Abstract
Physical activity is predictive of better cognitive performance and lower risk of Alzheimer’s disease (AD). The apolipoprotein E gene (APOE) is a susceptibility gene for AD with the e4 allele being associated with a greater risk of AD. Cross-sectional and prospective research shows that physical activity is predictive of better cognitive performance for those at greater genetic risk for AD. However, the moderating role of APOE on the effects of a physical activity intervention on cognitive performance has not been examined. The purpose of this manuscript is to justify the need for such research and to describe the design, methods, and recruitment tactics used in the conductance of a study designed to provide insight as to the extent to which cognitive benefits resulting from an 8-month physical activity program are differentiated by ApoEe4 status. The effectiveness of the recruitment strategies and the feasibility of recruiting ApoE e4 carriers are discussed.
Keywords: exercise, cognition, aging, brain-derived neurotrophic factor, Alzheimer’s Disease, apolipoprotein E, ApoE
Introduction
The global prevalence of Alzheimer’s disease (AD) is predicted to reach 65.7 million by 2030 (Alzheimer's Disease International, 2009). Despite worldwide research efforts, a cure for AD has not been identified. Thus, it is critical to identify preventive strategies that reduce the risk of or delay the onset of AD (Emery, 2011). This has important public health implications because a therapy that delays the development of AD by 5 years could reduce its risk by 50% (Brookmeyer, Gray, & Kawas, 1998). Physical activity holds promise as a behavioral therapy that benefits cognitive performance. Non-experimental prospective studies have shown that physical activity is associated with better cognitive performance and a decreased risk of dementia (Abbott et al., 2004; Hamer & Chida, 2009; J. M. Kim et al., 2010; Lindsay et al., 2002). Quasi-experimental and experimental evidence indicates that participation in physical activity improves cognitive performance for samples of cognitively normal adults (Angevaren, Aufdemkampe, Verhaar, Aleman, & Vanhees, 2008; Colcombe & Kramer, 2003; Heyn, Abreu, & Ottenbacher, 2004), patients with mild cognitive impairment (MCI) (Baker et al., 2010), and persons with memory complaints (Lautenschlager et al., 2008). Thus the extant literature supports the potential role of physical activity in the maintenance of cognitive performance.
A question of particular interest relative to AD is if the effects of physical activity on cognitive performance differ as a function of a person’s genetic risk for AD. There is evidence that this is the case with respect to one particular gene that is predictive of AD. Apolipoprotein E (ApoE) is a strong susceptibility gene for AD (Bu, 2009; Cedazo-Minguez, 2007; Farrer et al., 1997; Gomez-Isla et al., 1996; J. Kim, Basak, & Holtzman, 2009; Myers et al., 1996). Compared with non-carriers, persons with one ApoE epsilon 4 (e4) allele have 3-4 times (Bertram & Tanzi, 2008; Kapur, Sharad, Kapoor, & Bala, 2006) and those with two ApoE e4 alleles have 5-18 times greater risk of AD (Alzheimer's Association Working Group, 1996). Additionally, differences in preclinical biomarkers of AD (e.g., neurofibrillary tangles) as a function of ApoE genotype appear in young to middle age (Kok et al., 2009; Morris et al., 2010). Intriguingly, results from cross-sectional (Deeny et al., 2008; Etnier et al., 2007) and non-experimental prospective studies (Niti, Yap, Kua, Tan, & Ng, 2008; Podewils et al., 2005; Rovio et al., 2005; Schuit, Feskens, Launer, & Kromhout, 2001) suggest that ApoE moderates the relationship between physical activity or aerobic fitness and cognition, with larger benefits of physical activity typically reported for ApoEe4 carriers (Deeny et al., 2008; Etnier, Sibley, Caselli, & Tessier, 2003; Niti et al., 2008; Rovio et al., 2005; Schuit et al., 2001). Additionally, using a rodent model, Nichol et al. (2009) provided experimental evidence that participation in exercise causes cognitive benefits that are larger for ApoEe4 carriers than non-carriers. Thus, ApoE genotype may be a determinant of who benefits the most from a physical activity intervention. However, there is no published study with humans in which the effects of a physical activity intervention on cognition have been tested relative to ApoE genotype.
In addition to not fully understanding who benefits the most from PA, we also do not fully understand how physical activity benefits cognitive performance. Circulating brain-derived neurotrophic factor (BDNF) is one plausible mechanism of the effect of physical activity on cognitive performance. BDNF can be measured centrally in the brain (cBNDF), or peripherally in the blood (pBDNF). BDNF is a neurotrophic factor that is important for neuronal survival, growth, and maintenance and has been implicated in the consolidation of memory in animals (Johnston & Rose, 2001; Mu, Li, Yao, & Zhou, 1999; Patterson et al., 1996; Tang et al., 1998) and in humans (Egan, Weinberger, & Lu, 2003; Hariri et al., 2003). Although the results are not unequivocal, there is evidence that BDNF crosses the blood-brain barrier (Pan, Banks, Fasold, Bluth, & Kastin, 1998; Poduslo & Curran, 1996) and there is recent evidence that cBDNF correlates with pBDNF (Rasmussen et al., 2009; Seifert et al., 2010). With regards to AD, pBDNF decreases during the course of AD (Laske et al., 2006) and higher pBDNF is associated with slower decline in AD (Laske et al., 2011). Importantly, in response to physical activity, increases in pBDNF are observed (Ferris, Williams, & Shen, 2007; Gold et al., 2003; Griffin et al., 2011; Vega et al., 2006) and there is evidence that brain tissue is the major contributor to the concentration of BDNF in the periphery (Rasmussen et al., 2009). Although the effects of chronic physical activity on BDNF are not consistent (Knaepen, Goekint, Heyman, & Meeusen, 2010; Zoladz & Pilc, 2010), there is some evidence that chronic physical activity increases pBDNF (Baker et al., 2010; Knaepen et al., 2010; Seifert et al., 2010; Zoladz et al., 2008) and a recent systematic review of randomized control trials conducted with older adults supports that chronic exercise increases resting levels of pBDNF (Coelho et al., 2013). Animal research also supports pBDNF as a mediator of the effects of physical activity on cognitive performance (Cotman & Berchtold, 2002; Cotman & Engesser-Cesar, 2002; Nichol et al., 2009; Vaynman, Ying, & Gomez-Pinilla, 2003, 2004), and Nichol et al. (2009) reported that changes in levels of BDNF receptors in response to physical activity were only evident for ApoEe4 carriers. In sum, this evidence supports the possibility that pBDNF is a mechanism which could explain the effects of physical activity on cognitive performance (how) and which could potentially explain the moderating role of ApoE on this effect (who).
The purpose of this manuscript is to describe the design, methods, and recruitment strategies for the Physical Activity and Alzheimer’s Disease Study (the PAAD study). The PAAD study was designed to identify the extent to which changes in cognitive performance that result from 8-months of physical activity differ as a function of a person’s ApoE genotype. Given that there is no published study in which the moderating role of ApoE has been tested relative to a physical activity intervention, the provision of a detailed description of this study is meant to give guidance to future research in this area. Considerations made in the design of the study, detailed methods, recruitment efforts and effectiveness, and feasibility information regarding the recruitment of ApoEe4 carriers for the study are presented herein. This information is intended to inform subsequent research exploring physical activity relative to genetic risk factors in general and relative to ApoE in specific.
Materials and Methods
Overview
Older adults with a family history of AD were recruited to participate in an 8-month physical activity program. To minimize experimenter and participant burden, research staff initially assessed an interested person’s eligibility for the study during a telephone interview. Eligible participants then visited the University of North Carolina at Greensboro campus for baseline testing which consisted of additional screening instruments, provision of demographic information, performance of the cognitive test battery, and provision of a buccal sample for ApoE genotyping. Participants who qualified for the study were invited in three cohorts to participate in the physical activity program. These individuals completed a pre-test in the two weeks immediately prior to enrollment in the physical activity program, a mid-test during the two-weeks surrounding the completion of 4-months of exercise, and a post-test during the two weeks immediately following the 8-months of exercise. At these sessions, participants provided blood samples to assess resting pBDNF, performed the cognitive test battery, and performed a submaximal aerobic fitness test. The physical activity program was individualized and progressive and consisted of walking and strength training exercises.
We did not include a no-treatment control group in the current study. This decision was based upon the exploratory nature of this initial study and the financial and time limits of the funding mechanism. This decision is further motivated by the considerable past research (see Angevaren et al., 2008; Colcombe & Kramer, 2003) that has shown that PA interventions have a positive effect on the cognitive performance of older adults. In our current study, we intend to focus on whether ApoE genotype moderates and pBDNF mediates changes in cognitive performance as a function of physical activity participation. We hypothesize that both ApoEe4 carriers and non-carriers will show improvements in cognitive performance across the exercise intervention, but that improvements will be greater for ApoEe4 carriers than for non-carriers. We also hypothesize that changes in resting pBDNF across time will partially mediate observed changes in cognitive performance over time. If findings support the hypotheses, future work will include a no-treatment control group so that conclusions regarding causality will be possible.
Participants
Cognitively normal, community-dwelling adults aged 50-65 years with a self-reported family history of AD were recruited for the study. Adults older than 65 years were not recruited because this would likely result in an over-representation of ApoEe4 non-carriers (Frikke-Schmidt, Nordestgaard, Agerholm-Larsen, Schnohr, & Tybjaerg-Hansen, 2000; McKay et al., 2011). Recruiting persons with a family history of AD was a strategy used to increase the numbers of volunteers who were ApoEe4 carriers. ApoEe4 carriers make up approximately 23% of the general U.S. population age 60 and older (Raber, Huang, & Ashford, 2004). However, based upon our own (Etnier et al., 2007) and other research (Caselli, 2008), recruiting adults with a family history of AD was expected to increase the percent of ApoEe4 carriers to >30%. Cognitive normality was a requirement because we did not want to include persons who were already experiencing clinical cognitive decline.
Recruitment
Recruitment was conducted through a variety of methods particularly targeting audiences in the desired age range and, when possible, persons who might be expected to have a family history of AD. Participants were asked how they had learned about the study to provide information regarding the most effective recruitment avenues.
Genetic information
Although the withholding of genetic information collected as a part of a research study is an issue that is currently under debate (Lee & Lin, 2013), participants in this study were informed that based upon ethical considerations and in compliance with current recommendations (Alzheimer's Association Working Group, 1996), they would not be given information about their genotype. This is because there is currently no treatment for AD and having information about a heightened genetic risk for AD might lead to mental distress. Genotype information was linked to an ID# and was not made available to participants or to intervention or research staff who interacted directly with participants.
Inclusion criteria
Inclusion criteria were assessed during a telephone interview. Eligible participants had to self-report having one first degree family member (mother, father, sister, brother) or two second degree relatives (grandparent, aunt, uncle, niece, nephew) who had been diagnosed with AD. Participants also had to be between 50-65 years of age, able to communicate in English, and sedentary (30 min of moderate intensity physical activity fewer than 5x/week for the last 3 months assessed by self-report) as defined by the Guidelines of the American College of Sports Medicine (ACSM) (2010). We did not include currently active adults because they are less likely to benefit from the intervention, and they are likely to experience ceiling effects on their cognitive performance that would inhibit our ability to test whether ApoEe4 carriers differentially benefit from the intervention.
Exclusion criteria
Participants were excluded if they met the criteria for clinical cognitive impairment, had contraindications to being physically active, self-reported any history of confounding illnesses or the use of medications that influence cognition, or had uncorrectable visual impairment or hearing loss that impacted their ability to be in the study.
Clinical cognitive impairment
Clinical cognitive impairment (MCI, AD, or other forms of dementia) was assessed in two steps. First, during the telephone interview, the modified Telephone Interview for Cognitive Status (TICS-m) was administered. This is a 13-item telephone-administered screening instrument with scores ranging from 0-50. The TICS-m has acceptable sensitivity and specificity in the detection of dementia (Manly et al., 2011) and amnestic MCI (Cook, Marsiske, & McCoy, 2009). Participants who scored <36 were excluded (Cook et al., 2009). Second, at baseline, participants completed the Folstein Mini-Mental Status Exam (MMSE), a 30-point screening tool for global cognitive functioning. Concurrent validity with the Wechsler Adult Intelligence Scale (r=0.66-0.78) and test-retest reliability (r=0.83-0.98) have been established (Folstein, Folstein, & McHugh, 1975). Participants had to score at least 27 and at least 1 out of 3 on the recall subtest to be included in the study (Crum, Anthony, Bassett, & Folstein, 1993). This cut-point is well above the conventional cut-points used to indicate possible cognitive impairment (score >24 for individuals with > 8th grade education or >21 for <8th grade education) and was chosen to reduce the likelihood of including persons with existing cognitive impairment (MCI, dementia) (Caselli et al., 2009).
Contraindications to physical activity
Contraindications to physical activity were also assessed in two stages. This was first assessed during the telephone interview using the American Heart Association/ACSM Health/Fitness Facility Preparticipation Screening Questionnaire (PrepartQ) as per the Guidelines of the ACSM (2010). The second assessment was performed using a Medical Health History (MHH) which was completed during the baseline visit. Participants were judged as unable to perform PA if they reported functional limitations, use of medications that suppress heart rate [i.e., beta-blockers, calcium-channel blockers], active and/or symptomatic heart disease, pulmonary, or metabolic diseases. Additionally, all participants were required to submit a permission to participate form that was signed by their physician.
Confounding conditions
The MHH was also used to identify confounding illnesses or medications that affect cognition. Confounding illnesses that resulted in exclusion included neurologic (e.g., traumatic brain injury, prior stroke, myelopathy, myopathy, peripheral neuropathy), psychiatric (e.g., active major depression, any history of schizophrenia or bipolar disorder), or active severe or functionally disabling neurologic or medical diseases (e.g., Parkinson’s disease, active treatment for cancer), or any other conditions that might limit exercise or pose a danger to the patient.
Uncorrectable visual impairment or hearing loss
This was assessed subjectively during the baseline testing when participants interacted with the research staff.
Peripheral brain-derived neurotrophic factor
Standard protocols were followed with regard to BDNF collection, storage, assays, and analyses. Blood samples were collected from an arm vein at rest (Knaepen et al., 2010). Plasma and serum were separated by centrifugation. Samples were stored at −80° F and analyses for pBDNF will be conducted as cohorts complete the post-test using the Quantikine BDNF enzyme-linked immunosorbent assay (ELISA) (R & D Systems, Minneapolis, MN).
Cognitive Test Battery
We selected tests to assess cognitive performance across a range of cognitive domains and to specifically include tests assessing cognitive abilities sensitive to the early stages of dementia (Lezak, Howieson, & Loring, 2004) and to physical activity interventions (Colcombe & Kramer, 2003). All measures have been used extensively, have well-established psychometrics, are not reliant on a particular reading level, and have age-appropriate normative data adjusted for education level (when relevant). Information processing speed was assessed with the Wechsler Adult Intelligence Scale III (WAIS-III) Digit Symbol Task (raw score). Memory (immediate recall, delayed recall, and retroactive and proactive interference) was measured using the Auditory Verbal Learning Test. Delayed visuospatial memory and constructional praxis were measured using the Rey-Osterrieth Complex Figure Test. Attention was assessed using the Paced Auditory Serial Addition Test (3- and 2-second tests) and forward and backward Digit Span measures from the WAIS-III (raw scores). Executive function was measured using the Trail-Making Test A and B and the Stroop Test. Visuospatial perception was assessed with the Judgment of Line Orientation. Perceptions of the ability to perform real-world cognitive activities was measured with the Pincus Cognitive Symptoms Inventory.
Submaximal Aerobic Fitness
Aerobic fitness is estimated using a submaximal exercise test. Participants walk as quickly and as far as possible around a marked track during a 6-minute period. This submaximal measure of aerobic fitness is appropriate for use with older adults and provides an accurate estimate of VO2peak (Steele, 1996).
Covariates
Identifying individual difference variables that discriminate levels of responsiveness to the intervention may provide important insights for subsequent research. Although sample size limitations will preclude our ability to statistically examine the effects of covariates on the relationship between physical activity and cognitive performance, data on variables that have been identified as important in past research (Abbott et al., 2004; Lytle, Vander Bilt, Pandav, Dodge, & Ganguli, 2004; Weuve et al., 2004; Yaffe, Barnes, Nevitt, Lui, & Covinsky, 2001) were collected at baseline and will be explored to potentially provide guidance for future research. These include: age, education, smoking, medications and supplements, body mass index (BMI), menopausal status, hormone therapy use, and cardiovascular risk factors. Additionally, participants completed the Community Healthy Activities Model Program for Seniors (CHAMPS) Questionnaire (Stewart et al., 2001; Stewart et al., 1997) to assess PA level at baseline so that initial activity level could be considered as a potential covariate. The CHAMPS includes leisure, work, housework, and social activities and has established 3-month stability and construct validity (Harada, Chiu, King, & Stewart, 2001).
Procedures
Interested individuals were contacted by project staff by telephone. After hearing a description of the study, individuals who remain interested answered questions to ascertain initial eligibility for the study. Responses were entered into a web form created specifically for the PAAD study. This form included the script for the experimenters, recorded the data, and was programmed to evaluate participants’ responses using logic statements to determine whether or not a participant qualified for the study and to direct the experimenter to the appropriate final information screen (i.e, eligible or not eligible). Eligible participants were scheduled for baseline testing. Participants who were not eligible were told why they were excluded and, as appropriate depending upon their reason for exclusion, were encouraged to consult with their personal physician and were given contact information for a geriatric nurse practitioner on the research team.
This study was approved by the university’s Institutional Review Board, and participants read and signed an approved informed consent document at the baseline testing session. Participants had their height and weight measured, provided demographic information and information regarding potential covariates, completed the Geriatric Depression Scale (GDS), the PrepartQ, the MHH, and performed the MMSE. They then performed the cognitive test battery and provided two buccal samples (i.e., cheek swabs) that were used to assess ApoE genotype
At the pre-test, mid-test, and post-test, participants first had their blood drawn for the BDNF sample. To control for diurnal and dietary variations in pBDNF, participants were asked to fast for 8 hours and testing begins before 9:00 am. Participants provided a blood sample to assess resting pBDNF. They were then offered a light breakfast that was made available throughout the testing session. Participants then performed the cognitive test battery which was followed by performance of the submaximal exercise test. All experimenters who interacted with participants were unaware of participants’ ApoE genotype.
PA intervention
The design of the PA intervention is based upon meta-analytic evidence (Colcombe & Kramer, 2003) indicating that the largest effects of physical activity on cognition in older adults are observed for programs that include both aerobic and strength training (g=0.59) and last longer than 6 months (g=0.67). Thus, the PA intervention consisted of aerobic exercise (walking) and strength training offered in 35-60 minute sessions.
Exercise sessions were offered from 8-9 am and 12-1 pm on Monday, Wednesday, and Friday and from 6-7 pm on Monday, Tuesday, and Thursday. Subjects were asked to attend 3x/week for 8 months. Each subject walked at a target heart rate of 60% of heart rate reserve calculated using the Karvonen method and a 10-sec heart rate was recorded after 10 min of walking at every session. Periodically, individual data were examined so that the target heart rate could be adjusted, if necessary, for that individual. For strength training, elastic Thera-Bands were used. Subjects began with bands which provided the least resistance, completing one set of 10 repetitions for each of 10-12 exercises. As they demonstrated that they could complete 15 repetitions for any given exercise in proper form, they progressed to the next higher resistance band for that exercise dropping back to eight repetitions and progressing to 15 repetitions over time at the new resistance. Detailed records were kept at every exercise session reflecting the distance and time walked (recorded by the experimenter) and the strength training exercises performed (exercise, band color, sets, repetitions recorded by the participant). Given that we needed to instruct the participants on the proper form for the strength training exercises and on how to record their data, sessions during the first 2 weeks of the program consisted of approximately 20 min of strength training and 15 min of walking. The duration of the strength training portion increased quickly as participants became familiar with the exercises and the duration of walking increased as participants increased their walking capacity. By the 3rd week, sessions included 50-60 min of exercise made up of 20 minutes of walking and approximately 30-40 minutes of strength training. This PA program was chosen because it is inexpensive, safe, and exportable into community older adult programs and into the homes of older adults.
Statistical Analyses
Descriptive data are reported relative to recruitment efforts and outcomes. Feasibility of recruiting a sample for participation in an 8-month physical activity program conducted on the university campus is considered by examining descriptive data to understand reasons for not being included in the study and reasons for dropping out of the study. Feasibility of recruiting sufficient ApoEe4 carriers for statistical comparisons was tested by using a χ2 analysis to compare the proportion of carriers vs non-carriers in the sample to that of the general population. Our ability to recruit a sample of participants representative of the community was assessed in several ways. First, representativeness of the final sample was assessed by comparing drop-outs to those who enrolled in the exercise program. Second, representativeness was considered by comparing the characteristics of participants who expressed interest in the study to those of the population of older adults in the community. Third, comparisons were made between participants enrolled in the exercise program and the population of older adults in the community. In these comparisons, analyses of variance (ANOVA) were used for MMSE, BMI, education, and GDS scores and chi-square analyses were used for ApoE genotype, age group, sex, race, marital status, income, employment, and smoking status.
Results
Recruitment
Recruitment of participants began in October 2012 and continued for 11 months. These efforts were categorized (denoted in brackets) to help identify the most effective recruitment avenues. First described are the recruitment avenues that did not require a direct cost (assessment of experimenter time in the creation of advertising or in the conductance of interviews was not considered as this was built into the grant in terms of percent effort).
The study description was published in a newsletter sent via email to persons involved in the Community A+ Program for Disabled Adults, Adult Health Day Center, In-Home-Aid, and Home Care Agency on two occasions, to approximately 300-400 caregivers in approximately 30-40 agencies on one occasion, and to 94 Adult Day Care members on one occasion [email]. Employers with business offices in the Greensboro area and that employed > 1000 employees in the Triad (Greensboro, Highpoint, Winston-Salem) area (n=16) were identified from the local Business Journal’s 2012 Book of Lists and were contacted to request that information regarding the PAAD study be distributed to employees [email]. The researchers gave presentations on physical activity and health and handed out flyers at a Rotary Club meeting and at an Alzheimer’s support group meeting [talks]. A story describing the study appeared in the three free circulations: Women Go and Do magazine, the Triad Retirement Guide, and the University’s campus newspaper [free ad]. A public service announcement appeared on the local PBS television station and one of the researchers was interviewed on a local television station during the morning news [tv]. NPR aired an interview with the primary investigator twice during the morning commute and once during the evening commute (on a single day) [free ad].
Other recruitment avenues had a direct cost associated with them. Flyers (cost including printing and postage = $263) were delivered through the mail to approximately 200 caregivers on two occasions, were handed out to approximately 400 participants in the Alzheimer’s Association walk (cost for table = $125), and were posted in four Department of Social Services facilities, 14 senior living facilities, 3 physician offices, 3 home health care providers, and 24 churches or synagogues [flyers]. Advertisements for the study were published in the local newspaper on 6 occasions at a total cost of $1784 [paper] and in the local Business Journal on two occasions at a total cost of $746 [journal]. Advertisements were aired on the 2 radio stations that have the highest average rating for adult listeners aged 50-64 years. These advertisements were aired on the radio stations 39 times over a weekly period at a cost of $1089 (WPAW) and 26 times in one week at a cost of $976 (WQMG) [radio]. Advertisements were also aired on the local NPR station 10 times during the morning commute and 10 times during the evening commute over a two-week period (cost = $1000) [radio]. A domain was purchased (cost = $18) and was made accessible through the department’s home page, the University facebook page, and a facebook page created for the study [web]. This web page included study information and a webform that participants could submit to express their interest in the study and contact information. The webpage and webform were created by a staff person at no cost to the study. A click-through was posted for 2 months on a website hosted by the Senior Living Guide ($200) which allowed participants to click to the study web page [SLG].
Data regarding recruitment provided the following information relative to the sources through which participants learned of the study (see Table 1). As is evident from the data, the most effective recruiting method was advertising in the local newspaper. This method of recruitment resulted in the largest number of contacts for the study and also an estimated cost per person that equaled the mean cost for all participants for the study. Notably, the number of participants who reported hearing about the study in the newspaper was larger following the first (n=29) as compared to the second running (n=15) of the advertisement which may suggest diminishing returns. The advertisements on the radio were also effective in that a large number of contacts were obtained, but at a much higher estimated cost per person as compared to the newspaper ads. Of additional interest, the distribution of flyers and the use of a study web page were effective methods of recruitment and the cost per participant was relatively low. The free advertising on the television through the public service advertisement and the interview was also effective and of course this is of great value since there were no costs associated other than the time of the principal investigator who had percent effort built into the grant. Lastly, many participants reported that they heard of the study by word of mouth. Although we cannot identify the particular source that resulted in their knowledge of the study, this does likely suggest the value in using a wide spectrum of recruitment avenues to create a conversation about the study in the community.
Table 1.
Information regarding how participants learned of the study
| Source | Number of participants |
Total cost | Estimated cost per person |
|---|---|---|---|
| Newspaper | 44 | $1784 | $41 |
| Radio | 21 | $3065 | $117 |
| Word of mouth | 22 | ||
| Flyer | 13 | $388 | $30 |
| Webpage | 13 | $18 | $1 |
| Television | 12 | ||
| Free advertisement | 4 | ||
| 4 | |||
| Researcher talk | 1 | ||
| Business journal | 1 | $746 | $746 |
| SLG | 0 | $200 | |
| Can’t remember | 5 | ||
| Not recorded | 11 | ||
|
| |||
| Total | 151 | $6201 | $41 |
Note. Participants for whom the source of information was “not recorded” did not complete any or enough of the telephone interview to be asked this question.
Feasiblity
One of the important considerations in conducting this study in XXXX County and the Triad (XXXX, XXXX, XXXX) area was to determine whether or not it is feasible to recruit sufficient numbers of individuals who meet the eligibility requirements, who are able to attend the physical activity program on a university campus 3 days per week for 8 months, and who (as a sample) provide a good representation of ApoEe4 carriers and non-carriers.
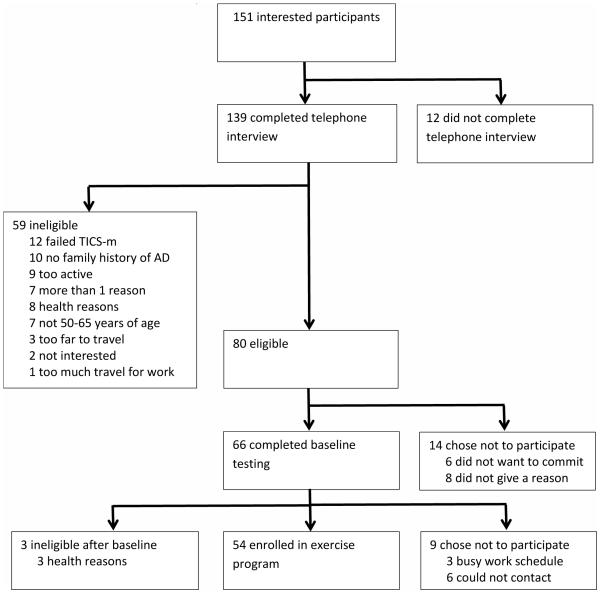
The consort flow diagram (see Figure 1) provides information regarding sample size relative to inclusion/exclusion criteria and interest in participating. The largest percentage of participants was lost as a result of not meeting criteria identified during the telephone interview (42%). Of this group, most did not meet the minimum score for inclusion on the TICS-m (20%), had no family history of AD (17%), or were already too physically active (15%). Of the 80 who were eligible for the study after the telephone interview, 66 completed the baseline testing on campus while 14 decided that they were no longer interested in participating in the study. Of the 66 who completed baseline testing, 54 began the exercise program, 9 chose not to participate, and 3 had to be excluded for health reasons identified during baseline testing (i.e., asthma, major depression, diabetes).
Figure 1.
Consort Flow Diagram.
With regards to genotype, data relative to each specific genotype are presented in Table 2. The recruitment technique resulted in a sample of 54 exercisers consisting of 18 carriers (e3/e4, e4/e4) and 36 non-carriers (e2/e3, e2/e4, e3/e3). Persons with the e2/e4 genotype were considered non-carriers because of evidence that the e2 allele (which is protective against AD) mitigates the risk typically conferred by the e4 allele (Raber et al., 2004). In comparison to the expected distribution of carriers vs. non-carriers (23% vs. 77%) in the U.S. population age 60 and older (Raber et al., 2004), this sample consisted of significantly more carriers (34%) than expected, χ2 (1, n=54) = 6.83, p<.05.
Table 2.
Genotype information for the sample
| Source | % of Baseline (n=66) |
% Exercising (n=54) |
|---|---|---|
| e2/e3 | 14% | 15% |
| e2/e4 | 8% | 9% |
| e3/e3 | 48% | 42% |
| e3/e4 | 24% | 28% |
| e4/e4 | 6% | 6% |
Sample representativeness
Analyses were conducted to identify whether or not there were differences between the 12 participants who were baseline tested, but did not participate in the exercise program and the 54 participants who enrolled in the exercise program. Results of ANOVAs indicated that there were no significant differences between the groups in MMSE, F(1,65)=0.47, p>.05, BMI, F(1,65)=2.95, p>.05, education, F(1,65)=0.001, p>.05, or GDS, F(1,65)=3.13, p>.05. Chi-square analyses showed that there were no significant differences (p>.05) in the distribution of ApoE genotype, χ2(4, n=66)=5.20, sex, χ2(1, n=66)=2.99, marital status, χ2 (2,n=66)=2.24, income, χ2 (5, n=66)=5.82, or employment, χ2 (4, n=66)=1.37, between the groups. There was a significant difference in smoking status, χ2 (2, n=66)=7.92, p<.05, between the groups. However, upon removal of the 3 participants who were excluded from the study due to health reasons, this difference was no longer statistically significant, χ2 (2, n=63)=3.30, p>.05.
Comparisons were also made between the sample who contacted us with interest in participating and data from the 2005-2009 American Community Survey for 50-64 year olds in XXXX County to ascertain the extent to which the recruiting efforts were reaching a representative sample of county residents. Results indicated that there were no significant differences (p>.05) between contacts and XXX County residents for age group, χ2(2, n=111)=2.70, or for ethnicity, χ2(1, n=125)=0.04. However, there were significant differences in race, χ2(4, n=127)=19.68, p<.001, and sex, χ2(1,n=126)=28.77, p<.001. The contacts included more Whites and American Indian or Alaskan natives and fewer Black or African Americans and Asians than expected. The contacts also included more women and fewer men than expected.
Lastly, data were compared between the participants who started the exercise program and relevant population data to identify the extent to which the exercisers are representative of the community of adults ages 50-64. In particular, data on sex, race, ethnicity, and age group (50-54, 55-59, 60-64) were compared to data from the 2005-2009 American Community Survey which provides data for adults between the ages of 50-64 in XXXX County. Similar to the comparison between the contacts and the county data, these results indicated that the exercisers were representative with regards to age group and ethnicity, but were not representative with regards to race or sex (differences in the same direction as previously described).
Demographics for the contacts and for the final sample of 54 participants are displayed in Table 3.
Table 3.
Demographics for the participants who expressed interest in the study (n=127 completed telephone screening) and for those who enrolled in the exercise program relative to data from the 2005-2009 census for XXXX County, XX
| Contacts | Exercisers | XXXX County | |||
|---|---|---|---|---|---|
| Variable | n | % | n | % | % |
| Sex*±! | |||||
| Female | 97 | 76% | 43 | 80% | 51% |
| Male | 30 | 24% | 11 | 20% | 48% |
| Race*±! | |||||
| White | 91 | 72% | 47 | 87% | 63% |
| Black or African American | 26 | 20% | 6 | 11% | 32% |
| American Indian or Alaskan native | 5 | 4% | 0 | 1% | |
| Asian | 1 | 1% | 0 | 3% | |
| Multiracial | 2 | 1.5% | 0 | 1% | |
| Not Reported | 2 | 1.5% | 1 | 2% | -- |
| Ethnicity* | |||||
| Not Hispanic | 118 | 93% | 54 | 94% | |
| Hispanic | 8 | 6% | 0 | ||
| Not reported | 1 | 1% | 0 | 6% | |
| Age Group*^ | |||||
| <50 | 11 | 9% | 4 | 7% | |
| 50-54 | 34 | 27% | 12 | 22% | |
| 55-59 | 45 | 35% | 20 | 37% | |
| 60-64 | 32 | 25% | 17 | 32% | |
| >=65 | 5 | 4% | 1 | 2% | |
| Marital status | |||||
| Married or living with partner | 39 | 72% | |||
| Divorced, separated, or widowed | 10 | 19% | |||
| Never married | 5 | 9% | |||
| Educational status (highest level attained) | |||||
| High school | 6 | 11% | |||
| Associate’s degree | 2 | 4% | |||
| Bachelor’s degree | 26 | 48% | |||
| Graduate degree | 20 | 37% | |||
| Income status | |||||
| <$20,000 | 2 | 4% | |||
| $20,000-$39,999 | 1 | 2% | |||
| $40,000-$59,999 | 6 | 11% | |||
| $60,000-$79,999 | 14 | 26% | |||
| $80,000-$99,999 | 10 | 18% | |||
| >$100,000 | 21 | 39% | |||
| Employment status | |||||
| Fulltime employment | 37 | 68% | |||
| Part-time employment | 3 | 6% | |||
| Homemaker | 4 | 7% | |||
| Retired | 7 | 13% | |||
| Unemployed | 3 | 6% | |||
| Smoking status | |||||
| Never smoked | 37 | 68% | |||
| Past smoker (all quit >16 yrs ago) | 16 | 30% | |||
| Currently smoke (1-2 packs/mo) | 1 | 2% | |||
| Menstrual status (women only) | |||||
| Regular | 3 | 7% | |||
| Irregular | 3 | 7% | |||
| Post-menopausal | 38 | 86% | |||
Note.
=County data from the 2005-2009 American Community Survey 5-year estimates for adults age 50-64;
=significant differences between contacts and XXXX County data;
=significant differences between exercisers and XXXX County data;
Percentages for age groups for the county reflect the number of adults within that specific age group relative to the total number of adults between 50-64.
Discussion
Data collected during the recruitment and initial testing of participants provide guidance for future research of a similar nature. In particular, a total of $6201 was spent on recruitment over 11 months and this resulted in contact by 151 interested people of whom ultimately 54 individuals qualified and remained willing to participate in the study. Considering the 54 enrolled in the exercise program, the cost per person for recruitment was ~$115. It should be remembered, however, that there were many additional efforts that did not cost money and that may have contributed to the number of participants who reported hearing about the study by word of mouth. Given that 14% of those who expressed interest in the study heard about the study by word of mouth, there is support for the need to use all recruitment avenues possible to ensure that as many eligible people as possible become aware of the study. The data from recruitment efforts can also provide guidance as to how to increase the number of people enrolled in the study. For example, the targeted enrollment number for the study was 60. Thus, the data indicate that spending approximately ~$700 more on recruitment may have yielded the targeted enrollment.
Evidence indicates that it is feasible to recruit eligible persons between the ages of 50-65 yrs to participate in an on-campus exercise program for 3 days per week for 8 months. Importantly, the recruited sample consisted of 34% ApoEe4 carriers. Given that the primary research question is to explore differences in the cognitive benefits of the 8-month physical activity program as a function of e4 carrier status, obtaining an acceptable percentage of carriers was critical. The goal in using this recruitment technique was to recruit a sample of which 30% were carriers, hence the strategy of recruiting persons with a family history of AD was effective.
The sample recruited for this study is representative of the community in XXXX County with regards to age group and ethnicity, but was not representative in terms of sex and race. This may limit the generalizability of the findings of this study. With regards to sex, significantly more women were recruited relative to the general population. It is typical of exercise interventions with sedentary older adults to have a larger percentage of female participants as compared to male participants. This is evidenced from observations in 37 randomized controlled trials with older adults (M=68.4 yrs) indicating that approximately 65% of participants have been female. With regards to race, a significantly smaller percentage of our sample consisted of African Americans than would be expected with random sampling of the community. This limitation may have been because we did not advertise effectively to this population. However, given that we targeted our advertisements to persons with a family history of AD, this disparity may also be explained by evidence that African Americans have a lower perceived risk of AD than non-Latino whites (Chung, Mehta, Shumway, Alvidrez, & Perez-Stable, 2009).
The use of the telephone interview was of value in terms of minimizing the burden on potential participants and on research staff. In particular, the telephone interview allowed for the identification of 59 volunteers who were ineligible prior to asking them to visit the university for baseline testing. This was important from the perspective of the grant because it saved approximately 120 hours of time for testing (3 weeks of full time effort) and approximately $700 ($2 for parking and $10 for a gift card for completion of testing). The efficacy of the telephone interview is also evidenced by the fact that only 3 of the 80 (~4%) found eligible after the telephone interview had to be excluded from the study for health reasons. The relatively large number of participants who chose not to participate after the baseline interview (n=14) may indicate that more information regarding the requirements of the study need to be shared during the telephone interview so that participants can more accurately judge their interest in participating in the study.
In sum, initial data support our ability to recruit participants with a family history of AD who meet inclusion criteria and do not meet exclusion criteria and are willing to participate in an 8-month physical activity program. The percentage of ApoEe4 carriers approximated our expectations (obtained 34%, expected 23%), hence giving us sufficient carriers to explore the moderating effect of this variable on the cognitive benefits observed in response to the physical activity program. Generalizability of the results will be limited by our inability to enroll representative numbers of men and African Americans in the study. Future research conducted in this area will need to consider the incorporation of additional or different strategies to improve in this regard.
Acknowledgements
Research reported in this publication was supported by the National Institute of Aging of the National Institutes of Health under award number R21 AG040310-01.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors wish to thank Meg Haas, Devin Buecker, Wade Rothrock, Valen Zhao, and Melvin Gaddy for their assistance with implementing the physical activity program and/or with data entry. The authors also thank the PAAD study participants for their commitment to this study.
References
- Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. Journal of the American Medical Association. 2004;292(12):1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association Working Group Apolipoprotein E genotyping in Alzheimer's disease. Lancet. 1996;347(9008):1091–1095. [PubMed] [Google Scholar]
- Alzheimer's Disease International . In: World Alzheimer Report. Prince M, Jackson J, editors. UKI; London: 2009. [Google Scholar]
- American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. 8th Lippincott Williams & Wilkins; Philadelphia, PA: 2010. [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systematic Reviews. 2008;3:CD005381. doi: 10.1002/14651858.CD005381.pub3. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Archives of Neurology. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nature Reviews Neuroscience. 2008;9(10):768–778. doi: 10.1038/nrn2494. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. American Journal of Public Health. 1998;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nature Reviews Neuroscience. 2009;10(5):333–344. doi: 10.1038/nrn2620. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ. Recruiting efficacy for ApoE genotypes. 2008 Dec 9; 2008. [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Reiman EM. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. New England Journal of Medicine. 2009;361(3):255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedazo-Minguez A. Apolipoprotein E and Alzheimer's disease: molecular mechanisms and therapeutic opportunities. Journal of Cellular and Molecular Medicine. 2007;11(6):1227–1238. doi: 10.1111/j.1582-4934.2007.00130.x. doi: 10.1111/j.1582-4934.2007.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Mehta K, Shumway M, Alvidrez J, Perez-Stable EJ. Risk perception and preference for prevention of Alzheimer's disease. Value Health. 2009;12(4):450–458. doi: 10.1111/j.1524-4733.2008.00482.x. doi: 10.1111/j.1524-4733.2008.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho FG, Gobbi S, Andreatto CA, Corazza DI, Pedroso RV, Santos-Galduroz RF. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Archives of Gerontology and Geriatrics. 2013;56(1):10–15. doi: 10.1016/j.archger.2012.06.003. doi: 10.1016/j.archger.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Cook SE, Marsiske M, McCoy KJ. The use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. Journal of Geriatric Psychiatry and Neurology. 2009;22(2):103–109. doi: 10.1177/0891988708328214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neuroscience. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exercise and Sport Science Reviews. 2002;30(2):75–79. doi: 10.1097/00003677-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. Journal of the American Medical Association. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- Deeny SP, Poeppel D, Zimmerman JB, Roth SM, Brandauer J, Witkowski S, Hatfield BD. Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biological Psychology. 2008;78(2):179–187. doi: 10.1016/j.biopsycho.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Weinberger DR, Lu B. Schizophrenia, III: brain-derived neurotropic factor and genetic risk. American Journal of Psychiatry. 2003;160(7):1242. doi: 10.1176/appi.ajp.160.7.1242. [DOI] [PubMed] [Google Scholar]
- Emery VO. Alzheimer disease: Are we intervening too late? Journal of Neural Transmission. 2011;118(9):1361–1378. doi: 10.1007/s00702-011-0663-0. doi: 10.1007/s00702-011-0663-0. [DOI] [PubMed] [Google Scholar]
- Etnier JL, Caselli RJ, Reiman EM, Alexander GE, Sibley BA, Tessier D, McLemore EC. Cognitive performance in older women relative to ApoE-ε4 genotype and aerobic fitness. Medicine and Science in Sports and Exercise. 2007;39(1):199–207. doi: 10.1249/01.mss.0000239399.85955.5e. doi: PMID: 17218903. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Medicine and Science in Sports and Exercise. 2007;39(4):728–734. doi: 10.1249/mss.0b013e31802f04c7. doi: 10.1249/mss.0b013e31802f04c700005768-200704000-00020 [pii] [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frikke-Schmidt R, Nordestgaard BG, Agerholm-Larsen B, Schnohr P, Tybjaerg-Hansen A. Context-dependent and invariant associations between lipids, lipoproteins, and apolipoproteins and apolipoprotein E genotype. Journal of Lipid Research. 2000;41(11):1812–1822. [PubMed] [Google Scholar]
- Gold SM, Schulz KH, Hartmann S, Mladek M, Lang UE, Hellweg R, Heesen C. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. Journal of Neuroimmunology. 2003;138(1-2):99–105. doi: 10.1016/s0165-5728(03)00121-8. doi: S0165572803001218 [pii] [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, West HL, Rebeck GW, Harr SD, Growdon JH, Locascio JJ, Hyman BT. Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer's disease. Annals of Neurology. 1996;39(1):62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- Griffin EW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiology & Behavvior. 2011;104(5):934–941. doi: 10.1016/j.physbeh.2011.06.005. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychological Medicine. 2009;39(1):3–11. doi: 10.1017/S0033291708003681. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Medicine and Science in Sports and Exercise. 2001;33(6):962–970. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. Journal of Neuroscience. 2003;23(17):6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Archives of Physical Medicien and Rehabilitation. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Johnston AN, Rose SP. Memory consolidation in day-old chicks requires BDNF but not NGF or NT-3; an antisense study. Brain Research. 2001;88(1-2):26–36. doi: 10.1016/s0169-328x(01)00016-x. [DOI] [PubMed] [Google Scholar]
- Kapur S, Sharad S, Kapoor M, Bala K. ApoE Genotypes: Risk factor for Alzheimer’s Disease. Journal, Indian Academy of Clinical Medicine. 2006;7(2):118–122. [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63(3):287–303. doi: 10.1016/j.neuron.2009.06.026. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Bae KY, Kim SW, Yang SJ, Park KH, Yoon JS. Role of BDNF val66met polymorphism on the association between physical activity and incident dementia. Neurobiology of Aging. 2010;32(3):551–e5. doi: 10.1016/j.neurobiolaging.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Medicine. 2010;40(9):765–801. doi: 10.2165/11534530-000000000-00000. doi: 10.2165/11534530-000000000-000004 [pii] [DOI] [PubMed] [Google Scholar]
- Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, Karhunen PJ. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Annals of Neurology. 2009;65(6):650–657. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- Laske C, Stellos K, Hoffmann N, Stransky E, Straten G, Eschweiler GW, Leyhe T. Higher BDNF serum levels predict slower cognitive decline in Alzheimer's disease patients. The International Journal of Neuropsychopharmacology. 2011;14(3):399–404. doi: 10.1017/S1461145710001008. doi: S1461145710001008 [pii]10.1017/S1461145710001008. [DOI] [PubMed] [Google Scholar]
- Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E, Schott K. Stage-dependent BDNF serum concentrations in Alzheimer's disease. Journal of Neural Transmission. 2006;113(9):1217–1224. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. Journal of the American Medical Association. 2008;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- Lee M, Lin JC. Overcoming the obstacles to returning genomic research results. Genetics Research. 2013;95(2-3):45–50. doi: 10.1017/S0016672313000050. doi: 10.1017/S0016672313000050. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th Oxford University Press; New York: 2004. [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. American Journal of Epidemiology. 2002;156(5):445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Disease & Associated Disorders. 2004;18(2):57–64. doi: 10.1097/01.wad.0000126614.87955.79. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Schupf N, Stern Y, Brickman AM, Tang MX, Mayeux R. Telephone-based identification of mild cognitive impairment and dementia in a multicultural cohort. Archives of Neurology. 2011;68(5):607–614. doi: 10.1001/archneurol.2011.88. doi: 68/5/607 [pii]10.1001/archneurol.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay GJ, Silvestri G, Chakravarthy U, Dasari S, Fritsche LG, Weber BH, Patterson CC. Variations in apolipoprotein E frequency with age in a pooled analysis of a large group of older people. American Journal of Epidemiology. 2011;173(12):1357–1364. doi: 10.1093/aje/kwr015. doi: kwr015 [pii]10.1093/aje/kwr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Annals of Neurology. 2010;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Research. 1999;835(2):259–265. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- Myers RH, Schaefer EJ, Wilson PW, D'Agostino R, Ordovas JM, Espino A, Wolf PA. Apolipoprotein E epsilon4 association with dementia in a population-based study: The Framingham study. Neruology. 1996;46(3):673–677. doi: 10.1212/wnl.46.3.673. [DOI] [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimer's & Dementia: The Journal of the Alzeimer's Association. 2009;5(4):287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niti M, Yap KB, Kua EH, Tan CH, Ng TP. Physical, social and productive leisure activities, cognitive decline and interaction with APOE-epsilon 4 genotype in Chinese older adults. International Psychogeriatrics. 2008;20(2):237–251. doi: 10.1017/S1041610207006655. doi: S1041610207006655 [pii]10.1017/S1041610207006655. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553–1561. doi: 10.1016/s0028-3908(98)00141-5. doi: S0028390898001415 [pii] [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16(6):1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. American Journal of Epidemiology. 2005;161(7):639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Molecular Brain Research. 1996;36(2):280–286. doi: 10.1016/0169-328x(95)00250-v. doi: 0169328X9500250V [pii] [DOI] [PubMed] [Google Scholar]
- Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiology of Aging. 2004;25(5):641–650. doi: 10.1016/j.neurobiolaging.2003.12.023. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Experimental Physiology. 2009;94(10):1062–1069. doi: 10.1113/expphysiol.2009.048512. doi: expphysiol.2009.048512 [pii]10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. The Lancet Neurology. 2005;4(11):705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Medicine and Science in Sports and Exercise. 2001;33(5):772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B, Secher NH. Endurance training enhances BDNF release from the human brain. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2010;298(2):R372–377. doi: 10.1152/ajpregu.00525.2009. doi: 00525.2009 [pii]10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- Steele B. Timed walking tests of exercise capacity in chronic cardiopulmonary illness. Journal of Cardiopulmonary Rehabilitation and Prevention. 1996;16(1):25–33. doi: 10.1097/00008483-199601000-00003. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Medicine and Science in Sports and Exercise. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, Sepsis PG, King AC, McLellan BY, Roitz K, Ritter PL. Evaluation of CHAMPS, a physical activity promotion program for older adults. Annals of Behavioral Medicine. 1997;19(4):353–361. doi: 10.1007/BF02895154. [DOI] [PubMed] [Google Scholar]
- Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Mayeux R. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. Journal of the American Medical Association. 1998;279(10):751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122(3):647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. The European Journal of Neuroscience. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vega SR, Struder HK, Wahrmann BV, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Research. 2006;1121(1):59–65. doi: 10.1016/j.brainres.2006.08.105. [DOI] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. Journal of the American Medical Association. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Archives of Internal Medicine. 2001;161(14):1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. Journal of Physiology and Pharmacology. 2010;61(5):533–541. [PubMed] [Google Scholar]
- Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. Journal of Physiology and Pharmacology. 2008;59(Suppl 7):119–132. [PubMed] [Google Scholar]