in two-bottle choice paradigms. Most, if not all, drugs of abuse have been shown to activate the mesolimbic reward system to enhance dopamine (DA) transmission in the striatum (Sulzer, 2011), albeit via distinctly different mechanisms. Drugs such as cocaine and amphetamine act in part within the striatum via their effects on the DA transporter of axon terminals. Opiates and benzodiazepines target the inhibitory interneurons in ventral tegmental area (VTA) to disinhibit DA cell firing and indirectly promote DA release (Tan et al., 2010; Luscher and Malenka, 2011). In contrast to these well-characterized drugs of abuse, the manner in which alcohol alters DA physiology remains unclear and controversial (Morikawa and Morrisett, 2010).
There have been many previous studies of alcohol action in VTA in vitro, many of which were quite elegant in their methodology. As a result, numerous synaptic and intrinsic targets for EtOH have been suggested in recent years (Brodie et al., 1990; Okamoto et al., 2006; Nimitvilai et al., 2013). The significance of some of these findings has been difficult to interpret because of the universal observation that very high concentrations of EtOH application have been required to observe responses. Typically 80–100 mM EtOH has been used, a level of the drug that would result in unconsciousness or death in naïve animals, although tolerated in some alcoholics. The question therefore remains as to how the VTA responds to modest levels of EtOH that are typically associated with social intoxication. Indeed, low and moderate concentrations of EtOH (equivalent to several glasses of wine in humans) have been little studied since the early days of the field (Gessa et al., 1985), yet there is no question that these provide more accurate models of early-stage drinking.
It has been suggested by some authors that these difficulties have been amplified by other problems, such as ambiguous identification of DA neurons in VTA (Margolis et al., 2006; Ungless and Grace, 2012). Microdialysis studies in freely-moving rats have demonstrated that low doses of EtOH injection (0.5-g/kg i.p.) preferentially stimulated DA release in the nucleus accumbens (Di Chiara and Imperato, 1985). Furthermore, precise mapping of DA release sites using cyclic voltammetry in vivo after acute EtOH injection (0.5-g/kg injected intravenously) revealed the existence of “hot spots” of EtOH-responsive regions in nucleus accumbens core and shell, as well as clearly unresponsive regions nearby (Robinson et al., 2009). The anatomical basis for the heterogeneity of these EtOH responses is unknown, but a reasonable supposition is that this may originate in the cell bodies in the VTA.
Considerable attention has recently focused on the concept of regional heterogeneity of VTA cells, which had formerly been lumped together as a homogenous population of DA neurons bearing considerable similarity to the neighboring DA cell population in the substantia nigra (SN) pars compacta (Neuhoff et al., 2002; Ungless et al., 2004; Bjorklund and Dunnett, 2007; Lammel et al., 2008; Borgkvist et al., 2011; Lammel et al., 2014; Marinelli and McCutcheon, 2014). Unlike the nigral DA cells, however, the identification of DA cells in VTA by physiological criteria or pharmacology alone appears to be insufficient (Margolis et al., 2006). It is now generally accepted that verification of tyrosine hydroxylase (TH) expression is necessary to confirm DA identity (Fields et al., 2007; Ungless and Grace, 2012).
VTA cells appear to exhibit regional differences in responses to other drugs of abuse, including opioids (Ford et al., 2006; Margolis et al., 2008), nicotine (Ericson et al., 2008; Zhao-Shea et al., 2011), and cocaine (Lammel et al., 2011). Retrograde labeling studies have demonstrated that midline VTA DA cells are most sensitive to cocaine, and that the axons of these DA cells project to the medial shell of NAc and prefrontal cortex (Lammel et al., 2011). It has been suggested that responsiveness to EtOH may also exhibit regional differences (Robinson et al., 2009).
Here we undertook a simple but careful study of the alcohol responses of a large sample of 81 DA neurons in the mouse VTA, in an attempt to locate the cells that might be most sensitive to EtOH, in order to facilitate future characterization of target molecules. We selected a technologically simple, yet highly stable recording technique (“loose-patch” recording of action potentials) that obviates any problems associated with cytoplasmic dialysis during long recordings (Carta et al., 2004) and prepared midbrain slices from a transgenic mouse line (TH-GFP) expressing green fluorescent protein under the TH promoter (Sawamoto et al., 2001) in order to facilitate the identification of the DA neuron phenotype. Our results show that EtOH can accelerate DA neuron firing of a subpopulation of medial VTA DA neurons.
EXPERIMENTAL PROCEDURES
All animal procedures were performed following NIH guidelines, and were approved by the Institutional Animal Care and Use Committee at the Columbia University Medical Center. Wild-type C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, MA, USA). Wild-type and TH-GFP mice, in which neuronal GFP expression showed >87% co-localization with TH immunoreactivity (Sawamoto et al., 2001), were sacrificed at 3–12 weeks of age, and their brains removed for acute slice recordings.
Electrophysiological recordings in brain slice
Coronal midbrain slices (250- μm-thick) were prepared using a vibratome (Leica VT1200; Nussloch, Germany) with VTA between bregma −3.0 to −3.8 mm (primarily near bregma −3.5 mm). Brains were submerged in ice-cold cutting solution containing (in mM): 100 glucose, 75 NaCl, 26 NaHCO3, 2.5 KCl, 2 MgCl2-6H20, 1.25 NaH2PO4-6H20, and 0.7 CaCl2. Slices were allowed to recover in the solution for 30 min at 34 °C and then transferred to a recording solution (artificial cerebrospinal fluid, ACSF) containing (in mM): 119 NaCl, 26.2 NaHCO3, 2.4 CaCl2, 1.8 KCl, 1.2 MgCl2-6H20, 1.0 NaH2PO4-6H20, and 10 glucose. The recording chamber temperature was maintained at 32 °C (± 2 °C) with an in-line heater and temperature controller (Warner Instruments, Hamden, CT, USA).
Extracellular “on-cell” recordings were obtained with pipettes (tip resistance 2–4 MΩ) pulled from borosilicate glass (G150F-4, Warner Instruments) on a P-97 Flaming-Brown micropipette puller (Sutter Instruments) and filled with ACSF solution. Seal resistances ranged from 10 MΩ to 1 GΩ, but most recordings were between 10 and −30 MΩ and were monitored in voltage-clamp mode at a command potential of 0 mV throughout the recordings. In a subset of experiments, whole-cell patch clamp recordings were performed with pipettes (tip resistance 3–4 MΩ) filled with internal solution containing (in mM): 115 K-gluconate, 20 KCl, 10 HEPES, 2 MgCl2, 2 ATP-Mg, 2 ATP-Na2, 0.3 GTP-Na, (pH=7.3, ~290 mOsm).
DA neurons expressing GFP were visualized under a 40x water immersion objective by fluorescence and DIC optics (Olympus, Waltham, MA, USA). Voltage-clamp and whole cell current-clamp recordings were performed with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA) and digitized at 10 kHz with a Digidata 1332 (Molecular Devices). Data were acquired using Clampex 8 software (Molecular Devices). For whole-cell patch-clamp recordings, the resting membrane potential Vm, spontaneous firing frequency (FF), and input resistance (measured by −100 pA, 100 ms duration hyperpolarizing pulses) were monitored throughout the recording. Only cells in which a baseline FF was stable for at least 5 min were retained and analyzed for tonic firing. The coefficient of variation (CV) of interspike intervals (ISIs) was calculated as CV=(SD of ISIs)/mean ISI. The resting Vm in these neurons was arbitrarily measured at the trough of the after-hyperpolarization. All drugs were purchased from Sigma Aldrich (St. Louis, MO, USA) and Tocris Bioscience (Minneapolis, MN, USA) unless otherwise specified.
Imaging of TH-gfp expression
Acute coronal slices (prepared as above) were placed in the bath chamber of a multiphoton microscope and continuously perfused with ACSF bubbled with carbogen. VTA cells were visualized at >30-μm depth in the slice under a 10× objective using a Prairie Ultima Multiphoton Microscopy System (Prairie Technologies, Middleton, WI, USA). TH-GFP was excited at 900 nm with a Mai Tai laser and visualized using an emission range of 440–500 nm. Images were acquired with Prairie v 4.0 software scanning 10-μm steps through a z-stack of 200 μm, and image stacks were flattened using Image J software and tiled in Photoshop CS3 (Adobe).
For Fig. 1B, acute brain slices (150-μm-thick) were cut on a vibratome as described above and stained with 4′, 6-diamidino-2-phenylindole (DAPI) (Calbiochem, Billerica, MA, USA) diluted at 1:5000 in phosphate buffered saline (PBS) (Sigma) for 5 minutes. Slices were mounted on glass slides for imaging with a confocal microscope (Nikon Eclipse Ti) under 10× and 20× objective lenses. Images were acquired with NIS Elements software.
Fig. 1.
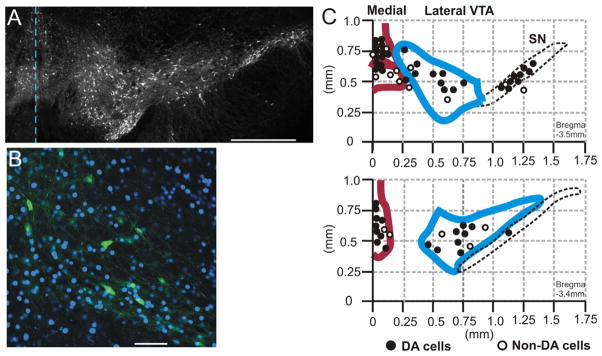
Recording loci within the ventral midbrain. (A) Acute coronal brain slice (−3.4 mm from bregma) of TH-GFP mouse as prepared for electrophysiological recording under fluorescence. The broken line indicates the midline. GFP expression in living DA neurons can be observed in neurons of the medial and lateral VTA, and SN. Scale bar=500 μm. (B) Lateral VTA from TH-GFP brain slice with TH-GFP+ cells (green) and nuclei stained with DAPI (blue). Scale bar=100 μm. (C) Map of recording locations in coronal midbrain slices. Top: A caudal slice (posterior to bregma −3.5 mm). Bottom: A more rostral slice (at bregma −3.4 mm). Recordings were in the medial VTA (red), lateral VTA (blue), and the substantia nigra pars compacta (black dotted line). Filled circles represent TH+putative DA cells, and open circles indicate TH-putative non-DA cells. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Statistics
Statistical analysis was performed in Prism 6 (GraphPad Software, La Jolla, CA, USA). The distribution of firing frequencies did not always pass the Kolmogorov–Smirnov normality test, and a non-parametric Mann– Whitney test was used to compare spontaneous FF distributions (in Fig. 2). A non-parametric Mann–Whitney test was used to compare control recordings with EtOH-treated recordings as% change in FF above baseline (mean ± SE). Correlation coefficients were calculated by a Pearson analysis. Significance level was p<0.05.
Fig. 2.
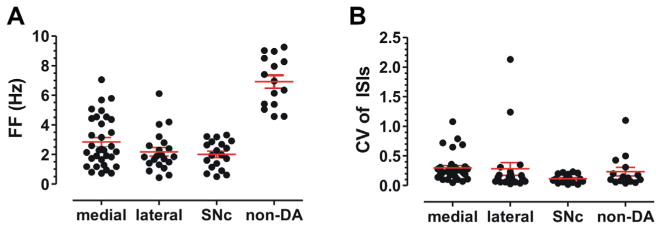
Spontaneous firing rates of VTA DA and non-DA neurons. (A) Midline VTA DA cells fired at a mean frequency of 2.8 ± 0.3 Hz (n=33), while lateral VTA DA cells fired at 2.2 ± 0.3 Hz (n=21), and substantia nigra DA cells fired at 2.0 ± 0.2 Hz (n=19). This is in contrast to a population identified as non-DA neurons which fired at a mean frequency of 6.9 ± 0.4 Hz (n=15). (B) CV of ISIs was more variable in medial VTA (mean CV=0.3) and lateral VTA (mean CV=0.3) than in SNc (mean CV=0.1).
RESULTS
Spontaneous firing pattern properties of VTA DA and non-DA neurons
Electrical extracellular recordings were made from 81 VTA DA neurons identified by TH-GFP fluorescence. To obtain an unbiased representation of all VTA neurons, the entire VTA region was sampled, as shown in Fig. 1. We recorded from “medial VTA” cells within 250 μm of the midline, including the rostral linear nucleus (RLi) and interfascicular (IF) nucleus (red outline in Fig. 1C), and from the “lateral VTA” consisting of the parabrachial pigmented (PBP) nucleus, paranigral nucleus (PN), and rostral part of VTA (VTAR) (blue outline in Fig. 1C). DA neurons in VTA slices were spontaneously active, with a mean FF of 2.3 ± 0.2 Hz (n=81), and the measure of firing regularity (expressed as the CV in inter-spike intervals) was 0.28.
Within the VTA, DA neurons of medial VTA showed spontaneous firing rates similar to lateral VTA and SN DA cells. As shown in Fig. 2A, medial VTA DA cells fired at mean FF of 2.8 ± 0.3 Hz SEM (n=33) versus lateral VTA DA cells that fired at 2.2 ± 0.3 Hz (n=21). SN DA cells fired at a mean frequency of 2.0 ± 0.2 Hz (n=19). The FF of these three populations of DA neurons was not statistically different from each other, as FF was somewhat heterogeneous even within these subgroups (p>0.05, Mann–Whitney test). In contrast, VTA non-DA neurons fired at a mean frequency of 6.9 ± 0.4 Hz (n=15) with a CV of ISI=0.23 ± 0.07. The FF of non-DA neurons was significantly faster than all DA cell groups (p<0.0001).
Consistent with previous in vitro studies (Neuhoff et al., 2002; Margolis et al., 2006; Margolis et al., 2012), the midline VTA DA neurons (CV of ISI=0.30 ± 0.04, n=32) and lateral VTA DA neurons (CV of ISI=0.28 ± 0.11, n=21) exhibited significantly more variability in FF than the SNc DA neurons (CV of ISI=0.12 ± 0.02, n=18) (p<0.0001, Mann–Whitney test), as shown in Fig. 2B. Thus, VTA neurons appear to have unique spontaneous firing properties distinct from nigral DA neurons.
To determine whether DA neuron firing frequencies were stable over long duration extracellular recording conditions, we recorded from a subset of VTA DA cells for 30 min during saline (ACSF) application. The firing rates of these TH-GFP-positive cells were measured at the time points 13, 22, and 30 min after baseline (Fig. 3A). A representative recording of stable firing rate is shown in Fig. 3B, with a baseline FF of 4.5 Hz that showed a maximal 12% increase after 22 min. For the entire VTA DA population, we observed a mean increase in FF of 13 ± 9%, 18 ± 10%, and 23 ± 18%, respectively, after 13, 22, and 30 min (n=27), as shown in Fig. 3C, D. These firing rates were remarkably stable, and any changes observed were not statistically significant (p>0.05).
Fig. 3.
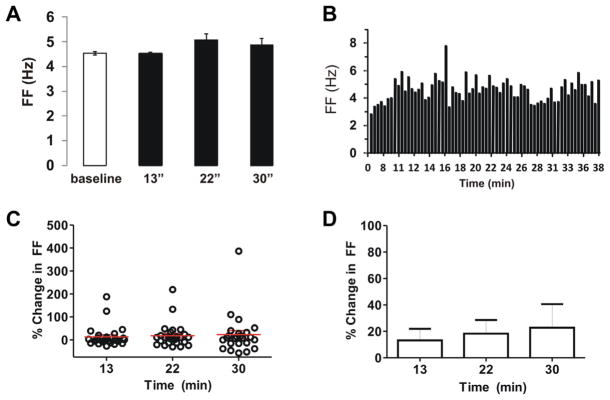
Control recordings show stability of firing rate in extracellular “on-cell” patch mode. (A) Representative trace of DA neuron in medial VTA (quinpirole-sensitive cell) during “on-cell” recording. Baseline FF was 4.5 Hz, with changes of −0.4%, 12%, and 7% after 13, 22, and 30 min, respectively. (B) Spike ratemeter of same cell shows FF over 40 min. (C, D) Population data of FF changes for DA neurons monitored at 13, 22, and 30 min after baseline recording. The mean increases in FF were 13%, 18%, and 23%, respectively (n=27). These changes were not significant (p>0.05).
A subset of identified midbrain DA neurons responds to EtOH
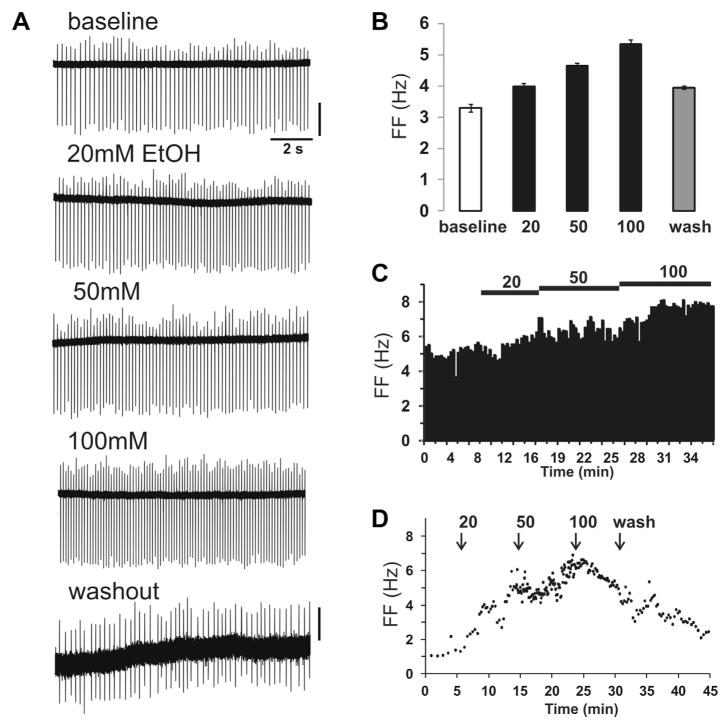
Responses to acute EtOH (bath-applied) were measured in VTA DA neurons and some of these neurons exhibited a concentration-dependent increase in FF. Fig. 4A shows a representative recording from a medial VTA neuron in the IF nucleus (TH-GFP+, quinpirole-sensitive cell) that displayed a concentration-dependent increase of firing in response to EtOH. Spontaneous FF of 3.3 Hz increased by 21%, 41%, and 62% with increasing [EtOH] (20, 50, and 100 mM), respectively (Fig. 4B, C). These changes were reversible upon washout of EtOH. Other DA neurons showed even more robust changes with EtOH, showing an increase of FF by 200%, 329%, and 299% at 20, 50, and 100 mM concentrations, respectively (Fig. 4D).
Fig. 4.
Acute EtOH responses in VTA DA cells. (A) Representative trace of extracellular recording from DA neuron in medial VTA (IF nucleus, quinpirole-sensitive cell) with a baseline FF 3.3 Hz. (Scale bars, top: 40 pA, bottom: 20 pA). (B) This cell had a robust concentration-dependent increase of FF (21%, 41%, and 62%) with increasing [EtOH] (20, 50, 100 mM), respectively. Washout completely reversed this effect. (C) Spike ratemeter showing entire recording from baseline to EtOH application. (D) Spontaneous firing of a medial VTA DA cell with baseline FF of 1.3 Hz. EtOH increased the FF to 3.9 Hz at 20 mM, 5.5 Hz at 50 mM, and 5.1 Hz at 100 mM. Washout restored FF to 2.3 Hz but was incomplete after 15 min.
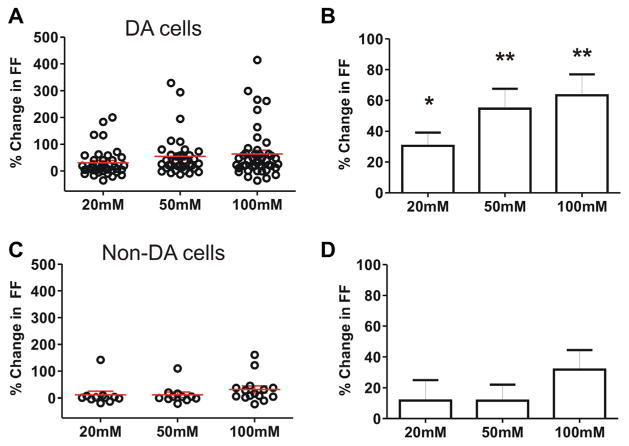
For the entire population of VTA DA neurons, the mean increase in FF was 31 ± 9% at 20 mM (n=38), 55 ± 13% at 50 mM (n=35), and 63 ± 14% at 100 mM EtOH (n=46) (Fig. 5A, B). All three doses of EtOH were significant compared to control recordings in Fig. 3 (at 20 mM EtOH p<0.05; at 50 and 100 mM EtOH p<0.01 Mann–Whitney test). There was considerable variability in the responses of VTA DA cells, and half of the cells showed less than 40% change in FF at 100 mM EtOH. Several DA cells showed a robust increase of firing rate in EtOH (maximum: 200% increase at 20 mM, and 415% increase at 100 mM) as illustrated by Fig. 4D, while others showed a reduced firing rate (minimum: 35% decrease at 100 mM). It thus appeared that some DA cells in naïve mice are uniquely sensitive to acute EtOH at low concentrations, while many DA cells in VTA do not react at all to EtOH.
Fig. 5.
Population data for responses of all VTA neurons to acute EtOH VTA DA and non-DA cells were tested for EtOH responses at three concentrations (20, 50, and 100 mM). (A, B) For TH-positive DA cells, the mean increase in FF was 31% at 20 mM (n=38, p<0.05), 55% at 50 mM (n=35, p<0.01), and 64% at 100 mM (n=46, p<0.01). (C, D) TH-negative neurons had a mean increase in FF of 12% at 20 mM (n=11), 12% at 50 mM (n=11), and 32% at 100 mM (n=15).
Reversal of EtOH effects on DA cells occurred after 15–30 min in 18 out of 25 washout recordings, such that FF resumed within 30% of baseline. Complete washout of EtOH was not possible for all long-duration recordings (60 min) due to instability of seal resistance.
EtOH is a highly membrane-permeable substance, and is known to equilibrate fully and rapidly across biological cell membranes, and so does not create an osmotic gradient. We nonetheless tested whether an increase in extracellular osmolarity might alter firing rates of DA neurons. The osmolarity of our ACSF was measured as 295 mOsm (0 mM EtOH), 316 mOsm with 20 mM EtOH added, 346 mOsm with 50 mM EtOH added, and 394 mOsm with 100 mM EtOH added (Fiske osmometer, Norwood, MA, USA). In a series of control experiments, we added sucrose to ACSF to concentrations of 12, 48, and 94 mM, respectively, corresponding to the same osmotic equivalent as 20, 50, and 100 mM EtOH. DA neuron firing rates showed a mean change in FF of 0.7 ± 7.0% (n=8) with 48 mM sucrose (346 mOsm), and a −25 ± 19% decrease in FF at 94 mM sucrose (n=2). Firing rates were thus unchanged or slower when sucrose was added. We conclude that the FF increases observed in VTA in response to EtOH were not due to changes in osmolarity during the experiment.
We measured the FF of DA cells by loose-patch extracellular recordings, using both the voltage-clamp (command voltage=0 mV) and current-clamp (holding current=0 pA) configurations to compare baseline FF and EtOH responses, and determined that recording mode did not alter these parameters (p>0.05, n=66 voltage-clamp; n=25 current-clamp). In addition, to further determine whether recording configuration has any impact on FF of DA cells, we performed whole-cell current-clamp recordings from midbrain DA cells. The resting Vm of VTA DA cells was −54 ± 3 mV (n=7) and Vm for SNc DA cells was −56 ± 2 mV (n=11). VTA cells showed spontaneous firing with a mean FF of 2.6 ± 0.5 Hz (n=6), and SNc cells had mean FF of 2.0 ± 0.3 Hz (n=10). These values were similar to our extracellular recordings (e.g., voltage-clamped at a holding potential of 0 mV) from Fig. 2, and suggest that resting membrane properties were unaltered by the recording configuration.
We measured the amplitude of the “sag” in the electrotonic potential elicited by a hyperpolarizing current injection. VTA neurons recorded in lateral and medial regions had considerable variability in sag amplitudes during a −200 pA injection, with a mean of 15 ± 5 mV, as compared to SNc neurons with mean of 20 ± 2 mV. As noted by others, the VTA shows greater heterogeneity in resting and active membrane properties than the SNc, and this consideration may be fundamental in understanding how EtOH affects these DA cell regions.
Based on studies suggesting that VTA DA neurons with elevated basal excitability have stronger responses to cocaine (Lammel et al., 2011), we tested whether the baseline FF of DA neurons were correlated with EtOH responses. Baseline FF of VTA DA cells was plotted versus changes in FF with EtOH at 20 mM (Pearson r=−0.25), 50 mM (Pearson r=−0.26), and 100 mM (Pearson r=−0.20). There was no significant correlation between FF and EtOH responses. This suggests that the mechanisms that regulate spontaneous firing rates may be distinct from those mediating EtOH responsiveness.
VTA non-DA neurons were unresponsive to low doses of EtOH
We then tested the responses of VTA non-DA neurons to acute EtOH in vitro. These non-DA neurons (putative GABA) were identified by lack of TH-GFP under fluorescence in TH-GFP mice (in which GFP expression had >87% co-localization with TH immunoreactivity) (Sawamoto et al., 2001). Since some GFP-negative neurons may be dopaminergic, additional criteria for identification included: (a) lack of response to quinpirole (1 μM, bath), and (b) spontaneous FF >4 Hz. Extracellular recordings were obtained from non-DA cells, and we tested EtOH responses. Baseline spontaneous FF of non-DA cells in VTA was 6.9 ± 0.4 Hz (n=15). Non-DA neurons exhibited small changes in FF, with a mean increase in FF of 12 ± 13% at 20 mM (n=11), 12 ± 10% at 50 mM (n=11), and 32 ± 13% at 100 mM EtOH (n=15), as shown in Fig. 5C, D. These changes were not significantly different from controls (p>0.05). Thus, unlike the DA neurons in VTA, VTA non-DA neurons were not sensitive to low amounts of EtOH and did not exhibit a concentration- dependent response. These observations also suggested that FF was very stable over long recording times.
Medial and lateral VTA DA cells differ in sensitivity to EtOH
We postulated that the VTA DA cells most responsive to EtOH may be located at the midline, analogous to the observations made with cocaine (Lammel et al., 2011). To test this hypothesis, we recorded cells at VTA midline and tested responses to three concentrations of EtOH. Medial VTA neurons were located in the RLi and IF nuclei within 250 μM of the midline, as outlined in Fig. 1B.
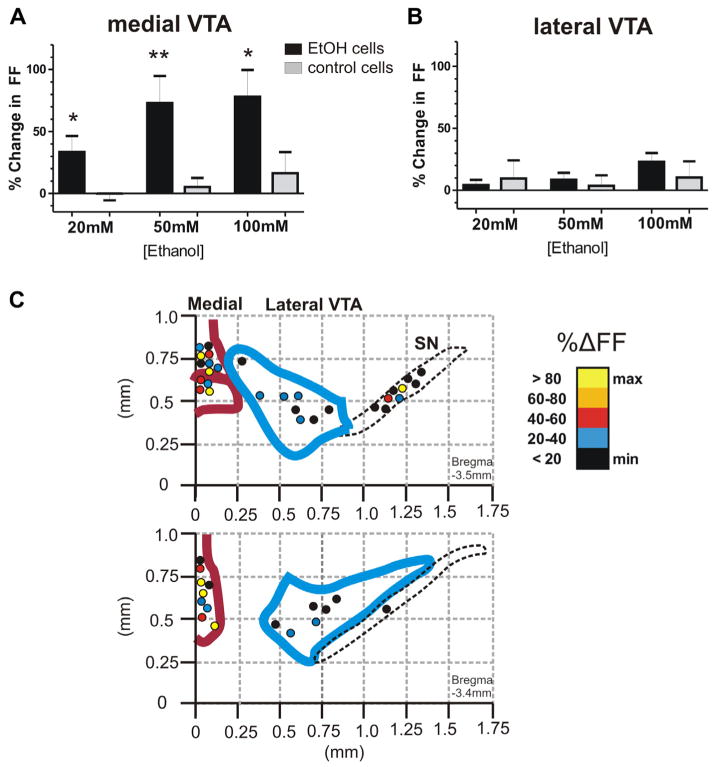
The mean increase in FF was 34 ± 13% at 20 mM (p<0.05), 73 ± 22% at 50 mM (p<0.01), and 78 ± 22% at 100 mM EtOH (p<0.05, n=19), as summarized in Fig. 6A. All doses of EtOH significantly increased firing rates compared to medial VTA control cells with no EtOH added. Of the midline DA cells recorded, (n=7/19) 37% showed >40% change in FF at 20 mM EtOH, (n=10/19) 53% responded at 50 mM, and (n=11/19) 58% responded at 100 mM. Thus, over a third to half of all DA cells located at the midline VTA nuclei respond to EtOH with greater than a 40% increase in FF. In the medial VTA, one third of cells tested with quinpirole (1 μM) were unresponsive (n=9/27). The medial DA cells most strongly excited by EtOH (>40% increase of FF) included a mix of quinpirole-sensitive (n=6/10), and insensitive cells (n=4/10).
Fig. 6.
Medial and lateral VTA DA cells differ in sensitivity to ethanol. Medial VTA and lateral VTA DA cells were tested for responses to three doses of EtOH. (A) For medial VTA, the mean increase in FF with EtOH (black bars) was 34 ± 13% at 20 mM, 73 ± 22% at 50 mM, and 78 ± 22% at 100 mM (n=19). These changes were significant (p<0.05 at 20 and 100 mM, p<0.01 at 50 mM EtOH) as compared to medial VTA control neurons without EtOH (gray bars) at corresponding time points during the recording. (B) In the lateral VTA, the mean increase in FF with EtOH was 4 ± 4% at 20 mM, 8 ± 6% at 50 mM, and 23 ± 7% at 100 mM (n=11). These changes were not significant compared to lateral VTA control neurons without EtOH (gray bars). (C) Map of recording locations from TH+DA cells in the VTA and SN. Top: A caudal slice (posterior to bregma −3.5 mm). Bottom: A more rostral slice (at bregma −3.4 mm). Color scale represents the% change in FF with EtOH (50 mM).
In comparison, DA cells in the lateral VTA (PBP nucleus) exhibited a mean increase in FF of 4 ± 4% at 20 mM, 8 ± 6% at 50 mM, and 23 ± 7% at 100 mM EtOH (n=13), as shown in Fig. 6B. These changes were not significant (p>0.05). In the lateral VTA, only (n=1/12) 8% showed >40% response in FF at 20 mM, (n=0/10) none at 50 mM, and (n=3/16) 19% at 100 mM EtOH. In the lateral VTA, all DA cells tested with quinpirole showed sensitivity to this agonist (n=14/14).
SNc DA cells were the least responsive to EtOH, and nearly half of these cells were in fact slightly inhibited by high levels of EtOH (100 mM) (n=4/10). SNc cells exhibited a mean change in FF of 14 ± 9% at 20 mM, 29 ± 22% at 50 mM, and 21 ± 29% at 100 mM EtOH (n=10). These changes were not significant (p>0.05), and did not appear to be concentration-dependent.
DISCUSSION
We report that pharmacologically relevant levels of EtOH (20 mM corresponding to a blood alcohol level of 0.09, or 3–4 glasses of wine for humans) increased neuronal firing in a subset of medial VTA DA neurons. VTA neurons in vivo receive strong glutamatergic and cholinergic drive from brainstem laterodorsal (LDTg) and pedunculopontine (PPTg) tegmental nuclei, and inhibitory drive from rostromedial (RmTg) tegmentum, which has been suggested to regulate DA neurons responses to drugs of abuse (Lodge and Grace, 2006; Geisler and Wise, 2008; Sesack and Grace, 2010; Lecca et al., 2012; Watabe-Uchida et al., 2012). The strong response to EtOH we find in a subset of medial VTA neurons is however present in coronal ventral midbrain slices that are largely deafferented from excitatory and inhibitory inputs. In contrast to in vivo recordings in which SN GABA cells were potently inhibited by EtOH in vivo (Mereu and Gessa, 1985), we did not observe significant inhibitory or excitatory effects of EtOH on non-DA cell firing in our brain slices. A possible contribution of synaptic inputs to the EtOH effect in vitro remains to be determined, including the possibility of transmitter release from excitatory and inhibitory terminals.
The identification of a specific subset of EtOH responsive medial VTA neurons contrasts with some previous studies. In particular, Ih-containing DA cells of the VTA in vitro have to date been reported to exhibit minimal responses to lower concentrations of EtOH. Using extracellular recordings similar to ours, Brodie and colleagues reported that 20 mM EtOH increased FF by less than 20% above baseline (Brodie et al., 1990). Okamoto et al. showed that 25 mM EtOH only caused a 5% increase in one of five DA cells tested, while 50 and 100 mM EtOH increased FF by 19% and 18% above baseline, respectively (Okamoto et al., 2006). Note however that this study included only VTA DA cells that resemble nigral DA cells (calbindin-negative, Ih – positive cells) and that they reported no difference between SNc and VTA responses to EtOH. In contrast, our study revealed a 33% increase at 20 mM EtOH in VTA. If Ih were indeed the primary target underlying the mechanism of EtOH excitation, SNc cells would be expected to show the largest response, but we find that SNc DA cells are the least EtOH-responsive subset of ventral midbrain DA neurons.
Two groups have reported that Ih regulates FF in VTA DA cells, as the Ih inhibitor ZD7288 (30 μM) decreased spontaneous firing by −33% (McDaid et al., 2008), and −28% (Okamoto et al., 2006). Other studies, however, elicited different findings: Neuhoff et al. found in VTA DA neurons that express Ih that ZD7288 (30 μM) had no effect on pacemaking (Neuhoff et al., 2002). Khaliq and Bean, moreover, demonstrated that 3 mM CsCl did not stop or slow pacemaking in medial VTA neurons that express Ih from TH-GFP mice (Khaliq and Bean, 2010). These contrasting results may reflect the heterogeneity of DA neurons, and the significance of Ih for pacemaking in VTA DA neurons remains unresolved at this time.
The robust responses to EtOH reported here were recorded from identified TH-GFP-positive DA neurons. These recordings included a large number of medial VTA DA cells reported to be Ih – negative (Neuhoff et al., 2002; Lammel et al., 2008) and highly sensitive to acute cocaine (Lammel et al., 2011), and are thought to project to the medial shell of nucleus accumbens and PFC. Of the medial VTA DA neurons that we identified as high responders to EtOH, one third were quinpirole-insensitive and may therefore be PFC-projecting cells that are likely to have been missed in previous studies of EtOH response. Our data thus suggest that EtOH sensitivity may be mediated, at least in part, by a subset of midbrain DA neurons that lack D2 receptors (Ford, 2014).
The DA neurons of the medial VTA appear to be unique in their high level of expression of the synaptic vesicle glutamate transporter vGluT2 (Hnasko et al., 2010; Yamaguchi et al., 2011; Gorelova et al., 2012; Li et al., 2013; Trudeau et al., 2014). Our recordings were primarily in posterior VTA at the level of the prominent fibers of the oculomotor nerve (Bregma 3.52 mm, Interaural 0.28 mm) (Paxinos and Franklin, 2001). Distinct subnuclei within the VTA have been described by immunostaining and are readily identifiable (Del-Fava et al., 2007; Ferreira et al., 2008; Fu et al., 2012). Differential responsiveness of anterior and posterior VTA has also been reported to impact alcohol drinking behavior (Rodd et al., 2005; Melon and Boehm, 2011), and the idea that these represent distinct populations of DA cells warrants further study in vitro.
In summary, our results identify a subset of DA neurons that are activated by levels of alcohol attained during social intoxication. These observations may have future impact for the treatment of alcoholic patients by addressing the relevant neural targets for the initial rewarding effects prior to addiction onset. There are currently no widely effective therapies for treating alcoholism, and improved clinical approaches may be possible in future with the advent of novel therapies using drugs or gene therapy approaches targeted to the sub-populations within the medial VTA to interact with the (as yet unknown) molecular targets of EtOH within the midbrain.
Acknowledgments
We thank Dr. Daniela Pereira for assistance in multiphoton imaging and Dr. Jessica Wu for image analysis. We especially thank Vanessa Morales and Candace Castagna for their excellent technical support. We also thank Dr. Anders Borgkvist for his valuable feedback on the manuscript. This work was supported in part by NIH/National Institute on Alcohol Abuse and Alcoholism grant AA019801 to NLH and DS, the Parkinson’s and JPB Foundations to DS, NIH/National Institute on Drug Abuse grants DA07418 and DA10154 to DS, and by NIH fellowship 5T32DA016224 to AM. LMP was funded by an “Atracció de talent” grant from the Universitat de València.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- CV
coefficient of variation
- DA
dopamine
- EtOH
ethanol
- FF
firing frequency
- GABA
gamma-aminobutyric acid
- HEPES
(4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid
- IF
interfascicular nucleus of VTA
- Ih
hyperpolarization-activated non-selective cation current
- ISI
interspike interval
- PBP
parabrachial pigmented nucleus of VTA
- RLi
rostral linear nucleus of VTA
- TH
tyrosine hydroxylase
- TH-GFP
transgenic mouse line
- SN
substantia nigra
- Vm
membrane potential
- VTA
ventral tegmental area
Footnotes
AUTHOR CONTRIBUTIONS
NLH, DS and AM: conception and experimental design, data interpretation; AM and LMP: performed electrophysiology experiments and data analysis; EMA: confocal imaging of TH-gfp mice; AM, DS and NLH: writing of the manuscript.
References
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Mrejeru A, Sulzer D. Multiple personalities in the ventral tegmental area. Neuron. 2011;70:803–805. doi: 10.1016/j.neuron.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del-Fava F, Hasue RH, Ferreira JG, Shammah-Lagnado SJ. Efferent connections of the rostral linear nucleus of the ventral tegmental area in the rat. Neuroscience. 2007;145:1059–1076. doi: 10.1016/j.neuroscience.2006.12.039. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol. 1985;115:131–132. doi: 10.1016/0014-2999(85)90598-9. [DOI] [PubMed] [Google Scholar]
- Ericson M, Lof E, Stomberg R, Chau P, Soderpalm B. Nicotinic acetylcholine receptors in the anterior, but not posterior, ventral tegmental area mediate ethanol-induced elevation of accumbal dopamine levels. J Pharmacol Exp Ther. 2008;326:76–82. doi: 10.1124/jpet.108.137489. [DOI] [PubMed] [Google Scholar]
- Ferreira JG, Del-Fava F, Hasue RH, Shammah-Lagnado SJ. Organization of ventral tegmental area projections to the ventral tegmental area-nigral complex in the rat. Neuroscience. 2008;153:196–213. doi: 10.1016/j.neuroscience.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282C:13–22. doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Yuan Y, Halliday G, Rusznak Z, Watson C, Paxinos G. A cytoarchitectonic and chemoarchitectonic analysis of the dopamine cell groups in the substantia nigra, ventral tegmental area, and retrorubral field in the mouse. Brain Struct Funct. 2012;217:591–612. doi: 10.1007/s00429-011-0349-2. [DOI] [PubMed] [Google Scholar]
- Geisler S, Wise RA. Functional implications of glutamatergic projections to the ventral tegmental area. Rev Neurosci. 2008;19:227–244. doi: 10.1515/revneuro.2008.19.4-5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Mulholland PJ, Chandler LJ, Seamans JK. The glutamatergic component of the mesocortical pathway emanating from different subregions of the ventral midbrain. Cereb Cortex. 2012;22:327–336. doi: 10.1093/cercor/bhr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Bean BP. Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. J Neurosci. 2010;30:7401–7413. doi: 10.1523/JNEUROSCI.0143-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76(Pt B):351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology. 2012;37:1164–1176. doi: 10.1038/npp.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang HL, Morales M. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct Funct. 2013;218:1159–1176. doi: 10.1007/s00429-012-0452-z. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008;28:12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Toy B, Himmels P, Morales M, Fields HL. Identification of rat ventral tegmental area GABAergic neurons. PLoS One. 2012;7:e42365. doi: 10.1371/journal.pone.0042365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, McCutcheon JE. Heterogeneity of dopamine neuron activity across traits and states. Neuroscience. 2014;282C:176–197. doi: 10.1016/j.neuroscience.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, McElvain MA, Brodie MS. Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: involvement of barium-sensitive potassium currents. J Neurophysiol. 2008;100:1202–1210. doi: 10.1152/jn.00994.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Enrico P, Peana AT, Diana M. Acetaldehyde mediates alcohol activation of the mesolimbic dopamine system. Eur J Neurosci. 2007;26:2824–2833. doi: 10.1111/j.1460-9568.2007.05887.x. [DOI] [PubMed] [Google Scholar]
- Melon LC, Boehm SL., 2nd GABAA receptors in the posterior, but not anterior, ventral tegmental area mediate Ro15-4513- induced attenuation of binge-like ethanol consumption in C57BL/6J female mice. Behav Brain Res. 2011;220:230–237. doi: 10.1016/j.bbr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu G, Gessa GL. Low doses of ethanol inhibit the firing of neurons in the substantia nigra, pars reticulata: a GABAergic effect? Brain Res. 1985;360:325–330. doi: 10.1016/0006-8993(85)91249-1. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Morrisett RA. Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol. 2010;91:235–288. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Arora DS, You C, McElvain M, Brodie MS. Phorbol ester reduces ethanol excitation of dopaminergic neurons of the ventral tegmental area: involvement of protein kinase C theta. Front Integr Neurosci. 2013;7:96. doi: 10.3389/fnint.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA., USA: Academic Press; 2001. [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin Exp Res. 2009;33:1187–1196. doi: 10.1111/j.1530-0277.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ. Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. J Pharmacol Exp Ther. 2005;315:648–657. doi: 10.1124/jpet.105.084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K, Nakao N, Kobayashi K, Matsushita N, Takahashi H, Kakishita K, Yamamoto A, Yoshizaki T, Terashima T, Murakami F, Itakura T, Okano H. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci U S A. 2001;98:6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Luscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Hnasko TS, Wallen-Mackenzie A, Morales M, Rayport S, Sulzer D. The multilingual nature of dopamine neurons. Prog Brain Res. 2014;211:141–164. doi: 10.1016/B978-0-444-63425-2.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35:422–430. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, McIntosh JM, Grady SR, Marks MJ, Gardner PD, Tapper AR. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology. 2011;36:1021–1032. doi: 10.1038/npp.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]